Abstract
Hyperinsulinemia increases sympathetic nerve activity and contributes to cardiovascular dysfunction in obesity and diabetes. Neurons of the hypothalamic paraventricular nucleus regulate sympathetic nerve activity through mono- and poly-synaptic connections to preganglionic neurons in the spinal cord. The purpose of the present study was to determine whether hypothalamic paraventricular nucleus neurons mediate the sympathetic response to insulin. Hyperinsulinemic-euglycemic clamps were performed in α-chloralose-anesthetized, male Sprague-Dawley rats (280–420 g) by an infusion of insulin (3.75 mU/kg/min) and 50% dextrose (0.75–2.0 ml/h) for 120 min. At 90 min, insulin significantly increased lumbar sympathetic nerve activity without any change in renal sympathetic nerve activity, heart rate, or blood glucose levels. Inhibition of the hypothalamic paraventricular nucleus with bilateral injection of the GABAA receptor agonist muscimol completely reversed the sympathoexcitatory response. However, direct injection of insulin into the hypothalamic paraventricular nucleus did not alter lumbar sympathetic nerve activity and thereby suggests insulin activates neurons upstream of the hypothalamic paraventricular nucleus. Interestingly, the sympathetic response to insulin was eliminated by hypothalamic paraventricular nucleus injection of the melancortin 3/4 receptor antagonist SHU9119 but unaffected by the angiotensin II type 1 receptor antagonist losartan. A final set of experiments suggests activation of hypothalamic paraventricular nucleus neurons during hyperinsulinemia increases glutamatergic drive to the rostral ventrolateral medulla. Collectively, these findings indicate insulin activates a melanocortin-dependent pathway to the hypothalamic paraventricular nucleus that increases glutamatergic drive to the rostral ventrolateral medulla and alter cardiovascular function.
Keywords: hyperinsulinemia, blood pressure, obesity, pro-opiomelancortin, angiotensin II
Introduction
Insulin contributes to cardiovascular dysfunction in obesity and type II diabetes 1, 2. These consequences are partly mediated by the ability of insulin to act within the central nervous system to elevate sympathetic nerve activity (SNA) 3–6 and alter baroreflex function 7, 8. In both humans and rodents, an acute hyperinsulinemic-euglycemic clamp elevates muscle or lumbar SNA, respectively 3–6. Intracerebroventricular (ICV) administration of insulin in rodents produces similar responses 4. These sympathoexcitatory effects of insulin are prevented by lesion of the anteroventral third ventricle region 9, attenuated by ICV administration of a phosphoinositol 3 kinase inhibitor 10 or angiotensin II type I (AT1) receptor antagonist losartan 11, and are absent in melanocortin-4 receptor deficient mice 12. Blockade of brain melanocortin receptors also prevents the central anorexic effect of insulin 13. Despite these observations, there is a paucity of knowledge regarding the central neural circuitry that mediates the sympathetic and cardiovascular effects of insulin.
Our laboratory recently reported that blockade of glutamatergic receptors in the rostral ventrolateral medulla (RVLM) completely reversed the sympathoexcitatory response to insulin 5. A primary source of glutamatergic input to the RVLM arises from neurons in the hypothalamic paraventricular nucleus (PVN) 14, 15. PVN neurons play a pivotal role in the regulation of SNA and arterial blood pressure (ABP) through mono-and poly-synaptic pathways via the medulla and thoracic and lumbar segments of the spinal cord. Activation of PVN neurons elevates SNA and ABP 16–20, and previous studies have reported that altered PVN neurotransmission contributes to elevated SNA and ABP in several experimental models of hypertension 21. Interestingly, insulin receptors are widely expressed throughout the hypothalamus including the PVN 22. Moreover, PVN neurons also express an abundance of melancortin-4 23 and AT1 24 receptors. Therefore, the purpose of the present study was to initially determine whether PVN neurons mediate the sympathoexcitatory response to insulin, and then subsequently identify the specific mechanism(s) within PVN that elevates SNA during hyperinsulinemia.
Methods
Animals
All of the experimental procedures conformed to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee at the Pennsylvania State College of Medicine. Male Sprague-Dawley rats (250–420 g, Charles River Laboratories) were housed in a temperature controlled room (22±1°C) with a 12-hour:12-hour light-dark cycle. Rats were fed standard chow (Harlan Teklad Global Diet 2018) and given access to deionized water.
General Procedures
Rats were anesthetized with isoflurane (2–3% in 100% O2) and prepared for recordings of ABP, lumbar and/or renal SNA as described elsewhere 5, 25 (see online supplement at http://hyper.ahajournals.org). Animals were artificially-ventilated with oxygen-enriched room air. End-tidal CO2 and body temperature were maintained at 4–4.5% and 37±1 °C, respectively. Rats were placed into a stereotaxic head frame, and a craniotomy was performed to remove bone overlying the cortex to allow access to the PVN. After all surgical procedures were completed, anesthesia was replaced by α-chloralose (50 mg/kg bolus followed by 25 mg/kg/hr, IV). The level of anesthesia was examined by the lack of a withdrawal reflex to a foot pinch. Animals were allowed to stabilize >1 hr before experiments began.
Hyperinsulinemic-Euglycemic Clamp
Baseline values of SNA and ABP were recorded for 20 min. Then, insulin (3.75 mU/kg/min, IV; Humulin R) and a 50% dextrose solution (0.25–2.0 mL/hr, IV) were infused for 150 min. This dose of insulin has been reported previously in our laboratory to produce plasma insulin levels equivalent to those of rats fed a moderately high-fat diet for 13 weeks and/or obese Zucker rats 5. Blood glucose was measured from a drop of arterial blood every 10 min using a standard glucometer (One Touch Ultra). The dextrose infusion rate was adjusted to maintain euglycemia. Control animals were infused with equal volumes of isotonic saline.
