Abstract
Cromolyn, widely characterized as a “mast cell stabilizer”, has been used in mice to investigate the biological roles of mast cells in vivo. However, it is not clear to what extent cromolyn can either limit the function of mouse mast cells or influence biological processes in mice independently of effects on mast cells. We confirmed that cromolyn (at 10 mg/kg in vivo or 10 – 100 μM in vitro) can inhibit IgE-dependent mast cell activation in rats in vivo (measuring Evans blue extravasation in passive cutaneous anaphylaxis and increases in plasma histamine in passive systemic anaphylaxis) and in vitro (measuring peritoneal mast cell β-hexosaminidase release and prostaglandin D2 synthesis). However, under the conditions tested, cromolyn did not inhibit those mast cell-dependent responses in mice. In mice, cromolyn also failed to inhibit the ear swelling or leukocyte infiltration at sites of passive cutaneous anaphylaxis. Nor did cromolyn inhibit IgE-independent degranulation of mouse peritoneal mast cells induced by various stimulators in vitro. At 100 mg/kg, a concentration ten times higher than that which inhibited passive systemic anaphylaxis in rats, cromolyn significantly inhibited the increases in plasma concentrations of mouse mast cell protease-1 (but not of histamine) during passive systemic anaphylaxis, but had no effect on the reduction in body temperature in this setting. Moreover, this concentration of cromolyn (100 mg/kg) also inhibited LPS-induced TNF production in genetically mast cell-deficient C57BL/6-KitW-sh/W-sh mice in vivo. These results question cromolyn’s effectiveness and selectivity as an inhibitor of mast cell activation and mediator release in the mouse.
Keywords: Anaphylaxis, cromolyn, disodium cromoglycate, DSCG, mast cell, mouse, rat
Mast cells have long been regarded as exceptionally efficient initiators and amplifiers of certain innate and acquired immune responses, especially IgE-dependent acute responses to challenge with specific antigen.1–5 In addition to their well-known functions in IgE-dependent responses, mast cells also can secrete mediators in response to a variety of other signals including products of pathogens, components of animal venoms,6–7 products of complement activation, neurotransmitters,8 vascular factors,9 and stem cell factor (SCF), suggesting that mast cells have a much larger spectrum of potential roles in health and disease than was originally thought.1–5
One approach for understanding the roles of mast cells in health and disease would be to employ drugs that can effectively and selectively inhibit the function of this cell in vivo. Cromolyn (cromolyn sodium, sodium cromoglycate, SCG, disodium cromoglycate, DSCG) is often used as such an agent. Although its mechanism of action has not been clarified, cromolyn has been used clinically as an anti-asthma drug for more than 30 years.10 Clinical studies showed that inhalation of cromolyn by subjects with allergic asthma blocked allergen-induced bronchospasm, presumably by inhibiting the release of mediators from mast cells,11 an idea that was supported by the drug’s ability to inhibit the degranulation of rat peritoneal mast cells in response to challenge with IgE and specific antigen in vitro.12–15 For this reason, cromolyn is often characterized clinically as a “mast cell stabilizer”,16 and this drug is now used for the treatment of other diseases thought to involve mast cell activation, including allergic rhinitis, allergic conjunctivitis, and mastocytosis.17
Although the early studies of cromolyn were performed predominantly in rats and humans, many groups are now employing this agent to “stabilize” mast cells or to suppress mast cell functions in mice. Indeed, treatment of mice with cromolyn in vivo has become commonplace as part of efforts to investigate roles of mast cells in mouse models of host defense or disease that are thought to be independent of IgE.18–29 However, surprisingly, there is little published information supporting the conclusion that cromolyn is either an effective or selective “stabilizer” (i.e., inhibitor) of the activation of mast cell populations in the mouse.
On the contrary, one group reported that cromolyn (at 200 mg/kg) did not inhibit Evans blue extravasation associated with IgE-dependent PCA reactions in mice, one of the most common assays used to measure mast cell function in vivo.30 Moreover, other groups reported that treatment with cromolyn (at 1 – 100 μM31 or 1 – 100 μg/mL,15 respectively) at the time of antigen challenge did not inhibit IgE plus antigen-induced degranulation31 or cysteinyl leukotriene release15 in mouse bone-marrow-derived cultured mast cells (BMCMCs) in vitro. However, Marquardt et al. reported that long term culture of mouse BMCMCs with cromolyn, as opposed to short-term treatment with the drug, resulted in a significant inhibition of IgE- and antigen-induced mediator release by BMCMCs, but the basis for the difference in the response of these cells to short-term versus long-term treatment with cromolyn was not defined.31 In addition, Forbes et al. reported that repeated treatment of mice with cromolyn inhibited the increases in blood concentrations of the mast cell-associated mediator, mouse mast cell protease-1 (mMCP-1), a product of mucosal mast cells, in mice that genetically overexpressed IL-9.32 Taken together, such studies raise the possibility that cromolyn might have inhibitory effects on some mast cell functions (such as the release of MCP-1 by mucosal mast cells32) but not others (e.g., IgE-dependent activation of skin mast cells30), and that the effects of the drug on even the same type of mast cells may depend on the conditions of cromolyn exposure.15,31
However, there are very few reports analyzing the effects of cromolyn on mouse mast cells, and we are aware of no reports on the effects of cromolyn on mouse peritoneal mast cells (PMCs) – the mast cell type most thoroughly investigated in studies of the effects of cromolyn on mast cell activation in the rat.12–15 Accordingly, we think that it is difficult to draw any conclusion about the effectiveness or selectivity of cromolyn as a mast cell stabilizer in mice other than more studies of this topic are needed. By contrast, there have been many reports showing that cromolyn can inhibit rat PMC degranulation12–15 or prostaglandin D2 (PGD2) synthesis in vitro,33 as well as reduce the extent of IgE-dependent degranulation of rat skin mast cells in passive cutaneous anaphylaxis (PCA) reactions in vivo13,15,30.
In the present study, we used rats and rat PMCs as positive controls for experiments designed to investigate in detail the effectiveness of cromolyn as an inhibitor of mouse mast cell activation in vivo and in vitro. These studies included testing the drug’s ability to interfere with mouse PMC activation by IgE and specific antigen or by multiple different agents which can elicit IgE-independent mast cell activation in vitro. Because it has been well-established that pre-treatment of rat PMCs with cromolyn before stimulating the cells to degranulate substantially reduced the inhibitory effect of cromolyn on PMC degranulation (an effect of the drug called “tachyphylaxis”12,13), we focused primarily on the effects of cromolyn treatment observed when the agent is administered at the time of mast cell activation. We also used genetically mast cell-deficient mice to assess whether the actions of this agent are selective for mast cells in vivo.
MATERIALS AND METHODS
Animals
KitW-sh/+ mice generously provided by Peter Besmer (Memorial Sloan-Kettering Cancer Center) were backcrossed with C57BL/6J mice (Jackson Laboratories) for more than 11 generations to produce mast cell-deficient C57BL/6-KitW-sh/W-sh (herein: KitW-sh/W-sh) mice.34 Cpa3-Cre; Mcl-1+/+ and mast cell- and basophil-deficient Cpa3-Cre; Mcl-1fl/fl mice were bred in our laboratory.35 C57BL/6J mice or Wistar rats were purchased from Jackson Laboratories or Charles River, respectively. All animal care and experimentation was conducted in accordance with the “Guide for the Care and Use of Laboratory Animals” prepared by the Institute of Laboratory Animal Resources, National Research Council, and published by the National Academy Press (revised, 1996) and with the approval of the Stanford University Committee on Animal Welfare.
