Abstract
The presence of acellular hemoglobin (Hb) within the circulation is generally viewed as a pathological state that can result in toxic consequences. Haptoglobin (Hp), a globular protein found in the plasma, binds with high avidity the αβ dimers derived from the dissociation of Hb tetramer and thus helps clear free Hb. More recently there have been compelling indications that the redox properties of the Hp bound dimer (Hb–Hp) may play a more active role in controlling toxicity by limiting the potential tissue damage caused by propagation of the free-radicals generated within the heme containing globin chains. The present study further examines the potential protective effect of Hp through its impact on the production of nitric oxide (NO) from nitrite through nitrite reductase activity of the Hp bound αβ Hb dimer. The presented results show that the Hb dimer in the Hb–Hp complex has oxygen binding, CO recombination and spectroscopic properties consistent with an Hb species having properties similar to but not exactly the same as the R quaternary state of the Hb tetramer. Consistent with these observations is the finding that the initial nitrite reductase rate for Hb–Hp is approximately ten times that of HbA under the same conditions. These results in conjunction with the earlier redox properties of the Hb–Hp are discussed in terms of limiting the pathophysiological consequences of acellular Hb in the circulation.
Keywords: Nitrite reductase, Haptoglobin, Hemoglobin
Introduction
The presence of acellular hemoglobin within the circulation can trigger a cascade of processes with profound toxic consequences on surrounding tissues. Acellular Hb in the vasculature can occur due to acute or chronic hemolytic conditions that result in the release of free Hb from the red blood cell or from the infusion of Hb based oxygen carriers (HBOCs). The origins of the toxic effects are still not fully understood. One potential mechanism of toxicity is believed to be the result of the propensity of acellular Hbs to create or enhance oxidative stress largely due to the formation of globin-associated free radicals that are capable of initiating lipid peroxidation thereby triggering an inflammatory cascade [1,2]. The other perspective is that acellular Hbs are highly effective at scavenging endothelial generated NO which can trigger or exacerbate pathological states through decreased tissue perfusion and decreased scavenging of reactive oxygen species (ROS) [3–6]. The toxic consequences of NO scavenging are likely to be especially serious when there is a backdrop of endothelial dysfunction that is already causing both a drop in NO bio-availability and an increase in oxidative stress [7].
Haptoglobin (Hp), an acellular globular protein found in the plasma plays a major role in mediating toxicity due to acellular Hb [8–10]. Hp is a hetero-dimer composed of α (9 kDa)-and β (33 kDa)-subunits and in humans exists in two allelic forms; Hp1 and Hp2, which differ only in their respective α chains. The α1-chain in Hp1 carries one free sulfhydryl group, while α2 in Hp2 is longer and carries two sulfhydryl groups. Polymeric structures with variable numbers of associated Hp dimers are found in serum from individuals. Sera of individuals in which Hp molecules comprise either two α1β units (Hp1–1), a variable number of α2β units (Hp1–2), or two α1β units and a variable number of α2β units (Hp2–2) [11]. Hp seemingly binds with high avidity αβ dimers derived from the dissociation of the Hb tetramer and thus participates in acellular Hb clearance without damage to the kidneys. Recently, it was demonstrated that Hp can play a more active role in minimizing acellular Hb derived toxicity by stabilizing reactive tyrosine radicals within the Hp bound globin thus reducing the likelihood of Hb-induced lipid peroxidation and inflammation [12].
The Hb–Hp complex might also have additional properties that could limit toxicity based on the functional properties of the Hp bound αβ globin dimer. In solution, the functional properties of the αβ dimer are similar to that of the R state tetramer [13–16]. There is however a phenomenon termed quaternary enhancement based on a modest enhancement of the oxygen affinity and ligand binding properties of the αβ dimer when the dimer is incorporated into the R quaternary state of the Hb tetramer [17–24]. If the Hp bound dimer has R state functional properties, it is anticipated that the quaternary structure sensitive rate for the formation of NO from nitrite via a nitrite reductase reaction between a five coordinate ferrous heme (AKA deoxy heme) and nitrite should also be enhanced. Several studies show that an R state deoxy heme reacts much faster than a T state deoxy heme [25–30]. Increased production of NO by acellular Hb’s with high oxygen affinity has been proposed as a mechanism to compensate for NO scavenging by acellular Hbs [31]. This mechanism has been proposed [31,32] as the basis for why certain PEGylated HBOCs are not vasoactive [33–35] despite an NO scavenging capacity comparable to other Hb based oxygen carriers (HBOCs) that promote vasoconstriction [33].
This manuscript describes studies designed to determine whether the hemes in the Hb–Hp complex exhibit spectroscopic and functional properties consistent with the Hp complexed globin αβ dimer having R state-like properties including an enhanced rate for the nitrite reductase reaction. Functional properties include measurements of oxygen affinity, geminate and solvent phase CO recombination and nitrite reductase activity (initial rates). The geminate recombination is additionally useful in that the geminate yield (fraction of the photodissociated population undergoing geminate recombination) for the dimer is lower than that of the R state tetramer (quaternary enhancement effect) [20] but still much greater than the near zero geminate yield for the low affinity T state tetramer [36]. Thus, the geminate recombination can help establish whether the Hp bound dimer exhibits that same reduction in geminate yield as seen for the unbound solution phase dimer. In addition to the functional studies, the visible (Q bands) [37,38] and near IR (Band III) [22,29,39–44] absorption spectrum of the fully deoxygenated ferrous derivative is also used to evaluate whether the deoxy hemes manifest spectra that are consistent with an R-like species. In all cases, the results are consistent with an R-like heme species both functionally and spectroscopically. The geminate yield is consistent with an Hp bound dimer functionally similar to the unbound dimer in solution.
Materials and methods
Sample preparation
Human hemoglobin A (HbA)
Hp was a kind gift from Bio Products Laboratory (BPL), Hertfordshire, England. Typical size exclusion HPLC separation profiles of Hp samples used in this study shows the following molecular weight distribution (60% dimer, 21% trimer and 19% larger forms). In Hp1–1 individuals, Hp will be present in its simplest form, consisting of dimers (two α and two β-chains, connected by disulfide bridges) with a molecular weight of 98 kDA. In Hp2–1 and Hp2–2 individual’s trimers, termers and polymers are present with molecular weights up to ~700 kDa. Adult human Hb (HbA0) was prepared by ammonium sulfate precipitation, stripped of endogenous organic phosphates and purified by anion-exchange FPLC as previously described [45].
Hemoglobin–haptoglobin (Hb–Hp) complex preparation
Adult human Hb, purified by FPLC chromatography and stripped of anionic effectors as described in Methods, was passed through a superdex-200 column for removal of catalase using 50 mM potassium phosphate buffer at pH 7.4 using the protocol described earlier [45]. For complex formation, the purified Hb was mixed with Hp in a 1:1.5 ratio (60 μM in heme). The complex was further purified using a BioSep 3000 column using 50 mM potassium phosphate buffer at pH 7.4 (Phenomenex, Torrence, CA). MALDE-MS was used to verify the Hb–Hp complex formation as described previously [46].