At 90 min, one of several compounds was bilaterally injected into the PVN: 1) the GABAA receptor agonist muscimol (5mM), 2) the melanocortin 3/4 receptor antagonist SHU9119 (0.5 mM), 3) the AT1 receptor antagonist losartan (40 mM ), or 4) artificial cerebrospinal fluid (aCSF). Volumes (60 nL) were injected over 5 s using single-barrel glass micropipettes (OD: 30–50 μm) connected to a pneumatic picopump and lowered into the PVN using the following coordinates with reference to bregma: 1.7–1.8 mm caudal, 8.0–8.2 mm ventral, and 0.5–0.7 mm lateral. Doses of various receptor antagonists were based on previous studies 5, 19, 26, 27 and/or confirmed in preliminary experiments to block sympathoexcitatory responses to the respective agonist.
In a final set of experiments, microinjections were performed in the PVN and RVLM of the same animal. The incisor bar was positioned at −11 mm. At 75 min after the start of the hyperinsulinemic-euglycemic clamp, muscimol (5mM) or aCSF was injected bilaterally into the PVN with a micropipette angled 15° rostral from the vertical plane using the same coordinates as described above. At 90 min, the ionotropic glutamate receptor antagonist kynurenic acid (KYN, 25 mM) was injected into the RVLM as described previously in our laboratory 5, 25.
Injection sites were marked with 0.2% rhodamine or FITC beads added to the respective drug. At the end of the experiments, animals were perfused transcardially with 4% paraformaldehyde. Brains were post-fixed in 4% paraformaldehyde and sectioned at 100 μm. Histological analysis was performed using a Nikon Eclipse 90i microscope with appropriate filters.
Central Insulin Injection
To determine whether insulin directly acts on PVN neurons to increase lumbar SNA, various concentrations of insulin (5, 0.5, 0.05, or 0.0005 μU per nL, 60 nL) were microinjected bilaterally into the PVN. ABP and lumbar SNA were recorded for 60 minutes, and blood glucose measured every 30 minutes. The insulin concentrations were based on previous studies using ICV injection of insulin 4, 5, 10 and recalculated because of a minimum 10-fold dilution attributable to the CSF volume of the 3rd ventricle. Animals received 1–2 dose of insulin separated by a minimum of 90 min. For purposes of comparison, insulin (50 mU per 2 μL) was injected into the 3rd ventricle using the following coordinates in reference to bregma: 1.0–1.5 mm caudal, 9.0 mm ventral, 0.5–0.7 mm lateral from the midline, 4° angle from the midsagital plane. At the end of experiments, Evan’s Blue Dye (0.5%, 1 μL) was injected into the 3rd ventricle using the same coordinates; proper placement of the injection was confirmed by presence of the dye in the 3rd and 4th ventricle.
Data Analysis
All data are expressed as mean ± SE. Changes in rectified and integrated (5 s time constant) SNA were calculated by subtracting the background noise after hexamethonium (30 mg/kg, IV). For all variables, 5-minute segments at each time point were compared to three baseline period measurements. All data were analyzed by a 1-or 2- way ANOVA, with repeated measures when appropriate. All post hoc tests were performed with independent or paired t tests with a layered Bonferroni correction. A P<0.05 was statistically significant.
Results
Inhibition of the PVN Reverses the Sympathoexcitatory Response to Insulin
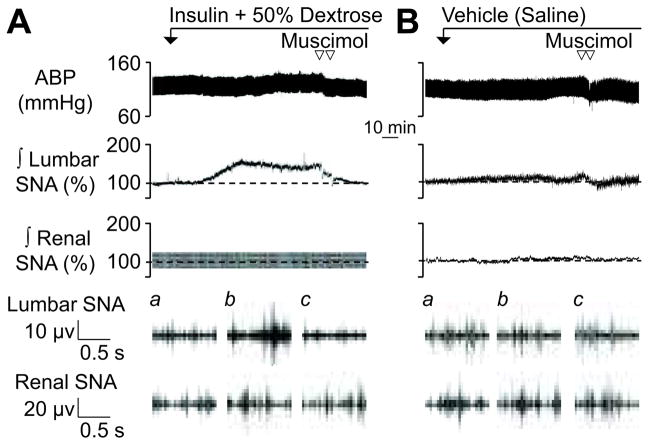
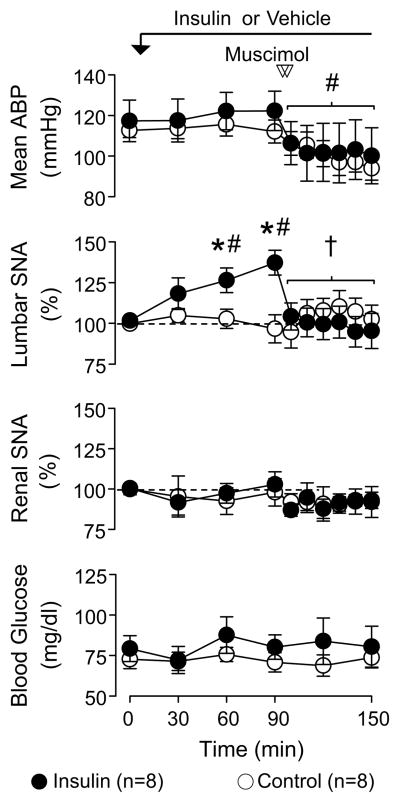
A major goal of the present study was to determine whether the PVN mediates the sympathoexcitatory response to insulin during euglycemia. Figure 1 illustrates a representative example of the sympathetic-cardiovascular response to insulin before and after PVN injection of muscimol or aCSF. Figure 2 summarizes group data. As previously reported 3, 5, a hyperinsulinemic-euglycemic clamp significantly increased lumbar SNA without a change renal SNA or heart rate (data not shown). Although mean ABP was not different between groups, there was a small significant increase in mean ABP of hyperinsulinemic versus control rats at 90 min (Δ: 6±2 vs 0±1 mmHg, respectively; P<0.05). Infusion of saline did not significantly alter any variable.
Figure 1.
