Abstract
Background
We compared brain-map correlations of relative cerebral glucose metabolism (rCMRglu) with psychopathologic factors derived from the self-rated Beck Depression Inventory (BDI) and factors from the clinician-rated Hamilton Depression Rating Scale (HDRS) factors, seeking an anatomic basis for differences in self and clinician ratings.
Methods
[18F]-FDG Positron Emission Tomography generated rCMRglu, SPM-estimated, voxel-level, brain correlation maps with BDI factor scores and HDRS factor scores in medication-free major depressive disorder.
Results
Regional brain correlates of BDI are more extensive than HDRS, even when adjusting for variance accounted for by the HDRS. Factors comprising the BDI were associated with distinct cortical and subcortical regions. The degree of overlap in factor correlation brain maps is explained by the variance shared by BDI and HDRS factor scores.
Conclusion
Self and clinician-rated aspects of depression have common and distinct neuroanatomic correlates that reflect correlations between rating scales, but correlations between glucose metabolism and self-rated depression were anatomically more extensive in this sample. Findings highlight the importance and biological underpinnings of these subjective features of major depression.
Keywords: Depression, PET, FDG, Factor analysis, Brain regions
1. Introduction
Clinician ratings of the symptom severity of major depression have been the gold standard for evaluation of efficacy of antidepressant treatments for more than 40 years. However, subjective aspects of the disorder may be more relevant for social and functional recoveries, long-term adjustment, treatment adherence, or associated behaviors, such as suicide attempts. Subjective severity of depression is only modestly correlated with clinician-rated severity (shared variance range is 10–25%) (Adda et al., 1997; Akdemir et al., 2001; Hautzinger, 1991; Uehara et al., 2005; Wichowicz et al., 2004), responds more slowly to antidepressant treatment (Adda et al., 1997; Ambrosini et al., 1999; Posternak and Miller, 2001; Thase et al., 1991), and is a stronger predictor of suicide attempts (Grunebaum et al., 2005; Mann et al., 2008, 1999; Oquendo et al., 2007; Oquendo et al., 2004). We have previously reported that in untreated major depression, component factors of the clinician-rated Hamilton Depression Rating Scale correlate with glucose metabolism in distinct brain regions (Milak et al., 2005). Factor analysis of the Hamilton Depression Rating Scale (HDRS) and Beck Depression Inventory (BDI) indicates that the BDI captures subjective “cognitive factors” that are only minimally represented in the HDRS. When these scales are combined for factor analyses, the two scales tend to yield orthogonal factors (Steer et al., 1987; Uher et al., 2008).
These differences raise the question as to whether subjective perception of depression severity and its components involves different neural circuits or brain regions compared with clinician ratings, that may explain the limited correlation between the scales, as well as differences between these set of symptoms in their temporal response to antidepressant treatment. Therefore, we compared correlation maps of Positron Emission Tomography (PET) [18F]-FDG relative regional cerebral metabolic rates of glucose in untreated major depression, at the voxel level, with total score and factor scores of HDRS and BDI. We sought to determine whether the total score and the factor scores for the clinician-rated scale and the self-rated scale correlate with different brain regions. We hypothesized that correlations with the “objective” clinician-rated measure would be stronger, and would likely incorporate regions associated with “subjective” self-rated symptoms. The patient sample and the PET scan data were almost the same as our previously published study on correlates of the HDRS (Milak et al., 2005).
2. Methods
2.1. Subjects
40 depressed patients (Table 1) who met DSM-IV (American Psychiatric Association, 1994) criteria for current Major Depressive Disorder, and with a score of 16 or greater on the 17-item Hamilton Depression Rating Scale (HDRS) (Hamilton, 1960), were enrolled into the study after giving written informed consent as approved by the Institutional Review Board. Comorbidities: 1) One subject had dysthymia. 2) Five subjects had comorbid anxiety disorders (3 PTSD, 2 panic disorder, 1 simple phobia). 3) No subject had current substance abuse or dependence, 14 of 40 subjects had past histories of substance dependence (11 alcohol [3 of these with additional cocaine, 2 with additional marijuana] and 3 marijuana). 4) Sixteen of 40 subjects had a past suicide attempt. Patients were administered the HDRS and Beck Depression Inventory (BDI) (Beck and Beamesderfer, 1974) within minutes of each other on the day of the PET scan. Demographics, past psychiatric and medical histories, developmental and family histories were recorded on the Columbia Baseline Demographic Form. Three subjects who had been in our previously published study of the HDRS and regional brain metabolism (Milak et al., 2005) were not included here because they did not complete the BDI.
Table 1.
Demographic and clinical data for subjects undergoing Positron Emission Tomography (N = 40).
| Mean | SD | |
|---|---|---|
| Age (y) | 38.8 | 13.5 |
| Education (y) | 15.5 | 2.8 |
| Age at 1st MDE (y) | 24.5 | 15.0 |
| Number of MDEs | 3.7 | 2.5 |
| GAF | 43.9 | 10.2 |
| % Female | 57.5 |
Abbreviations: MDE = Major Depressive Episode; GAF = Global Assessment of Functioning; Min = minimum; Max = maximum.
2.2. Depression rating
Subjects were administered both the Hamilton Depression Rating Scale (HDRS, 24-item scale) and Beck Depression Inventory (BDI), as part of an extensive clinical assessment including both diagnostic interviews and multiple self-report rating scales. Total scores were computed, as well as factor scores derived from a previously reported factor analysis of both scales (Grunebaum et al., 2005). Correlations between rCMRglu and the five factor scores from the HDRS (depressed mood, loss of motivation, anxiety, insomnia, and thought disturbance) have previously been reported (Milak et al., 2005). Factor scores derived for the BDI were labeled: 1. Subjective Depression (items 1, 2, 4, 12, 13, 15, 17 and 21; corresponding to ratings of sadness, loss of future orientation, dissatisfaction, indecisiveness, concern about appearance, work inhibition, feeling tired and loss of libido), 2. Self-Blame (items 3, 5, 6, 7 and 8; ratings of sense of failure, feelings of guilt, feeling punished, sense of disappointment, and self-consciousness), and 3. Somatic Complaints (items 16, 18 and 19; ratings of sleep disturbance, appetite disturbance and weight loss).
2.3. PET studies
This was a treatment-seeking sample, all of whom were unmedicated at the time of their scans. Subjects who were not medication free when first recruited were tapered off their medication such that they were medication free for a minimum of 14 days prior to scanning (6 weeks in the case of fluoxetine and one month in the case of oral antipsychotics). Patients were free of medical illnesses based on history, physical examination and laboratory tests. Pregnant females were excluded. Pre-menopausal female subjects were studied within five days after onset of menses.
Scan details were described previously (Milak et al., 2005). A bolus of approximately 10 mCi [18F]-FDG was administered intravenously. Subjects gazed at cross hairs in a dimmed room during the first 15 min of the 18F-FDG distribution phase, rested quietly for another 15 min before moving to the scanner where they lay supine for 10 more minutes prior to the emission scan.
2.4. Image analysis
Statistical analysis was performed using Statistical Parametric Mapping (SPM 99) (Institute of Neurology, University College of London, UK) implemented in Matlab 5 (Math-works Inc., USA) (Friston et al., 1995). The summed reconstructed frames were spatially transformed into the MNI (Montreal Neurological Institute, McGill University, CA) standard template to remove the inter-subject structural variability (Friston et al., 1995). Affine transformation was performed to determine the 12 optimal parameters to register the brain PET to the MNI template. Spatially normalized images were smoothed by convolution with an isotropic Gaussian kernel of 12 mm. The effects of global metabolism were controlled by normalizing individual voxel counts to total gray matter counts (global normalization and proportional scaling in SPM). To determine the regions where rCMRglu correlates with factors of BDI, a voxel-level correlation analysis was performed using the general linear model. Height threshold was set a priori to p < 0.01 and extent threshold was set to p < 0.05 after correction for multiple comparisons by SPM. Stereotaxic coordinates reported are Talairach Atlas (Talairach and Tournoux, 1988) coordinates, converted from MNI coordinates (Brett, 1999).
