Abstract
Lay Abstract
We investigated brain chemistry of the primary region of the brain involved in auditory processing in adults with autism spectrum disorder (ASD). Due to the highly heritable nature of ASD and the lack of prior brain chemistry data on unaffected first-degree relatives, we also enrolled parents of children with ASD (pASD), comparing both groups to a healthy adult control group. The technique used to quantify chemical signals was magnetic resonance spectroscopy (MRS), which we used to assess the concentration of auditory glutamate, the primary excitatory brain neurotransmitter, as well as other metabolites that assess neuronal integrity and metabolism. We found significantly higher levels of auditory glutamate in persons with ASD. In addition, increases in two other metabolites, n-acetyl-aspartate (NAA), and creatine (Cr), were observed in the ASD group. No differences were observed in the pASD group in any MRS measurement. We interpret the glutamate finding as suggestive of an increase in brain excitability, and the NAA and Cr findings as indicative of a change in brain energy metabolism in ASD.
Scientific Abstract
Increased glutamate levels have been reported in the hippocampal and frontal regions of persons with autism using proton magnetic resonance spectroscopy (1H-MRS). Although autism spectrum disorders (ASD) are highly heritable, MRS studies have not included relatives of persons with ASD. We therefore conducted a study to determine if glutamate levels are elevated in people with autism and parents of children with autism.
Single-voxel, point resolved spectroscopy (PRESS) data were acquired at 3T for left and right hemisphere auditory cortical voxels in 13 adults with autism, 15 parents of children with autism, and 15 adult control subjects. The primary measure was Glx. Additional measures included n-acetyl-aspartate (NAA), choline (Cho), myoinositol (mI) and creatine (Cr).
The autism group had significantly higher Glx, NAA and Cr concentrations than the control subjects. Parents did not differ from control subjects on any measures. No significant differences in Cho or mI levels were seen among groups. No reliable correlations between autism symptom measures and MRS variables were seen after Bonferroni correction for multiple comparisons.
The elevation in Glx in autism is consistent with prior MRS data in the hippocampus and frontal lobe and may suggest increased cortical excitability. Increased NAA and Cr may indicate brain metabolism disturbances in autism. In the current study, we found no reliable evidence of a familial effect for any spectroscopy measure. This may indicate that these metabolites have no heritable component in autism, the presence of a compensatory factor in parents, or sample specific limitations such the participation of singleton families.
Keywords: glutamate, n-acetyl-aspartate, creatine, spectroscopy, auditory cortex
Introduction
Autism spectrum disorders (ASD) are clinically defined by impairments of social interaction, communication, and restricted/stereotyped behaviors. The prevalence for ASD, which includes Autistic Disorder, Asperger’s Syndrome and Pervasive Developmental Disorder – Not Otherwise Specified, is estimated to be as high as 1.1 percent (Kogan et al., 2009). While diagnosable medical conditions, including genetic syndromes, are estimated to account for as many as 10% of cases, the majority of cases remain idiopathic (Kielinen, Rantala, Timonen, Linna, & Moilanen, 2004; Schaefer & Lutz, 2006).
One perspective on the pathophysiology of ASD is the excitation/inhibition imbalance (EI) theory (Rubenstein & Merzenich, 2003), which proposes that relatively high ratios of excitatory to inhibitory neuronal processes could explain a reasonable portion of the ASD phenotype, particularly including the observed increased prevalence of seizure disorders (Tuchman, Cuccaro, & Alessandri, 2010). Evidence suggesting the possibility of higher than normal neuronal excitability in ASD comes from several lines of evidence, including higher than normal serum glutamate (Shinohe et al., 2006), increased AMPA-type glutamate receptor mRNA (Purcell, Jeon, Zimmerman, Blue, & Pevsner, 2001), increased metabotropic glutamate receptor expression (Fatemi, Folsom, Kneeland, & Liesch, 2011), increased hippocampal glutamate concentration using in vivo proton magnetic resonance spectroscopy (1H-MRS) (Page et al., 2006) and genetic evidence in ASD pointing to the neurexin-neuroligin gene superfamily (Szatmari et al., 2007), whose proteins are important in regulating glutamatergic synapse formation (Chih, 2005). Additional genetic evidence comes from family-based association of the single nucleotide polymorphisms in glutamate transporter genes SLC1A1 and SLC1A2 in ASD (Jacob et al., 2011; Szatmari, et al., 2007). Collectively, these data have come to support what has been termed the so-called “Hyperglutamate Theory” of ASD (Fatemi, 2008), which is consistent with the EI hypothesis.
Conversely, evidence for GABAergic inhibitory deficits converges from a variety of methods and has been of interest for some time (e.g., Hussman, 2001). Blatt et al. (2001) reported significantly reduced GABAA-receptor binding in high binding regions of the hippocampus. This has been extended recently to several areas of cortex and cerebellum (Fatemi, Reutiman, Folsom, & Thuras, 2009). A study of children and adolescents with ASD reported elevated plasma GABA levels (Dhossche et al., 2002). Reduced protein levels of several GABA receptor subunits have been reported in frontal cortex in ASD (Fatemi, et al., 2009). Messenger RNA levels of glutamate decarboxylase (GAD), the enzyme that converts glutamate to GABA and is highly related to intraneuronal GABA, have been reported to be reduced by about 40% in cerebellar Purkinje cells in persons with autism (Yip, Soghomonian, & Blatt, 2007) and up to 50% in parietal and/or cerebellar tissues (Fatemi et al., 2002). Reduced GAD expression also implies a corresponding increase in glutamate neurotransmitter levels. Indeed, Fatemi et al. (2008) suggest this possibility as a potential underpinning of the higher glutamate levels found in autism in their Hyperglutamate theory.
Measurement of in-vivo glutamate and GABA neurotransmitter levels in human subjects is possible using 1H-MRS methods. Page et al. (2006) reported higher hippocampal glutamate concentration in persons with autism. To date, there has only been a single study of GABA in ASD using MRS methods, likely due to the necessity and resulting challenges of using spectral editing techniques that are not needed to measure glutamate. In this study, GABA concentration was lower in the frontal lobe in ASD, and there was a corresponding decrease in the ratio of GABA to glutamate, in line with the predictions based on reduced GAD expression (Harada et al., 2010).
Aside from glutamate, which is normally combined with glutamine in spectroscopy studies (i.e., glutamate+glutamine, aka Glx), standard 1H-MRS studies can also reveal concentrations of other important molecules, including N-acetyl-aspartate (NAA), creatine (Cr), myo-inositol (mI) and choline (Cho). Studies in persons with autism generally have revealed lower NAA concentration (DeVito et al., 2007; Hardan et al., 2008; Hashimoto et al., 1997; Otsuka, Harada, Mori, Hisaoka, & Nishitani, 1999). NAA levels are usually interpreted as a marker of neuronal density and/or mitochondrial function (Clark, 1998), which might suggest decreased neuronal density in ASD, altered mitochondrial function, or both. The former interpretation, however, is somewhat tempered by post-mortem tissue studies that suggest regional variation of neuronal density in ASD, with higher density in regions of the limbic system, including the hippocampus, and reductions in the Purkinje cells of the cerebellum (M. Bauman & Kemper, 1985, 1994; Ritvo et al., 1986). Although Bailey et al. (1998) have reported some changes in neocortical neuronal density, consistent findings have not yet been observed for neocortical regions (e.g., see review by M. L. Bauman & Kemper, 2005).
