Abstract
Dicer, an enzyme involved in microRNA maturation, is required for proper embryo gastrulation and tissue morphogenesis during mammalian development. Using primary cultures of fibroblasts and pre-adipocytes, we have previously shown that Dicer is essential for early stages of adipogenic cell differentiation. In this present study, we have utilized Dicer-conditional mice to explore a role for Dicer and microRNA biogenesis in the terminal differentiation of adipocytes in vivo and in the formation of white and brown adipose tissue. Deletion of Dicer in differentiated adipocytes in Dicer-conditional, aP2-Cre transgenic mice reduced the level of various adipogenic-associated transcripts and inhibited lipogenesis in white adipocytes, resulting in a severe depletion of white adipose tissue in mice. In contrast, Dicer was not required in vivo for lipogenesis in brown adipose or for brown fat formation. However, Dicer deletion in brown adipose did decrease the expression of genes involved in thermoregulation. The results of our study provide genetic evidence of a role for microRNA molecules in regulating adipogenesis and reveal distinct requirements for Dicer in the formation of white and brown adipose tissue.
Keywords: Dicer, microRNA, adipocyte, white adipose, brown adipose
INTRODUCTION
Mesenchymal precursor cells can differentiate into either white or brown adipocytes during mammalian development to form either white adipose tissue (WAT) or brown adipose tissue (BAT) (Park, 2008; Lefterova and Lazar, 2009; Seale et al, 2009). White adipocytes secrete hormones such as leptin to regulate food intake and whole body metabolism, whereas brown adipocytes help regulate body temperature by expressing uncoupling protein 1 (UCP-1) to disassociate oxidative phosphorylation and mitochondrial electron transport from ATP production (Seale et al, 2009; Nedergaard et al, 2005; Wijers et al, 2009). Although both white and brown adipocytes accumulate and release fatty acids, brown adipocytes contain less overall lipids than white adipocytes and are rich in large mitochondria that give these cells their characteristic brown color (Cannon and Nedergaard, 2004). Brown adipocytes are scattered throughout WAT in rodents, but these mammals also retain large postnatal deposits of BAT in the anterior intrascapular region (Cannon and Nedergaard, 2004). In humans, brown fat surrounds the heart and major vessels at birth, and recent evidence indicates that mature brown adipocytes are also present the upper regions of the adult torso (Nedergaard et al, 2007; Cypess et al, 2009; vanMarken et al, 2009).
A temporal cascade of adipogenic transcription factors governs differentiation of mesenchymal cells into mature white or brown adipocytes. Transcription factors important in promoting adipogenesis include the peroxisome proliferator-activated receptor-γ (PPARγ, members of the CCAAT enhancer-binding protein (C/EBP) family, Kruppel-like factor (KLF) family members, and sterol regulatory element binding proteins (SREBPs).(Lefterova and Lazar, 2009; Seale et al, 2009; Rosen and MacDougald, 2006; Brey et al, 2009; White and Stephens, 2010). In addition, PRDM16 was recently identified as a transcription factor selectively expressed in brown adipose that is required for brown adipose development (Seale et al, 2007; Kajimura et al, 2008).
In addition to transcription factors, microRNA molecules have also been implicated as important regulators of adipogenic differentiation. MicroRNAs (miRNAs) are small, single-stranded, non-coding RNA molecules that are transcribed in the nucleus, processed and exported into the cytoplasm, and matured by a final cleavage by a ribonuclease III-like cellular enzyme known as Dicer. The mature 20–24 nucleotide miRNAs are then incorporated into the RNA-induced silencing complex (RISC) and target the RISC to the 3′untranslated region of cognate messenger RNAs. This binding inhibits translation of targeted mRNAs, resulting in reduced levels of the mRNA-encoded protein (Bartel, 2009; Bushati and Cohen, 2007; Valencia-Sanchez et al, 2006; Ghildiyal and Zamore, 2009).
Recently, numerous studies employing cell lines and primary cells from human and mouse have demonstrated the differential expression of select miRNAs during adipocyte differentiation (Gerin et al, 2010; Kim et al, 2010; Huang et al, 2010; Karbiener et al, 2009; Sun et al, 2009; Xie et al, 2009; Kennell et al, 2008; Qin et al, 2010; Wang et al, 2008; Kajimoto et al, 2006; Kim et al, 2009; Esau et al, 2004). In general, data from these in vitro studies indicate that increased expression or inhibition of certain miRNAs either inhibited or accelerated adipogenic differentiation. We have also recently demonstrated that loss of Dicer activity and miRNA biogenesis in primary cultures of fibroblasts and pre-adipocytes inhibits PPARγ expression and adipocyte differentiation independent of the ability of miRNAs to regulate cell growth (Mudhasani et al, 2010). Collectively, these reports highlight an important role for miRNA molecules in regulating the early stages of pre-adipocyte to adipocyte differentiation. However, little is known regarding a role for Dicer or miRNAs in the terminal differentiation of adipocytes or in the formation of adipose tissues in vivo. Moreover, a role for miRNA in regulating brown adipocyte differentiation is unexplored. To directly assess in vivo the requirement for miRNAs in terminal adipocyte differentiation and in the formation of WAT and BAT, we crossed mice bearing Dicer-conditional alleles (Mudhasani et al, 2008) with aP2-Cre transgenic mice containing the Cre recombinase gene placed under transcriptional control of the adipogenic fatty acid binding protein (Fabp) promoter (He et al, 2003). The resulting Dicer-conditional, aP2-Cre mice undergo loss of Dicer function in post-differentiated adipocytes. Analysis of aP2-Cre, Dicer-conditional mice reveals that Dicer is required in post-differentiated adipocytes to generate WAT but not BAT, and that miRNA biogenesis plays selective roles in vivo in the function of white and brown adipose tissue.
