Abstract
Purpose
Exposure to ionizing radiation is an established risk factor for breast cancer. Radiation exposure during infancy, childhood, and adolescence confers the highest risk. Although radiation is a proven mammary carcinogen, it remains unclear where it acts in the complex multistage process of breast cancer development. In this study, we investigated the long-term pathophysiologic effects of ionizing radiation at a dose (2 Gy) relevant to fractionated radiotherapy.
Methods and Materials
Adolescent (6–8 weeks old; n = 10) female C57BL/6J mice were exposed to 2 Gy total body γ-radiation, the mammary glands were surgically removed, and serum and urine samples were collected 2 and 12 months after exposure. Molecular pathways involving estrogen receptor-α (ERα) and phosphatidylinositol-3-OH kinase (PI3K)-Akt signaling were investigated by immunohistochemistry and Western blot.
Results
Serum estrogen and urinary levels of the oncogenic estrogen metabolite (16αOHE1) were significantly increased in irradiated animals. Immunostaining for the cellular proliferative marker Ki-67 and cyclin-D1 showed increased nuclear accumulation in sections of mammary glands from irradiated vs. control mice. Marked increase in p85α, a regulatory sub-unit of the PI3K was associated with increase in Akt, phospho-Akt, phospho-BAD, phospho-mTOR, and c-Myc in irradiated samples. Persistent increase in nuclear ERα in mammary tissues 2 and 12 months after radiation exposure was also observed.
Conclusions
Taken together, our data not only support epidemiologic observations associating radiation and breast cancer but also, specify molecular events that could be involved in radiation-induced breast cancer.
Keywords: Radiation, Estradiol, Estrogen, Estrogen receptor α, Estrogen metabolite
Introduction
On the basis of epidemiologic studies conducted on atomic bomb survivors and on patients receiving radiotherapy, ionizing radiation exposure has been established as a breast cancer risk factor (1). Breast cancer risk after radiation exposure is considered to be age dependent, with maximum risk for adolescent women. Studies have also shown that diagnostic and therapeutic radiation exposure can lead to cumulative breast tissue doses ranging from 0.02 to 2.8 Gy with increase in breast cancer risk (2, 3). Furthermore, in a population-based case study, Ma et al. have shown that diagnostic radiation exposure of mammary glands increases the risk of developing breast cancer by twofold relative to control groups (4). Importantly, fractionated radiotherapy for cancer, including breast cancer, commonly uses a daily dose of 2 Gy, and breast cancer radiotherapy to patients under 45 years of age has been suggested to enhance the risk for the development of cancer in the contralateral breast, with a relative risk of 1.59 (2).
Estrogen exposure is also important in the context of radiation-induced mammary cancer, and studies in rats have shown that estrogen promotes radiation-induced carcinogenesis (5). Furthermore, several downstream estrogen metabolites have been proposed as markers for the risk of mammary carcinogenesis. The 16α-hydroxy metabolite 16α-hydroxyestrone (16α-OHE1) is biologically active, whereas the 2-hydroxy metabolite 2-hydroxyestrone (2-OHE1) is not (6). Interestingly, of these two metabolites, only 16α-OHE1 has a significant affinity for the estrogen receptor (ER), and low urinary 2-OHE:16α-OHE ratios have been reported to be associated with an increased risk of breast cancer (6, 7).
Estrogen signaling and ERα are strongly associated with breast cancer initiation and progression, either by directly activating estrogen-responsive genes such as cyclin-D1 and c-Myc or by activating other proproliferative and prosurvival pathways such as phosphatidylinositol-3-OH kinase (PI3K)-Akt. Studies have demonstrated that membrane ERα interacts with p85α, one of the regulatory subunits of PI3K (8), and activation of PI3K results in the phosphorylation of its principal downstream effector Akt. Enhanced Akt activity observed in several cancers is linked to decreased apoptosis and increased cell growth (9). Akt suppresses cell death by direct phosphorylation and consequent inhibition of pro-apoptotic proteins like Bcl2-associated death promoter (BAD) and promotes cell growth by activating downstream effectors such as mammalian target of rapamycin (mTOR). Moreover, Cyclin D1, a known proto-oncogene, is stabilized after Akt-mediated phosphorylation of its negative regulator glycogen synthase kinase 3β (GSK3β), which may further aid in cellular proliferation. Even though radiation is a breast cancer risk factor and estrogen/ER enhances cell proliferation, the precise mechanisms by which radiation plays a role in the complex multistage signaling processes responsible for breast cancer initiation and progression are not yet fully explored.
In the present study, we investigated the long-term effects of acute whole-body exposure to 2 Gy γ-radiation on the mammary glands of 6- to 8-week-old female C57BL/6J mice. Our observations showed that radiation exposure caused a sustained increase in the levels of circulating serum estrogen and upregulation of ERα levels and the activation of the PI3K-Akt proliferative pathway in mammary glands. Radiation exposure also induced long-term shifts in the levels and ratio of estrogen metabolites, with implications for epithelial cell proliferation in the mammary glands.
Methods and Materials
Mice
Female C57BL/6J mice 6 to 8 weeks old were purchased from Jackson Laboratories (Bar Harbor, ME). All animals were housed in an air- and temperature-controlled room with 12-hour dark and light cycle maintained at 22°C in 50% humidity. The mice received certified rodent diet along with filtered water ad libitum. All animal procedures were performed according to protocols approved by the Institutional Animal Care and Use Committee.
