Abstract
The nuclear factor-erythroid 2-related factor 2 (Nrf2) plays a critical role in protecting various tissues against inflammation, which is a potential risk factor for colorectal and other cancers. Our previously published mouse model work showed that Nrf2 helps protect against dextran sulfate sodium (DSS)–induced colitis/inflammation, and others have shown that Nrf2 helps protect against inflammation-associated colorectal carcinogenesis (aberrant crypt foci). The present study extended these important earlier findings by exploring the role of Nrf2 in colitis-associated colorectal cancer in a mouse model involving azoxymethane/DSS–induced colorectal carcinogenesis in Nrf2 knockout mice. Azoxymethane/DSS–treated Nrf2 knockout mice had increased incidence, multiplicity, and size of all colorectal tumors, including adenomas, versus treated wild-type (WT) mice, and the proportion of tumors that were adenocarcinoma was much higher in knockout (80%) versus WT (29%) mice. Compared with WT mice, knockout mice also had increased markers of inflammation in tumor tissue (cyclooxygenase-2 and 5-lipoxygenase expressions and prostaglandin E2 and leukotriene B4 levels) and in inflamed colonic mucosa (nitrotyrosine expression), supporting the association of knockout mouse tumor formation with inflammation. The phase II detoxifying/antioxidant enzymes NAD(P)H-quinone reductase 1 and UDP-glucurosyltransferase 1A1 were elevated in the normal mucosa of WT, but not Nrf 2 knockout, mice treated with azoxymethane/DSS. Our findings show that Nrf2 plays a critical role in protecting against inflammation-associated colorectal cancer.
Chronic inflammation has been identified as a potential risk factor for colorectal and other cancers. Inflammatory bowel diseases are forms of chronic, recurrent colitis, most commonly Crohn’s disease and ulcerative colitis, and many epidemiologic and clinical studies have shown that inflammatory bowel disease increases the risk of colorectal cancer (1-5). Inflammation, or colitis, can be triggered by environmental insults such as a gastrointestinal microbial infection or toxic chemical irritation. Driven by a cascade of cytokine and chemokine interactions, inflammatory cells such as neutrophils, macrophages, and lymphocytes infiltrate the wounded colonic mucosa and create oxidative stress, which in turn generates reactive oxygen and nitrogen species. Reactive oxygen and nitrogen species are thought to be a major factor underlying the contribution of chronic inflammation to neoplastic transformation (5).
Nuclear factor-erythroid 2-related factor 2 (Nrf2) is a basic leucine zipper redox-sensitive transcriptional factor that plays a central role in transcriptional regulation of antioxidant and/or detoxifying genes. We recently reported that Nrf2-deficient mice are more susceptible to dextran sulfate sodium (DSS)–induced inflammation/colitis compared with wild-type (WT) mice (6). This increased susceptibility was associated with decreased expression of antioxidant/phase II detoxifying enzymes and concomitant increases in proinflammatory cytokines and other biomarkers (6). These findings illustrated the importance of Nrf2 in protecting or restoring intestinal integrity after a wound to the colonic mucosa. Others have shown that Nrf2-deficient mice are also more susceptible to inflammation-associated preinvasive colorectal carcinogenesis (aberrant crypt foci) versus WT mice (7). We conducted the present study to extend these previous findings by addressing the question of whether colitis-associated colorectal cancer risk is increased in Nrf2 knockout mice.
Materials and Methods
Reagents
DSS was purchased from MP Biomedicals. Azoxymethane was obtained from the National Cancer Institute Chemical Carcinogen Reference Standard Repository (Midwest Research Institute). Rabbit polyclonal antibodies against NAD(P)H-quinone reductase 1 and UDP-glucurosyltransferase 1A1 and goat polyclonal antibodies against actin and heme oxygenase 1 were purchased from Santa Cruz Biotechnology. Anti–cyclooxygenase-2 (COX-2) and anti–5-lipoxygenase (5-LOX) antibodies were purchased from Cayman Chemical.
