Abstract
The core features of risk for alcohol use disorders (AUD), including behavioral disinhibition, affective dysregulation, and executive dysfunction, map onto distinct neural circuits that have been found to be abnormal in the offspring of alcohol dependent individuals. Components of the cerebellothalamocortical system and the extended limbic network may provide the underpinnings for the behavioral and emotional dysfunction observed in individuals at heightened risk for AUD. In addition, abnormalities in these structures appear to be altered in individuals with the predisposition for other psychiatric conditions that may share a similar genetic diathesis. This review proposes several neurobehavioral mechanisms of genetic vulnerability that may account for phenotypic characteristics in individuals at risk for AUD.
Keywords: Neuropsychology, High risk offspring, MRI, Limbic system, Cerebellum, Amygdala
Introduction
Premorbid behavioral, cognitive, and psychobiological risk factors have been observed in children and youth with a family history of alcoholism that are predictive of early initiation of drinking behavior or subsequent AUD (Crum et al. 2008; Hill et al. 2008; Iacono et al. 2008; Johnson and Leff 1999; Kramer et al. 2008; Kuo et al. 2008; Porjesz and Rangaswamy 2007). Notably, individuals at heightened risk for AUD commonly display deficits in response inhibition, cognitive control, and emotional regulation (i.e. aggressiveness/ irritability/mood lability), and often suffer from comorbid conditions (e.g. anxiety disorder, ADHD, conduct disorder, major depression, and antisocial personality disorder) that share features of behavioral and affective dysregulation. Also, offspring of alcoholics have been reported to have diverse neuropsychological deficits, and tend to exhibit electrophysiological abnormalities. Emerging literature suggests that these neurophysiological differences may be related to inherited variation in brain structures that are part of the neurocircuitry responsible for these differences. Although multiple pathways to alcohol use and abuse during adolescence and young adulthood have been identified, the mechanisms of heightened risk have not been fully elucidated. The purpose of this review is to elaborate on the neural underpinnings of risk for AUD.
The offspring of alcohol dependent individuals are at increased risk for alcohol and drug dependence in young adulthood over that seen in the general population (Bohman 1978; Cloninger et al. 1981; Goodwin et al. 1973; Kendler et al. 2008). Twin and adoption studies reveal that the heritability for alcohol dependence is between 40–60% (Enoch and Goldman 1999; Heath et al. 1991, 1997; Kendler et al. 1994, 1997; Knopik et al. 2004). The substantial heritability of alcohol dependence also implies a need to search for mechanisms of transmission across generations. Among the mechanisms suggested have been genetically-mediated tendencies toward novelty seeking or harm avoidance (Cloninger 1987), disinhibition (Begleiter and Porjesz 1999; Tarter et al. 2003), under controlled behaviors including impulsivity and aggressiveness (Sher 1991; Sher and Trull 1994), and reduced response to alcohol (Schuckit 1994; Schuckit and Smith 1997). The purpose of this review is to summarize the neurocircuitry that may be responsible for variations in these behaviors in offspring with a family history of AUD.
Due to the substantial effects that prenatal alcohol exposure has on brain development (reviewed by Spadoni et al. 2007), as well as the chronic effects of alcohol consumption on brain structure and function (reviewed by Oscar-Berman and Marinkovic 2007; Sullivan and Pfefferbaum 2005), it has been a challenge to delineate the neurobiological risk for AUD from the consequences of alcohol on brain systems. Therefore, it is useful to focus on studies of high risk individuals with minimal alcohol exposure to identify possible etiologic mechanisms for the development of AUD. Neurobiological factors that have been documented prior to alcohol exposure in the offspring of alcoholics and the offspring from families with multiplex alcohol dependence are more likely to be causally related to risk for AUD.
First, potential developmental pathways leading to alcohol use disorders will be identified by synthesizing the behavioral attributes observed in individuals at high risk for AUD. Next, neuropsychological and neurophysiological characteristics of offspring of alcoholics will be reviewed for possible identification of predisposing risk factors. Finally, neural underpinnings of these core deficits are proposed, and alterations in brain circuitry in individuals at elevated risk for AUD are discussed. A neural diathesis model will be introduced as a framework for future endeavors in the research and treatment of AUD.
Pathways to Alcohol Use Disorders
The regular use of alcohol emerges in adolescence and young adulthood. Whether or not this use becomes problematic appears to be related to a number of neurobiological and psychosocial factors. With a view toward integrating biological/genetic theories of etiology with environmental hypotheses, a vulnerability conceptualization was developed (Hill et al. 1987a). Other early conceptualizations of AUD have been offered that further emphasized interactions between biogenetic aspects of personality/termperament, environment, and behavioral domains of influence (Sher 1991; Bates and Labouvie 1994; Jessor et al. 1991). In a recent review, Masten et al. (2008) notes the factors that have consistently predicted the age of regular drinking onset including a family history of alcohol abuse, parents' antisocial behavior, mother's depression, poor parenting, prenatal exposure to alcohol, child antisocial behavior, and child self-regulation problems. Moreover, early onset to begin drinking is an important predictor of adult alcohol problems.
Early Onset to Begin Drinking
The age of onset to begin regular drinking is an important predictor of age of first alcohol problem and subsequent alcohol dependence (Hawkins et al. 1997; Grant and Dawson 1997), as well as greater severity and persistence of problems with illicit drugs (Kandel et al. 1992). For individuals that initiated drinking prior to age 14 years, the likelihood of adult alcohol dependence was 40%, four times more likely than individuals who began drinking at 20 years or older (Grant and Dawson 1997). Sartor et al. (2007) also reported that individuals that drank before age 14 years were more than twice as likely to become alcohol dependent than those trying alcohol after age 16 years.
A number of factors such as early adverse childhood experiences (Rothman et al. 2008; Waltrop et al. 2007), familial density of alcoholism (Hill and Yuan 1999; Hill et al. 2000a), extraversion (Hill and Yuan 1999; Hill et al. 2000a), as well as markers of altered neurodevelopment including P300 amplitude trajectories and delays in attaining age-appropriate postural sway (Hill et al. 2000a) predict earlier age of drinking onset. Earlier onset of drinking also appears to be related to the presence of behaviors often characterized as “disinhibited” or belonging to the externalizing domain.
Externalizing Pathway
Disinhibited behavioral problems at age 11 years, including oppositionality, hyperactivity, impulsivity, and inattention, predict an earlier onset of drinking (McGue et al. 2001a, b). Greater externalizing problems indicative of behavioral under-control have been reported to be related to earlier age at first drink (Kuperman et al. 2005). There is also abundant evidence that behavioral under-control is an important determinant of later development of substance use disorders (SUD) (reviewed by Stice et al. 1998; Zucker 2008). Behavioral under-control observed as early as 3 years is predictive of alcohol-related problems at 21 years (Caspi et al. 1996), and in adolescents mediates the relationship between family history of alcoholism and young adult SUD (King and Chassin 2004). Risk for AUD appears to be associated with under-controlled behaviors that may derive from certain personality traits including impulsivity, novelty and sensation-seeking, and extraversion (Sher et al. 1991; Sher and Trull 1994). Interestingly, extraversion in adolescence completely mediates the relationship between family history of alcoholism in multiplex families and earlier onset to begin drinking seen in these offspring when compared to controls (Hill et al. 2000a).
Externalizing disorders including ADHD, conduct disorder, oppositional defiant disorder, and antisocial personality disorder (ASPD) are common among children, adolescents, and young adults with a family history of alcohol dependence (Clark et al. 1997; Earls and Powell 1988; Earls et al. 1988; Hill and Muka 1996; Hill et al. 1999a; Hill et al. 2008; Kuperman et al. 1999; Merikangas et al. 1998; Ohannessian et al. 2004; Reich et al. 1993). In high-risk youth, these disorders generally precede the initiation of alcohol use and are predictive of subsequent abuse and dependence (Chassin et al. 1999; Hill et al. 2000a; Hill et al. 2008; King and Chassin 2008; Marshal et al. 2007). Prospective longitudinal studies in populations not selected for familial risk for AUD further suggest that AUD is often secondary to childhood externalizing behavioral problems (Biederman et al. 1997, 1998). For example, Mannuzza et al. (1993) found that boys diagnosed with ADHD in childhood had significantly higher rates of SUD in adolescence and young adulthood. Ten year follow-up of 6–17 year old boys with ADHD has confirmed that higher rates of adult SUD occur in association with an earlier ADHD diagnosis (Biederman et al. 2008).
Importantly, conduct disorder is observed in approximately 30% to 50% of adolescents with ADHD (Biederman et al. 1991). Several prospective studies have found that the relation between ADHD and SUD is no longer significant after controlling for conduct disorder (Barkley et al. 2004; August et al. 2006). However, Elkins et al. (2007) showed that ADHD was a significant prospective predictor of SUD even after taking into account a diagnosis of conduct disorder. Furthermore, population-based studies indicate that conduct disorder and oppositional defiant disorder increase the likelihood of developing a SUD (Nock et al. 2006, 2007). Ohannessian et al. (1995) found that conduct disorder during childhood and antisocial behavior during adulthood are among the most powerful predictors of adult SUD compared to other psychiatric disorders.
The association between SUD and conduct disorder in adolescence appears to be consistent with Cloninger's conceptualization of two types of alcohol dependence that vary in the degree to which antisocial personality disorder is seen. Studying a sample of Swedish men, Cloninger found that the “type II” alcoholics could be characterized by an early onset of problematic drinking and greater ASPD (Cloninger et al. 1981). Type I alcoholics, on the other hand, were characterized by a relatively late onset of alcohol problems associated with high levels of anxiety, introversion, and harm avoidance. Although several other typologies have been suggested (Babor et al. 1992; Hill 1992; Moss et al. 2007), most include mention of a severity dimension and personality characteristics including ASPD. Although the more severe form of alcohol dependence (Type II) has been associated with greater ASPD, a severe form seen in multiplex families (families with multiple alcoholic members) appears to exist in the absence of ASPD (Hill 1992) that is characterized by an early onset of alcohol dependence suggesting a third typology.
Internalizing Pathway
Although studies of offspring of AUD individuals typically have emphasized externalizing psychopathology, Kellam et al. (1980) first proposed that the presence of internalizing disorders might predispose children to developing AUD. Internalizing disorders have not been studied as often in association with familial risk for AUD, though a few early studies have suggested an elevation in internalizing symptoms in offspring of alcohol dependent individuals (Earls et al. 1988; Hill and Muka 1996; Reich et al. 1993). More recent reports also find offspring at elevated risk for internalizing disorders (Hill et al. 2008). It should be noted that greater incidence of internalizing psychopathology may be associated with the more severe form of alcohol dependence seen in offspring from multiplex families (Hill et al. 2008) than in offspring selected for the presence of single alcohol dependent parent (Clark et al. 2004). Epidemiological studies confirm that a family history of AUD increases the risk for both major depression and/or AUD, with comorbid AUD and depression most notable in individuals with greater familial loading for alcohol dependence (Dawson and Grant 1998).
Anxiety and stress-reactive personality traits such as harm avoidance are frequently observed in the offspring of alcoholics, as are low self esteem, negative affectivity, and impairments in emotional regulation (Fine et al. 1976; Finn et al. 1997; Moos and Billings 1982; Ohannessian and Hesselbrock 1995; Steinhausen et al. 1984). Hill et al. (1999b) found that 5 year old high-risk children from multiplex alcohol dependent families exhibit a higher frequency of behavioral inhibition in a peer play setting modeled after the observational paradigm developed by Kagan et al. (1984). Behavioral inhibition is a moderately stable temperamental characteristic characterized by a restrained, cautious, or fearful reaction to unfamiliar people, places, or objects. Behaviorally inhibited children are described as shy and avoidant, and are at greater risk for developing an overanxious disorder in adolescence (Rosenbaum et al. 1991; Schwartz et al. 1999).
Children who are more likely to exhibit one or more anxiety disorders and/or clinical depression may be at increased risk for developing AUD (Cockerham et al. 1989; Crum et al. 2008; Vaglum et al. 1987; reviewed by Wesner 1990). Internalizing problems in childhood, particularly childhood separation anxiety disorder, have been found to be associated with later development of alcoholism (Brückl et al. 2007). Also, the time from first drink to a diagnosis of alcoholism is decreased in individuals with a childhood history of generalized anxiety disorder (Sartor et al. 2007), consistent with the idea that the emotional distress associated with depression and anxiety may drive adolescent substance use (Newcomb and Bentler 1989).
Summary: Pathways to AUD
It is important to consider the diverse developmental pathways of risk whereby individuals ultimately develop an AUD because each of the pathways may be associated with alterations in varying neural circuits. A diagnosis of a disruptive behavior disorder (e.g. ADHD, conduct disorder, oppositional defiant disorder) increases the likelihood of an AUD in young adulthood among offspring with an alcoholic parent. Offspring of alcoholics also are at greater risk for developing internalizing disorders (anxiety disorders, major depressive disorder and related mood disorders) that influence the likelihood of future substance use problems. Because the neural circuitry associated with ADHD, conduct disorder, ASPD, anxiety, and depression most likely differs in structural or functional characteristics, it is important to assess comorbid conditions in offspring who are at risk for AUD because of their familial/genetic background. Uniformity of results should probably not be expected for high risk offspring unless personal histories of psychiatric disorders are controlled. However, the prefrontal cortex and regions of the limbic system are good candidate regions because they have been observed to be abnormal in individuals with externalizing and internalizing disorders and in offspring of alcoholics.
Also, it is noteworthy that the set of variables associated with the initiation of drinking behavior may not be the same as those related to problematic use. Externalizing problems, antisocial behavior, and peer influences appear to be more strongly associated with the exposure and initial use of alcohol, while family history of alcoholism and parental psychopathology may play a more salient role in transition to dependence (Fite et al. 2006; Pagan et al. 2006; Rhee et al. 2003; Rose et al. 2001, 2004).
Risk Factors for AUD from Neuropsychological, Neurophysiological, and Neuroimaging Studies
In addition to behavioral and emotional difficulties, offspring of alcoholics have been reported to have poor linguistic ability, deficits in problem solving and abstract reasoning, poor visuospatial and perceptual-motor ability, and decreased attention span (Drejer et al. 1985; Ozkaragoz et al. 1997; reviewed by Pihl et al. 1990). Background factors such as socioeconomic status may influence the extent to which these deficits are observed, so that extensive neuropsychological deficits are not seen in offspring of alcoholic parents with higher socioeconomic status (Hill et al. 1999a). High risk offspring tend to display impulsivity and disinhibition during cognitive paradigms designed to require thoughtful planning and inhibitory control. Also, reduced amplitude of the visual P300 component of the event-related potential appears to be commonly observed. Heightened neuroendocrine responses to threatening stimuli also appear to characterize those with familial loading for alcohol dependence. Finally, differential reactions to alcohol administration in the form of a low level response to the effects of alcohol are prominent among the offspring of alcoholics (Schuckit 1994; Schuckit et al. 1996).
Although abundant neuroimaging studies have documented the effects of alcohol on the brain in both chronic and abstinent alcoholics (reviewed by Oscar-Berman and Marinkovic 2007; Sullivan and Pfefferbaum 2005; Mann et al. 2001), there have been limited studies using MRI to investigate neural mechanisms of possible risk in high risk offspring who have not developed an AUD. Moreover, fMRI studies of offspring with a family history of AUD have used a variety of cognitive paradigms with small heterogeneous samples, thereby making it difficult to generalize findings across studies. Aside from these limitations, the studies provide some evidence of functional deficits that generally precede alcohol use and may underlie behavioral and cognitive deficits among offspring with a family history of AUD.
Attention and Executive Dysfunction
It has been frequently observed that offspring from families with a history of AUD exhibit deficient performance on attention and working memory tasks. Children with high familial density of AUD show poorer performance compared with children with a negative family history on the Digit Span subtest of the WISC-R, a test requiring focused attention (Corral et al. 1999). Tapert and Brown (2000) found that family history of alcohol dependence was an independent predictor of deficient performance on attention tasks during adolescence after accounting for previous alcohol use. During vigilance tasks, high risk youth show decreased blood-oxygenation level dependent (BOLD) response in bilateral regions of the middle frontal and cingulate gyri (Spadoni et al. 2008). On working memory tasks, sons of male alcohol dependent individuals have been shown to perform more poorly compared to control subjects (Harden and Pihl 1995; Peterson et al. 1992). However, in sons of multi-generational AUD families, working memory deficits were more likely due to attention problems or other co-existing mental health issues (Wiers et al. 1998).