Irradiation and experimental groups
The mice (n = 10 per group) were exposed to 2 Gy whole-body γ-radiation (137Cs; dose rate 1 Gy/min). After irradiation, the mice were returned to their home cages and monitored regularly. The irradiation experiments were repeated three times, serum and urine samples were obtained, and mammary glands were surgically removed 2 and 12 months after each exposure for further analysis. In all experiments the control mice were sham irradiated.
Urine collection
For urine collection we followed the procedure described previously (10). Briefly, mice (n = 10) were placed individually in metabolic cages (Nalgene, Tecniplast, Exton, PA) at specified time points (2 and 12 months after exposure) for 24 hours. Urine was collected from all the three irradiation experiments, and all the samples were stored at −80°C before further analysis.
Tissue and blood collection
The mice were killed with CO2, blood samples were collected by cardiac puncture with sterile 1-mL syringes and 25-gauge needles, and the mammary glands were dissected en bloc with the fat pad from all the mice (n = 10) in each experimental group. The separated serum samples were flash frozen and stored at −80°C for further analysis. For immunohistochemistry, number four mammary glands were surgically removed from the right side, laid individually on glass slides, and fixed in 10% buffered formalin, and sections 4 µm thick were prepared from paraffin-embedded tissues. The mammary glands on the left side of the animal were flash frozen in liquid N2 and stored at −80°C for molecular assay.
Serum estradiol estimation
Serum estradiol concentrations in control and treated mice (n = 10 for each group) were measured by using an estradiol assay kit (Cayman Chemicals, Ann Arbor, MI) according to the manufacturer’s protocol. This is a competitive assay, measurements were performed in triplicates, and the assay has a detection limit of approximately 20 pg/mL.
Estrogen metabolite assay
Estrogen metabolites (2OHE1 and 16αOHE1) were measured in the urine of mice (n = 10) with the Estramet kit (Immuna Care, Bethlehem, PA) according to manufacturer’s instructions. This is a competitive, solid-phase enzyme immunoassay, each measurement was done in triplicate, and measurements of estradiol and its metabolite were repeated in two other irradiation experiments. The results are expressed as nanograms of metabolites per milliliter of urine.
Immunohistochemistry
Immunostaining (sections from 5 mice) for Ki67 (Catalog No. Sc15402; dilution 1:50; Santa Cruz Biotechnology, Santa Cruz, CA), ERα (Catalog No. 04-227; dilution 1:50; Millipore, Billerica, CA), and cyclin D1 (Catalog No. 04-1151; dilution 1:150; Millipore) was performed after antigen retrieval in pH 6.0 citrate buffer (Dako, Carpinteria, CA). SuperPicture Third-Generation IHC detection kit (Catalog No. 87-9673; Invitrogen, Carlsbad, CA) was used for signal detection and color development. Anatomically equivalent parts of the gland were compared, and a total of 500 nuclei in the mammary ducts of 10 to 15 randomly selected fields were scored for Ki67, ERα, and cyclin D1–positive stained cells by two observers who were blinded to the experimental groups. The results are expressed as percent of the total cells in the fields. Images were captured by bright field microscopy at a magnification of ×60, and at least 10 images were captured from each slide. To determine the specificity of the staining, appropriate controls were run in parallel with the experimental slides.
Western blot
Mammary tissues (pooled samples from 5 mice) were homogenized in ice-cold protein extraction buffer (0.5% sodium deoxycholate, 0.5% NP-40, 10 mM EDTA in phosphate-buffered saline containing protease inhibitor cocktail [Sigma, St. Louis, MO]). The homogenates were centrifuged at 12000 × g at 4°C for 10 minutes, and supernatants were collected. Equal amounts of protein were resolved by SDS-PAGE, transferred onto polyvinylidene fluoride membrane, and incubated with appropriate antibody Akt (Catalog No. Sc-52981; dilution 1:400, Santa Cruz Biotechnology); p-Akt Ser-473 (Catalog No. 4058S; dilution 1:1000; Cell Signaling Technology, Danvers, MA); p-GSK3β Ser-9 (Catalog No. 9336S; dilution 1:1000; Cell Signaling Technology); p85α (Catalog No. 556399, dilution 1:1000; BD Biosciences, San Jose, CA); p-mTOR-ser2448 (Catalog No. 2971S; dilution 1:500; Cell Signaling Technology); p-BAD-ser136 (Catalog No. 9295S; dilution 1:1000; Cell Signaling Technology); c-Myc (Catalog No. Sc-40; dilution 1:400; Santa Cruz Biotechnology); β-actin (Catalog No. Sc-47778; dilution 1:2000; Santa Cruz Biotechnology); and developed with HRP conjugated secondary antibody and ECL detection system. Images were captured on photographic films and scanned, and representative results were displayed. Densitometric quantification of Western blot results was performed by using ImageJ v1.44 software.
Statistical analysis
To determine differences between the two groups, statistical analysis was performed with a two-tailed paired Student’s t test and p < 0.05 was taken as statistically significant. Error bars represent ± standard errors of the mean (SEM).