MATERIALS AND METHODS
Mice and tissue work
Dicer-conditional (Dicerc/c) mice have been described previously (32). The aP2-Cre transgenic mouse model (B6.Cg-Tg(Fabp4-cre)1Rev/J) (He et al, 2003) was obtained from the Jackson Laboratory (stock number 005069). Tissue fixing, sectioning, and hematoxylin and eosin (H&E) staining was performed by the UMMS DERC Morphology Core. Oil Red O (ORO) staining and Masson’s trichome staining of adipose tissues were performed as described (43). Scanning electron microscopy was performed by the UMMS Electron Microscopy Core. All mice were maintained and used in accordance with federal guidelines and those set by the University of Massachusetts Animal Care and Use Committee.
RNA Isolation and Analysis
Total RNA from cultured cells or tissue samples was isolated using Trizol method (Invitrogen). For real-time PCR analysis, RNA was reverse transcribed using the Superscript II reverse transcriptase enzyme (Invitrogen) and used in quantitative PCR reactions (Qiagen master mix containing SYBR-green fluorescent dye [ABI]). Relative expression of mRNAs was determined after normalization with 36B4 levels using the ΔΔ-Ct method. Q-PCR was performed using the ABI-9300 PCR machine. Quantitative PCR (q-PCR) analysis for miRNA levels was done using mirVana qRT-PCR miRNA detection kit (Ambion) Data for let-7a miRNA was normalized with U6 expression levels. Primers used for PCR reactions are as follows: PPARγ2 (f) – gcatggtgccttcgctgatgc, PPARγ2 (r) – aggcctgttgtagagctgggt, SREBP1 (f) – gatgtgcgaactggacacag, SREBP1 (r) – catagggggcgtcaaacag, PGC-1α (f) – gaagtggtgtagcgaccaatc, PGC-1α (r) – aatgagggcaatccgtcttca, aP2(f) – aaggtgaagagcatcatcaccct, aP2 (r) – tcacgcsctttcataacacattcc, FAS (f) – ggaggtggtgatagccggtat, FAS (r) – tgggtaatccatagagcccag, Glut4 (f) – gtgactggaacactggtccta, Glut4 (r) – ccagccacgttgcattgtag, Cox8b (f) – gaaccatgaagccaacgact, Cox8b (r) – gcgaagttcacagtggttcc, UCP-1 (f) – ccgaaactgtacagcggtct, UCP-1(r) – taagccggctgagatcttgt, Cidea (f) – tgctcttctgtatcgcccagt, Cidea (r) – gccgtgttaaggaatctgctg, PDRM16 (f) – cagcacggtgaagccattc, PDRM16 (r) – gcgtgcatccgcttgtg.
RESULTS
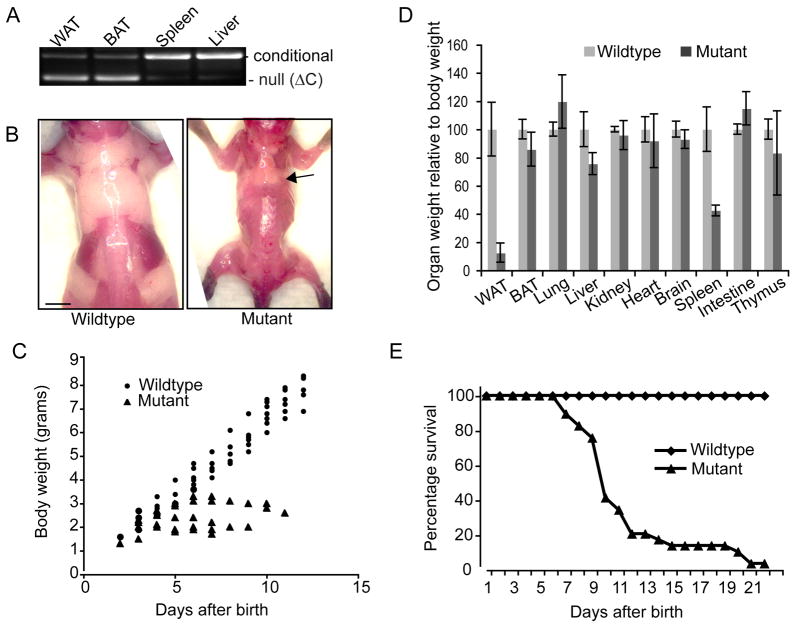
We have previously demonstrated that Dicer is required for the induction of Peroxisome Profilator Activator Receptor-γ (PPARγ) expression in cultured pre-adipocytes and the subsequent adipogenic differentiation of these cultured cells (Mudhasani et al, 2010). To document a role for Dicer and miRNA biogenesis in the terminal differentiation of adipocytes and in the development of adipose tissue in vivo, we crossed Dicer-conditional mice that we generated previously (Mudhasani et al, 2008) with aP2-Cre transgenic mice (He et al, 2003). These Cre transgenic mice were previously found to express Cre recombinase at high levels during adipogenic differentiation and in WAT and BAT in embryonic and adult mice, as well as low levels of leaky Cre expression in brain, skin, spinal cord, limb mesenchyme, and bone cartilage progenitors during development (Urs et al, 2006). Dicerc/+ mice bearing the aP2-Cre transgene were crossed with Dicerc/c mice, and mice that were both Dicerc/c and aP2-Cre were born in expected Mendelian numbers. However, these Dicerc/c, aP2-Cre mice were approximately 50% smaller than either non-transgenic, Dicerc/c littermates or wildtype mice, and displayed a sporadic and mild shivering-like behavior. Analysis of the Dicer alleles revealed that Dicer was deleted in the WAT and BAT of Dicerc/c; aP2-Cre mice (Figure 1A) but not in control tissues. All Dicerc/c; aP2-Cre mice (“mutant”) were noticeably more slender at 1–2 weeks of age than the non-Cre transgenic, Dicerc/c mice (“wildtype”) (Figure 1B), and necropsies revealed that the mutant mice were either severely depleted or completely devoid of WAT. In contrast, the Dicer-mutant mice displayed normal amounts of intrascapular BAT (Figures 1B and 1D). Dicerc/+; aP2-Cre mice were mated with Dicerc/c mice, and litter-matched offspring were weighed daily starting on the second day after birth. Subsequent genotyping determined that all mutant Dicerc/c; aP2-Cre siblings had greatly reduced bodyweights relative to the wildtype offspring (Figure 1C).
