Abstract
Metabolic syndrome (MetS) is characterized by central obesity, hypertension, insulin resistance, and hypercholesterolemia. Hypothalamic-pituitary-adrenal (HPA) axis activity is frequently abnormal in MetS, and excessive cortisol exposure may be implicated in metabolic derangements. We investigated the hypothesis that cortisol and adrenocorticotropic hormone (ACTH) responses to a standardized neuroendocrine challenge test would be associated with indices of MetS in a community sample of healthy adults. Healthy adults, 125 men and 170 women, without significant medical problems or chronic medications were recruited from the community. Participants completed the dexamethasone/corticotropin-releasing hormone (Dex/CRH) test, and anthropometric measurements, blood pressure, glycosylated hemoglobin (HbA1c), and cholesterol were measured. Participants reported on their history of early life stress and recent stress, as well as mood and anxiety symptoms. Cortisol and ACTH responses to the Dex/CRH test were negatively associated with measures of central adiposity (p < 0.001) and blood pressure (p < 0.01), and positively associated with HDL cholesterol (p < 0.01). These findings remained significant after controlling for body mass index (BMI). Measures of stress and anxiety and depressive symptoms were negatively correlated with cortisol and ACTH responses in the Dex/CRH test but were not related to MetS indices. That altered HPA axis function is linked to MetS components even in a healthy community sample suggests that these processes may be involved in the pathogenesis of MetS. Identification of premorbid risk processes might allow for detection and intervention prior to the development of disease.
Keywords: metabolic syndrome, HPA axis, cortisol, ACTH, corticotrophin-releasing hormone
Introduction
The metabolic syndrome (MetS) is characterized by central obesity, hypertension, insulin resistance, and hypercholesterolemia. MetS affects approximately one quarter of the population of North America, and is a major risk factor for cardiovascular disease, diabetes, and all-cause mortality [1]. The pathogenesis of this syndrome is uncertain, but dysfunction of the hypothalamic-pituitary-adrenal axis and altered cortisol metabolism may be involved. Cushing’s Syndrome, characterized by endogenous or exogenous hypercortisolism, is also characterized by abdominal obesity, insulin resistance, abnormal fasting glucose levels, hypertension, and dyslipidemia; this has led to the hypothesis that excessive glucocorticoid exposure may be responsible for MetS. As discussed in recent reviews (e.g., [2,3]) glucocorticoids are centrally involved in glucose, lipid, and protein metabolism, and function to provide a substrate for oxidative metabolism by activating lipolysis, proteolysis, and hepatic glucose production. Glucocorticoids increase glucose and insulin levels and promote insulin resistance via actions on hepatic glucose output and on several components of the insulin signaling cascade. Lipid metabolism is also altered by excessive glucocorticoid exposure; in visceral adipose tissue, glucocorticoids increase triglyceride synthesis, lipoprotein lipase activity, and differentiation of cells into mature adipocytes [2]. The enzyme 11β-hydroxysteroid dehydrogenase-1 (11β-HSD1) catalyses the conversion of inactive cortisone to cortisol, and is most prevalent in liver and adipose tissue. In rodent models and human studies of obesity, 11β-HSD1 is increased in adipose tissue. Increased activity of this enzyme in adipose tissue in rodents induces central adiposity, dyslipidemia, and insulin resistance [2]. In addition, stress exposure and activation of the HPA axis stimulate the sympathetic nervous system to secrete endogenous catecholamines, which can lead to hypertension and associated cardiovascular risk factors [3].
Basal cortisol concentrations have been measured in studies of children and adults with central adiposity or high body mass index (BMI), a key component of MetS. Findings have been mixed, with several studies showing that circulating or urinary cortisol concentrations are decreased [4–12], and others finding elevated cortisol levels [13–17]. Although most work has focused on obesity, some studies have examined other features of MetS, with most finding evidence of elevated circulating cortisol [18–22]. A study of a large group of middle-aged men showed that anthropometric, metabolic, and hemodynamic factors of MetS were linked to perceived stress-related cortisol in men with normal diurnal cortisol curves, and these associations were even stronger in men characterized by low morning cortisol and low diurnal variability [23].
A few investigations have examined dynamic measures of HPA axis activity using predominantly small samples of overweight or obese individuals. Enhanced cortisol secretion in response to acute psychological stressors has been documented [24–26]. The cortisol awakening response (CAR) has been reported to be blunted in men and women [6], or to be enhanced in men [19,27] in association with central adiposity. One study found reduced cortisol upon awakening but a greater awakening response among men with MetS [28]. Findings from studies that used pharmacologic probes of HPA function have also been mixed. Reduced cortisol suppression by dexamethasone (Dex) among individuals with higher body mass or obesity has been reported in some investigations [7,11,23], but others found enhanced suppression [10,29]. Using CRH stimulation, 2 studies found significantly reduced cortisol responses in relation to obesity or central adiposity [8,30]. Others have found no relationship [31], or positive associations with cortisol or ACTH responses to CRH [12,13,32,33] or the CRH-arginine vasopressin test [34]. In the one study that examined responses to the Dex/CRH test, there was no association between markers of MetS and cortisol or ACTH responses [12].