Examples of ABP and rectified and integrated lumbar (∫ lumbar) and renal (∫ renal) SNA during PVN injection of the GABAA agonist muscimol in rats receiving (A) a hyperinsulinemic-euglycemic clamp or (B) saline infusion. Traces for raw lumbar and renal SNA represent (a) baseline, (b) 90 min, and (c) post-injection. Group data are presented in Figure 2.
Figure 2.
Mean ± SEM of mean ABP, lumbar SNA, renal SNA, and blood glucose before and after PVN injection of muscimol in rats receiving a hyperinsulinemic-euglycemic-clamp or saline infusion. *P<0.05 insulin vs saline, †P<0.05 vs 90 min values for insulin rats, #P<0.05 vs baseline values
Inhibition of the PVN via bilateral microinjection of the GABAA receptor agonist muscimol significantly reduced lumbar SNA and mean ABP in hyperinsulinemic rats (Figures 1 and 2). In fact, injection of muscimol reduced lumbar SNA to levels that were not different from baseline values or those of saline-infused rats. Although muscimolsignificantly reduced mean ABP in both groups, the magnitude of the decrease in mean ABP was significantly greater in hyperinsulinemic versus saline rats (−21±4 vs −10±3 mmHg, P<0.05). Injection of muscimol did not significantly alter renal SNA (Figures 1 and 2) or heart rate (data not shown). It is noteworthy that injection sites located outside the PVN did not reverse the sympathoexcitatory response to insulin (lumbar SNA at 90 min: 141±10% vs 120 min: 133±9%, n=7; for histology, see online supplement at http://hyper.ahajournals.org).
Bilateral injection of aCSF did not significantly alter any variable in either hyperinsulinemic or saline rats (data not shown).
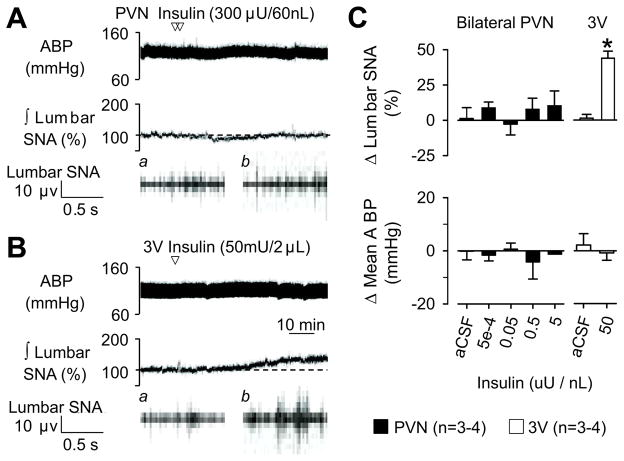
PVN Injection of Insulin Does Not Alter Lumbar SNA
Previous studies indicate that insulin receptors are expressed within the PVN 22. To determine whether insulin may act directly on PVN neurons to increase SNA, we analyzed lumbar SNA responses to microinjection of insulin into the PVN. As illustrated in Figure 3, bilateral injection of various insulin concentrations into the PVN failed to produce a significant increase in lumbar SNA or mean ABP. However, injection of insulin into the adjacent 3rd ventricle significantly elevated lumbar SNA.
Figure 3.
Examples of ABP, ∫ lumbar SNA, and raw lumbar SNA during injection of insulin into the (A) PVN bilaterally or (B) 3rd ventricle (3V). Arrow denotes injection. Traces for raw lumbar SNA represent (a) baseline and (b) 60 min. (C) Summary data (mean ± SEM). There were no differences in baseline mean ABP (116±4 vs 116±3) or heart rate (368±9 vs 385±8) of animals receiving a PVN versus 3V injection, respectively. *P<0.01 vs aCSF
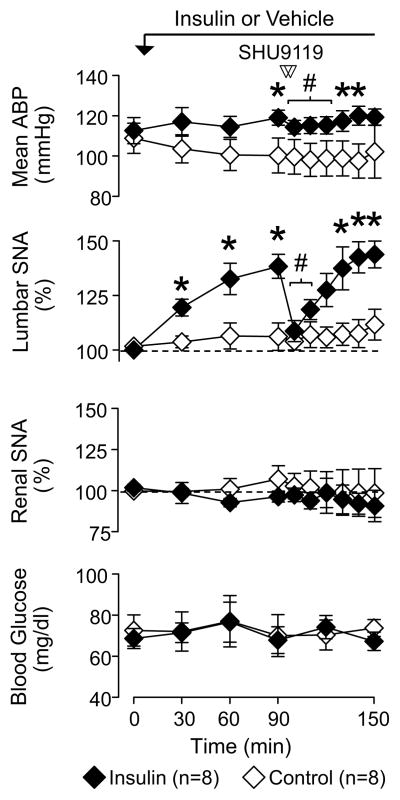
Blockade of PVN Melanocortin 3/4 but not AT1 Receptors Reverses the Sympathoexcitatory Response to Hyperinsulinemia
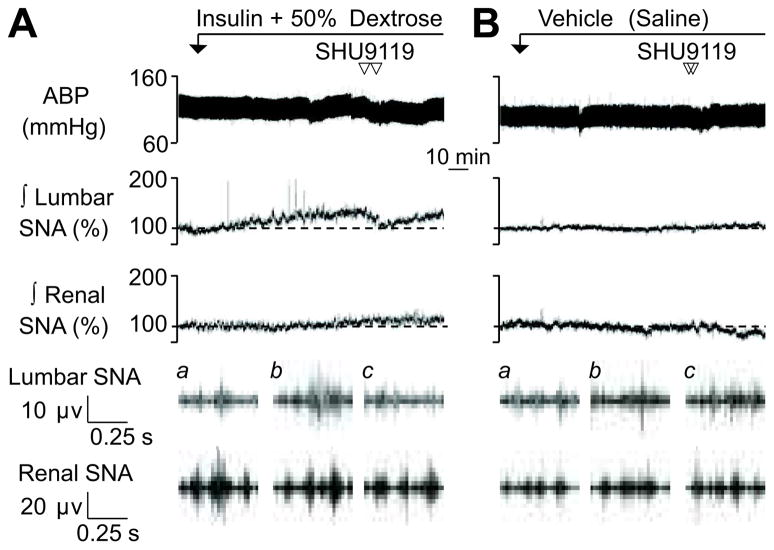
Since inhibition of the PVN reversed the sympathoexcitatory response to insulin but PVN injection of insulin did not raise lumbar SNA, additional experiments sought to determine the specific mechanism(s) within PVN. Bilateral injection of the melanocortin 3/4 receptor antagonist SHU9119 significantly lowered lumbar SNA and mean ABP in hyperinsulinemic rats (Figures 4 and 5). In fact, injection of SHU9119 reduced lumbar SNA to levels that were not different versus baseline values or those of control rats (109±5% vs 100±1% vs 104±3%, respectively). SHU9119 did produce a small fall in mean ABP of hyperinsulinemic versus control rats (Δ: −5±1 vs −1±1 mmHg, respectively; P<0.05), but did not significantly alter renal SNA (Figures 4 and 5) or heart rate (data not shown) in hyperinsulinemic rats. Both lumbar SNA and mean ABP returned to pre-injection values between 120–140 min. Injection of SHU9119 did not alter any variable of control rats. It is noteworthy that injection sites located outside the PVN did not alter lumbar SNA (90 min: 142±11% vs 100 min: 144±13%, n=4; for histology, see online supplement at http://hyper.ahajournals.org).
