Abstract
The purpose of this study was to compare the hepatic and small intestinal metabolism, and examine bioavailability and gastro-intestinal first-pass effects of Kaempferol in the rats. Liver and small intestinal microsomes fortified with either NADPH or UDPGA were incubated with varying concentrations of Kaempferol for upto 120 minutes. Based on the values of the kinetic constants (Km and Vmax), the propensity for UDPGA-dependent conjugation as compared to NADPH-dependent oxidative metabolism was higher for both hepatic and small intestinal microsomes. Male Sprague-Dawley rats were administered Kaempferol intravenously (IV) (10, 25 mg/kg) or orally (100, 250 mg/kg). Gastro-intestinal first pass effects were observed by collecting portal blood after oral administration of 100 mg/kg Kaempferol. Pharmacokinetic parameters were obtained by Noncompartmental analysis using WinNonlin. After IV administration, the plasma concentration-time profiles for 10 and 25 mg/kg were consistent with high clearance (~ 3 L/hr/kg) and large volumes of distribution (8-12 L/kg). The disposition was characterized by a terminal half-life value of 3-4 hours. After oral administration the plasma concentration-time profiles demonstrated fairly rapid absorption (tmax ~ 1-2 hours). The area under the curve (AUC) values after IV and oral doses increased proportional to the dose. The bioavailability (F) was poor at ~ 2%. Analysis of portal plasma after oral administration revealed low to moderate absorption. Taken together, the low F of Kaempferol is attributed in part to extensive first-pass metabolism by glucuronidation and other metabolic pathways in the gut and in the liver.
Keywords: Pharmacokinetics, Kaempferol, metabolism, flavonoids, liver, small intestine
1. Introduction
Kaempferol (3, 4′, 5, 7-tetrahydroxyflavone) is a flavonoid that is widely distributed in onion, kale, endive and tea. It has been shown to exhibit cancer chemopreventive efficacy against many types of cancers including hepatocellular carcinoma, breast, lung and prostate. Possible mechanisms of chemoprevention by Kaempferol include inhibition of carcinogen activating genes and enzymes, enhancing carcinogen detoxification by inducing the phase II detoxifying genes and enzymes [1]. Its abilities to scavenge free radicals and modulate several signaling pathways leading ultimately to cancer cell apoptosis has also been documented [2-4].
To better understand the in vivo pharmacological properties of any chemopreventive agent, it is important to have a thorough understanding of its metabolism and pharmacokinetics. The metabolism of Kaempferol has been studied to some extent. Quercetin has been identified as the product of oxidative metabolism while the 7-O-glucuronide has been identified as the major product of conjugative metabolism using rat liver microsomes [5-7]. However, the differences between hepatic and intestinal metabolism and disposition of Kaempferol remained largely unknown.
In this report we compare the rates of oxidative and glucuronide conjugative metabolism between rat liver and small intestinal microsomes. In addition we have carried out pharmacokinetic studies to examine the bioavailability (F) of Kaempferol in the rats. The results from our current study examining the rat liver and small intestinal metabolism coupled with its in vivo disposition would yield better insights into the pharmacokinetics of Kaempferol.
2. Experimental methods
2.1 Materials
Kaempferol was purchased from Indofine Chemicals (Hillsborough, New Jersey). All solvents were of HPLC grade (Fisher Scientific)
2.2 Incubations with NADPH or UDPGA fortified microsomes
NADPH-Dependent Phase I Metabolism
Liver and small intestinal microsomes were prepared as described elsewhere [8]. Phase I metabolism of Kaempferol was studied using Sprague-Dawley rat liver microsomes (RLM) and small intestinal microsomes (RSiM). Incubations in a final volume of 200 μL consisted of microsomes (0.1 mg protein/ml) suspended in 100 mM potassium phosphate buffer (pH 7.4) and varying concentrations of Kaempferol. The reaction was initiated by the addition of 5 mM NADPH. Reactions were carried out for various time points up to 120 minutes at 37°C and stopped by addition of equal volume of cold methanol containing the internal standard (IS), Biochanin A. After vortex-mixing and spinning in a centrifuge (14,000 rpm for 10 min), supernatants were analyzed by HPLC. Turnover was calculated based on the formation of Quercetin using a standard curve derived by incubating Quercetin with cold microsomes. Km and Vmax values were determined at the end of 45 minute incubation obtained using WinNonlin (Pharsight, Mountainview, CA).
UDPGA-dependent Phase II metabolism
Phase II metabolism of Kaempferol was studied using Sprague-Dawley rat liver and small intestinal microsomes. Incubations in a final volume of 200 μL consisted of microsomes (0.1 mg protein/ml) suspended in 100 mM potassium phosphate buffer (pH 7.4) with alamethicin (1 μg/10 μg protein) and varying concentrations of Kaempferol. The reaction was initiated by the addition of 2 mM UDPGA. Reactions were carried out for various time points up to 120 minutes at 37°C and stopped by addition of equal volume of cold methanol containing the IS. The samples were vortex-mixed followed by centrifugation at 14,000 rpm for 10 minutes and the supernatants were analyzed by HPLC. Turnover was calculated as the individual glucuronide peak area divided by the sum of glucuronide and parent peak areas. Both Km and Vmax parameters were determined at the end of 45 minute incubation by using WinNonlin (Pharsight, Mountainview, CA).