Stress exposure is a risk factor for obesity and MetS [3]. Moreover, chronic or extreme stress can alter HPA axis function, and psychiatric conditions, including major depression, have been linked to both HPA axis abnormalities and MetS [35]. However, the existing literature on neuroendocrine function in obesity has generally not included measures of stress exposure. The foregoing summary establishes that glucocorticoids are likely to play a significant role in MetS, but findings are mixed with respect to circulating cortisol and HPA axis responses to challenge in relation to central obesity and other signs of MetS. Only a few studies have examined the HPA axis response to neuroendocrine challenge tasks in relation to MetS. The mixed nature of reported findings may be influenced by small sample sizes, a focus on obesity without measurement of other MetS features (e.g., measurements of central adiposity), or neglect of potentially relevant factors such as stress and adversity. Due to the mixed findings and limitations of previous studies, we tested a non-directional hypothesis that cortisol and ACTH responses to neuroendocrine challenge would be associated with subsyndromal parameters of MetS in a large group of healthy adults, and tested for the influence of important covariates.
Subjects and Methods
Subjects
Two hundred ninety-five healthy adults, 125 males and 170 females, 18–61 years (mean ± SD, 30.30 ± 11.04), were recruited from the community using flyers and advertisements for a larger study of stress reactivity in healthy adults. Exclusions included working night shifts, acute/unstable medical illness, bipolar disorder, any psychotic disorder, substance abuse or dependence, and a history of brain injury, seizure disorder, or endocrine disease. Also excluded were individuals undergoing treatment with drugs that might influence HPA axis function or MetS; including psychotropics, antihypertensives, corticosteroids, cholesterol-lowering agents, ketoconazole, and metyrapone. Oral contraceptives (OCs) were allowed. The study was approved by the Butler Hospital Institutional Review Board, and subjects gave voluntary written informed consent to participate.
Metabolic syndrome measures
To assess abdominal adiposity, waist/hip ratio (WHR) was assessed in the standing position, with waist circumference (cm) measured at the midpoint of the lowest rib and the iliac crest and hip circumference measured at the widest point at the level of the greater trochanters, and sagittal trunk diameter (cm) was measured in the supine position. Weight and height were measured with light clothing without shoes, and BMI was calculated [weight (kg)/ht (m)2]. Systolic and diastolic blood pressures were measured. A subset of the sample (n = 185) had nonfasting blood drawn for laboratory tests of MetS. Total cholesterol, high-density lipoprotein (HDL), and glycosylated hemoglobin (HbA1c) concentrations were used to assess lipids and glucose regulation, respectively, as they are not influenced by recent food intake [36].
In this healthy community sample, we anticipated that participants would have subclinical indices of MetS, so our central analyses used the continuous measures of MetS features. Cut-points for MetS features established by the WHO [37] were also used for descriptive purposes and to examine additive effects of MetS features. A summary variable was developed, with one point for each of the following: 1) central adiposity (WHR > 0.9, waist circumference > 68.6 cm, or trunk diameter > 27 cm); 2) BMI > 30; 3) HDL cholesterol < 35 mg/dl; 4) blood pressure > 140/90 mm Hg; and 5) HbA1c ≥ 6.0 % (used as a cut-point for abnormal glucose regulation even though this is not included in the WHO criteria). Because only a subset (n = 185) of the participants had blood drawn for HDL and HbA1c, this index of risk likely represents an underestimate of the degree of risk for the participants with missing data.
Dex/CRH test
This test was scheduled for a time without anticipated life stressors and in the event of an unanticipated stressor, the test was rescheduled. The night before the test, a single oral dose of Dex 1.5 mg was self-administered at 23:00 h. The following day, subjects arrived at 12:00 h and were given lunch. Following application of a topical anesthetic cream containing lidocaine 2.5 % and prilocaine 2.5 % (EMLA™), an indwelling intravenous (IV) catheter was inserted in the forearm at 13:00 h by a research nurse with extensive experience with IV catheter placement. Subjects remained in a semi-recumbent position throughout the procedure except to use the bathroom. They were permitted to read or watch pre-selected films that did not contain emotionally-charged material. Vital signs were monitored throughout the test. After a blood sample was drawn at 14:59 h, at 15:00 h, CRH (corticorelin ovine triflutate, Acthrel, Ferring Pharmaceuticals, Inc.) 100 mcg reconstituted in 2 ml 0.9 % sodium chloride was infused intravenously over 30 s. Additional blood samples were drawn at 15:30 h, 15:45 h, 16:00 h, 16:15 h, and 17:00 h and assayed for cortisol. A subset of participants (n = 207) had ACTH assays performed on the samples drawn at 14:59 h, 15:30 h, 16:00 h, 16:30 h, and 17:00 h. Of these, 112 participants also had the laboratory tests for MetS.
Trier social stress test (TSST)
A subset of individuals (n = 79) participated in the TSST [24], a standardized laboratory psychosocial stress protocol (see [38] for a detailed description). The TSST consists of an anticipation period followed by a 10-min role play, during which the subject must deliver a monologue speech about her/his qualifications for a chosen vocation and perform mental calculation and recitation of serial subtraction by 13’s in front of a panel of “judges”. Plasma samples for cortisol (n = 79) and ACTH (n = 60) assay were obtained through an intravenous catheter before, during, and after the stressor. These participants completed the anthropometric and blood pressure MetS measures, but did not have laboratory MetS variables measured.
