Abstract
Genetic polymorphisms of IRF5 are associated with an increased risk of lupus in humans. Here, we examined the role of IRF5 in the pathogenesis of pristane-induced lupus in mice. The pathological response to pristane in IRF5−/− mice shared many features with IFN-I receptor (IFNAR) −/− and TLR7−/− mice: production of anti-Sm/RNP autoantibodies, glomerulonephritis, generation of Ly6Chi monocytes, and IFN-I production all were greatly attenuated. Lymphocyte activation following pristane injection was greatly diminished in IRF5−/− mice and helper T cell differentiation was deviated from TH1 in wild type mice toward TH2 in IRF5−/− mice. TH cell development was skewed similarly in TLR7−/− or IFNAR−/− mice, suggesting that IRF5 alters T cell activation and differentiation by affecting cytokine production. Indeed, production of IFN-I, IL-12, and IL-23 in response to pristane was markedly decreased, whereas IL-4 increased. Unexpectedly, plasmacytoid dendritic cells (pDC) were not recruited to the site of inflammation in IRF5−/− or MyD88−/− mice, but were recruited normally in IFNAR−/− and TLR7−/− mice. In striking contrast to wild type mice, pristane did not stimulate local expression of CCL19 and CCL21 in IRF5−/− mice, suggesting that IRF5 regulates chemokine-mediated pDC migration independently of its effects on IFN-I. Collectively, these data indicate that altered production of IFN-I and other cytokines in IRF5−/− mice prevents pristane from inducing lupus pathology by broadly affecting T and B lymphocyte activation/differentiation. Additionally, we uncovered a new, IFN-I independent, role of IRF5 in regulating chemokines involved in the homing of pDCs and certain lymphocyte subsets.
Introduction
The transcription factor IRF5 is a member of the interferon regulatory factor family with a key role in toll-like receptor (TLR)-stimulated production of proinflammatory cytokines such as IL-12, IL-23, IL-6, and TNFα (1), activation of Type I interferon genes (2, 3), regulation of apoptosis (4), and development of B cells (5, 6). In humans, there are multiple IRF5 isoforms resulting from alternative splicing of the IRF5 gene (7–10). In contrast, murine IRF5 is expressed as a single transcript (11). Certain genetic polymorphisms of IRF5 are strongly associated with an increased risk of developing systemic lupus erythematosus (SLE) in humans and the IRF5 haplotype helps to define the risk for SLE (7, 10, 12–15). IRF5 also contributes to the pathogenesis of lupus in mouse models. In the FcγRIIB−/−Yaa and FcγRIIB−/− lupus models, IRF5 is required for autoantibody production and renal disease (16). The mechanism appears to be partly independent of IFN-I production, but additional mechanisms have not been defined. IRF5 deficiency also abolishes anti-Sm/RNP antibodies and reduces anti-dsDNA autoantibodies and inflammatory cytokine production while decreasing renal disease and improving survival in MRL/lpr mice (17). Although IFN-I ameliorates lupus in the MRL/lpr model (18), lupus induced by pristane is mediated by signaling through the Type I interferon receptor (IFNAR) and TLR7 (19). In a recent study, autoantibody production and renal disease were abolished in pristane-treated IRF5−/− mice, an effect ascribed to a B cell-intrinsic IRF5 requirement for class switching to IgG2a, the predominant autoantibody isotype in the pristane lupus model (6). The present study was carried out to further define the mechanisms by which IRF5 influences the development of autoimmune disease in mice. We present evidence that the effects of IRF5 on the induction of autoimmune disease in pristane-treated mice is more complex than previously believed, with interferon -dependent or -independent effects on multiple cell lineages including B and T lymphocytes, monocyte/macrophages, and plasmacytoid dendritic cells (pDC).
Materials and Methods
Mice and pristane treatment
Mice were bred and maintained under specific pathogen free (SPF) conditions at the University of Florida Animal Facility. IRF5−/− mice on a C57BL/6 (B6) background were provided by Dr. Katherine Fitzgerald (University of Massachusetts, MA) with permission from Dr. Tak Mak (University of Toronto, Canada) and were back-crossed to B6 for at least 10 generations. B6 MyD88−/− mice were provided by Dr. Lyle Moldawer (Department of Surgery, University of Florida). TLR7−/− mice on a BALB/c background were acquired from Oriental Bioservices (Kyoto, Japan) and IFNAR−/− mice backcrossed 9 generations onto a BALB/c background were provided by Dr. Joan Durbin (Nationwide Children’s Hospital, Ohio State University, Columbus OH), respectively. Wild type BALB/cJ, C57BL/6 and BALB/C X B6 F1 CB6F1/J mice were purchased from Jackson Laboratory (Bar Harbor, ME). Mice received a single intraperitoneal (I.P.) injection of 0.5 mL of pristane (2,6,10,14 tetramethylpentadecane, TMPD, Sigma, St. Louis, MO) filtered through a 0.25 µm filter or left untreated as controls These studies were approved by the Institutional Animal Care and Use Committee.