The current study was aimed at extending the finding of increased glutamate concentration in ASD to the auditory cortex, where we and others have previously reported electrophysiological abnormalities in gamma-band oscillations in ASD suggestive of changes in EI (Gandal et al., 2010; D. C. Rojas, Maharajh, Teale, & Rogers, 2008; Donald C. Rojas et al., 2011; Wilson, Rojas, Reite, Teale, & Rogers, 2007). We therefore predicted increased Glx concentration in persons with ASD relative to healthy control subjects. In addition, due to the high heritability of ASD and the presence of auditory gamma-band abnormalities in first-degree relatives of persons with ASD, we also examined the possibility that there would be corresponding alteration in Glx in a group of parents of children with ASD.
Methods and Materials
Participants
Thirteen individuals with ASD (7 Autistic Disorder, 6 Asperger Syndrome), ages 25 to 48, participated in the study. Each met DSM-IV criteria for ASD, as determined by consensus of the Autism Diagnostic Observation Schedule (Lord et al., 2000), the Autism Diagnostic Interview, Revised (ADI-R: Lord, Rutter, & Le Couteur, 1994) and DSM-IV diagnosis by an experienced clinical psychologist (SH). Six of the ASD group participants were medicated at the time of the study. Five were taking selective serotonin reuptake inhibitor (SSRI) medications, 2 were on atypical antipsychotic medications and 1 was taking a selective norepinephrine reuptake inhibitor.
A second group was comprised of 15 participants who were parents of a child with autistic disorder (pASD). Each pASD participant had one child who met the same criteria for autism as the ASD group. None of the children of the pASD group were participants in the ASD group because they were too young for the MRI scans at the time of parent recruitment. Three pASD participants were medicated at the time of the study. Three were taking SSRI medications and one was also taking lithium.
Finally, a third group of participants was comprised a healthy control group (HC), which included 15 adults with no personal or family history of developmental disability or other neurological disorder. All control participants were medication free. All participants, including those with autism, had full scale IQs greater than or equal to 80 as determined by assessment with the Wechsler Abbreviated Scale of Intelligence (WASI: (Psychological Corporation, 1999)). Table 1 provides additional details concerning the sample. All signed informed consent to participate in the experiment consistent with the guidelines of the Colorado Multiple Institution Review Board.
Table 1.
Sample Demographics
| HC | pASD | ASD | Results | |
|---|---|---|---|---|
| Age | 41.08 (6.77) | 41.22 (6.87) | 36.89 (6.80) | n.s. |
| Gender (M/F) | 6/9 | 11/4 | 9/4 | n.s. |
| FSIQ | 114.67 (11.82) | 110.07 (11.53) | 103.55 (16.31) | n.s. |
| SRS | 35.23 (29.61) | 33.38 (17.08) | 88.62 (42.66) | ASD > HC; ASD > pASD |
| AQ | 16.40 (6.11) | 15.79 (6.09) | 29.44 (8.6) | ASD > HC; ASD > pASD |
| SES | 42.40 (12.61) | 47.13 (11.68) | 44.11 (12.56) | n.s. |
Numbers in parentheses are standard deviations.
p < .05.
Degrees of freedom = 39 for all t-tests. FSIQ = Full scale IQ. AQ = Autism-Spectrum Quotient. SRS = Social Responsiveness Scale. SES = Socioeconomic status (Hollingshead, 1975).
Behavioral measures
In addition to the diagnostic measures and WASI, several additional measures were included to assess aspects of the autism phenotype that have been found to be present in first-degree relatives. The Autism-Spectrum Quotient (AQ: Baron-Cohen, Wheelwright, Skinner, Martin, & Clubley, 2001) is a self-administered scale of autism symptoms with scores ranging from 0–50 (higher scores are more indicative of traits associated with autism). The Social Responsiveness Scale (SRS: Constantino & Todd, 2005) is an informant-based (spouse/partner/parent) measure of reciprocal social behaviors. Scores on the SRS range from 0 (no problems) to 195 (severe problems) indicating degree of social reciprocity problems. Two HC group subjects were missing the SRS. One parent and 2 ASD group participants did not have AQ assessments and their data are excluded from any analyses involving those two variables below. The Hollingshead 4-factor index of social position (Hollingshead, 1975) was obtained as a measure of socioeconomic status (SES), with higher values indicating higher SES.
MR and MRS Acquisitions
MR images and spectra were acquired using a 3.0T GE Signa HDx whole body, long-bore MR scanner (GE Healthcare, Waukesha, WI). Subjects were imaged in the supine position using a GE 8-channel phased array head coil. Subjects were allowed to watch a movie during the exams using MR compatible goggles and headphones (Resonance Technology Inc., Northridge, CA) during the procedure, which aided in compliance and helped minimize subject discomfort and motion. The imaging protocol for localization of the spectroscopy voxels included an initial 3-plane scout, followed by an oblique axial T2-weighted FSE (FOV 22 cm, TE/TR = 95/5000 ms, echo train length (ETL)= 20, slice thickness/gap = 3/0 mm, ~20 slices, matrix 512 × 256, 1 nex, flow compensation (slice), time = 45 s), angled to the AC-PC (anterior commissure - posterior commissure) line and a sagittal series with similar parameters. An oblique coronal image was then prescribed off the axial series to be parallel to the plane of Heschl’s gyrus, the first transverse temporal gyrus (TE/TR 105/2000 ms, ETL = 26, slice thickness = 3.0, 1 slice, matrix 512 × 256, 1 nex, time = 16 s). This image was then used to place the spectroscopy voxel, aided by the axial and sagittal scout series, such that the spectroscopy voxel was angled to the auditory cortex. To increase voxel placement reliability, Heschl’s gyrus was identified using criteria previously published by our group for volumetry (D. C. Rojas, Teale, Sheeder, Simon, & Reite, 1997). GE’s PROBE-P (PRESS) sequence was used for the MRS acquisitions with parameters TR/TE= 2000/30, 192 averages, phase cycling nex =8, voxel size ~ 25×15×20 mm, time = 432 s. The voxel placement and example spectrum are shown in Figure 1. A separate prescan and shim were used for each hemisphere.
Figure 1.