Figure 4.
Examples of ABP and ∫ lumbar and renal SNA during PVN injection of SHU9119 in rats receiving (A) a hyperinsulinemic-euglycemic clamp or (B) saline infusion. Traces for raw lumbar and renal SNA represent (a) baseline, (b) 90 min, and (c) post-injection. Summary data are presented in Figure 5.
Figure 5.
Mean ± SEM of mean ABP, lumbar and renal SNA, and blood glucose before and after PVN injection of the melanocortin 3/4 receptor antagonist SHU9119 in rats receiving a hyperinsulinemic-euglycemic-clamp or saline infusion. *P<0.05 insulin vs saline, †P<0.05 vs 90 min values for insulin rats.
An additional set of experiments was performed to determine whether the sympathoexcitatory response to ICV administration of insulin was also dependent upon melancortin 3/4 receptor activation within the PVN. ICV administration of insulin (50 mU per 2 μL, n=4) significantly increased lumbar SNA (90 min: 142±10%, P<0.05) and heart rate (0 min: 404±33 bpm vs 90 min: 432±24, Δ: 28±10 bpm, P<0.05) without a change in mean ABP (0min: 96±2 vs 90 min: 99±2 mmHg) or renal SNA (90 min: 99±2%). Bilateral microinjection of SHU9119 significantly reduced lumbar SNA (116±5%, P<0.05) without a significant change in mean ABP (Δ: 0±5 mmHg), renal SNA (Δ: 1±4 %), or heart rate (Δ: −5±3 bpm). Bilateral injection of SHU9119 into the PVN did not affect any variable after ICV injection of aCSF (data not shown).
To test whether PVN injection of SHU9119 non-specifically attenuates all sympathoexcitatory responses evoked from the PVN, N-Methyl-D-aspartic acid (NMDA, 5 mM, 60 nL) was microinjected into the PVN before and 10 min after injection of SHU9119 or aCSF. Unilateral injection of NMDA significantly increased lumbar and renal SNA, heart rate, and mean ABP; however, the magnitude of these responses were unaffected by PVN injection of SHU9119 (Table). In addition, PVN injection of SHU9119 did not affect the sympathoexcitatory response to PVN injection of the GABA receptor antagonist gabazine (2mM per 60 nL, data not shown). PVN Injection of SHU9119 or aCSF did not alter baseline variables.
Table.
PVN Injection of SHU9119 (0.5mM, 60nL) did not alter the sympathoexcitatory responses evoked by PVN injection of NMDA (5mM, 60nL).
| Time | n | Mean ABP (mmHg) | Heart Rate (bpm) | Lumbar SNA (%) | Renal SNA (%) |
|---|---|---|---|---|---|
| aCSF (60 nL) | |||||
| Baseline Values | 5 | 114±9 | 424±23 | -------- | -------- |
| Δ (Before Treatment) | 14±3 | 35±7 | 117±6 | 124±6 | |
| Δ (10 min post) | 19±3 | 41±8 | 122±9 | 128±4 | |
| SHU9119 (0.5 mM, 60 nL) | |||||
| Baseline Values | 5 | 111±6 | 425±24 | -------- | -------- |
| Δ (Before Treatment) | 14±3 | 59±23 | 118±9 | 131±11 | |
| Δ (10 min post) | 19±2 | 55±17 | 128±8 | 131±10 | |
Values are mean ± SEM. NMDA injections were separated by >30 min. Injection of SHU9119 did not attenuate the sympathoexcitatory response to PVN injection of NMDA.
In marked contrast to SHU9119, PVN injection of losartan did not affect the sympathoexcitatory response to insulin (data not shown). A hyperinsulinemic-euglycemic clamp significantly raised lumbar SNA at 90 min (133±2%, n=6), but bilateral injection of losartan did not lower lumbar SNA at 100 min (131±8%), 110 min (145±15%), or 120 min (151±18%). Microinjection of losartan into the PVN did not alter mean ABP in hyperinsulinemic rats (0 min: 115±8 vs 90 min: 120±9 vs 110 min: 119±6 mmHg), and losartan did not affect any variable in control rats (data not shown).