Results
Radiation exposure causes sustained increase in serum estradiol and urinary oncogenic 16αOHE1 metabolite
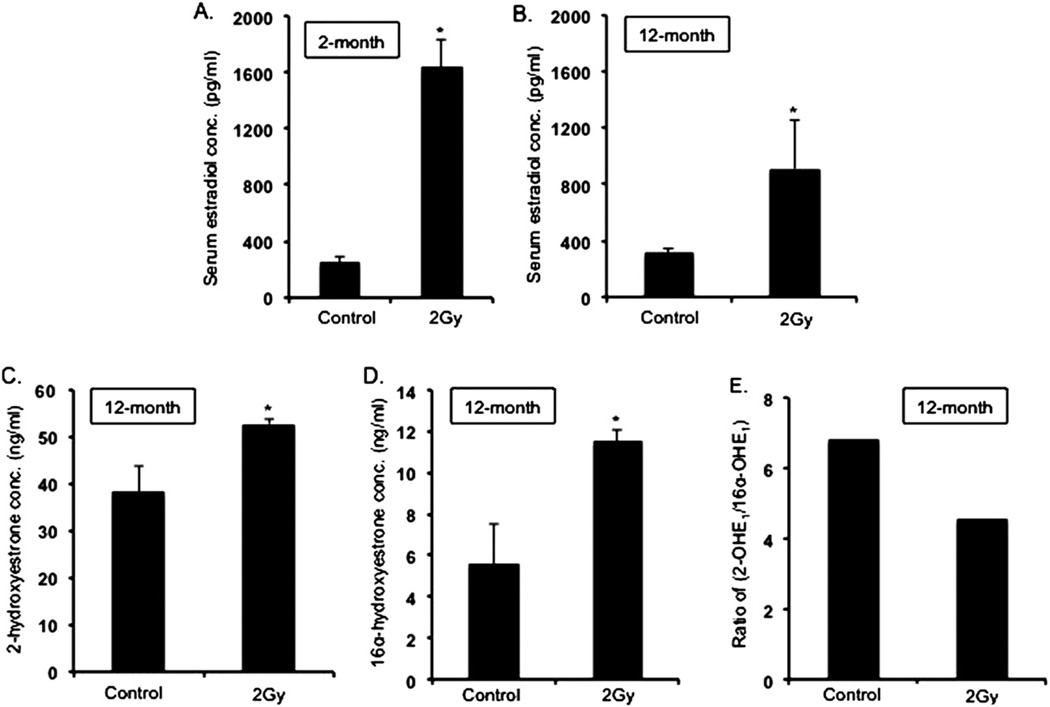
Serum estradiol concentrations in the irradiated groups measured 2 month after radiation were approximately fivefold higher (p < 0.002) (Fig. 1A) than in the unirradiated control groups (estradiol concentrations in the control groups: 282.25 pg/mL ± 16.28 SEM), and even 12 months after radiation, a twofold increase (p < 0.004) in estradiol concentration was observed in irradiated mice (Fig. 1B). Evaluation of estradiol metabolites in urine samples 12 months after radiation exposure revealed significantly higher concentrations of both 2OHE1 and 16αOHE1 in the irradiated groups than in the unirradiated mice (p < 0.04 compared with control mice for both metabolites) (Fig. 1C, D). However, there was a decrease in the 2OHE1:16αOHE1 ratio (Fig. 1E), indicating a greater increase of the 16αOHE1 than the 2OHE1 metabolite.
Fig. 1.
(A) Serum estradiol level in 2-month postirradiation samples. (B) Serum estradiol level in 12-month postirradiation samples. (C, D) Urinary estrogen metabolites 2OHE1 and 16αOHE1 in 12-month postirradiation samples. (E) Ratio of 2OHE1/16αOHE1 in 12-month postirradiation samples. *p < 0.05.
Long-term increase of ERα expression in irradiated mammary tissues
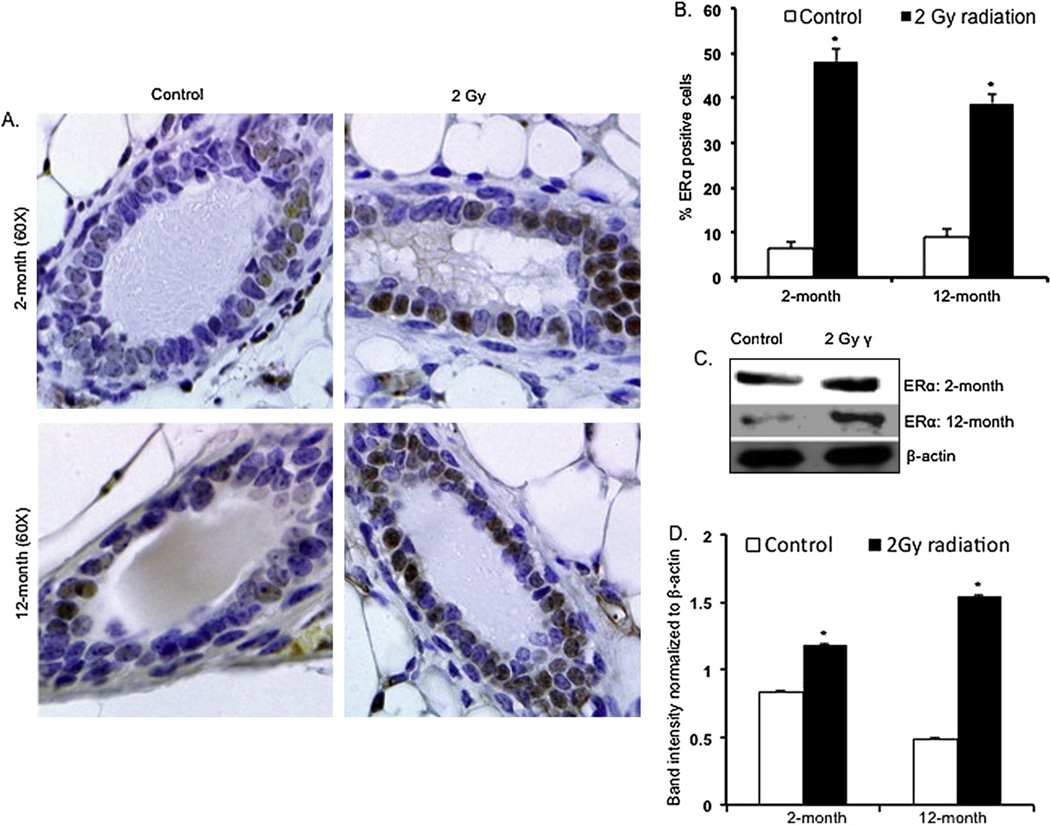