Animal care and treatment
The male Nrf2(−/−) knockout mice used in this study were essentially of the C57BL/6 strain (through backcrossing) and have been described in detail elsewhere (6). Control mice were age-matched male C57BL/6 WT mice purchased from Hilltop Lab Animals, Inc. Five- to six-week-old mice were used and housed at Rutgers Animal Facility and maintained under 12-h light/dark cycles. All animals received water and food ad libitum. After 1 wk of acclimatization, mice were divided into four groups: (a) control WT (n = 6); (b) control Nrf2(−/−) (n = 6); (c) WT + azoxymethane/DSS (n = 15); and (d) Nrf2(−/−) + azoxymethane/DSS (n = 15). Mice were injected s.c. with a single dose of azoxymethane (10 mg/kg body weight) in 100 μL of normal saline or vehicle alone (100 μL normal saline, control group 1) and water ad libitum. Two weeks after the azoxymethane injection, the mice were given 1% DSS (w/v) in water as the sole source of drinking fluid for 1 wk, after which the DSS was stopped and drinking fluid was shifted back to drinking water, which continued until the end of experiment (20 wk). All animal use procedures were in accordance with the National Academy of Sciences Guide for the Care and Use of Laboratory Animals and were approved by the Rutgers Institutional Animal Care and Use Committee, which is accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care.
Immunohistochemistry
Immunohistochemical staining of nitrotyrosine has previously been described (6). The expression of nitrotyrosine was scored as the number of positive cells per field at ×400 magnification. Four fields were randomly selected for the scoring.
Western blotting
Large bowel tissues and tumors dissected from mice of each treatment group were weighed and pooled. The protein extraction and Western blotting methods were done as previously described (6).
Measurement of prostaglandin E2 and leukotriene B4 levels in the small intestine
Frozen intestinal polyps and normal mucosa were quickly excised from the small intestine, stored at −80°C, cut in the microtome at −20°C, and homogenized using a Polytron homogenizer in an ice-cold homogenizing buffer containing protease inhibitors (0.2 mmol/L phenyl methyl sulfonyl fluoride and 2.5 μg/mL of pepstatin, aprotinin, and leupeptin), 10 μmol/L nordihydroguaiaretic acid (a LOX inhibitor), and 10 μmol/L indomethacin (a COX inhibitor; ref. 8). The LOX and COX inhibitors were added to stabilize the levels of prostaglandin E2 (PGE2) and leukotriene B4 (LTB4) during sample processing. The homogenates were then transferred to a clean tube and centrifuged at 14,000 × g for 10 min at 4°C. The supernatant was aliquoted and stored at −80°C until further analysis. To analyze the PGE2 and LTB4 levels, a 100-μL homogenate supernatant was acidified with 15 μL of 0.1 N HCl, vortexed for 1 min after adding 1 mL of ethyl acetate, and then centrifuged at 10,000 rpm for 3 min at room temperature. The organic layer was collected and evaporated under nitrogen gas. The dried sample was reconstituted in a 1-mL enzyme immunoassay buffer. The reconstituted samples were diluted appropriately and the concentrations of PGE2 and LTB4 were measured using an enzyme immunoassay kit (Cayman Chemical) according to the manufacturer’s protocol.
Statistical considerations
Student’s t test was used for simple group means analysis (nitrotyrosine score), and Fisher’s exact test was used to analyze the incidence of tumors and anal bleeding. For between-group comparisons, one-way ANOVA and post-hoc Tukey’s test were used. P < 0.05 was considered to be statistically significant.