There is further evidence of deficits on classical neuropsychological tests of executive function [e.g. Wisconsin Card Sorting Test (WCST), Stroop Task] in the offspring of alcoholics. Sons of alcoholic fathers make greater perseverative errors on the WCST compared to offspring of control subjects (Peterson et al. 1992), reflecting an inability to inhibit response patterns based on experimenter feedback. Perseverative errors appear to be a relatively common difficulty in impulsive/compulsive disorders including addiction (Goldstein and Volkow 2002). Only children with multi-generational alcoholism and not all children with a family history of alcoholism show impaired performance on the WCST over time (Corral et al. 2003). Performance on the Stroop task is impaired in individuals with a family history of AUD compared to persons with no family history (Diaz et al. 2008), and this difference appears to be more common in those with antisocial tendencies (Lovallo et al. 2006). Consistently, children of antisocial alcoholics display relatively poorer attention, working memory, and abstract planning abilities compared to children from control families (Poon et al. 2000). Performance deficits on the Stroop task may be related to decreased activation in frontolimbic circuitry that has been observed in adolescents with a positive family history (Silveri et al. 2009). Possibly, WCST performance involves decreased activation of frontolimbic circuitry among offspring of alcoholics, though studies addressing this issue have not been done.
Giancola and Moss (1998) report that mild executive dysfunction in alcoholics and those at-risk for alcohol dependence by family history appears greater in individuals with comorbid ASPD, ADHD, or conduct disorder. Compromised executive function may be an underlying etiologic substrate of disorders of behavioral dysregulation and not limited to risk for alcohol dependence. This is consistent with the documented executive function deficits in individuals with ADHD, conduct disorder, and ASPD (Barkley et al. 1992; Doyle 2006; Herba et al. 2006; Pajer et al. 2008; Stevens et al. 2003).
Executive Deficits in Motor Control
Barkley (2004) has argued that motor control is part of executive function. Therefore, intact executive function would appear to be crucial for competent motor control (e.g. Seitz et al. 2000). There is some indication of deficits in gross and fine motor coordination in the offspring from families of alcoholics. Lipscomb et al. (1979) were the first to identify greater body sway in offspring from families with an alcoholic parent. These initial observations that individuals with a family history of alcoholism are more likely to exhibit impairments in postural stability were supported by subsequent studies (Hegedus et al. 1984; Hill et al. 1987b; Hill and Steinhauer 1993). Because postural control improves with age, decrements in the rate of improvement with age seen in high risk offspring may reflect a subtle developmental delay (Hill et al. 2000b). A recent report finds that greater sway at age 15 coupled with lower amplitude of P300 before age 13 increases the risk for developing SUD in young adulthood 8-fold (Hill et al. 2009b). Using data from the Danish Longitudinal Study of Alcoholism, Manzardo et al. (2005) found that insufficient motor tone several days following birth and delays in age to sitting and walking significantly predicted those individuals that received a diagnosis of lifetime AUD. These investigators suggest that cerebellar abnormalties may be associated with these observed differences in motor tone that appear to predict the occurrence of AUD.
Saccadic and smooth pursuit eye movements have been postulated to play a role in organizing and integrating visual forms in the environment. The delay in voluntary occulomotor navigation reflects deficits in the neural systems that underlie the executive control of eye movements. Habeyck et al. (2006) found that children of fathers with an AUD exhibited a higher rate of saccadic errors on the most difficult tasks associated with antisaccadic eye movement. A follow-up neuro-imaging study of youth at high risk for developing SUD found that the inhibition of eye movement response was related to activation in the frontal cortex (McNamee et al. 2008). Likewise, a number of studies investigating eye movements in subjects with ADHD have identified deficits in inhibiting responses (i.e. premature saccades; Ross et al. 1994), directional movement (i.e. antisaccades; Mostofsky et al. 2001), and stopping an already initiated response (i.e. countermanding saccade task; Hanisch et al. 2006). Dysfunction in oculomotor control has been identified in boys with ADHD and their unaffected brothers, suggesting that saccade deficits may be a potential endophenotype for ADHD (Rommelse et al. 2008).
Disinhibition
Neuropsychological inhibition has been operationalized by a number of cognitive paradigms that measure the ability to inhibit a behavior, stop a response once it has been initiated, or delay the choice of a rewarding item. Commonly, stop tasks or go/no-go tasks have been used to quantify neuropsychological inhibition. During a stop task in which a subject is instructed to respond as quickly as possible only on trials with a no-stop tone, children with a family history of AUD were slower in responding and also had difficulty suppressing responses to stop tones (Nigg et al. 2004). Using a go/no-go task, Saunders et al. (2008) found that high risk offspring with a greater tendency toward impulsivity and norm violation were more likely to make commission errors. BOLD activation in the middle frontal gyrus during response inhibition on a go/no-go task has been shown to be decreased among high risk offspring (Schweinsburg et al. 2004).
A multi-dimensional construct of neurobehavioral disinhibition consisting of indicators of difficult temperament, executive dysfunction, and externalizing behaviors has been reported to discriminate between high risk and low risk offspring of AUD individuals during childhood and adolescence (Tarter et al. 2003). However, it should be noted that the parents of the studied offspring had a high degree of comorbid antisocial behaviors due to the selection process used to include the parent-offspring pairs. Also, difficult temperament judged by parents may be unreliable due to parental expectations that bias parent report of offspring (Mangelsdorf et al. 2000). Also, it seems plausible that parents with greater likelihood of psychopathology will be less tolerant of their children's perceived behavioral infractions. Not surprisingly, parental SUD predicts neurobehavioral disinhibition in their offspring, and neurobehavioral disinhibition during adolescence in the offspring of alcoholics is predictive of SUD in young adulthood (Tarter et al. 2004). King et al. (2009) constructed an index of behavioral disinhibition comprised of measures of delinquency and peer deviance, antisocial attitudes, impulsive traits, and substance use in the adolescent offspring of adoptive and non-adoptive parents. A history of parental AUD was associated with higher levels of adolescent disinhibition, particularly when reared by their biological alcoholic parents.
Alternatively, physiological measures of neural disinhibition have been studied extensively in the offspring of alcoholics. There is a long-established literature on differences in resting EEG in individuals at risk for AUD (reviewed by Porjesz et al. 2005). It has been suggested that low amplitude of event-related potentials (ERPs) and other event-related oscillations (EROs) in high risk children may reflect an overall reduction in central nervous system inhibition. P300 amplitude is one salient predictor of age of onset to begin drinking (Hill et al. 2000a). A consensus seems to have emerged that the amplitude of the P300 component of the ERP reflects a predisposition to have a disinhibited temperament that is related to risk for SUD (Iacono and McGue 2006; Porjesz and Rangaswamy 2007). However, the lower P300 amplitude associated with earlier onset to begin drinking may be associated with generalized disinhibition that is associated with early onset of a number of deviant behaviors (McGue et al. 2001a, b).
Begleiter et al. (1984) were the first to show P300 amplitude differences between high and low risk 10 year old boys who performed a visual ERP task. Numerous cross-sectional studies of ERP abnormalities in the offspring of alcohol dependent individuals and in offspring from multiplex families confirmed that reduced P300 amplitude was a plausible endophenotype for risk for AUD (Costa et al. 2000; Hesselbrock et al. 1993; Hill and Steinhauer 1993; Porjesz et al. 1998; Rangaswamy et al. 2007). Availability of data for children and adolescents studied yearly with ERP provided the first evidence that observed risk group differences might be due to altered developmental trajectories of P300 amplitude in offspring from families with high densities of alcohol dependence. Mixture analysis of trajectories suggests that those individuals with a pattern characterized by low P300 amplitude across childhood and adolescence have greater risk for externalizing disorders (Hill and Shen 2002). These findings support risk group differences in mechanisms of neural inhibition (Hill et al. 1995, 1999c).
Additionally, youth with a family history of AUD display reduced EROs on go/no-go tasks predominantly at parietal-occipital and centro-parietal regions (Cohen et al. 1997; Kamarajan et al. 2005, 2006). Children of alcoholics tend to have reduced gamma band activity in parietal regions while processing the target stimulus during a visual oddball task (Padmanabhapillai et al. 2006). Similarly, high risk children from multiplex families have reduced delta and theta band EROs to the target stimulus during the same task (Rangaswamy et al. 2007). A significant reduction of delta and theta EROs has been postulated to underlie the decreased P3 amplitude often observed in the offspring of alcohol dependent individuals (Porjesz et al. 2005).
Dysfunction in Decision Making and Affective Responsivity
Young adults with a family history of AUD have been characterized as having an excessive sensitivity to reward, in that they tend to pay greater attention to monetary gains when making decisions on the Iowa Gambling Test compared with individuals with no family history of alcoholism (Lovallo et al. 2006). A follow-up fMRI study of high risk individuals performing the Iowa Gambling Task has revealed greater activation in the left anterior cingluate gyrus and left caudate in the absence of performance differences on this task (Acheson et al. 2009). Investigating the recruitment of motivational circuitry by monetary rewards using fMRI in adolescents at high and low risk for AUD, Bjork et al. (2008) found BOLD signal did not differ in the ventral striatum, nucleus accumbens, and mesofrontal cortical regions between risk groups. Decisions that led to the loss of rewards, though, evoked more extensive right anterior insula activation in controls compared to adolescents with a family history of SUD.
Evidence for diminished affective responsivity was found in an fMRI study in which offspring from multiplex for alcohol dependence families exhibited diminished BOLD response in the right medial temporal gyrus in comparison to controls when asked to judge facial affect during a theory of mind (ToM) task (Hill et al. 2007b). Young adults with a family history of AUD, and who had been characterized as more disinhibited than young adults without a family history, were more likely to display reduced amygdala activation to fearful faces (Glahn et al. 2007). During a similar passive viewing task of emotional stimuli, Heitzeg et al. (2008) found less bilateral activation of the orbitofrontal gyrus and left insula in a group of offspring of alcoholics with a pattern of problematic drinking during adolescence. Compared to both a non-drinking control group and a high risk group that had refrained from alcohol use during adolescence, the problem drinking group had greater activation in medial prefrontal cortex and decreased activation in the striatum and amygdala. However, no differences were observed between the control and non-drinking high risk groups. Overall, these studies generally suggest differential sensitivity to rewards and affective cues in individuals at risk for AUD.
Autonomic Hyperarousal
Offspring of alcoholics tend to have higher baseline heart rates (Harden and Pihl 1995; Hill et al. 1992), and show increased cardiovascular reactivity to aversive stimuli (Finn and Pihl 1988; Finn et al. 1990; Harden and Pihl 1995; Peterson et al. 1993; Stewart et al. 1992). Also, offspring from families with AD individuals may be hypersensitive to the effects of alcohol on cardiovascular activity (Finn and Pihl 1988; Peterson et al. 1993; Shuckit 1994; Schuckit et al. 1996). Alcohol may serve to dampen heart rate and electrodermal reactivity to stress more in young adults with a family history of alcoholism than in offspring without a family history (Finn et al. 1990; Peterson et al. 1993, 1996; Stewart et al. 1992). Slowing of heart rate may be associated with increased perception of relaxation making alcohol more rewarding to high risk offspring.
Several studies suggest that high risk individuals display a blunted neuroendocrine response to stress (Dai et al. 2002; Dawes et al. 1999; Sorocco et al. 2006), similar to those observed in children with ADHD (Hastings et al. 2009; King et al. 1998; Shin and Lee 2007), conduct disorder (Fairchild et al. 2008; McBurnett et al. 2005) and ASPD (O'Leary et al. 2007). It has been reported that 10- to 12-year-old offspring of alcoholics show reduced reactivity of the hypothalamic-pituitary-adrenocortical (HPA) system to a potentially anxiety-provoking situation and an attenuated return to baseline cortisol levels (Moss et al. 1995, 1999). In contrast, other studies have shown that cortisol response to psychosocial stress is significantly increased in offspring with a family history of AUD compared to those with no family history of AUD (Uhart et al. 2006; Zimmermann et al. 2004). These contradictory findings may be due to varying psychological comorbidity, previous drug and alcohol use, gender distribution, and personality traits all of which that have been shown to influence HPA functioning. Interestingly, conduct disorder symptoms in children may contribute to the diminished cortisol secretion in response to an anticipated stressor suggesting that neuroendocrine response in offspring of AUD individuals is complex and often appears in a multitude of forms (Moss et al. 1995).
In offspring of alcohol dependent individuals, ethanol consumption results in significantly lower adrenocorticotropic hormone (ACTH) and cortisol levels compared to control subjects that are predictive of future AUD (Schuckit 1998; Schuckit et al. 1996; Schuckit and Smith 1997). An alcohol-induced attenuation of the ACTH response to stress has also been noted in high risk subjects (Zimmermann et al. 2004), while ethanol consumption prior to a stress task attenuated the stress-induced increase is ACTH and cortisol levels in both high and low risk groups (Dai et al. 2002, 2007).
Vulnerable Brain Systems in Individuals at Risk for AUD
A consideration of the brain regions involved in affective regulation, executive function, and autonomic reactivity suggests a primary role for the limbic, cerebellothalamocortical, and HPA systems. These regions may play an important role as neural underpinnings of risk for AUD. The cerebellum, thalamus, hypothalamus, and regions of the prefrontal cortex are particularly susceptible to alcohol (Harper et al. 2003; reviewed by Harper and Matsumoto 2005), and the interconnected circuits among these systems define the neural substrates of intrinsic motivation, reinforcement, and reward in addiction (Koob 1996, 2003). The forthcoming discussion describes components of these overlapping systems that have been found to be vulnerable in individuals at risk for AUD.
Cerebellothalamocortical System
The cerebellum has been most notably associated with the coordination of motor functions, particularly the movement of voluntary muscles and the maintenance of balance and postural stability. Although the cerebellum plays a prominent role in motor functions, the cerebellum is also involved in planning and reasoning abilities, assisting the prefrontal cortex in these functions (Strick et al. 2009). The cerebellum is extensively interconnected to the neocortex through both feedforward and feedback mechanisms (Fig. 1). In addition, physiological observations support the existence of pathways to and from the amygdala, hippocampus, and posterior hypothalamus (Anand et al. 1959; Harper and Heath 1973; Schmahmann and Pandya 1997). These pathways have been shown to subserve a number of executive functions involved in affective regulation (Baillieux et al. 2008).
Fig. 1.

According to Schmahmann and Pandya (1997), the cerebrocerebellar circuit is composed of a feedforward limb that includes the corticopontine and pontocerebellar mossy fiber projections, and a feedback loop that contains the cerebellothalamic and thalamocortical pathways. The corticopontine pathway originates in the Vb layer of cerebral cortex and carries associative, sensory, and motor information from cortical neurons to neurons in the ventral pons. This information is directed to the cerebellum, and then projected to the thalamus through midbrain neurons of the red nucleus. Afferent pathways originating in the thalamus and projecting to the cortex complete the feedback loop
Hill et al. (2007a) found a significant increase in grey matter and a tendency for total volume of the cerebellum to be increased in adolescents and young adults at high risk for AUD. Age regression of the grey matter volumes suggested a slower reduction in grey matter volumes among the offspring of alcoholics that may indicate a delay in grey matter pruning or slower maturational increases in white matter. Benegal et al. (2007) also found differences in cerebellar volume in high risk alcohol-naive subjects using both region of interest and voxel-based morphometric analyses. Compared to controls, high risk subjects also had decreased grey matter volume in the thalamus, superior frontal gyrus, and cingulate gyrus.
Cerebellar abnormalities are associated with deficits in posture, volitional movements, balance, and gait (reviewed by Steinlin 2008). Cerebellar vermian volume in chronic alcoholics correlates with postural sway, with longer duration of sobriety related to improved balance (Sullivan et al. 2006). Hyperkinetic movements that are a hallmark of the primarily hyperactive subtype of ADHD are likely to be associated with a nonlinear volume loss in the cerebellar vermis (Mackie et al. 2007). Given the high prevalence of alcohol abuse in patients with ADHD, these disorders may share a genetic liability in the form of morphometric abnormalities of the cerebellum that underlie the documented premorbid deficits in posture and gait. As noted previously, motor deficits have been identified in the offspring of alcohol dependent individuals prior to prolonged alcohol exposure.
Cerebellar output to ocular muscles via motor neurons in the brainstem control saccadic and smooth pursuit eye movements (reviewed by Thier and Ilg 2005; Krauzlis 2004). Eye movement irregularities in individuals at heightened risk for AUD suggest that these pathways may also be susceptible. This is consistent with oculomotor deficits in ADHD, further suggesting that cerebellar dysfunction may be a common neurobiological substrate for AUD and ADHD. Taken together, static ataxia and deficient control of eye movements may be present prior to prolonged alcohol consumption in subjects with a family history of AUD or those with behavioral predispositions to AUD. Longitudinal data from high risk offspring with slower derailment of postural control suggest this may be so (Hill et al. 2000b). Recent evidence suggests that there is an eight-fold increase in risk for SUD in those above the median for sway and below the median for P300 amplitude (Hill et al. 2009b).