Figure 1. Deletion of Dicer in aP2-Cre transgenic mice inhibits the formation of WAT.
(A) PCR genotyping reveals deletion of Dicer conditional alleles specifically in the WAT and BAT of aP2-Cre, Dicer-conditional mice. (B) Dorsal view of adipose deposits in Dicer wildtype and mutant mice at 2 weeks of age. Dicer ablated mice retain intrascapular brown adipose tissue but are devoid of WAT. Scale bar represents 250μm. (C) Weight of Dicer-wildtype and Dicer-ablated (mutant) littermates from day 2 to day 14 post birth. (D) Weight of various organs relative to overall bodyweight in Dicer-wildtype and Dicer-ablated (mutant) mice at 8–10 days after birth. Bars represent standard deviation (SD). (E) Kaplan-Meyer survival plot of Dicer-wildtype and Dicer-ablated (mutant) mice.
Organs were harvested from six mutant and wildtype mice at 8–10 days after birth. The weight of each organ was recorded relative to the overall bodyweight of the mouse from which it was harvested (Figure 1D). No differences were found in the weight of various tissues relative to overall body mass in Dicer-mutant mice with two exceptions: WAT was severely reduced in Dicer-mutant mice (about 10–15% control levels), and the spleen exhibited a 50% reduction in weight. There was no reduction in the mass of BAT relative to overall bodyweight, even though Dicer was deleted in this tissue (Figure 1A). To confirm that the reduction in WAT was not a simple reflection of the overall reduced size of Dicerc/c; aP2-Cre mice, tissue weights of four (10-day old) Dicerc/c; aP2-Cre mice and four (6-day old) Dicerc/c control mice were analyzed. Each of these pups each weighed the same (5.7 ± 0.2 grams) at the time of analysis, regardless of genotype. Dicerc/c; aP2-Cre mice displayed juvenile lethality, with nearly all (98%) of the Dicerc/c; aP2-Cre mice dying between one and three weeks of age (Figure 1E). Two Dicerc/c; aP2-Cre mice did live beyond weaning and lived to one year of age. However, these mice remained smaller than age-matched wildtype mice. While retaining normal amounts of intrascapular BAT, these surviving Dicerc/c, aP2-Cre mice also remained very slender and did not exhibit any increase in WAT mass throughout their lives.