Passive cutaneous anaphylaxis (PCA) reaction
Rats (female, 8 weeks) and mice (male or female, 8 weeks) were sensitized by intradermal (i.d.) injection of anti-DNP IgE (αDNP clone ε26, generously provided by Dr. Fu-Tong Liu, UC Davis; 2.5 ng for rat dorsal skin; 10 ng for mouse ear pinnae) in saline (0.9% sodium chloride; 50 μL for rats; 10 μL for mice). Twenty four h later, DNP-HSA (Sigma; 1 mg/kg for rats; 10 mg/kg for mice) was injected i.v. with or without cromolyn sodium salt (MP Biomedicals). Mouse ear thickness was measured with a dial thickness gauge (G-1A, Ozaki, Tokyo, Japan). Mouse ear pinnae collected from mice killed by exposure to CO2 6 h after challenge were fixed in 10% formalin for preparation of paraffin sections stained with hematoxylin and eosin, and leukocyte numbers were quantified by light microscopy as per mm of horizontal field length of the ear (by an observer not aware of the identity of the individual sections). For measuring Evans blue extravasation, Evans blue (Sigma; 10 mg/kg for rats; 100 mg/kg for mice) was injected i.v. with DNP-HSA.36 Thirty min later, skin areas were photographed and cut out. Evans blue dye was extracted by incubating the skin samples in DMSO (1 mL for rat samples; 0.5 mL for mouse samples) for 24 h at 37°C, and then O.D. 650 nm was measured.
Passive systemic anaphylaxis (PSA) reaction
Rats (female, 8 weeks) and mice (male, 8 weeks) were sensitized i.v. with anti-DNP IgE (1 μg/kg for rats; 100 μg/kg for mice37). Twenty four h later, DNP-HSA (1 mg/kg for rats; 10 mg/kg for mice37) was injected i.v. with or without cromolyn. Ninety sec later, blood was collected and the plasma histamine or mouse mast cell protease-1 (mMCP-1) concentrations were measured using histamine or mMCP-1 ELISA kits (Beckman coulter or eBioscience, respectively). Body temperature was measured with a rectal thermometer (Physitemp Instrument, Inc., NJ).
Preparation of PMCs
Whole peritoneal cells were collected in RPMI medium (GIBCO; with 1 mg/mL BSA and 10 units/mL heparin) from rat (female, 12 weeks) or mouse (female, retired bleeder) peritoneal cavities. The cells were mounted on 0.235 g/mL histodenz (Sigma), and were centrifuged at 400 xg (7 min for rats; 15 min for mice). The cells at the bottom of the tube were collected. More than 90% cells were PMCs by toluidine blue metachromatic staining. For stimulation with antigen (DNP-HSA), PMCs were cultured for 24 h with anti-DNP IgE in Opti-MEM (GIBCO; with 10% FBS) prior to stimulation with DNP-HSA. For other stimuli (recombinant mouse SCF was purchased from Peprotech, all other materials from Sigma), the PMCs were used immediately after isolation.
Measurement of β-hexosaminidase release
β-Hexosaminidase release was measured as described previously.6,36 The PMCs were resuspended (5 × 105 cells/mL) in Tyrode’s buffer6 then treated with the stimuli in the absence or presence (added simultaneously) of cromolyn at 37°C. The reactions were stopped on ice 3 min later. The percentage of degranulation was calculated, taking the O.D. 405 nm of 0.5% triton X-100-treated cell sample as 100%.
Measurement of PGD2 production
PMCs sensitized with IgE were resuspended (5 × 105 cells/mL) in Tyrode’s buffer (without BSA), then treated with 100 ng/mL DNP-HSA in the absence or presence (added simultaneously [except Supplementary Figure S6]) of cromolyn at 37°C. The reactions were stopped on ice 10 min later and PGD2 concentrations in the supernatants were measured by a PGD2 EIA kit (Cayman chemical company).
LPS challenge in vivo and measurement of TNF
Vehicle (sterile, non-pyrogenic 0.9% sodium chloride, Hospira) or cromolyn (100 mg/kg in vehicle) was administered to mice (male, 8 weeks) by i.p. injection 30 min before challenge i.p. with 0.1 mg/kg LPS (LPS from E. coli, Serotype EH100, Enzo Life Sciences). Blood samples were collected 90 min after challenge and plasma was collected by centrifugation. TNF concentrations in plasma were measured using a mouse TNF ELISA kit (BD Biosciences).
LPS challenge in vitro
Spleen cells were collected from the spleens of mice (male, 8 weeks), treated with ACK lysing buffer for 5 min, and then passed through 70 μm cell strainers. Peritoneal cells collected from mouse peritoneal cavities were washed twice and resuspended in RPMI (with 10% FBS). The spleen cells (5 × 106 cells/mL) or the peritoneal cells (5 × 10 5 cells/mL) were treated with or without cromolyn for 15 min and then stimulated with 1 ng/mL LPS for 3 h at 37°C. TNF concentrations in supernatants were measured by an ELISA as described above.
Statistical analyses
Unless noted otherwise, all results are expressed as mean + or +/− S.D. and were evaluated for statistical significance (defined as p < 0.05) by unpaired Student’s t-test for comparisons between two groups (Supplementary Figure S1 and S3), by two-way ANOVA for comparisons between the time dependent curve of ear thickness (Figure 1c and S4c) and body temperature (Figure 2c), or by one-way ANOVA followed by Bonferroni test for comparisons between more than two groups (all other figures).
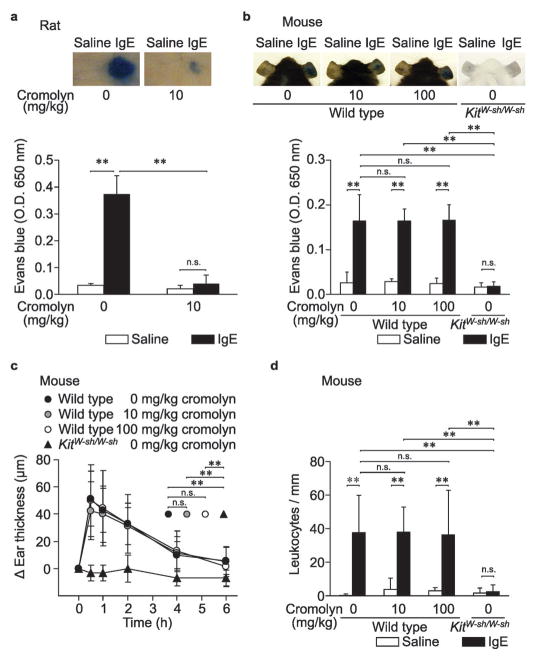
Figure 1.
Effects of cromolyn on PCA reactions in vivo. Female rats (a) or male mice (b – d) injected i.d. with vehicle (saline) or anti-DNP IgE (IgE) were challenged i.v. with DNP-HSA with or without cromolyn (injected simultaneously); we then measured Evans blue extravasation (a and b), ear swelling (c) and leukocyte migration (d). N = 4 rats (a), 5 mice (b) and 7 – 8 mice (c and d) per group from at least 2 independent experiments. ** p < 0.01, * p < 0.05; n.s.: p > 0.05.