Correlation maps between clinical ratings and rCMRglu were generated in SPM. The first of these compared the relationship between BDI total score and rCMRglu with that between HDRS (24 item) total score and rCMRglu. BDI and HDRS scores were then statistically adjusted to remove their shared variance, via linear regression. This provided a measure of depression severity that was unique to each scale. This unique variance was then compared to rCMRglu to determine if the residual variance in either scale was independently associated with underlying metabolism. Brain regional correlation maps with each scale and its factor scores were compared for overlap, then the degree of overlap was compared to the degree to which scale scores correlated with each other. Finally, associations between the 3 factors of the BDI and rCMRglu that measure different aspects of psychopathology were examined, to determine if these factors could be distinguished by their correlation with different brain regions.
3. Results
3.1. BDI scores: correlation with HDRS and component factors
In an expanded cohort of 246 depressed subjects evaluated by the same research team, BDI and HDRS scores were modestly correlated (r = 0.41, p < 0.001; 17.0% shared variance). A correlation matrix of factor scores derived separately from the BDI and HDRS (Table 2) show modest correlations even across the scales (Grunebaum et al., 2005). Overall, the factor structures of the two scales are mostly different.
Table 2.
Correlations between the 24-item Hamilton Depression Rating Scale (HDRS-24) and the Beck Depression Inventory (BDI) and their respective factor scores (N=246).
| BF1 | BF2 | BF3 | BDI total | ||
|---|---|---|---|---|---|
| HF1 | Pearson correlation | 0.40 | 0.44 | 0.10 | 0.40 |
| Psychic depression |
p (2-tailed) | 0.00 | 0.00 | 0.11 | 0.00 |
| % variance shared | 16% | 19% | 1% | 16% | |
| HF2 | Pearson correlation | 0.32 | 0.01 | 0.40 | 0.26 |
| Loss of motivated behavior |
p (2-tailed) | 0.00 | 0.93 | 0.00 | 0.00 |
| % variance shared | 10% | 0% | 16% | 7% | |
| HF3 | Pearson correlation | 0.12 | 0.22 | 0.12 | 0.18 |
| Psychosis | p (2-tailed) | 0.07 | 0.00 | 0.07 | 0.01 |
| % variance shared | 1% | 5% | 1% | 3% | |
| HF4 | Pearson correlation | 0.08 | 0.06 | 0.20 | 0.13 |
| Anxiety | p (2-tailed) | 0.21 | 0.35 | 0.00 | 0.04 |
| % variance shared | 1% | 0% | 4% | 2% | |
| HF5 | Pearson correlation | −0.10 | 0.07 | 0.37 | 0.10 |
| Sleep disturbance |
p (2-tailed) | 0.88 | 0.29 | 0.00 | 0.11 |
| % variance shared | 1% | 0% | 14% | 1% | |
| HDRS-24 | Pearson correlation | 0.37 | 0.33 | 0.36 | 0.41 |
| Total score | p (2-tailed) | 0.00 | 0.00 | 0.00 | 0.00 |
| % variance shared | 14% | 11% | 13% | 17% |
Abbreviations: HF1–5 = HDRS factor 1–5; BF1–3 = BDI factor 1–3.
In the sample that received PET scans (N = 40) (mean BDI = 31.6 ± 10.0; mean HDRS-24 = 29.9 ± 6.3), the correlation matrix was similar to the expanded sample above (Table 3). BDI and HDRS total scores were correlated moderately. The BDI Subjective Depression factor correlated with Loss of Motivation factor and with the Thought Disturbance factor. The BDI Self-Blame factor correlated with Depressed Mood. BDI Somatic Complaint correlated with HDRS Loss of Motivation and Thought Disturbance.
Table 3.
Correlations between the 24-item Hamilton Depression Rating Scale (HDRS-24) and the Beck Depression Inventory (BDI) and their respective factor scores (N = 40, PET subjects only).
| BF1 | BF2 | BF3 | BDI total | ||
|---|---|---|---|---|---|
| HF1 | Pearson correlation | 0.24 | 0.40 | 0.11 | 0.33 |
| Psychic depression |
p (2-tailed) | 0.14 | 0.01 | 0.52 | 0.04 |
| % variance shared | 6% | 16% | 1% | 11% | |
| HF2 | Pearson correlation | 0.40 | −0.04 | 0.60 | 0.37 |
| Loss of motivated |
p (2-tailed) | 0.01 | 0.79 | 0.00 | 0.02 |
| % variance shared | 16% | 0% | 35% | 14% | |
| behavior | |||||
| HF3 | Pearson correlation | 0.33 | 0.24 | 0.34 | 0.36 |
| Psychosis | p (2-tailed) | 0.04 | 0.14 | 0.03 | 0.02 |
| % variance shared | 11% | 6% | 11% | 13% | |
| HF4 | Pearson correlation | −0.03 | 0.23 | 0.09 | 0.12 |
| Anxiety | p (2-tailed) | 0.86 | 0.15 | 0.57 | 0.45 |
| % variance shared | 0% | 5% | 1% | 2% | |
| HF5 | Pearson correlation | 0.14 | 0.26 | 0.25 | 0.26 |
| Sleep disturbance |
p (2-tailed) | 0.38 | 0.11 | 0.11 | 0.11 |
| % variance shared | 2% | 7% | 6% | 7% | |
| HDRS-24 | Pearson correlation | 0.41 | 0.44 | 0.50 | 0.55 |
| Total score | p (2-tailed) | 0.01 | 0.00 | 0.00 | 0.00 |
| % variance shared | 17% | 20% | 25% | 30% |
Abbreviations: HF1–5 = HDRS factor 1–5; BF1–3 = BDI factor 1–3.
3.2. Neuroanatomic correlates of the BDI total score
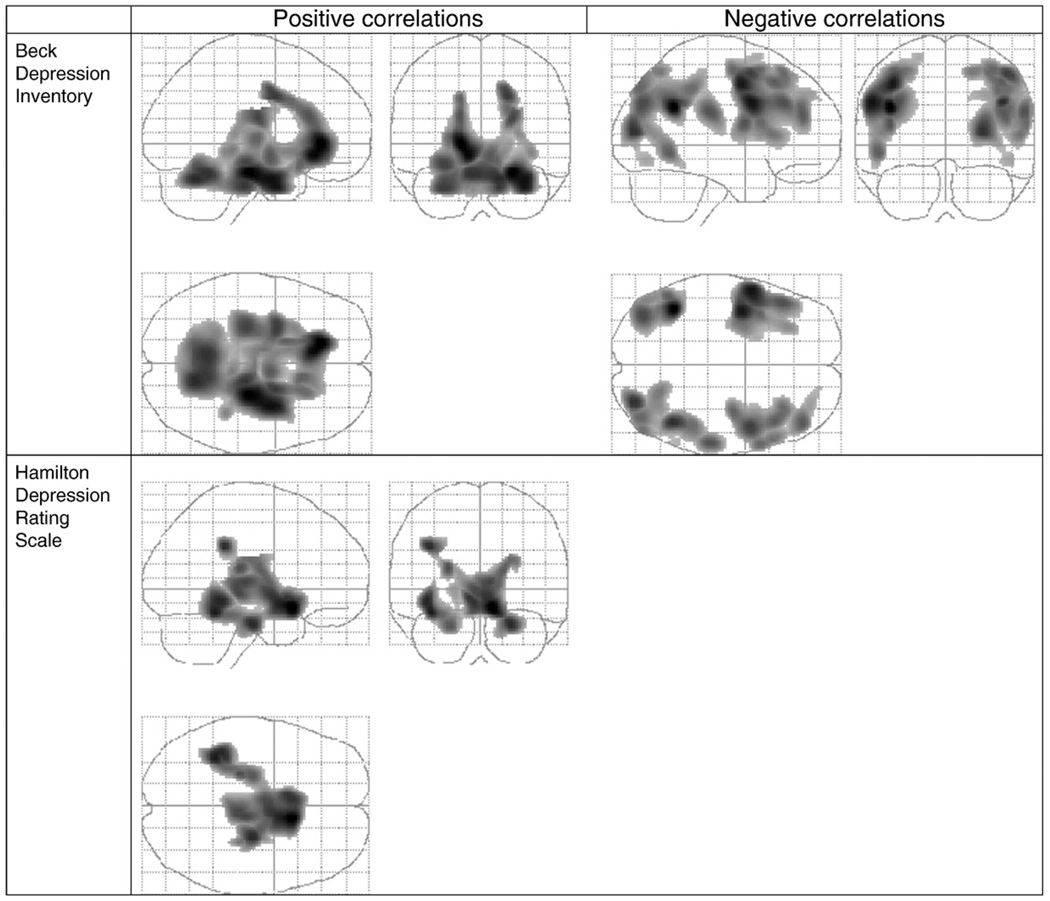
Table 5 summarizes the brain regions that have positive and negative correlations with BDI total score and factor scores. Fig. 1 depicts regions listed in Table 5 that are significantly correlated with the BDI total score and the HDRS total score for the same subjects, for comparison purposes.