Real-time quantitative PCR (Q-PCR)
Q-PCR was performed as previously described (20, 21). In brief, total RNA was extracted from 106 peritoneal cells using TRIzol reagent (Invitrogen, Carlsbad, CA) and cDNA was synthesized using the Superscript II First-Strand Synthesis kit (Invitrogen) according to the manufacturer's protocol. SYBR green Q-PCR analysis was performed using an Opticon II thermocycler (Bio-Rad, Hercules, CA). Amplification conditions were as follows: 95°C for 10 min, followed by 45 cycles of 94°C for 15 s, 60°C for 25 s, and 72°C for 25 s. After the final extension (72°C for 10 min), a melting-curve analysis was performed to ensure specificity of the products. Primer sequences are listed as follows: ISG-15 Forward: GAGCTAGAGCCTGCAGCAAT, Reverse: TAAGACCGTCCTGGAGCACT; IRF7 Forward: ACAGCACAGGGCGTTTTATC, Reverse: GAGCCCAGCATTTTCTCTTG; Mx-1 Forward: GATCCGACTTCACTTCCAGATGG, Reverse: CATCTCAGTGGTAGTCCAACCC; CXCL5 Forward: CCCCTTCCTCAGTCATAGCC, Reverse: TGGATTCCGCTTAGCTTTCT; YM-2 Forward: CTGGGTAATGAGTGGGTTGG, Reverse: ACGTCCCTGGTGACAGAAAG; YM-1 Forward: TGAAGGAGCCACTGAGGTCT, Reverse: CACGGCACCTCCTAAATTGT; Fizz1 Forward: TGCTGGGATGACTGCTACTG, Reverse: AGCTGGGTTCTCCACCTCTT; IL23 P19 Forward: CATGGGGCTATCAGGGAGTA, Reverse: GACCCACAAGGACTCAAGGA.
Flow cytometry
Flow cytometry was performed as described previously (20). Before surface staining, peritoneal or peripheral blood cells were incubated with anti-mouse CD16/32 (Fc Block, BD Bioscience, San Jose, CA) for 10 min. Cells then were stained with an optimized amount of primary antibody or the appropriate isotype control for 10 min at room temperature before washing and resuspending in PBS supplemented with 0.1% BSA. Ten thousand to 50,000 events per sample were acquired using a CYAN ADP flow cytometer (Beckman Coulter, Hialeah, FL) and analyzed with FCS Express 3 (De Novo Software, Ontario, Canada). The following conjugated antibodies were used: anti-CD8-APC, anti-Ly6G-PE, anti-B220-APC-CY7, anti-CD11c-APC, anti-B220-FITC, anti-Ly6C-FITC, CD69-FITC, CD69-PE-CY7, CD25-PE (BD Bioscience), anti-H2-Kb-APC, anti-H2-Dd-Biotin, avidin-PE-CY7, anti-CD11b-Brilliant Violet, anti-PDCA-1-biotin (Biolegend, San Diego, CA), anti-CD4-PE-CY7, CCR7-PE (eBioscience, San Diego, CA). For morphological analysis, 106 peritoneal cells were cytospun onto glass slides and stained using the Hema3 kit (modified Wright stain; Fisher Scientific, Pittsburgh, PA).
Intracellular cytokine staining
Intracellular IFN-γ, IL-4 and IL-17 were detected after culturing peritoneal cells or splenocytes at a concentration of 2.5 × 106 cells/ml in RPMI medium with 10% FBS, PMA (50 ng/mL), and ionomycin (1 µg/ml), and GolgiStop (BD; 5 µg/ml) for 6 h at 37°C in a 5% CO2 incubator. After stimulation, cells were surface stained, fixed in Fixation/Permeabilization buffer (eBioscience), washed in Perm/Wash buffer (eBioscience), and then stained with PE-labeled anti–IFNγ, APC-labeled anti-IL-4 (BD Biosciences), APC-labeled anti-IL-17 (eBioscience), or isotype controls, according to the manufacturer’s protocol. Cells were washed twice in Perm/Wash buffer and resuspended in PBS and analyzed by flow cytometry. Data were analyzed with FCS Express 3 software (De Novo Software).