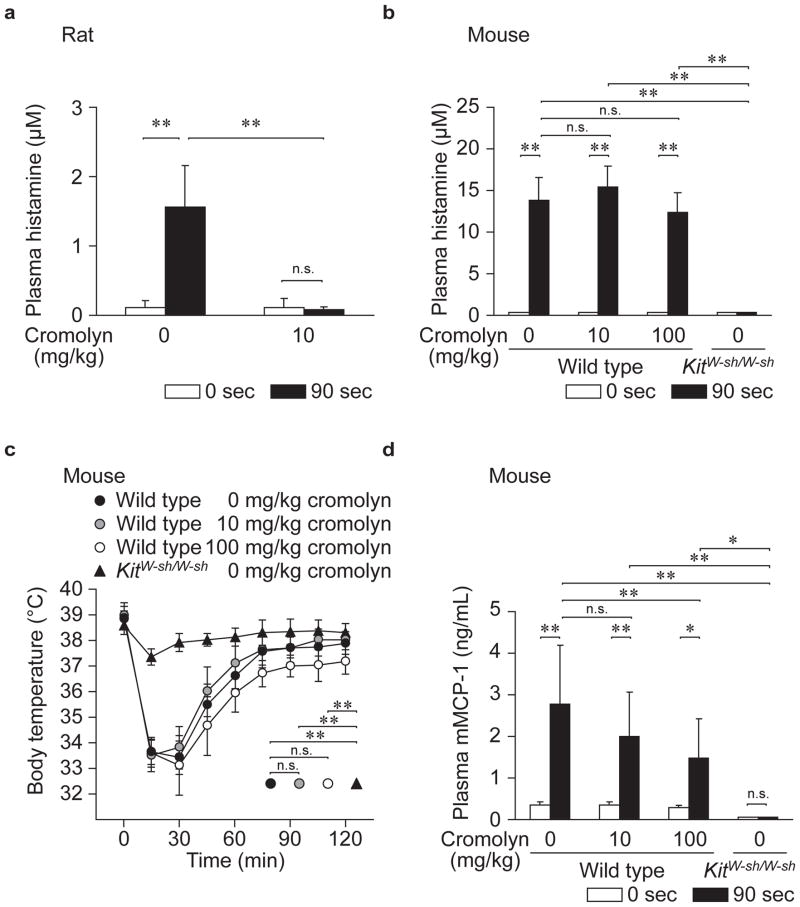
Figure 2.
Effect of cromolyn on PSA reactions in vivo. Female rats (a) or male mice (b – d) sensitized i.v. with anti-DNP IgE were challenged i.v. with DNP-HSA with or without cromolyn (injected simultaneously); we then measured plasma histamine (a and b), body temperature (c) and plasma mMCP-1 (d). N = 4 rats (a), and 5 – 10 mice (b – d) per group from at least 2 independent experiments. ** p < 0.01, * p < 0.05; n.s.: p > 0.05.
RESULTS
Cromolyn can effectively inhibit activation of rat, but not mouse, mast cells in vivo
First, we sought to confirm the well-established inhibitory effect of cromolyn on rat PCA reactions (Table 1).13,15,30 In accordance with previously published reports, we found that cromolyn (10 mg/kg) almost completely inhibited Evans blue extravasation induced by local IgE-dependent mast cell degranulation in rats (Figure 1a). We then tested PCA reactions in mice. The different concentrations of reagents (i.e., IgE, DNP-HSA, and Evans blue) and the different skin regions used to induce PCA reactions (i.e., dorsal skin or ear pinnae) that we used routinely for studies in rats or mice were chosen based on preliminary experiments that took into account species-specific variation in the amounts needed to elicit and assess robust reactions (Supplementary Figure S1). As expected, Evans blue extravasation was not observed when we tried to elicit PCA reactions in mast cell-deficient KitW-sh/W-sh mice (Figure 1b), confirming that this reaction is dependent on the presence of skin mast cells.38 However, treatment with 10 mg/kg cromolyn did not inhibit the Evans blue extravasation at PCA reaction sites in wild type mice (Figure 1b). Even when administered at 100 mg/kg (a dose ten times higher than that used in rats), cromolyn did not significantly inhibit IgE-dependent PCA reactions in male mice (Figure 1b). Because we tested PCA in female rats, we also confirmed that there was no inhibitory effect of cromolyn (at 10 or 100 mg/kg) on PCA-associated Evans blue extravasation in female wild type mice (Supplementary Figure S2).
Table 1.
Ability of acute exposure to cromolyn to inhibit features of PCA or PSA responses in vivo or mast cell degranulation in vitro in rats and mice. The table shows results obtained when cromolyn was injected either simultaneously with13,15 or immediately before13,30 antigen challenge in vivo, or added either simultaneously with12–15,31 or 5 min before12,39 antigen challenge in vitro. All of the results reported below from this report are from experiments in which cromolyn was administered simultaneously with antigen.
| Previous reports | This report | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Rat | Mouse | Rat | Mouse | |||
| In vivo | PCA | Evans blue extravasation | Yes13,15,30 | No30 | Yes (10 mg/kg) | No (10 – 100 mg/kg) |
| Ear swelling | No (10 – 100 mg/kg) | |||||
| Leukocyte infiltration | No (10 – 100 mg/kg) | |||||
| PSA | Histamine release | Yes (10 mg/kg) | No (10 – 100 mg/kg) | |||
| Temperature decrease | No (10 – 100 mg/kg) | |||||
| mMCP-1 release | Yes (only at 100 mg/kg) | |||||
| In vitro | PMCs | Yes12–15 | Yes (10 – 100 μM) | No (1 – 100 μM) | ||
| BMCMCs | No39 | No15,31 | No (1 – 100 μM) | |||
| Mucosal mast cells | No14 | |||||
Ear swelling and numbers of dermal leukocytes also can be measured to evaluate PCA reactions in mice. In IgE-injected KitW-sh/W-sh mice, there were no significant changes in either ear thickness (Figure 1c) or skin leukocyte numbers (Figure 1d) after antigen challenge, confirming that both are largely or entirely dependent on the presence of skin mast cells. However, in support of our Evans blue extravasation results (Figure 1b), treatment with 10 or 100 mg/kg cromolyn failed to inhibit either the ear swelling (Figure 1c) or the local leukocyte response (Figure 1d) associated with PCA reactions in wild type mice.
We also measured plasma histamine concentrations 90 sec after inducing IgE-dependent PSA, again using higher concentrations of IgE and antigen in mice than rats in order to elicit robust reactions in each species (mice did not detectably respond to the concentrations of reagents that elicited a reaction in rats; see Supplementary Figure S3). When IgE-sensitized rats were challenged with antigen together with cromolyn (10 mg/kg), the antigen-induced increase in plasma histamine was completely inhibited (Figure 2a). Antigen challenge did not increase plasma histamine levels in IgE-sensitized KitW-sh/W-sh mice (Figure 2b), confirming that this finding is primarily dependent on mast cells. However, administration of 10 or 100 mg/kg cromolyn together with antigen failed to inhibit the increase in plasma histamine in IgE-sensitized wild type mice (Figure 2b). We also measured body temperature (Figure 2c) and plasma mMCP-1 concentration (Figure 2d) after inducing IgE-dependent PSA. Again, antigen challenge did not result in decreased body temperature (Figure 2c) or increased plasma mMCP-1 levels (Figure 2d) in IgE-sensitized KitW-sh/W-sh mice, confirming that these findings are primarily dependent on mast cells. However, administration of 10 or 100 mg/kg cromolyn together with antigen failed to inhibit the decrease in body temperature in wild type mice (Figure 2c). On the other hand, at the higher dose tested (100 mg/kg), cromolyn did significantly inhibit the increase in plasma mMCP-1 associated with IgE-dependent PSA in wild type mice (Figure 2d).
In some previous studies, cromolyn was administered repeatedly to mice.23,27,32 Therefore, we tested the effect of repeated administration of cromolyn (Supplementary Figure S4a) on IgE-dependent Evans blue dye extravasation (Supplementary Figure S4b) and ear swelling (Supplementary Figure S4c) in PCA reactions, and on increases in plasma histamine (Supplementary Figure S4d) and mMCP-1 (Supplementary Figure S4e) in PSA. In support of our finding in Figure 2d, the repeated administration of cromolyn inhibited the increases in plasma mMCP-1 concentrations associated with PSA (Supplementary Figure S4e). However, repeated treatment with cromolyn at 100 mg/kg failed to inhibit other features of the IgE-dependent reactions (Supplementary Figure S4b – d), including the increases in plasma histamine associated with IgE-dependent PSA (Supplementary Figure S4d).