Table 5.
Regions in which relative regional cerebral glucose metabolism shows significant correlations with Beck Depression Inventory (BDI) total and factor scores.
| Region | Beck total (+) |
BF1 (+) |
BF2 (+) |
BF3 (+) |
Beck total (−) |
BF1 (−) |
BF3 (−) |
|---|---|---|---|---|---|---|---|
| Right mesiotemporal cortex | AMY, HIP, 34, 28 |
28, 35 | HIP, AMY, 28, 30*, 34*, 35, 36*, 38* |
HIP, AMY | |||
| Left mesiotemporal cortex | AMY, HIP, 34, 28 |
* | HIP, 35, 36* | HIP, AMY | |||
| Ventral medial prefrontal cortex | 10, 25, 47 | BL 25 | 11*, 25 | ||||
| Dorsal medial and lateral prefrontal cortex |
BL 6, 8, 9, 44, 45, 46 |
BL 6, 8, 9, 44, 45, 46 |
BL 4*, 6, 8, 9, 10*, 44, 45 |
||||
| Ventral anterior cingulate | BL 25, 32 | BL 25, 32 | BL 32, 25 | 25, 32 | |||
| Pre-genual cingulate | BL 32, 33 | BL 32, 33 | * | 32, 33 | |||
| Dorsal anterior cingulate | BL 24 | BL 24 | * | 24 | |||
| Dorsal posterior cingulate | |||||||
| Insula | R anterior inferior | ||||||
| Left parietal cortex | 7, 19, 40 | 19, 40 | 1*, 2*, 3*, 4*, 7, 19, 39, 40 |
||||
| Right parietal cortex | 7, 19, 40 | 7, 19, 40 | 2, 7, 19, 40 | ||||
| Left temporal cortex | 19, 37, 39 | 19, 22, 39 | 19, 20, 21, 37, 39 |
||||
| Right temporal cortex | 19, 37, 39 | 19, 21, 22, 37, 39, 40 |
39 | ||||
| Left occipital cortex | 19 | 19 | 19, 39 | ||||
| Right occipital cortex | 19 | 19 | 19, 39 | ||||
| Thalamus | BL VAN | BL VLN, epithalamus |
|||||
| Hypothalamus | BL | BL* | |||||
| Caudate | ventral striatum | ||||||
| Putamen | BL | BL ventral* | |||||
| Globus pallidus | BL* | ||||||
| Midbrain | PAG | PAG* | |||||
| Pons | BL ventral | BL ventral* | |||||
| Cerebellum | BL anterior lobe | BL anterior lobe | BL Vermis* | BL anterior lobe |
Abbreviations: + and − = positive and negative correlations; BL = bilateral; numbers are Brodmann areas; PAG = periaqueductal gray; HIP = hippocampus; AMY = amygdala; VLN = Ventral Lateral Nucleus of the thalamus; VAN = ventral anterior nucleus of the thalamus; *areas not correlating with other factors.
Fig. 1.
Beck Depression Inventory (BDI) and Hamilton Depression Rating Scale (HDRS) total score correlations with relative regional cerebral metabolic rate for glucose (rCMRglu). Regions shown as a volume in a glass brain. Left upper panel: positive correlation with BDI. Right upper panel: negative correlation with BDI. Lower panel positive correlations with HDRS. No regions had significant negative correlations with the total HDRS score.
3.2.1. BDI positive correlations
A single contiguous brain region was identified (17,045 voxels, cluster level p < 0.001; partial R = 0.60; global maximum at Talairach (xyz) coordinates of −16, 33, −2), that includes mostly ventral cortical and subcortical structures including mesiotemporal cortex, ventro-medial prefrontal cortex, peri-genual cingulate, the diencephalon, midbrain, pons and cerebellum.
3.2.2. BDI negative correlations (Fig. 1)
Four clusters of 2981, 5190, 4378 and 4227 voxels respectively, are identified (cluster level p ≤ 0.001: partial R: 0.63, 0.61, 0.57 and 0.55 respectively at −42 −51 28, −55 5 29, 44 −51 32 and 46 6 49 Talairach coordinates, respectively), including dorso-medial and dorsolateral prefrontal cortex and the occipito- and parieto-temporal cortices, bilaterally.
3.3. Orthogonalized BDI and HDRS scores: correlations with rCMRglu
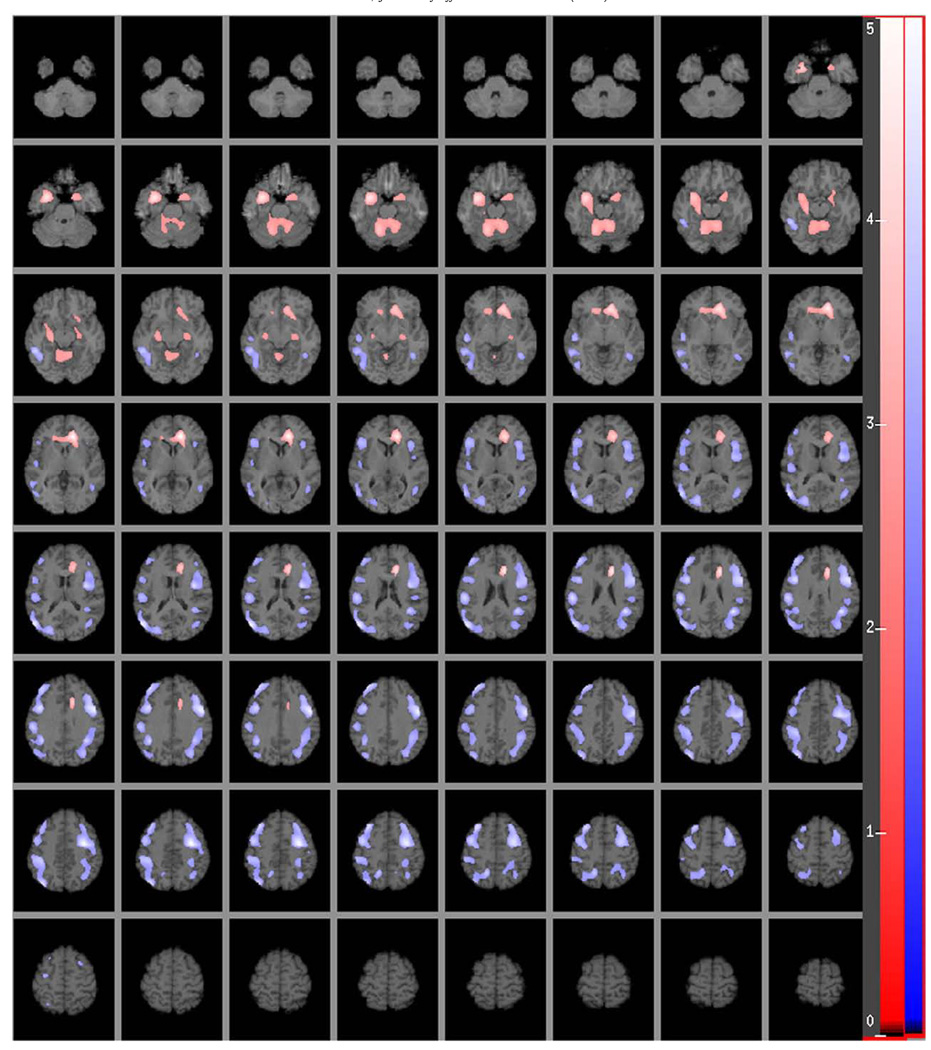
Analysis of the relationship between adjusted HDRS score (variance that it shares with BDI removed) and rCMRglu produced no significant associations, suggesting that all of the variability in HDRS score that was associated with rCMRglu is subsumed by its correlation with BDI score. In contrast, analysis of adjusted BDI score (variance it shares with HDRS removed) and rCMRglu produced a number of significant associations, which are depicted in Fig. 2.
Fig. 2.