Immunohistochemical analysis of ERα in the mammary tissues of irradiated mice showed greater nuclear staining than in the control samples at both the 2-month and 12-month time points (Fig. 2A). Quantification of the immunohistochemical results showed, in comparison with controls, a statistically significant increase in ERα-positive nuclei in the irradiated samples (p < 0.003 for 2-month and p < 0.004 for 12-month samples) (Fig. 2B). Western blot analysis showed increased levels of ERα 2 months and 12 months after irradiation (Fig. 2C), and densitometry showed significant differences in ERα levels between irradiated and unirradiated samples (Fig. 2D) (p < 0.003 for 2-month and p < 0.0005 for 12-month samples).
Fig. 2.
(A) Mammary gland sections showing immunohistochemical staining for ERα in 2-month and 12-month postirradiation samples. (B) Quantitation of ERα-positive nuclei in 2-month and 12-month postirradiation samples. (C, D) Western blot analysis for ERα in 2-month and 12-month postirradiation samples. *p < 0.05.
Proliferative marker proteins in mammary gland after radiation exposure
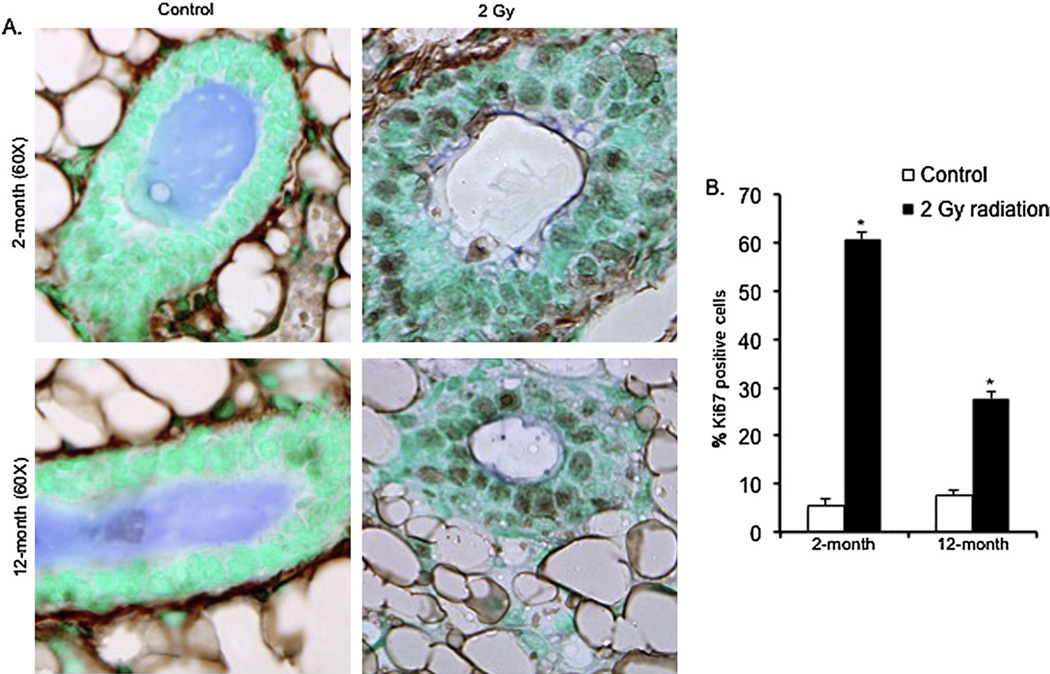
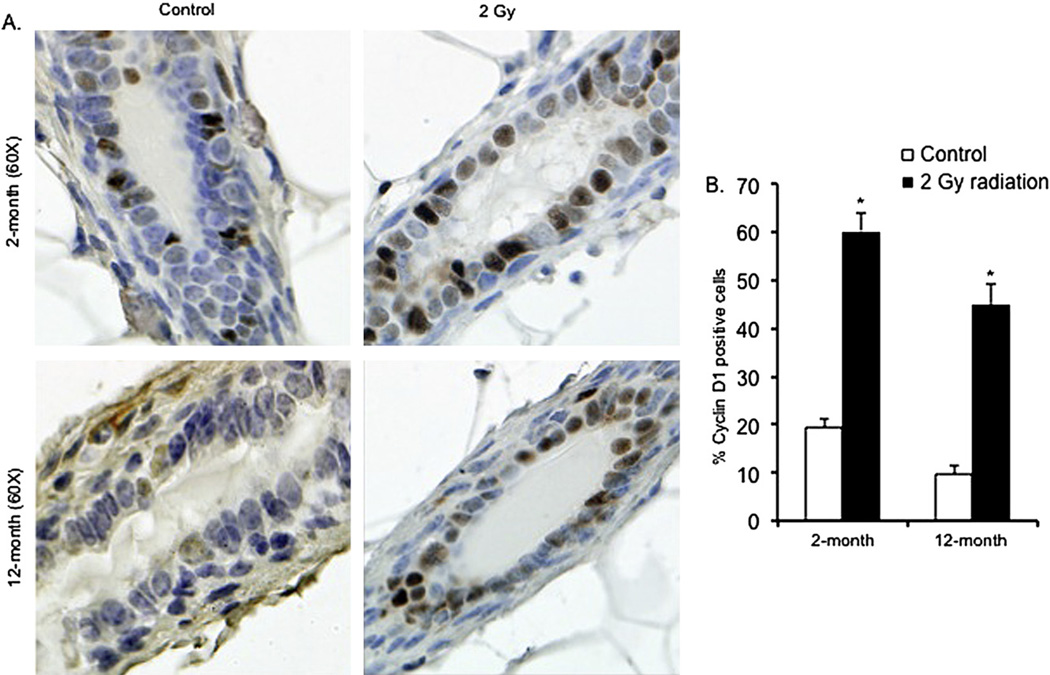
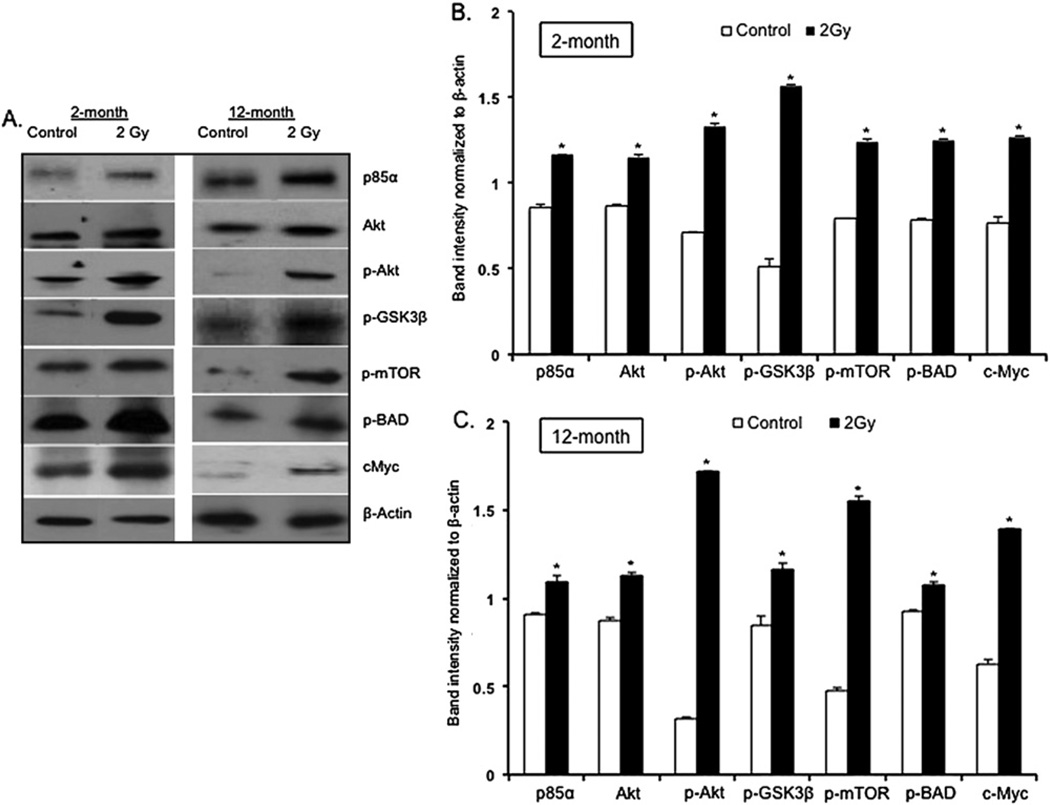
Positive staining of cells for the proliferation marker Ki-67 was increased in samples from mice 2 months and 12 months after irradiation (Fig. 3A). Counting of Ki-67-positive nuclei revealed that in comparison