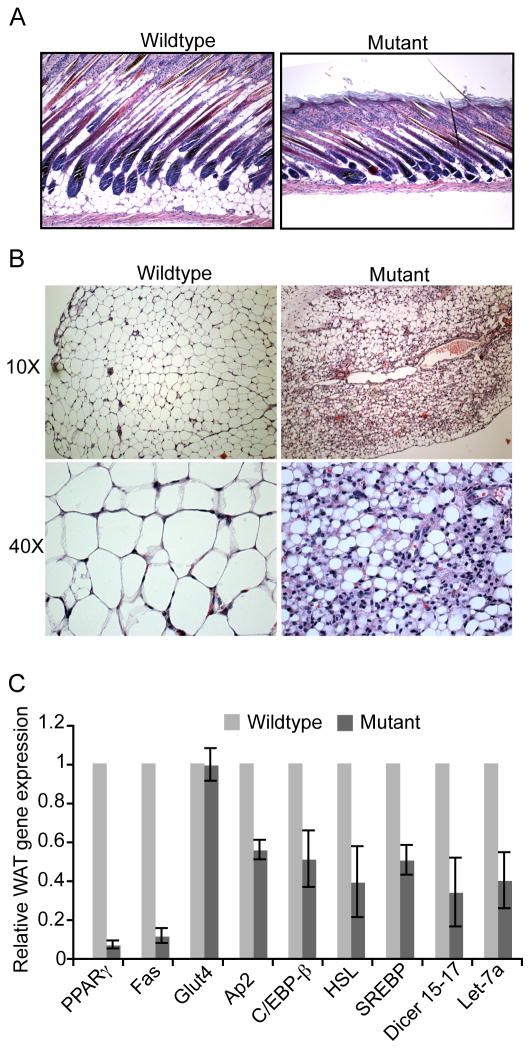
To further document the effects of Dicer ablation on WAT, the epidermis was isolated from 12-day old Dicerc/c mice and Dicerc/c; aP2-Cre mice, fixed and sectioned, and the tissue was hematoxylin and eosin (H&E) stained. There is a clear reduction in subcutaneous adipose tissue present in mice ablated for Dicer (Figure 2A). WAT also was recovered from the lower abdomen of 10–12 day-old Dicerc/c mice and Dicerc/c; aP2-Cre mice. H&E staining of fixed tissue sections reveals that the Dicer-mutants did not have overtly reduced adipocyte cellularity, but clearly exhibited a greatly reduced amount of fat accumulation within each individual adipocyte (Figure 2B). To confirm alterations in adipogenic functions of these cells, RNA was harvested from the WAT of (10-day old) Dicer-wild type and Dicer-mutant mice, and quantitative PCR was performed using primers for various gene transcripts associated with adipocyte differentiation, including those encoding transcriptional regulators such as PPARγ2, the CCAAT/enhancer binding protein β (C/EBPβ), and the Sterol Regulatory Element Binding Protein 1(SREBP1), and transcripts encoding factors regulating fat synthesis (Fas), lipid transport (aP2), lipid hydrolysis (hormone sensitive lipase [HSL]), and glucose transport (Glut4). The WAT expression levels of most adipogenic genes were reduced to 50–90% of that observed in controls (Figure 2C). Analysis of Dicer transcript levels revealed that, as expected, Dicer was reduced in the WAT of Dicerc/c; aP2-Cre mice. However, some Dicer transcripts were still detected, likely due either to incomplete deletion of the Dicer conditional allele in WAT or due to contaminating wildtype, non-adipopse cells in the WAT. Let-7a is a representative miRNA species that is highly expressed in adipocyte precursors (Sun et al, 2009; Kajimoto et al, 2006). The levels of mature Let-7a RNA in WAT following Dicer deletion correlated with the reduction of Dicer expression in this tissue, confirming that Dicer expression and miRNA biogenesis are compromised in Dicerc/c; aP2-Cre WAT.
Figure 2. Dicer is required for the formation and function of WAT in vivo.
(A) H&E stain of the epidermis from 12-day old Dicer-wildtype mice and Dicer-mutant mice. Scale bar represents 1000μm. (B) H&E stain of abdominal WAT of Dicer-wildtypemice and Dicer-mutant mice. Figures shown at 10X (top) and 40X (bottom) magnifications. Scale bars represent 100μm and 10μm, respectively. (C) Quantitative PCR results of adipogenic-related transcripts present in WAT of 10-day old Dicer-wildtype mice and Dicer-mutant mice. The average value for each wildtype transcript was set at 1. Bars represent SD values obtained from eight sibling pairs wherein each pair consists of one mutant and one wildtype mouse.
Scanning electron micrographs (SEM) of WAT in Dicerc/c control mice (+) and Dicerc/c; aP2-Cre mice (−) were performed on day 12 sibling mice to confirm reduced WAT in the Dicer mutant mice (Figure 3). Although these data confirmed a large reduction of WAT tissue in Dicerc/c; aP2-Cre mice, the SEM unexpectedly also revealed a large increase in the density of the extra-cellular basement membrane surrounding the WAT (Figure 3A) in Dicer mutant mice. This extra-cellular matrix is composed primarily of collagen fibers and must be effectively remodeled during WAT deposition in mice (Chun et al, 2006). To confirm increased collagen in the basement membrane in Dicer-mutant mice, we performed Masson’s trichome staining of WAT deposits taken from day 12 Dicerc/c control (+) and Dicerc/c; aP2-Cre mice (−) mice. This stain detects collagen in the basement membrane surrounding adipocytes and in the space separating WAT depots within the tissue (Khan et al, 2009; Kawaguchi et al, 2007). The results (Figure 3B) confirm thickened collagen fibers in Dicer-mutant WAT, suggesting a possible role for mature miRNA molecules in remodeling of the WAT extra cellular matrix.
Figure 3. Dicer plays a role in modeling of the extra-cellular basement matrix of WAT.
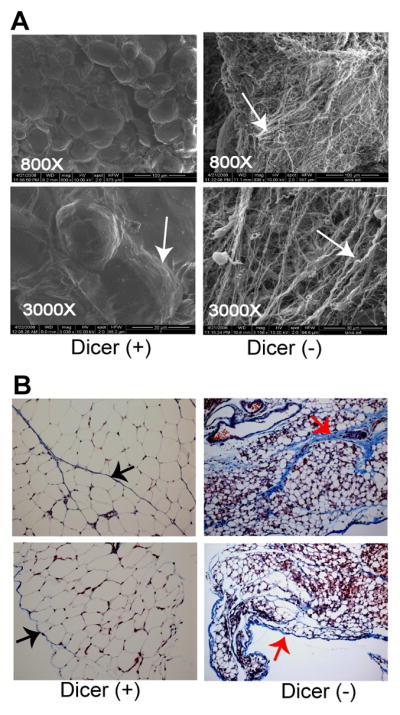
(A) Scanning electron micrograph of WAT from 12-day old Dicer-conditional mice either lacking the aP2-Cre transgene [Dicer (+)] or bearing the aP2-Cre transgene [Dicer (−)]. White arrows indicate the collagen fibers that normally form a thin basement membrane around the adipose tissue. Magnification of each sample is given. (B) Masson’s trichome staining of WAT of 12-day old Dicer-conditional mice either lacking the aP2-Cre transgene [Dicer (+)] or bearing the aP2-Cre transgene [Dicer (−)]. Collagen stains blue, the cytoplasm red, and the nuclei black. Arrows point to collagen in the basement membrane surrounding the adipocytes and in the spaces separating the depots. Magnification of Dicer (+) and Dicer (−) samples: top panels = 20X, bottom panels= 40X.