Relative regional cerebral metabolic rate for glucose (rCMRglu) correlation with the residuals of the Beck Depression Inventory (BDI) scores while controlling for the variance explained by the Hamilton Depression Rating Scale (HDRS). The color scales in the figure indicate the strength (t score) of the correlation (t score maps are overlaid on a series of transaxial slices (2 mm apart) of a coregistered MRI scan from ~38 mm below to ~72 mm above a line connecting the anterior and posterior commissure). Red to light red regions are uniquely positively correlated with BDI total score residuals, dark to light blue regions are uniquely negatively correlated with BDI total score residuals. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.4. Neuroanatomic correlations with BDI component factors: 1. Subjective Depression
3.4.1. Positive correlation
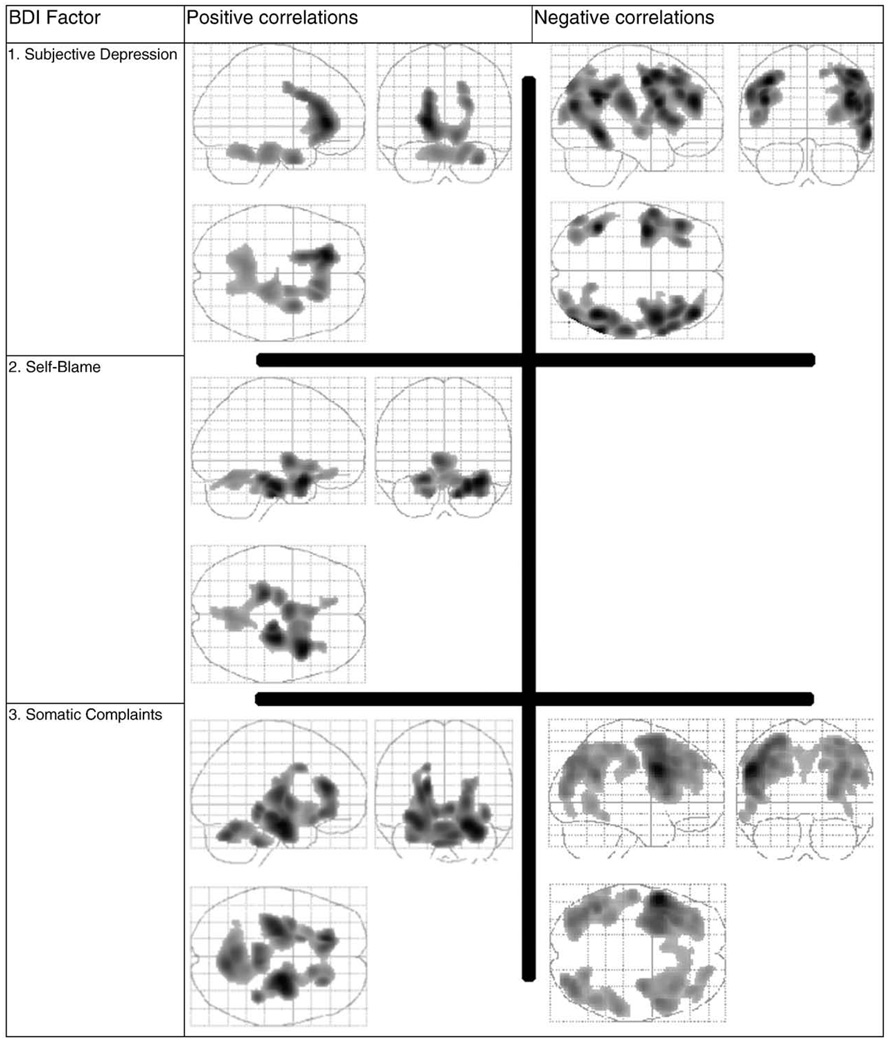
Two clusters of 3388 and 2564 voxels correlate with Subjective Depression (cluster level p ≤ 0.004; partial R: 0.62, and 0.51 respectively at −18 31 2 and 34 1 −27 Talairach xyz coordinates, respectively). These brain regions include the right mesiotemporal cortex, ventro-medial prefrontal cortex, anterior cingulate and the anterior lobes of the cerebellum (Figs. 3 and 4). Parts of these regions are unique to Subjective Depression and do not overlap with regions that correlate with the other two factors (Fig. 4).
Fig. 3.
Regions shown as a volume in a glass brain. Maps of correlations of relative regional glucose metabolic rate (rCMRglu) in major depression with severity of depression as measured by the three Beck Depression Inventory (BDI)-derived factor scores.
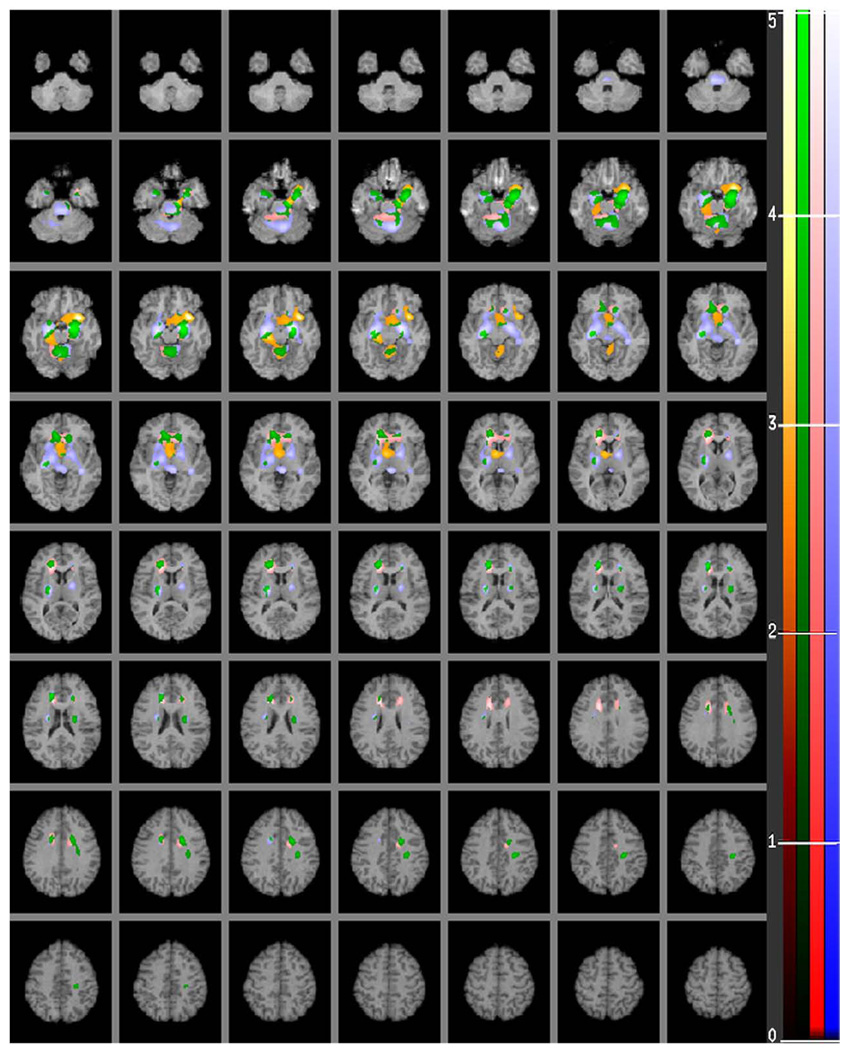
Fig. 4.
A human brain map of positive correlations of relative regional glucose metabolic rate (rCMRglu) in major depression with severity of depression as measured by factors 1, 2 and 3 derived from the Beck Depression Inventory (BDI). The color scales in the figure indicate the strength (t score) of the correlation (t score maps are overlaid on a series of transaxial slices (2 mm apart) of a coregistered MRI scan from 38 mm below to 72 mm above a line connecting the anterior and posterior commissure). Red to light red regions are uniquely positively correlated with Subjective Depression, brown to light orange regions are uniquely positively correlated with Self-Blame, blue to light blue regions are uniquely positively correlated with Somatic Complaint factor and green to light green colored areas depict regions that correlate with factors 1, 2 and/or 3. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.4.2. Negative correlation
Four clusters of 5542, 4487, 3390 and 1519 voxels respectively correlate negatively with Subjective Depression (cluster level p ≤ 0.001, p ≤ 0.001, p ≤ 0.001, p ≤ 0.04 respectively: partial R: −0.56, −0.61, −0.56 and −0.56 respectively at 44 −51 34, 42 44 22, −38 −242 and −42 −49 28 Talairach (xyz) coordinates respectively). These areas include: dorsolateral prefrontal cortex, occipito- and parieto-temporal association cortices (Fig. 3 and Table 5) and areas exclusive correlated negatively with Subjective Depression are shown in Table 5.