ββ XL P2K HbA
This high oxygen affinity cross linked derivative of the HbA tetramer (prepared and provided by members of Professor S. Acharya’s research group at the Albert Einstein College of Medicine) is stabilized through a bifunctional maleimide-PEG (2K) linker (bis mal-PEG) that crosslinks the two β chains through a maleimide reaction with the two β Cys 93 sulfydryls [47]. This cross linked Hb has similar functional properties to other Hbs (e.g. NEM Hb) that have the same thiol modification but without crosslinking [18,32,48,49]. The maleimide modification increases oxygen affinity and shifts the allosteric equilibrium towards the R state. The cross linked derivative is used to eliminate any possible issue of dimer formation thus allowing a comparison of the Hb–Hp complex with a high oxygen affinity tetramer that has been shown to be vasodilatory in the presence of low levels nitrite (Cabrales and Friedman, unpublished results).
Preparation of solution phase samples of HbA and Hb–Hp for kinetic and spectroscopic study
Deoxy heme derivatives of HbA and Hp–Hb for kinetic and spectroscopic solution studies (~0.2 mM heme) were prepared from the initial oxygenated derivatives by exhaustive purging with Ar in an oxygen free glove box, and adding a modest excess of dithionite (>1:1 dithionite:heme) as necessary. The visible to near IR optical absorption spectra of samples in Bis–Tris buffer, pH 7.0, were scanned to establish the ligation and redox status of the heme. COHb samples were prepared by exposing the deoxy samples to buffer saturated CO gas, until the absorption spectrum revealed complete conversion to the COHb derivative. Aquomet samples were prepared by oxidizing Hb using K3Fe(CN)6, which was subsequently removed using a spin column prepared from G10 Sephadex in the same buffer. In some instances L-cysteine was used to reduce the aquomet samples in order to compare deoxy heme populations generated from the oxy heme plus dithionite or aquomet heme plus L-cysteine.
Oxygen equilibrium measurements
Oxygen equilibrium binding studies were carried out using a Hemox Analyzer (TCS Scientific, New Hope, Pennsylvania, US) [50]. Sample deoxygenation and reoxygenation was performed using pure nitrogen gas and air, respectively. Oxygen tensions were measured using a Clark Model 5331 O2 Probe (Yellow Springs Instrument Company, Yellow Springs, Ohio, US). Oxygen equilibrium curves (OECs) were generated by measuring O2 fractional saturation by optical absorbance spectroscopy using a dual-wavelength spectrophotometer at varied O2 partial pressures. Hb and Hb–Hp complex solutions (heme concentrations in the range of 60–75 μM) were used in 10 mM potassium phosphate/0.1 M NaCl, pH 7.4 at 37 °C. All solutions contained 4 μl of the Hayashi mixture [50] of reducing enzymes to minimize Hb oxidation during measurements. Data obtained from these studies were fit using the nonlinear least-squares method and Adair equations built-into the Hemox Analyzer software (P50 Plus, Version 1.2).
Geminate and solvent phase CO recombination kinetics
CO recombination traces from the CO heme derivatives of HbA and Hb–Hp were generated and displayed on a log–log plot of normalized absorbance (NA) versus time as previously described and discussed in details [36,51–54]. Photodissociation of the CO from the heme is triggered using a 7 ns pulse at 532 nm from a Nd:YAG laser. The recombination trace over several decades in time starting from 10 ns out to 1 s is generated from a change in absorption using the several milliwatt 442 nm output of a CWHeCd laser at as a probe of the evolving populations undergoing CO recombination.
Nitrite reductase activity at pH 7.0
Deoxygenated Hb samples were prepared in solution at pH 7.0 in 0.05 M Bis–Tris; ~0.2 mM in heme with the exception that an excess of dithionite was added to a concentration of 1 mM. Nitrite was added in a slight excess of 1.1:1(heme:nitrite) to deoxy Hb) to initiate the reaction. Under these conditions, the nitrite reductase reaction is modified in that the met (Hb+) product generated from the nitrite reductase reaction is reduced back to deoxy Hb by the excess dithionite (Eq. (1)):
| (1) |
The deoxy Hb binds NO yielding the ferrous NO derivative of Hb (NOHb). As a result, the observed reaction consists overwhelmingly of the loss of deoxy Hb and the formation of NOHb as previously described [29,55]. Dithionite at this concentration does not reduce nitrite to NO [55,56].
The change in absorption as a function of time at 430 nm (Sôret band for deoxy HbA) was monitored on a Lambda 2 (Perkin Elmer, Norwalk, CT). The choice of the 430 nmb and to follow the initial rate of decay of the starting deoxy species in solution is based on the relatively large extinction coefficient for deoxyHbat this wavelength, −133,000 M−1-cm−1 compared to (NOHb, ~8400, and met Hb, ~4000). The initial linear portion was used to calculate initial rates.
The Q-band absorption spectra (~480–800 nm) was also scanned and deconvoluted into populations of products using authentic standard basis sets and a program in Mathcad, V14.0 (Parametric Technology Corporation, Needham, MA). The deoxy heme population was plotted as a function of time to provide an alternative approach to generate the initial nitrite reductase rates.
Initial rates for the nitrite reductase reaction were generated for the Hb–Hp complex, HbA and ββ XL P2K HbA.
Nitrite reductase activity at pH 7.4 in the presence of L35, a potent allosteric effector
The nitrite reductase reaction rate in HbA has been shown to increase as the reaction progresses. The enhancement is directly attributable to the allosteric behavior of the Hb tetramer [27]. Initially at the start of the nitrite reductase reaction, the heme population for the Hb is fully deoxygenated which for HbA results in a population that is overwhelmingly T state. The T state has a ten fold slower rate than the R state. As the reaction progresses, the population becomes a mix of Hbs containing an increasing population of met hemes and NO ferrous hemes as well as a decreasing population of deoxy hemes. This population progression shifts the allosteric equilibrium towards the R quaternary state. The remaining deoxy hemes within the R state tetramers will manifest the approximately ten fold enhancement in the nitrite reductase rate. The introduction of an allosteric effector that stabilizes the T over R quaternary structure will inhibit or eliminate the above described progressive enhancement in rate. In contrast, the dimer is anticipated to be relatively insensitive to the addition of allosteric effectors and hence show minimal variation in the kinetic trace associated with the nitrite reductase reaction. L35 (2-[4-(3,5-dichlorophenylureido)phenoxy]-2-methylpropionic acid), is a potent allosteric effector [57] that is still fully operational at high pH [58] (unlike inositol hexaphosphate and diphosphoglycerate) which allows for the monitoring of its impact at pH 7.4 where the nitrite reductase reaction is slower thus allowing a more clear temporal window to assess allosteric effects.