Because deficits in executive function and behavioral control are frequently observed in the offspring of alcoholics, the prefrontal cortex, as one component of the cerebellothalamocortical system, is likely to be integrally involved in alcoholism risk. Recent reviews have highlighted the role of the prefrontal cortex in behavioral control (Tanji and Hoshi 2008), working memory (Badre and Wagner 2007), behavioral planning (Tanji and Hoshi 2001), and decision making (Fellows 2007). Neuropsychological studies of high risk offspring of alcohol dependent individuals underscore deficits in these domains. Also, neuroimaging studies of high risk subjects, though much fewer in number, implicate involvement of the prefrontal cortex, particularly the inferior and middle frontal gyri. This supports a lack of prefrontal regulation on mechanisms of attention, behavioral control, and inhibition. Not surprisingly, structural abnormalities in the cerebellothalamocortical system are present before the initiation of alcohol use in the offspring of alcoholics (Benegal et al. 2007; Hill et al. 2007a).
The Limbic System
The limbic system remains the pre-eminent mechanism of emotion. Heimer and colleagues (Alheid and Heimer 1988; de Olmos and Heimer 1999; Heimer et al. 1991) proposed an anatomical framework that delineates limbic regions into functional—anatomical systems consisting of the basal forebrain, “extended amygdala”, and limbic lobe. In doing so, this revised conceptualization of the limbic system more appropriately address the complexity of the basal forebrain and extended amygdala as primary output channels for activities originating from the neocortex (Heimer 2003; Heimer and Van Hoesen 2006). An abbreviated schematic of this extended limbic network is provided in Fig. 2.
Fig. 2.
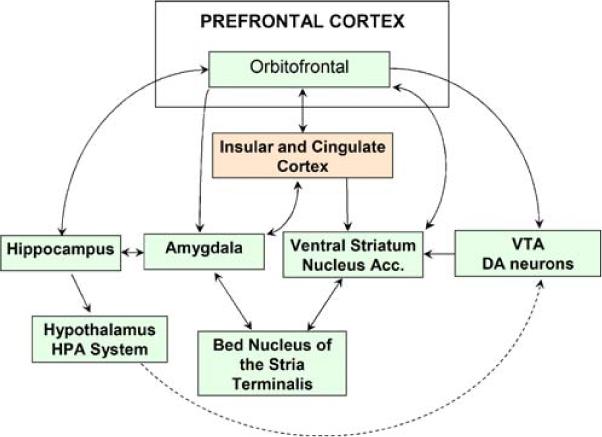
The extended limbic network consists of the basal forebrain, extended amygdala, and limbic lobe as described by Heimer and Van Hoesen (2006). To aid in visualization, not all structures or established projections are displayed. Regions of the basal forebrain, which includes areas usually referred to as the ventral striatum and nucleus accumbens, receive input from the extended amygdala, limbic lobe, and dopaminergic neurons of the ventral tegmental area. The extended amygdala includes the central and medial nuclei of the amygdala, their extensions to the bed nucleus of the stria terminalis, as well as downstream projections to the ventral striatum and nucleus accumbens. The extended amygdala has abundant associative connections with the hypothalamus as well as greater limbic lobe, including the hippocampus, cingulate and insular cortex, lateral basal cortical amygdala, and OFC
The broadly defined extended amygdala, which includes the bed nucleus of the stria terminalis and the central medial amygdala have been implicated in behavioral motivation and reward (Alheid and Heimer 1988). These nuclei are fundamental in avoidance learning, and projections from the extended amygdala to the lateral and medial divisions of the hypothalamus also support their role in the regulation of autonomic and somatosensory activities. These functions have been studied extensively with regard to their role in appetitive mechanisms and addiction. Thus, the extended amygdala is in a unique position to coordinate inputs from multiple limbic lobe regions in order to provide behavioral responses though its output channels (Heimer 2003). Dysfunction of the extended amygdala has been proposed to underlie changes in behavioral motivation for rewarding objects that manifest as (a) impulsive acts driven by pleasure and gratification and the positive reinforcing effects of drugs of abuse and (b) compulsive behavior that is produced through negative reinforcement to reduce the negative emotional state arising from substance dependence (Koob 2003).
The limbic lobe, on the other hand, is restricted to portions of the cortical mantle on the medial side of each hemisphere (Heimer and Van Hoesen 2006). The limbic lobe encompasses the hippocampus and parahippocampal gyri, lateral basal cortical amygdala, cingulate and insular cortices, as well as their pathways to the orbitofrontal cortex (OFC). The cingulate cortex has been implicated in attention, emotional processing, decision-making, and motor initiation (reviewed by Paus 2001), while the insular cortex is involved in processing of multimodal sensory information (reviewed by Nagai et al. 2007). The OFC, particularly in the right hemisphere, appears to be a neural substrate for mechanisms of inhibition and has been implicated in substance use disorders (reviewed by Dom et al. 2005). OFC projections to the basolateral amygdala provide local inhibitory influences through GABAergic neurotransmission. Failure to regulate the amygdala due to aberrant OFC volume or projections may cause impairment in learning to inhibit a prepotent response to obtain reward, or difficulty adjusting to new reward contingencies (e.g. Man et al. 2009).
Multiple components of the extended limbic network have been found to be structurally and/or functionally deficient in the offspring of alcohol dependent individuals. Particularly, offspring from multiplex families of alcoholic individuals with minimal prior exposure to alcohol have decreased volume of the orbitofrontal cortex in the right hemisphere (Hill et al. 2009). They also show reduced right amygdala volume compared to control subjects before drinking begins (Hill et al. 2001). Reduced grey matter has been reported for the amygdala and hippocampus in offspring from an Indian population of multigenerational families of alcoholics (Benegal et al. 2007). Since these regions are involved in emotion regulation and inhibition, the documented abnormalities in the offspring of alcoholics may correspond to the behavioral and affective dysfunction often observed in these individuals. Additionally, many of these regions have been found to be abnormal in psychiatric disorders that may share a diathesis for AUD.
An inverse relationship between grey matter volumes of the OFC and measures of impulsivity has been shown in healthy adults (Matsuo et al. 2008). Children and adults with ADHD who exhibit disinhibited and impulsive behaviors have smaller OFC volumes compared with healthy subjects (Carmona et al. 2005; Hesslinger et al. 2002). Aspects of the extended limbic network have been found to be abnormal in individuals with major depression (reviewed by Drevets et al. 2008). Similarly, a meta-analysis of functional imaging studies supports a connection between increased activation of the amygdala and insula and anxiety disorders (Etkin and Wager 2007).
There appears to be a distinct pattern of anatomical asymmetry, particularly in the OFC, that is functionally important in behavioral and emotional regulation. Unilateral right hemisphere damage to the ventrolateral prefrontal cortex (PFC) is associated with severe deficits in social/emotional and descision-making processes (Tranel et al. 2005). Greater involvement of the right than left prefrontal cortex is seen in tasks involving response selection and inhibition, with suboptimal response inhibition in children and adolescents is related to insufficient recruitment of the right ventrolateral PFC (Rubia et al. 2001). This is consistent with a subsequent study that found that boys with conduct disorder had lower activation of the right orbitofrontal cortex compared with healthy controls subjects and those with ADHD (Rubia et al. 2008). Moreover, volumetric differences in adolescents at high risk for AUD appear to be limited to the right OFC (Hill et al. 2009).
Although the functional and psychopathologic consequences of hemispheric differences remain uncertain, these findings emphasize the importance of the lateralization of the limbic lobe in the pathophysiology of risk for AUD as well as in disorders that are frequently observed in the offspring of alcoholics. It is possible that differences in the hemispheric lateralization of the OFC in a number of psychiatric disorders reflect a failure to regulate the amygdala and hence a vulnerability to over and under-controlled behaviors.
The HPA System
As noted earlier, individuals at risk for AUD tend to exhibit anomalies in their response to aversive stimuli as indicated by elevations in heart rate and blunted cortisol levels. In the presence of alcohol, the HPA system seems to be differentially affected by stress in high risk youth compared to control subjects. The HPA system regulates multiple components of the body's autonomic response to stress and prepares mechanisms in the brain for responsive action and survival (de Kloet et al. 1999). The HPA system is an important regulatory system that acts through hormone cascades to support both behavioral and physiological responses to threat (de Kloet et al. 1998). Alteration in HPA functioning associated with alcoholism risk may negatively impact an individual's ability to respond to internal and external challenges (i.e. cope with stress). In addition, cognitive deficits are associated with HPA system dysfunction (e.g. Cushings disease), so it is equally plausible that altered neurohormone levels in the offspring of alcoholics can disrupt cognitive processes (Starkman et al. 2001).
Figure 3 displays a schematic of the HPA system. The system involves regions within the brain including the hypothalamus that produces corticotropin-releasing hormone (CRH), and the pituitary that releases adrenocorticotropin hormone (ACTH). Cortisol and other glucocorticoid hormones are produced and secreted outside the brain by the adrenal glands. The release of CRH from the hypothalamus stimulates ACTH release from the pituitary that triggers the secretion of glucocorticoids, including cortisol, from the adrenal glands. In turn, a negative feedback effect on the system occurs at the level of the pituitary as well as other brain sites including the hypothalamus, hippocampus, and regions in the frontal cortex (Diorio et al. 1993). Therefore, abnormalities in the brain structures associated with negative feedback on the HPA system may produce maladaptive patterns of hormone secretion that influences behavioral and emotional regulation.
Fig. 3.
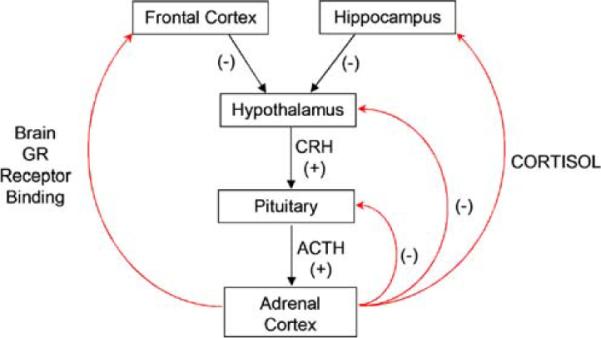
A schematic representation of the hypothalamic-pituitary-adrenocortical (HPA) system. Cortisol acts on the brain through binding to glucocorticoid receptors (GRs). Binding to these receptors in some regions of the brain, including the hippocampus, cingulate, and frontal cortex triggers a negative feedback system that dampens release of corticotropin-releasing hormone (CRH) and adrenocorticotropic hormone (ACTH), thus modulating HPA activity
The hippocampus plays a role in modulating activity of the HPA axis via a negative feedback system that involves glucocorticoid binding to receptors in the hippocampus (Herman et al. 2005; Jacobson and Sapolsky, 1991). In rodents, smaller hippocampal volume is associated with heightened glucocorticoid levels and blunted negative feedback (Hibberd et al. 2000; Meaney et al. 1995, 1996). Consistent with the role of the hippocampus in modulating HPA activity, hippocampal volume is typically found to be inversely correlated with glucocorticoid levels in humans (Tessner et al. 2007; Wiedenmayer et al. 2006). In addition, the medial frontal cortex and anterior cingulate is involved in glucocorticoid regulation (Brake et al. 2000; Diorio et al. 1993; Sullivan and Gratton, 2002), and it appears that smaller cingulate cortex volumes may be associated with HPA axis dysregulation (MacLullich et al. 2006).
There is an extensive body of literature to support a relationship between neuroendocrine abnormalities of the HPA system and various forms of psychopathology (Pariante and Lightman 2008; Stansbury and Gunnar 1994; Susman 2006; Walker et al. 2008). More specifically, differences in basal cortisol levels as well as the adrenal response to stress have been noted in studies of childhood behavioral problems (Klimes-Dougan et al. 2001; Shirtcliff et al. 2005). Heightened cortisol secretion has been linked to internalizing symptoms, including anxiety and withdrawal (Brown et al. 1996; Colomina et al. 1997; Schmidt et al. 1997; Windle 1994). Baseline and post-dexamethasone elevation in cortisol levels is associated with depression in adults (Nelson et al. 1997; Rush et al. 1996), adolescents (Foreman and Goodyer 1988), and prepubertal children (Puig-Antioch et al. 1989). Conversely, attenuated activity of the HPA system has been identified as a risk factor for antisocial behavior (Susman 2006).
Possible Neural Diatheses for Alcohol Use Disorders
The emergence of alcohol use disorders, as with other psychiatric disorders that follow a developmental course, are preceded by a number of neurobiological and psychosocial risk factors that are often predictive of outcome. Identified in this review are two likely pathways to the emergence of alcohol use and subsequent abuse and dependence: (1) A constellation of externalizing behaviors representative of deficits in executive control and behavioral inhibition and (2) a trajectory of internalizing problems undermined by mechanisms of affective dysregulation. Importantly, these behavioral and emotional characteristics are present before the onset of alcohol use and appear to be subserved by genetically-mediated neural circuits in the offspring of alcoholics.
The preponderance of the data suggests that the externalizing pathway to alcohol dependence may be partially determined by variation in the functioning of circuitry involved in executive control and behavioral inhibition that includes connectivity of the cerebellum, thalamus, and prefrontal cortex which generates a cortico-thalamo-cerebellar feedback loop. This circuitry has also been implicated in frequently co-occurring conditions including ADHD (Nigg and Casey 2005). The internalizing pathway is associated with mood problems, negative affect, and a hypersensitivity to rewards/stress reflective of alterations in the OFC, extended amygdala, hypothalamus, and the HPA system. Impairments in emotional conditioning due to abnormalities of the extended limbic network (including hippocampal and hypothalamic feedback of the HPA system) have been postulated in developmental models of ASPD (Blair 2001; Blair et al. 2006; Crowe and Blair 2008; Susman 2006) and affective disorders (Monk 2008). The neural circuits of the internalizing and externalizing pathways are highlighted in Fig. 4. Notably, the pathways are interconnected and further communicate through the cingulate gyrus and insular cortex.
Fig. 4.
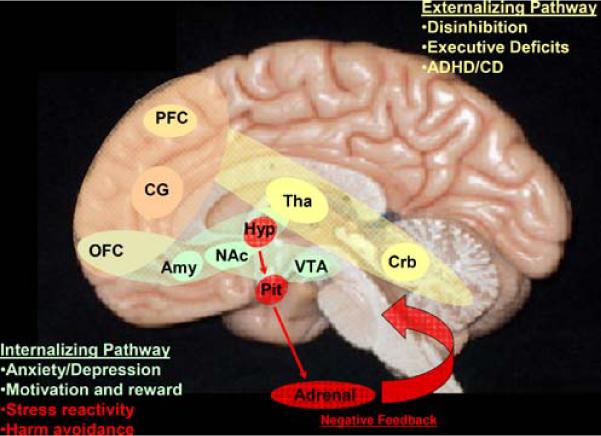
A schematic of the pathways that are of primary relevance to the core features of risk for alcohol dependence, particularly behavioral and affective dysfunction. The pathways are color coded to assist in visualization. The externalizing pathway to alcohol dependence (yellow) is associated with deficits in executive function and in inhibition and may share a common genetic diathesis with ADHD and conduct disorder. The brain regions of the externalizing pathway consist of the cerebellum (Crb), thalamus (Tha), and prefrontal cortex (PFC) which comprise the cerebellothalamocortical feedback system. The internalizing pathway to alcohol dependence (green) is associated with affective dysregulation (i.e. depression and anxiety-related traits), deficient mechanisms of motivation and reward (i.e. impulsivity and dysfunction in decision-making), and autonomic hyperarousal (i.e. harm avoidance and stress reactivity) through overlapping pathways of the HPA system (shown in red). The internalizing pathway includes the amygdala (Amy), orbitofrontal cortex (OFC), and hypothalamus (Hyp) and pituitary (Pit) of the HPA axis, as well as the brain regions of the mesolimbic dopamine pathway (NAc = nucleus accumbens; VTA = ventral tegmental area). The cingulate gyrus (CG) connects these pathways
The model proposed here encompasses potential neural systems underlying the risk for AUD and serves as a framework for understanding the complex traits associated with alcoholism risk. The nature of the inborn variations in brain structure and function associated with risk for AUD and other comorbid conditions requires further study. At present, it appears that premorbid dysfunction of the cerebellothalocortical system may predispose an individual to an externalizing pathway to AUD that is prompted by difficulties with behavioral control and inhibition. Following the reinforcing pleasurable effects of alcohol exposure, behavioral under control may lead to an inability to restrain future consumption. Alternatively, abnormalities in the extended limbic network, including the orbitofrontal cortex, amygdala, and hypothalamus may drive the negative affective characteristics shown to elicit alcohol abuse, thereby making the anxiolytic properties of ethanol more gratifying. Complementary, failure to regulate the HPA axis may influence an individual's behavioral response to novelty and anxiety-related behaviors in the presence of stress, motivating the use of alcohol to achieve sedation.