3.5. Neuroanatomic correlations with BDI component factors: 2. Self-Blame
3.5.1. Positive correlation
A single contiguous brain region is identified (17,045 voxels) that correlates positively with Self-Blame (cluster level p < 0.001; partial R = 0.54; global maximum at Talairach (xyz) coordinates of 34, 9, −19) (see Figs. 3 and 4, Table 5). It encompasses bilateral mesiotemporal cortex, ventro-medial prefrontal cortex, ventral anterior cingulate, right anterior insula, thalamus, hypothalamus, ventral striatum and the cerebellar vermis. Regions of correlation exclusive to Self-Blame are presented in Fig. 4. The Self-Blame factor shows no negative correlations with rCMRglu.
3.6. Neuroanatomic correlations with BDI component factors: 3. Somatic Complaint
3.6.1. Positive correlation
Two clusters of 9259 and 2613 voxels respectively correlate positively with Somatic Complaint (cluster level p ≤ 0.003 n: partial R: 0.56, and 0.51 respectively at 24 −11 −16 and 8 −65 −22 Talairach (xyz) coordinates respectively). These areas include the bilateral mesiotemporal cortex, hippocampus, amygdala, anterior cingulate, thalamus, epithalamus, ventral lentiform nuclei, pontine nuclei and the anterior lobes of the cerebellum (Figs. 3 and 4).
3.6.2. Negative correlation
Three clusters of 10,225, 5137 and 3398 voxels respectively, correlate negatively with Somatic Complaint (cluster level p ≤ 0.001: partial R: −0.68, −0.56, and −0.53 respectively at −51 7 31, −44 −51 28 and 38 −72 37 Talairach (xyz) coordinates respectively). In contrast with brain areas exclusively negatively correlated with Subjective Depression, which are predominantly right hemispheric cortical regions, the areas that are exclusively negatively correlated with the Somatic Complaint Factor are predominantly left hemispheric cortical regions (see Table 5).
3.7. BDI and HDRS factor correlations and physical overlap between associated brain regions
In order to evaluate the relationship of the BDI and HDRS factors, the individual factor totals from each subject were subjected to correlation analysis (see Table 2 this is expanded sample). Generally, modest correlations were found; only eight of the twenty-eight possible pairs showed correlations with shared variance ranging between 15 and 39% (see Table 2). The 3rd and 4th HDRS factors (anxiety and psychosis respectively) had no rCMRglu correlations in the brain (Milak et al., 2005). In order to evaluate the biological relevance of these correlations between the different factors we determined the correlation between the variances shared by different factors and the degree to which the brain areas associated with the different factors physically overlap (see Table 4). The overall shared variance (r2) for the correlation between shared variances in the factor scores and the percent anatomical overlap between factors and associated brain areas is 58% (see Table 4).
Table 4.
Beck Depression Inventory (BDI) and Hamilton Depression Rating Scale (HDRS) Factor Overlap. This demonstrates that the percent variances shared by BDI and HDRS factors (column 3) are highly correlated with the degree to which the brain areas associated with those factors physically overlap (column 4). This suggests that these associated brain regions differ from each other not by chance but rather due to the relationship in severity between factors (variance not shared with other factors).
| Factor A |
Factor B |
%variance shared (r2) |
%physical overlap |
p | r2 | p | r2 |
|---|---|---|---|---|---|---|---|
| H1 | H2 | 0.00 | 0.50 | 0.027 | 0.845 | 1.13E–06 | 0.58 |
| H1 | H5 | 0.05 | 8.33 | ||||
| H1 | B1 | 0.06 | 1.81 | ||||
| H1 | B2 | 0.16 | 17.02 | ||||
| H1 | B3 | 0.01 | 1.58 | ||||
| H2 | H1 | 0.00 | 0.70 | 0.000 | 0.998 | ||
| H2 | H5 | 0.00 | 0.00 | ||||
| H2 | B1 | 0.16 | 27.71 | ||||
| H2 | B2 | 0.00 | 0.00 | ||||
| H2 | B3 | 0.35 | 57.02 | ||||
| H5 | H1 | 0.05 | 11.30 | 0.202 | 0.468 | ||
| H5 | H2 | 0.00 | 0.00 | ||||
| H5 | B1 | 0.02 | 8.87 | ||||
| H5 | B2 | 0.07 | 10.29 | ||||
| H5 | B3 | 0.06 | 40.48 | ||||
| B1 | H1 | 0.06 | 1.03 | 0.009 | 0.924 | ||
| B1 | H2 | 0.16 | 13.25 | ||||
| B1 | H5 | 0.02 | 3.03 | ||||
| B1 | B2 | 0.14 | 5.11 | ||||
| B1 | B3 | 0.39 | 49.47 | ||||
| B2 | H1 | 0.16 | 26.31 | 0.424 | 0.221 | ||
| B2 | H2 | 0.00 | 0.00 | ||||
| B2 | H5 | 0.07 | 13.21 | ||||
| B2 | B1 | 0.14 | 19.37 | ||||
| B2 | B3 | 0.03 | 30.37 | ||||
| B3 | H1 | 0.01 | 0.66 | 0.025 | 0.852 | ||
| B3 | H2 | 0.35 | 18.43 | ||||
| B3 | H5 | 0.06 | 9.11 | ||||
| B3 | B1 | 0.39 | 33.76 | ||||
| B3 | B2 | 0.03 | 5.49 |
4. Discussion
Self-Rated BDI depression correlates both positively and negatively with more extensive brain regions compared with clinician-rated HDRS scores. This is seen most clearly in Fig. 2, which shows only the brain region correlations with BDI that are independent of the regions correlating with the HDRS score. Notably, there are extensive negative correlations of BDI score with widespread bilateral dorsal cortical areas encompassing prefrontal, occipital, parietal and temporal association cortices; and positive correlations with mesiotemporal and cingulate cortical regions (see Figs. 1 and 2). Conversely, we detected no correlations between clinician-rated HDRS total score and any brain region, after controlling for the variance in relative regional brain activity accounted for by the BDI score.
Similarly, we found positive correlations of BDI scores with not only basal ganglia and subgenual cingulate gyrus, but also extending through the anterior and dorsal anterior cingulate, areas that do not correlate with the HDRS. The negative correlations of the BDI total score with dorsal prefrontal cortex and positive correlations with ventral structures and components of the limbic system are consistent with the proposed involvement of these regions in an affect regulation circuit that is dysfunctional in depression (Mayberg et al., 1997; Mayberg et al., 1999a; Seminowicz et al., 2004). An inverse correlation between subgenual cingulate and right dorsolateral prefrontal activity is reported in both depression and recovery (Mayberg et al., 1999b). In ventral limbic structures rCMRglu can be elevated by induced sadness (Mayberg et al., 1999b) and decreased by treatment-related reductions in depressive symptoms (Brody et al., 2001).
BDI total score correlated with metabolism in dorsal prefrontal cortex, supra- and pre-genual anterior cingulate and paracingulate gyrus, whereas HDRS showed no association with metabolic activity in these structures. Differences in these anatomical correlates may be related to the role of these structures in processing subjective emotional states and experiences (Gusnard et al., 2001) and with representation of mental states of self (Frith and Frith, 1999) and self-reflecting thoughts (Johnson et al., 2002). Therapies such as cognitive therapy that target negative perceptual sets, self-esteem and are effective antidepressants should be evaluated in terms of effects on activity in these brain regions.
We also observed that the factors derived from the BDI correlated with different brain regions and the extent to which those regions overlap is highly correlated with the degree to which these factor scores correlate with each other (Table 4) and thereby provides further evidence that these brain regions are involved in depression.
4.1. BDI factor scores: 1. Subjective Depression
Subjective Depression is positively correlated with rCMRglu in rostral and ventral anterior cingulates, but not left mesiotemporal cortex and diencephalon (see Fig. 4) that correlate with Self-Blame and Somatic Complaint factors. The correlation is preserved even after excluding regional correlations with these factors (Fig. 4). The anterior cingulate comprises different sub-regions (Devinsky et al., 1995; Vogt et al., 1992). The “affective” ventral division of anterior cingulate (BA: 25, 32, and 33) has connections with amygdala, nucleus accumbens, ventro-medial prefrontal cortex, anterior insula and brainstem, whereas the more dorsal, “cognitive division” (BA: 24 and 32) is connected to parietal cortex, supplemental motor cortex and dorsolateral prefrontal cortex (Devinsky et al., 1995). It is this more dorsal part that correlates with Subjective Depression severity and not clinician-rated depression scores.