with control mice, the number of positive staining nuclei was approximately 11-fold higher in the 2-month postirradiation samples (p < 0.0002) and was approximately threefold higher in the 12-month postirradiation samples (p < 0.005) (Fig. 3B). In comparison with control mice, a significant increase in the number of positively stained cells in the irradiated mammary tissues was also observed for cyclin D1 at both the 2-month (~threefold increase; p < 0.004) and the 12-month (~fivefold increase; p < 0.007) postexposure time points (Fig. 4A, B). Western blot analysis showed that in both the 2-month and 12-month samples, in comparison with control samples, there was a significant increase in p85α, the regulatory subunit of PI3K (p < 0.004 for 2-month and p < 0.009 for 12-month samples). In irradiated samples, compared with unirradiated controls, we also observed a significant increase in Akt (p < 0.004 for 2-month and p < 0.0009 for 12–month samples) and phospho-Akt (p-Akt; p < 0.0008 for 2-month and p < 0.0003 for 12-month samples), and an increase in downstream Akt targets phospho-GSK3β (p-GSK3β; p < 0.0003 for 2-month and p < 0.004 for 12-month samples), phospho-mTOR (p-TOR; p < 0.0008 for 2-month and p < 0.0002 for 12-month samples), phospho-BAD (p-BAD; p < 0.001 for 2-month and p < 0.007 for 12-month samples), and c-Myc (p < 0.0003 for both the 2-month and 12-month samples) (Fig. 5A, B, C).