Results
Oxygen affinity measurements
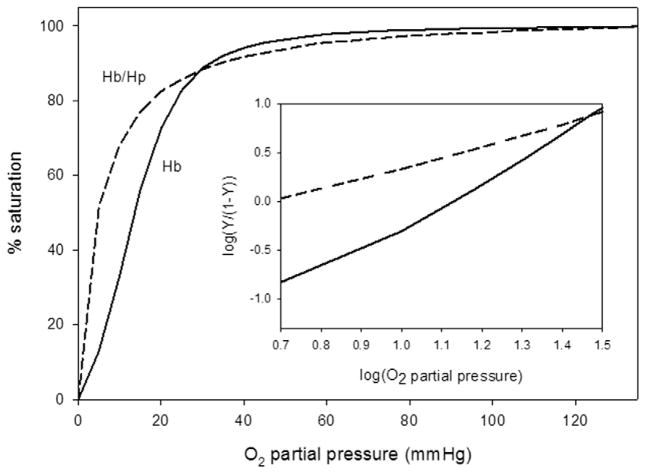
Fig. 1 shows representative oxygen equilibrium curves (OECs) together with their corresponding Hill plots for uncomplexed Hb and for the purified Hb–Hp complex. The Hb–Hp complex showed significantly increased O2-binding affinity relative to uncomplexed Hb (P50 = 4.59 versus 13.51 mm Hg in 10 mM potassium phosphate/0.1 M NaCl, pH 7.4 at 37 °C (see also Table 1). Cooperativity of O2 binding (n1/2 = 2.37 for HbA) was lost and subunit heterogeneity was evident in the Hill plots of the Hb–Hp complex (n1/2 = 1.15). We have recently reported and in accord with the elevated O2 affinity, the apparent first order rate of O2 dissociation from HbA and the Hb–Hp complex as captured in a stopped flow spectrophotometric method. Comparison of the process for Hb and the Hb–Hp complex, carried out under identical conditions, showed Hb to have an apparent rate constant for O2 dissociation of 58 s−1 at the mid-point of the process, while that of the Hb–Hp complex was 25 s−1 (Banerjee et al., unpublished data). Both equilibrium and kinetic data are consistent with recently published work by our group [59] and with that of earlier studies using slightly different preparations of haptoglobin solutions [13,60].
Fig. 1.
Representative plots showing O2 binding equilibrium curves (OECs) for the 60 μM (in heme) solutions of the Hb–Hp complex (– – – –) and for uncomplexed Hb (– –) measured 10 mM potassium phosphate/0.1 M NaCl, pH 7.4 at 37 °C. The average P50 and n50 values derived from three separate experiments are reported in Table 1. Insert for this figure shows the respective Hill plots for each curve.
Table 1.
Comparison of initial nitrite reductase rates for HbA and Hb–HP. Conditions: 0.2 mM heme, 1:1.1 heme:nitrite; 0.05 M Bis–Tris, pH 7.0 + dithionite (>1:1 heme). Rates derived from changes in the 430 nm deoxy band monitored as a function of time. The rates for HbA and Hb–Hp are averages of two independent runs. The conditions for the oxygen affinity and cooperativity measurements are given in the text. The P50 and n50 (Hill coefficient) values for the crosslinked Hb are taken from an earlier work [48].
| Sample | Rate (μM s−1) | P50 (mm Hg) | n50 |
|---|---|---|---|
| Hb–Hp | 0.111 ± 0.001 | 4.79 ± 0.21 | 1.06 ± 0.081 |
| Hb | 0.022 ± 0.01 | 13.63 ± 0.11 | 2.38 ± 0.015 |
| XL P2K Hb | 0.087 | 6.5 | 2.1 |
Geminate and solution phase CO recombination
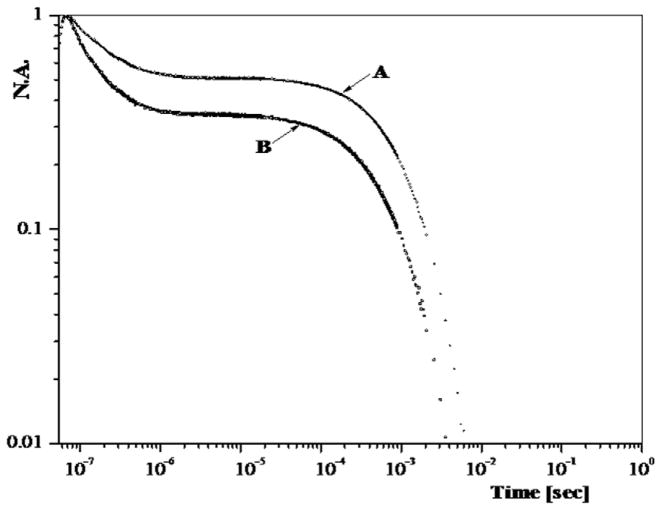
Fig. 2 displays on a log normalized absorbance/log time scale the normalized kinetic traces for the recombination of CO to HbA and Hb–Hp subsequent to photodissociation on a 7 ns time scale of the corresponding starting fully CO saturated derivatives. The recombination occurring on the sub-microsecond to second time scale is assigned to geminate recombination; whereas, the slower recombination is assigned to the solvent (bimolecular) recombination. The solvent phase recombination traces are consistent with R like species [18]. Under these experimental conditions (CO saturated buffer, low temperature and incomplete photolysis), there is limited evolution of the photoproduct population from R to T for the tetramer which accounts for the absence of the very much slower T state solvent phase. Both traces exhibit solvent phase recombination traces that are R-like. The tetramer exhibits a slightly faster rate for that process which might be due to quaternary enhancement. The geminate yield for the Hb tetramer is larger (~.6 versus .4) than for the Hb/Hb complex. This difference in the geminate yield is consistent with the difference seen between R state COHA and the unbound CO αβ dimer [20]. There are however multiple R states for the HbA tetramer [61–63] that exhibit similar differences in the geminate yield [19,58]; however, the known properties of the Hb–Hp complex overwhelmingly favor the dimer as the basis for the lower geminate yield seen for the complex. At the concentrations being used in the present study, the population of unbound dimers present in the COHbA sample is minimal. Under these conditions, T state CO derivatives of HbA derived from either mutants or sol–gel entrapment protocols exhibit a kinetic trace with a geminate yield at or below 0.1 and a solvent phase that is at least ten times slower than those shown in Fig. 2 [36,52,54], thus ruling out the possibility that the Hb–Hp complex might have T state properties when fully liganded. Although the reduced geminate yield and the R-like solvent phase recombination for the Hb–Hp sample are consistent with an αβ dimer, a similar pattern is seen for maleimide modified tetramers [18].
Fig. 2.
CO recombination traces displayed on a log normalized absorbance (NA) versus log time plot for COHbA (trace B) and CO Hb–Hp (trace A). The heme concentration for both samples was on the order of 0.45 mM in CO saturated 50 mM BisTris buffer at pH 7. The kinetics were generated from samples maintained at 3.5 °C. The kinetic phase occurring on the sub-microsecond time scale is geminate recombination whereas the slower recombination is attributed to solvent phase (AKA bimolecular) recombination.