Results
Susceptibility to azoxymethane/DSS–induced colon carcinogenesis and cancer increase in Nrf2 knockout mice
Nrf2 knockout mice were more susceptible to azoxymethane/DSS–induced colorectal carcinogenesis and cancer compared with WT mice. The incidence of colonic tumors increased by ~40% in Nrf2 knockout mice in association with increased rectal bleeding and prolapsed rectums (~60% increased) compared with WT counterparts (Fig. 1). The number of tumors per mouse also increased significantly in Nrf2 knockout mice (Table 1). Subsequent histologic evaluations of tumors (>4 mm2) revealed that 80% of tumors in Nrf2 knockout mice were adenocarcinomas, versus 29% in the control mice (Table 2). The number of nitrotyrosine-positive cells (primarily stroma cells) was significantly higher in the colonic mucosa of Nrf2 knockout mice versus WT mice after azoxymethane/DSS exposure (Fig. 2A and B). These collective results strongly indicate that Nrf2 plays an indispensable role in protecting colonic mucosa against inflammation and carcinogenesis.
Fig. 1.
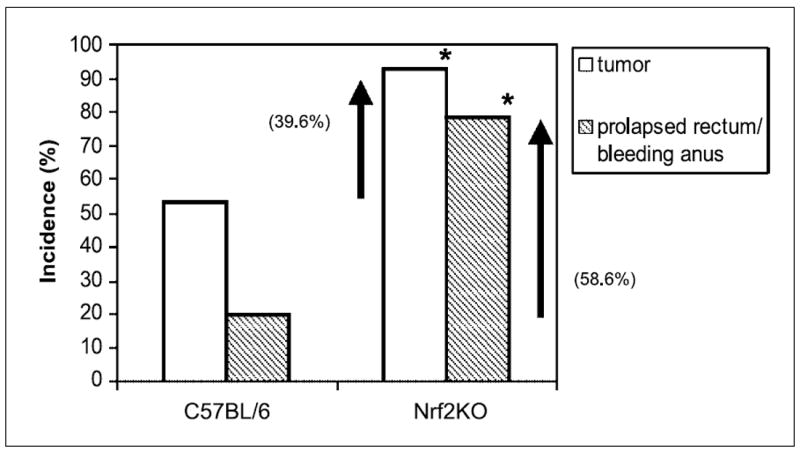
Incidence of colonic tumors and prolapsed rectum/bleeding anus in C57BL/6 versus Nrf2 knockout (KO) mice after azoxymethane (AOM)/DSS treatment (20 wks). The incidence of colonic tumors per mouse and prolapsed rectum/bleeding anus was significantly higher in Nrf2 knockout mice compared with WT C57BL/6 mice. Fisher’s exact test was used to compare the difference between groups.
*, P < 0.01.
Table 1.
Incidence, tumor multiplicity, tumor size, and percentage of prolapsed rectum/anus bleeding after AOM/DSS treatment (20 wk)
| Group (no. mice) | Tumor incidence (%) | Tumor/mouse (mean ± SE) | Tumor size (mm2)
|
Prolapsed rectum/bleeding of anus (%) | ||
|---|---|---|---|---|---|---|
| <4 | 4-16 | >16 | ||||
| WT + H2O (6) | 0 | 0.00 ± 0.00 | 0 | 0 | 0 | 0 |
| Nrf2 KO + H2O (6) | 0 | 0.00 ± 0.00 | 0 | 0 | 0 | 0 |
| WT + AOM/DSS (15) | 53.3 | 1.00 ± 0.40 | 7 | 4 | 4 | 20.0 |
| Nrf2 KO + AOM/DSS (15) | 92.9* | 1.86 ± 0.33 | 9 | 11 | 6 | 78.6* |
P < 0.05 compared with WT + AOM/DSS
Abbreviations: KO, knockout; AOM, azoxymethane.
Table 2.
Pathologic classification of tumors
| Group | Adenomas (%) | Adenocarcinomas (%) |
|---|---|---|
| WT + AOM/DSS | 71 | 29 |
| Nrf2 KO + AOM/DSS | 20 | 80 |
Fig. 2.