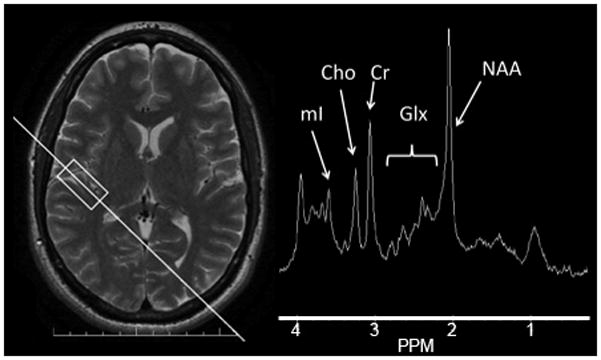
1H-MRS example. Image on left is axial localizer image illustrating voxel of interest (rectangle) and prescription plane for additional localizer (not shown) for spectroscopy voxel. Voxel is aligned with first transverse temporal gyrus (Heschl’s gyrus). Spectrum obtained from the prescribed voxel is shown on the right, with arrows showing the signals of interest in the study.
All spectra in the study were processed using the LCModel software, version 6.2-1Q (Provencher, 1993), which uses Bayesian analysis starting with solution spectra basis sets to provide estimates of metabolite concentrations without operator bias. The unsuppressed water signal, collected automatically by the PROBE sequence, was used by LCModel as a reference for the metabolite quantification. Although the repetition time (TR) used in this study was too short for fully relaxed spectra, relaxation corrections were not applied, since T1 and T2 were not measured in individuals and may vary in pathology compared to normal brain. Relaxation weighting in LCModel results depends on the difference between the relaxation constants in vivo and in the basis set. The main effect is that the T2 weighting of water in vivo leads to metabolite concentrations being overestimated. For this reason, the reported concentrations should be considered as being in “institutional units” rather than absolutely determined. LC Model also provides error estimates for each metabolite (given as % standard deviations (SD) but actually Cramer-Rao lower bounds (CRLB)). LCModel estimates with errors higher than 20% are generally not considered reliable. Reliable values, with a range of errors of 2–3% for total NAA (n-acetyl aspartate + n-acetyl aspartyl glutamate), total choline (choline, glycerophosphocholine and phosphocholine, Cho), total creatine (phosphocreatine and creatine, Cr), myoinositol (mI), errors between 6–8% for the combined glutamate+glutamine (Glx), and errors betwen 8–10% for glutamate (Glu), were obtained from these analyses. The largest error for any value from any spectra used in this study was <15%. The average signal to noise (SNR) ratio for the spectra in the study (obtained from LCModel output) was 32.5.
Statistical Analyses
SPSS version 19 was used for statistical analysis. Reviewer concern over the modest sample size in the study motivated us to conduct statistical analyses using non-parametric statistical tests to avoid assumptions about normality of the distribution. All null hypothesis significance tests were conducted at p < .05 for significance. For 2-group comparisons, the Mann-Whitney U test was employed, and for 3-group comparisons, a Kruskal-Wallis one-way analysis of variance by ranks was used, followed by Dunn’s multiple comparison tests. Tests of proportion were conducted using chi-square tests. Correlations were computed using Spearman Rank Order Correlation coefficients.
Results
Sample Characteristics
There were no significant differences in age between groups, H(2) = 3.55, p > .1. Because age has been found in some prior studies to correlate with metabolite concentrations, we computed correlation coefficients between all MRS variables and age. None of the correlations were significant (all p > .05), even without Bonferroni correction for multiple comparisons. No significant differences between groups were present for SES, H(2) = .61, p = .74.
Gender differences between groups were examined using a chi-square test. Although there were more males in the ASD group than the other two groups, no significant differences in the gender distribution were present, χ2 (df = 2) = 4.08, p > .1. Although there were not enough males and females in each group to examine the impact of gender on MRS variables within a factorial design, gender irrespective of group was analyzed as a potential confound in analyses described below.
IQ and Autism Symptom Scales
There was also no full-scale IQ difference between groups, H(2) = 1.67, p > .4. The SRS was significantly different between groups, H(2) = 12.80, p = .002, with scores for both HC and pASD groups significantly lower than those of the ASD group (p = .006 for both pairwise comparisons). The AQ also differed significantly between groups, H(2) = 13.53, p = .001, with scores for both HC and pASD groups significantly lower than ASD (p = .003 and .004 respectively).
We also computed correlation coefficients between full scale IQ, the SRS and AQ scores and all MRS variables to assess relationships between metabolites, intellectual ability and symptom dimensions. Significant correlations, uncorrected for multiple comparisons, were present between left NAA and the SRS (ρ = .35, p = .03) left glutamate and the AQ (ρ = .38, p = .02). No correlation remained significant after Bonferroni correction.
MR Spectroscopy
For group differences, each spectroscopy concentration variable (left and right NAA, MI, Glx, Cho, Cr) was entered into separate Kruskal-Wallis tests. We examined the impact of gender on spectroscopy measures by separately analyzing each MRS variable in a Mann-Whitney U Test between genders. There were no significant effects of gender on MRS measures (p > .1 for all cases). Figure 2 illustrates the mean and standard deviation for all spectroscopy measures in each group.
Figure 2.
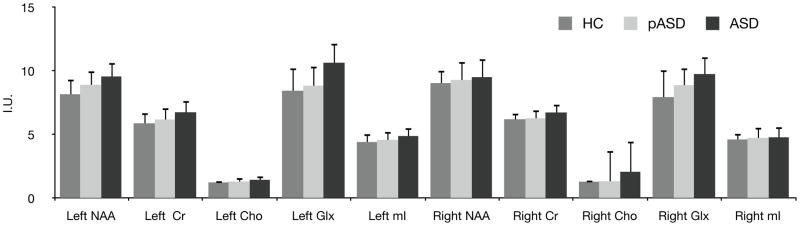
Mean +/− SD 1H-MRS Metabolite Concentrations. Cho = choline; Cr = creatine; Glx = Glutamate + Glutamine; mI = myo inositol; NAA = n-acetyl-aspartate.
Glx and Glutamate
Left hemisphere Glx differed significantly among groups, H(2) = 12.16, p = .002. Subjects in the ASD group had higher left Glx concentrations than either the HC group (p = .003) or the pASD group (p = .02). Because of a prior study suggesting that antipsychotic medications could decrease Glx in persons with schizophrenia (Szulc et al., 2011), we reanalyzed the Glx data excluding the 2 individuals with ASD who were taking atypical antipsychotics and the left hemisphere results remained significant. Right hemisphere Glx also differed significantly between groups, H(2) = 8.63, p = .01. Post-hoc tests indicated that the ASD subjects had higher Glx than HC subjects, p = .01. Glx concentration for individual subjects is shown separately in Figure 3 along with identification of medicated individuals in the study.
Figure 3.