Assays
Plasma ACTH was assayed in duplicate in 200 μl plasma samples using immunoradiometric assay (Wilkinson CW, Raff H, 2006; Scantibodies Laboratory, Santee, CA, USA). The minimum detectable ACTH concentration was 2 pg/ml, and intra- and interassay coefficients of variation (CV) were 4.6 % and 5.3 %, respectively. Plasma cortisol concentrations from 244 participants were assayed in duplicate using the Gamma Coat cortisol I-125 coated-tube radioimmunoassay (RIA) kit (INCSTAR Corp., Still-water, MN, USA). Intra- and interassay CVs observed for quality assessment samples (3 and 20 μg/dl) were less than 5 % and 10 %, respectively. Plasma cortisol concentrations from 51 participants were measured in duplicate with the double antibody DSL-2000 Cortisol Radioimmunoassay Kit (Diagnostic Systems Laboratories, Webster, TX, USA) according to the manufacturer’s instructions. The antiserum has 100 % cross-reactivity with cortisol and only 0.38 % cross-reactivity with Dex. The intra- and interassay coefficients of variation are 5.3 % and 7.0 %, respectively. Using a 25 μl sample, the minimum detectable level of cortisol is 4.8 nmol/l. The 2 assays were highly correlated [r(27) = 0.95, p < 0.001]. Area under the curve over time (AUC) for cortisol and ACTH in the challenge tests was calculated. In addition, post-Dex, pre-CRH values in the Dex/CRH test were examined to determine the effects of Dex on cortisol and ACTH concentrations. These cortisol and ACTH variables were positively skewed and therefore log-transformed to meet normal distribution requirements for statistical tests.
HDL cholesterol was assayed using the Roche HDL-C plus 3rd Generation kit (Roche Diagnostics, Indianapolis, IN, USA); the range of detection was 5–200 mg/dl. Total cholesterol was determined using the Enzymatic Olympus AU5400 kit (Olympus America Inc, Center Valley, PA, USA); the range of detection was 40–1000 mg/dl. HbA1c was assayed using the Roche Integra COBAS Integra 800 Tinaquant HbA1c Gen.2 (Roche Diagnostics, Indianapolis, IN, USA); the range of detection was 3.4–18.0 %.
Measures of behavior and stress exposure
The Structured Clinical Interview for DSM-IV was used to diagnose psychiatric disorders. Subclinical symptoms of depression and anxiety were assessed with the Inventory of Depressive Symptomatology (self-report version; IDS-SR) and the State-Trait Anxiety Questionnaire (STAI). Measures of stress and resilience included the Childhood Trauma Questionnaire, 28-item version (CTQ) total score and Emotional Abuse subscale (which we previously linked to attenuated cortisol responses to the Dex/CRH test in a large subset of this sample [39]), the Perceived Stress Scale (PSS), and the Connor-Davidson Resilience Scale (CD-RISC).
Statistical analysis
Data were analyzed with SPSS version 19; all tests were 2-tailed tests and alpha was set to 0.05. Outliers greater than 3 standard deviations were Winsorized to equal the next highest value for the following measures: blood pressure (n = 4), total cholesterol (n = 1), HbA1c (n = 2), BMI (n = 5), sagittal trunk diameter (n = 4) and WHR (n = 1). Raw data were used for determination of MetS cut-points and in the figures. Partial correlations, controlling for age and sex, were used to test associations of cortisol and ACTH with signs of MetS; additional models controlled for other potentially important covariates. A repeated measures general linear model tested the effect of the number of MetS risk factors on hormone responses to the Dex/CRH test.
Results
Participant characteristics
As expected in this sample, few participants met the criteria for 3 or more MetS signs (n = 7), but many participants had 1 (n = 44) or 2 (n = 30) abnormal MetS factors (Table 1). Participants met criteria for current major Axis I psychiatric disorders as follows: major depressive disorder (n = 33), other depressive disorder (n = 20), post-traumatic stress disorder (n = 4), other anxiety disorder (n = 16).
Table 1.
Metabolic characteristics of the sample.
| MetS risk factor | Range | Mean | At risk (n) |
|---|---|---|---|
| Blood pressure (mm Hg) | 6 | ||
| Systolic | 88–159 | 122 ± 14 | |