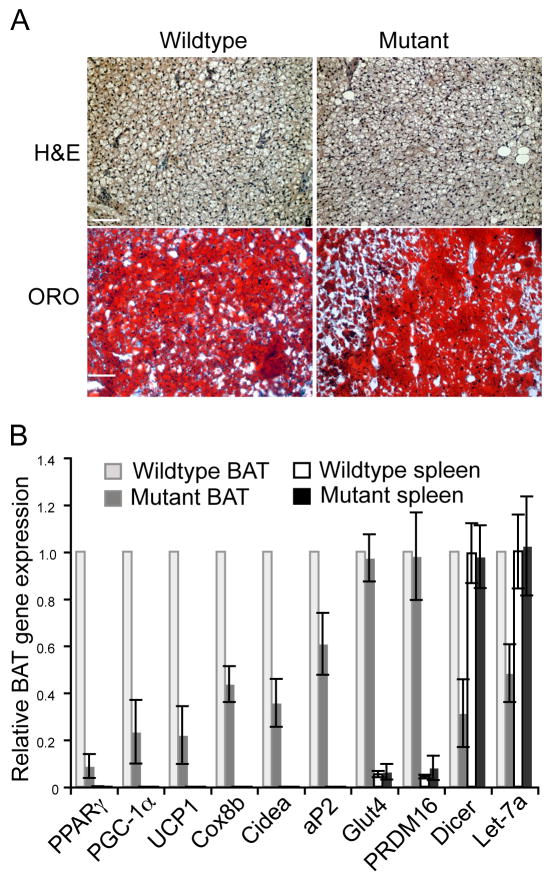
In contrast to large reduction in WAT observed in Dicer-mutant mice, there was no difference between Dicerc/c control mice and Dicerc/c; aP2-Cre mice in the appearance or weight of intrascapular BAT (Figures 1B and 1D). Sectioning and staining of BAT harvested from 10–12 day old Dicerc/c mice and Dicerc/c, aP2-Cre mice revealed no differences in relative cellularity of the tissue (Figure 4, top panels), and analysis of ORO staining of BAT isolated from juvenile mutant and wildtype mice revealed no difference in lipogenesis in the Dicer-ablated brown adipocytes (Figure 4A, bottom panels). Analysis of the expression levels of genes involved in BAT differentiation such as the positive regulatory domain containing 16 (PRDM16) gene (14) and certain lipogenic markers such as aP2 and Glut4 also revealed little or no difference in mutant BAT and wildtype BAT (Figure 4B), although PPARγ–2 and FAS transcript levels were downregulated in mutant BAT. Interestingly, loss of Dicer did strongly impact the expression of several BAT genes involved in mitochondrial oxidation and thermogenesis, including PPARγ Coactivator 1α (PGC-1α), UCP-1, Cyctochrome C oxidase subunit 8b (Cox8b), and Cell death-inducing DNA fragmentation factor[d]-like effector a (Cidea) (Uldry et al, 2006; Nedergaard et al, 2001; Puri et al, 2008). As seen in the WAT of these mice, Dicer transcripts and Let-7a were greatly reduced in Dicer-mutant BAT (Figure 2A & 4B) but not in wildtype or Dicer-deleted control tissues such as spleen (Figure 4B, right-most panels). These data suggest that miRNAs do not regulate the formation of BAT or BAT-mediated biogenesis functions, but do regulate the expression levels of BAT-associated thermogenic genes.
Figure 4. Dicer is not required for the formation of BAT in vivo.
(A) H&E and ORO staining reveal that lipogenesis is not compromised in the intrascapular BAT of Dicer-ablated (mutant) mice. Scale bar represents 80μm (B) Quantitative PCR results of adipogenic and thermogenic-related transcripts present in BAT of 10-day old Dicer-wildtypemice and Dicer-mutant mice. Analysis of Dicer-wildtype and Dicer-mutant spleen was performed as control. The average value for each wildtype transcript was set at 1. Bars represent SD values obtained from eight sibling pairs wherein each pair consists of one mutant and one wildtype mouse.