Fig. 3.
(A) Ki-67 immunostaining in 2-month and 12-month postexposure samples. (B) Quantitation of Ki-67–positive nuclei in 2-month and 12-month postirradiation samples. *p < 0.05.
Fig. 4.
(A) Cyclin-D1 immunostaining in 2-month and 12-month postexposure samples. (B) Quantitation of Cyclin-D1–positive nuclei in 2-month and 12-month postirradiation samples. *p < 0.05.
Fig. 5.
(A) Western blot analysis of PI3K–Akt pathway in 2-month and 12-month postexposure samples. (B, C) Quantification of Western blots in 2-month and 12-month postexposure samples. *p < 0.05.
Discussion
Our results demonstrate that exposure to 2 Gy whole-body radiation not only caused a long-term increase in serum estrogen levels but also increased the levels of urinary estrogen metabolites, and it shifted the ratio of those metabolites in a manner that has been associated with increased risk of breast cancer. Not only is the radiation dose of 2 Gy relevant to radiotherapy, but also it is important to understand the effects of cumulative radiation dose from diagnostic procedures like roentgenography and computerized tomography, which over the years have alarmingly increased by ~600% (11). Although much less than 2 Gy, the radiation dose from diagnostic procedures is expected, according to the prevailing linear no-threshold model, to show trends similar to our observations. However, further investigation using human samples will be required to confirm our assumptions.
Epidemiologic data have revealed a strong correlation between radiation exposure and breast cancer risk, and, interestingly, our data demonstrating persistent, radiation-induced increase in serum estrogen levels mirror observations made in studies of hormone levels in atomic bomb survivors (12, 13). Studies in atomic bomb survivor cohorts found higher estradiol levels in the survivor cohort than in the nonexposed women, and report that radiation exposure was correlated with increased levels of bioavailable estradiol and breast cancer incidence (12, 13). Although the basal estrogen levels in our study (282.25 ± 16.28 SEM) are similar to what has been reported for C57BL/6J mice (14), our observations demonstrate that mice irradiated (2 Gy) in the beginning of their reproductive life (6–8 weeks) had an increase of approximately twofold in their serum estradiol level even 1 year after radiation exposure. An association of increased circulating estrogen level with breast cancer risk has been established in population-based studies and in laboratory studies (15, 16). Importantly, increased estradiol levels in the sera of atomic bomb survivors collected from premenopausal and postmenopausal women after radiation exposure were significantly associated with breast cancer risk, with an odds ratio of 2.3 for premenopausal and 2.1 for postmenopausal women (13). We believe that the increased serum estrogen levels that have been observed after radiation exposure must be due to long-term alterations in the estrogen biosynthetic pathways, either in the ovaries or in the peripheral tissues, and will require additional studies to establish the underlying mechanisms. Importantly, our results do not rule out the possibility that radiation exposure might also positively alter the hypothalamic–pituitary axis to enhance estrogen biosynthesis and release. Although exogenous estrogen has been shown to act synergistically in radiation-related mammary carcinogenesis, and radiation has been shown to induce ERα in rats (5, 17), here we show, for the first time to our knowledge, that radiation exposure led to sustained elevated level of endogenous estradiol at 2 months and 12 months in mice, with implications for carcinogenesis.
It is noteworthy that not only estrogen but also its metabolites have been implicated in breast cancer initiation and progression (6, 7). Here we show that radiation exposure to mice led to increase in urinary estrogen metabolites, which could be due to postradiation induction of cytochrome P450, which is involved in metabolizing exogenous and endogenous chemicals and is expressed not only in liver but also locally in the mammary glands and needs further investigation. Our assumption, based on the available literature, is that increased urinary estrogen metabolite, especially 16α-OHE, may expose these mice to an enhanced risk of developing mammary cancer later (6, 7).
Apart from the increase in estrogen and its metabolites, we also observed that radiation exposure led to greater ERα expression in mammary glands both 2 months and 12 months after exposure. It is now well known that estrogen influences the development of breast cancer. Indeed, the proliferative effects of estrogen on tissues are mediated via ER, and long-term exposure to increased levels of estrogen has been shown to enhance nuclear ER staining (18). Relevant to our observations on estrogen level and ERα, Miyoshi et al. in an epidemiologic study have shown that women with higher estrogen levels have a higher chance of developing ER-positive breast cancer with increased levels of ER (19). An increase in ERα levels in mammary glands, shown by our results, could not only lead to an increase in its direct effects on genome but could also open the possibility of its coordinated crosstalk with other cellular growth and proliferative pathways.