Cromolyn can effectively inhibit activation of rat, but not mouse, mast cells in vitro
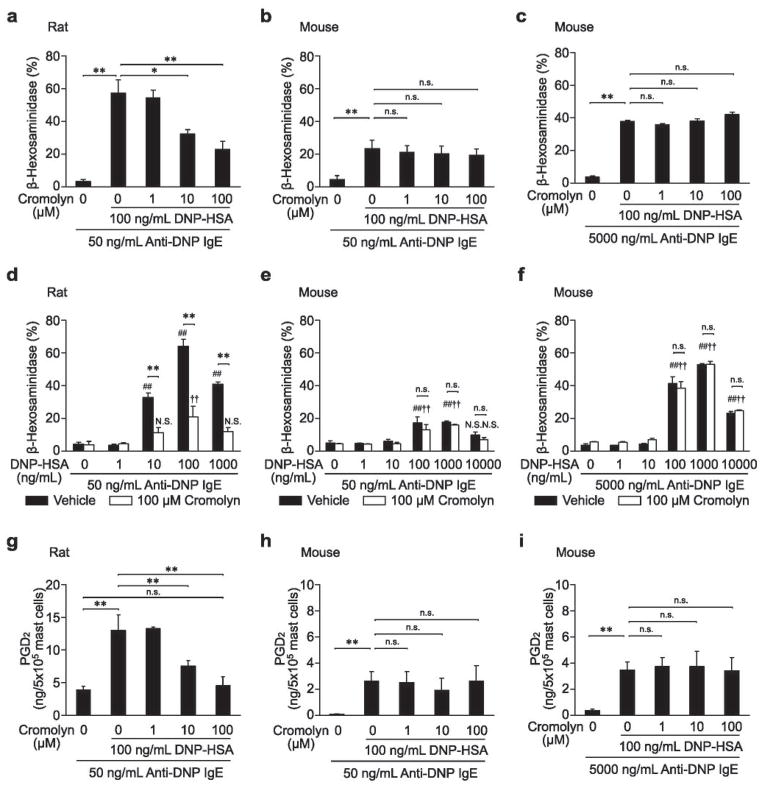
To study the effects of cromolyn on rat or mouse mast cell activation more directly, we assessed mouse PMC in vitro. In preliminary experiments, we confirmed that a higher concentration of IgE was needed to induce high levels of β-hexosaminidase release in mouse PMCs (Supplementary Figure S5b) than in rat PMCs (Supplementary Figure S5a) in vitro. Therefore, in tests of mouse PMCs, we used both low and high concentrations of IgE (i.e., 50 ng/mL [the same concentration as for rat PMC experiments] and 5000 ng/mL [in order to induce more robust activation of mouse PMCs]). First, we tried to confirm the well-established inhibitory effect of cromolyn on IgE- and antigen-induced degranulation in rat PMCs (Table 1).12–15 In accordance with previously published reports,12,13 we found that pretreating rat PMCs with cromolyn before adding DNP-HSA reduced the inhibitory effect of cromolyn on PMC degranulation (so called “tachyphylaxis”; Supplementary Figure S6). In order to avoid this phenomenon, we added cromolyn simultaneously with DNP-HSA challenge. The simultaneous addition of cromolyn (10 – 100 μM) inhibited antigen-induced IgE-dependent degranulation of rat PMCs in a concentration-dependent manner (Figure 3a). Cromolyn at 100 μM also inhibited β-hexosaminidase release induced by different antigen concentrations in rat PMCs (Figure 3d). However, treatment of mouse PMCs with cromolyn (at 1 – 100 μM) did not inhibit antigen- and IgE-induced degranulation (Figure 3b, c, e and f). Similarly, cromolyn (10 – 100 μM) inhibited PGD2 synthesis in rat (Figure 3g), but not mouse (Figure 3h and i), PMCs after challenge with IgE and antigen. We also tried to confirm the lack of effect of cromolyn on mouse BMCMC degranulation when the agent was administered at the time of antigen challenge. In accord with a prior report,31 treatment with cromolyn (at 1– 100 μM) at the time of antigen challenge did not inhibit antigen- and IgE-induced degranulation in mouse BMCMCs (Supplementary Figure S7). Moreover, in our hands, maintaining BMCMCs with cromolyn (1 μM to 1 mM) for up to 8 days also did not result in any inhibition of the cells’ degranulation in response to IgE and antigen (data not shown).
Figure 3.
Effects of cromolyn on IgE-dependent PMC degranulation (a – f) or PGD2 synthesis (g – i) in vitro. IgE-sensitized PMCs from female rats (a, d and g) or mice (b, c, e, f, h and i) were stimulated with DNP-HSA with or without cromolyn (added simultaneously). N = 3 – 10 per group from 1 – 2 independent experiments. ## p < 0.01, # p < 0.05, †† p < 0.01, † p < 0.05; N.S.: not significantly different versus corresponding values (vehicle or 100 μM cromolyn) for groups not treated with stimuli. ** p < 0.01, * p < 0.05; n.s.: p > 0.05.
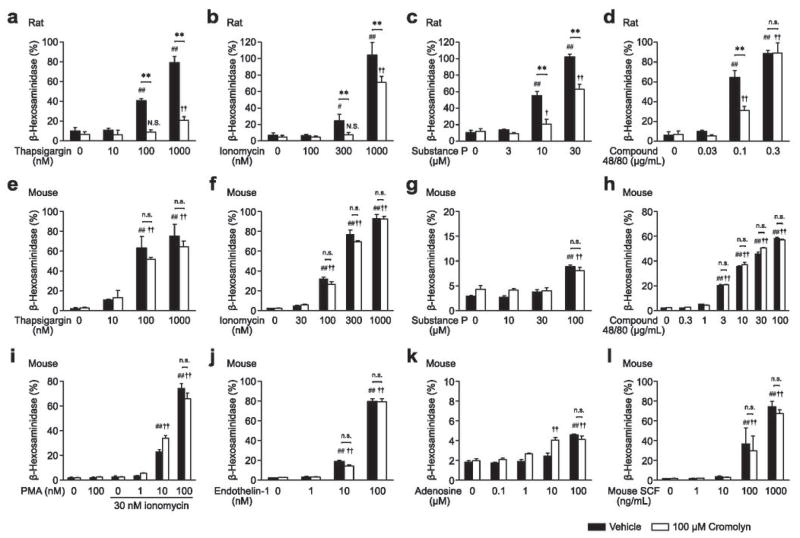
Although cromolyn did not inhibit IgE-dependent degranulation in mouse PMCs (Figure 3), the possibility remained that cromolyn might be able to inhibit IgE-independent degranulation induced by other activators. To investigate this possibility, we tested the ability of cromolyn to inhibit β-hexosaminidase release following stimulation with a variety of agents known to activate PMCs in vitro. We tested thapsigargin (Figure 4a and e), ionomycin (Figure 4b and f), substance P (Figure 4c and g), compound 48/80 (Figure 4d and h), phorbol 12-myristate 13-acetate (PMA) with ionomycin (Figure 4i), endothelin-1 (Figure 4j), adenosine (Figure 4k), and mouse SCF (Figure 4l). All agents induced β-hexosaminidase release from both rat and mouse PMCs in a dose dependent manner (Figure 4a – l). In rat PMCs (Figure 4a – d), despite differences in cromolyn sensitivity at different concentrations of certain stimuli (e.g., compound 48/80), the simultaneous addition of 100 μM cromolyn inhibited the degranulation induced by each of the stimuli tested. However, we observed no significant inhibitory effects of 100 μM cromolyn on the stimulus-induced degranulation responses of mouse PMCs to any of the agents we tested (Figure 4e – l).