The current model provides some indication of the neuroanatomical circuits that may underlie the core features of risk for AUD. Others have proposed similar brain regions associated with AUD, including the cerebellothalamocortical system (Sullivan 2003; Sullivan et al. 2003), extended amygdala (Koob and Le Moal 2008), and mesocorticolimbic dopamine system (Comings and Blum 2000; Blum et al. 2000; Bowirrat and Oscar-Berman, 2005; Makris et al. 2008). However, these conceptualizations have been largely based on the effects of chronic alcohol exposure on brain systems. The present review has focused on identifying the neural circuitry that may provides clues concerning how familial genetic loading for alcohol dependence is translated into higher risk for succeeding generations to become alcohol dependent as well. In doing so, we have described findings obtained in those with familial loading for AUD who have had minimal alcohol exposure at the time of examination. Consequently, the findings described do imply that abnormalities of the cerebellothalamocortical, extended limbic network, and HPA system may precede the initiation of alcohol use and are therefore particularly important in the etiology of AUD.
Directions for Future Research
Genetic variation may result in differences in the constitution and functioning of neural mechanisms that underlie the phenotypic manifestations of risk for AUD. Numerous studies have linked structural and functional brain alterations to genetic variations associated with neurotransmitter/transporter synthesis, receptor organization, and brain growth (Frodl et al. 2004, 2008; Nemoto et al. 2006; reviewed by Lipsky and Marini 2007; Peper et al. 2007). Further, genetic variations associated with risk for AUD have also been recognized as etiological factors in ADHD, conduct disorder, depression, and anxiety disorders. Therefore, it is likely that there are specific genetic mechanisms of risk for AUD, as well as common genetic diatheses that exist between AUD, conduct disorder, depression, anxiety, ASPD, and ADHD. Genetic factors may mediate the neural circuitry comprising the externalizing and internalizing pathways to AUD.
To date, genetic mechanisms of risk in the offspring of alcoholic parents or families with high density of alcoholism remain relatively unknown. Alcohol dependence has been linked to regions on chromosome 1, 2, 3, 4, 7, and 8 (Foroud et al. 2000; Hill et al. 2004; Reich et al. 1998; Williams et al. 1999). A recent review from the Collaborative Study on the Genetics of Alcoholism (COGA; Edenberg and Foroud 2006) have identified variations associated with alcohol dependence in a number of genes including ADH1B and ALDH2 (alcohol metabolism genes), GABRA2 and GABRG3 (GABA receptor genes), and CHRM2 (muscarinic acetylcholine receptor gene). However, several of these finding have not been confirmed, and the processes by which these genetic risk factors influence the development of AUD have yet to be determined.
Emerging evidence provides a potential clue to the mechanisms through which genes increase risk for AUD. For example, variations of the GABRA2 gene have been linked to conduct disorder in a high-risk sample of offspring of alcoholics (Dick et al. 2006). These results have recently been replicated in an independent sample of offspring from multiplex families for alcohol dependence (Hill et al. unpublished results). This suggests that GABA receptors are important for the behavioral attributes associated with externalizing disorders that may increase the likelihood of developing an AUD in young adulthood. Additionally, analyses of the COGA data indicate that the GABRA2 gene is associated with EEG oscillations and alcohol dependence, suggesting GABRA2 might influence susceptibility to alcohol dependence by modulating neural inhibition (Edenberg et al. 2004). Lastly, Hill et al. (2009a) were the first to report a statistically significant association between variation of the serotonin transporter (5-HTT) and brain-derived neurotrophic factor (BDNF) genes and volume of the OFC in the right hemisphere among high risk offspring. Therefore, variations in genes are likely to influence behavioral outcome by altering the structure and function of specific brain systems.
Ultimately, understanding how genes affect behavioral pathways and neural systems will be particularly important in future studies of offspring of alcoholics and other disorders that may share a common genetic diathesis. Even though the processes by which genetic variations influence brain structure and function remain elusive, it appears that neurotrophic factors are likely targets for future research given the pre-existing morphological differences in those at elevated risk for AUD. GABA is particularly influential in the signaling of growth factors like BDNF and other neuronal growth-associated proteins during neural maturation. Since the development of the human brain proceeds through a series of stages, understanding the nature and activity of growth factors during these periods in brain development will be crucial to understanding the structural and functional outcomes that are often used as the dependent variables in neuroimaging genetics studies of risk for psychiatric disorders.
The field of neuroimaging genetics provides a promising approach for elucidating mechanisms of genetic susceptibility in relation to brain structure and function (Tan et al. 2008). Also, the field of epigenetics has highlighted the presence of heritable changes in gene expression that occurs independently of alterations in primary DNA sequence (Kiefer, 2007). Brain development is under epigenetic control, and alterations in brain structure and subsequent function may be contributed to differential promoter DNA methylation patterns, histone changes, and chromosomal interactions in the absence of allelic variations. Epigenetic mechanisms may account for one-third to one-half of known genetic alterations, and they may provide an understanding of the molecular underpinnings of long-term changes in the structure and function of the brain (Weinhold 2006). It is likely that the changes in the neural structures of the externalizing and internalizing behavioral pathways to AUD are modulated by a variety of genetic mechanisms. Accommodating the influences of genetic variation and epigenetic events will be important in future models of AUD.
Acknowledgements
This research was supported by the National Institute on Alcohol Abuse and Alcoholism (NIAAA) Grants AA 005909, AA 008082, AA 015168 to SYH.
Footnotes
Disclosures The authors have no financial conflicts of interest related to this paper.
References
- Acheson A, Robinson JL, Glahn DC, Lovallo WR, Fox PT. Differential activation of the anterior cingulate cortex and caudate nucleus during a gambling simulation in persons with a family history of alcoholism: studies from the Oklahoma Family Health Patterns Project. Drug and Alcohol Dependence. 2009;100(1–2):17–23. doi: 10.1016/j.drugalcdep.2008.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alheid GF, Heimer L. New perspectives in basal forebrain organization of special relevance for neuropsychiatric disorders: the striatopallidal, amygdaloid, and corticopetal components of substantia innominata. Neuroscience. 1988;27(1):1–39. doi: 10.1016/0306-4522(88)90217-5. [DOI] [PubMed] [Google Scholar]
- Anand BK, Malhotra CL, Singh B, Dua S. Cerebellar projections to limbic system. Journal of Neurophysiology. 1959;22(4):451–457. doi: 10.1152/jn.1959.22.4.451. [DOI] [PubMed] [Google Scholar]
- August GJ, Winters KC, Realmuto GM, Fahnhorst T, Botzet A, Lee S. Prospective study of adolescent drug use among community samples of ADHD and non-ADHD participants. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2006;45(7):824–832. doi: 10.1097/01.chi.0000219831.16226.f8. [DOI] [PubMed] [Google Scholar]
- Babor TF, Hofmann M, DelBoca FK, Hesselbrock V, Meyer RE, Dolinsky ZS, et al. Types of alcoholics I. Evidence for an empirically derived typology based on indicators of vulnerability and severity. Archives of General Psychiatry. 1992;49(8):599–608. doi: 10.1001/archpsyc.1992.01820080007002. [DOI] [PubMed] [Google Scholar]
- Badre D, Wagner AD. Left ventrolateral prefrontal cortex and the cognitive control of memory. Neuropsychologia. 2007;45(13):2883–2901. doi: 10.1016/j.neuropsychologia.2007.06.015. [DOI] [PubMed] [Google Scholar]
- Baillieux H, De Smet HJ, Paquier PF, De Deyn PP, Mariën P. Cerebellar neurocognition: insights into the bottom of the brain. Clinical Neurology and Neurosurgery. 2008;110(8):763–773. doi: 10.1016/j.clineuro.2008.05.013. [DOI] [PubMed] [Google Scholar]
- Barkley RA. The executive functions and self regulation: An evolutionary neuropsychological perspective. Neuropsychology Review. 2004;11:1–20. doi: 10.1023/a:1009085417776. [DOI] [PubMed] [Google Scholar]
- Barkley RA, Grodzinsky G, DuPaul GJ. Frontal lobe functions in attention deficit disorder with and without hyperactivity: a review and research report. Journal of Abnormal Child Psychology. 1992;20(2):163–188. doi: 10.1007/BF00916547. [DOI] [PubMed] [Google Scholar]
- Barkley RA, Fischer M, Smallish L, Fletcher K. Young adult follow-up of hyperactive children: antisocial activities and drug use. Journal of Child Psychology and Psychiatry. 2004;45(2):195–211. doi: 10.1111/j.1469-7610.2004.00214.x. [DOI] [PubMed] [Google Scholar]
- Bates ME, Labouvie EW. Familial alcoholism and personality-environment fit. A developmental study of risk in adolescents. Annals of the New York Academy of Sciences. 1994;708:202–213. doi: 10.1111/j.1749-6632.1994.tb24713.x. [DOI] [PubMed] [Google Scholar]
- Begleiter H, Porjesz B. What is inherited in the predisposition toward alcoholism? A proposed model. Alcoholism, Clinical and Experimental Research. 1999;23(7):1125–1135. doi: 10.1111/j.1530-0277.1999.tb04269.x. [DOI] [PubMed] [Google Scholar]
- Begleiter H, Porjesz B, Bihari B, Kissin B. Event-related brain potentials in boys at risk for alcoholism. Science. 1984;225(4669):1493–1496. doi: 10.1126/science.6474187. [DOI] [PubMed] [Google Scholar]
- Benegal V, Antony G, Venkatasubramanian G, Jayakumar PN. Gray matter volume abnormalities and externalizing symptoms in subjects at high risk for alcohol dependence. Addiction Biology. 2007;12(1):122–132. doi: 10.1111/j.1369-1600.2006.00043.x. [DOI] [PubMed] [Google Scholar]
- Biederman J, Newcorn J, Sprich S. Comorbidity of attention deficit hyperactivity disorder with conduct, depressive, anxiety, and other disorders. American Journal of Psychiatry. 1991;148(5):564–577. doi: 10.1176/ajp.148.5.564. [DOI] [PubMed] [Google Scholar]
- Biederman J, Wilens T, Mick E, Faraone SV, Weber W, Curtis S, et al. Is ADHD a risk factor for psychoactive substance use disorders? Findings from a four-year prospective follow-up study. Journal of Amer Acadedmy of Child and Adolescent Psychiatry. 1997;36(1):21–29. doi: 10.1097/00004583-199701000-00013. [DOI] [PubMed] [Google Scholar]
- Biederman J, Wilens TE, Mick E, Faraone SV, Spencer T. Does attention-deficit hyperactivity disorder impact the developmental course of drug and alcohol abuse and dependence? Biological Psychiatry. 1998;44(4):269–273. doi: 10.1016/s0006-3223(97)00406-x. [DOI] [PubMed] [Google Scholar]
- Biederman J, Petty CR, Dolan C, Hughes S, Mick E, Monuteaux MC, et al. The long-term longitudinal course of oppositional defiant disorder and conduct disorder in ADHD boys: findings from a controlled 10-year prospective longitudinal follow-up study. Psychological Medicine. 2008;38(7):1027–1036. doi: 10.1017/S0033291707002668. [DOI] [PubMed] [Google Scholar]
- Bjork JM, Knutson B, Hommer DW. Incentive-elicited striatal activation in adolescent children of alcoholics. Addiction. 2008;103(8):1308–1319. doi: 10.1111/j.1360-0443.2008.02250.x. [DOI] [PubMed] [Google Scholar]
- Blair RJ. Neurocognitive models of aggression, the antisocial personality disorders, and psychopathy. Journal of Neurology, Neurosurgery and Psychiatry. 2001;71(6):727–731. doi: 10.1136/jnnp.71.6.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair RJ, Peschardt KS, Budhani S, Mitchell DG, Pine DS. The development of psychopathy. Journal of Child Psychology and Psychiatry. 2006;47(3–4):262–276. doi: 10.1111/j.1469-7610.2006.01596.x. [DOI] [PubMed] [Google Scholar]
- Blum K, Braverman ER, Holder JM, Lubar JF, Monastra VJ, Miller D, et al. Reward deficiency syndrome: a biogenetic model for the diagnosis and treatment of impulsive, addictive, and compulsive behaviors. Journal of Psychoactive Drugs. 2000;32:1–112. doi: 10.1080/02791072.2000.10736099. [DOI] [PubMed] [Google Scholar]
- Bohman M. Some genetic aspects of alcoholism and criminality. A population of adoptees. Archives of General Psychiatry. 1978;35(3):269–276. doi: 10.1001/archpsyc.1978.01770270019001. [DOI] [PubMed] [Google Scholar]
- Bowirrat A, Oscar-Berman M. Relationship between dopaminergic neurotransmission, alcoholism, and Reward Deficiency syndrome. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2005;132B(1):29–37. doi: 10.1002/ajmg.b.30080. [DOI] [PubMed] [Google Scholar]
- Brake WG, Flores G, Francis D, Meaney M, Srivastava LK, Gratton A. Enhanced nucleus accumbens dopamine and plasma corticosterone stress responses in adult rats with neonatal excitotoxic lesions to the medial prefrontal cortex. Neuroscience. 2000;96:687–695. doi: 10.1016/s0306-4522(00)00002-6. [DOI] [PubMed] [Google Scholar]
- Brown LL, Tomarken AJ, Orth DN, Loosen PT, Kalin NH, Davidson RJ. Individual differences in repressive-defensiveness predict basal salivary cortisol. Journal of Personality and Social Psychology. 1996;70:362–371. doi: 10.1037//0022-3514.70.2.362. [DOI] [PubMed] [Google Scholar]
- Brückl TM, Wittchen HU, Höfler M, Pfister H, Schneider S, Lieb R. Childhood separation anxiety and the risk of subsequent psychopathology: Results from a community study. Psychotherapy and Psychosomatics. 2007;76(1):47–56. doi: 10.1159/000096364. [DOI] [PubMed] [Google Scholar]
- Carmona S, Vilarroya O, Bielsa A, Trèmols V, Soliva JC, Rovira M, et al. Global and regional gray matter reductions in ADHD: a voxel-based morphometric study. Neuroscience Letters. 2005;389:88–93. doi: 10.1016/j.neulet.2005.07.020. [DOI] [PubMed] [Google Scholar]
- Caspi A, Moffitt TE, Newman DL, Silva PA. Behavioral observations at age 3 years predict adult psychiatric disorders. Longitudinal evidence from a birth cohort. Archives of General Psychiatry. 1996;53(11):1033–1039. doi: 10.1001/archpsyc.1996.01830110071009. [DOI] [PubMed] [Google Scholar]
- Chassin L, Pitts SC, DeLucia C, Todd M. A longitudinal study of children of alcoholics: predicting young adult substance use disorders, anxiety, and depression. Journal Abnormal Psychology. 1999;108(1):106–119. doi: 10.1037//0021-843x.108.1.106. [DOI] [PubMed] [Google Scholar]
- Clark DB, Moss HB, Kirisci L, Mezzich AC, Miles R, Ott P. Psychopathology in preadolescent sons of fathers with substance use disorders. Journal of Amer Academy of Child and Adolescent Psychiatry. 1997;36(4):495–502. doi: 10.1097/00004583-199704000-00012. [DOI] [PubMed] [Google Scholar]
- Clark DB, Cornelius J, Wood DS, Vanukov M. Psychopathology risk transmission in children of parents with substance use disorders. American Journal of Psychiatry. 2004;161:685–691. doi: 10.1176/appi.ajp.161.4.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloninger CR. Neurogenetic adaptive mechanisms in alcoholism. Science. 1987;236:410–416. doi: 10.1126/science.2882604. [DOI] [PubMed] [Google Scholar]