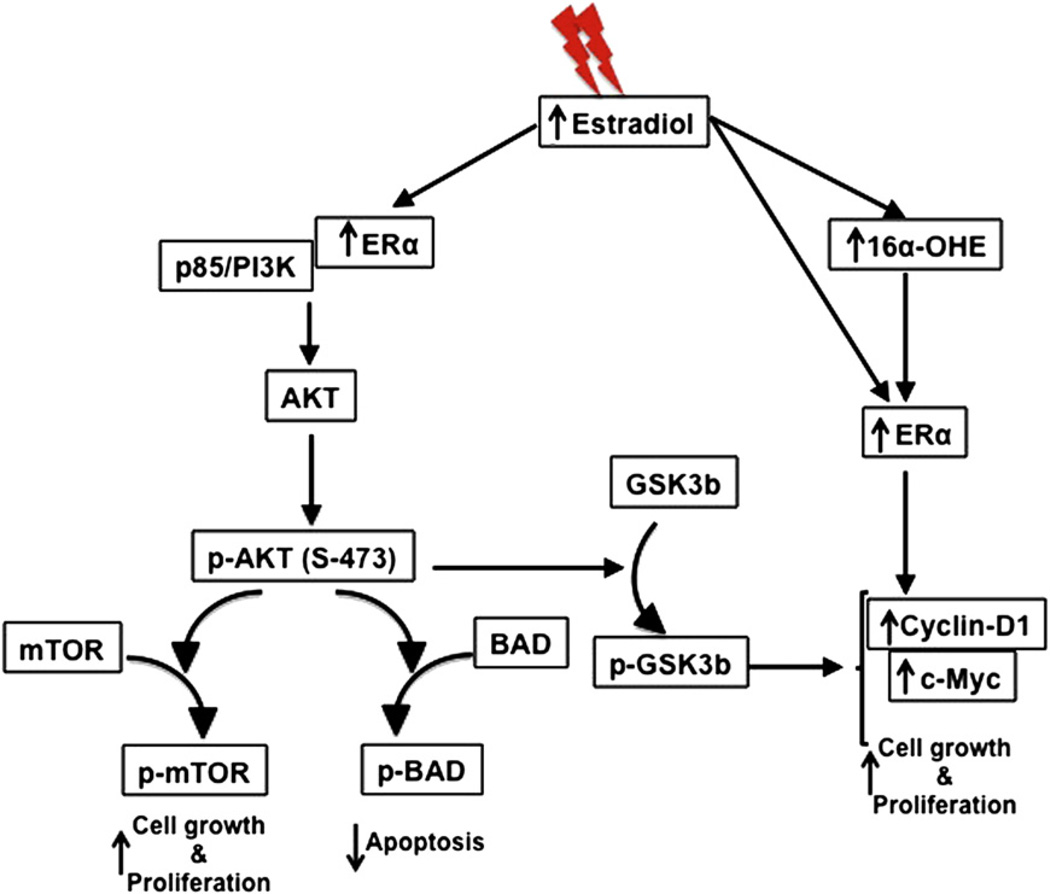
Estrogen through the classic genomic pathway binds to nuclear ER, which in turn binds to estrogen response element in the genome to regulate cellular proliferation and differentiation (20). By contrast, membrane-associated ER, through interaction with other signaling molecules, is known to impart cell growth and differentiation functions of estrogen via alternate pathways (20). The activation of PI3K by membrane-associated ERα (20), combined with our observation that p85α is increased after radiation exposure, led us to believe that increased estrogen level and enhanced ERα could be working in tandem to activate the PI3K-Akt pathway in mammary glands after exposure to radiation. Our results show a marked increase of Akt and p-Akt, which in turn phosphorylate downstream targets to block cell death and enhance cell growth and proliferation. This is evident in the inactivation of proapoptotic BAD along with the activation of cellular growth and proliferative markers Ki67, cyclin D1, mTOR, and cMyc in irradiated mammary glands. Although we did not see any mammary tumorigenesis in these mice within the study timeframe, the changes in cellular function toward proliferation is indicative of carcinogenic precursor events. Our data reveal that radiation exposure at 2 Gy led to long-term upregulation of cell growth and proliferation signaling locally at the cellular level, and elevation of estrogen levels and its estrogenic metabolite levels at the systemic level. Taking these results together, we propose (Fig. 6) that radiation-induced elevated estrogen and its estrogenic metabolite resulted on one hand to increased ERα activity, which could directly induce proliferative signals via c-Myc and cyclin D1, and on the other to activation of the proliferative PI3K/Akt pathway with implications for mammary carcinogenesis.
Fig. 6.
Summary of radiation-induced long-term alterations, local and systemic, in relation to mammary glands.
Summary.
Radiation is a known risk factor for breast cancer. However, long-term alterations at the molecular level require exploration. Effects of ionizing radiation on modifications at the hormonal and tissue levels were assessed 2 months and 12 months after radiation exposure. Exposure to 2 Gy radiation led to increases in serum estradiol and urinary estrogen metabolite levels. Radiation exposure also caused increased estrogen receptor expression and activation of PI3K-Akt pathway in mouse mammary glands.
Acknowledgments
Supported in part by NASA Grant No. NNX07AH70G.
Footnotes
Conflict of interest: none.