Figure 4.
Effect of cromolyn on IgE-independent degranulation of PMCs in vitro. PMCs from female rats (a – d) or mice (e – l) were stimulated with thapsigargin (a and e), ionomycin (b and f), substance P (c and g), compound 48/80 (d and h), PMA with ionomycin (i), endothelin-1 (j), adenosine (k), or mouse SCF (l), with or without cromolyn (added simultaneously). N = 3 – 6 per group from 1 – 2 independent experiments. ## p < 0.01, # p < 0.05, †† p < 0.01, † p < 0.05; N.S. not significantly different versus corresponding values (vehicle or 100 μM cromolyn) for groups not treated with stimuli. ** p < 0.01, * p < 0.05; n.s.: p > 0.05.
Cromolyn can inhibit LPS-induced TNF production in both wild type and mast cell deficient mice
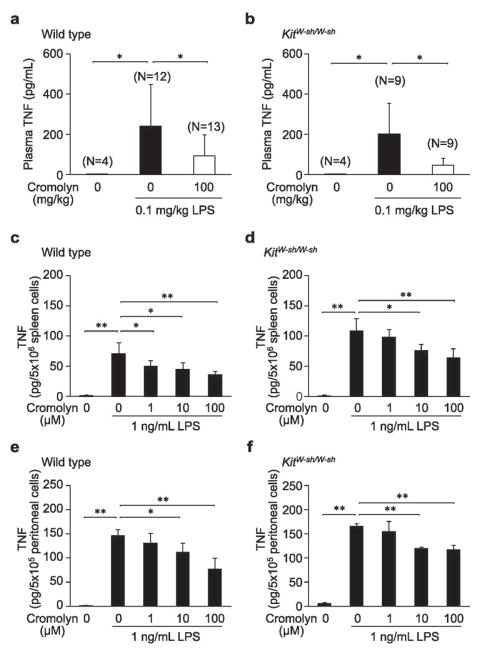
Finally, we investigated whether cromolyn might have detectable effects in genetically mast cell-deficient mice. Because KitW-sh/W-sh mice virtually lack mast cells, any effects of cromolyn observed in these mice would indicate an effect of the agent on other cell types. In wild type mice, increased levels of plasma TNF were detectable 90 min after injection of the mice with 0.1 mg/kg LPS (Figure 5a). Administration of 100 mg/kg cromolyn significantly inhibited the increase in plasma TNF levels in wild type mice (Figure 5a). In KitW-sh/W-sh mice, injection of LPS induced increases in plasma TNF to almost the same levels observed in wild type mice (Figure 5b), indicating that such LPS-induced TNF production can occur independently of mast cells. Moreover, administration of 100 mg/kg cromolyn significantly inhibited the increase in plasma TNF in KitW-sh/W-sh mice (Figure 5b). These results indicate that cromolyn can inhibit LPS-induced TNF production in mice in a mast cell-independent manner in vivo.
Figure 5.
Effects of cromolyn on LPS-induced TNF production in C57BL/6J wild type or mast cell-deficient KitW-sh/W-sh mice in vivo (a and b) or in vitro (c – f). Male wild type (a) or KitW-sh/W-sh (b) mice were injected i.p. with vehicle or 100 mg/kg cromolyn 30 min before i.p. challenge with LPS. Spleen cells (c and d) or peritoneal cells (e and f) from wild type (c and e) or KitW-sh/W-sh (d and f) mice incubated for 15 min with or without cromolyn were stimulated with LPS. N = 4 – 13 mice per group from 3 (a) or 2 (b) independent experiments or 4 per group from one of 2 – 3 independent experiments, each of which gave similar results (c – f). ** p < 0.01, * p < 0.05.
We next investigated the effects of cromolyn on LPS-induced TNF production in vitro. TNF was detectable in the supernatants of wild type spleen cells (Figure 5c) and peritoneal cells (Figure 5e) 3 h after stimulation with 1 ng/mL LPS, and the addition of cromolyn (1 – 100 μM) inhibited such TNF production in a dose-dependent manner (Figure 5c and e). Treatment of spleen cells (Figure 5d) and peritoneal cells (Figure 5f) from KitW-sh/W-sh mice with 1 ng/mL LPS also induced TNF production. Moreover, cromolyn (1 – 100 μM) inhibited LPS-induced TNF production by KitW-sh/W-sh spleen or peritoneal cells in a dose-dependent manner (Figure 5d and f). We also tested the effect of cromolyn on these reactions in vitro using Kit-independent mast cell- (and basophil-) deficient Cpa3-Cre; Mcl-1fl/fl mice35 (Supplementary Figure S8). In accordance with our data from KitW-sh/W-sh mice, we found that cromolyn (1 – 100 μM) inhibited LPS-induced TNF production by spleen or peritoneal cells from both Cpa3-Cre; Mcl-1+/+ (littermate control) mice and Cpa3-Cre; Mcl-1fl/fl (mast cell- and basophil-deficient) mice in a dose-dependent manner. Taken together, our findings indicate that cromolyn can inhibit LPS-induced TNF production in mice in a mast cell-independent manner.
DISCUSSION
We confirmed the well-established inhibitory effect of cromolyn on IgE-dependent PCA reactions in rats in vivo13,15,30 (Figure 1, Table 1) and on IgE- and antigen-dependent degranulation and mediator release in rat PMCs in vitro12–15 (Figure 3, Table 1). In contrast to rat PMCs, it was reported that cromolyn added 5 min before or at the time of antigen challenge did not inhibit IgE- and antigen-induced degranulation of either rat BMCMCs39 or mucosal type mast cells isolated from rat intestine14 in vitro (Table 1). These findings suggest that, in rats, different mast cell populations may exhibit different sensitivity to cromolyn. In addition, we also confirmed the previously reported phenomenon that cromolyn pretreatment induces “tachyphylaxis” in rat PMCs12,13 (Supplementary Figure S6). This phenomenon shows that, even in rats, cromolyn can inhibit mast cell degranulation only under certain conditions of administration.
In mice, it has been reported that cromolyn treatment immediately before30 or at the time of15,31 antigen challenge did not inhibit either IgE-dependent PCA reactions in vivo30 or the activation of mouse BMCMCs in vitro15,31 (Table 1). Since, as noted above, different mast cell populations might exhibit different sensitivity to cromolyn in rats, it is possible that the drug also might vary in its ability to inhibit the activity of different populations of mouse mast cells in vivo or in vitro. We detected no inhibitory effects of cromolyn (either given once at the time of antigen challenge or administered every 12 hours starting 2.5 days before antigen challenge) on the increases in vascular permeability, tissue swelling, or leukocyte infiltration at sites of IgE-dependent PCA reactions (Figure 1b – d, Supplementary Figure S4b and c) or on the increases in plasma histamine (Figure 2b and Supplementary Figure S4d) or decreases in body temperature (Figure 2c) associated with IgE-dependent PSA reactions in mice in vivo. However, we found that the increases in plasma levels of mMCP-1 associated with IgE-dependent PSA reactions were significantly inhibited by treatment of mice with a high dose (100 mg/kg) of cromolyn, either given once at the time of antigen challenge (Figure 2d) or administered repeatedly starting 2.5 days before antigen challenge (Supplementary Figure S4e). Since mMCP-1 is a serine protease which is thought to be stored and secreted in a tissue-specific manner by mucosal mast cells,40 this result suggests that, under the conditions tested, cromolyn can inhibit the activation of mucosal type mast cells in mice. Forbes et al. reported that cromolyn inhibited the increases in blood concentrations of mMCP-1 in transgenic mice that overexpressed IL-9.32 Taken together, our results and those of Forbes et al.,32 suggest that, in mice, as well as in rats, different mast cell populations may exhibit different sensitivity to cromolyn.