- Cloninger CR, Bohman M, Sigvardsson S. Inheritance of alcohol abuse. Cross-fostering analysis of adopted men. Archives of General Psychiatry. 1981;38(8):861–868. doi: 10.1001/archpsyc.1981.01780330019001. [DOI] [PubMed] [Google Scholar]
- Cockerham WC, Kunz G, Lueschen G. Alcohol use and psychological distress: a comparison of Americans and West Germans. International Journal of the Addictions. 1989;24(10):951–961. doi: 10.3109/10826088909047322. [DOI] [PubMed] [Google Scholar]
- Cohen HL, Porjesz B, Begleiter H, Wang W. Neurophysiological correlates of response production and inhibition in alcoholics. Alcoholism, Clinical and Experimental Research. 1997;21(8):1398–1406. [PubMed] [Google Scholar]
- Colomina MT, Canals J, Carbajo G, Domingo JL. Salivary cortisol in a young population: Relationship with psychopathological disorders. Research Communications in Giological Psychology and Psychiatry. 1997;22:1–10. [Google Scholar]
- Comings DE, Blum K. Reward deficiency syndrome: genetic aspects of behavioral disorders. Progress in Brain Research. 2000;126:325–341. doi: 10.1016/S0079-6123(00)26022-6. [DOI] [PubMed] [Google Scholar]
- Corral MM, Holguín SR, Cadaveira F. Neuropsycho-logical characteristics in children of alcoholics: familial density. Journal of Studies on Alcohol. 1999;60(4):509–513. doi: 10.15288/jsa.1999.60.509. [DOI] [PubMed] [Google Scholar]
- Corral M, Holguín SR, Cadaveira F. Neuropsychological characteristics of young children from high-density alcoholism families: a three-year follow-up. Journal of Studies on Alcohol. 2003;64(2):195–199. doi: 10.15288/jsa.2003.64.195. [DOI] [PubMed] [Google Scholar]
- Costa L, Bauer L, Kuperman S, Porjesz B, O'Connor S, Hesselbrock V, et al. Frontal P300 decrements, alcohol dependence, and antisocial personality disorder. Biological Psychiatry. 2000;47(12):1064–1071. doi: 10.1016/s0006-3223(99)00317-0. [DOI] [PubMed] [Google Scholar]
- Crowe SL, Blair RJ. The development of antisocial behavior: what can we learn from functional neuroimaging studies? Development and Psychopathology. 2008;20(4):1145–1159. doi: 10.1017/S0954579408000540. [DOI] [PubMed] [Google Scholar]
- Crum RM, Green KM, Storr CL, Chan YF, Ialongo N, Stuart EA, et al. Depressed mood in childhood and subsequent alcohol use through adolescence and young adulthood. Archives of General Psychiatry. 2008;65(6):702–712. doi: 10.1001/archpsyc.65.6.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai X, Thavundayil J, Gianoulakis C. Response of the hypothalamic-pituitary-adrenal axis to stress in the absence and presence of ethanol in subjects at high and low risk of alcoholism. Neuropsychopharmacology. 2002;27(3):442–452. doi: 10.1016/S0893-133X(02)00308-1. [DOI] [PubMed] [Google Scholar]
- Dai X, Thavundayil J, Santella S, Gianoulakis C. Response of the HPA-axis to alcohol and stress as a function of alcohol dependence and family history of alcoholism. Psycho-neuroendocrinology. 2007;32(3):293–305. doi: 10.1016/j.psyneuen.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Dawes MA, Dorn LD, Moss HB, Yao JK, Kirisci L, Ammerman RT, et al. Hormonal and behavioral homeostasis in boys at risk for substance abuse. Drug and Alcohol Dependence. 1999;55(1–2):165–176. doi: 10.1016/s0376-8716(99)00003-4. [DOI] [PubMed] [Google Scholar]
- Dawson DA, Grant BF. Family history of alcoholism and gender: their combined effects on DSM-IV alcohol dependence and major depression. Journal of Studies on Alcohol. 1998;59(1):97–106. doi: 10.15288/jsa.1998.59.97. [DOI] [PubMed] [Google Scholar]
- de Kloet E, Vreugdenhil E, Oitzl M, Joels M. Brain corticoisteriod receptor balance in health and disease. Endocrine Reviews. 1998;19(3):269–301. doi: 10.1210/edrv.19.3.0331. [DOI] [PubMed] [Google Scholar]
- de Kloet E, Oitzl M, Joels M. Stress and cognition: are coricosteroids good or bad guys? Trends in Neuroscience. 1999;22(10):422–426. doi: 10.1016/s0166-2236(99)01438-1. [DOI] [PubMed] [Google Scholar]
- de Olmos JS, Heimer L. The concepts of the ventral striatopallidal system and extended amygdala. Annals of the New York Academy of Sciences. 1999;877:1–32. doi: 10.1111/j.1749-6632.1999.tb09258.x. [DOI] [PubMed] [Google Scholar]
- Díaz R, Gual A, García M, Arnau J, Pascual F, Cañuelo B, et al. Children of alcoholics in Spain: from risk to pathology. Results from the ALFIL program. Social Psychiatry and Psychiatric Epidemiology. 2008;43(1):1–10. doi: 10.1007/s00127-007-0264-2. [DOI] [PubMed] [Google Scholar]
- Dick DM, Bierut L, Hinrichs A, Fox L, Bucholz KK, Kramer J, et al. The role of GABRA2 in risk for conduct disorder and alcohol and drug dependence across developmental stages. Behavior Genetics. 2006;36:577–590. doi: 10.1007/s10519-005-9041-8. [DOI] [PubMed] [Google Scholar]
- Diorio D, Viau V, Meaney M. The role of the medial prefrontal cortex (cingulate cortex) in the regulation of hypothalamic—pituitary—adrenal responses to stress. Journal of Neuroscience. 1993;13:3839–3847. doi: 10.1523/JNEUROSCI.13-09-03839.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dom G, Sabbe B, Hulstijn W, van den Brink W. Substance use disorders and the orbitofrontal cortex: systematic review of behavioural decision-making and neuroimaging studies. British Journal of Psychiatry. 2005;187:209–220. doi: 10.1192/bjp.187.3.209. [DOI] [PubMed] [Google Scholar]
- Doyle AE. Executive functions in attention-deficit/hyperactivity disorder. Journal of Clinical Psychiatry. 2006;67:21–26. [PubMed] [Google Scholar]
- Drejer K, Theilgaard A, Teasdale TW, Schulsinger F, Goodwin DW. A prospective study of young men at high risk for alcoholism: neuropsychological assessment. Alcoholism, Clinical and Experimental Research. 1985;9(6):498–502. doi: 10.1111/j.1530-0277.1985.tb05590.x. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Furey ML. Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Structure and Function. 2008;213(1–2):93–118. doi: 10.1007/s00429-008-0189-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earls F, Powell J. Patterns of substance use and abuse in inner-city adolescent medical patients. Yale Journal of Biology & Medicine. 1988;61(3):233–242. [PMC free article] [PubMed] [Google Scholar]
- Earls F, Reich W, Jung KG, Cloninger CR. Psychopathology in children of alcoholic and antisocial parents. Alcoholism, Clinical and Experimental Research. 1988;12(4):481–487. doi: 10.1111/j.1530-0277.1988.tb00230.x. [DOI] [PubMed] [Google Scholar]
- Edenberg HJ, Foroud T. The genetics of alcoholism: identifying specific genes through family studies. Addiction Biology. 2006;11(3–4):386–396. doi: 10.1111/j.1369-1600.2006.00035.x. [DOI] [PubMed] [Google Scholar]
- Edenberg HJ, Dick DM, Xuei X, Tian H, Almasy L, Bauer LO, et al. Variations in GABRA2, encoding the alpha 2 subunit of the GABA(A) receptor, are associated with alcohol dependence and with brain oscillations. American Journal of Human Genetics. 2004;74:705–714. doi: 10.1086/383283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkins IJ, McGue M, Iacono WG. Prospective effects of attention-deficit/hyperactivity disorder, conduct disorder, and sex on adolescent substance use and abuse. Archives of General Psychiatry. 2007;64(10):1145–1152. doi: 10.1001/archpsyc.64.10.1145. [DOI] [PubMed] [Google Scholar]
- Enoch MA, Goldman D. Genetics of alcoholism and substance abuse. Psychiatric Clinics of North America. 1999;22(2):289–299. doi: 10.1016/s0193-953x(05)70077-0. [DOI] [PubMed] [Google Scholar]
- Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. American Journal of Psychiatry. 2007;164(10):1476–1488. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairchild G, van Goozen SH, Stollery SJ, Brown J, Gardiner J, Herbert J, et al. Cortisol diurnal rhythm and stress reactivity in male adolescents with early-onset or adolescence-onset conduct disorder. Biological Psychiatry. 2008;64(7):599–606. doi: 10.1016/j.biopsych.2008.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellows LK. Advances in understanding ventromedial prefrontal function: the accountant joins the executive. Neurology. 2007;68(13):991–995. doi: 10.1212/01.wnl.0000257835.46290.57. [DOI] [PubMed] [Google Scholar]
- Fine EQ, Yudin LW, Holmes J, Heinemann S. Behavioral disorders in children with parental alcoholism. Annals of the New York Academy of Sciences. 1976;273:507–517. doi: 10.1111/j.1749-6632.1976.tb52922.x. [DOI] [PubMed] [Google Scholar]
- Finn PR, Pihl RO. Risk for alcoholism: a comparison between two different groups of sons of alcoholics on cardiovascular reactivity and sensitivity to alcohol. Alcoholism, Clinical and Experimental Research. 1988;12(6):742–747. doi: 10.1111/j.1530-0277.1988.tb01338.x. [DOI] [PubMed] [Google Scholar]
- Finn PR, Zeitouni NC, Pihl RO. Effects of alcohol on psychophysiological hyperreactivity to nonaversive and aversive stimuli in men at high risk for alcoholism. Journal of Abnormal Psychology. 1990;99(1):79–85. doi: 10.1037//0021-843x.99.1.79. [DOI] [PubMed] [Google Scholar]
- Finn PR, Sharkansky EJ, Viken R, West TL, Sandy J, Bufferd GM. Heterogeneity in the families of sons of alcoholics: the impact of familial vulnerability type on offspring characteristics. Journal of Abnormal Psychology. 1997;106(1):26–36. doi: 10.1037//0021-843x.106.1.26. [DOI] [PubMed] [Google Scholar]
- Fite PJ, Colder CR, O'Connor RM. Childhood behavior problems and peer selection and socialization: risk for adolescent alcohol use. Addictive Behaviors. 2006;31(8):1454–1459. doi: 10.1016/j.addbeh.2005.09.015. [DOI] [PubMed] [Google Scholar]
- Foreman DM, Goodyer IM. Salivary cortisol hypersecretion in juvenile depression. Journal of Child Psychology and Psychiatry and Allied Disciplines. 1988:311–320. doi: 10.1111/j.1469-7610.1988.tb00719.x. [DOI] [PubMed] [Google Scholar]
- Foroud T, Edenberg HJ, Goate A, Rice J, Flury L, Koller DL, et al. Alcoholism susceptibility loci: confirmation studies in a replicate sample and further mapping. Alcoholism, Clinical and Experimental Research. 2000;24(7):933–945. [PubMed] [Google Scholar]
- Frodl T, Meisenzahl EM, Zill P, Baghai T, Rujescu D, Leinsinger G, et al. Reduced hippocampal volumes associated with the long variant of the serotonin ransporter polymorphism in major depression. Archives of General Psychiatry. 2004;61(2):177–183. doi: 10.1001/archpsyc.61.2.177. [DOI] [PubMed] [Google Scholar]
- Frodl T, Koutsouleris N, Bottlender R, Born C, Jäger M, Mörgenthaler M, et al. Reduced gray matter brain volumes are associated with variants of the serotonin transporter gene in major depression. Molecular Psychiatry. 2008;13(12):1093–1101. doi: 10.1038/mp.2008.62. [DOI] [PubMed] [Google Scholar]
- Giancola PR, Moss HB. Executive cognitive functioning in alcohol use disorders. Recent Developments in Alcoholism. 1998;14:227–251. doi: 10.1007/0-306-47148-5_10. [DOI] [PubMed] [Google Scholar]
- Glahn DC, Lovallo WR, Fox PT. Reduced amygdala activation in young adults at high risk of alcoholism: studies from the Oklahoma family health patterns project. Biological Psychiatry. 2007;61(11):1306–1309. doi: 10.1016/j.biopsych.2006.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. American Journal of Psychiatry. 2002;59(10):1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin DW, Schulsinger F, Hermansen L, Guze SB, Winokur G. Alcohol problems in adoptees raised apart from alcoholic biological parents. Archives of General Psychiatry. 1973;28(2):238–243. doi: 10.1001/archpsyc.1973.01750320068011. [DOI] [PubMed] [Google Scholar]
- Grant BF, Dawson DA. Age at onset of alcohol use and its association with DSM-IV alcohol abuse and dependence: results from the National Longitudinal Alcohol Epidemiologic Survey. Journal of Substance Abuse. 1997;9:103–110. doi: 10.1016/s0899-3289(97)90009-2. [DOI] [PubMed] [Google Scholar]
- Habeych ME, Folan MM, Luna B, Tarter RE. Impaired oculomotor response inhibition in children of alcoholics: The role of attention deficit hyperactivity disorder. Drug and Alcohol Dependence. 2006;82(1):11–17. doi: 10.1016/j.drugalcdep.2005.07.012. [DOI] [PubMed] [Google Scholar]
- Hanisch C, Radach R, Holtkamp K, Herpertz-Dahlmann B, Konrad K. Oculomotor inhibition in children with and without attention-deficit hyperactivity disorder (ADHD) Journal of Neural Transmission. 2006;113(5):671–684. doi: 10.1007/s00702-005-0344-y. [DOI] [PubMed] [Google Scholar]
- Harden PW, Pihl RO. Cognitive function, cardiovascular reactivity, and behavior in boys at high risk for alcoholism. Journal of Abnormal Psychology. 1995;104(1):94–103. doi: 10.1037//0021-843x.104.1.94. [DOI] [PubMed] [Google Scholar]
- Harper JW, Heath RG. Anatomic connections of the fastigial nucleus to the rostral forebrain in the cat. Experimental Neurology. 1973;39(2):285–292. doi: 10.1016/0014-4886(73)90231-8. [DOI] [PubMed] [Google Scholar]
- Harper C, Matsumoto I. Ethanol and brain damage. Current Opinion in Pharmacology. 2005;5(1):73–78. doi: 10.1016/j.coph.2004.06.011. [DOI] [PubMed] [Google Scholar]
- Harper C, Dixon G, Sheedy D, Garrick T. Neuro-pathological alterations in alcoholic brains. Studies arising from the New South Wales Tissue Resource Centre. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2003;27(6):951–961. doi: 10.1016/S0278-5846(03)00155-6. [DOI] [PubMed] [Google Scholar]
- Hastings PD, Fortier I, Utendale WT, Simard LR, Robaey P. Adrenocortical Functioning in Boys with Attention-Deficit/Hyperactivity Disorder: Examining Subtypes of ADHD and Associated Comorbid Conditions. J Abnorm Child Psychol. 2009 Jan 9; doi: 10.1007/s10802-008-9292-y. ahead of print. [DOI] [PubMed] [Google Scholar]
- Hawkins JD, Graham JW, Maguin E, Abbott R, Hill KG, Catalano RF. Exploring the effects of age of alcohol use initiation and psychosocial risk factors on subsequent alcohol misuse. Journal of Studies Alcohol. 1997;58(3):280–290. doi: 10.15288/jsa.1997.58.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath AC, Meyer J, Jardine R, Martin NG. The inheritance of alcohol consumption patterns in a general population twin sample: II Determinants of consumption frequency and quantity consumed. Journal of Studies Alcohol. 1991;52(5):425–433. doi: 10.15288/jsa.1991.52.425. [DOI] [PubMed] [Google Scholar]
- Heath AC, Bucholz KK, Madden PA, Dinwiddie SH, Slutske WS, Bierut LJ, et al. Genetic and environmental contributions to alcohol dependence risk in a national twin sample: consistency of findings in women and men. Psychological Medicine. 1997;27(6):1381–1396. doi: 10.1017/s0033291797005643. [DOI] [PubMed] [Google Scholar]
- Hegedus AM, Tarter RE, Hill SY, Jacob T, Winsten NE. Static ataxia: a possible marker for alcoholism. Alcoholism, Clinical and Experimental Research. 1984;8(6):580–582. doi: 10.1111/j.1530-0277.1984.tb05735.x. [DOI] [PubMed] [Google Scholar]
- Heimer L. A new anatomical framework for neuropsychiatric disorders and drug abuse. American Journal of Psychiatry. 2003;160:1726–1739. doi: 10.1176/appi.ajp.160.10.1726. [DOI] [PubMed] [Google Scholar]
- Heimer L, Van Hoesen GW. The limbic lobe and its output channels: implications for emotional functions and adaptive behavior. Neuroscience and Biobehavioral Reviews. 2006;30(2):126–147. doi: 10.1016/j.neubiorev.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Heimer L, Zahm DS, Churchill L, Kalivas PW, Wohltmann C. Specificity in the projection patterns of accumbal core and shell in the rat. Neuroscience. 1991;41(1):89–125. doi: 10.1016/0306-4522(91)90202-y. [DOI] [PubMed] [Google Scholar]