The anterior and rostral cingulate evaluate motivational content of stimuli and generate goal-directed responses that balance the salience of the response against other considerations like longer-term risk or other logic considerations, such as in the Stroop task (Carter et al., 2000; Devinsky et al., 1995). Responses to stimuli are affected by emotional cues in stimuli (Lane et al., 1997) and selective attention (Whalen et al., 1998). We also find that this region correlates positively with severity of loss of energy, drive and interest. Patients with cingulate lesions display avolition, apathy and amotivation much like components of the Subjective Depression factor (Degos et al., 1993). Higher relative glucose metabolism in this region may reflect a dysfunctional state in the anterior cingulate leading to avolition.
The negative correlations with dorsal cortical rCMRglu are consistent with studies of brain injuries. Reduced goal-directed behavior due to amotivation or apathy is also associated with frontal cortical dysfunction or lesions (Andersson and Bergedalen, 2002). Subjective Depression only, but not Self-Blame or Somatic Complaints, has negative correlations predominantly with right hemispheric cortical regions. This observation appears to contradict the dual-system model of emotion, such as the valence asymmetry hypothesis (Hellige, 1993) which posits that the left hemisphere is involved with positive emotions and the right hemisphere is involved with negative emotions, and is in agreement with recent metaanalyses (Murphy et al., 2003; Wager et al., 2003) that also failed to find support for single and dual-system models of emotion like the valence asymmetry hypothesis. Consistent with our findings, other studies have reported that lower rCBF in dorsolateral frontal cortex in depression correlates with psychomotor slowing, poverty of speech and cognitive impairment (Bench et al., 1993; Bench et al., 1992; Dolan et al., 1994; Dolan et al., 1993; Mayberg et al., 1994).
4.2. BDI factor scores: 2. Self-Blame
The Self-Blame Factor correlates with parts of the mesio-temporal cortex (BA: 30, 34, 36, 38, and 11), ventral anterior nucleus of thalamus, hypothalamus and cerebellar vermis, all brain regions having no correlations with Subjective Depression or Somatic Complaints factors (see Table 5).
Self-Blame in MDD has previously been reported to correlate with lower absolute metabolism bilaterally in frontal cortex and supracallosal cingulate, and with higher rCMRglu in dorsal right insula, dorsal regions of left middle and superior temporal gyri, right cerebellum (anterior lobe, vermis), and mesencephalon (Dunn et al., 2002). These regions have also been implicated in recognizing facial emotion (George et al., 1993), transformations of the body-in-space (Bonda et al., 1995), anticipatory anxiety (Reiman et al., 1989), and in interpretation of figurative aspects of language (Bench et al., 1992; Bottini et al., 1994). Self-Blame may involve impaired processing of environmental cues regarding affect conveyed either by language or facial expressions leading the individual to assign negative self-appraisals.
Active maintenance of negative emotion is associated with increased amygdala activity (Schaefer et al., 2002)and detection of fearful faces is associated with amygdala over activation in major depression, that is reversed by treatment (Sheline et al., 2001). The effortful reduction of subjective experience of negative affect via reappraisal of negative social emotions is associated with decreased activation of the amygdala and medial orbito-frontal cortex and increased activation of the lateral and medial prefrontal regions (Ochsner et al., 2002), and is the opposite of the pattern of activation found here and in other studies of major depression (Drevets, 2000; Mayberg, 1997; Mayberg et al., 1999b). Thus, Self-Blame may be understood as resulting from a failure of regulation of negative self-directed emotions and its correlation with activity in these regions may be part of the biological underpinning of the symptoms covered by Self-Blame factor.
The BDI Self-Blame factor is also the component of this scale that can distinguish individuals with a past history of suicide attempt (Grunebaum et al., 2005).
4.3. BDI factor scores: 3. Somatic Complaints
Somatic Complaints, but not Subjective Depression or Self-Blame, is positively correlated with activity in deeper brain structures including ventro-lateral thalamus, epithalamus, putamen, globus pallidus, periaqueductal gray, midbrain, superior colliculi and ventral pons, but not the hypothalamus. Items loading on Somatic Complaints were insomnia, weight loss, loss of appetite and somatic concerns. The thalamus monitors physical state via sensory inputs and may mediate the somatic concerns including appetite (Killgore et al., 2003; McGilchrist et al., 1993; Reilly, 1998). In addition, Somatic Complaints factor also positively correlated with the anterior cingulate (Table 5 and Fig. 4). Higher normalized rCBF in cingulate was previously found to correlate with somatic symptoms (Bench et al., 1993). Moreover, subgenual anterior cingulate (Devinsky et al., 1995; Paus, 2001) is involved in autonomic and conditioned responses to emotionally salient stimuli, while the subgenual ventral PFC (Rolls, 2000) is implicated in the evaluation and regulation of autonomic and endocrine response to negatively charged emotional stimuli such as fear (Sullivan and Gratton, 1999). Our study suggests that exaggerated thalamic responses to sensory inputs may underlie somatic symptoms associated with Somatic Complaints factor.
4.4. Orthogonalized BDI and HDRS score correlations with rCMRglu
To remove shared variance between the HDRS and BDI as well as HDRS and rCMRglu, we partialled out HDRS scores from both the BDI scores and rCMRglu. The residuals of these BDI scores show both extensive positive and negative correlations with the residuals of cerebral metabolic activity (see Fig. 2). This suggests that the self-rated BDI captures biologically relevant components of depression severity and related neural circuitry that the HDRS does not. In contrast, residualized HDRS scores (removing variance shared with BDI) do not correlate with cerebral metabolic activity, suggesting that all regional activity variance due to HDRS scores, in this sample, is accounted for in the BDI score.
This finding suggests that measuring depression severity solely via the HDRS will miss clinically important and biologically relevant aspects of depressive symptomatology.
4.5. Limitations
One limitation of this study is that we examined a resting state and not changes in brain activity in the context of a cognitive or mood challenge. However, identifying psycho-pathological correlates of resting activity in brain regions in MDD suggests functional studies of cognition and mood regulation to further evaluate the role of these brain regions in the pathogenesis of major depression.
4.6. Consequences for measurement of treatment effects
The degree of overlap in brain region correlates of the BDI and HDRS is strongly associated with the degree of correlation between HDRS and BDI factor scores (Steer et al., 1987). The subjective experience of major depression, as reflected by the self-reported BDI, appears to relate to a more extensive array of brain structures thought to be responsible for the perception, production and/or regulation of emotion compared with the clinician-rated HDRS factors. HDRS shows associations primarily with brain structures thought to be responsible for autonomic or neurovegetative responses to emotionally salient stimuli (Milak et al., 2005). The subjective sense of depression may be more deeply rooted in altered brain physiology than previously suspected.
The emphasis on “objectivity” in clinical ratings of depression may overlook fundamental aspects of the disorder related to the subjective experience of major depression that this study shows also involve brain regions that differ from those associated with clinician-rated depression severity. The time course of clinical response to antidepressant treatment also appears to be different with clinician-rated depression responding more quickly than subjective severity of major depression (Adda et al., 1997; Ambrosini et al., 1999; Posternak and Miller, 2001; Thase et al., 1991).
The BDI and its correlated brain regions and the HDRS and its correlated brain regions need to be evaluated as potential independent predictors of clinical response to various antidepressant therapies. Our findings illustrate that the subjective symptoms of depression have distinct biological correlates that are not captured by clinician ratings.
Acknowledgements
Dr. Shuhua Li assisted with the polychoric statistics.
Role of funding source
Funding for this study was provided by NIMH Grants MH40695, MH62185, RR00645 and NARSAD; the funding agencies had no further role in study design or in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Footnotes
Conflict of interest
Dr. Mann has unrelated grants from Novartis and GSK, all other authors declare that they have no conflicts of interest.