2.3 Animal treatment
Male Sprague Dawley rats (250-300 gms) bearing indwelling jugular vein and/or portal vein cannulae were obtained from Hilltop Labs (Scottsdale, PA). The animals were housed at the Animal Care facility at Rutgers University under 12 hour light-dark cycles with free access to food and water. All procedures and protocols were approved by Institutional Animal Care and Use Committee at Rutgers University. The animals were allowed to acclimatize for 3 days before commencement of the study during which time they were put on an antioxidant free AIN-76A diet (Dyets Inc, PA). On the day of the study, the cannulae were exteriorized on the dorsal side of the neck and connected to a long polyethylene tube wrapped in a wire coil (Instech, Plymouth, PA) for blood collection. Heparinized saline (50 U/ml) was used to flush the cannula. Rats (n=4) were administered Kaempferol (10 mg/kg and 25 mg/kg) in a vehicle composition consisting of cremaphor/ tween-80/ PEG/ ethanol and water (2:1:1:1:5) intravenously (IV) in a final injection volume of 0.15 ml. Rats (n=5) were fasted overnight and administered Kaempferol (100 mg/kg) in a similar vehicle composition by oral gavage in a final volume of 0.7 ml. Likewise an oral dose of 250 mg/kg was also administered to the rats (n=4), however, due to solubility issues Kaempferol was suspended in corn oil. Blood samples (0.2 ml) were withdrawn at regular time intervals for up to 24 hours following which 0.2 ml of heparinized saline was flushed into the cannula. To assess gastro-intestinal first pass effects, male rats bearing indwelling portal vein cannulae were used. Rats (n=3) were administered Kaempferol (100 mg/kg) in a similar vehicle composition by oral gavage in a final volume of 0.7 ml. Portal blood was withdrawn at regular intervals for up to 24 hours. All the blood samples were immediately centrifuged to obtain plasma samples. The plasma samples were then acidified with 50 μL of acetic acid (0.5 mol/L) and stored at −20 °C until further analysis.
In addition, urine from each animal was also collected over a period of 96 hours after IV and PO dosing. The time intervals for urine collection were as follows 0-4, 4-8, 8-24, 24-48, 48-72 and 72-96 hours. The volume of urine collected at each time interval was recorded. The urine samples were also stored at −20 °C until further analysis.
2.4 Sample preparation
One aliquot of plasma (50 μl) spiked with 0.1 μg/ μl Biochanin A as internal standard (IS) was extracted twice with a mixture of ethylacetate: methanol (95:5 v/v). The layers were separated by centrifugation 4,000g for 10 minutes. The combined organic extracts were dried under nitrogen followed by reconstitution in 100 μl of mobile phase B. Another aliquot of plasma (50 μl) was incubated with 1 × 105 U/l β-glucuronidase / sulfatase (crude extract from Helix pomatia, Sigma-Aldrich, MO) at 37°C overnight followed by addition of IS and subsequent extraction as described earlier. Similar extraction procedure using a 50 μl urine aliquot was followed for extraction of Kaempferol and its metabolites. The absolute recovery of Kaempferol was approximately 89%. The absolute recoveries for Quercetin, Isorhamnetin and Biochanin A were found to be 92%, 88% and 71%, respectively.
2.5 HPLC conditions
A Shimadzu HPLC system (SCL-10A VP) consisting of a binary pump (FCV-10AL VP), an autosampler (SIL-10AD VP) that was maintained at 4°C, and a UV-Vis detector (SPD-10AV VP). Reversed-phase chromatography was performed with an analytical Shimadzu C18 column (240 mm × 2.0 mm, 5-μm, Shimadzu, MD) protected with a SecurityGuard™ cartridge system (Phenomenex) and a 0.45-μm in-line filter. The aqueous phase (Mobile phase A) and organic phase (Mobile phase B) were water: acetonitrile: trifluoroacetic acid (90:10:0.1 v/v/v) and acetonitrile: water: trifluoroacetic acid (95:5:0.1 v/v/v), respectively. The flow rate was set at 0.7 ml/min. Chromatographic separation was achieved using a linear gradient elution from 0-25 minutes from 25 % Mobile phase B to 100 % Mobile phase B. Mobile phase B was maintained at 100 % for an additional 2.5 minutes followed by return to initial mobile phase conditions in 2.5 minutes. Peaks of the HPLC chromatograms were evaluated using Class-VP program (7.1.1, Shimadzu, MD).
The various flavonols extracted from the plasma samples were identified by comparing the retention times with those of the authentic standards. The retention times for M1 (Quercetin), Kaempferol, M2 (Isorhamnetin) were found to be 8.8 ± 0.2, 10.3 ± 0.3, and 11.4 ± 0.2 minutes, respectively. Peaks were evaluated and considered if the peak height was at least five times higher than the baseline.