Absorption spectra
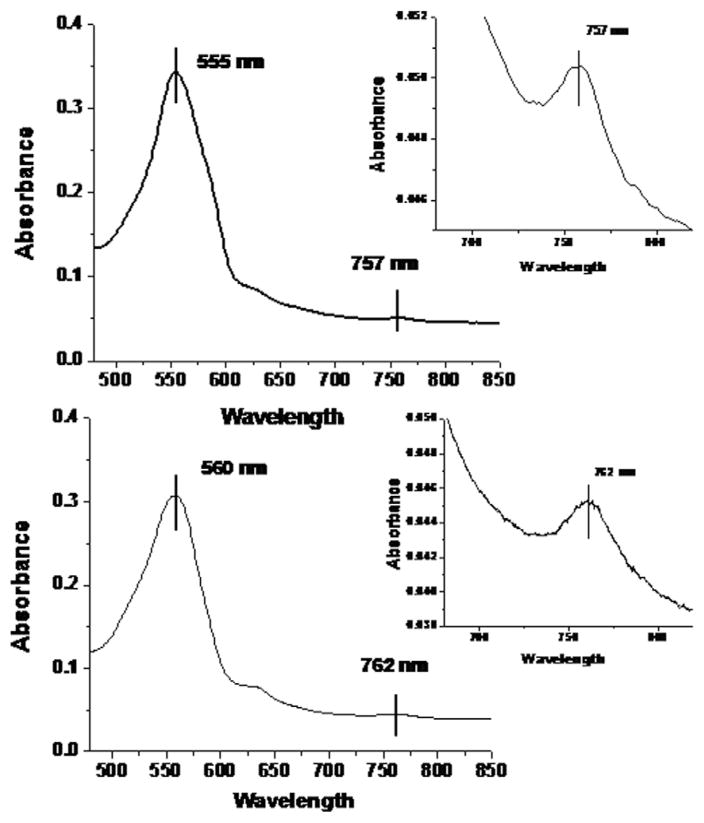
The oxygen binding measurements and the CO recombination studies indicate that the hemoglobin species associated with the Hb–Hp complex is consistent with an Hp bound stabilized αβ dimer. If this assessment is indeed correct one would expect that this species would be reflected in the structure sensitive features of the heme absorption spectrum. Two features of the absorption spectrum of the “deoxy” heme of Hb are especially revealing. The so called Q band centered at 555 nm for the deoxy hemes from the equilibrium ligand free T state Hb population shifts to ~558 nm or higher for deoxy hemes associated with ligand free R state Hbs generated using mutagenic/chemical modifications, sol–gel trapping, and time resolved spectroscopy of photodissociated R state COHb’s. Similarly, the proximal strain sensitive near IR band (Band III) at ~755 nm for equilibrium deoxy T state hemes is known to shift to 760 nm and higher (765 nm for the nanosecond R state photoproduct of COHbA) for the corresponding R state deoxy hemes. Fig. 3 compares the Q band and Band III spectra of the deoxy derivatives of HbA and Hb–Hp under similar conditions. Both the Q band and Band III show the spectral shifts indicative of an R-like environment for the hemes in the Hb–Hp complex. The identical shifts were observed for deoxy samples generated using either sodium dithionite or L-cysteine [29]to generate the deoxy species from either nitrogen purged ferrous samples (starting as oxy) or aquomet derivatives, respectively. The peak positions for the fully deoxygenated derivative of ββ XL P2K HbA were 555 and 758 nm for the Q band and the near IR band, respectively. This cross linked Hb has enhanced oxygen affinity but still manifests cooperativity in its oxygen binding albeit at a reduced level compared to HbA [48] and very similar to other PEG-maleimide modified Hbs [32,48,49].
Fig. 3.
Comparison of the visible (Q band) and near IR (Band III) absorption spectra of deoxy hemes from HbA and Hb–Hp. The deoxy derivatives were prepared by purging the oxy derivatives with Ar and then adding dithionite; (>2:1 heme (0.24 mM) in 0.05 M Bis–Tris, pH 7.0). Very similar spectra were obtained by using L-cysteine to reduce aquomet derivatives.
Nitrite reductase activity
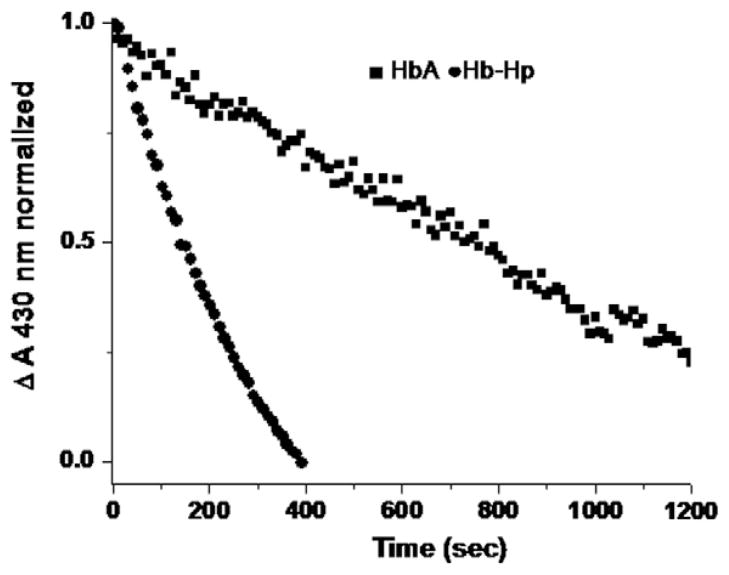
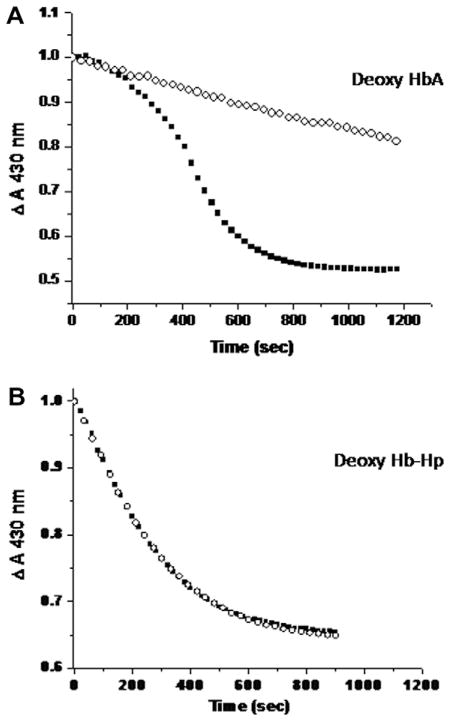
A representative comparison between HbA and Hb–Hp of the initial rate of decay of the starting fully deoxy heme population subsequent to addition of nitrite (1:1.1 heme:nitrite) is shown in Fig. 4. It can be seen that the initial rate for the Hb–Hp complex is considerably faster than that for HbA. The kinetic traces are generated from the change in the absorbance at 430 nm which preferentially reports on the deoxy population. Under the conditions of this measurement (a slight excess dithionite is added to insure no build up of met Hb during the course of the reaction), the presented time course exclusively probes the decay of the deoxy population at the expense of a build up of the ferrous NO heme species. Parallel measurements in which the entire spectrum is scanned, confirms and unambiguously demonstrates that the deoxy population decays as the ferrous NO heme population builds up. In the absence of the added dithionite, there is also a progressive build up of met heme and ferrous NO heme as anticipated based on the nitrite reductase reaction in question and the many previously reported results. The rates from the single wavelength kinetic measurements and from analysis of the overall spectral changes are all consistent in showing that under the same conditions the initial rate for the Hb–Hp complex is roughly ten times that of the HbA sample as displayed in Table 1 along with the corresponding P50 and n1/2 values described above. The shown rates are the average for two measurements for each sample. A very similar order of magnitude difference for the rates is seen for similar samples (both at pH 7.4 and at pH 7.0) probed using the full Q band absorption as the basis for deriving the initial decay of the deoxy population (not shown). Table 1 also contains the initial rate for ββ XL P2K HbA which is comparable to that of the Hb–Hp complex. An enhancement of this magnitude is also reported for other maleimide modified Hbs both with [32,49] and without [27] attached PEG. We compared a large series of PEGylated Hbs to maleimide modified Hbs and find that modification of β93 through the maleimide reagent induces comparable rate enhancements either with or without attachment of PEG (unpublished results).