PVN Provides Glutamatergic Drive to the RVLM During Hyperinsulinemia
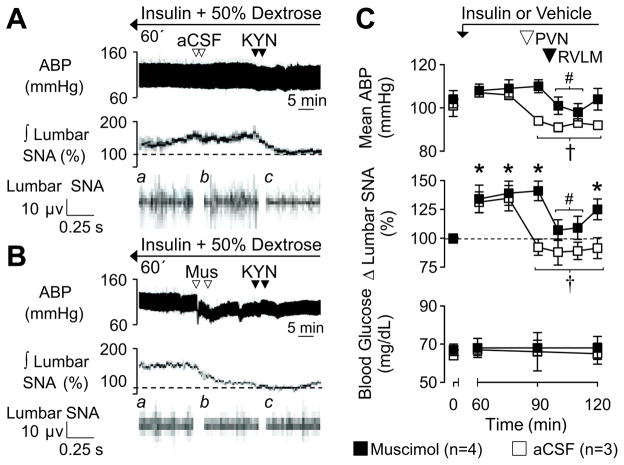
Our laboratory has previously reported that blockade of glutamatergic receptors in the RVLM reverses the sympathoexcitatory response to insulin 5. Therefore, we hypothesized that PVN neurons provide this glutamatergic drive to the RVLM. In hyperinsulinemic-euglycemic rats, PVN injection of aCSF at 75 min did not affect lumbar SNA or mean ABP; however, RVLM injection of KYN significantly lowered lumbar SNA and mean ABP (Figure 6). Prior inhibition of the PVN by injection of muscimol at 75 min significantly lowered lumbar SNA and mean ABP but also prevented any effect of subsequent injection of KYN into the RVLM at 90 min. In control rats (n=4), injection of muscimol into the PVN and KYN into the RVLM did not alter lumbar SNA or mean ABP (data not shown).
Figure 6.
Examples of ABP and ∫ lumbar SNA during PVN injection of (A) aCSF or (B) muscimol at 75 min followed by RVLM injection of KYN at 90 min in hyperinsulinemic rats. Note that the hyperinsulinemic-euglyemic clamp began 60 min earlier. Traces for raw lumbar SNA represent (a) 60 min, (b) post-PVN injection, and (c) post-RVLM injection. (C) Mean ± SEM of mean ABP, lumbar SNA, and blood glucose before and after PVN and RVLM injections. Mus, muscimol; *P<0.05 vs baseline, †P<0.05 vs 60 min (PVN muscimol group), # P<0.05 vs 75 min (PVN aCSF group)
Discussion
Despite the ability of insulin to elevate SNA and alter cardiovascular function 1, 2, there is a paucity of knowledge regarding the central neural mechanisms that mediate these effects. The present study provides several novel findings: 1) inhibition of the PVN completely reversed the sympathoexcitatory response to insulin, 2) direct injection of insulin into the PVN did not elevate lumbar SNA, 3) blockade of PVN melancortin 3/4 but not AT1 receptors eliminated the sympathoexcitatory response to insulin, and 4) inhibition of the PVN prevented the fall in lumbar SNA and mean ABP to RVLM injection of KYN in hyperinsulinemic rats. Collectively, these findings indicate insulin activates a melanocortin-dependent pathway to the PVN, and activation of PVN neurons increases glutamatergic drive to the RVLM via a direct or indirect pathway to alter cardiovascular function.
The PVN plays a pivotal role in the regulation of SNA and ABP through mono-and poly-synaptic pathways to sympathetic-regulatory neurons in the medulla and thoracic and lumbar segments of the spinal cord. The present findings clearly demonstrate that inhibition of the PVN completely reversed the sympathoexcitatory response to insulin. However, direct injection of insulin into the PVN failed to alter lumbar SNA or ABP despite the sympathoexcitatory response to insulin injections into the adjacent 3rd ventricle. These findings indicate that structures outside or upstream of the PVN and perhaps along the 3rd ventricle detect changes in circulating or CSF insulin. Although insulin receptors are expressed within PVN, previous studies have not localized expression to any specific cell population 22, 28. Therefore, the lack of a sympathoexcitatory response to direct injection of insulin into the PVN may be explained by the expression of insulin receptors on cell groups distinct from sympathetic-regulatory neurons.
PVN neurons express a number of receptors including those previously reported to mediate the anorexic and sympathetic and/or pressor effects of central insulin 11–13, 23, 24. Here, blockade of PVN melancortin 3/4 but not AT1 receptors reversed the increase in lumbar SNA of hyperinsulinemic rats. However, injection of SHU9119 did not affect the sympathoexcitatory response to PVN injection of NMDA (or gabazine). These observations suggest the effect of SHU9119 to reverse the sympathoexcitatory response to insulin cannot be attributed to a general inactivation of PVN neurons but reflects the antagonism of melanocortin 4 receptors. Furthermore, injections of SHU9119 (or muscimol) located outside the PVN (~200–400 μm) did not reduce SNA or mean ABP thereby suggesting the actions of these drugs can be attributed to receptors on PVN neurons. Interestingly, lumbar SNA and mean ABP did return to pre-injection levels at 120–140 min. This observation is consistent with previous studies 29 and unpublished data in our laboratory that show injection of SHU9119 effectively blocks melanocortin receptors for ~30–40 min.
Proopiomelanocortin (POMC) neurons are confined to the arcuate nucleus and nucleus of the solitary tract 30, 31. Although insulin receptor binding/expression is more abundant within the arcuate nucleus 22, 28, the contribution of these structures to the sympathetic-cardiovascular actions of insulin has not been determined. Manipulation of hypothalamic insulin receptors does attenuate the impact of insulin on energy homeostasis 32–34 thereby suggesting that insulin may act on neurons within the arcuate nucleus. This notion is supported by several recent electrophysiological studies in vitro 35, 36, although acute insulin application was reported to hyperpolarize POMC cells through a phosphoinositol 3 kinase-dependent mechanism 35. It is noteworthy that inhibition of hypothalamic phosphoinositol 3 kinase has been reported to prevent insulin-induced increases in SNA 10. Moreover, preliminary data in our laboratory suggests that inhibition of the arcuate nucleus reverses the sympathoexcitatory response to insulin, and direct injection of insulin into the arcuate nucleus raises SNA. However, future studies that directly antagonize insulin receptors or knock-down insulin receptor expression will be needed to determine whether arcuate neurons detect changes in circulating insulin levels to subsequently alter cardiovascular function.