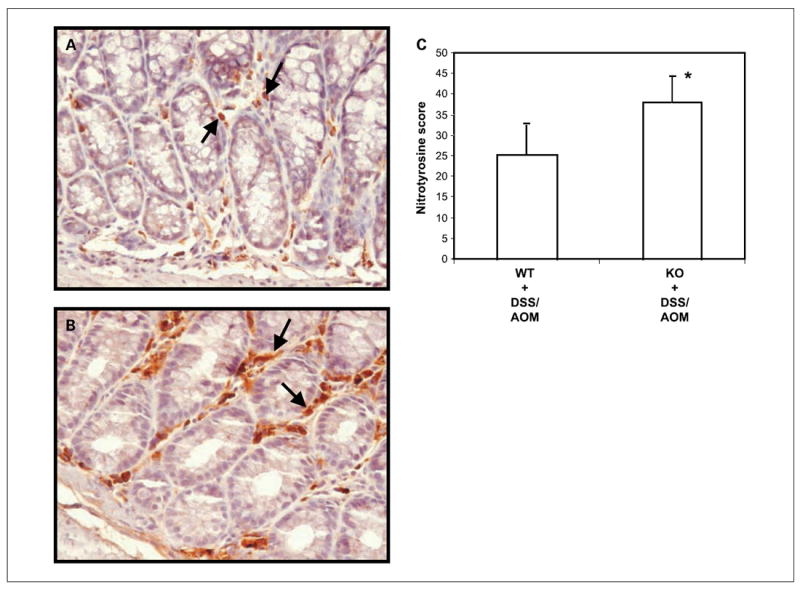
Nitrotyrosine staining of colonic mucosa. Representative slide showing the expression of nitrotyrosine in colonic tissues of C57BL/6 (A) and Nrf2 knockout (B) mice after azoxymethane/DSS treatment (20 wk). Positive immunoreactivity of nitrotyrosine was observed predominantly in the stroma cells (arrow). Increased nitrotyrosine expression was observed in Nrf2 knockout mice as compared with WT C57BL/6 mice. Columns, mean of number of positive nitrotyrosine cells per field (40×); bars, SD. *, P < 0.05 (Student’s t test).
Association of increased susceptibility of Nrf2 knockout mice to azoxymethane/DSS–induced colon carcinogenesis with increased expressions of COX-2, 5-LOX, and proinflammatory metabolites of arachidonic acids
Proinflammatory biomarkers such as COX-2 and 5-LOX were overexpressed in tumors compared with their expression in adjacent apparently normal mucosa. Furthermore, COX-2 and 5-LOX expressions were higher in tumors of Nrf2 knockout mice than in tumors of WT mice (Fig. 3A). The expressions of PGE2 and LTB4, two downstream products of COX-2 and 5-LOX metabolism of arachidonic acid, were also higher in tumors of Nrf2 knockout mice than in tumors of WT mice (Fig. 3B and C).
Fig. 3.
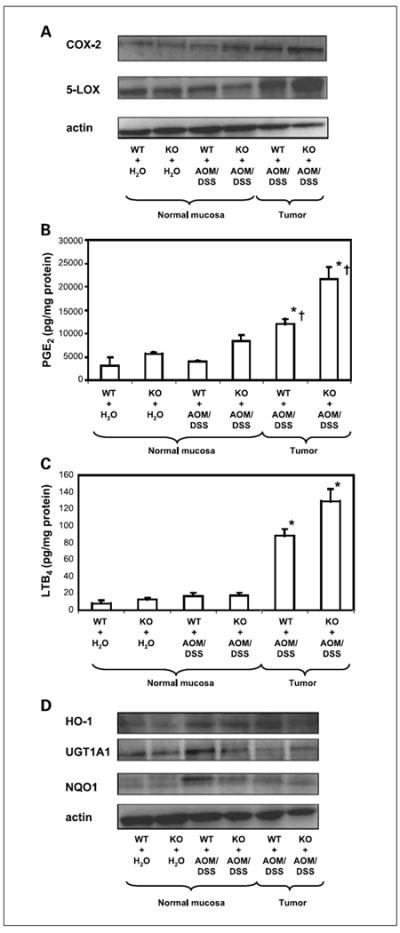
The expression of proinflammatory and phase II detoxifying/antioxidant enzymes in the normal mucosa and tumors of Nrf2 knockout mice compared with WT C57BL/6J mice. A, expressions of COX-2 and 5-LOX were significantly elevated in the tumors of Nrf2 knockout mice when compared with normal tissues of knockout mice and tumors of WT mice. The levels of PGE2 (B) and LTB4 (C) were also higher in the tumors of Nrf2 knockout mice. Columns, mean; bars, SD.