DISCUSSION
Dicer-conditional mice, aP2-Cre transgenic mice displayed reduced levels of Dicer and Dicer activity in WAT and BAT, were significantly smaller and more slender than control littermates, and typically died within 3 weeks after birth (just prior to weaning). Although these mice displayed normal amounts of brown fat depots, they failed to develop WAT. That a small percentage of these mice did not succumb to early lethality yet still failed to develop WAT as they aged demonstrates that the early lethality in the majority of the Dicerc/c; aP2-Cre mice does not account for the inability of these mice to form WAT. These results also demonstrate that the early lethality exhibited by most Dicerc/c; aP2-Cre mice is unlinked to the lack of WAT development, a finding in keeping with the results of other studies of viable mouse models with reduced adipose deposits (Garg, 2004; Asterholm et al, 2007). Rather, it is likely that leaky aP2-Cre transgene expression (Urs et al, 2006) is inducing sporadic Dicer ablation in non-adipose tissues, resulting in smaller sized mice with compromised viability. Although the cellularity of WAT in Dicerc/c, aP2-Cre mice and in control mice was similar, overall WAT was severely reduced in the Dicer-mutant mice due to a large decrease in lipid storage within the individual white adipocytes. These findings suggest that pre-adipocytes can undergo differentiation in the mouse model, but the white adipocytes fail to undergo or to maintain terminal differentiation following loss of Dicer. Although we have previously reported that Dicer loss inhibits pre-adipogenic differentiation (Mudhasani et al, 2010) in primary cells, the presence of adipocytes within the WAT in Dicerc/c; aP2-Cre mice is in keeping with the expression pattern of the aP2 promoter, as this lipogenic promoter is normally induced by the PPARγ transcription factor during the terminal stages of adipogenic differentiation (He et al, 2003). Interestingly, loss of Dicer also led to reduced levels of PPARγ-2 in the WAT. These results suggest either that Dicer is required for maintenance of terminal adipogenic differentiation or that Dicer-loss and the subsequent reduction of C/EBP factors results in downregulation of PPARγ-2 expression. Furthermore, loss of Dicer also impacted the ability of the white adipose to remodel the extra cellular matrix. Although remodeling of collagen has been previously reported to be necessary for proper formation of WAT in mice (Khan et al, 2009), it is unlikely that this is the only role for Dicer and mature miRNA in adipogenesis, as pre-adipocytes ablated for Dicer display defects in lipogenesis when grown either in two-dimensional plating (Mudhasani et al, 2010) or when cultured in 3D using 2mg/ml of collagen type-1 gel matrix (data not shown).
In contrast to WAT, Dicer was not required for the formation or maintenance of BAT in the mutant mice, even though the expression of genes such as PPAR-γ2 and FAS were similarly downregulated in this adipose tissue following Dicer ablation. These data support the accumulating evidence for differential regulation of WAT and BAT development. Interestingly, although the formation of BAT and lipogenesis in BAT was not affected in Dicer-ablated mice, the transcript levels of other genes that regulate thermogenesis (PGC-1α, UCP1, Cox8b and Cidea) were much reduced, suggesting that ablation of Dicer in BAT may affect BAT thermogenic functions. In keeping with this finding, we observed sporadic shivering in juvenile Dicerc/c, aP2-Cre mice. Although this phenotype might be due to leaky aP2-Cre expression and Dicer loss in other (non-adipose) tissues, it is possible that the shivering mutant mice were attempting to generate body heat. Further analysis of the Dicer/aP2-Cre mice will be necessary to determine the precise cause for this phenotype and for the premature lethality observed in this model.
We have determined that Dicer is required for the formation of WAT but not BAT in vivo, although BAT functions appear to be altered in Dicerc/c, aP2-Cre mice. These results offer genetic evidence for distinct roles of miRNAs in white and brown adipogenesis. As Dicer ablation should impact the maturation of most miRNA species, multiple adipogenic functions are likely impacted by the loss of miRNA biogenesis in these tissues. Identification of the specific miRNAs involved in regulating adipocyte differentiation and in adipose formation should facilitate the identification of target molecules involved in these processes, and add to our understanding of the molecular mechanisms involved in the regulation of adipogenesis.
Acknowledgments
Grant Support: This work was supported by grants from the National Institutes of Health to ANI (DK079239 and DK084278) and to (DK073324 and CA077735). RM was supported by an American Heart Award (0625823T) and by NIH-T32CA130807. Core facilities used in this study were partially supported by program project grant P30DK32529 from the National Institute of Diabetes and Digestive and Kidney Diseases. MPC, ANI, and SNJ are members of UMass DERC (DK32520).
We thank Zhiqing Zhu for technical assistance with the Dicer colony work, Gregory Hendricks for assistance with electron microscopy, and Charlene Baron for help with manuscript preparation.