| Diastolic | 45–97 | 69 ± 10 | |
| HDL cholesterol (mg/dl) | 24–127 | 58 ± 15 | 10 |
| Total cholesterol (mg/dl) | 100–339 | 176 ± 35 | |
| Central Adiposity | |||
| WHR | 0.7–1.1 | 0.8 ± 0.1 | 50 |
| Sagittal trunk diameter (cm) | 5.3–10.3 | 7.6 ± 1.0 | 4 |
| Waist circumference (cm) | 52.0–134.6 | 83.1 ± 11.8 | 54 |
| BMI | 17.0–37.5 | 25.5 ± 4.1 | 41 |
| HbA1c, mmol/mol (NG-SP%) | 27–45 (4.6–6.3) | 33 ± 0.9 (5.2 ± 0.3) | 5 |
| Total number of risk factors | |||
| 0 | 214 | ||
| 1 | 44 | ||
| 2 | 30 | ||
| 3 | 5 | ||
| 4 | 2 | ||
At risk cut points are 140/90 for blood pressure, < 35 mg/dl for HDL cholesterol, > 0.9 for waist-to-hip ratio (WHR), > 27 cm for sagittal trunk diameter, > 94 cm for waist circumference, > 30 for body mass index (BMI), and ≥6.0 % for glycosylated hemoglobin (HbA1c). Total number of risk factors refers to the sum of MetS risk factors, including blood pressure, HDL, any measure of central adiposity, BMI, and HbA1c
Primary analyses
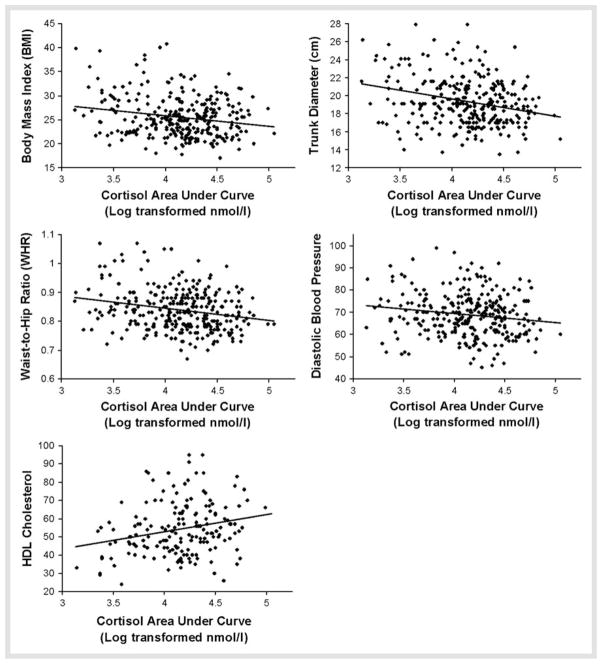
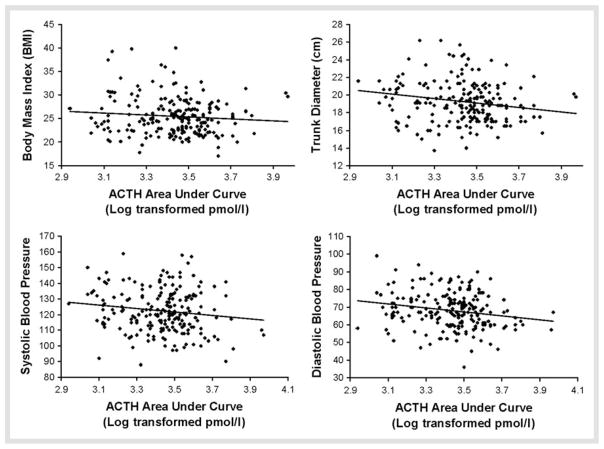
Table 2 shows partial correlations, controlling for age and sex, testing associations of MetS parameters with cortisol and ACTH AUC in the Dex/CRH test. Cortisol responses were inversely associated with measures of central adiposity and diastolic blood pressure, and positively associated with HDL cholesterol. Scatter plots depicting these relationships are shown in Fig. 1. The post-Dex, pre-CRH cortisol values also showed significant negative relationships with BMI [r(201) = −0.24, p < 0.001] and trunk diameter [r(285) = −0.23, p < 0.001], and a positive partial correlation with HDL [r(173) = 0.18, p < 0.05]. Similarly, ACTH responses to the test were inversely associated with measures of central adiposity and systolic and diastolic blood pressure (Table 2, Fig. 2). Post-Dex and pre-CRH ACTH values also showed negative associations with systolic [r (205) = −0.15, p < 0.05] and diastolic [r(205) = −0.16, p < 0.05] blood pressure, and a trend-level inverse correlation with trunk diameter [r(205) = −0.12, p = 0.08].
Table 2.
Correlations between MetS variables and results of Dex/CRH.
| MetS variable | Dex/CRH Test | |
|---|---|---|
| Cortisol AUC | ACTH AUC | |
| BMI | −0.20† | −0.16* |
|
| ||
| Trunk diameter | −0.27† | −0.20*** |
|
| ||
| Waist/Hip ratio | −0.18*** | −0.11 |
|
| ||
| Waist circumference | −0.16** | −0.06 |
|
| ||
| Systolic blood pressure | −0.06 | −0.19** |
|
| ||
| Diastolic blood pressure | −0.14* | −0.18** |
|
| ||
| Total cholesterol | 0.02 | 0.09 |
|
| ||
| HDL cholesterol | 0.22** | 0.07 |
|
| ||
| HbA1c | −0.06 | −0.07 |
AUC: area under the curve over time; ACTH: adrenocorticotropic hormone; BMI: body mass index; HDL cholesterol: high-density lipoprotein cholesterol; HbA1c: glycosylated hemoglobin.
p < 0.05,
p < 0.01,
p < 0.005,
p < 0.001
Fig. 1.
Scatter plots for significant correlations of Dex/CRH cortisol area under curve and MetS variables.
Fig. 2.
Scatter plots for significant correlations of Dex/CRH ACTH area under curve and MetS variables.
A repeated measures general linear model, controlling for age and sex, showed that the number of MetS indices was predictive of lower cortisol [F(2, 290) = 5.01, p < 0.01; Fig. 3a] and ACTH [F(2, 202) = 3.58, p < 0.05; Fig. 3b] responses to the Dex/CRH test. In the analysis of the anthropometric and blood pressure variables available in the subset of participants that completed the TSST (n = 70), BMI and trunk diameter were inversely associated with cortisol response (r = −0.36, p < 0.001 and r = −0.36, p < 0.005; respectively; Fig. 4). These MetS indices were not correlated with ACTH responses (n = 60).
Fig. 3.
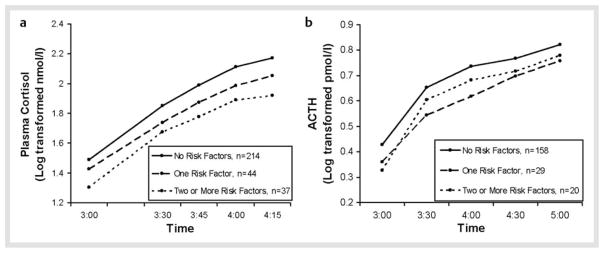
Number of metabolic risk factors and neuroendocrine responses to the Dex/CRH test. a General linear model, controlling for age and sex, showed a main effect of number of metabolic risk factors on cortisol response to the Dex/CRH test [F(2, 290) = 5.01, p < 0.01]. b General linear model, controlling for age and sex, showed a main effect of number of metabolic risk factors on ACTH response to the test [F(2, 202) = 3.58, p < 0.05].