We have previously reported that ~85% of RVLM-projecting PVN neurons express the vesicular glutamate transporter-2 mRNA 14. Since blockade of RVLM ionotropic glutamate receptors reversed the sympathetic response to insulin 5, we hypothesized that this increased glutamatergic drive originated from the PVN. Indeed, the present findings are consistent with this hypothesis as inhibition of the PVN prevented the fall in lumbar SNA after RVLM injection of KYN. While these data highlight the importance of the PVN and RVLM as a circuit to mediate the sympathoexcitatory effects of insulin, these findings do not conclusively prove that a direct pathway from the PVN to the RVLM is involved.
Several studies clearly indicate the insulin acutely alters SNA and baroreflex function in animals and humans 3–8. However, the role of insulin in cardiovascular dysfunction in obesity-induced hypertension or type II diabetes remains controversial. In rodents, a hyperinsulinemic-euglycemic clamp acutely raises SNA 3–5 and chronically raises ABP 37. However, a chronic infusion of insulin in dogs produced opposite hemodynamic changes in cardiac output and peripheral resistance and does not elevate ABP 38. In humans, findings from the Normative Aging Study suggest a correlation between obesity, insulin levels and blood pressure 2, 39, and plasma insulin concentrations have been correlated with muscle SNA 40, 41. However, other studies have not observed a relationship between plasma insulin concentrations versus muscle SNA and/or renal NE spillover in obese populations 42, 43 or after modest weight gain in non-obese individuals 44. Therefore, the precise role of insulin in cardiovascular dysfunction during obesity-induced hypertension or type II diabetes remains unclear. However, it is unlikely that one factor, by itself, mediates the pathogenesis of complex diseases such as the metabolic syndrome.
Perspective
Accumulating evidence indicates the important of the central melancortin system as a pivotal mediator of obesity-induced hypertension. First, the sympathoexcitatory responses to acute leptin and insulin administration are abolished by ICV administration of SHU9119 45 or in melanocortin-4 deficient mice 12. Interestingly, melancortin-4 deficient mice are normotensive despite obesity, hyperinsulinemia, and hyperleptinemia 46. Chronic ICV administration of SHU9119 lowers ABP in a rodent model of diet-induced obesity 47. Finally, the prevalence of hypertension is significantly lower in obese humans with melanocortin-4 receptor deficiency 48. These observations together with the present findings suggest melanocortin receptors on PVN sympathetic-regulatory neurons may represent a novel therapeutic target for obesity-related hypertension. However, future studies are needed to specifically determine whether such mechanisms in the PVN mediate elevated SNA and ABP in obesity and identify the specific populations of PVN neurons involved.
Supplementary Material
Acknowledgments
Sources of Funding
This work was supported by a National Institutes of Health National Heart, Lung, and Blood Institute Grant HL090826 (S.D.S.).
Footnotes
Disclosures
The authors have no disclosures.
References
- 1.Esler M, Straznicky N, Eikelis N, Masuo K, Lambert G, Lambert E. Mechanisms of sympathetic activation in obesity-related hypertension. Hypertension. 2006;48:787–796. doi: 10.1161/01.HYP.0000242642.42177.49. [DOI] [PubMed] [Google Scholar]
- 2.Landsberg L. Insulin-mediated sympathetic stimulation: role in the pathogenesis of obesity-related hypertension (or, how insulin affects blood pressure, and why) J Hypertens. 2001;19:523–528. doi: 10.1097/00004872-200103001-00001. [DOI] [PubMed] [Google Scholar]
- 3.Morgan DA, Balon TW, Ginsberg BH, Mark AL. Nonuniform regional sympathetic nerve responses to hyperinsulinemia in rats. Am J Physiol Regul Integr Comp Physiol. 1993;264:R423–7. doi: 10.1152/ajpregu.1993.264.2.R423. [DOI] [PubMed] [Google Scholar]
- 4.Muntzel MS, Morgan DA, Mark AL, Johnson AK. Intracerebroventricular insulin produces nonuniform regional increases in sympathetic nerve activity. Am J Physiol Regul Integr Comp Physiol. 1994;267:R1350–R1355. doi: 10.1152/ajpregu.1994.267.5.R1350. [DOI] [PubMed] [Google Scholar]
- 5.Bardgett ME, McCarthy JJ, Stocker SD. Glutamatergic receptor activation in the rostral ventrolateral medulla mediates the sympathoexcitatory response to hyperinsulinemia. Hypertension. 2010;55:284–290. doi: 10.1161/HYPERTENSIONAHA.109.146605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anderson EA, Hoffman RP, Balon TW, Sinkey CA, Mark AL. Hyperinsulinemia produces both sympathetic neural activation and vasodilation in normal humans. J Clin Invest. 1991;87:2246–2252. doi: 10.1172/JCI115260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pricher MP, Freeman KL, Brooks VL. Insulin in the brain increases gain of baroreflex control of heart rate and lumbar sympathetic nerve activity. Hypertension. 2008;51:514–520. doi: 10.1161/HYPERTENSIONAHA.107.102608. [DOI] [PubMed] [Google Scholar]