LITERATURE CITED
- Asterholm IW, Halberg N, Scherer PE. Mouse Models of Lipodystrophy Key reagents for the understanding of the metabolic syndrome. Drug Discov Today Dis Models. 2007;4:17–24. doi: 10.1016/j.ddmod.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brey CW, Nelder MP, Hailemariam T, Gaugler R, Hashmi S. Kruppel-like family of transcription factors: an emerging new frontier in fat biology. Int J Biol Sci. 2009;5:622–636. doi: 10.7150/ijbs.5.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushati N, Cohen SM. microRNA functions. Annu Rev Cell Dev Biol. 2007;23:175–205. doi: 10.1146/annurev.cellbio.23.090506.123406. [DOI] [PubMed] [Google Scholar]
- Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004;84:277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- Chun TH, Hotary KB, Sabeh F, Saltiel AR, Allen ED, Weiss SJ. A pericellular collagenase directs the 3-dimensional development of white adipose tissue. Cell. 2006;125:577–591. doi: 10.1016/j.cell.2006.02.050. [DOI] [PubMed] [Google Scholar]
- Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, Kuo FC, Palmer EL, Tseng YH, Doria A, Kolodny GM, Kahn CR. Identification and importance of brown adipose tissue in adult humans. N Engl J Med. 2009;360:1509–1517. doi: 10.1056/NEJMoa0810780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esau C, Kang X, Peralta E, Hanson E, Marcusson EG, Ravichandran LV, Sun Y, Koo S, Perera RJ, Jain R, Dean NM, Freier SM, Bennett CF, Lollo B, Griffey R. MicroRNA-143 regulates adipocyte differentiation. J Biol Chem. 2004;279:52361–52365. doi: 10.1074/jbc.C400438200. [DOI] [PubMed] [Google Scholar]
- Garg A. Acquired and inherited lipodystrophies. N Engl J Med. 2004;350:1220–1234. doi: 10.1056/NEJMra025261. [DOI] [PubMed] [Google Scholar]
- Gerin I, Bommer GT, McCoin CS, Sousa KM, Krishnan V, Macdougald OA. Roles for miRNA-378/378* in adipocyte gene expression and lipogenesis. Am J Physiol Endocrinol Metab. 2010;299:E198–206. doi: 10.1152/ajpendo.00179.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghildiyal M, Zamore PD. Small silencing RNAs: an expanding universe. Nat Rev Genet. 2009;10:94–108. doi: 10.1038/nrg2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He W, Barak Y, Hevener A, Olson P, Liao D, Le J, Nelson M, Ong E, Olefsky JM, Evans RM. Adipose-specific peroxisome proliferator-activated receptor gamma knockout causes insulin resistance in fat and liver but not in muscle. Proc Natl Acad Sci U S A. 2003;100:15712–15717. doi: 10.1073/pnas.2536828100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Zhao L, Xing L, Chen D. MicroRNA-204 regulates Runx2 protein expression and mesenchymal progenitor cell differentiation. Stem Cells. 2010;28:357–364. doi: 10.1002/stem.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajimoto K, Naraba H, Iwai N. MicroRNA and 3T3-L1 pre-adipocyte differentiation. RNA. 2006;12:1626–1632. doi: 10.1261/rna.7228806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajimura S, Seale P, Tomaru T, Erdjument-Bromage H, Cooper MP, Ruas JL, Chin S, Tempst P, Lazar MA, Spiegelman BM. Regulation of the brown and white fat gene programs through a PRDM16/CtBP transcriptional complex. Genes Dev. 2008;22:1397–1409. doi: 10.1101/gad.1666108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karbiener M, Fischer C, Nowitsch S, Opriessnig P, Papak C, Ailhaud G, Dani C, Amri EZ, Scheideler M. microRNA miR-27b impairs human adipocyte differentiation and targets PPARgamma. Biochem Biophys Res Commun. 2009;390:247–251. doi: 10.1016/j.bbrc.2009.09.098. [DOI] [PubMed] [Google Scholar]
- Kawaguchi N, Xu X, Tajima R, Kronqvist P, Sundberg C, Loechel F, Albrechtsen R, Wewer UM. ADAM12 protease induces adipogenesis in transgenic mice. Am J Pathol. 2002;160:1895–1903. doi: 10.1016/S0002-9440(10)61136-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennell JA, Gerin I, MacDougald OA, Cadigan KM. The microRNA miR-8 is a conserved negative regulator of Wnt signaling. Proc Natl Acad Sci U S A. 2008;105:15417–15422. doi: 10.1073/pnas.0807763105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan T, Muise ES, Iyengar P, Wang ZV, Chandalia M, Abate N, Zhang BB, Bonaldo P, Chua S, Scherer PE. Metabolic dysregulation and adipose tissue fibrosis: role of collagen VI. Mol Cell Biol. 2009;29:1575–1591. doi: 10.1128/MCB.01300-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YJ, Hwang SJ, Bae YC, Jung JS. MiR-21 regulates adipogenic differentiation through the modulation of TGF-beta signaling in mesenchymal stem cells derived from human adipose tissue. Stem Cells. 2009;27:3093–3102. doi: 10.1002/stem.235. [DOI] [PubMed] [Google Scholar]
- Kim SY, Kim AY, Lee HW, Son YH, Lee GY, Lee JW, Lee YS, Kim JB. miR-27a is a negative regulator of adipocyte differentiation via suppressing PPARgamma expression. Biochem Biophys Res Commun. 2010;392:323–328. doi: 10.1016/j.bbrc.2010.01.012. [DOI] [PubMed] [Google Scholar]
- Lefterova MI, Lazar MA. New developments in adipogenesis. Trends Endocrinol Metab. 2009;20:107–114. doi: 10.1016/j.tem.2008.11.005. [DOI] [PubMed] [Google Scholar]