Fig. 4.
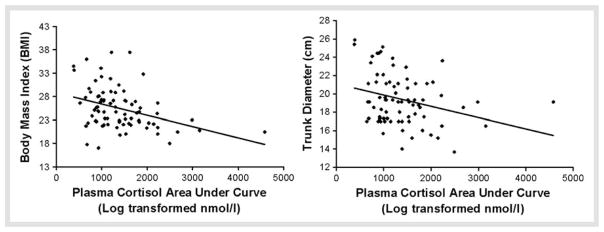
Scatter plots for significant correlations of TSST plasma cortisol area under curve and significant correlations with metabolic variables.
Analyses of potential confounds
Controlling for type of cortisol assay, diagnosis (current and lifetime diagnoses of major depression, PTSD, or any Axis I disorder), or OC use did not alter the findings. Controlling for BMI changed only a few associations with cortisol: diastolic blood pressure became trend-level (p = 0.094), waist circumference was no longer significant, and in the small TSST sample, trunk diameter was no longer significant.
Next, we explored whether measures of subclinical mood or anxiety symptoms or stress or adversity could account for our findings. Total scores on the CTQ were not linked to measures of neuroendocrine response, but emotional abuse was associated with lower cortisol [r(290) = −0.12, p < 0.05] and marginally lower ACTH [r(203) = −0.13, p = 0.061] responses to the Dex/CRH test. Recent perceived stress was also inversely associated with ACTH responses [r(202) = −0.17, p < 0.01], whereas total resiliency was positively correlated with cortisol [r(240) = 0.13, p < 0.05] and ACTH [r(173) = 0.25, p = 0.001] responses. Depressive symptoms were inversely correlated with ACTH responses to the Dex/CRH test [r(203) = −0.16, p < 0.05] and cortisol responses to the TSST [r(75) = −0.23, p < 0.05]. Cortisol responses to the Dex/CRH test were also inversely associated with trait anxiety [r(201) = −0.19, p < 0.01] and marginally with state anxiety [r(199) = −0.14, p = 0.056]. None of these measures was associated with MetS signs, so they cannot account for the association between MetS indices and neuroendocrine responses.
Discussion
These findings add to a growing literature on dysregulation of HPA axis function in relation to central obesity and other features of MetS. We studied healthy adults without known diabetes or cardiovascular disease, and relatively few participants had clinically significant elevations in these metabolic parameters or met our criteria for MetS. Nonetheless, we found linear relationships of individual MetS parameters with cortisol and ACTH responses, suggesting that our findings may largely reflect subclinical processes involved in the pathogenesis of MetS. Identification of premorbid risk processes might permit early detection and intervention prior to the development of MetS and related diseases.
It has been hypothesized that excessive cortisol exposure, with the attendant metabolic and tissue alterations that promote MetS, is causally involved in this syndrome. Several prior investigations documented that central obesity and other aspects of MetS were associated with elevated basal or provoked cortisol concentrations; however, our findings are consistent with other work finding reduced circulating cortisol concentrations and attenuated responses to the CRH stimulation test. Our observations can be reconciled with the hypothesis that cortisol is causally involved in MetS by the recognition that circulating cortisol concentrations might not reflect local glucocorticoid activity. In addition to being preferentially expressed in adipose tissue, 11βl-HSD1 is reduced in liver of obese humans and rodents [2,40]. 5α-Reductase activity, which inactivates cortisol, is enhanced in liver in obese humans and rodents [2]. Several studies of obesity have documented increased cortisol clearance and reduced conversion from cortisone [2]. Thus, in obesity, circulating cortisol may be decreased due to increased cortisol clearance, but local effects of cortisol in adipose tissue on tissue composition, dyslipidemia, and insulin resistance may be enhanced.
That we found an association of MetS characteristics with 1) lower ACTH in addition to cortisol responses to challenge, and 2) lower cortisol and ACTH responses to Dex prior to administration of CRH, suggests the possibility that enhanced glucocorticoid receptor sensitivity may be a mechanism of the association between MetS signs and attenuated neuroendocrine responses to the Dex/CRH test. MetS could develop in response to exaggerated effects of cortisol in the periphery, while enhanced central negative feedback would lead to reduced cortisol and ACTH responses to the test. Multiple abnormalities in the HPA system have been associated with obesity, and it remains unclear whether there is a primary mechanism or multiple alterations that might give rise to MetS.
Relative hypocortisolism or reduced cortisol response to provocation has been associated with other somatic conditions, including chronic fatigue, fibromyalgia, and asthma, as well as atypical depression and PTSD [41]. Although the mechanism is unknown, hypersecretion due to chronic stress or genetic influences with subsequent compensatory stress hyporeactivity has been proposed. Our group and others have found that healthy adults with a history of early life stress have blunted cortisol responses to psychosocial and neurobiological stress tests [39,42–46]. A recent longitudinal study found that childhood adversity increased the risk of developing MetS risk factors and elevations of an inflammatory marker in adulthood [47]. Despite this strong evidence, stress exposure has generally not been examined in studies of neuroendocrine abnormalities in obesity or MetS. In the current investigation, participants with a history of childhood emotional abuse had reduced cortisol responses to the Dex/CRH test, but history of early life stress or exposure to stress in the past month did not explain the links we found between hormone reactivity and parameters of MetS.