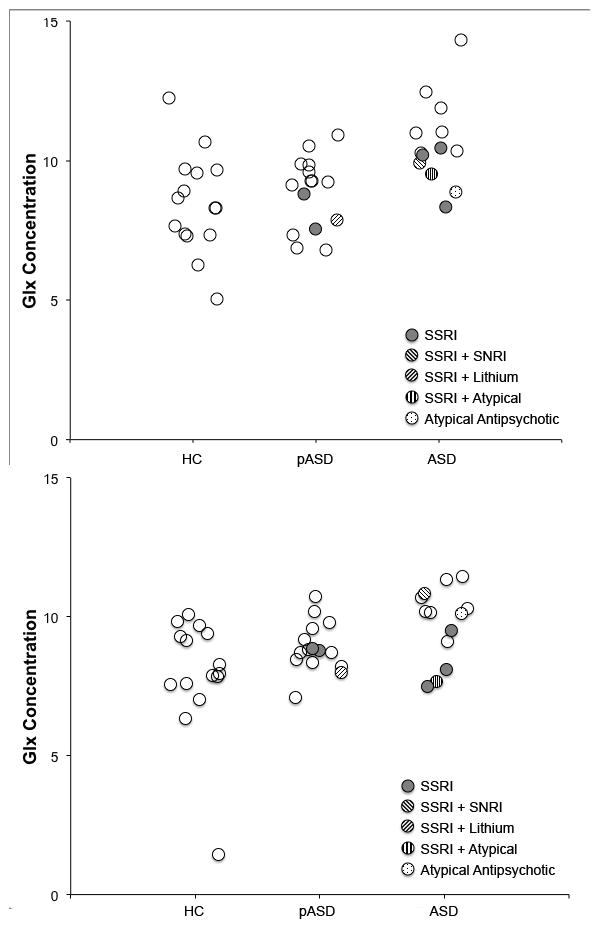
Glx concentration dot plots for left (top) and right (bottom) auditory voxels. Individuals taking various medications at the time of scan are identified. Dots are semi-transparent so that no medicated subjects are hidden by overlap with another dot.
Because the separate estimates of glutamate were deemed reliable from LCModel analyses (see Methods), we also analyzed the glutamate data separately from Glx. Left hemisphere glutamate differed significantly between groups, H(2) = 16.86, p < .001, with both HC and pASD groups having lower glutamate than the ASD group (p < .001 and p < .02 respectively). Right hemisphere glutamate also differed between groups, H(2) = 9.09, p = .01, with the only significant post-hoc test between HC and ASD groups (p = .008).
NAA
Left NAA differed significantly among groups, H(2) = 7.78, p = .02. Subjects in the ASD group had higher NAA concentrations than the HC group (p = .02), but not the pASD group (p = .77). Right NAA did not significantly differ between groups, H(2) = 1.60, p = .45.
Cr
Left Cr differed significantly among groups, H(2) = 8.47, p < .02. Subjects in the ASD group had higher Cr concentrations than both the HC group (p < .02) but not the pASD group (p = .1). Right Cr did not differ significantly among groups, but trended toward a significant difference, H(2) = 5.05, p = .08.
Cho
There were no significant differences for left Cho, H(2) = 4.14, p = .13 or right Cho, H(2) = 3.90, p = .14.
mI
There were no significant differences observed for left mI, H(2) = 3.84, p = .15, or right mI, H(2) = .58, p = .75.
Discussion
We observed significant group effects in the spectroscopy data for Glx, NAA, Cr, all of which were elevated in the ASD subjects (Glx in both hemispheres, NAA and Cr only in the left hemisphere). The elevation in Glx seen in the ASD group corresponds well with prior MRS data in the hippocampus (Page, et al., 2006) and frontal lobe (Harada, et al., 2010), as well as with elevations in serum glutamate levels (Shinohe, et al., 2006). A recent MRS study observed increased Glx in the pregenual region of the anterior cingulate gyrus (Bejjani et al., 2012). One study, however, reported lower Glx concentrations in a much younger sample (DeVito, et al., 2007). Of note, however, that study also used a significantly longer TE (135 ms) than either the current study (30 ms) or that of Page et al. (2006) (35 ms). Values for Glx are less reliable at longer echo times as much of the signal has undergone significant T2 decay by 135 ms. Bernardi et al. (2011) reported reduced Glx concentration in the right anterior cingulate cortex, but did not examine the hippocampus or auditory cortex. It is possible that Glx findings in autism, similar to MRI volumetric changes and other aspects of the ASD phenotype, are age specific. Previous spectroscopy studies in healthy individuals have reported that Glx concentration is negatively correlated with age (Kaiser, Schuff, Cashdollar, & Weiner, 2005; Sailasuta, Ernst, & Chang, 2008). However, the Bernardi et al. study was conducted in young, high-functioning adults with autism spectrum disorder (Bernardi, et al., 2011) and the DeVito et al. study with younger children (DeVito, et al., 2007), both of which found reduced Glx. Taken together with the current results, for which we did not observe age correlations, this suggests that Glx findings may be regionally specific in ASD. Bernardi et al. (Bernardi, et al., 2011) also examined Glx signals in the thalamus, temporo-parietal junction, inter-parietal sulcus and found no significant differences in those regions, supportive of this interpretation. It seems clear from studies that examine multiple regions that there is significant variation in Glx concentration in healthy controls (e.g., see Bernardi, et al., 2011; Sailasuta, et al., 2008). Separate glutamate and glutamine measures are rarely reported in the ASD literature, even at higher field strengths. Since our glutamate data appeared reliable, we also analyzed it and found it to be higher in ASD, suggesting that our Glx measure was driven at least in part by higher glutamate. Further study will be needed to assess the regional specificity of Glx, glutamate and other metabolite changes in ASD. The time to acquire the single voxel spectroscopy data for each region is a technical shortcoming for acquisition of multiple regions in challenging patient populations. Since we limited our acquisition to left and right auditory cortex, which have previously not been the focus of MRS studies of any metabolite in ASD, our findings cannot address this issue.
We also observed significantly higher NAA in the auditory regions bilaterally in ASD subjects. The literature for NAA in autism, like that of Glx and other metabolites, is somewhat mixed. Although the majority of MRS studies have reported lower NAA in at least one studied region in autism (Chugani, Sundram, Behen, Lee, & Moore, 1999; DeVito, et al., 2007; Endo et al., 2007; Friedman et al., 2006; Gabis et al., 2008; Hardan, et al., 2008; Hisaoka, Harada, Nishitani, & Mori, 2001; Levitt et al., 2003; Otsuka, et al., 1999), this is not a universal finding among regions even within the same study. To our knowledge, higher NAA has been reported in only one prior study (Murphy et al., 2002), although other studies do not find NAA differences between ASD and comparison groups (Bernardi, et al., 2011; Harada, et al., 2010; Kleinhans et al., 2009; Page, et al., 2006; Vasconcelos et al., 2008; Zeegers, van der Grond, van Daalen, Buitelaar, & van Engeland, 2007). Ages of participants are widely variable among the published studies, but could also potentially contribute to the mixed findings for NAA, as NAA (NAA/Cr and NAA/Cho) is reported to increase dramatically from 1 month to 3 years, with changes observed until 16 years (van der Knaap et al., 1990). Age effects have been noted in previous NAA studies (O’Brien et al., 2010), and a recent meta-analysis of spectroscopy studies in autism found that while there was evidence of frontal lobe NAA reduction in children with ASD, the reduction was not evident in adult samples (Aoki, Kasai, & Yamasue, 2012). Reporting of NAA ratios to Cr and/or Cho is common among the cited studies, and there is some evidence for increases in Cr and Cho in ASD (Levitt, et al., 2003; Vasconcelos, et al., 2008). In addition, there is wide variance in the sites for voxel placements, and since most of the studies done have used single voxel techniques rather than magnetic resonance spectroscopic imaging (MRSI) studies, the issue of regional variance in both healthy and autism samples places an important constraint on pooling observations about metabolites across studies. Our NAA results suggest that for this sample of subjects, both auditory cortices had differences in neuronal numbers, mitochondrial function or another anatomical difference in that region of the brain.