References
- 1.Ronckers CM, Erdmann CA, Land CE. Radiation and breast cancer: A review of current evidence. Breast Cancer Res. 2005;7:21–32. doi: 10.1186/bcr970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boice JDJ, Harvey EB, Blettner M, et al. Cancer in the contralateral breast after radiotherapy for breast cancer. N Engl J Med. 1992;326:781–785. doi: 10.1056/NEJM199203193261201. [DOI] [PubMed] [Google Scholar]
- 3.Brenner DJ, Doll R, Goodhead DT, et al. Cancer risks attributable to low doses of ionizing radiation: Assessing what we really know. Proc Natl Acad Sci U S A. 2003;100:13761–13766. doi: 10.1073/pnas.2235592100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ma H, Hill CK, Bernstein L, et al. Low-dose medical radiation exposure and breast cancer risk in women under age 50 years overall and by estrogen and progesterone receptor status: Results from a case-control and a case-case comparison. Breast Cancer Res Treat. 2008;109:77–90. doi: 10.1007/s10549-007-9625-5. [DOI] [PubMed] [Google Scholar]
- 5.Segaloff A, Maxfield WS. The synergism between radiation and estrogen in the production of mammary cancer in the rat. Cancer Res. 1971;31:166–168. [PubMed] [Google Scholar]
- 6.Ursin G, London S, Stanczyk FZ, et al. A pilot study of urinary estrogen metabolites (16alpha-OHE1 and 2-OHE1) in postmenopausal women with and without breast cancer. Environ Health Perspect. 1997;105(Suppl 3):601–605. doi: 10.1289/ehp.97105s3601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Im A, Vogel VG, Ahrendt G, et al. Urinary estrogen metabolites in women at high risk for breast cancer. Carcinogenesis. 2009;30:1532–1535. doi: 10.1093/carcin/bgp139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kazi AA, Molitoris KH, Koos RD. Estrogen rapidly activates the PI3K/AKT pathway and hypoxia-inducible factor 1 and induces vascular endothelial growth factor A expression in luminal epithelial cells of the rat uterus. Biol Reprod. 2009;81:378–387. doi: 10.1095/biolreprod.109.076117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bose S, Chandran S, Mirocha JM, et al. The Akt pathway in human breast cancer: A tissue-array-based analysis. Mod Pathol. 2006;19:238–245. doi: 10.1038/modpathol.3800525. [DOI] [PubMed] [Google Scholar]
- 10.Tyburski JB, Patterson AD, Krausz KW, et al. Radiation metabolomics. 1. Identification of minimally invasive urine biomarkers for gamma-radiation exposure in mice. Radiat Res. 2008;170:1–14. doi: 10.1667/RR1265.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mettler FAJ, Bhargavan M, Faulkner K, et al. Radiologic and nuclear medicine studies in the United States and worldwide: Frequency, radiation dose, and comparison with other radiation sources—1950–2007. Radiology. 2009;253:520–531. doi: 10.1148/radiol.2532082010. [DOI] [PubMed] [Google Scholar]
- 12.Grant EJ, Neriishi K, Cologne J, et al. Associations of ionizing radiation and breast cancer-related serum hormone and growth factor levels in cancer-free female A-bomb survivors. Radiat Res. 2011;176:678–687. doi: 10.1667/rr2631.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kabuto M, Akiba S, Stevens RG, et al. A prospective study of estradiol and breast cancer in Japanese women. Cancer Epidemiol Biomarkers Prev. 2000;9:575–579. [PubMed] [Google Scholar]
- 14.Zhang L, Fishman MC, Huang PL. Estrogen mediates the protective effects of pregnancy and chorionic gonadotropin in a mouse model of vascular injury. Arterioscler Thromb Vasc Biol. 1999;19:2059–2065. doi: 10.1161/01.atv.19.9.2059. [DOI] [PubMed] [Google Scholar]
- 15.Cheriyath V, Kuhns MA, Jacobs BS, et al. G1P3, an interferon- and estrogen-induced survival protein contributes to hyperplasia, tamoxifen resistance and poor outcomes in breast cancer. Oncogene. 2011 doi: 10.1038/onc.2011.393. In press. [DOI] [PubMed] [Google Scholar]
- 16.Lai JN, Wu CT, Chen PC, et al. Increased risk for invasive breast cancer associated with hormonal therapy: A nation-wide random sample of 65,723 women followed from 1997 to 2008. PLoS ONE. 2011;6 doi: 10.1371/journal.pone.0025183. e25183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Imaoka T, Nishimura M, Iizuka D, et al. Pre- and postpubertal irradiation induces mammary cancers with distinct expression of hormone receptors, ErbB ligands, and developmental genes in rats. Mol Carcinog. 2011;50:539–552. doi: 10.1002/mc.20746. [DOI] [PubMed] [Google Scholar]
- 18.Liao DZ, Pantazis CG, Hou X, et al. Promotion of estrogen-induced mammary gland carcinogenesis by androgen in the male Noble rat: Probable mediation by steroid receptors. Carcinogenesis. 1998;19:2173–2180. doi: 10.1093/carcin/19.12.2173. [DOI] [PubMed] [Google Scholar]
- 19.Miyoshi Y, Tanji Y, Taguchi T, et al. Association of serum estrone levels with estrogen receptor-positive breast cancer risk in postmenopausal Japanese women. Clin Cancer Res. 2003;9:2229–2233. [PubMed] [Google Scholar]
- 20.Bjornstrom L, Sjoberg M. Mechanisms of estrogen receptor signaling: convergence of genomic and nongenomic actions on target genes. Mol Endocrinol. 2005;19:833–842. doi: 10.1210/me.2004-0486. [DOI] [PubMed] [Google Scholar]