2.6 Pharmacokinetic analysis
The pharmacokinetic parameters were determined using the bolus intravenous input (Model 201) and Extravascular input (Model 200) non compartmental analysis module of WinNonlin version 4.1 (Pharsight, Mountain View, CA). The area under the plasma concentration time curve (AUC) was calculated using linear trapezoidal method. The slope of the apparent terminal phase was estimated by log-linear estimation using the last three data points and the apparent elimination rate constant (λ) was derived from the slope. The apparent elimination half-life (t1/2) was calculated as 0.693/ λ. Systemic total body clearance (Cltotal) following IV dosing was calculated as Dose/AUC0-∞. Metabolite ratio (MR) was calculated by dividing AUCglucuronides by the AUCKaempferol.
2.7 Statistics
Experimental values are expressed as mean ± standard error (S.E). Statistical analysis was performed using Student’s t test. A p value of < 0.05 was considered significant.
3. Results
3.1 In vitro metabolism of Kaempferol
The rates of phase I oxidation and phase II conjugation for Kaempferol as a function of both time and concentration were measured in both small intestinal microsomes (RSiM)and rat liver microsomes (RLM). The corresponding kinetic parameters are listed in Table 1. Vmax values were consistently higher in the hepatic microsomes compared to the small intestinal microsomes. The Michelis-Menten constant Km was generally smaller for the hepatic microsomes as compared to the small intestinal microsomes, thus, signifying the more important role of hepatic over intestinal metabolism. Furthermore, higher propensity for phase II UDPGA-mediated conjugation reactions as compared to phase I NADPH mediated oxidative reactions was also evident. Thus metabolic clearance due to conjugation appears to be more efficient than the phase I oxidation.
Table 1. Kinetic constants obtained from Phase I oxidative and Phase II conjugation reactions, mean ± S.E.
| Km (μM) | Vmax (nmol of product/min/mg protein) | ||
|---|---|---|---|
| Phase I oxidative metabolism | |||
| RLM * | 14.3 ± 1.2 | 95.7 ± 9.1 | |
| RSiM * | 28.1 ± 1.6 | 58.5 ± 2.4 | |
| Phase II conjugation | |||
| RLM | †G2 | 20 ± 1.1 | 112.2 ± 17.2 |
| †G3 | 8.8 ± 0.8 | 1736 ± 53.6 | |
| RSiM | G2 | 14.6 ± 3.6 | 229 ± 26.2 |
| G3 | 25.3 ± 2.4 | 152 ± 16 |
Rat liver microsomes (RLM); rat small intestinal microsomes (RSiM)
see Figure 1 for the prominent glucuronide peaks G2 and G3.
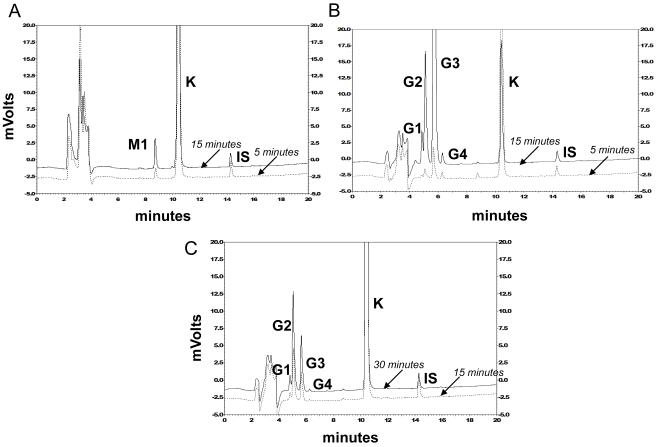
The chromatograms depicting the in vitro metabolic profiles are shown in Figure 1. The metabolic profiles generated by rat liver and small intestinal microsomes which were fortified with NADPH were identical, resulting in the formation of a single metabolite M1 (rt: 8.8 ± 0.2 minutes). The rate for formation of M1 however was slower in small intestinal microsomes. Based on retention time match, M1 was tentatively identified to be Quercetin. Phase II UDPGA-mediated conjugation reaction led to the formation of 4 glucuronide peaks (G1-G4). These peaks were identified to be glucuronide peaks following glucuronidase incubation resulting in their disappearance (results not shown). G1 and G4 were minor peaks and their peak areas did not change significantly with time. G2 (rt: 5.1 ± 0.1 min) and G3 (rt: 5.7 ± 0.1 min) were the more predominant peaks.
Figure 1.
HPLC elution profiles following NADPH- or UDPGA-dependent metabolism (A) 0.1 mg/ml rat liver microsomes fortified with 5 mM NADPH at 37° C for 5 or 15 minutes. (B) 0.1 mg/ml rat liver microsomes fortified with 2 mM UDPGA at 37° C for 5 or 15 minutes (C) 0.7 mg/ml rat small intestinal microsomes fortified with 2 mM UDPGA at 37° C for 15 or 30 minutes. For details of procedure, see Materials and Methods section.