The Cr peak at 3.03 ppm contains contributions from both creatine and phosphocreatine, both involved with cellular energy production and creating ATP. In most studied conditions, the overall levels of Cr as measured with MRS tend to not be significantly altered from normal. This stability is why many spectroscopy studies use ratios of metabolites compared to Cr, such as NAA/Cr (Soares & Law, 2009). Our finding of elevated Cr indicates suggests, however, that the overall cellular energy metabolism may be elevated in the ASD group. Previous studies have also reported significantly elevated Cr in ASD, in the hippocampal/amygdala complex (Page, et al., 2006) and the medial prefrontal cortex (Murphy, et al., 2002). Creatine is not synthesized in the brain, but rather in the liver, and is then transported to the brain. It has been shown in vitro that glial cells contain a two-to fourfold higher concentrations of creatine than do neurons (Urenjak, Williams, Gadian, & Noble, 1993). Therefore, higher levels of creatine may indicate that the glial/neuron ratio is higher in the ASD group than in the control group. As mentioned, it is common practice as well in proton spectroscopy studies of autism to use Cr as a reference signal for NAA. As others have reported increased Cr signal in ASD (Levitt, et al., 2003; Page, et al., 2006), taken together with the current findings this suggests caution in using ratio measurements for spectroscopy measurements in autism studies without examination of potential group differences in both measures comprising the ratio.
In the current study, we found no statistically reliable evidence of a familial effect for any of the 1H MRS measures, although we note that for the main findings the means for the parent group are in between the control and autism groups. The lack of significance may be due to several factors, including little to no familial component to auditory metabolites in autism, compensatory factors in first-degree relatives, sample specific limitations such as the use of primarily singleton families, or smaller effect sizes. Sample size was modest, which motivated the use of non-parametric statistics (results were identical, however, when using a general linear model approach). Nonetheless, the predicted intermediate effect size for first-degree relatives may require a larger sample. It is also noteworthy that the parent sample in this study did not exhibit elevated SRS and AQ scores, suggesting that may not have a high loading of autism-like traits (e.g., BAP). Previous studies have shown that the BAP is expressed more strongly in multiplex autism families than in singleton families (Losh, Childress, Lam, & Piven, 2008; Virkud, Todd, Abbacchi, Zhang, & Constantino, 2009). As with any potentially heritable component, it would be expected that not all 1st degree relatives would exhibit the deficit, so the lack of finding here could also be due to lack of statistical power to detect an effect. The use of larger samples, directly comparing relatives from singleton and multiplex families, would be desirable.
Overall, the principal finding of the study, increased auditory glutamate in autism, is consistent with the predictions of imbalanced amino-acid neurotransmission suggested by the EI imbalance theory of ASD (Rubenstein & Merzenich, 2003). Although MRS of GABA, the major CNS inhibitory amino-acid transmitter, is more technologically challenging than Glx/glutamate, evidence for GABAergic dysfunction is high in ASD (Coghlan et al., 2012). The only published 1H-MRS paper on GABA to date in ASD suggests decreased GABA concentration and reduced glutamate to GABA ratio in the frontal lobe (Harada, et al., 2010). Current interest in EI theory has also sparked renewed focus on pharmacological interventions with putative action on either glutamatergic, GABAergic, or both transmitter systems (Chez et al., 2007; Erickson et al., 2011; Gürkan & Hagerman, 2012). Advances in 1H-MRS, particularly with respect to GABA, and application to ASD in conjunction with clinical trials designed to affect EI balance, is encouraged for future consideration.
Limitations
One limitation in our study is that we did not obtain fully relaxed spectra in our subjects, and the reported concentration differences could possibly be due to differences in the relaxation parameter T1. This is common in spectroscopy studies as the very long repetition time (TR) required for fully relaxed spectra (~ 10 secs) results in prohibitively long acquisitions. However, the TR used here (2500ms) is somewhat longer than is commonly used for spectroscopy studies at 3T (2000ms), and thus our results have somewhat less T1 weighting than many other studies.
Another limitation is that we did not assess voxel compositions for possible variations in water content and/or gray white matter volume between subjects. The increases in Glx, NAA and Cr, combined with lack of change for mI and Cho, could be consistent with increased neuronal density in the auditory cortices of ASD. MR morphometry and neuropathological studies of the auditory cortices could help clarify this possibility, although MR morphometry cannot currently distinguish between neuronal and non-neuronal cell types.
A final limitation was that nearly half of the participants in the autism group were medicated, while none in the control group were, at the time of their MRI scan. The effect of medications on proton spectroscopy is not well understood. SSRI treatment is common in ASD. Two prior studies have examined SSRI effects on 1H-MRS. Kaymak et al. (2009) examined single voxel 1H-MRS at baseline and following 8-weeks of antidepressant treatment (various SSRI medications) in dorsolateral prefrontal cortex, reporting an increase in mI/Cr ratio after treatment in medication-naïve individuals with major depressive disorder. NAA/Cr and Cho/Cr ratios, the other metabolites considered in that study, were not significantly affected by SSRI treatment. Taylor et al. (2010) reported no effect of 10 days of citalopram treatment (an SSRI used by 3 of our participants) on medial prefrontal cortex Glx, glutamate, mI, Cho, NAA or Cr in a placebo-controlled study involving 23 participants with no history of mental illness. Taken together, the 2 studies suggest few, if any, effects of SSRI medications on 1H-MRS metabolites included in our study and differing between groups. For antipsychotic medications, one study examined the effect of medications (quetiapine and haloperidol) on NAA, Cho and Cr in a medication trial in schizophrenia patients (Bustillo et al., 2008). No changes in these three metabolites were noted after initiation of antipsychotic medications. A recent study by Szulc et al. (2011) observed a decrease in temporal lobe Glx/Cr ratio after antipsychotic treatment (varying medications) in schizophrenia patients. Exclusion of the 2 individuals with ASD who were taking antipsychotic medications at the time of study, however, did not alter the significant increase in Glx observed in the ASD group relative to controls. Although it does not appear that SSRI medications alter 1H-MRS metabolites, some caution is warranted in interpreting all of the differences we observed as attributable entirely to ASD diagnosis. In particular, ASD studies should be careful about antipsychotic medication effects on Glx.
Acknowledgments
Grant information: NIH/NIMH grant R01 MH082820 and NIH/NCRR Colorado CTSI grant UL1 RR025780.