- Heitzeg MM, Nigg JT, Yau WY, Zubieta JK, Zucker RA. Affective circuitry and risk for alcoholism in late adolescence: differences in frontostriatal responses between vulnerable and resilient children of alcoholic parents. Alcoholism, Clinical and Experimental Research. 2008;32(3):414–426. doi: 10.1111/j.1530-0277.2007.00605.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herba CM, Tranah T, Rubia K, Yule W. Conduct problems in adolescence: three domains of inhibition and effect of gender. Developmental Neuropsychology. 2006;30(2):659–695. doi: 10.1207/s15326942dn3002_2. [DOI] [PubMed] [Google Scholar]
- Herman JP, Ostrander MM, Mueller NK, Figueiredo H. Limbic system mechanisms of stress regulation: hypothalamo-pituitary-adrenocortical axis. Biological Psychiatry. 2005;29(8):1201–1213. doi: 10.1016/j.pnpbp.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Hesselbrock V, Bauer L, O'Connor S, Gillen R. Reduced P300 amplitude in relation to family history of alcoholism and antisocial personality disorder among young men at risk for alcoholism. Alcohol and Alcoholism. 1993;(Supplement, 2):95–100. [PubMed] [Google Scholar]
- Hesslinger B, Tebartz van Elst L, Thiel T, Haegele K, Hennig J, Ebert D. Frontoorbital volume reductions in adult patients with attention deficit hyperactivity disorder. Neuroscience Letters. 2002;328:319–321. doi: 10.1016/s0304-3940(02)00554-2. [DOI] [PubMed] [Google Scholar]
- Hibberd C, Yau JL, Seckl JR. Glucocorticoids and the ageing hippocampus. Journal of Anatomy. 2000;197:553–562. doi: 10.1046/j.1469-7580.2000.19740553.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SY. Absence of paternal sociopathy in the etiology of severe alcoholism: is there a type III alcoholism? Journal of Studies Alcohol. 1992;53(2):161–169. doi: 10.15288/jsa.1992.53.161. [DOI] [PubMed] [Google Scholar]
- Hill SY, Muka D. Childhood psychopathology in children from families of alcoholic female probands. Journal of the American Academy of Child and Adolescent Psychiatry. 1996;35(6):725–733. doi: 10.1097/00004583-199606000-00012. [DOI] [PubMed] [Google Scholar]
- Hill SY, Steinhauer SR. Postural sway in children from pedigrees exhibiting a high density of alcoholism. Biological Psychiatry. 1993;33(5):313–325. doi: 10.1016/0006-3223(93)90320-d. [DOI] [PubMed] [Google Scholar]
- Hill SY, Yuan H. Familial density of alcoholism and onset of adolescent drinking. Journal of Studies on Alcohol. 1999;60(1):7–17. doi: 10.15288/jsa.1999.60.7. [DOI] [PubMed] [Google Scholar]
- Hill SY, Shen S. Neurodevelopmental patterns of visual P3b in association with familial risk for alcohol dependence and childhood diagnosis. Biological Psychiatry. 2002;51(8):621–631. doi: 10.1016/s0006-3223(01)01301-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SY, Steinhauer SR, Zubin J. Biological markers for alcoholism: A vulnerability model conceptualization. In: Rivers PC, editor. Alcohol and Addictive Behavior, Nebraska Symposium on Motivation, 1986. University of Nebraska Press; Lincoln and London: 1987a. pp. 207–256. [PubMed] [Google Scholar]
- Hill SY, Armstrong J, Steinhauer SR, Baughman T, Zubin J. Static ataxia as a psychobiological marker for alcoholism. Alcoholism, Clinical and Experimental Research. 1987b;11(4):345–348. doi: 10.1111/j.1530-0277.1987.tb01323.x. [DOI] [PubMed] [Google Scholar]
- Hill SY, Steinhauer SR, Zubin J. Cardiac responsivity in individuals at high risk for alcoholism. Journal of Studies Alcohol. 1992;53(4):378–388. doi: 10.15288/jsa.1992.53.378. [DOI] [PubMed] [Google Scholar]
- Hill SY, Steinhauer S, Lowers L, Locke J. Eight-year longitudinal follow-up of P300 and clinical outcome in children from high-risk for alcoholism families. Biological Psychiatry. 1995;37(11):823–827. doi: 10.1016/0006-3223(95)00041-E. [DOI] [PubMed] [Google Scholar]
- Hill SY, Locke J, Lowers L, Connolly J. Psychopathology and achievement in children at high risk for developing alcoholism. Journal of the American Academy of Child and Adolescent Psychiatry. 1999a;38(7):883–891. doi: 10.1097/00004583-199907000-00019. [DOI] [PubMed] [Google Scholar]
- Hill SY, Lowers L, Locke J, Snidman N, Kagan J. Behavioral inhibition in children from families at high risk for developing alcoholism. Journal of the American Academy of Child and Adolescent Psychiatry. 1999b;38(4):410–417. doi: 10.1097/00004583-199904000-00013. [DOI] [PubMed] [Google Scholar]
- Hill SY, Shen S, Locke J, Steinhauer SR, Konicky C, Lowers L, et al. Developmental delay in P300 production in children at high risk for developing alcohol-related disorders. Biological Psychiatry. 1999c;46(7):970–981. doi: 10.1016/s0006-3223(99)00032-3. [DOI] [PubMed] [Google Scholar]
- Hill SY, Shen S, Lowers L, Locke J. Factors predicting the onset of adolescent drinking in families at high risk for developing alcoholism. Biological Psychiatry. 2000a;48(4):265–275. doi: 10.1016/s0006-3223(00)00841-6. [DOI] [PubMed] [Google Scholar]
- Hill SY, Shen S, Locke J, Lowers L, Steinhauer S, Konicky C. Developmental changes in postural sway in children at high and low risk for developing alcohol-related disorders. Biological Psychiatry. 2000b;47(6):501–511. doi: 10.1016/s0006-3223(99)00175-4. [DOI] [PubMed] [Google Scholar]
- Hill SY, De Bellis MD, Keshavan MS, Lowers L, Shen S, Hall J, et al. Right amygdala volume in adolescent and young adult offspring from families at high risk for developing alcoholism. Biological Psychiatry. 2001;49(11):894–905. doi: 10.1016/s0006-3223(01)01088-5. [DOI] [PubMed] [Google Scholar]
- Hill SY, Shen S, Zezza N, Hoffman EK, Perlin M, Allan W. A genome wide search for alcoholism susceptibility genes. American Journal of Medical Genetics Part B: Neuropsychiatr Genetics. 2004;128B(1):102–113. doi: 10.1002/ajmg.b.30013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SY, Muddasani S, Prasad K, Nutche J, Steinhauer SR, Scanlon J, et al. Cerebellar volume in offspring from multiplex alcohol dependence families. Biological Psychiatry. 2007a;61(1):41–47. doi: 10.1016/j.biopsych.2006.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SY, Kostelnik B, Holmes B, Goradia D, McDermott M, Diwadkar V, et al. fMRI BOLD response to the eyes task in offspring from multiplex alcohol dependence families. Alcoholism, Clinical and Experimental Research. 2007b;31(12):2028–2035. doi: 10.1111/j.1530-0277.2007.00535.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SY, Shen S, Lowers L, Locke-Wellman J, Matthews AG, McDermott M. Psychopathology in offspring from multiplex alcohol dependence families with and without parental alcohol dependence: a prospective study during childhood and adolescence. Psychiatry Research. 2008;160(2):155–166. doi: 10.1016/j.psychres.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SY, Wang S, Kostelnik B, Carter H, Holmes B, McDermott M, et al. Disruption of orbitofrontal cortex laterality in offspring from multiplex alcohol dependence families. Biological Psychiatry. 2009a;65(2):129–136. doi: 10.1016/j.biopsych.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SY, Steinhauer S,R, Locke-Wellman J, Ulrich R. Childhood risk factors for young adult substance dependence in offspring from multiplex alcohol dependence families: A prospective study. 2009b doi: 10.1016/j.biopsych.2009.05.030. doi:10.1016/j.biopsych.2009.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacono WG, McGue M. Association between P3 event-related brain potential amplitude and adolescent problem behavior. Psychophysiology. 2006;43(5):465–469. doi: 10.1111/j.1469-8986.2006.00422.x. [DOI] [PubMed] [Google Scholar]
- Iacono WG, Malone SM, McGue M. Behavioral disinhibition and the development of early-onset addiction: common and specific influences. Annual Review of Clinical Psychology. 2008;4:325–348. doi: 10.1146/annurev.clinpsy.4.022007.141157. [DOI] [PubMed] [Google Scholar]
- Jacobson L, Sapolsky R. The role of the hippocampus in feedback regulation of the hypothalamic—pituitary—adrenocortical axis. Endocrine Reviews. 1991;12:118–134. doi: 10.1210/edrv-12-2-118. [DOI] [PubMed] [Google Scholar]
- Jessor R, Donovan JE, Costa F. Beyond Adolescence: Problem Behavior and Young Adult Development. Cambridge University Press; New York: 1991. [Google Scholar]
- Johnson JL, Leff M. Children of substance abusers: overview of research findings. Pediatrics. 1999;103(5 Pt 2):1085–1099. [PubMed] [Google Scholar]
- Kagan J, Reznick JS, Clarke C, Snidman N, Garcia-Coll C. Behavioral inhibition to the unfamiliar. Child Development. 1984;55:2212–2225. [Google Scholar]
- Kamarajan C, Porjesz B, Jones KA, Choi K, Chorlian DB, Padmanabhapillai A, et al. Alcoholism is a disinhibitory disorder: neurophysiological evidence from a Go/No-Go task. Biological Psychology. 2005;69(3):353–373. doi: 10.1016/j.biopsycho.2004.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamarajan C, Porjesz B, Jones K, Chorlian D, Padmanabhapillai A, Rangaswamy M, et al. Event-related oscillations in offspring of alcoholics: neurocognitive disinhibition as a risk for alcoholism. Biological Psychiatry. 2006;59(7):625–634. doi: 10.1016/j.biopsych.2005.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel DB, Yamaguchi K, Chen K. Stages of progression in drug involvement from adolescence to adulthood: further evidence for the gateway theory. Journal of Studies Alcohol. 1992;53(5):447–457. doi: 10.15288/jsa.1992.53.447. [DOI] [PubMed] [Google Scholar]
- Kellam SG, Ensminger ME, Simon MB. Mental health in first grade and teenage drug, alcohol, and cigarette use. Drug and Alcohol Dependence. 1980;5(4):273–304. doi: 10.1016/0376-8716(80)90003-4. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Neale MC, Heath AC, Kessler RC, Eaves LJ. A twin-family study of alcoholism in women. American Journal of Psychiatry. 1994;151(5):707–715. doi: 10.1176/ajp.151.5.707. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Prescott CA, Neale MC, Pedersen NL. Temperance board registration for alcohol abuse in a national sample of Swedish male twins, born 1902 to 1949. Archives of General Psychiatry. 1997;54(2):178–184. doi: 10.1001/archpsyc.1997.01830140090015. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Schmitt E, Aggen SH, Prescott CA. Genetic and environmental influences on alcohol, caffeine, cannabis, and nicotine use from early adolescence to middle adulthood. Archives of General Psychiatry. 2008;65(6):674–682. doi: 10.1001/archpsyc.65.6.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiefer JC. Epigenetics in development. Developmental Dynamics. 2007;236(4):1144–1156. doi: 10.1002/dvdy.21094. [DOI] [PubMed] [Google Scholar]
- King KM, Chassin L. Mediating and moderated effects of adolescent behavioral undercontrol and parenting in the prediction of drug use disorders in emerging adulthood. Psychology of Addictive Behaviors. 2004;18(3):239–249. doi: 10.1037/0893-164X.18.3.239. [DOI] [PubMed] [Google Scholar]
- King KM, Chassin L. Adolescent stressors, psycho-pathology, and young adult substance dependence: a prospective study. Journal of Studies on Alcohol and Drugs. 2008;69(5):629–638. doi: 10.15288/jsad.2008.69.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King JA, Barkley RA, Barrett S. Attention-deficit hyperactivity disorder and the stress response. Biological Psychiatry. 1998;44(1):72–74. doi: 10.1016/s0006-3223(97)00507-6. [DOI] [PubMed] [Google Scholar]
- King SM, Keyes M, Malone SM, Elkins I, Legrand LN, Iacono WG, McGue M. Parental alcohol dependence and the transmission of adolescent behavioral disinhibition: a study of adoptive and non-adoptive families. Addiction. 2009 Feb 10; doi: 10.1111/j.1360-0443.2008.02469.x. ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimes-Dougan B, Hastings PD, Granger DA, Usher BA, Zahn-Waxler C. Adrenocortical activity in at-risk and normally developing adolescents: individual differences in salivary cortisol basal levels, diurnal variation, and responses to social challenges. Development Psychopathology. 2001;13(3):695–719. doi: 10.1017/s0954579401003157. [DOI] [PubMed] [Google Scholar]
- Knopik VS, Heath AC, Madden PA, Bucholz KK, Slutske WS, Nelson EC, et al. Genetic effects on alcohol dependence risk: re-evaluating the importance of psychiatric and other heritable risk factors. Psychological Medicine. 2004;34(8):1519–1530. doi: 10.1017/s0033291704002922. [DOI] [PubMed] [Google Scholar]
- Koob GF. Hedonic valence, dopamine and motivation. Molecular Psychiatry. 1996;1(3):186–189. [PubMed] [Google Scholar]
- Koob GF. Neuroadaptive mechanisms of addiction: studies on the extended amygdala. European Neuropsychopharmacology. 2003;13(6):442–452. doi: 10.1016/j.euroneuro.2003.08.005. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Addiction and the brain antireward system. Annual Review of Psychology. 2008;59:29–53. doi: 10.1146/annurev.psych.59.103006.093548. [DOI] [PubMed] [Google Scholar]
- Kramer JR, Chan G, Dick DM, Kuperman S, Bucholz KK, Edenberg HJ, et al. Multiple-domain predictors of problematic alcohol use in young adulthood. Journal of Studies on Alcohol and Drugs. 2008;69(5):649–659. doi: 10.15288/jsad.2008.69.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauzlis RJ. Recasting the smooth pursuit eye movement system. Journal of Neurophysiology. 2004;91(2):591–603. doi: 10.1152/jn.00801.2003. [DOI] [PubMed] [Google Scholar]
- Kuo PH, Aggen SH, Prescott CA, Kendler KS, Neale MC. Using a factor mixture modeling approach in alcohol dependence in a general population sample. Drug and Alcohol Dependence. 2008;98(1–2):105–114. doi: 10.1016/j.drugalcdep.2008.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuperman S, Schlosser SS, Lidral J, Reich W. Relationship of child psychopathology to parental alcoholism and antisocial personality disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 1999;38:686–692. doi: 10.1097/00004583-199906000-00015. [DOI] [PubMed] [Google Scholar]
- Kuperman S, Chan G, Kramer JR, Bierut L, Bucholz KK, Fox L, et al. Relationship of age of first drink to child behavioral problems and family psychopathology. Alcoholism, Clinical and Experimental Research. 2005;29(10):1869–1876. doi: 10.1097/01.alc.0000183190.32692.c7. [DOI] [PubMed] [Google Scholar]
- Lipscomb T, Carpenter J, Nathan P. Static ataxia: a predictor of. alcoholism? British Journal of Addiction. 1979;74:289–294. doi: 10.1111/j.1360-0443.1979.tb01350.x. [DOI] [PubMed] [Google Scholar]
- Lipsky RH, Marini AM. Brain-derived neurotrophic factor in neuronal survival and behavior-related plasticity. Annals of the New York Academy of Sciences. 2007;1122:130–143. doi: 10.1196/annals.1403.009. [DOI] [PubMed] [Google Scholar]
- Lovallo WR, Yechiam E, Sorocco KH, Vincent AS, Collins FL. Working memory and decision-making biases in young adults with a family history of alcoholism: studies from the Oklahoma family health patterns project. Alcoholism, Clinical and Experimental Research. 2006;30(5):763–773. doi: 10.1111/j.1530-0277.2006.00089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackie S, Shaw P, Lenroot R, Pierson R, Greenstein DK, Nugent TF, 3rd, et al. Cerebellar development and clinical outcome in attention deficit hyperactivity disorder. American Journal of Psychiatry. 2007;164(4):647–655. doi: 10.1176/ajp.2007.164.4.647. [DOI] [PubMed] [Google Scholar]
- MacLullich AM, Ferguson KJ, Wardlaw JM, Starr JM, Deary IJ, Seckl JR. Smaller left anterior cingulate cortex volumes are associated with impaired hypothalamic—pituitary—adrenal axis regulation in healthy elderly men. Journal of Clinical Endocrinology and Metabolism. 2006;91:1591–1594. doi: 10.1210/jc.2005-2610. [DOI] [PubMed] [Google Scholar]