References
- Adda C, Lefevre B, Reimao R. Narcolepsy and depression. Arq. Neuropsiquiatr. 1997;55:423–426. doi: 10.1590/s0004-282x1997000300012. [DOI] [PubMed] [Google Scholar]
- Akdemir A, Turkcapar MH, Orsel SD, Demirergi N, Dag I, Ozbay MH. Reliability and validity of the Turkish version of the Hamilton Depression Rating Scale. Compr. Psychiatry. 2001;42:161–165. doi: 10.1053/comp.2001.19756. [DOI] [PubMed] [Google Scholar]
- Ambrosini PJ, Wagner KD, Biederman J, Glick I, Tan C, Elia J, Hebeler JR, Rabinovich H, Lock J, Geller D. Multicenter open-label sertraline study in adolescent outpatients with major depression. J. Am. Acad. Child Adolesc. Psychiatry. 1999;38:566–572. doi: 10.1097/00004583-199905000-00018. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association, T.F.o.D.-I. Diagnostic and Statistical Manual of Mental Disorders: DSM-IV. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- Andersson S, Bergedalen AM. Cognitive correlates of apathy in traumatic brain injury. Neuropsychiatry Neuropsychol. Behav. Neurol. 2002;15:184–191. [PubMed] [Google Scholar]
- Beck AT, Beamesderfer A. Assessment of depression: the depression inventory. Mod. Probl. Pharmacopsychiatry. 1974;7:151–169. doi: 10.1159/000395074. [DOI] [PubMed] [Google Scholar]
- Bench CJ, Friston KJ, Brown RG, Scott LC, Frackowiak RS, Dolan RJ. The anatomy of melancholia-focal abnormalities of cerebral blood flow in major depression. Psychol. Med. 1992;22:607–615. doi: 10.1017/s003329170003806x. [DOI] [PubMed] [Google Scholar]
- Bench CJ, Friston KJ, Brown RG, Frackowiak RS, Dolan RJ. Regional cerebral blood flow in depression measured by positron emission tomography: the relationship with clinical dimensions. Psychol. Med. 1993;23:579–590. doi: 10.1017/s0033291700025368. [DOI] [PubMed] [Google Scholar]
- Bonda E, Petrides M, Frey S, Evans A. Neural correlates of mental transformations of the body-in-space. Proc. Natl. Acad. Sci. U. S. A. 1995;92:11180–11184. doi: 10.1073/pnas.92.24.11180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottini G, Corcoran R, Sterzi R, Paulesu E, Schenone P, Scarpa P, Frackowiak RS, Frith CD. The role of the right hemisphere in the interpretation of figurative aspects of language. A positron emission tomography activation study. Brain. 1994;117(Pt 6):1241–1253. doi: 10.1093/brain/117.6.1241. [DOI] [PubMed] [Google Scholar]
- Brett M. The MNI brain and the Talairach atlas. 1999 [Google Scholar]
- Brody AL, Saxena S, Mandelkern MA, Fairbanks LA, Ho ML, Baxter LR. Brain metabolic changes associated with symptom factor improvement in major depressive disorder. Biol. Psychiatry. 2001;50:171–178. doi: 10.1016/s0006-3223(01)01117-9. [DOI] [PubMed] [Google Scholar]
- Carter CS, Macdonald AM, Botvinick M, Ross LL, Stenger VA, Noll D, Cohen JD. Parsing executive processes: strategic vs. evaluative functions of the anterior cingulate cortex. Proc. Natl. Acad. Sci. U. S. A. 2000;97:1944–1948. doi: 10.1073/pnas.97.4.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degos JD, da Fonseca N, Gray F, Cesaro P. Severe frontal syndrome associated with infarcts of the left anterior cingulate gyrus and the head of the right caudate nucleus. A clinico-pathological case. Brain. 1993;116(Pt 6):1541–1548. doi: 10.1093/brain/116.6.1541. [DOI] [PubMed] [Google Scholar]
- Devinsky O, Morrell MJ, Vogt BA. Contributions of anterior cingulate cortex to behaviour. Brain. 1995;118(Pt 1):279–306. doi: 10.1093/brain/118.1.279. [DOI] [PubMed] [Google Scholar]
- Dolan RJ, Bench CJ, Liddle PF, Friston KJ, Frith CD, Grasby PM, Frackowiak RS. Dorsolateral prefrontal cortex dysfunction in the major psychoses; symptom or disease specificity? J. Neurol. Neurosurg. Psychiatry. 1993;56:1290–1294. doi: 10.1136/jnnp.56.12.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolan RJ, Bench CJ, Brown RG, Scott LC, Frackowiak RS. Neuropsychological dysfunction in depression: the relationship to regional cerebral blood flow. Psychol. Med. 1994;24:849–857. doi: 10.1017/s0033291700028944. [DOI] [PubMed] [Google Scholar]
- Drevets WC. Functional anatomical abnormalities in limbic and prefrontal cortical structures in major depression. Prog. Brain Res. 2000;126:413–431. doi: 10.1016/S0079-6123(00)26027-5. [DOI] [PubMed] [Google Scholar]
- Dunn RT, Kimbrell TA, Ketter TA, Frye MA, Willis MW, Luckenbaugh DA, Post RM. Principal components of the Beck Depression Inventory and regional cerebral metabolism in unipolar and bipolar depression. Biol. Psychiatry. 2002;51:387–399. doi: 10.1016/s0006-3223(01)01244-6. [DOI] [PubMed] [Google Scholar]
- Friston K, Holmes A, Worsley K, Poline J, Frith C, Frackowiak R. Statistical parametric maps in functional imaging: a general linear approach. Hum. Brain Mapp. 1995;2:189–210. [Google Scholar]
- Frith CD, Frith U. Interacting minds—a biological basis. Science. 1999;286:1692–1695. doi: 10.1126/science.286.5445.1692. [DOI] [PubMed] [Google Scholar]
- George MS, Ketter TA, Gill DS, Haxby JV, Ungerleider LG, Herscovitch P, Post RM. Brain regions involved in recognizing facial emotion or identity: an oxygen-15 PET study. J. Neuropsychiatry Clin. Neurosci. 1993;5:384–394. doi: 10.1176/jnp.5.4.384. [DOI] [PubMed] [Google Scholar]
- Grunebaum MF, Keilp J, Li S, Ellis SP, Burke AK, Oquendo MA, Mann JJ. Symptom components of standard depression scales and past suicidal behavior. J. Affect. Disord. 2005;87:73–82. doi: 10.1016/j.jad.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proc. Natl. Acad. Sci. U. S. A. 2001;98:4259–4264. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. J. Neurol. Neurosurg. Psychiatry. 1960;23(56–62):56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hautzinger M. The Beck Depression Inventory in clinical practice. Nervenarzt. 1991;62:689–696. [PubMed] [Google Scholar]
- Hellige JB. Hemispheric Asymmetry: What's Right and What's Left. Cambridge, Mass: Harvard University Press; 1993. [Google Scholar]
- Johnson SC, Baxter LC, Wilder LS, Pipe JG, Heiserman JE, Prigatano GP. Neural correlates of self-reflection. Brain. 2002;125:1808–1814. doi: 10.1093/brain/awf181. [DOI] [PubMed] [Google Scholar]
- Killgore WD, Young AD, Femia LA, Bogorodzki P, Rogowska J, Yurgelun-Todd DA. Cortical and limbic activation during viewing of high- versus low-calorie foods. NeuroImage. 2003;19:1381–1394. doi: 10.1016/s1053-8119(03)00191-5. [DOI] [PubMed] [Google Scholar]
- Lane RD, Reiman EM, Ahern GL, Schwartz GE, Davidson RJ. Neuroanatomical correlates of happiness, sadness, and disgust. Am. J. Psychiatry. 1997;154:926–933. doi: 10.1176/ajp.154.7.926. [DOI] [PubMed] [Google Scholar]
- Mann JJ, Waternaux C, Haas GL, Malone KM. Toward a clinical model of suicidal behavior in psychiatric patients. Am. J. Psychiatry. 1999;156:181–189. doi: 10.1176/ajp.156.2.181. [DOI] [PubMed] [Google Scholar]
- Mann JJ, Ellis SP, Waternaux CM, Liu X, Oquendo MA, Malone KM, Brodsky BS, Haas GL, Currier D. Classification trees distinguish suicide attempters in major psychiatric disorders: a model of clinical decision making. J. Clin. Psychiatry. 2008;69:23–31. doi: 10.4088/jcp.v69n0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayberg HS. Limbic-cortical dysregulation: a proposed model of depression. J. Neuropsychiatry Clin. Neurosci. 1997;9:471–481. doi: 10.1176/jnp.9.3.471. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Lewis PJ, Regenold W, Wagner HN., Jr Paralimbic hypoperfusion in unipolar depression. J. Nucl. Med. 1994;35:929–934. [PubMed] [Google Scholar]