Differences in rates of formation for G2 and G3 were observed between rat liver and small intestinal microsomes. G3 (rt: 5.7 ± 0.1 min) appeared to be the predominant peak in the liver microsomes while G2 (rt: 5.1 ± 0.1 min) was the predominant peak in the small intestinal microsomes.
3.2 Pharmacokinetics and oral bioavailability of Kaempferol in rats
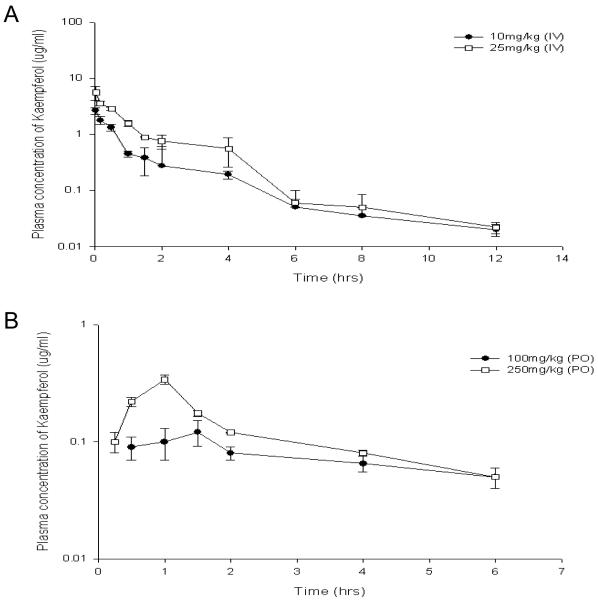
The pharmacokinetic parameters following IV and oral doses of Kaempferol are listed in Table 2. Figure 2A shows the plasma concentration time-course of Kaempferol following IV administration (10 mg/kg and 25 mg/kg). The plasma concentrations following administration of both doses dropped to 50% in approximately 3-4 hours followed by a steady decline up to ~12 hours. No free Kaempferol even following the higher dose (25 mg/kg) was detected at 24 hours. The dose normalized AUC values were independent of the doses and were not significantly different from each other (p = 0.181). The Cltotal values were high (~ 3 L/hr/kg) compared to the hepatic blood flow [9]. The Vd values was relatively large (12 ± 0.4 L/kg in 10 mg/kg treated group and 8.2 ± 0.2 L/kg in 25 mg/kg) indicating high tissue distribution. Figure 2B shows the plasma concentration time-course of Kaempferol following oral doses of 100 mg/kg and 250 mg/kg. The tmax for both oral doses ranged between 1-1.5 hour followed by a steady decline up to 6 hours. No free Kaempferol was detected at time points beyond six hours. The dose normalized AUC values in orally treated rats did not exactly superimpose each other although this difference was not found to be statistically significant (p = 0.054). The bioavailable fraction after oral doses relative to the IV 10 mg/kg AUC values was found to be ~2 % of the administered doses.
Table 2. Pharmacokinetic parameters following IV and PO administration of Kaempferol, mean ± S.E.
| IV (10 mg/kg), n=4 |
IV (25 mg/kg), n=4 |
PO (100 mg/kg), n=5 |
PO (250 mg/kg), n=4 |
|
|---|---|---|---|---|
| AUC 0-∞ (hr*μg/ml) | 3.2 ± 0.3 | 6.7 ± 0.2 | 0.76 ± 0.1 | 1.3 ± 0.04 |
|
AUC 0-∞ /Dose (hr*μg*kg/ml*mg) |
0.32 ± 0.027 † | 0.27 ± 0.007 † | 0.0076 ± 0.001 § | 0.0052 ± 0.016 § |
| Vd (L/kg) | 12.7 ± 0.5 | 8.2 ± 0.2 | ||
| t1/2 (hr) | 4.1 ± 0.2 | 3.5 ± 0.2 | ||
| Cmax (μg/ml) | 253.9 ± 48 | 190 ± 29.4 | ||
| Tmax (hr) | 1.5 ± 0.8 | 1.0 | ||
| Cltotal(L/hr/kg) | 3.2 ± 0.1 | 3.7 ± 0.3 | ||
| Clrenal (L/hr/kg) | 2.3 ± 0.16 | 2.5 ± 0.7 | ||
| Clnonrenal (L/hr/kg) | 0.8 ± 0.1 | 1.3 ± 0.4 | ||
| F (%) | 2.7 ± 0.2 a | 1.9 ± 0.1 a |
Statistically insignificant based on Student’s t test (p = 0.181). p values <0.05 were considered significant.
Statistically insignificant based on Student’s t test (p = 0.054). p values <0.05 were considered significant.
The IV AUC for 10 mg/kg dose was used to determine F, together with the 100 and 250 mg/kg PO doses.
Figure 2.