*, significantly different from normal mucosa of mice with same phenotype.
†, significantly different from tumors of mice with different phenotypes.
D, expression of phase II detoxifying/antioxidant enzymes in the normal mucosa and tumors of Nrf2 knockout mice compared with WT C57BL/6J mice.
Expressions of antioxidant/phase II detoxifying enzymes in normal mucosa versus tumors of azoxymethane/DSS–treated mice
As in our previous studies (6, 9), the expressions of the antioxidant/phase II detoxifying enzymes NAD(P)H-quinone reductase 1, UDP-glucurosyltransferase 1A1, and heme oxygenase 1 were elevated in the normal mucosa of azoxymethane/DSS–treated WT mice, but only heme oxygenase 1 was elevated in the normal mucosa of azoxymethane/DSS–treated Nrf2 knockout mice (Fig. 3D). The expressions of these markers were either totally diminished [NAD(P)H-quinone reductase 1] or reduced (UDP-glucurosyltransferase 1A1) in the tumors of both WT and Nrf2 knockout mice (Fig. 3D).
Discussion
Our present data clearly show that Nrf2 played a critical role in protecting against colitis/inflammation-associated neoplastic transformation. Nrf2 knockout mice had a significantly greater incidence, multiplicity, and size of colorectal cancers than did control WT mice. Prolapsed rectums and rectal bleeding also were significantly more frequent in Nrf2 knockout mice. The striking significantly higher proportion of adenocarcinomas among Nrf2 knockout tumors (80%) than among WT tumors (29%) further confirms the greater severity of colorectal carcinogenesis in Nrf2 knockout mice. Our findings show that Nrf2 plays a critical role in protecting against inflammation-associated colorectal cancer and thus support and extend a recently reported study showing that Nrf2 knockout increases susceptibility to inflammation-associated aberrant crypt foci (7).
Although strong data support the association between chronic inflammation and cancer, the biological basis of this association is not fully understood; our present data throw some light on this association. Positive associations have been found between the inflammatory diseases hepatitis and hepatocellular carcinoma (10), pancreatitis and pancreatic cancer (11), Helicobacter pylori infection and gastric cancer (12), schistosomiasis and bladder cancer (13), primary sclerosing cholangitis and cholangiocarcinoma (14), and between inflammatory bowel diseases and colorectal cancer (4). Oxidative stress has been identified as a possible culprit in the influence of inflammation on cancer development. Our present study joins other recent studies (6, 15, 16), including our study showing that Nrf2-deficient mice were more susceptible to DSS-induced colitis (6), in suggesting that Nrf2 may play a central role in regulating inflammation because Nrf2 is an integral regulator of detoxification and antioxidation mechanisms.
Our findings on the inflammation markers COX-2, 5-LOX, PGE2, and LBT4 (19, 20) support the association of knockout mouse tumor formation with inflammation. COX-2 and 5-LOX expressions and PGE2 and LBT4 levels were increased in Nrf2 knockout versus WT tumor tissue. These markers also were higher in tumor versus normal tissue either in knockout or WT mice. Furthermore, expression of the inflammation marker nitrotyrosine, which indicates cell damage, inflammation, and nitrite oxide production (data not shown), was significantly increased in the colonic mucosa of mice treated with azoxymethane/DSS (versus not treated) overall and, more importantly, of Nrf2 knockout (versus WT) mice. Increased COX-2 expression has been observed in colitis-associated cancers of ulcerative colitis patients (20).