Supported by NIH/NIMH grant R01 MH082820 and by NIH/NCRR Colorado CTSI grant UL1 RR025780. Contents are the authors’ sole responsibility and do not necessarily represent official NIH views.
Footnotes
The authors of the manuscript declare that they have no conflict of interests to report regarding this manuscript.
References
- Aoki Y, Kasai K, Yamasue H. Age-related change in brain metabolite abnormalities in autism: a meta-analysis of proton magnetic resonance spectroscopy studies. Translational psychiatry. 2012;2:e69. doi: 10.1038/tp.2011.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey A, Luthert P, Dean A, Harding B, Janota I, Montgomery M, et al. A clinicopathological study of autism. Brain: a journal of neurology. 1998;121(Pt 5):889–905. doi: 10.1093/brain/121.5.889. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Wheelwright S, Skinner R, Martin J, Clubley E. The autism-spectrum quotient (AQ): evidence from Asperger syndrome/high-functioning autism, males and females, scientists and mathematicians. J Autism Dev Disord. 2001;31(1):5–17. doi: 10.1023/a:1005653411471. [DOI] [PubMed] [Google Scholar]
- Bauman M, Kemper TL. Histoanatomic observations of the brain in early infantile autism. Neurology. 1985;35(6):866–874. doi: 10.1212/wnl.35.6.866. [DOI] [PubMed] [Google Scholar]
- Bauman M, Kemper TL. Neuroanatomic observations of the brain in autism. In: Bauman M, Kemper TL, editors. The Neurobiology of Autism. Baltimore: Johns Hopkins University Press; 1994. pp. 119–145. [Google Scholar]
- Bauman ML, Kemper TL. Neuroanatomic observations of the brain in autism: a review and future directions. Int J Dev Neurosci. 2005;23(2–3):183–187. doi: 10.1016/j.ijdevneu.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Bejjani A, O’Neill J, Kim JA, Frew AJ, Yee VW, Ly R, et al. Elevated Glutamatergic Compounds in Pregenual Anterior Cingulate in Pediatric Autism Spectrum Disorder Demonstrated by(1)H MRS and (1)H MRSI. PloS one. 2012;7(7):e38786. doi: 10.1371/journal.pone.0038786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardi S, Anagnostou E, Shen J, Kolevzon A, Buxbaum JD, Hollander E, et al. In vivo 1H-magnetic resonance spectroscopy study of the attentional networks in autism. Brain Res Brain Res Rev. 2011;1380:198–205. doi: 10.1016/j.brainres.2010.12.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatt GJ, Fitzgerald CM, Guptill JT, Booker AB, Kemper TL, Bauman ML. Density and distribution of hippocampal neurotransmitter receptors in autism: an autoradiographic study. Journal of Autism & Developmental Disorders. 2001;31(6):537–543. doi: 10.1023/a:1013238809666. [DOI] [PubMed] [Google Scholar]
- Bustillo JR, Rowland LM, Jung R, Brooks WM, Qualls C, Hammond R, et al. Proton magnetic resonance spectroscopy during initial treatment with antipsychotic medication in schizophrenia. Neuropsychopharmacology. 2008;33(10):2456–2466. doi: 10.1038/sj.npp.1301631. [DOI] [PubMed] [Google Scholar]
- Chez MG, Burton Q, Dowling T, Chang M, Khanna P, Kramer C. Memantine as adjunctive therapy in children diagnosed with autistic spectrum disorders: an observation of initial clinical response and maintenance tolerability. Journal of Child Neurology. 2007;22(5):574–579. doi: 10.1177/0883073807302611. [DOI] [PubMed] [Google Scholar]
- Chih B. Control of Excitatory and Inhibitory Synapse Formation by Neuroligins. Science. 2005;307(5713):1324–1328. doi: 10.1126/science.1107470. [DOI] [PubMed] [Google Scholar]
- Chugani DC, Sundram BS, Behen M, Lee ML, Moore GJ. Evidence of altered energy metabolism in autistic children. Prog Neuropsychopharmacol Biol Psychiatry. 1999;23(4):635–641. doi: 10.1016/s0278-5846(99)00022-6. [DOI] [PubMed] [Google Scholar]
- Clark JB. N-acetyl apartate: a marker for neuronal loss or mitochondrial dysfunction. Developmental Neuroscience. 1998;20:271–276. doi: 10.1159/000017321. [DOI] [PubMed] [Google Scholar]
- Coghlan S, Horder J, Inkster B, Mendez MA, Murphy DG, Nutt DJ. GABA System Dysfunction in Autism and Related Disorders: From Synapse to Symptoms. Neuroscience and Biobehavioral Reviews. 2012 doi: 10.1016/j.neubiorev.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantino JN, Todd RD. Intergenerational transmission of subthreshold autistic traits in the general population. Biol Psychiatry. 2005;57(6):655–660. doi: 10.1016/j.biopsych.2004.12.014. [DOI] [PubMed] [Google Scholar]
- DeVito TJ, Drost DJ, Neufeld RW, Rajakumar N, Pavlosky W, Williamson P, et al. Evidence for cortical dysfunction in autism: a proton magnetic resonance spectroscopic imaging study. Biol Psychiatry. 2007;61(4):465–473. doi: 10.1016/j.biopsych.2006.07.022. [DOI] [PubMed] [Google Scholar]
- Dhossche D, Applegate H, Abraham A, Maertens P, Bland L, Bencsath A, et al. Elevated plasma gamma-aminobutyric acid (GABA) levels in autistic youngsters: stimulus for a GABA hypothesis of autism. Med Sci Monit. 2002;8(8):PR1–6. [PubMed] [Google Scholar]
- Endo T, Shioiri T, Kitamura H, Kimura T, Endo S, Masuzawa N, et al. Altered Chemical Metabolites in the Amygdala-Hippocampus Region Contribute to Autistic Symptoms of Autism Spectrum Disorders. Biol Psychiatry. 2007 doi: 10.1016/j.biopsych.2007.05.015. [DOI] [PubMed] [Google Scholar]
- Erickson CA, Early M, Stigler KA, Wink LK, Mullett JE, McDougle CJ. An Open-Label Naturalistic Pilot Study of Acamprosate in Youth with Autistic Disorder. JOURNAL OF CHILD AND ADOLESCENT PSYCHOPHARMACOLOGY. 2011;21(6):565–569. doi: 10.1089/cap.2011.0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatemi SH. The hyperglutamatergic hypothesis of autism. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(3):911. doi: 10.1016/j.pnpbp.2007.11.004. author reply 912–913. [DOI] [PubMed] [Google Scholar]
- Fatemi SH, Folsom TD, Kneeland RE, Liesch SB. Metabotropic glutamate receptor 5 upregulation in children with autism is associated with underexpression of both Fragile X mental retardation protein and GABAA receptor beta 3 in adults with autism. Anat Rec (Hoboken) 2011;294(10):1635–1645. doi: 10.1002/ar.21299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatemi SH, Halt AR, Stary JM, Kanodia R, Schulz SC, Realmuto GR. Glutamic acid decarboxylase 65 and 67 kDa proteins are reduced in autistic parietal and cerebellar cortices. Biol Psychiatry. 2002;52(8):805–810. doi: 10.1016/s0006-3223(02)01430-0. [DOI] [PubMed] [Google Scholar]