Mean Plasma concentrations in male rats following (A) intravenous (10 and 25 mg/kg, n=4 each) and (B) oral (100 and 250 mg/kg, n=5 and 4 respectively) administration of Kaempferol. Blood samples were drawn up to 24 h. Bars represent mean ± S.E. For details of procedures, see Materials and Methods section.
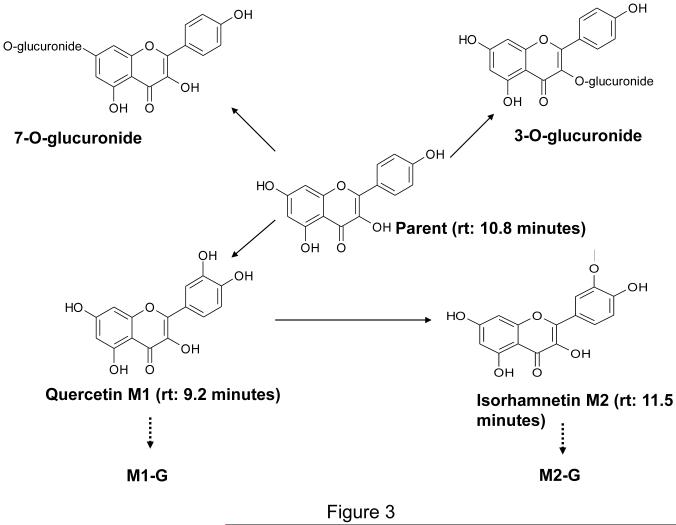
Conjugated Kaempferol and two major metabolites M1 (Quercetin) and M2 (identified as Isorhamnetin which is 3′-O-methylated Quercetin) and their glucuronides (M1-G and M2-G, respectively) were found in the systemic plasma. Similar metabolites were found in the portal plasma after oral administration of 100 mg/kg Kaempferol. Figure 3 depicts the major metabolites identified.
Figure 3.
Potential metabolites of Kaempferol in the rat.
Urine analysis following IV Kaempferol revealed significant amounts of parent - Kaempferol and its glucuronide metabolites. Insignificant amounts of M1 and M2 were observed. Urine from rats administered Kaempferol orally demonstrated the presence of both M1 and M2 in addition to the parent Kaempferol and its glucuronide metabolites. Approximately 16-18 % and 3-4 % of the dose was excreted as parent Kaempferol after IV and oral administration, respectively.
4. Discussion
Hepatic and intestinal conjugation with subsequent excretion of phase II conjugates has been shown to be an important component of first-pass metabolism for many flavonoids [8, 10-11]. Several studies including those using Caco-2 cells showed that intestinal conjugation followed by excretion of the conjugates depends on the native structure of the flavonoid [11, 12]. Thus significant differences exist in the conjugation and excretion rates between liver and small intestine and also between various flavonoids bearing close structural similarity. The role of the liver and small intestine in metabolizing Quercetin is well appreciated and all the metabolites are well characterized too [13]. While the in vitro oxidation of Kaempferol to Quercetin has been reported earlier [5], the differences between hepatic and intestinal disposition of Kaempferol are largely unknown. This study was therefore aimed to obtain an appreciation for the role of small intestine in metabolizing Kaempferol both in vitro and in vivo.
Liver and small intestinal microsomes fortified with UDPGA revealed a four peaks pattern with two major predominant peaks G2 and G3. Yodogawa et al. reported a similar four peaks pattern for glucuronides formation using liver subcellular fractions and the major glucuronide potentially being the 7-O-glucuronide [7]. Based on this data, G3 is most likely the 7-O-glucuronide - the formation of which is catalyzed by UDP-glucuronosyltransferase (UGT) and occurs via SN2 mechanism characterized by nucleophilic attack of the hydroxyl group on the pyranose acid ring of UDPGA. Resonance structures are easily formed for hydroxyl groups on positions other than 3 position on ring C. Resonance structures may weaken the nucleophilicity thus making the hydroxyl on position 3 a good candidate for glucuronidation [14]. Hence it is possible that G2 is the 3-O-glucuronide. However, MS/MS techniques that can accurately elucidate the metabolite structures are needed to ascertain the identities of these metabolites. Chen et al. reported that, in human liver microsomes, the two predominant glucuronide peaks formed by Kaempferol were catalyzed by different isoforms of UGT, namely UGT1A3 and UGT1A9 [6]. Moreover, tissue specific differences in expression of UGTs between liver and small intestine have also been reported earlier [15, 16]. Thus, differences in the expression patterns of UGT isoforms between liver and small intestine could offer an explanation for the different rates of formation of G2 and G3 between liver and small intestine in the rats.