A growing body of evidence indicates that the loss of antioxidant/phase II detoxifying enzymes, coupled with increases in reactive oxygen and nitrogen species, is detrimental to normal colonic homeostasis. We previously showed that decreased expression of antioxidant/phase II detoxifying enzymes could help explain why Nrf2 knockout mice are more susceptible to DSS-induced colitis (6). The present study also showed that the expressions of some antioxidant/phase II detoxifying enzymes, including NAD(P)H-quinone reductase 1 and UDP-glucurosyltransferase 1A1, were elevated in the normal mucosa of WT, but not Nrf2 knockout, mice treated with azoxymethane/DSS. The expression of heme oxygenase 1 was increased in the normal mucosa of treated WT and Nrf2 knockout mice. It is not surprising that heme oxygenase 1 was induced in Nrf2 knockout mice because other transcription factors (besides Nrf2) that regulate heme oxygenase 1, such as hypoxia-inducible factor 1 (17) and activator protein 1 (18), were presumably still present in Nrf2 knockout mice. In summary, these data suggest that decreased expression of the detoxifying enzymes NAD(P)H-quinone reductase 1 and UDP-glucurosyltransferase 1A1, coupled with enhanced inflammation (described above), could be important events that accelerate neoplastic transformation in the colonic tissues of Nrf2 knockout mice, further suggesting that these defensive enzymes are important for protecting colonic mucosa against colitis-associated colorectal carcinogenesis.
The present study indicates that Nrf2 knockout mice were more susceptible to colitis/inflammation-associated colorectal cancer in our model of colitis and cancer induction. Primary contributors to this increased susceptibility were impaired antioxidant/detoxifying mechanisms coupled with enhanced proinflammatory arachidonic acid metabolism. This work adds an important link to the chain of related recent findings on the effects of Nrf2 on the development of colitis/inflammation (6), colitis-associated aberrant crypt foci (16), and colitis-associated colorectal cancer.
Acknowledgments
Grant support: NIH grant R01 CA-073674.
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Eaden JA, Abrams KR, Mayberry JF. The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut. 2001;48:526–35. doi: 10.1136/gut.48.4.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ekbom A, Helmick C, Zack M, Adami HO. Ulcerative colitis and colorectal cancer. A population-based study. N Engl J Med. 1990;323:1228–33. doi: 10.1056/NEJM199011013231802. [DOI] [PubMed] [Google Scholar]
- 3.Friedman S, Rubin PH, Bodian C, et al. Screening and surveillance colonoscopy in chronic Crohn’s colitis. Gastroenterology. 2001;120:820–6. doi: 10.1053/gast.2001.22449. [DOI] [PubMed] [Google Scholar]
- 4.Choi PM, Zelig MP. Similarity of colorectal cancer in Crohn’s disease and ulcerative colitis: implications for carcinogenesis and prevention. Gut. 1994;35:950–4. doi: 10.1136/gut.35.7.950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Itzkowitz SH, Yio X. Inflammation and cancer IV. Colorectal cancer in inflammatory bowel disease: the role of inflammation. Am J Physiol Gastrointest Liver Physiol. 2004;287:G7–17. doi: 10.1152/ajpgi.00079.2004. [DOI] [PubMed] [Google Scholar]
- 6.Khor TO, Huang MT, Kwon KH, et al. Nrf2-deficient mice have an increased susceptibility to dextran sulfate sodium-induced colitis. Cancer Res. 2006;66:11580–4. doi: 10.1158/0008-5472.CAN-06-3562. [DOI] [PubMed] [Google Scholar]