Several limitations of this study should be noted. While the effects we observed across different components of MetS and different tests of HPA function are consistent, the magnitude of the effects was modest. Multiple factors may influence the neuroendocrine association with MetS, and each may have a relatively small contribution. This study was limited by the use of measures that are robust to the nonfasting state. Our sample included few participants with MetS, and therefore can only address subclinical processes. Inflammatory mediators also play an important role in obesity and MetS and future work should address these influences as well as genetic and epigenetic factors. Finally, the Dex/CRH test is a sensitive probe of HPA axis activation, but it does not allow assessment of specific components of the HPA axis. Tests that specifically target individual regulatory mechanisms in this system as well as measures of basal cortisol concentrations would provide further insight as to the mechanisms underlying these findings.
Acknowledgments
Funding for this study was provided by K23 MH067947 (ART) and R01 MH068767 (LLC).
References
- 1.Anagnostis P, Athyros VG, Tziomalos K, Karagiannis A, Mikhailidis DP. Clinical review: The pathogenetic role of cortisol in the metabolic syndrome: a hypothesis. J Clin Endocrinol Metab. 2009;94:2692–2701. doi: 10.1210/jc.2009-0370. [DOI] [PubMed] [Google Scholar]
- 2.Morton NM. Obesity and corticosteroids: 11beta-hydroxysteroid type 1 as a cause and therapeutic target in metabolic disease. Mol Cell Endocrinol. 2010;316:154–164. doi: 10.1016/j.mce.2009.09.024. [DOI] [PubMed] [Google Scholar]
- 3.Rosmond R. Role of stress in the pathogenesis of the metabolic syndrome. Psychoneuroendocrinology. 2005;30:1–10. doi: 10.1016/j.psyneuen.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 4.Duclos M, Marquez Pereira P, Barat P, Gatta B, Roger P. Increased cortisol bioavailability, abdominal obesity, and the metabolic syndrome in obese women. Obes Res. 2005;13:1157–1166. doi: 10.1038/oby.2005.137. [DOI] [PubMed] [Google Scholar]
- 5.Filipovsky J, Ducimetiere P, Eschwege E, Richard JL, Rosselin G, Claude JR. The relationship of blood pressure with glucose, insulin, heart rate, free fatty acids and plasma cortisol levels according to degree of obesity in middle-aged men. J Hypertens. 1996;14:229–235. doi: 10.1097/00004872-199602000-00012. [DOI] [PubMed] [Google Scholar]
- 6.Lasikiewicz N, Hendrickx H, Talbot D, Dye L. Exploration of basal diurnal salivary cortisol profiles in middle-aged adults: associations with sleep quality and metabolic parameters. Psychoneuroendocrinology. 2008;33:143–151. doi: 10.1016/j.psyneuen.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 7.Ljung T, Andersson B, Bengtsson BA, Bjorntorp P, Marin P. Inhibition of cortisol secretion by dexamethasone in relation to body fat distribution: a dose-response study. Obes Res. 1996;4:277–282. doi: 10.1002/j.1550-8528.1996.tb00546.x. [DOI] [PubMed] [Google Scholar]
- 8.Ljung T, Holm G, Friberg P, Andersson B, Bengstson BA, Svensson J, Dallman M, McEwen B, Bjorntorp P. The activity of the hypothalamic-pituitary-adrenal axis and the sympathetic nervous system in relation to waist/hip circumference ratio in men. Obes Res. 2000;8:487–495. doi: 10.1038/oby.2000.61. [DOI] [PubMed] [Google Scholar]
- 9.Marniemi J, Kronholm E, Aunola S, Toikka T, Mattlar CE, Koskenvuo M, Ronnemaa T. Visceral fat and psychosocial stress in identical twins discordant for obesity. J Intern Med. 2002;251:35–43. doi: 10.1046/j.1365-2796.2002.00921.x. [DOI] [PubMed] [Google Scholar]
- 10.Rask E, Olsson T, Soderberg S, Andrew R, Livingstone DE, Johnson O, Walker BR. Tissue-specific dysregulation of cortisol metabolism in human obesity. J Clin Endocrinol Metab. 2001;86:1418–1421. doi: 10.1210/jcem.86.3.7453. [DOI] [PubMed] [Google Scholar]
- 11.Rutters F, Nieuwenhuizen AG, Lemmens SG, Born JM, Westerterp-Plantenga MS. Hypothalamic-pituitary-adrenal (HPA) axis functioning in relation to body fat distribution. Clin Endocrinol. 2010;72:738–743. doi: 10.1111/j.1365-2265.2009.03712.x. [DOI] [PubMed] [Google Scholar]
- 12.Ward AM, Syddall HE, Wood PJ, Dennison EM, Phillips DI. Central hypothalamic-pituitary-adrenal activity and the metabolic syndrome: Studies using the corticotrophin-releasing hormone test. Metabolism. 2004;53:720–726. doi: 10.1016/j.metabol.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 13.Pasquali R, Cantobelli S, Casimirri F, Capelli M, Bortoluzzi L, Flamia R, Labate AM, Barbara L. The hypothalamic-pituitary-adrenal axis in obese women with different patterns of body fat distribution. J Clin Endocrinol Metab. 1993;77:341–346. doi: 10.1210/jcem.77.2.8393881. [DOI] [PubMed] [Google Scholar]