The kinetic constants obtained from the in vitro metabolism reveal that rates of glucuronidation are much higher than that for phase I oxidative metabolism. The in vivo data parallel the in vitro findings since extensive glucuronidation of the parent compound occurred after both IV and oral administrations. DuPont et al. studied the pharmacokinetics and excretion kinetics of Kaempferol in humans after administration of endive soup [17]. They reported a reentry peak in the plasma concentration time curve indicating potential enterohepatic recirculation. Kaempferol mainly existed in the conjugated form both in plasma and urine [17]. This parallels the observations we made following IV and oral doses of Kaempferol. Secondary peaks were not very evident from the plasma concentration-time profiles however Kaempferol mainly existed as the glucuronide metabolites. In addition to the conjugated forms, Kaempferol also underwent oxidative conversion to Quercetin, which was further subject to methylation yielding Isorhamnetin. Both Quercetin and Isorhamnetin could also be glucuronidated. This metabolic route however appears to be uncommon in humans since no such metabolism is reported to occur in humans [18-20]. Preliminary data from our laboratory (unpublished results) using human liver microsomes fortified with NADPH also showed no peak corresponding to Quercetin. Since there is no evidence for the formation of hydroxylated metabolite Quercetin, the likelihood of formation of Isorhamnetin as metabolite M2 in humans is probably minimal.
The bioavailability (F) of any drug depends on the extent of absorption and the rate of oral clearance (including all possible routes of clearances). The amount of the drug that can be taken up by the intestinal cells into mesenteric architecture and into the liver depends on various factors including lipophilicity, permeability (Peff), and uptake and/or efflux by transporters. Crespy et al. studied the splanchnic metabolism of several flavonoids including Kaempferol. They reported that Kaempferol demonstrated a relatively high (66 to 86%) net transfer directed toward the serosal side. The net absorption of Kaempferol (8.50 ± 0.40 nmol/min) was high owing to the less abundant secretion of its conjugates into the lumen. On the other hand, total Kaempferol conjugates excreted in the bile were also high [21]. This parallels our study since we observed measurable amounts of the free Kaempferol in the portal blood that could be subjected to further sequential hepatic metabolism. The AUCKaempferol in portal plasma was approximately 10 times that for systemic plasma (7.0 ± 0.5 versus 0.76 ± 0.1 hr*μg/ml) while the AUCglucuronides in portal plasma was approximately 1.5 times the AUCglucuronides in systemic plasma (4.3 ± 1.1 versus 2.81 ± 0.69 hr*μg/ml).
Based on the data we present here, Kaempferol undergoes low to moderate absorption. The oral F of Kaempferol is very low (~2%) and this atleast in part is due to extensive first-pass metabolism by phase I oxidative metabolism and phase II glucuronidation in the intestine as well as in the liver.
5. Conclusion
The biological activities of flavonoids, in addition to their underlying inherent mechanisms of action, rely on the activity of their metabolites due to rapid and extensive biotransformation. Information regarding which metabolites appear in the plasma and in what amounts is crucial for proper evaluation of their biological activity. Based on the data we present here and that has been reported by several other researchers, the most common metabolites or biotransformation products of flavonoids appear to the methyl, sulfate or glucuronide conjugates [22-24]. Variable rates of formation for these conjugates have been observed depending on the species and organ/ site of metabolism [25-30]. Thus, the relative contribution of methylation, sulfation or glucuronidation may vary according to the nature of the flavonoid, species and site of metabolism. Regardless, the capacity to undergo conjugation appears high for a majority of flavonoids thus making them poorly bioavailable (~ 2 – 20 %) [31, 22]. Hence, in order to develop these polyphenols as better cancer chemopreventive agents, research to overcome their poor bioavailability is imminent.
Table 3. Comparison of systemic and portal plasma AUC values for Kaempferol and Kaempferol glucuronides following PO dose of 100 mg/kg, mean ± S.E.
| AUC Kaempferol (hr*μg/ml) |
AUC glucuronides (hr*μg/ml) |
Metabolite ratio (MR) † |
|
|---|---|---|---|
| Systemic plasma | 0.76 ± 0.095 | 2.81 ± 0.69 | 3.7 § |
| Portal plasma | 7.0 ± 0.5 | 4.3 ± 1.1 | 0.61 |
Metabolite ratio calculated as (AUC glucuronides)/ (AUC Kaempferol)
Statistically significant from Portal plasma MR value (p = 0.0029) based on Students t test. p values <0.05 were considered significant.
Acknowledgement
This study was supported in part by NIH R01-CA118947.