- Fatemi SH, Reutiman TJ, Folsom TD, Thuras PD. GABA(A) receptor downregulation in brains of subjects with autism. J Autism Dev Disord. 2009;39(2):223–230. doi: 10.1007/s10803-008-0646-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman SD, Shaw DW, Artru AA, Dawson G, Petropoulos H, Dager SR. Gray and white matter brain chemistry in young children with autism. Arch Gen Psychiatry. 2006;63(7):786–794. doi: 10.1001/archpsyc.63.7.786. [DOI] [PubMed] [Google Scholar]
- Gabis L, Wei H, Azizian A, DeVincent C, Tudorica A, Kesner-Baruch Y, et al. 1H-Magnetic Resonance Spectroscopy Markers of Cognitive and Language Ability in Clinical Subtypes of Autism Spectrum Disorders. Journal of Child Neurology. 2008;23(7):766–774. doi: 10.1177/0883073808315423. [DOI] [PubMed] [Google Scholar]
- Gandal MJ, Edgar JC, Ehrlichman RS, Mehta M, Roberts TPL, Siegel SJ. Validating γ Oscillations and Delayed Auditory Responses as Translational Biomarkers of Autism. Biological Psychiatry. 2010;68(12):1100–1106. doi: 10.1016/j.biopsych.2010.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gürkan CK, Hagerman RJ. Targeted treatments in autism and fragile X syndrome. Research in Autism Spectrum Disorders. 2012;6(4):1311–1320. doi: 10.1016/j.rasd.2012.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada M, Taki MM, Nose A, Kubo H, Mori K, Nishitani H, et al. Non-Invasive Evaluation of the GABAergic/Glutamatergic System in Autistic Patients Observed by MEGA-Editing Proton MR Spectroscopy Using a Clinical 3 Tesla Instrument. Journal of Autism and Developmental Disorders. 2010;41(4):447–454. doi: 10.1007/s10803-010-1065-0. [DOI] [PubMed] [Google Scholar]
- Hardan AY, Minshew NJ, Melhem NM, Srihari S, Jo B, Bansal R, et al. An MRI and proton spectroscopy study of the thalamus in children with autism. Psychiatry Res. 2008;163(2):97–105. doi: 10.1016/j.pscychresns.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T, Tayama M, Miyazaki M, Yoneda Y, Yoshimoto T, Harada M, et al. Differences in brain metabolites between patients with autism and mental retardation as detected by in vivo localized proton magnetic resonance spectroscopy. J Child Neurol. 1997;12(2):91–96. doi: 10.1177/088307389701200204. [DOI] [PubMed] [Google Scholar]
- Hisaoka S, Harada M, Nishitani H, Mori K. Regional magnetic resonance spectroscopy of the brain in autistic individuals. Neuroradiology. 2001;43(6):496–498. doi: 10.1007/s002340000520. [DOI] [PubMed] [Google Scholar]
- Hollingshead AB. Four factor index of social position. New Haven, CT: Yale University; 1975. [Google Scholar]
- Hussman JP. Suppressed GABAergic inhibition as a common factor in suspected etiologies of autism. J Autism Dev Disord. 2001;31(2):247–248. doi: 10.1023/a:1010715619091. [DOI] [PubMed] [Google Scholar]
- Jacob S, Brune CW, Badner JA, Ernstrom K, Courchesne E, Lord C, et al. Family-based association testing of glutamate transporter genes in autism. Psychiatr Genet. 2011;21(4):212–213. doi: 10.1097/YPG.0b013e328341a323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser LG, Schuff N, Cashdollar N, Weiner MW. Age-related glutamate and glutamine concentration changes in normal human brain: 1H MR spectroscopy study at 4 T. Neurobiology of Aging. 2005;26(5):665–672. doi: 10.1016/j.neurobiolaging.2004.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaymak SU, Demir B, Oguz KK, Senturk S, Ulug B. Antidepressant effect detected on proton magnetic resonance spectroscopy in drug-naive female patients with first-episode major depression. Psychiatry and clinical neurosciences. 2009;63(3):350–356. doi: 10.1111/j.1440-1819.2009.01951.x. [DOI] [PubMed] [Google Scholar]
- Kielinen M, Rantala H, Timonen E, Linna SL, Moilanen I. Associated medical disorders and disabilities in children with autistic disorder: a population-based study. Autism. 2004;8(1):49–60. doi: 10.1177/1362361304040638. [DOI] [PubMed] [Google Scholar]
- Kleinhans NM, Richards T, Weaver KE, Liang O, Dawson G, Aylward E. Brief Report: Biochemical Correlates of Clinical Impairment in High Functioning Autism and Asperger’s Disorder. Journal of Autism and Developmental Disorders. 2009;39(7):1079–1086. doi: 10.1007/s10803-009-0707-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogan MD, Blumberg SJ, Schieve LA, Boyle CA, Perrin JM, Ghandour RM, et al. Prevalence of parent-reported diagnosis of autism spectrum disorder among children in the US, 2007. Pediatrics. 2009;124(5):1395–1403. doi: 10.1542/peds.2009-1522. [DOI] [PubMed] [Google Scholar]
- Levitt JG, O’Neill J, Blanton RE, Smalley S, Fadale D, McCracken JT, et al. Proton magnetic resonance spectroscopic imaging of the brain in childhood autism. Biol Psychiatry. 2003;54(12):1355–1366. doi: 10.1016/s0006-3223(03)00688-7. [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Leventhal BL, DiLavore PC, et al. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord. 2000;30(3):205–223. [PubMed] [Google Scholar]
- Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview - Revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders. 1994;25(5):659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Losh M, Childress D, Lam K, Piven J. Defining key features of the broad autism phenotype: A comparison across parents of multiple- and single-incidence autism families. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2008;147B(4):424–433. doi: 10.1002/ajmg.b.30612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy DG, Critchley HD, Schmitz N, McAlonan G, Van Amelsvoort T, Robertson D, et al. Asperger syndrome: a proton magnetic resonance spectroscopy study of brain. Arch Gen Psychiatry. 2002;59(10):885–891. doi: 10.1001/archpsyc.59.10.885. [DOI] [PubMed] [Google Scholar]
- O’Brien FM, Page L, O’Gorman RL, Bolton P, Sharma A, Baird G, et al. Maturation of limbic regions in Asperger syndrome: a preliminary study using proton magnetic resonance spectroscopy and structural magnetic resonance imaging. Psychiatry Res. 2010;184(2):77–85. doi: 10.1016/j.pscychresns.2010.08.007. [DOI] [PubMed] [Google Scholar]