- 7.Osburn WO, Karim B, Dolan PM, et al. Increased colonic inflammatory injury and formation of aberrant crypt foci in Nrf2-deficient mice upon dextran sulfate treatment. Int J Cancer. 2007;121:1883–91. doi: 10.1002/ijc.22943. [DOI] [PubMed] [Google Scholar]
- 8.Ju J, Hong J, Zhou JN, et al. Inhibition of intestinal tumorigenesis in Apcmin/+ mice by (−)-epigallocatechin-3-gallate, the major catechin in green tea. Cancer Res. 2005;65:10623–31. doi: 10.1158/0008-5472.CAN-05-1949. [DOI] [PubMed] [Google Scholar]
- 9.Xu C, Huang MT, Shen G, et al. Inhibition of 7,12-dimethylbenz(a)anthracene-induced skin tumori-genesis in C57BL/6 mice by sulforaphane is mediated by nuclear factor E2-related factor 2. Cancer Res. 2006;66:8293–6. doi: 10.1158/0008-5472.CAN-06-0300. [DOI] [PubMed] [Google Scholar]
- 10.Harris CC. Solving the viral-chemical puzzle of human liver carcinogenesis. Cancer Epidemiol Biomarkers Prev. 1994;3:1–2. [PubMed] [Google Scholar]
- 11.Bansal P, Sonnenberg A. Pancreatitis is a risk factor for pancreatic cancer. Gastroenterology. 1995;109:247–51. doi: 10.1016/0016-5085(95)90291-0. [DOI] [PubMed] [Google Scholar]
- 12.Asaka M, Takeda H, Sugiyama T, Kato M. What role does Helicobacter pylori play in gastric cancer? Gastroenterology. 1997;113:S56–60. doi: 10.1016/s0016-5085(97)80013-3. [DOI] [PubMed] [Google Scholar]
- 13.Rosin MP, Anwar WA, Ward AJ. Inflammation, chromosomal instability, and cancer: the schistosomiasis model. Cancer Res. 1994;54:1929s–33s. [PubMed] [Google Scholar]
- 14.Lewis JT, Talwalkar JA, Rosen CB, Smyrk TC, Abraham SC. Prevalence and risk factors for gallbladder neoplasia in patients with primary sclerosing cholangitis: evidence for a metaplasia-dysplasia-carcinoma sequence. Am J Surg Pathol. 2007;31:907–13. doi: 10.1097/01.pas.0000213435.99492.8a. [DOI] [PubMed] [Google Scholar]
- 15.Rangasamy T, Cho CY, Thimmulappa RK, et al. Genetic ablation of Nrf2 enhances susceptibility to cigarette smoke-induced emphysema in mice. J Clin Invest. 2004;114:1248–59. doi: 10.1172/JCI21146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thimmulappa RK, Lee H, Rangasamy T, et al. Nrf2 is a critical regulator of the innate immune response and survival during experimental sepsis. J Clin Invest. 2006;116:984–95. doi: 10.1172/JCI25790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ockaili R, Natarajan R, Salloum F, et al. HIF-1 activation attenuates postischemic myocardial injury: role for heme oxygenase-1 in modulating microvascular chemokine generation. Am J Physiol Heart Circ Physiol. 2005;289:H542–8. doi: 10.1152/ajpheart.00089.2005. [DOI] [PubMed] [Google Scholar]
- 18.Kiemer AK, Bildner N, Weber NC, Vollmar AM. Characterization of heme oxygenase 1 (heat shock protein 32) induction by atrial natriuretic peptide in human endothelial cells. Endocrinology. 2003;144:802–12. doi: 10.1210/en.2002-220610. [DOI] [PubMed] [Google Scholar]
- 19.Kuehl FA, Jr, Egan RW. Prostaglandins, arachidonic acid, and inflammation. Science. 1980;210:978–84. doi: 10.1126/science.6254151. [DOI] [PubMed] [Google Scholar]
- 20.Agoff SN, Brentnall TA, Crispin DA, et al. The role of cyclooxygenase 2 in ulcerative colitis-associated neoplasia. Am J Pathol. 2000;157:737–45. doi: 10.1016/S0002-9440(10)64587-7. [DOI] [PMC free article] [PubMed] [Google Scholar]