- 8.Young CN, Deo SH, Chaudhary K, Thyfault JP, Fadel PJ. Insulin enhances the gain of arterial baroreflex control of muscle sympathetic nerve activity in humans. J Physiol. 2010;588:3593–3603. doi: 10.1113/jphysiol.2010.191866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muntzel M, Beltz T, Mark AL, Johnson AK. Anteroventral third ventricle lesions abolish lumbar sympathetic responses to insulin. Hypertension. 1994;23:1059–1062. doi: 10.1161/01.hyp.23.6.1059. [DOI] [PubMed] [Google Scholar]
- 10.Rahmouni K, Morgan DA, Morgan GM, Liu X, Sigmund CD, Mark AL, Haynes WG. Hypothalamic PI3K and MAPK differentially mediate regional sympathetic activation to insulin. J Clin Invest. 2004;114:652–628. doi: 10.1172/JCI21737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nakata T, Takeda K, Hatta T, Kiyama M, Moriguchi J, Miki S, Kawa T, Morimoto S, Nakamura K, Uchida A, Itoh H, Sasaki S, Nakagawa M. Blockade of angiotensin II receptors inhibits the increase in blood pressure induced by insulin. J Cardiovasc Pharmacol. 1998;31:248–252. doi: 10.1097/00005344-199802000-00010. [DOI] [PubMed] [Google Scholar]
- 12.Rahmouni K, Haynes WG, Morgan DA, Mark AL. Role of melanocortin-4 receptors in mediating renal sympathoactivation to leptin and insulin. J Neurosci. 2003;23:5998–6004. doi: 10.1523/JNEUROSCI.23-14-05998.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Benoit SC, Air EL, Coolen LM, Strauss R, Jackman A, Clegg DJ, Seeley RJ, Woods SC. The catabolic action of insulin in the brain is mediated by melanocortins. J Neurosci. 2002;22:9048–9052. doi: 10.1523/JNEUROSCI.22-20-09048.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stocker SD, Simmons JR, Stornetta RL, Toney GM, Guyenet PG. Water deprivation activates a glutamatergic projection from the hypothalamic paraventricular nucleus to the rostral ventrolateral medulla. J Comp Neurol. 2006;494:673–685. doi: 10.1002/cne.20835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang Z, Bertram D, Coote JH. The role of glutamate and vasopressin in the excitation of RVL neurones by paraventricular neurones. Brain Res. 2001;908:99–103. doi: 10.1016/s0006-8993(01)02593-8. [DOI] [PubMed] [Google Scholar]
- 16.Martin DS, Haywood JR. Sympathetic nervous system activation by glutamate injections into the paraventricular nucleus. Brain Res. 1992;577:261–267. doi: 10.1016/0006-8993(92)90282-e. [DOI] [PubMed] [Google Scholar]
- 17.Martin DS, Segura T, Haywood JR. Cardiovascular responses to bicuculline in the paraventricular nucleus of the rat. Hypertension. 1991;18:48–55. doi: 10.1161/01.hyp.18.1.48. [DOI] [PubMed] [Google Scholar]
- 18.Chen QH, Haywood JR, Toney GM. Sympathoexcitation by PVN-injected bicuculline requires activation of excitatory amino acid receptors. Hypertension. 2003;42:725–731. doi: 10.1161/01.HYP.0000085197.20043.44. [DOI] [PubMed] [Google Scholar]
- 19.Chen QH, Toney GM. Responses to GABA-A receptor blockade in the hypothalamic PVN are attenuated by local AT1 receptor antagonism. Am J Physiol Regul Integr Comp Physiol. 2003;285:R1231–R1239. doi: 10.1152/ajpregu.00028.2003. [DOI] [PubMed] [Google Scholar]
- 20.Deering J, Coote JH. Paraventricular neurones elicit a volume expansion-like change of activity in sympathetic nerves to the heart and kidney in the rabbit. Exp Physiol. 2000;85:177–186. [PubMed] [Google Scholar]
- 21.Guyenet PG. The sympathetic control of blood pressure. Nat Rev Neurosci. 2006;7:335–346. doi: 10.1038/nrn1902. [DOI] [PubMed] [Google Scholar]
- 22.Werther GA, Hogg A, Oldfield BJ, McKinley MJ, Figdor R, Allen AM, Mendelsohn FA. Localization and characterization of insulin receptors in rat brain and pituitary gland using in vitro autoradiography and computerized densitometry. Endocrinology. 1987;121:1562–1570. doi: 10.1210/endo-121-4-1562. [DOI] [PubMed] [Google Scholar]
- 23.Kishi T, Aschkenasi CJ, Lee CE, Mountjoy KG, Saper CB, Elmquist JK. Expression of melanocortin 4 receptor mRNA in the central nervous system of the rat. J Comp Neurol. 2003;457:213–235. doi: 10.1002/cne.10454. [DOI] [PubMed] [Google Scholar]
- 24.Song K, Allen AM, Paxinos G, Mendelsohn FA. Mapping of angiotensin II receptor subtype heterogeneity in rat brain. J Comp Neurol. 1992;316:467–484. doi: 10.1002/cne.903160407. [DOI] [PubMed] [Google Scholar]
- 25.Adams JM, Madden CJ, Sved AF, Stocker SD. Increased dietary salt enhances sympathoexcitatory and sympathoinhibitory responses from the rostral ventrolateral medulla. Hypertension. 2007;50:354–359. doi: 10.1161/HYPERTENSIONAHA.107.091843. [DOI] [PubMed] [Google Scholar]
- 26.Adams JM, McCarthy JJ, Stocker SD. Excess dietary salt alters angiotensinergic regulation of neurons in the rostral ventrolateral medulla. Hypertension. 2008;52:932–937. doi: 10.1161/HYPERTENSIONAHA.108.118935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kawabe T, Chitravanshi VC, Kawabe K, Sapru HN. Cardiovascular effects of adrenocorticotropin microinjections into the rostral ventrolateral medullary pressor area of the rat. Brain Res. 2006;1102:117–126. doi: 10.1016/j.brainres.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 28.Marks JL, Porte D, Jr, Stahl WL, Baskin DG. Localization of insulin receptor mRNA in rat brain by in situ hybridization. Endocrinology. 1990;127:3234–3236. doi: 10.1210/endo-127-6-3234. [DOI] [PubMed] [Google Scholar]