- Makris N, Oscar-Berman M, Jaffin SK, Hodge SM, Kennedy DN, Caviness VS, et al. Decreased volume of the brain reward system in alcoholism. Biological Psychiatry. 2008;64(3):192–202. doi: 10.1016/j.biopsych.2008.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Man MS, Clarke HF, Roberts AC. The role of the orbitofrontal cortex and medial striatum in the regulation of prepotent responses to food rewards. Cerebral Cortex. 2009;19(4):899–906. doi: 10.1093/cercor/bhn137. [DOI] [PubMed] [Google Scholar]
- Mangelsdorf SC, Schoppe SJ, Buur H. The meaning of parental reports: A contextual approach to the study of temperament and behavior problems in childhood. In: Molfese VJ, Molfese DL, editors. Temperament and Personality Development Across the Lifespan. Erlbaum; London UK: 2000. pp. 121–140. [Google Scholar]
- Mann K, Agartz I, Harper C, Shoaf S, Rawlings RR, Momenan R, et al. Neuroimaging in alcoholism: ethanol and brain damage. Alcoholism, Clinical and Experimental Research. 2001;25:104S–109S. doi: 10.1097/00000374-200105051-00019. [DOI] [PubMed] [Google Scholar]
- Mannuzza S, Klein RG, Bessler A, Malloy P, LaPadula M. Adult outcome of hyperactive boys. Educational achievement, occupational rank, and psychiatric status. Archives of General Psychiatry. 1993;50(7):565–576. doi: 10.1001/archpsyc.1993.01820190067007. [DOI] [PubMed] [Google Scholar]
- Manzardo AM, Penick EC, Knop J, Nickel EJ, Hall S, Jensen P, et al. Developmental differences in childhood motor coordination predict adult alcohol dependence: proposed role for the cerebellum in alcoholism. Alcoholism, Clinical and Experimental Research. 2005;29(3):353–357. doi: 10.1097/01.alc.0000156126.22194.e0. [DOI] [PubMed] [Google Scholar]
- Marshal MP, Molina BS, Pelham WE, Cheong J. Attention-deficit hyperactivity disorder moderates the life stress pathway to alcohol problems in children of alcoholics. Alcoholism, Clinical and Experimental Research. 2007;31(4):564–574. doi: 10.1111/j.1530-0277.2007.00340.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masten AS, Faden VB, Zucker RA, Spear LP. Underage drinking: a developmental framework. Pediatrics. 2008;121(Suppl 4):S235–S251. doi: 10.1542/peds.2007-2243A. [DOI] [PubMed] [Google Scholar]
- Matsuo K, Nicoletti M, Nemoto K, Hatch JP, Peluso MA, Nery FG, Soares JC. A voxel-based morphometry study of frontal gray matter correlates of impulsivity. Hum Brain Mapp. 2008 May 8; doi: 10.1002/hbm.20588. ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBurnett K, Raine A, Stouthamer-Loeber M, Loeber R, Kumar AM, Kumar M, et al. Mood and hormone responses to psychological challenge in adolescent males with conduct problems. Biological Psychiatry. 2005;57(10):1109–1116. doi: 10.1016/j.biopsych.2005.01.041. [DOI] [PubMed] [Google Scholar]
- McGue M, Iacono WG, Legrand LN, Malone S, Elkins I. Origins and consequences of age at first drink. I. Associations with substance-use disorders, disinhibitory behavior and psychopathology, and P3 amplitude. Alcoholism, Clinical and Experimental Research. 2001a;25(8):1156–1165. [PubMed] [Google Scholar]
- McGue M, Iacono WG, Legrand LN, Elkins I. Origins and consequences of age at first drink II. Familial risk and heritability. Alcoholism, Clinical and Experimental Research. 2001b;25(8):1166–1173. [PubMed] [Google Scholar]
- McNamee RL, Dunfee KL, Luna B, Clark DB, Eddy WF, Tarter RE. Brain activation, response inhibition, and increased risk for substance use disorder. Alcoholism, Clinical and Experimental Research. 2008;32(3):405–413. doi: 10.1111/j.1530-0277.2007.00604.x. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, O'Donnell D, Rowe W, Tannenbaum B, Steverman A, Walker M, et al. Individual differences in hypothalamic—pituitary—adrenal activity in later life and hippocampal aging. Experimental Gerontology. 1995;30:229–251. doi: 10.1016/0531-5565(94)00065-b. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Diorio J, Francis D, Widdowson J, LaPlante P, Caldji D, et al. Early environmental regulation of forebrain glucocorticoid receptor gene expression: implications for adrenocortical responses to stress. Developmental Neuroscience. 1996;18:49–72. doi: 10.1159/000111395. [DOI] [PubMed] [Google Scholar]
- Merikangas KR, Stolar M, Stevens DE, Goulet J, Preisig MA, Fenton B, et al. Familial transmission of substance use disorders. Archives of General Psychiatry. 1998;55(11):973–979. doi: 10.1001/archpsyc.55.11.973. [DOI] [PubMed] [Google Scholar]
- Monk CS. The development of emotion-related neural circuitry in health and psychopathology. Development and Psychopathol. 2008;20(4):1231–1250. doi: 10.1017/S095457940800059X. [DOI] [PubMed] [Google Scholar]
- Moos RH, Billings AG. Children of alcoholics during the recovery process: alcoholic and matched control families. Addictive Behaviors. 1982;7(2):155–163. doi: 10.1016/0306-4603(82)90040-5. [DOI] [PubMed] [Google Scholar]
- Moss HB, Vanyukov MM, Martin CS. Salivary cortisol responses and the risk for substance abuse in prepubertal boys. Biological Psychiatry. 1995;38(8):547–555. doi: 10.1016/0006-3223(94)00382-D. [DOI] [PubMed] [Google Scholar]
- Moss HB, Vanyukov M, Yao JK, Kirillova GP. Salivary cortisol responses in prepubertal boys: the effects of parental substance abuse and association with drug use behavior during adolescence. Biological Psychiatry. 1999;45(10):1293–1299. doi: 10.1016/s0006-3223(98)00216-9. [DOI] [PubMed] [Google Scholar]
- Moss HB, Chen CM, Ye HY. Subtypes of alcohol dependence in a nationally representative sample. Drug and Alcohol Dependence. 2007;91:149–158. doi: 10.1016/j.drugalcdep.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostofsky SH, Lasker AG, Cutting LE, Denckla MB, Zee DS. Oculomotor abnormalities in attention deficit hyperactivity disorder: a preliminary study. Neurology. 2001;57(3):423–430. doi: 10.1212/wnl.57.3.423. [DOI] [PubMed] [Google Scholar]
- Nagai M, Kishi K, Kato S. Insular cortex and neuropsychiatric disorders: a review of recent literature. Europian Psychiatry. 2007;22(6):387–394. doi: 10.1016/j.eurpsy.2007.02.006. [DOI] [PubMed] [Google Scholar]
- Nelson JC, Davis JM. DST studies in psychotic depression: A meta-analysis. American Journal of Psychiatry. 1997;154:1497–1503. doi: 10.1176/ajp.154.11.1497. [DOI] [PubMed] [Google Scholar]
- Nemoto K, Ohnishi T, Mori T, Moriguchi Y, Hashimoto R, Asada T, et al. The Val66Met polymorphism of the brain-derived neurotrophic factor gene affects age-related brain morphology. Neuroscience Letters. 2006;397(1–2):25–29. doi: 10.1016/j.neulet.2005.11.067. [DOI] [PubMed] [Google Scholar]
- Newcomb MD, Bentler PM. Substance use and abuse among children and teenagers. American Psychologist. 1989;44(2):242–248. doi: 10.1037//0003-066x.44.2.242. [DOI] [PubMed] [Google Scholar]
- Nigg JT, Casey BJ. An integrative theory of attention-deficit/ hypractivity disorder based on the cognitive and affective neurosciences. Development and Psychopathol. 2005;17(3):785–806. doi: 10.1017/S0954579405050376. [DOI] [PubMed] [Google Scholar]
- Nigg JT, Glass JM, Wong MM, Poon E, Jester JM, Fitzgerald HE, et al. Neuropsychological executive functioning in children at elevated risk for alcoholism: findings in early adolescence. Journal of Abnormal Psychology. 2004;113(2):302–314. doi: 10.1037/0021-843X.113.2.302. [DOI] [PubMed] [Google Scholar]
- Nock MK, Kazdin AE, Hiripi E, Kessler RC. Prevalence, subtypes, and correlates of DSM-IV conduct disorder in the National Comorbidity Survey Replication. Psychological Medicine. 2006;36(5):699–710. doi: 10.1017/S0033291706007082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nock MK, Kazdin AE, Hiripi E, Kessler RC. Lifetime prevalence, correlates, and persistence of oppositional defiant disorder: results from the National Comorbidity Survey Replication. Journal of Child Psychology and Psychiatry. 2007;48(7):703–713. doi: 10.1111/j.1469-7610.2007.01733.x. [DOI] [PubMed] [Google Scholar]
- Ohannessian CM, Hesselbrock VM. Temperament and personality typologies in adult offspring of alcoholics. Journal of Studies on Alcohol. 1995;56(3):318–327. doi: 10.15288/jsa.1995.56.318. [DOI] [PubMed] [Google Scholar]
- Ohannessian CM, Stabenau JR, Hesselbrock VM. Childhood and adulthood temperament and problem behaviors and adulthood substance use. Addictive Behaviors. 1995;20(1):77–86. doi: 10.1016/0306-4603(94)00047-3. [DOI] [PubMed] [Google Scholar]
- Ohannessian CM, Hesselbrock VM, Kramer J, Kuperman S, Bucholz KK, Schuckit MA, et al. The relationship between parental alcoholism and adolescent psychopathology: a systematic examination of parental comorbid psychopathology. Journal of Abnormal Psychology. 2004;32:519–533. doi: 10.1023/b:jacp.0000037781.49155.a6. [DOI] [PubMed] [Google Scholar]
- O'Leary MM, Loney BR, Eckel LA. Gender differences in the association between psychopathic personality traits and cortisol response to induced stress. Psychoneuroendocrinology. 2007;32(2):183–191. doi: 10.1016/j.psyneuen.2006.12.004. [DOI] [PubMed] [Google Scholar]
- Oscar-Berman MM, Marinkovic K. Alcohol: effects on neurobehavioral functions and the brain. Neuropsychology Review. 2007;17(3):239–257. doi: 10.1007/s11065-007-9038-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozkaragoz T, Satz P, Noble EP. Neuropsychological functioning in sons of active alcoholic, recovering alcoholic, and social drinking fathers. Alcohol. 1997;14(1):31–37. doi: 10.1016/s0741-8329(96)00084-5. [DOI] [PubMed] [Google Scholar]
- Padmanabhapillai A, Tang Y, Ranganathan M, Rangaswamy M, Jones KA, Chorlian DB, et al. Evoked gamma band response in male adolescent subjects at high risk for alcoholism during a visual oddball task. International Journal of Psychophysiology. 2006;62(2):262–271. doi: 10.1016/j.ijpsycho.2006.05.012. [DOI] [PubMed] [Google Scholar]
- Pagan JL, Rose RJ, Viken RJ, Pulkkinen L, Kaprio J, Dick DM. Genetic and environmental influences on stages of alcohol use across adolescence and into young adulthood. Behavior Genetics. 2006;36(4):483–497. doi: 10.1007/s10519-006-9062-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pajer K, Chung J, Leininger L, Wang W, Gardner W, Yeates K. Neuropsychological function in adolescent girls with conduct disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2008;47(4):416–425. doi: 10.1097/CHI.0b013e3181640828. [DOI] [PubMed] [Google Scholar]
- Pariante CM, Lightman SL. The HPA axis in major depression: classical theories and new developments. Trends in Neurosciences. 2008;31(9):464–468. doi: 10.1016/j.tins.2008.06.006. [DOI] [PubMed] [Google Scholar]
- Paus T. Primate anterior cingulate cortex: where motor control, drive and cognition interface. Nature Reviews Neuroscience. 2001;2(6):417–424. doi: 10.1038/35077500. [DOI] [PubMed] [Google Scholar]
- Peper JS, Brouwer RM, Boomsma DI, Kahn RS, Hulshoff Pol HE. Genetic influences on human brain structure: a review of brain imaging studies in twins. Human Brain Mapping. 2007;28(6):464–473. doi: 10.1002/hbm.20398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson JB, Finn PR, Pihl RO. Cognitive dysfunction and the inherited predisposition to alcoholism. Journal of Studies on Alcohol. 1992;53(2):154–160. doi: 10.15288/jsa.1992.53.154. [DOI] [PubMed] [Google Scholar]
- Peterson JB, Pihl RO, Séguin JR, Finn PR, Stewart SH. Heart-rate reactivity and alcohol consumption among sons of male alcoholics and sons of non-alcoholics. Journal of Psychiatry & Neuroscience. 1993;18(4):190–198. [PMC free article] [PubMed] [Google Scholar]
- Peterson JB, Pihl RO, Gianoulakis C, Conrod P, Finn PR, Stewart SH, et al. Ethanol-induced change in cardiac and endogenous opiate function and risk for alcoholism. Alcoholism, Clinical and Experimental Research. 1996;20(9):1542–1552. doi: 10.1111/j.1530-0277.1996.tb01697.x. [DOI] [PubMed] [Google Scholar]
- Pihl RO, Peterson J, Finn P. Inherited predisposition to alcoholism: characteristics of sons of male alcoholics. Journal of Abnormal Psychology. 1990;99(3):291–301. doi: 10.1037//0021-843x.99.3.291. [DOI] [PubMed] [Google Scholar]
- Poon E, Ellis DA, Fitzgerald HE, Zucker RA. Intellectual, cognitive, and academic performance among sons of alcoholics, during the early school years: differences related to subtypes of familial alcoholism. Alcoholism, Clinical and Experimental Research. 2000;24(7):1020–1027. [PubMed] [Google Scholar]
- Porjesz B, Rangaswamy M. Neurophysiological endophenotypes, CNS disinhibition, and risk for alcohol dependence and related disorders. ScientificWorldJournal. 2007;7:131–141. doi: 10.1100/tsw.2007.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porjesz B, Begleiter H, Reich T, Van Eerdewegh P, Edenberg HJ, Foroud T, et al. Amplitude of visual P3 event-related potential as a phenotypic marker for a predisposition to alcoholism: preliminary results from the COGA Project Collaborative Study on the Genetics of Alcoholism. Alcoholism, Clinical and Experimental Research. 1998;22(6):1317–1323. [PubMed] [Google Scholar]
- Porjesz B, Rangaswamy M, Kamarajan C, Jones KA, Padmanabhapillai A, Begleiter H. The utility of neurophysiological markers in the study of alcoholism. Clinical Neurophysiology. 2005;116(5):993–1018. doi: 10.1016/j.clinph.2004.12.016. [DOI] [PubMed] [Google Scholar]
- Puig-Antich J, Goetz D, Davies M, Kaplan T. A controlled family history study of prepubertal major depressive disorder. Archives of General Psychiatry. 1989;46:406–418. doi: 10.1001/archpsyc.1989.01810050020005. [DOI] [PubMed] [Google Scholar]
- Rangaswamy M, Jones KA, Porjesz B, Chorlian DB, Padmanabhapillai A, Kamarajan C, et al. Delta and theta oscillations as risk markers in adolescent offspring of alcoholics. International Journal of Psychophysiology. 2007;63(1):3–15. doi: 10.1016/j.ijpsycho.2006.10.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich W, Earls F, Frankel O, Shayka JJ. Psychopathology in children of alcoholics. Journal of the American Academy of Child and Adolescent Psychiatry. 1993;32(5):995–1002. doi: 10.1097/00004583-199309000-00017. [DOI] [PubMed] [Google Scholar]
- Reich T, Edenberg HJ, Goate A, Williams JT, Rice JP, Van Eerdewegh P, et al. Genome-wide search for genes affecting the risk for alcohol dependence. American Journal of Medical Genetics. 1998;81(3):207–215. [PubMed] [Google Scholar]
- Rhee SH, Hewitt JK, Young SE, Corley RP, Crowley TJ, Stallings MC. Genetic and environmental influences on substance initiation, use, and problem use in adolescents. Archives of General Psychiatry. 2003;60(12):1256–1264. doi: 10.1001/archpsyc.60.12.1256. [DOI] [PubMed] [Google Scholar]
- Rommelse NN, Van der Stigchel S, Witlox J, Geldof C, Deijen JB, Theeuwes J, et al. Deficits in visuo-spatial working memory, inhibition and oculomotor control in boys with ADHD and their non-affected brothers. Journal of Neural Transmission. 2008;115(2):249–260. doi: 10.1007/s00702-007-0865-7. [DOI] [PubMed] [Google Scholar]
- Rose RJ, Dick DM, Viken RJ, Pulkkinen L, Kaprio J. Drinking or abstaining at age 14? A genetic epidemio-logical study. Alcoholism, Clinical and Experimental Research. 2001;25(11):1594–1604. [PubMed] [Google Scholar]
- Rose RJ, Dick DM, Viken RJ, Pulkkinen L, Kaprio J. Genetic and environmental effects on conduct disorder and alcohol dependence symptoms and their covariation at age 14. Alcoholism, Clinical and Experimental Research. 2004;28(10):1541–1548. doi: 10.1097/01.alc.0000141822.36776.55. [DOI] [PubMed] [Google Scholar]