- Mayberg HS, Brannan SK, Mahurin RK, Jerabek PA, Brickman JS, Tekell JL, Silva JA, McGinnis S, Glass TG, Martin CC, Fox PT. Cingulate function in depression: a potential predictor of treatment response. NeuroReport. 1997;8:1057–1061. doi: 10.1097/00001756-199703030-00048. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Liotti M, Brannan SK, McGinnis S, Mahurin RK, Jerabek PA, Silva JA, Tekell JL, Martin CC, Lancaster JL, Fox PT. Reciprocal limbic-cortical function and negative mood: converging PET findings in depression and normal sadness. Am. J. Psychiatry. 1999a;156:675–682. doi: 10.1176/ajp.156.5.675. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Liotti M, Brannan SK, McGinnis S, Mahurin RK, Jerabek PA, Silva JA, Tekell JL, Martin CC, Lancaster JL, Fox PT. Reciprocal limbic-cortical function and negative mood: converging PET findings in depression and normal sadness. Am. J. Psychiatry. 1999b;156:675–682. doi: 10.1176/ajp.156.5.675. [DOI] [PubMed] [Google Scholar]
- McGilchrist I, Goldstein LH, Jadresic D, Fenwick P. Thalamo-frontal psychosis. Br. J. Psychiatry. 1993;163:113–115. doi: 10.1192/bjp.163.1.113. [DOI] [PubMed] [Google Scholar]
- Milak MS, Parsey RV, Keilp J, Oquendo MA, Malone KM, Mann JJ. Neuroanatomic correlates of psychopathologic components of major depressive disorder. Arch. Gen. Psychiatry. 2005;62:397–408. doi: 10.1001/archpsyc.62.4.397. [DOI] [PubMed] [Google Scholar]
- Murphy FC, Nimmo-Smith I, Lawrence AD. Functional neuroanatomy of emotions: a meta-analysis. Cogn. Affect. Behav. Neurosci. 2003;3:207–233. doi: 10.3758/cabn.3.3.207. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Bunge SA, Gross JJ, Gabrieli JD. Rethinking feelings: an FMRI study of the cognitive regulation of emotion. J. Cogn. Neurosci. 2002;14:1215–1229. doi: 10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
- Oquendo MA, Galfalvy H, Russo S, Ellis SP, Grunebaum MF, Burke A, Mann JJ. Prospective study of clinical predictors of suicidal acts after a major depressive episode in patients with major depressive disorder or bipolar disorder. Am. J. Psychiatry. 2004;161:1433–1441. doi: 10.1176/appi.ajp.161.8.1433. [DOI] [PubMed] [Google Scholar]
- Oquendo MA, Bongiovi-Garcia ME, Galfalvy H, Goldberg PH, Grunebaum MF, Burke AK, Mann JJ. Sex differences in clinical predictors of suicidal acts after major depression: a prospective study. Am. J. Psychiatry. 2007;164:134–141. doi: 10.1176/appi.ajp.164.1.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T. Primate anterior cingulate cortex: where motor control, drive and cognition interface. Nat. Rev. Neurosci. 2001;2:417–424. doi: 10.1038/35077500. [DOI] [PubMed] [Google Scholar]
- Posternak MA, Miller I. Untreated short-term course of major depression: a meta-analysis of outcomes from studies using wait-list control groups. J. Affect. Disord. 2001;66:139–146. doi: 10.1016/s0165-0327(00)00304-9. [DOI] [PubMed] [Google Scholar]
- Reilly S. The role of the gustatory thalamus in taste-guided behavior. Neurosci. Biobehav. Rev. 1998;22:883–901. doi: 10.1016/s0149-7634(98)00015-3. [DOI] [PubMed] [Google Scholar]
- Reiman EM, Fusselman MJ, Fox PT, Raichle ME. Neuroanatomical correlates of anticipatory anxiety. Science. 1989;243:1071–1074. doi: 10.1126/science.2784226. [DOI] [PubMed] [Google Scholar]
- Rolls ET. Precis of the brain and emotion. Behav. Brain Sci. 2000;23:177–191. doi: 10.1017/s0140525x00002429. discussion 192–233. [DOI] [PubMed] [Google Scholar]
- Schaefer SM, Jackson DC, Davidson RJ, Aguirre GK, Kimberg DY, Thompson-Schill SL. Modulation of amygdalar activity by the conscious regulation of negative emotion. J. Cogn. Neurosci. 2002;14:913–921. doi: 10.1162/089892902760191135. [DOI] [PubMed] [Google Scholar]
- Seminowicz DA, Mayberg HS, McIntosh AR, Goldapple K, Kennedy S, Segal Z, Rafi-Tari S. Limbic-frontal circuitry in major depression: a path modeling metanalysis. NeuroImage. 2004;22:409–418. doi: 10.1016/j.neuroimage.2004.01.015. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Barch DM, Donnelly JM, Ollinger JM, Snyder AZ, Mintun MA. Increased amygdala response to masked emotional faces in depressed subjects resolves with antidepressant treatment: an fMRI study. Biol. Psychiatry. 2001;50:651–658. doi: 10.1016/s0006-3223(01)01263-x. [DOI] [PubMed] [Google Scholar]
- Steer R, Beck A, Riskind J, Brown G. Relationships between the Beck Depression Inventory and the Hamilton Psychiatric Rating Scale for Depression in depressed outpatients. J. Psychopathol. Behav. Assess. 1987;9:327–339. [Google Scholar]
- Sullivan RM, Gratton A. Lateralized effects of medial prefrontal cortex lesions on neuroendocrine and autonomic stress responses in rats. J. Neurosci. 1999;19:2834–2840. doi: 10.1523/JNEUROSCI.19-07-02834.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar Stereotaxic Atlas of the Human Brain: 3-Dimensional Proportional System — an Approach to Cerebral Imaging. New York, NY: Thieme Medical Publishers; 1988. [Google Scholar]
- Thase ME, Simons AD, Cahalane J, McGeary J, Harden T. Severity of depression and response to cognitive behavior therapy. Am. J. Psychiatry. 1991;148:784–789. doi: 10.1176/ajp.148.6.784. [DOI] [PubMed] [Google Scholar]
- Uehara T, Sato T, Sakado K. Correlations among depression rating scales and a self-rating anxiety scale in depressive outpatients. Psychiatry on-line. 2005 [Google Scholar]
- Uher R, Farmer A, Maier W, Rietschel M, Hauser J, Marusic A, Mors O, Elkin A, Williamson RJ, Schmael C, Henigsberg N, Perez J, Mendlewicz J, Janzing JG, Zobel A, Skibinska M, Kozel D, Stamp AS, Bajs M, Placentino A, Barreto M, McGuffin P, Aitchison KJ. Measuring depression: comparison and integration of three scales in the GENDEP study. Psychol. Med. 2008;38:289–300. doi: 10.1017/S0033291707001730. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Finch DM, Olson CR. Functional heterogeneity in cingulate cortex: the anterior executive and posterior evaluative regions. Cereb. Cortex. 1992;2:435–443. doi: 10.1093/cercor/2.6.435-a. [DOI] [PubMed] [Google Scholar]
- Wager TD, Phan KL, Liberzon I, Taylor SF. Valence, gender, and lateralization of functional brain anatomy in emotion: a meta-analysis of findings from neuroimaging. NeuroImage. 2003;19:513–531. doi: 10.1016/s1053-8119(03)00078-8. [DOI] [PubMed] [Google Scholar]
- Whalen PJ, Bush G, McNally RJ, Wilhelm S, McInerney SC, Jenike MA, Rauch SL. The emotional counting Stroop paradigm: a functional magnetic resonance imaging probe of the anterior cingulate affective division. Biol. Psychiatry. 1998;44:1219–1228. doi: 10.1016/s0006-3223(98)00251-0. [DOI] [PubMed] [Google Scholar]
- Wichowicz H, Sumila A, Stolcman M. Assessment of presence and degree of depression in elderly, chronic ill patients: application selected scales advantages and disadvantages of this method of diagnosis. Przegl. Lek. 2004;61:1374–1377. [PubMed] [Google Scholar]