Given the potency of cromolyn as an inhibitor of rat PMC degranulation,12–15 and given our inability to find any prior reports on the effects of cromolyn on degranulation of mouse PMCs (Table 1), we were especially interested to test the effects of this agent on mouse PMCs. We found that cromolyn did not effectively inhibit mouse PMC degranulation in response to IgE and specific antigen or to any of the various other activation stimuli we tested (Figures 3 and 4). Given that both PMCs and skin mast cells are considered connective tissue type mast cells, our results in experiments with mouse PMCs in vitro (Figures 3 and 4) and analyzing IgE-dependent PCA reactions in mice in vivo (Figure 1) support the conclusion that cromolyn is not a potent inhibitor of connective tissue mast cell activation in the mouse.
Studies with cromolyn illustrate two limitations that apply to many pharmacological approaches. First, there appear to be clear species-dependent differences in sensitivity to the drug. We confirmed that treatment with cromolyn (at 10 mg/kg in vivo or at 10 – 100 μM in vitro) at the time of antigen challenge significantly inhibited IgE-dependent rat mast cell activation (Figures 1 – 4, Table 1). In mice, we found that the increase in plasma concentrations of mMCP-1 associated with IgE-dependent PSA was significantly inhibited by 100 mg/kg cromolyn administered at the time of antigen challenge in vivo (Figure 2d), but we did not detect inhibition by cromolyn of any of the other mast cell responses we tested, using the drug at 10 or 100 mg/kg in vivo and at 1 – 100 μM in vitro (Figures 1 – 4, Table 1). It also has been reported that cromolyn, at 0.1 – 100 mg/kg in vivo and 1 – 100 μg/mL in vitro, failed to inhibit guinea pig PCA reactions in vivo or histamine release from chopped guinea pig lung in vitro.15 In humans, a high concentration (1 mM) of cromolyn was required to achieve even modest inhibition of mediator release in vitro from mast cells isolated from lung,41 tonsils or intestines,42 or from cultured human mast cells derived from umbilical cord blood cells.43 Moreover, human skin mast cells42 or cultured human mast cells derived from blood buffy coats44 were unresponsive to this agent in vitro.
Second, cromolyn’s mechanism of action still is not understood, including the basis for the phenomenon of “tachyphylaxis” illustrated in Supplementary Figure S6 or the explanation for the different effects of short-term versus long-term exposure to cromolyn on IgE- and antigen-dependent mediator release by BMCMCs that have been reported by Marquardt et al.31 However, several lines of evidence indicate that the effects of cromolyn are not restricted specifically to mast cells. It has been reported that cromolyn can bind directly to some ubiquitous proteins, for instance, heat-shock protein 90 (Hsp90),45 S100 proteins,46,47 or G-protein-coupled receptor 35 (GPR35).48 Considering that Hsp90 can interact with variety of proteins including those involved in hormone signaling, intracellular signal transduction, and cell cycle control,45,49 that S100 proteins are expressed in pancreatic,46,47 breast,50 lung51 and colon52 cancer cells and that GPR35 is expressed in leukocytes, monocytes, neutrophils and T cells,48,53 the cellular targets of cromolyn are unlikely to be restricted to mast cells.
Indeed, by testing genetically mast cell-deficient KitW-sh/W-sh or Cpa3-Cre; Mcl-1fl/fl mice, we found that cromolyn (at 100 mg/kg in vivo and 10 – 100 μM in vitro) can inhibit LPS-induced TNF production in a mast cell-independent manner in vivo and in vitro (Figure 5 and Supplementary Figure S8). Notably, acute treatment with the same high dose of cromolyn (100 mg/kg) which inhibited increases in plasma mMCP-1 in IgE-dependent PSA (Figure 2d) also resulted in a mast cell-independent inhibition of TNF production in mast cell-deficient KitW-sh/W-sh mice. In addition to our findings, there have been at least two other reports showing that cromolyn can inhibit certain immune responses in mast cell-deficient mice in vivo. In those reports, which employed WBB6F1-KitW/Wv mice rather than KitW-sh/W-sh or Cpa3-Cre; Mcl-1fl/fl mice, cromolyn inhibited both the neutrophil infiltration into the gastric mucosa that was observed after active anaphylaxis54 and capsaicin-induced infiltration of eosinophils into the conjunctiva55. Those results, and our findings that cromolyn can inhibit LPS-induced TNF release by genetically mast cell-deficient mice in vivo and by the spleen or peritoneal cells of such mice in vitro, are consistent with observations indicating that cromolyn can dose-dependently inhibit the activation of several different hematopoietic cell types. For example, cromolyn tested in vitro at concentrations as low as 10−7 M markedly reduced the formyl-methionyl-leucyl-phenylalanine-dependent activation of human peripheral blood neutrophils, eosinophils and monocytes, as well as the ability of highly purified human neutrophils or eosinophils to kill Schistosoma mansoni schistosomula,56 and also markedly reduced Sμ to Sε deletional switch recombination and IgE synthesis in highly purified human B cells.57
In conclusion, based on our results and those of other groups, we think that there are extensive species-dependent differences in the responsiveness of mast cell populations to the inhibitory effects of cromolyn and that skin and peritoneal mast cells in mice are substantially less responsive to this drug than are the corresponding mast cell populations in the rat. Moreover, cromolyn can inhibit biological processes in vivo in mast cell-deficient mice and, when tested in vitro, can inhibit the activation of multiple hematopoietic cell types other than mast cells. Because neither the critical molecular targets of cromolyn that explain its ability to inhibit rat mast cell degranulation have been clearly defined nor has its mechanism(s) of action been identified, we think it might be very difficult to clarify why this drug inhibits the degranulation of some types of mast cells but not others, to explain why the effects of the drug on some mast cell populations can differ based on the duration of exposure to the agent, or to define the mechanism(s) by which it can inhibit the activation of other cell types in mast cell-deficient mice. Also, we obviously have not tested cromolyn in all strains of mice, in all biological responses thought to require mast cells, or with all agents capable of inducing mast cell activation. Therefore, it is possible that short or long-term treatment with cromolyn might be able to inhibit functions of mouse mast cells observed in settings other than those studied in this report. Indeed, our data, like those reported by Forbes et al.,32 suggest that treatment with high doses of cromolyn can inhibit the activation of mouse mucosal mast cells. However, given the results presented in this report, including the finding that cromolyn can inhibit TNF release in mast cell-deficient mice in vivo, we think that one should not assume that the effects of cromolyn treatment of mice in vivo necessarily reflect actions of this drug on mast cells.
Supplementary Material
Acknowledgments
This study was supported by grants from the National Institutes of Health AI070813, AI023990, and CA072074 to S. J. Galli.
We thank Mariola Liebersbach for support with mouse breeding and Chen Liu for processing slides for histological analysis.
ABBREVIATIONS
- BMCMCs
bone-marrow-derived cultured mast cells
- DNP-HSA
2,4-dinitrophenyl human serum albumin
- GPR35
G-protein-coupled receptor 35
- Hsp90
heat-shock protein 90
- KitW-sh/W-sh
C57BL/6-KitW-sh/W-sh
- mMCP-1
mouse mast cell protease-1
- PCA
passive cutaneous anaphylaxis
- PGD2
prostaglandin D2
- PMA
phorbol 12-myristate 13-acetate
- PMCs
peritoneal mast cells
- PSA
passive systemic anaphylaxis
- SCF
stem cell factor
Footnotes
DISCLOSURE/DUALITY OF INTEREST
The authors state no conflict of interest.