- 14.Ward AM, Fall CH, Stein CE, Kumaran K, Veena SR, Wood PJ, Syddall HE, Phillips DI. Cortisol and the metabolic syndrome in South Asians. Clin Endocrinol. 2003;58:500–505. doi: 10.1046/j.1365-2265.2003.01750.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brunner EJ, Hemingway H, Walker BR, Page M, Clarke P, Juneja M, Shipley MJ, Kumari M, Andrew R, Seckl JR, Papadopoulos A, Checkley S, Rumley A, Lowe GD, Stansfeld SA, Marmot MG. Adrenocortical, autonomic, and inflammatory causes of the metabolic syndrome: nested case-control study. Circulation. 2002;106:2659–2665. doi: 10.1161/01.cir.0000038364.26310.bd. [DOI] [PubMed] [Google Scholar]
- 16.Marin P, Darin N, Amemiya T, Andersson B, Jern S, Bjorntorp P. Cortisol secretion in relation to body fat distribution in obese premenopausal women. Metabolism. 1992;41:882–886. doi: 10.1016/0026-0495(92)90171-6. [DOI] [PubMed] [Google Scholar]
- 17.Misra M, Bredella MA, Tsai P, Mendes N, Miller KK, Klibanski A. Lower growth hormone and higher cortisol are associated with greater visceral adiposity, intramyocellular lipids, and insulin resistance in overweight girls. Am J Physiol Endocrinol Metab. 2008;295:E385–E392. doi: 10.1152/ajpendo.00052.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sen Y, Aygun D, Yilmaz E, Ayar A. Children and adolescents with obesity and the metabolic syndrome have high circulating cortisol levels. Neuro Endocrinol Lett. 2008;29:141–145. [PubMed] [Google Scholar]
- 19.Steptoe A, Kunz-Ebrecht SR, Brydon L, Wardle J. Central adiposity and cortisol responses to waking in middle-aged men and women. Int J Obes Relat Metab Disord. 2004;28:1168–1173. doi: 10.1038/sj.ijo.0802715. [DOI] [PubMed] [Google Scholar]
- 20.Weigensberg MJ, Toledo-Corral CM, Goran MI. Association between the metabolic syndrome and serum cortisol in overweight Latino youth. J Clin Endocrinol Metab. 2008;93:1372–1378. doi: 10.1210/jc.2007-2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reinehr T, Andler W. Cortisol and its relation to insulin resistance before and after weight loss in obese children. Horm Res. 2004;62:107–112. doi: 10.1159/000079841. [DOI] [PubMed] [Google Scholar]
- 22.Phillips DI, Barker DJ, Fall CH, Seckl JR, Whorwood CB, Wood PJ, Walker BR. Elevated plasma cortisol concentrations: a link between low birth weight and the insulin resistance syndrome? J Clin Endocrinol Metab. 1998;83:757–760. doi: 10.1210/jcem.83.3.4634. [DOI] [PubMed] [Google Scholar]
- 23.Rosmond R, Dallman MF, Bjorntorp P. Stress-related cortisol secretion in men: relationships with abdominal obesity and endocrine, metabolic and hemodynamic abnormalities. J Clin Endocrinol Metab. 1998;83:1853–1859. doi: 10.1210/jcem.83.6.4843. [DOI] [PubMed] [Google Scholar]
- 24.Kirschbaum C, Pirke KM, Hellhammer DH. The ‘Trier Social Stress Test’ – a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28:76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- 25.Epel ES, McEwen B, Seeman T, Matthews K, Castellazzo G, Brownell KD, Bell J, Ickovics JR. Stress and body shape: stress-induced cortisol secretion is consistently greater among women with central fat. Psychosom Med. 2000;62:623–632. doi: 10.1097/00006842-200009000-00005. [DOI] [PubMed] [Google Scholar]
- 26.Mujica-Parodi LR, Renelique R, Taylor MK. Higher body fat percentage is associated with increased cortisol reactivity and impaired cognitive resilience in response to acute emotional stress. Int J Obs. 2009;33:157–165. doi: 10.1038/ijo.2008.218. [DOI] [PubMed] [Google Scholar]
- 27.Wallerius S, Rosmond R, Ljung T, Holm G, Bjorntorp P. Rise in morning saliva cortisol is associated with abdominal obesity in men: a preliminary report. J Endocrinol Invest. 2003;26:616–619. doi: 10.1007/BF03347017. [DOI] [PubMed] [Google Scholar]
- 28.Bengtsson I, Lissner L, Ljung T, Rosengren A, Thelle D, Wahrborg P. The cortisol awakening response and the metabolic syndrome in a population-based sample of middle-aged men and women. Metabolism. 2010;59:1012–1019. doi: 10.1016/j.metabol.2009.10.024. [DOI] [PubMed] [Google Scholar]
- 29.Pasquali R, Ambrosi B, Armanini D, Cavagnini F, Uberti ED, Del Rio G, de Pergola G, Maccario M, Mantero F, Marugo M, Rotella CM, Vettor R. Cortisol and ACTH response to oral dexamethasone in obesity and effects of sex, body fat distribution, and dexamethasone concentrations: a dose-response study. J Clin Endocrinol Metab. 2002;87:166–175. doi: 10.1210/jcem.87.1.8158. [DOI] [PubMed] [Google Scholar]
- 30.Kopelman PG, Grossman A, Lavender P, Besser GM, Rees LH, Coy D. The cortisol response to corticotrophin-releasing factor is blunted in obesity. Clin Endocrinol. 1988;28:15–18. doi: 10.1111/j.1365-2265.1988.tb01197.x. [DOI] [PubMed] [Google Scholar]