Fig. 4.
Representative time course for the decay of the deoxy heme populations (as monitored by the absorbance at 430 nm) of HbA and Hb–Hp subsequent to the addition of a slight excess of nitrite to the corresponding deoxy heme populations (1.1:1). The heme concentration for both samples was 0.24 mM in BisTris pH 7.0 buffer heme concentration. The initial rates (Table 1) were calculated based on the best linear fit to the presented data.
The impact of L35 on kinetic traces associated with the nitrite reductase reaction
Fig. 5 shows the time dependence of the Sôret band at 430 nm over the course of the nitrite reductase reaction at pH 7.4. In panel A it can be seen that in the absence of the allosteric effector, HbA exhibits the characteristic time dependent acceleration in rate attributable to the progressive build up of met heme and ferrous NO heme resulting in a shift in the allosteric equilibrium from T to R. In the presence of L35, a very potent allosteric effector, the allosteric transition is clearly no longer in evidence on the time scale of the reaction. The trace remains slow (T-like) over the full duration of the observed process. In contrast, as can be seen in panel B, L35 has no discernible effect on the fast kinetic trace from Hb–Hp. The fast kinetics are not only consistent with the hemes in the Hb–Hp complex being R like but also not subject to significant allosteric regulation as anticipated for the hemes within the allosterically inert dimer.
Fig. 5.
Time course for nitrite reductase reaction of: A. ■ deoxy HbA + 3:1 nitrite:heme; ○ deoxy HbA + 3:1 nitrite:heme + L35; and B. ■ Hb–Hp 3:1 nitrite:heme; ○ Hb–Hp 3:1 nitrite:heme + L35; all in 0.05 M Bis–Tris, pH 7.4 with signal being monitored at 430 nm.
Discussion
The above results are all consistent with the hemes in the Hb–Hp complex having largely unaltered functional properties attributable to a αβ dimer of HbA. Most significantly with respect to bioactivity related to the toxicity of acellular HbA, the complex exhibits an enhanced rate for the production of NO from nitrite via the nitrite reductase reaction of deoxy heme with nitrite. The approximately ten fold enhancement of the initial rate over HbA, is similar to the enhancements seen for high oxygen affinity PEGylated HBOCs that are not vasoactive (vasoconstriction, increased mean arterial blood pressure) when infused into animal models. We (unpublished results with Cabrales, UCSD) and others [32] see similar enhancements in the rate for both PEGylated and non-PEGylated high oxygen affinity Hbs that have been modified at the Cys beta 93 position with the introduction of a maleimide group on the beta 93 thiol. All of these modified Hbs exhibit a shift in stability towards the R quaternary state. Importantly, based on its functional similarity to the PEGylated Hbs with respect to nitrite reductase activity, the Hb–Hp complex generates heme populations that should have favorable vasoactivity properties without the need for chemical or mutagenic modifications. The increased production of NO from nitrite should act synergistically with the capacity of the complex to inhibit free radical damage from acellular Hb. Results obtained using IV infused nanoparticles that release low levels of NO over a sustained period within the circulation [64], show that at low levels, NO in the vasculature inhibits HBOC induced vasoconstriction [65], reduces inflammation [64] and limits the inflammatory cascade/macrophage activation that leads to hemorrhagic shock [66]. Together all of the results point to the Hb–Hp complex having a pivotal role in maintaining vascular homeostasis in the presence of the pro-inflammatory and provasoconstrictive stimulus associated with the presence of acellular HbA in the circulation.
Recent published animal studies showed that the infusion of Hb and after complexation with Hp prevented oxidative injuries to kidneys which normally accompany the infusion in animals of free and chemically modified Hbs [12]. Additionally, and unexpectedly the normal hypertensive responses seen in response to the infusion of free Hb were completely eliminated in two animal models of exchange transfusion [59]. Biochemically, we and others have also shown unequivocally that complexation with Hp shields key amino acids on the Hb molecule from oxidative damage triggered by the presence of oxidants such as H2O2 [12,67]. In contrast to uncomplexed Hb, the ferryl species formed in the Hb–Hp complex solution persisted throughout the experiment. In follow up EPR, photometric and spectroelectrochemical studies carried out by our group we showed that Hp stabilizes ferryl and its radical and kinetically rendering these damaging species inactive (Cooper et al.). Haptoglobin binding stabilizes hemoglobin ferryl iron and the globin radical on tyrosine β-145, Antioxidant and Redox Signaling (under revision). The redox potential (E1/2) as measured by spectroelectrochemistry showed that the Hb–Hp complex was +54 mV, showing it to be much more easily oxidized than uncomplexed Hb (E1/2 = +125 mV). The ~70 mV shift of the reduction potential for Hb–Hp system resulting in a less redox active molecule (Banerjee et al., Haptoglobin alters oxygenation and oxidation of hemoglobin and decreases propagation of peroxide-induced oxidative reactions Radical Biology and Medicine (under revision)).
Under normal circumstances, the endothelial cell layer, RBC-free plasma zone, unstirred plasma layer around RBC, and RBC membrane provide diffusion barriers, which impair rapid, unhindered interactions between RBC Hb and endothelial NO [68]. Upon hemolysis or infusion of cell-free Hb, these diffusion barriers are no longer effective in preventing free Hb from reaching and reacting with endothelial NO. The vasoactivity of cell-free Hb-based blood substitutes is attributed to NO scavenging by free Hb which triggers vasoconstriction and subsequent elevation of blood pressure. There are a number of biochemical scenarios in which the Hb–Hp complex is envisioned to provide a protection against vascular toxicity and blood pressure controlling mechanisms. First, the molecular size of the Hb–Hp complex (~180–200 A) unlike smaller Hb dimers (~15 A) [68] can provide a physical separation away from RBC free zone thus reducing the interaction of NO with the complex. Additionally, our current data clearly show that following Hp binding, the heme active sites of the Hb subunits have high reactivity with O2 and nitrite. The increase in nitrite reductase activity for the Hb–Hp complex is likely due to both an enhanced access of nitrite to the heme as seen with the other ligands (CO and O2) and a decrease in redox potential stemming from the R-like properties of the dimer. If there is adequate nitrite present, the Hb–Hp complex should be effective at regenerating NO under conditions where NO is being scavenged. Studies with NO releasing nanoparticles have shown that even minor sustained production of NO can counter inflammation in the circulation. It would appear that functional properties of the Hb–Hp complex all favor minimizing the inflammatory cascade that can be triggered by the introduction of acellular Hb.
Acknowledgments
Francine Wood for carrying out the oxygen equilibrium studies on the hemoglobin and the hemoglobin/haptoglobin complex solutions. Fantao Meng for contributing the ββXL P2K modified Hb. This work was supported through the NHLBI (NIH R21 HL106421) (JMF) and FJC, a Foundation of Philanthropic Funds (JMF).