List of abbreviations
- AUC
Area under the curve
- CLtotal
Total body clearance
- CLrenal
Renal clearance
- CLnon-renal
Non-renal clearance
- Vd
Volume of distribution
References
- [1].Moon YJ, Wang X, Morris ME. Dietary flavonoids: effects on xenobiotic and carcinogen metabolism. Toxicology In vitro. 2006;20:187–210. doi: 10.1016/j.tiv.2005.06.048. doi:10.1016/j.tiv.2005.06.048. [DOI] [PubMed] [Google Scholar]
- [2].Ramos S. Cancer chemoprevention and chemotherapy: dietary polyphenols and signaling pathways. Mol Nutr and Food Research. 2008;52:507–526. doi: 10.1002/mnfr.200700326. 10.1002/mnfr.200700326. [DOI] [PubMed] [Google Scholar]
- [3].Ramos S. Effects of dietary flavonoids on apoptotic pathways related to cancer chemoprevention. J Nutr Biochemistry. 2007;18:427–442. doi: 10.1016/j.jnutbio.2006.11.004. doi:10.1016/j.jnutbio.2006.11.004. [DOI] [PubMed] [Google Scholar]
- [4].Abdulla M, Gruber P. Role of diet modification in cancer prevention. Biofactors. 2000;12:45–51. doi: 10.1002/biof.5520120108. [DOI] [PubMed] [Google Scholar]
- [5].Nielsen SE, Breinholt V, Justesen U, Cornett C, Dragsted LO. In vitro biotransformation of flavonoids by rat liver microsomes. Xenobiotica. 1998;28:389–401. doi: 10.1080/004982598239498. [DOI] [PubMed] [Google Scholar]
- [6].Chen Y, Xie S, Chen S, Zeng S. Glucuronidation of flavonoids by UGT1A3 and UGT1A9. Biochemical Pharmacology. 2008;76:416–425. doi: 10.1016/j.bcp.2008.05.007. doi:10.1016/j.bcp.2008.05.007. [DOI] [PubMed] [Google Scholar]
- [7].Yodogawa S, Arakawa T, Sugihara N, Furuno K. Glucurono- and Sulfo-conjugation of kaempferol in rat liver subcellular preparations and cultured hepatocytes. Biol Pharm Bull. 2003;26:1120–1124. doi: 10.1248/bpb.26.1120. doi:10.1248/bpb.26.1120. [DOI] [PubMed] [Google Scholar]
- [8].Chen J, Lin H, Hu M. Metabolism of flavonoids via enteric recycling: Role of intestinal disposition. J Pharmacol and Exp Ther. 2003;304:1228–1235. doi: 10.1124/jpet.102.046409. 10.1124/jpet.102.046409. [DOI] [PubMed] [Google Scholar]
- [9].Davies B, Morris T. Physiological Parameters in laboratory animals and humans. Pharm Res. 1993;10:1093–1095. doi: 10.1023/a:1018943613122. [DOI] [PubMed] [Google Scholar]
- [10].Zhang J, Zuo Z, Lin G. Intestinal and hepatic glucuronidation of flavonoids. Mol Pharm. 2007;4:833–845. doi: 10.1021/mp700077z. 10.1021/mp700077z. [DOI] [PubMed] [Google Scholar]
- [11].Zhang L, Lin G, Zuo Z. Position preference on glucuronidation of mono-hydroxyflavones in human intestine. Life Sci. 2006;78:2772–2780. doi: 10.1016/j.lfs.2005.10.038. doi:10.1016/j.lfs.2005.10.038. [DOI] [PubMed] [Google Scholar]
- [12].Chen J, Lin H, Hu M. Absorption and metabolism of genistein and its five isoflavone analogs in human intestinal Caco-2 model. Cancer Chemo Pharmacol. 2005;55:159–169. doi: 10.1007/s00280-004-0842-x. 10.1007/s00280-004-0842-x. [DOI] [PubMed] [Google Scholar]
- [13].van der Woude H, Boersma MG, Vervoort J, Rietjens IM. Identification of 14 Quercetin phase II mono- and mixed conjugates and their formation by rat and human phase II in vitro model systems. Chem Res Toxicol. 2004;17:1520–1530. doi: 10.1021/tx049826v. 10.1021/tx049826v. [DOI] [PubMed] [Google Scholar]
- [14].Yin H, Bennett G, Jones JP. Mechanistic studies of uridine diphosphate glucuronosyltransferase. Chem-Bio Interact. 1994;90:47–58. doi: 10.1016/0009-2797(94)90110-4. [DOI] [PubMed] [Google Scholar]
- [15].Shelby MK, Cherrington NJ, Vansell NR, Klaassen CD. Tissue mRNA expression of the rat UDP-glucuronosyltransferase gene family. Drug Metab Dispos. 2003;31:326–333. doi: 10.1124/dmd.31.3.326. [DOI] [PubMed] [Google Scholar]
- [16].Radominska-Pandya A, Little JM, Pandya JT, Tephly TR, King CD, Barone GW, Raufman JP. UDP-glucuronosyltransferase in human intestinal mucosa. Biochim Biophys Acta. 1998;1394:199–208. doi: 10.1016/s0005-2760(98)00115-5. doi:10.1016/S0005-2760(98)00115-5. [DOI] [PubMed] [Google Scholar]