References
- 1.Metcalfe DD, Baram D, Mekori YA. Mast cells. Physiol Rev. 1997;77:1033–1079. doi: 10.1152/physrev.1997.77.4.1033. [DOI] [PubMed] [Google Scholar]
- 2.Kalesnikoff J, Galli SJ. New developments in mast cell biology. Nat Immunol. 2008;9:1215–1223. doi: 10.1038/ni.f.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abraham SN, St John AL. Mast cell-orchestrated immunity to pathogens. Nat Rev Immunol. 2010;10:440–452. doi: 10.1038/nri2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tsai M, Grimbaldeston M, Galli SJ. Mast cells and immunoregulation/immunomodulation. Adv Exp Med Biol. 2011;716:186–211. doi: 10.1007/978-1-4419-9533-9_11. [DOI] [PubMed] [Google Scholar]
- 5.Galli SJ, Tsai M. IgE and mast cells in allergic disease. Nat Med. 2012;18:693–704. doi: 10.1038/nm.2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Akahoshi M, Song CH, Piliponsky AM, et al. Mast cell chymase reduces the toxicity of Gila monster venom, scorpion venom, and vasoactive intestinal polypeptide in mice. J Clin Invest. 2011;121:4180–4191. doi: 10.1172/JCI46139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Metz M, Piliponsky AM, Chen CC, et al. Mast cells can enhance resistance to snake and honeybee venoms. Science. 2006;313:526–530. doi: 10.1126/science.1128877. [DOI] [PubMed] [Google Scholar]
- 8.Piliponsky AM, Chen CC, Nishimura T, et al. Neurotensin increases mortality and mast cells reduce neurotensin levels in a mouse model of sepsis. Nat Med. 2008;14:392–398. doi: 10.1038/nm1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maurer M, Wedemeyer J, Metz M, et al. Mast cells promote homeostasis by limiting endothelin-1-induced toxicity. Nature. 2004;432:512–516. doi: 10.1038/nature03085. [DOI] [PubMed] [Google Scholar]
- 10.Bernstein IL, Siegel SC, Brandon ML, et al. A controlled study of cromolyn sodium sponsored by the Drug Committee of the American Academy of Allergy. J Allergy Clin Immunol. 1972;50:235–245. doi: 10.1016/0091-6749(72)90017-6. [DOI] [PubMed] [Google Scholar]
- 11.Howell JB, Altounyan RE. A double-blind trial of disodium cromoglycate in the treatment of allergic bronchial asthma. Lancet. 1967;2:539–542. doi: 10.1016/s0140-6736(67)90499-0. [DOI] [PubMed] [Google Scholar]
- 12.Kusner EJ, Dubnick B, Herzig DJ. The inhibition by disodium cromoglycate in vitro of anaphylactically induced histamine release from rat peritoneal mast cells. J Pharmacol Exp Ther. 1973;184:41–46. [PubMed] [Google Scholar]
- 13.Thomson DS, Evans DP. Inhibition of immediate hypersensitivity reactions by disodium cromoglycate. Clin Exp Immunol. 1973;13:537–544. [PMC free article] [PubMed] [Google Scholar]
- 14.Pearce FL, Befus AD, Gauldie J, et al. Mucosal mast cells. II. Effects of anti-allergic compounds on histamine secretion by isolated intestinal mast cells. J Immunol. 1982;128:2481–2486. [PubMed] [Google Scholar]
- 15.Kobayashi K, Hiroi J, Kishi S, et al. Effects of quinotolast, a new orally active antiallergic drug, on experimental allergic models. Jpn J Pharmacol. 1993;63:73–81. doi: 10.1254/jjp.63.73. [DOI] [PubMed] [Google Scholar]
- 16.Orr TS, Cox JS. Disodium cromoglycate, an inhibitor of mast cell degranulation and histamine release induced by phospholipase A. Nature. 1969;223:197–198. doi: 10.1038/223197b0. [DOI] [PubMed] [Google Scholar]
- 17.Storms W, Kaliner MA. Cromolyn sodium: fitting an old friend into current asthma treatment. J Asthma. 2005;42:79–89. [PubMed] [Google Scholar]
- 18.Kobayashi Y, Okunishi H. Mast cells as a target of rheumatoid arthritis treatment. Jpn J Pharmacol. 2002;90:7–11. doi: 10.1254/jjp.90.7. [DOI] [PubMed] [Google Scholar]
- 19.Huang M, Pang X, Karalis K, et al. Stress-induced interleukin-6 release in mice is mast cell-dependent and more pronounced in Apolipoprotein E knockout mice. Cardiovasc Res. 2003;59:241–249. doi: 10.1016/s0008-6363(03)00340-7. [DOI] [PubMed] [Google Scholar]
- 20.Samoszuk M, Corwin MA. Mast cell inhibitor cromolyn increases blood clotting and hypoxia in murine breast cancer. Int J Cancer. 2003;107:159–163. doi: 10.1002/ijc.11340. [DOI] [PubMed] [Google Scholar]
- 21.Bot I, de Jager SC, Zernecke A, et al. Perivascular mast cells promote atherogenesis and induce plaque destabilization in apolipoprotein E-deficient mice. Circulation. 2007;115:2516–2525. doi: 10.1161/CIRCULATIONAHA.106.660472. [DOI] [PubMed] [Google Scholar]
- 22.Kneilling M, Hultner L, Pichler BJ, et al. Targeted mast cell silencing protects against joint destruction and angiogenesis in experimental arthritis in mice. Arthritis Rheum. 2007;56:1806–1816. doi: 10.1002/art.22602. [DOI] [PubMed] [Google Scholar]
- 23.Soucek L, Lawlor ER, Soto D, et al. Mast cells are required for angiogenesis and macroscopic expansion of Myc-induced pancreatic islet tumors. Nat Med. 2007;13:1211–1218. doi: 10.1038/nm1649. [DOI] [PubMed] [Google Scholar]
- 24.Costa R, Marotta DM, Manjavachi MN, et al. Evidence for the role of neurogenic inflammation components in trypsin-elicited scratching behaviour in mice. Br J Pharmacol. 2008;154:1094–1103. doi: 10.1038/bjp.2008.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nautiyal KM, Ribeiro AC, Pfaff DW, et al. Brain mast cells link the immune system to anxiety-like behavior. Proc Natl Acad Sci U S A. 2008;105:18053–18057. doi: 10.1073/pnas.0809479105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marques MJ, Ventura Machado R, Minatel E, et al. Disodium cromoglycate protects dystrophin-deficient muscle fibers from leakiness. Muscle Nerve. 2008;37:61–67. doi: 10.1002/mus.20892. [DOI] [PubMed] [Google Scholar]
- 27.Liu J, Divoux A, Sun J, et al. Genetic deficiency and pharmacological stabilization of mast cells reduce diet-induced obesity and diabetes in mice. Nat Med. 2009;15:940–945. doi: 10.1038/nm.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liao CH, Akazawa H, Tamagawa M, et al. Cardiac mast cells cause atrial fibrillation through PDGF-A-mediated fibrosis in pressure-overloaded mouse hearts. J Clin Invest. 2010;120:242–253. doi: 10.1172/JCI39942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sharma A, Abraham T, Sampaio A, et al. Sodium cromolyn reduces expression of CTGF, ADAMTS1, and TIMP3 and modulates post-injury patellar tendon morphology. J Orthop Res. 2011;29:678–683. doi: 10.1002/jor.21291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Braga F, Mota I. Homologous passive cutaneous anaphylaxis (PCA) in mice and heterologous PCA induced in rats with mouse IgE. Immunology. 1976;30:655–669. [PMC free article] [PubMed] [Google Scholar]
- 31.Marquardt DL, Walker LL, Wasserman SI. Cromolyn inhibition of mediator release in mast cells derived from mouse bone marrow. Am Rev Respir Dis. 1986;133:1105–1109. doi: 10.1164/arrd.1986.133.6.1105. [DOI] [PubMed] [Google Scholar]