References
- 1.Alayash AI. Setbacks in blood substitutes research and development: a biochemical perspective. Clin Lab Med. 2010;30:381–389. doi: 10.1016/j.cll.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 2.Cooper CE. Radical producing and consuming reactions of hemoglobin: how can we limit toxicity? Artif Organs. 2009;33:110–114. doi: 10.1111/j.1525-1594.2008.00694.x. [DOI] [PubMed] [Google Scholar]
- 3.Donadee C, Raat NJ, Kanias T, Tejero J, Lee JS, Kelley EE, Zhao X, Liu C, Reynolds H, Azarov I, Frizzell S, Meyer EM, Donnenberg AD, Qu L, Triulzi D, Kim-Shapiro DB, Gladwin MT. Nitric oxide scavenging by red blood cell microparticles and cell-free hemoglobin as a mechanism for the red cell storage lesion. Circulation. 2011;124:465–476. doi: 10.1161/CIRCULATIONAHA.110.008698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reiter CD, Wang X, Tanus-Santos JE, Hogg N, Cannon RO, 3rd, Schechter AN, Gladwin MT. Cell-free hemoglobin limits nitric oxide bioavailability in sickle-cell disease. Nat Med. 2002;8:1383–1389. doi: 10.1038/nm1202-799. [DOI] [PubMed] [Google Scholar]
- 5.Rother R, Bell L, Hillmen P, Gladwin M. The clinical sequelae of intravascular hemolysis and extracellular plasma hemoglobin: a novel mechanism of human disease. JAMA. 2005;293:1653–1662. doi: 10.1001/jama.293.13.1653. [DOI] [PubMed] [Google Scholar]
- 6.Dou Y, Maillett D, Eich R, Olson J. Myoglobin as a model system for designing heme protein based blood substitutes. Biophys Chem. 2002;98:127–148. doi: 10.1016/s0301-4622(02)00090-x. [DOI] [PubMed] [Google Scholar]
- 7.Alayash AI. Oxygen therapeutics: can we tame haemoglobin? Nat Rev Drug Discov. 2004;3:152–159. doi: 10.1038/nrd1307. [DOI] [PubMed] [Google Scholar]
- 8.Alayash AI. Haptoglobin: old protein with new functions. Clin Chim Acta. 2011;412:493–498. doi: 10.1016/j.cca.2010.12.011. [DOI] [PubMed] [Google Scholar]
- 9.Schaer DJ, Alayash AI. Clearance and control mechanisms of hemoglobin from cradle to grave. Antioxid Redox Signal. 2010;12:181–184. doi: 10.1089/ars.2009.2923. [DOI] [PubMed] [Google Scholar]
- 10.Buehler PW, D’Agnillo F, Schaer DJ. Hemoglobin-based oxygen carriers: from mechanisms of toxicity and clearance to rational drug design. Trends Mol Med. 2010;16:447–457. doi: 10.1016/j.molmed.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 11.Wicher KB, Fries E. Evolutionary aspects of hemoglobin scavengers. Antioxid Redox Signal. 2010;12:249–259. doi: 10.1089/ars.2009.2760. [DOI] [PubMed] [Google Scholar]
- 12.Buehler PW, Abraham B, Vallelian F, Linnemayr C, Pereira CP, Cipollo JF, Jia Y, Mikolajczyk M, Boretti FS, Schoedon G, Alayash AI, Schaer DJ. Haptoglobin preserves the CD163 hemoglobin scavenger pathway by shielding hemoglobin from peroxidative modification. Blood. 2009;113:2578–2586. doi: 10.1182/blood-2008-08-174466. [DOI] [PubMed] [Google Scholar]
- 13.Alfsen A, Chiancone E, Antonini E, Waks M, Wyman J. Studies on the reaction of haptoglobin with hemoglobin and hemoglobin chains. 3. Observations on the kinetics of the reaction of the haptoglobin–hemoglobin complexes with carbon monoxide. Biochim Biophys Acta. 1970;207:395–403. doi: 10.1016/s0005-2795(70)80003-4. [DOI] [PubMed] [Google Scholar]
- 14.Chiancone E, Antonini E, Brunori M, Alfsen A, Lavialle F. Kinetics of the reaction between oxygen and haemoglobin bound to haptoglobin. Biochem J. 1973;133:205–207. doi: 10.1042/bj1330205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nagel RL, Gibson QH. Kinetics of the reaction of carbon monoxide with the hemoglobin–haptoglobin complex. J Mol Biol. 1966;22:249–255. doi: 10.1016/0022-2836(66)90130-6. [DOI] [PubMed] [Google Scholar]
- 16.Nagel RL, Whittenberg JB, Ranney HM. Oxygen equilibria of the hemoglobin–aptoglobin complex. Biochim Biophys Acta. 1965;100:286–289. doi: 10.1016/0304-4165(65)90454-x. [DOI] [PubMed] [Google Scholar]
- 17.Rujan IN, Russu IM. Allosteric effects of chloride ions at the intradimeric alpha1beta1 and alpha2beta2 interfaces of human hemoglobin. Proteins. 2002;49:413–419. doi: 10.1002/prot.10240. [DOI] [PubMed] [Google Scholar]
- 18.Khan I, Dantsker D, Samuni U, Friedman AJ, Bonaventura C, Manjula B, Acharya SA, Friedman JM. Beta 93 modified hemoglobin: kinetic and conformational consequences. Biochemistry. 2001;40:7581–7592. doi: 10.1021/bi010051o. [DOI] [PubMed] [Google Scholar]
- 19.Huang J, Juszczak LJ, Peterson ES, Shannon CF, Yang M, Huang S, Vidugiris GV, Friedman JM. The conformational and dynamic basis for ligand binding reactivity in hemoglobin Ypsilanti (beta 99 asp–>Tyr): origin of the quaternary enhancement effect. Biochemistry. 1999;38:4514–4525. doi: 10.1021/bi982724h. [DOI] [PubMed] [Google Scholar]
- 20.Kwiatkowski LD, Hui HL, Wierzba A, Noble RW, Walder RY, Peterson ES, Sligar SG, Sanders KE. Preparation and kinetic characterization of a series of betaW37 variants of human hemoglobin A: evidence for high-affinity T quaternary structures. Biochemistry. 1998;37:4325–4335. doi: 10.1021/bi970866q. [DOI] [PubMed] [Google Scholar]
- 21.Doyle ML, Holt JM, Ackers GK. Effects of NaCl on the linkages between O2 binding and subunit assembly in human hemoglobin: titration of the quaternary enhancement effect. Biophys Chem. 1997;64:271–287. doi: 10.1016/s0301-4622(96)02235-1. [DOI] [PubMed] [Google Scholar]
- 22.Huang J, Ridsdale A, Wang J, Friedman JM. Kinetic hole burning, hole filling, and conformational relaxation in heme proteins: direct evidence for the functional significance of a hierarchy of dynamical processes. Biochemistry. 1997;36:14353–14365. doi: 10.1021/bi9700274. [DOI] [PubMed] [Google Scholar]
- 23.Doyle ML, Ackers GK. Cooperative oxygen binding, subunit assembly, and sulfhydryl reaction kinetics of the eight cyanomet intermediate ligation states of human hemoglobin. Biochemistry. 1992;31:11182–11195. doi: 10.1021/bi00160a032. [DOI] [PubMed] [Google Scholar]