- 29.Brown S, Chitravanshi VC, Sapru HN. Cardiovascular actions of adrenocorticotropin microinjections into the nucleus tractus solitarius of the rat. Neuroscience. 2006;143:863–874. doi: 10.1016/j.neuroscience.2006.08.026. [DOI] [PubMed] [Google Scholar]
- 30.Palkovits M, Mezey E, Eskay RL. Pro-opiomelanocortin-derived peptides (ACTH/beta-endorphin/alpha-MSH) in brainstem baroreceptor areas of the rat. Brain Res. 1987;436:323–338. doi: 10.1016/0006-8993(87)91676-3. [DOI] [PubMed] [Google Scholar]
- 31.Watson SJ, Akil H, Richard CW, 3rd, Barchas JD. Evidence for two separate opiate peptide neuronal systems. Nature. 1978;275:226–228. doi: 10.1038/275226a0. [DOI] [PubMed] [Google Scholar]
- 32.Grillo CA, Tamashiro KL, Piroli GG, Melhorn S, Gass JT, Newsom RJ, Reznikov LR, Smith A, Wilson SP, Sakai RR, Reagan LP. Lentivirus-mediated downregulation of hypothalamic insulin receptor expression. Physiol Behav. 2007;92:691–701. doi: 10.1016/j.physbeh.2007.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Obici S, Feng Z, Karkanias G, Baskin DG, Rossetti L. Decreasing hypothalamic insulin receptors causes hyperphagia and insulin resistance in rats. Nat Neurosci. 2002;5:566–572. doi: 10.1038/nn0602-861. [DOI] [PubMed] [Google Scholar]
- 34.Paranjape SA, Chan O, Zhu W, Horblitt AM, McNay EC, Cresswell JA, Bogan JS, McCrimmon RJ, Sherwin RS. Influence of insulin in the ventromedial hypothalamus on pancreatic glucagon secretion in vivo. Diabetes. 59:1521–1527. doi: 10.2337/db10-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hill JW, Williams KW, Ye C, Luo J, Balthasar N, Coppari R, Cowley MA, Cantley LC, Lowell BB, Elmquist JK. Acute effects of leptin require PI3K signaling in hypothalamic proopiomelanocortin neurons in mice. J Clin Invest. 2008;118:1796–1805. doi: 10.1172/JCI32964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Williams KW, Margatho LO, Lee CE, Choi M, Lee S, Scott MM, Elias CF, Elmquist JK. Segregation of acute leptin and insulin effects in distinct populations of arcuate proopiomelanocortin neurons. J Neurosci. 30:2472–2479. doi: 10.1523/JNEUROSCI.3118-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brands MW, Lee WF, Keen HL, Alonso-Galicia M, Zappe DH, Hall JE. Cardiac output and renal function during insulin hypertension in Sprague-Dawley rats. Am J Physiol Regul Integr Comp Physiol. 1996;271:R276–R281. doi: 10.1152/ajpregu.1996.271.1.R276. [DOI] [PubMed] [Google Scholar]
- 38.Brands MW, Mizelle HL, Gaillard CA, Hildebrandt DA, Hall JE. The hemodynamic response to chronic hyperinsulinemia in conscious dogs. Am J Hypertens. 1991;4:164–168. doi: 10.1093/ajh/4.2.164. [DOI] [PubMed] [Google Scholar]
- 39.Ward KD, Sparrow D, Landsberg L, Young JB, Vokonas PS, Weiss ST. Influence of insulin, sympathetic nervous system activity, and obesity on blood pressure: the Normative Aging Study. J Hypertens. 1996;14:301–308. doi: 10.1097/00004872-199603000-00005. [DOI] [PubMed] [Google Scholar]
- 40.Monroe MB, Van Pelt RE, Schiller BC, Seals DR, Jones PP. Relation of leptin and insulin to adiposity-associated elevations in sympathetic activity with age in humans. Int J Obes Relat Metab Disord. 2000;24:1183–1187. doi: 10.1038/sj.ijo.0801364. [DOI] [PubMed] [Google Scholar]
- 41.Scherrer U, Randin D, Tappy L, Vollenweider P, Jequier E, Nicod P. Body fat and sympathetic nerve activity in healthy subjects. Circulation. 1994;89:2634–2640. doi: 10.1161/01.cir.89.6.2634. [DOI] [PubMed] [Google Scholar]
- 42.Vaz M, Jennings G, Turner A, Cox H, Lambert G, Esler M. Regional sympathetic nervous activity and oxygen consumption in obese normotensive human subjects. Circulation. 1997;96:3423–3429. doi: 10.1161/01.cir.96.10.3423. [DOI] [PubMed] [Google Scholar]
- 43.Alvarez GE, Ballard TP, Beske SD, Davy KP. Subcutaneous obesity is not associated with sympathetic neural activation. Am J Physiol Heart Circ Physiol. 2004;287:H414–H418. doi: 10.1152/ajpheart.01046.2003. [DOI] [PubMed] [Google Scholar]
- 44.Gentile CL, Orr JS, Davy BM, Davy KP. Modest weight gain is associated with sympathetic neural activation in nonobese humans. Am J Physiol Regul Integr Comp Physiol. 2007;292:R1834–R1838. doi: 10.1152/ajpregu.00876.2006. [DOI] [PubMed] [Google Scholar]
- 45.Haynes WG, Morgan DA, Djalali A, Sivitz WI, Mark AL. Interactions between the melanocortin system and leptin in control of sympathetic nerve traffic. Hypertension. 1999;33:542–547. doi: 10.1161/01.hyp.33.1.542. [DOI] [PubMed] [Google Scholar]
- 46.Tallam LS, Stec DE, Willis MA, da Silva AA, Hall JE. Melanocortin-4 receptor-deficient mice are not hypertensive or salt-sensitive despite obesity, hyperinsulinemia, and hyperleptinemia. Hypertension. 2005;46:326–332. doi: 10.1161/01.HYP.0000175474.99326.bf. [DOI] [PubMed] [Google Scholar]
- 47.Dubinion JH, da Silva AA, Hall JE. Enhanced blood pressure and appetite responses to chronic central melanocortin-3/4 receptor blockade in dietary-induced obesity. J Hypertens. 28:1466–1470. doi: 10.1097/HJH.0b013e328339f20e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Greenfield JR, Miller JW, Keogh JM, Henning E, Satterwhite JH, Cameron GS, Astruc B, Mayer JP, Brage S, See TC, Lomas DJ, O’Rahilly S, Farooqi IS. Modulation of blood pressure by central melanocortinergic pathways. N Engl J Med. 2009;360:44–52. doi: 10.1056/NEJMoa0803085. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.