- 31.Trainer PJ, Faria M, Newell-Price J, Browne P, Kopelman P, Coy DH, Besser GM, Grossman AB. A comparison of the effects of human and ovine corticotropin-releasing hormone on the pituitary-adrenal axis. J Clin Endocrinol Metab. 1995;80:412–417. doi: 10.1210/jcem.80.2.7852498. [DOI] [PubMed] [Google Scholar]
- 32.Solano MP, Kumar M, Fernandez B, Jones L, Goldberg RB. The pituitary response to ovine corticotropin-releasing hormone is enhanced in obese men and correlates with insulin resistance. Horm Res. 2001;33:39–43. doi: 10.1055/s-2001-12625. [DOI] [PubMed] [Google Scholar]
- 33.Katz JR, Taylor NF, Goodrick S, Perry L, Yudkin JS, Coppack SW. Central obesity, depression and the hypothalamo-pituitary-adrenal axis in men and postmenopausal women. Int J Obes Relat Metab Disord. 2000;24:246–251. doi: 10.1038/sj.ijo.0801122. [DOI] [PubMed] [Google Scholar]
- 34.Pasquali R, Anconetani B, Chattat R, Biscotti M, Spinucci G, Casimirri F, Vicennati V, Carcello A, Labate AM. Hypothalamic-pituitary-adrenal axis activity and its relationship to the autonomic nervous system in women with visceral and subcutaneous obesity: effects of the corticotropin-releasing factor/arginine-vasopressin test and of stress. Metabolism. 1996;45:351–356. doi: 10.1016/s0026-0495(96)90290-5. [DOI] [PubMed] [Google Scholar]
- 35.Epel ES. Psychological and metabolic stress: a recipe for accelerated cellular aging? Hormones. 2009;8:7–22. doi: 10.14310/horm.2002.1217. [DOI] [PubMed] [Google Scholar]
- 36.Folsom AR, Kuba K, Leupker RV, Jacobs DR, Frantz ID., Jr Lipid concentrations in serum and EDTA-treated plasma from fasting and nonfasting normal persons, with particular regard to high-density lipoprotein cholesterol. Clin Chem. 1983;29:505–508. [PubMed] [Google Scholar]
- 37.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15:539–553. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 38.Tyrka AR, Wier LM, Anderson GM, Wilkinson CW, Price LH, Carpenter LL. Temperament and response to the Trier Social Stress Test. Acta Psychiatrica Scandinavica. 2007;115:395–402. doi: 10.1111/j.1600-0447.2006.00941.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carpenter LL, Tyrka AR, Ross NS, Khoury L, Anderson GM, Price LH. Effect of childhood emotional abuse and age on cortisol responsivity in adulthood. Biol Psychiatry. 2009;66:69–75. doi: 10.1016/j.biopsych.2009.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Inder WJ, Obeyesekere VR, Alford FP, Jang C. Skeletal muscle 11HSD1β activity of nondiabetic subjects is unaltered in central obesity-associated insulin resistance. Horm Metab Res. 2011;43:257–260. doi: 10.1055/s-0030-1269905. [DOI] [PubMed] [Google Scholar]
- 41.Raison CL, Miller AH. When not enough is too much: the role of insufficient glucocorticoid signaling in the pathophysiology of stress-related disorders. Am J Psychiatry. 2003;160:1554–1565. doi: 10.1176/appi.ajp.160.9.1554. [DOI] [PubMed] [Google Scholar]
- 42.Carpenter LL, Carvalho JP, Tyrka AR, Wier LM, Mello AF, Mello MF, Anderson GM, Wilkinson CW, Price LH. Decreased adrenocorticotropic hormone and cortisol responses to stress in healthy adults reporting significant childhood maltreatment. Biol Psychiatry. 2007;62:1080–1087. doi: 10.1016/j.biopsych.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carpenter LL, Tyrka AR, McDougle CJ, Malison RT, Owens MJ, Nemeroff CB, Price LH. Cerebrospinal fluid corticotropin-releasing factor and perceived early-life stress in depressed patients and healthy control subjects. Neuropsychopharmacology. 2004;29:777–784. doi: 10.1038/sj.npp.1300375. [DOI] [PubMed] [Google Scholar]
- 44.Elzinga BM, Roelofs K, Tollenaar MS, Bakvis P, van Pelt J, Spinhoven P. Diminished cortisol responses to psychosocial stress associated with lifetime adverse events a study among healthy young subjects. Psychoneuroendocrinology. 2008;33:227–237. doi: 10.1016/j.psyneuen.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 45.Tyrka AR, Wier L, Price LH, Ross N, Anderson GM, Wilkinson CW, Carpenter LL. Childhood parental loss and adult hypothalamic-pituitary-adrenal function. Biol Psychiatry. 2008;63:1247–1254. doi: 10.1016/j.biopsych.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Klaassens ER, van Noorden MS, Giltay EJ, van Pelt J, van Veen T, Zitman FG. Effects of childhood trauma on HPA-axis reactivity in women free of lifetime psychopathology. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:889–894. doi: 10.1016/j.pnpbp.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 47.Danese A, Moffitt TE, Harrington H, Milne BJ, Polanczyk G, Pariante CM, Poulton R, Caspi A. Adverse childhood experiences and adult risk factors for age-related disease: depression, inflammation, and clustering of metabolic risk markers. Arch Pediatr Adolesc Med. 2009;163:1135–1143. doi: 10.1001/archpediatrics.2009.214. [DOI] [PMC free article] [PubMed] [Google Scholar]