- 24.Mills FC, Ackers GK. Quaternary enhancement in binding of oxygen by human hemoglobin. Proc Natl Acad Sci USA. 1979;76:273–277. doi: 10.1073/pnas.76.1.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Crawford JH, Isbell TS, Huang Z, Shiva S, Chacko BK, Schechter AN, Darley-Usmar VM, Kerby JD, Lang JD, Jr, Kraus D, Ho C, Gladwin MT, Patel RP. Hypoxia, red blood cells and nitrite regulate NO-dependent hypoxic vasodilation. Blood. 2006;107:566–574. doi: 10.1182/blood-2005-07-2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang KT, Keszler A, Patel N, Patel RP, Gladwin MT, Kim-Shapiro DB, Hogg N. The reaction between nitrite and deoxyhemoglobin. Reassessment of reaction kinetics and stoichiometry. J Biol Chem. 2005;280:31126–31131. doi: 10.1074/jbc.M501496200. [DOI] [PubMed] [Google Scholar]
- 27.Huang Z, Shiva S, Kim-Shapiro DB, Patel RP, Ringwood LA, Irby CE, Huang KT, Ho C, Hogg N, Schechter AN, Gladwin MT. Enzymatic function of hemoglobin as a nitrite reductase that produces NO under allosteric control. J Clin Invest. 2005;115:2099–2107. doi: 10.1172/JCI24650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roche CJ, Dantsker D, Samuni U, Friedman JM. Nitrite reductase activity of sol–gel-encapsulated deoxyhemoglobin. Influence of quaternary and tertiary structure. J Biol Chem. 2006;281:36874–36882. doi: 10.1074/jbc.M603914200. [DOI] [PubMed] [Google Scholar]
- 29.Roche CJ, Malashkevich V, Balazs TC, Dantsker D, Chen Q, Moreira J, Almo SC, Friedman JM, Hirsch RE. Structural and functional studies indicating altered redox properties of hemoglobin E: IMPLICATIONS FOR PRODUCTION OF BIOACTIVE NITRIC OXIDE. J Biol Chem. 2011;286:23452–23466. doi: 10.1074/jbc.M110.183186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cantu-Medellin N, Vitturi DA, Rodriguez C, Murphy S, Dorman S, Shiva S, Zhou Y, Jia Y, Palmer AF, Patel RP. Effects of T- and R-state stabilization on deoxyhemoglobin–nitrite reactions and stimulation of nitric oxide signaling. Nitric Oxide. 2011;25:59–69. doi: 10.1016/j.niox.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cabrales P, Friedman J. Pegylated hemoglobins mechanisms to avoid vasoconstriction and maintain perfusion. Transfus Altern Transfus Med. 2007;9:281–293. [Google Scholar]
- 32.Lui FE, Dong P, Kluger R. Polyethylene glycol conjugation enhances the nitrite reductase activity of native and cross-linked hemoglobin. Biochemistry. 2008;47:10773–10780. doi: 10.1021/bi801116k. [DOI] [PubMed] [Google Scholar]
- 33.Tsai AG, Cabrales P, Manjula BN, Acharya SA, Winslow RM, Intaglietta M. Dissociation of local nitric oxide concentration and vasoconstriction in the presence of cell-free hemoglobin oxygen carriers. Blood. 2006;108:3603–3610. doi: 10.1182/blood-2006-02-005272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vandegriff KD, Malavalli A, Wooldridge J, Lohman J, Winslow RM. MP4, a new nonvasoactive PEG-Hb conjugate. Transfusion. 2003;43:509–516. doi: 10.1046/j.1537-2995.2003.00341.x. [DOI] [PubMed] [Google Scholar]
- 35.Winslow RM. MP4, a new nonvasoactive polyethylene glycol–hemoglobin conjugate. Artif Organs. 2004;28:800–806. doi: 10.1111/j.1525-1594.2004.07392.x. [DOI] [PubMed] [Google Scholar]
- 36.Samuni U, Juszczak L, Dantsker D, Khan I, Friedman AJ, Perez-Gonzalez-de-Apodaca J, Bruno S, Hui HL, Colby JE, Karasik E, Kwiatkowski LD, Mozzarelli A, Noble R, Friedman JM. Functional and spectroscopic characterization of half-liganded iron–zinc hybrid hemoglobin: evidence for conformational plasticity within the T state. Biochemistry. 2003;42:8272–8288. doi: 10.1021/bi020648j. [DOI] [PubMed] [Google Scholar]
- 37.Perutz MF, Ladner JE, Simon SR, Ho C. Influence of globin structure on the state of the heme I Human deoxyhemoglobin. Biochemistry. 1974;13:2163–2173. doi: 10.1021/bi00707a026. [DOI] [PubMed] [Google Scholar]
- 38.Antonini E, Brunori M. Hemoglobins and Myoglobins in Their Reactions With Ligands. North-Holland Publishing Co; Amsterdam: 1971. [Google Scholar]
- 39.Kiger L, Stetzkowski-Marden F, Poyart C, Marden MC. Correlation of carbon monoxide association rates and the position of absorption band III in hemoproteins. Eur J Biochem. 1995;228:665–668. doi: 10.1111/j.1432-1033.1995.tb20307.x. [DOI] [PubMed] [Google Scholar]
- 40.Sassaroli M, Rousseau DL. Time dependence of near-infrared spectra of photodissociated hemoglobin and myoglobin. Biochemistry. 1987;26:3092–3098. doi: 10.1021/bi00385a022. [DOI] [PubMed] [Google Scholar]
- 41.Chavez MD, Courtney SH, Chance MR, Kuila D, Nocek J, Hoffman BM, Friedman JM, Ondrias MR. Structural and functional significance of inhomogeneous line broadening of band III in hemoglobin and Fe–Mn hybrid hemoglobins. Biochemistry. 1990;29:4844–4852. doi: 10.1021/bi00472a014. [DOI] [PubMed] [Google Scholar]
- 42.Schiro G, Cammarata M, Levantino M, Cupane A. Spectroscopic markers of the T<–>R quaternary transition in human hemoglobin. Biophys Chem. 2005;114:27–33. doi: 10.1016/j.bpc.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 43.Rousseau DL, Friedman JM. Transient and cryogenic studies of photodissociated hemoglobin and myoglobin. In: Spiro TG, editor. Biological Applications of Raman Spectroscopy. John Wiley & Sons; New York: 1988. pp. 133–215. [Google Scholar]
- 44.Schiro G, Cupane A. Quaternary relaxations in sol–gel encapsulated hemoglobin studied via NIR and UV spectroscopy. Biochemistry. 2007;46:11568–11576. doi: 10.1021/bi701166m. [DOI] [PubMed] [Google Scholar]
- 45.Bonaventura C, Cashon R, Bonaventura J, Perutz M, Fermi G, Shih DT. Involvement of the distal histidine in the low affinity exhibited by Hb Chico (Lys beta 66—Thr) and its isolated beta chains. J Biol Chem. 1991;266:23033–23040. [PubMed] [Google Scholar]
- 46.Vallelian F, Pimenova T, Pereira CP, Abraham B, Mikolajczyk MG, Schoedon G, Zenobi R, Alayash AI, Buehler PW, Schaer DJ. The reaction of hydrogen peroxide with hemoglobin induces extensive alpha–globin crosslinking and impairs the interaction of hemoglobin with endogenous scavenger pathways. Free Radic Biol Med. 2008;45:1150–1158. doi: 10.1016/j.freeradbiomed.2008.07.013. [DOI] [PubMed] [Google Scholar]