- Otsuka H, Harada M, Mori K, Hisaoka S, Nishitani H. Brain metabolites in the hippocampus-amygdala region and cerebellum in autism: an 1H-MR spectroscopy study. Neuroradiology. 1999;41(7):517–519. doi: 10.1007/s002340050795. [DOI] [PubMed] [Google Scholar]
- Page LA, Daly E, Schmitz N, Simmons A, Toal F, Deeley Q, et al. In vivo 1H-magnetic resonance spectroscopy study of amygdala-hippocampal and parietal regions in autism. Am J Psychiatry. 2006;163(12):2189–2192. doi: 10.1176/appi.ajp.163.12.2189. [DOI] [PubMed] [Google Scholar]
- Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med. 1993;30(6):672–679. doi: 10.1002/mrm.1910300604. [DOI] [PubMed] [Google Scholar]
- Psychological Corporation. The Wechsler Abbreviated Scale of Intelligence. San Antonio, TX: Harcourt Brace and Company; 1999. [Google Scholar]
- Purcell AE, Jeon OH, Zimmerman AW, Blue ME, Pevsner J. Postmortem brain abnormalities of the glutamate neurotransmitter system in autism. Neurology. 2001;57(9):1618–1628. doi: 10.1212/wnl.57.9.1618. [DOI] [PubMed] [Google Scholar]
- Ritvo ER, Freeman BJ, Scheibel AB, Duong T, Robinson H, Guthrie D, et al. Lower Purkinje cell counts in the cerebella of four autistic subjects: initial findings of the UCLA-NSAC Autopsy Research Report. Am J Psychiatry. 1986;143(7):862–866. doi: 10.1176/ajp.143.7.862. [DOI] [PubMed] [Google Scholar]
- Rojas DC, Maharajh K, Teale P, Rogers SJ. Reduced neural synchronization of gamma-band MEG oscillations in first-degree relatives of children with autism. BMC Psychiatry. 2008;8:66. doi: 10.1186/1471-244X-8-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas DC, Teale P, Sheeder J, Simon J, Reite M. Sex-specific expression of Heschl’s gyrus functional and structural abnormalities in paranoid schizophrenia. Am J Psychiatry. 1997;154(12):1655–1662. doi: 10.1176/ajp.154.12.1655. [DOI] [PubMed] [Google Scholar]
- Rojas DC, Teale PD, Maharajh K, Kronberg E, Youngpeter K, Wilson LB, et al. Transient and steady-state auditory gamma-band responses in first-degree relatives of people with autism spectrum disorder. Molecular Autism. 2011;2(1):11. doi: 10.1186/2040-2392-2-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubenstein JL, Merzenich MM. Model of autism: increased ratio of excitation/inhibition in key neural systems. Genes Brain Behav. 2003;2(5):255–267. doi: 10.1034/j.1601-183x.2003.00037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sailasuta N, Ernst T, Chang L. Regional variations and the effects of age and gender on glutamate concentrations in the human brain. Magnetic Resonance Imaging. 2008;26(5):667–675. doi: 10.1016/j.mri.2007.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer GB, Lutz RE. Diagnostic yield in the clinical genetic evaluation of autism spectrum disorders. Genet Med. 2006;8(9):549–556. doi: 10.1097/01.gim.0000237789.98842.f1. [DOI] [PubMed] [Google Scholar]
- Shinohe A, Hashimoto K, Nakamura K, Tsujii M, Iwata Y, Tsuchiya KJ, et al. Increased serum levels of glutamate in adult patients with autism. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30(8):1472–1477. doi: 10.1016/j.pnpbp.2006.06.013. [DOI] [PubMed] [Google Scholar]
- Soares DP, Law M. Magnetic resonance spectroscopy of the brain: review of metabolites and clinical applications. Clinical Radiology. 2009;64(1):12–21. doi: 10.1016/j.crad.2008.07.002. [DOI] [PubMed] [Google Scholar]
- Szatmari P, Paterson AD, Zwaigenbaum L, Roberts W, Brian J, Liu XQ, et al. Mapping autism risk loci using genetic linkage and chromosomal rearrangements. Nat Genet. 2007;39(3):319–328. doi: 10.1038/ng1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szulc A, Galinska B, Tarasow E, Waszkiewicz N, Konarzewska B, Poplawska R, et al. Proton magnetic resonance spectroscopy study of brain metabolite changes after antipsychotic treatment. Pharmacopsychiatry. 2011;44(4):148–157. doi: 10.1055/s-0031-1279739. [DOI] [PubMed] [Google Scholar]
- Taylor MJ, Norbury R, Murphy S, Rudebeck S, Jezzard P, Cowen PJ. Lack of effect of citalopram on magnetic resonance spectroscopy measures of glutamate and glutamine in frontal cortex of healthy volunteers. Journal of Psychopharmacology. 2010;24(8):1217–1221. doi: 10.1177/0269881109105679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuchman R, Cuccaro M, Alessandri M. Autism and epilepsy: historical perspective. Brain Dev. 2010;32(9):709–718. doi: 10.1016/j.braindev.2010.04.008. [DOI] [PubMed] [Google Scholar]
- Urenjak J, Williams SR, Gadian DG, Noble M. Proton nuclear magnetic resonance spectroscopy unambiguously identifies different neural cell types. J Neurosci. 1993;13(3):981–989. doi: 10.1523/JNEUROSCI.13-03-00981.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Knaap MS, van der Grond J, van Rijen PC, Faber JA, Valk J, Willemse K. Age-dependent changes in localized proton and phosphorus MR spectroscopy of the brain. Radiology. 1990;176(2):509–515. doi: 10.1148/radiology.176.2.2164237. [DOI] [PubMed] [Google Scholar]
- Vasconcelos MM, Brito AR, Domingues RC, da Cruz LCH, Gasparetto EL, Werner J, et al. Proton Magnetic Resonance Spectroscopy in School-Aged Autistic Children. Journal of Neuroimaging. 2008;18(3):288–295. doi: 10.1111/j.1552-6569.2007.00200.x. [DOI] [PubMed] [Google Scholar]
- Virkud YV, Todd RD, Abbacchi AM, Zhang Y, Constantino JN. Familial aggregation of quantitative autistic traits in multiplex versus simplex autism. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2009;150B(3):328–334. doi: 10.1002/ajmg.b.30810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson TW, Rojas DC, Reite ML, Teale PD, Rogers SJ. Children and Adolescents with Autism Exhibit Reduced MEG Steady-State Gamma Responses. Biol Psychiatry. 2007;62(3):192–197. doi: 10.1016/j.biopsych.2006.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip J, Soghomonian JJ, Blatt GJ. Decreased GAD67 mRNA levels in cerebellar Purkinje cells in autism: pathophysiological implications. Acta Neuropathol (Berl) 2007;113(5):559–568. doi: 10.1007/s00401-006-0176-3. [DOI] [PubMed] [Google Scholar]
- Zeegers M, van der Grond J, van Daalen E, Buitelaar J, van Engeland H. Proton magnetic resonance spectroscopy in developmentally delayed young boys with or without autism. J Neural Transm. 2007;114(2):289–295. doi: 10.1007/s00702-006-0501-y. [DOI] [PubMed] [Google Scholar]