- Mudhasani R, Imbalzano AN, Jones SN. An essential role for Dicer in adipocyte differentiation. J Cell Biochem. 2010;110:812–816. doi: 10.1002/jcb.22625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudhasani R, Zhu Z, Hutvagner G, Eischen CM, Lyle S, Hall LL, Lawrence JB, Imbalzano AN, Jones SN. Loss of miRNA biogenesis induces p19Arf-p53 signaling and senescence in primary cells. J Cell Biol. 2008;181:1055–1063. doi: 10.1083/jcb.200802105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedergaard J, Bengtsson T, Cannon B. Unexpected evidence for active brown adipose tissue in adult humans. Am J Physiol Endocrinol Metab. 2007;293:E444–452. doi: 10.1152/ajpendo.00691.2006. [DOI] [PubMed] [Google Scholar]
- Nedergaard J, Golozoubova V, Matthias A, Shabalina I, Ohba K, Ohlson K, Jacobsson A, Cannon B. Life without UCP1: mitochondrial, cellular and organismal characteristics of the UCP1- ablated mice. Biochem Soc Trans. 2001;29:756–763. doi: 10.1042/0300-5127:0290756. [DOI] [PubMed] [Google Scholar]
- Nedergaard J, Ricquier D, Kozak LP. Uncoupling proteins: current status and therapeutic prospects. EMBO Rep. 2005;6:917–921. doi: 10.1038/sj.embor.7400532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park KW, Halperin DS, Tontonoz P. Before they were fat: adipocyte progenitors. Cell Metab. 2008;8:454–457. doi: 10.1016/j.cmet.2008.11.001. [DOI] [PubMed] [Google Scholar]
- Puri V, Ranjit S, Konda S, Nicoloro SM, Straubhaar J, Chawla A, Chouinard M, Lin C, Burkart A, Corvera S, Perugini RA, Czech MP. Cidea is associated with lipid droplets and insulin sensitivity in humans. Proc Natl Acad Sci U S A. 2008;105:7833–7838. doi: 10.1073/pnas.0802063105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin L, Chen Y, Niu Y, Chen W, Wang Q, Xiao S, Li A, Xie Y, Li J, Zhao X, He Z, Mo D. A deep investigation into the adipogenesis mechanism: profile of microRNAs regulating adipogenesis by modulating the canonical Wnt/beta-catenin signaling pathway. BMC Genomics. 2010;11:320. doi: 10.1186/1471-2164-11-320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen ED, MacDougald OA. Adipocyte differentiation from the inside out. Nat Rev Mol Cell Biol. 2006;7:885–896. doi: 10.1038/nrm2066. [DOI] [PubMed] [Google Scholar]
- Salma N, Xiao H, Mueller E, Imbalzano AN. Temporal recruitment of transcription factors and SWI/SNF chromatin-remodeling enzymes during adipogenic induction of the peroxisome proliferator-activated receptor gamma nuclear hormone receptor. Mol Cell Biol. 2004;24:4651–4663. doi: 10.1128/MCB.24.11.4651-4663.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seale P, Kajimura S, Spiegelman BM. Transcriptional control of brown adipocyte development and physiological function--of mice and men. Genes Dev. 2009;23:788–797. doi: 10.1101/gad.1779209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seale P, Kajimura S, Yang W, Chin S, Rohas LM, Uldry M, Tavernier G, Langin D, Spiegelman BM. Transcriptional control of brown fat determination by PRDM16. Cell Metab. 2007;6:38–54. doi: 10.1016/j.cmet.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun T, Fu M, Bookout AL, Kliewer SA, Mangelsdorf DJ. MicroRNA let-7 regulates 3T3-L1 adipogenesis. Mol Endocrinol. 2009;23:925–931. doi: 10.1210/me.2008-0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uldry M, Yang W, St-Pierre J, Lin J, Seale P, Spiegelman BM. Complementary action of the PGC-1 coactivators in mitochondrial biogenesis and brown fat differentiation. Cell Metab. 2006;3:333–341. doi: 10.1016/j.cmet.2006.04.002. [DOI] [PubMed] [Google Scholar]
- Urs S, Harrington A, Liaw L, Small D. Selective expression of an aP2/Fatty Acid Binding Protein 4-Cre transgene in non-adipogenic tissues during embryonic development. Transgenic Res. 2006;15:647–653. doi: 10.1007/s11248-006-9000-z. [DOI] [PubMed] [Google Scholar]
- Valencia-Sanchez MA, Liu J, Hannon GJ, Parker R. Control of translation and mRNA degradation by miRNAs and siRNAs. Genes Dev. 2006;20:515–524. doi: 10.1101/gad.1399806. [DOI] [PubMed] [Google Scholar]
- van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, Bouvy ND, Schrauwen P, Teule GJ. Cold-activated brown adipose tissue in healthy men. N Engl J Med. 2009;360:1500–1508. doi: 10.1056/NEJMoa0808718. [DOI] [PubMed] [Google Scholar]
- Wang Q, Li YC, Wang J, Kong J, Qi Y, Quigg RJ, Li X. miR-17-92 cluster accelerates adipocyte differentiation by negatively regulating tumor-suppressor Rb2/p130. Proc Natl Acad Sci U S A. 2008;105:2889–2894. doi: 10.1073/pnas.0800178105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White UA, Stephens JM. Transcriptional factors that promote formation of white adipose tissue. Mol Cell Endocrinol. 2010;318:10–14. doi: 10.1016/j.mce.2009.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijers SL, Saris WH, van Marken Lichtenbelt WD. Recent advances in adaptive thermogenesis: potential implications for the treatment of obesity. Obes Rev. 2009;10:218–226. doi: 10.1111/j.1467-789X.2008.00538.x. [DOI] [PubMed] [Google Scholar]
- Xie H, Lim B, Lodish HF. MicroRNAs induced during adipogenesis that accelerate fat cell development are downregulated in obesity. Diabetes. 2009;58:1050–1057. doi: 10.2337/db08-1299. [DOI] [PMC free article] [PubMed] [Google Scholar]