- 47.Manjula BN, Malavalli A, Smith PK, Chan NL, Arnone A, Friedman JM, Acharya AS. Cys-93-betabeta-succinimidophenyl polyethylene glycol, hemoglobin A. Intramolecular cross-bridging of hemoglobin outside the central cavity. J Biol Chem. 2000;275(2000):5527–5534. doi: 10.1074/jbc.275.8.5527. [DOI] [PubMed] [Google Scholar]
- 48.Hu T, Manjula BN, Li D, Brenowitz M, Acharya SA. Influence of intramolecular cross-links on the molecular, structural and functional properties of PEGylated haemoglobin. Biochem J. 2007;402:143–151. doi: 10.1042/BJ20061434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lui FE, Kluger R. Enhancing nitrite reductase activity of modified hemoglobin: bis-tetramers and their PEGylated derivatives. Biochemistry. 2009;48:11912–11919. doi: 10.1021/bi9014105. [DOI] [PubMed] [Google Scholar]
- 50.Jia Y, Wood F, Menu P, Faivre B, Caron A, Alayash AI. Oxygen binding and oxidation reactions of human hemoglobin conjugated to carboxylate dextran. Biochim Biophys Acta. 2004;1672:164–173. doi: 10.1016/j.bbagen.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 51.Khan I, Shannon CF, Dantsker D, Friedman AJ, Perez-Gonzalez-de-Apodaca J, Friedman JM. Sol–gel trapping of functional intermediates of hemoglobin: geminate and bimolecular recombination studies. Biochemistry. 2000;39:16099–16109. doi: 10.1021/bi000536x. [DOI] [PubMed] [Google Scholar]
- 52.Samuni U, Dantsker D, Juszczak LJ, Bettati S, Ronda L, Mozzarelli A, Friedman JM. Spectroscopic and functional characterization of T state hemoglobin conformations encapsulated in silica gels. Biochemistry. 2004;43:13674–13682. doi: 10.1021/bi048531d. [DOI] [PubMed] [Google Scholar]
- 53.Samuni U, Roche CJ, Dantsker D, Friedman JM. Conformational dependence of hemoglobin reactivity under high viscosity conditions: the role of solvent slaved dynamics. J Am Chem Soc. 2007;129:12756–12764. doi: 10.1021/ja072342b. [DOI] [PubMed] [Google Scholar]
- 54.Samuni U, Roche CJ, Dantsker D, Juszczak LJ, Friedman JM. Modulation of reactivity and conformation within the T-quaternary state of human hemoglobin: the combined use of mutagenesis and sol–gel encapsulation. Biochemistry. 2006;45:2820–2835. doi: 10.1021/bi050010i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Salhany JM. Kinetics of reaction of nitrite with deoxy hemoglobin after rapid deoxygenation or predeoxygenation by dithionite measured in solution and bound to the cytoplasmic domain of band 3 (SLC4A1) Biochemistry. 2008;47:6059–6072. doi: 10.1021/bi8000819. [DOI] [PubMed] [Google Scholar]
- 56.Grubina R, Basu S, Tiso M, Kim-Shapiro DB, Gladwin MT. Nitrite reductase activity of hemoglobin S (sickle) provides insight into contributions of heme redox potential versus ligand affinity. J Biol Chem. 2008;283:3628–3638. doi: 10.1074/jbc.M705222200. [DOI] [PubMed] [Google Scholar]
- 57.Lalezari I, Lalezari P, Poyart C, Marden M, Kister J, Bohn B, Fermi G, Perutz MF. New effectors of human hemoglobin: structure and function. Biochemistry. 1990;29:1515–1523. doi: 10.1021/bi00458a024. [DOI] [PubMed] [Google Scholar]
- 58.Peterson ES, Shinder R, Khan I, Juczszak L, Wang J, Manjula B, Acharya SA, Bonaventura C, Friedman JM. Domain-specific effector interactions within the central cavity of human adult hemoglobin in solution and in porous sol–gel matrices: evidence for long-range communication pathways. Biochemistry. 2004;43:4832–4843. doi: 10.1021/bi035481o. [DOI] [PubMed] [Google Scholar]
- 59.Boretti FS, Buehler PW, D’Agnillo F, Kluge K, Glaus T, Butt OI, Jia Y, Goede J, Pereira CP, Maggiorini M, Schoedon G, Alayash AI, Schaer DJ. Sequestration of extracellular hemoglobin within a haptoglobin complex decreases its hypertensive and oxidative effects in dogs and guinea pigs. J Clin Invest. 2009;119:2271–2280. doi: 10.1172/JCI39115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nagel RL, Rothman MC, Bradley TB, Jr, Ranney HM. Comparative haptoglobin binding properties of oxyhemoglobin and deoxyhemoglobin. J Biol Chem. 1965;240:4543–4545. [PubMed] [Google Scholar]
- 61.Silva MM, Rogers PH, Arnone A. A third quaternary structure of human hemoglobin A at 1.7-A resolution. J Biol Chem. 1992;267:17248–17256. [PubMed] [Google Scholar]
- 62.Mueser TC, Rogers PH, Arnone A. Interface sliding as illustrated by the multiple quaternary structures of liganded hemoglobin. Biochemistry. 2000;39:15353–15364. doi: 10.1021/bi0012944. [DOI] [PubMed] [Google Scholar]
- 63.Lukin JA, Kontaxis G, Simplaceanu V, Yuan Y, Bax A, Ho C. Quaternary structure of hemoglobin in solution. Proc Natl Acad Sci USA. 2003;100:517–520. doi: 10.1073/pnas.232715799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cabrales P, Han G, Roche C, Nacharaju P, Friedman AJ, Friedman JM. Sustained release nitric oxide from long-lived circulating nanoparticles. Free Radic Biol Med. 2010;49:530–538. doi: 10.1016/j.freeradbiomed.2010.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cabrales P, Han G, Nacharaju P, Friedman AJ, Friedman JM. Reversal of hemoglobin-induced vasoconstriction with sustained release of nitric oxide. Am J Physiol Heart Circ Physiol. 2011;300:H49–H56. doi: 10.1152/ajpheart.00665.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nachuraju P, Friedman AJ, Friedman JM, Cabrales P. Exogenous nitric oxide prevents cardiovascular collapse during hemorrhagic shock. Resuscitation. 2011;82:607–613. doi: 10.1016/j.resuscitation.2010.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pimenova T, Pereira CP, Gehrig P, Buehler PW, Schaer DJ, Zenobi R. Quantitative mass spectrometry defines an oxidative hotspot in hemoglobin that is specifically protected by haptoglobin. J Proteome Res. 2010;9:4061–4070. doi: 10.1021/pr100252e. [DOI] [PubMed] [Google Scholar]
- 68.Alayash AI. Haptoglobin: old protein with new functions. Clin Chim Acta. 2011;412:493–498. doi: 10.1016/j.cca.2010.12.011. [DOI] [PubMed] [Google Scholar]