- [17].DuPont MS, Day AJ, Bennett RN, Mellon FA, Kroon PA. Absorption of kaempferol from endive, a source of kaempferol-3-glucuronide, in humans. Eur J Clin Nut. 2004;58:947–954. doi: 10.1038/sj.ejcn.1601916. 10.1038/sj.ejcn.1601916. [DOI] [PubMed] [Google Scholar]
- [18].Nielsen SE, Kall M, Justesen U, Schou A, Dragsted LO. Human absorption and excretion of flavonoids after broccoli consumption. Cancer Lett. 1997;114:173–174. doi: 10.1016/s0304-3835(97)04654-5. doi:10.1016/S0304-3835(97)04654-5. [DOI] [PubMed] [Google Scholar]
- [19].Breinholt VM, Offord EA, Brouwer C, Nielsen SE, Brosen K, Friedberg T. In vitro investigation of cytochrome P450-mediated metabolism of dietary flavonoids. Food Chem. Toxicol. 2002;40:609–616. doi: 10.1016/s0278-6915(01)00125-9. doi:10.1016/S0278-6915(01)00125-9. [DOI] [PubMed] [Google Scholar]
- [20].DeVries JHM, Hollman PCH, Meyboom S, Buysman MNCP, Zock PL, van Staveren WA, Katan MB. Plasma concentrations and urinary excretion of the antioxidant flavonols quercetin and kaempferol as biomarkers for dietary intake. Am. J. Clin. Nutr. 1998;68:60–65. doi: 10.1093/ajcn/68.1.60. [DOI] [PubMed] [Google Scholar]
- [21].Crespy V, Morand C, Besson C, Cotelle N, Vezin H, Demigne C, Remesy C. The splanchnic metabolism of flavonoids highly differed according to the nature of the compound. Am J Physiol. 2003;284:G980–988. doi: 10.1152/ajpgi.00223.2002. doi: 10.1152/ajpgi.00223.2002. [DOI] [PubMed] [Google Scholar]
- [22].Scalbert A, Williamson G. Dietary intake and bioavailability of polyphenols. J. Nutr. 2000;130(Suppl. 8S):2073S–2085S. doi: 10.1093/jn/130.8.2073S. [DOI] [PubMed] [Google Scholar]
- [23].Manach C, Williamson G, Morand C, Scalbert A, Remesy C. Bioavailability and bioefficacy of polyphenols in humans I. Review of 97 bioavailability studies. Am J Clin Nutr. 2005;81(Suppl 1):230S–242S. doi: 10.1093/ajcn/81.1.230S. [DOI] [PubMed] [Google Scholar]
- [24].Williamson G, Manach C. Bioavailability and bioefficacy of polyphenols in humans II. Review of 93 intervention studies. Am J Clin Nutr. 2005;81(Suppl 1):243S–255S. doi: 10.1093/ajcn/81.1.243S. [DOI] [PubMed] [Google Scholar]
- [25].Graefe EU, Wittig J, Mueller S, Reithling AK, Uehleke B, Drewelow B, Pforte H, Jacobasch G, Derendorf H, Veit M. Pharmacokinetics and bioavailability of quercetin glycosides in humans. J. Clin Pharmac. 2001;41:492–499. doi: 10.1177/00912700122010366. [DOI] [PubMed] [Google Scholar]
- [26].Steensma A, Faassen-Peters MA, Noteborn HP, Reitjen IM. Bioavailability of genistein and its glycoside genistin as measured in the portal vein of freely moving unanaesthetized rats. J. Agri Food Chem. 2006;54:8006–8012. doi: 10.1021/jf060783t. doi: 10.1021/jf060783t. [DOI] [PubMed] [Google Scholar]
- [27].Kuhnle G, Spencer JP, Schroeter H, Shenoy B, Debnam ES, Srai SK, Rice-Evans C, Hahn U. Epicatechin and catechin are O-methylated and glucuronidated in the small intestine. Biochem Biophy Res Commun. 2000;277:507–512. doi: 10.1006/bbrc.2000.3701. doi:10.1006/bbrc.2000.3701. [DOI] [PubMed] [Google Scholar]
- [28].Jia X, Chen J, Lin H, Hu M. Disposition of flavonoids via enteric recycling: enzyme-transporter coupling affects metabolism of biochanin A and formononetin and excretion of their phase II conjugates. J. Pharmacol Exp Ther. 2004;310:1103–1113. doi: 10.1124/jpet.104.068403. doi: 10.1124/jpet.104.068403. [DOI] [PubMed] [Google Scholar]
- [29].Hu M, Chen J, Lin H. Disposition of flavonoids via recycling: mechanistic studies of disposition of apigenein in the Caco-2 cell culture model. J. Pharmacol Exp Ther. 2003;307:314–321. doi: 10.1124/jpet.103.053496. doi: 10.1124/jpet.103.053496. [DOI] [PubMed] [Google Scholar]
- [30].Zhang Y, Hendrich S, Murphy PA. Glucuronides are the main isoflavone metabolites in women. J Nutr. 2003;133:399–404. doi: 10.1093/jn/133.2.399. [DOI] [PubMed] [Google Scholar]
- [31].Hu M. Commentary: bioavailability of flavonoids and polyphenols: call to arms. Mol Pharm. 2007;4:803–806. doi: 10.1021/mp7001363. 10.1021/mp7001363. [DOI] [PMC free article] [PubMed] [Google Scholar]