- Rosenbaum JF, Biederman J, Hirshfeld DR, Bolduc EA, Faraone SV, Kagan J, et al. Further evidence of an association between behavioral inhibition and anxiety disorders: results from a family study of children from a non-clinical sample. Journal of Psychiatric Research. 1991;25:49–65. doi: 10.1016/0022-3956(91)90015-3. [DOI] [PubMed] [Google Scholar]
- Ross RG, Hommer D, Breiger D, Varley C, Radant A. Eye movement task related to frontal lobe functioning in children with attention deficit disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 1994;33(6):869–874. doi: 10.1097/00004583-199407000-00013. [DOI] [PubMed] [Google Scholar]
- Rothman EF, Edwards EM, Heeren T, Hingson RW. Adverse childhood experiences predict earlier age of drinking onset: results from a representative US sample of current or former drinkers. Pediatrics. 2008;122(2):e298–e304. doi: 10.1542/peds.2007-3412. [DOI] [PubMed] [Google Scholar]
- Rubia K, Taylor E, Smith AB, Oksanen H, Overmeyer S, Newman S. Neuropsychological analyses of impulsiveness in childhood hyperactivity. British Journal of Psychiatry. 2001;179:138–143. doi: 10.1192/bjp.179.2.138. [DOI] [PubMed] [Google Scholar]
- Rubia K, Smith AB, Halari R, Matsukura F, Mohammad M, Taylor E, et al. Disorder-Specific dissociation of orbitofrontal dysfunction in boys with pure conduct disorder during reward and ventrolateral prefrontal dysfunction in boys with pure ADHD during sustained attention. American Journal of Psychiatry. 2008;166(1):83–94. doi: 10.1176/appi.ajp.2008.08020212. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Giles DE, Schlesser MA, Orsulak PJ, Parker CR, Weissenburger JE, et al. The dexamethasone suppression test in patients with mood disorders. Journal of Clinical Psychiatry. 1996;57(10):470–484. doi: 10.4088/jcp.v57n1006. [DOI] [PubMed] [Google Scholar]
- Sartor CE, Lynskey MT, Heath AC, Jacob T, True W. The role of childhood risk factors in initiation of alcohol use and progression to alcohol dependence. Addiction. 2007;102(2):216–225. doi: 10.1111/j.1360-0443.2006.01661.x. [DOI] [PubMed] [Google Scholar]
- Saunders B, Farag N, Vincent AS, Collins FL, Jr., Sorocco KH, Lovallo WR. Impulsive errors on a Go-NoGo reaction time task: disinhibitory traits in relation to a family history of alcoholism. Alcoholism, Clinical and Experimental Research. 2008;32(5):888–894. doi: 10.1111/j.1530-0277.2008.00648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmahmann JD, Pandya DN. The cerebrocerebellar system. International Review of Neurobiology. 1997;41:31–60. doi: 10.1016/s0074-7742(08)60346-3. [DOI] [PubMed] [Google Scholar]
- Schmidt LA, Fox NA, Rubin KH, Sternberg EM, Gold PW, Smith CC, et al. Behavioral and neuroendocrine responses in shy children. Developmental Psychobiology. 1997;30(2):127–140. doi: 10.1002/(sici)1098-2302(199703)30:2<127::aid-dev4>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Schuckit MA. Low level of response to alcohol as a predictor of future alcoholism. American Journal of Psychiatry. 1994;151(2):184–189. doi: 10.1176/ajp.151.2.184. [DOI] [PubMed] [Google Scholar]
- Schuckit MA. Biological, psychological and environmental predictors of the alcoholism risk: a longitudinal study. Journal of Studies on Alcohol. 1998;59(5):485–494. doi: 10.15288/jsa.1998.59.485. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL. Assessing the risk for alcoholism among sons of alcoholics. Journal of Studies on Alcohol. 1997;58(2):141–145. doi: 10.15288/jsa.1997.58.141. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Tsuang JW, Anthenelli RM, Tipp JE, Nurnberger JI., Jr. Alcohol challenges in young men from alcoholic pedigrees and control families: a report from the COGA project. Journal of Studies on Alcohol. 1996;57(4):368–377. doi: 10.15288/jsa.1996.57.368. [DOI] [PubMed] [Google Scholar]
- Schwartz CE, Snidman N, Kagan J. Adolescent social anxiety as an outcome of inhibited temperament in childhood. Journal of the American Academy of Child and Adolescent Psychiatry. 1999;38:1008–1015. doi: 10.1097/00004583-199908000-00017. [DOI] [PubMed] [Google Scholar]
- Schweinsburg AD, Paulus MP, Barlett VC, Killeen LA, Caldwell LC, Pulido C, et al. An FMRI study of response inhibition in youths with a family history of alcoholism. Annals of the New York Academy of Sciences. 2004;1021:391–394. doi: 10.1196/annals.1308.050. [DOI] [PubMed] [Google Scholar]
- Seitz RJ, Stephan KM, Binkofski F. Control of action as mediated by the human frontal lobe. Experimental Brain Research. 2000;133(1):71–80. doi: 10.1007/s002210000402. [DOI] [PubMed] [Google Scholar]
- Sher KJ. Children of alcoholics : a critical appraisal of theory and research. University of Chicago Press; Chicago: 1991. [Google Scholar]
- Sher KJ, Trull TJ. Personality and disinhibitory psychopathology: alcoholism and antisocial personality disorder. Journal of Abnormal Psychology. 1994;103:92–102. doi: 10.1037//0021-843x.103.1.92. [DOI] [PubMed] [Google Scholar]
- Sher KJ, Walitzer KS, Wood PK, Brent EE. Characteristics of children of alcoholics: putative risk factors, substance use and abuse, and psychopathology. Journal of Abnormal Psychology. 1991;100(4):427–448. doi: 10.1037//0021-843x.100.4.427. [DOI] [PubMed] [Google Scholar]
- Shin DW, Lee SH. Blunted hypothalamo-pituitary-adrenal axis reactivity is associated with the poor intelligence performance in children with attention-deficit/hyperactivity disorder. Neuropediatrics. 2007;38(6):298–303. doi: 10.1055/s-2008-1062717. [DOI] [PubMed] [Google Scholar]
- Shirtcliff EA, Granger DA, Booth A, Johnson D. Low salivary cortisol levels and externalizing behavior problems in youth. Development and Psychopathol. 2005;17(1):167–184. doi: 10.1017/s0954579405050091. [DOI] [PubMed] [Google Scholar]
- Silveri MM, McCaffrey A, Rogowska J, Yurgelun-Todd DA. Frontolimbic responses in adolescents at risk for substance abuse: FMRI brain activation during Stroop performance and the viewing of fearful affect. Alcoholism, Clinical and Experimental Research. 2009;33(6):276A. doi: 10.1111/j.1530-0277.2010.01337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorocco KH, Lovallo WR, Vincent AS, Collins FL. Blunted hypothalamic-pituitary-adrenocortical axis responsivity to stress in persons with a family history of alcoholism. International Journal of Psychophysiology. 2006;59(3):210–217. doi: 10.1016/j.ijpsycho.2005.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spadoni AD, McGee CL, Fryer SL, Riley EP. Neuroimaging and fetal alcohol spectrum disorders. Neuroscience and Biobehavioral Reviews. 2007;31(2):239–245. doi: 10.1016/j.neubiorev.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spadoni AD, Norman AL, Schweinsburg AD, Tapert SF. Effects of family history of alcohol use disorders on spatial working memory BOLD response in adolescents. Alcoholism, Clinical and Experimental Research. 2008;32(7):1135–1145. doi: 10.1111/j.1530-0277.2008.00694.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stansbury K, Gunnar MR. Adrenocortical activity and emotion regulation. Monographs of the Society for Research in Child Development. 1994;59(2–3):108–134. [PubMed] [Google Scholar]
- Starkman M, Giordani B, Berent S, Schork M, Schteingart D. Elevated cortisol levels in Cushing's Disease are associated with cognitive decrements. Psychosomatic Medicine. 2001;63:985–993. doi: 10.1097/00006842-200111000-00018. [DOI] [PubMed] [Google Scholar]
- Steinhausen HC, Göbel D, Nestler V. Psychopathology in the offspring of alcoholic parents. Journal of the American Academy of Child Psychiatry. 1984;23(4):465–471. doi: 10.1016/s0002-7138(09)60326-5. [DOI] [PubMed] [Google Scholar]
- Steinlin M. Cerebellar disorders in childhood: cognitive problems. Cerebellum. 2008;7(4):607–610. doi: 10.1007/s12311-008-0083-3. [DOI] [PubMed] [Google Scholar]
- Stevens MC, Kaplan RF, Hesselbrock VM. Executive-cognitive functioning in the development of antisocial personality disorder. Addictive Behaviors. 2003;28(2):285–300. doi: 10.1016/s0306-4603(01)00232-5. [DOI] [PubMed] [Google Scholar]
- Stewart SH, Finn PR, Pihl RO. The effects of alcohol on the cardiovascular stress response in men at high risk for alcoholism: a dose response study. Journal of Studies on Alcohol. 1992;53(5):499–506. doi: 10.15288/jsa.1992.53.499. [DOI] [PubMed] [Google Scholar]
- Stice E, Barrera M, Jr., Chassin L. Prospective differential prediction of adolescent alcohol use and problem use:examining the mechanisms of effect. Journal of Abnormal Psychology. 1998;107(4):616–628. doi: 10.1037//0021-843x.107.4.616. [DOI] [PubMed] [Google Scholar]
- Strick PL, Dum RP, Fiez JA. Cerebellum and nonmotor function. Annual Review of Neuroscience. 2009;32:413–434. doi: 10.1146/annurev.neuro.31.060407.125606. [DOI] [PubMed] [Google Scholar]
- Sullivan EV. Compromised pontocerebellar and cerebello-thalamocortical systems: speculations on their contributions to cognitive and motor impairment in nonamnesic alcoholism. Alcoholism, Clinical and Experimental Research. 2003;27(9):1409–1419. doi: 10.1097/01.ALC.0000085586.91726.46. [DOI] [PubMed] [Google Scholar]
- Sullivan RM, Gratton A. Prefrontal cortical regulation of hypothalamic—pituitary—adrenal function in the rat and implications for psychopathology: side matters. Psychoneuroendocrinology. 2002;27:99–114. doi: 10.1016/s0306-4530(01)00038-5. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Pfefferbaum A. Neurocircuitry in alcoholism: a substrate of disruption and repair. Psychopharmacology (Berl) 2005;180(4):583–594. doi: 10.1007/s00213-005-2267-6. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Harding AJ, Pentney R, Dlugos C, Martin PR, Parks MH, et al. Disruption of frontocerebellar circuitry and function in alcoholism. Alcoholism, Clinical and Experimental Research. 2003;27(2):301–309. doi: 10.1097/01.ALC.0000052584.05305.98. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Rose J, Pfefferbaum A. Effect of vision, touch and stance on cerebellar vermian-related sway and tremor: a quantitative physiological and MRI study. Cerebral Cortex. 2006;16(8):1077–1086. doi: 10.1093/cercor/bhj048. [DOI] [PubMed] [Google Scholar]
- Susman EJ. Psychobiology of persistent antisocial behavior: stress, early vulnerabilities and the attenuation hypothesis. Neuroscience and Biobehavioral Reviews. 2006;30(3):376–389. doi: 10.1016/j.neubiorev.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Tan HY, Callicott JH, Weinberger DR. Intermediate phenotypes in schizophrenia genetics redux: is it a no brainer? Molecular Psychiatry. 2008;13(3):233–238. doi: 10.1038/sj.mp.4002145. [DOI] [PubMed] [Google Scholar]
- Tanji J, Hoshi E. Behavioral planning in the prefrontal cortex. Current Opinion in Neurobiology. 2001;11(2):164–170. doi: 10.1016/s0959-4388(00)00192-6. [DOI] [PubMed] [Google Scholar]
- Tanji J, Hoshi E. Role of the lateral prefrontal cortex in executive behavioral control. Physiological Reviews. 2008;88(1):37–57. doi: 10.1152/physrev.00014.2007. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Brown SA. Substance dependence, family history of alcohol dependence and neuropsychological functioning in adolescence. Addiction. 2000;95(7):1043–1053. doi: 10.1046/j.1360-0443.2000.95710436.x. [DOI] [PubMed] [Google Scholar]
- Tarter RE, Kirisci L, Mezzich A, Cornelius JR, Pajer K, Vanyukov M, et al. Neurobehavioral disinhibition in childhood predicts early age at onset of substance use disorder. American Journal of Psychiatry. 2003;160(6):1078–1085. doi: 10.1176/appi.ajp.160.6.1078. [DOI] [PubMed] [Google Scholar]
- Tarter RE, Kirisci L, Reynolds M, Mezzich A. Neurobehavior disinhibition in childhood predicts suicide potential and substance use disorder by young adulthood. Drug and Alcohol Dependence. 2004;76(Suppl):S45–S52. doi: 10.1016/j.drugalcdep.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Tessner KD, Walker EF, Dhruv S, Hochman K, Hamann S. The relation of cortisol levels with hippocampus volumes under baseline and challenge conditions. Brain Research. 2007;1179:70–78. doi: 10.1016/j.brainres.2007.05.027. [DOI] [PubMed] [Google Scholar]
- Thier P, Ilg UJ. The neural basis of smooth-pursuit eye movements. Current Opinion in Neurobiology. 2005;15(6):645–652. doi: 10.1016/j.conb.2005.10.013. [DOI] [PubMed] [Google Scholar]
- Tranel D, Damasio H, Denburg NL, Bechara A. Does gender play a role in functional asymmetry of ventromedial prefrontal cortex? Brain. 2005;128:2872–2881. doi: 10.1093/brain/awh643. [DOI] [PubMed] [Google Scholar]
- Uhart M, Oswald L, McCaul ME, Chong R, Wand GS. Hormonal responses to psychological stress and family history of alcoholism. Neuropsychopharmacology. 2006;31(10):2255–2263. doi: 10.1038/sj.npp.1301063. [DOI] [PubMed] [Google Scholar]
- Vaglum S, Vaglum P, Larsen O. Depression and alcohol consumption in non alcoholic and alcoholic women. A clinical study. Acta Psychiatrica Scand. 1987;75(6):577–584. doi: 10.1111/j.1600-0447.1987.tb02838.x. [DOI] [PubMed] [Google Scholar]
- Waldrop AE, Ana EJ, Saladin ME, McRae AL, Brady KT. Differences in early onset alcohol use and heavy drinking among persons with childhood and adulthood trauma. American Journal on Addictions. 2007;16(6):439–442. doi: 10.1080/10550490701643484. [DOI] [PubMed] [Google Scholar]
- Walker E, Mittal V, Tessner K. Stress and the hypothalamic pituitary adrenal axis in the developmental course of schizophrenia. Annual Reveiw of Clinical Psychology. 2008;4:189–216. doi: 10.1146/annurev.clinpsy.4.022007.141248. [DOI] [PubMed] [Google Scholar]
- Weinhold B. Epigenetics: the science of change. Environmental Health Perspectives. 2006;3:A160–A167. doi: 10.1289/ehp.114-a160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesner RB. Alcohol use and abuse secondary to anxiety. Psychiatric Clinics of North America. 1990;13(4):699–713. [PubMed] [Google Scholar]
- Wiedenmayer CP, Bansal R, Anderson GM, Zhu H, Amat J, Whiteman R, et al. Cortisol levels and hippocampus volumes in healthy preadolescent children. Biological Psychiatry. 2006;60:856–861. doi: 10.1016/j.biopsych.2006.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiers RW, Gunning WB, Sergeant JA. Is a mild deficit in executive functions in boys related to childhood ADHD or to parental multigenerational alcoholism? Journal of Abnormal Child Psychology. 1998;26(6):415–430. doi: 10.1023/a:1022643617017. [DOI] [PubMed] [Google Scholar]
- Williams JT, Begleiter H, Porjesz B, Edenberg HJ, Foroud T, Reich T, et al. Joint multipoint linkage analysis of multivariate qualitative and quantitative traits II. Alcoholism and event-related potentials. American Journal of Human Genetics. 1999;65(4):1148–1160. doi: 10.1086/302571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windle M. Temperamental inhibition and activation: Hormonal and psychosocial correlates and associated psychiatric disorders. Personality and Individual Differences. 1994;17:61–70. [Google Scholar]
- Zimmermann U, Spring K, Kunz-Ebrecht SR, Uhr M, Wittchen HU, Holsboer F. Effect of ethanol on hypothalamicpituitary-adrenal system response to psychosocial stress in sons of alcohol-dependent fathers. Neuropsychopharmacology. 2004;29(6):1156–1165. doi: 10.1038/sj.npp.1300395. [DOI] [PubMed] [Google Scholar]
- Zucker RA. Anticipating problem alcohol use developmentally from childhood into middle adulthood: what have we learned? Addiction. 2008;103(Suppl 1):100–108. doi: 10.1111/j.1360-0443.2008.02179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