- 32.Forbes EE, Groschwitz K, Abonia JP, et al. IL-9- and mast cell-mediated intestinal permeability predisposes to oral antigen hypersensitivity. J Exp Med. 2008;205:897–913. doi: 10.1084/jem.20071046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Komatsu H, Kojima M, Tsutsumi N, et al. Mechanism of inhibitory action of tranilast on the release of slow reacting substance of anaphylaxis (SRS-A) in vitro: effect of tranilast on the release of arachidonic acid and its metabolites. Jpn J Pharmacol. 1988;46:53–60. doi: 10.1254/jjp.46.53. [DOI] [PubMed] [Google Scholar]
- 34.Piliponsky AM, Chen CC, Grimbaldeston MA, et al. Mast cell-derived TNF can exacerbate mortality during severe bacterial infections in C57BL/6-KitW-sh/W-sh mice. Am J Pathol. 2010;176:926–938. doi: 10.2353/ajpath.2010.090342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lilla JN, Chen CC, Mukai K, et al. Reduced mast cell and basophil numbers and function in Cpa3-Cre; Mcl-1fl/fl mice. Blood. 2011;118:6930–6938. doi: 10.1182/blood-2011-03-343962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oka T, Hori M, Ozaki H. Microtubule disruption suppresses allergic response through the inhibition of calcium influx in the mast cell degranulation pathway. J Immunol. 2005;174:4584–4589. doi: 10.4049/jimmunol.174.8.4584. [DOI] [PubMed] [Google Scholar]
- 37.Olivera A, Mizugishi K, Tikhonova A, et al. The sphingosine kinase-sphingosine-1-phosphate axis is a determinant of mast cell function and anaphylaxis. Immunity. 2007;26:287–297. doi: 10.1016/j.immuni.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 38.Wershil BK, Mekori YA, Murakami T, et al. 125I-fibrin deposition in IgE-dependent immediate hypersensitivity reactions in mouse skin. Demonstration of the role of mast cells using genetically mast cell-deficient mice locally reconstituted with cultured mast cells. J Immunol. 1987;139:2605–2614. [PubMed] [Google Scholar]
- 39.Broide DH, Metcalfe DD, Wasserman SI. Functional and biochemical characterization of rat bone marrow derived mast cells. J Immunol. 1988;141:4298–4305. [PubMed] [Google Scholar]
- 40.Wastling JM, Knight P, Ure J, et al. Histochemical and ultrastructural modification of mucosal mast cell granules in parasitized mice lacking the beta-chymase, mouse mast cell protease-1. Am J Pathol. 1998;153:491–504. doi: 10.1016/s0002-9440(10)65592-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Church MK, Hiroi J. Inhibition of IgE-dependent histamine release from human dispersed lung mast cells by anti-allergic drugs and salbutamol. Br J Pharmacol. 1987;90:421–429. doi: 10.1111/j.1476-5381.1987.tb08972.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Okayama Y, Benyon RC, Rees PH, et al. Inhibition profiles of sodium cromoglycate and nedocromil sodium on mediator release from mast cells of human skin, lung, tonsil, adenoid and intestine. Clin Exp Allergy. 1992;22:401–409. doi: 10.1111/j.1365-2222.1992.tb03102.x. [DOI] [PubMed] [Google Scholar]
- 43.Shichijo M, Inagaki N, Nakai N, et al. The effects of anti-asthma drugs on mediator release from cultured human mast cells. Clin Exp Allergy. 1998;28:1228–1236. doi: 10.1046/j.1365-2222.1998.00394.x. [DOI] [PubMed] [Google Scholar]
- 44.Wang XS, Lau HY. Histamine release from human buffy coat-derived mast cells. Int Immunopharmacol. 2007;7:541–546. doi: 10.1016/j.intimp.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 45.Okada M, Itoh H, Hatakeyama T, et al. Hsp90 is a direct target of the anti-allergic drugs disodium cromoglycate and amlexanox. Biochem J. 2003;374:433–441. doi: 10.1042/BJ20030351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Arumugam T, Ramachandran V, Logsdon CD. Effect of cromolyn on S100P interactions with RAGE and pancreatic cancer growth and invasion in mouse models. J Natl Cancer Inst. 2006;98:1806–1818. doi: 10.1093/jnci/djj498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Okada M, Tokumitsu H, Kubota Y, et al. Interaction of S100 proteins with the antiallergic drugs, olopatadine, amlexanox, and cromolyn: identification of putative drug binding sites on S100A1 protein. Biochem Biophys Res Commun. 2002;292:1023–1030. doi: 10.1006/bbrc.2002.6761. [DOI] [PubMed] [Google Scholar]
- 48.Yang Y, Lu JY, Wu X, et al. G-protein-coupled receptor 35 is a target of the asthma drugs cromolyn disodium and nedocromil sodium. Pharmacology. 2010;86:1–5. doi: 10.1159/000314164. [DOI] [PubMed] [Google Scholar]
- 49.Freeman BC, Morimoto RI. The human cytosolic molecular chaperones hsp90, hsp70 (hsc70) and hdj-1 have distinct roles in recognition of a non-native protein and protein refolding. EMBO J. 1996;15:2969–2979. [PMC free article] [PubMed] [Google Scholar]
- 50.Wang G, Platt-Higgins A, Carroll J, et al. Induction of metastasis by S100P in a rat mammary model and its association with poor survival of breast cancer patients. Cancer Res. 2006;66:1199–1207. doi: 10.1158/0008-5472.CAN-05-2605. [DOI] [PubMed] [Google Scholar]
- 51.Beer DG, Kardia SL, Huang CC, et al. Gene-expression profiles predict survival of patients with lung adenocarcinoma. Nat Med. 2002;8:816–824. doi: 10.1038/nm733. [DOI] [PubMed] [Google Scholar]
- 52.Bertram J, Palfner K, Hiddemann W, et al. Elevated expression of S100P, CAPL and MAGE 3 in doxorubicin-resistant cell lines: comparison of mRNA differential display reverse transcription-polymerase chain reaction and subtractive suppressive hybridization for the analysis of differential gene expression. Anticancer Drugs. 1998;9:311–317. doi: 10.1097/00001813-199804000-00004. [DOI] [PubMed] [Google Scholar]
- 53.Wang J, Simonavicius N, Wu X, et al. Kynurenic acid as a ligand for orphan G protein-coupled receptor GPR35. J Biol Chem. 2006;281:22021–22028. doi: 10.1074/jbc.M603503200. [DOI] [PubMed] [Google Scholar]
- 54.Furuta GT, Wang ZS, Wershil BK. Gastric inflammation during systemic anaphylaxis: neutrophil recruitment in stomach wall of mice does not require mast cell participation. Dig Dis Sci. 1998;43:2021–2027. doi: 10.1023/a:1018851012940. [DOI] [PubMed] [Google Scholar]
- 55.Ebihara N, Nishikawa M, Murakami A. Disodium cromoglycate inhibits capsaicin-induced eosinophil infiltration of conjunctiva independent of mast cells. Jpn J Ophthalmol. 2006;50:205–210. doi: 10.1007/s10384-005-0314-9. [DOI] [PubMed] [Google Scholar]
- 56.Kay AB, Walsh GM, Moqbel R, et al. Disodium cromoglycate inhibits activation of human inflammatory cells in vitro. J Allergy Clin Immunol. 1987;80:1–8. doi: 10.1016/s0091-6749(87)80183-5. [DOI] [PubMed] [Google Scholar]
- 57.Loh RK, Jabara HH, Geha RS. Disodium cromoglycate inhibits Sμ → Sε deletional switch recombination and IgE synthesis in human B cells. J Exp Med. 1994;180:663–671. doi: 10.1084/jem.180.2.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.