Immunoglobulin and autoantibody ELISAs
Total immunoglobulin levels were measured as described (20). Briefly, microtiter plates (Nunc, Immobilizer Amino) were coated with goat anti-mouse L-chain (κ and λ specific) antibodies (Southern Biotechnology, Birmingham, AL). Sera were diluted 1:200,000 in 150 mM NaCl, 50 mM Tris-HCl pH 7.5, 2mM EDTA, 0.5% BSA, 0.3% NP40, 0.05% NaN3 (NET/NP40). Alkaline phosphatase-conjugated goat anti-mouse polyclonal antibodies against μ, γ1, γ2c, γ2b, or γ3 H-chain (Southern Biotechnology, 1:1000 dilutions) were used as secondary antibodies. Antigen capture ELISAs for anti-nRNP/Sm antibodies were performed as described (20), using mouse sera at a dilution of 1:400 and goat anti-mouse IgG second antibodies (Southern Biotechnology). Levels of anti-dsDNA antibodies were tested (1:400 serum dilution) by ELISA using S1 nuclease-treated calf thymus DNA as antigen as previously described (22). Anti-U1A (subset of anti-RNP) antibodies were tested by ELISA as described (23). Briefly, recombinant U1A antigen-coated wells were incubated with mouse sera (1:400 dilutions) followed by alkaline phosphatase-conjugated goat anti-mouse μ or γ1, γ2c, γ2b, or γ3 H-chain-specific antibodies. ELISAs were developed with p-nitrophenyl phosphate substrate (Sigma-Aldrich) and optical density at 405 nm (OD405) was read using a VersaMax microplate reader (Molecular Devices Corporation, Sunnyvale, CA).
Cytokine ELISAs
CCL2 (MCP-1) levels in peritoneal lavage fluid were measured using the Mouse MCP-1 OptEIA Set (BD Bioscience) following the manufacturer’s instructions. IL-12 p40/p70 in peritoneal lavage fluid was measured as described previously (23) using rat monoclonal antibody pairs for IL-12 (BD Biosciences). After incubation with biotinylated IL-12-specific antibodies, streptavidin-conjugated alkaline phosphatase (1:1000 dilution, Southern Biotechnology) was added for 30 minutes at 22°C and the reaction was developed with p-nitrophenyl phosphate substrate. OD405 of each sample was converted into cytokine concentrations based on the standard curves of recombinant cytokines using the Softmax Pro 4.3 program (Molecular Devices) with a four-parameter logistic equation.
Autoantibody analysis by immunoprecipitation
Immunoprecipitation of radiolabeled cell extracts to detect serum autoantibodies against nRNP/Sm was performed as previously described (20).
Assessment of glomerulonephritis
Glomerular cellularity was evaluated by counting the number of nuclei per glomerular cross-section after staining with hematoxylin and eosin (H&E) as described (24). For assessing renal immune complex deposition, 4-µm frozen sections were stained with FITC rat anti-mouse complement component C3 monoclonal antibody (Cedarlane) and examined by fluorescence microscopy (24).
Bone marrow chimeras
Bone marrow chimeras were generated as described (25). Briefly, lethally irradiated (1000 Rad) BALB/c X B6 F1 (CB6F1/J) mice were reconstituted with bone marrow from wild type CB6F1/J mice (H2-Kb and H2-Dd positive) mice mixed 1:1 with bone marrow from either wild type BALB/c or BALB/c IFNAR−/− mice (H2-Dd single positive). After 6 weeks, the reconstituted mice were treated with 0.5 ml of pristane i.p. and 3 weeks later the presence of activated (CD4+CD69+) T cells was determined in the peritoneum and spleen.
Statistical analysis
Statistical analyses were performed using Prism 4.0 software (GraphPad Software, Inc, La Jolla, CA). Differences between groups were analyzed by the unpaired Student's t test or the Mann-Whitney U-test. The data are shown as mean +/− SD for normally distributed data sets, and as median and interquartile range for non-normally distributed data sets. Student's t-test was used for normally distributed data, and the Mann-Whitney U test for non-normally distributed data. Normality was determined by D’agostino and Person omnibus normality test using Prism 4.0. All tests were two-sided, and P < 0.05 was considered significant.
Results
Intraperitoneal pristane injection induces autoantibodies in two phases: an early phase, peaking at ~ 2 weeks, of IgM anti-ssDNA and chromatin autoantibodies, and a later phase (3–6 months) in which autoantibodies characteristic of SLE are produced including IgG anti-Sm/RNP and anti-dsDNA (19). Most of these autoantibodies are IgG2a in BALB/c background mice (IgG2c in B6 background mice) or IgG2b. As pristane-induced lupus (autoantibodies and renal disease) is dependent on TLR7-stimulated IFN-I production (20), we examined the role of IRF5, a transcription factor downstream of TLR7 and MyD88, in pristane-induced IFN-I production.
IRF5 is critical for the pathogenesis of pristane-lupus
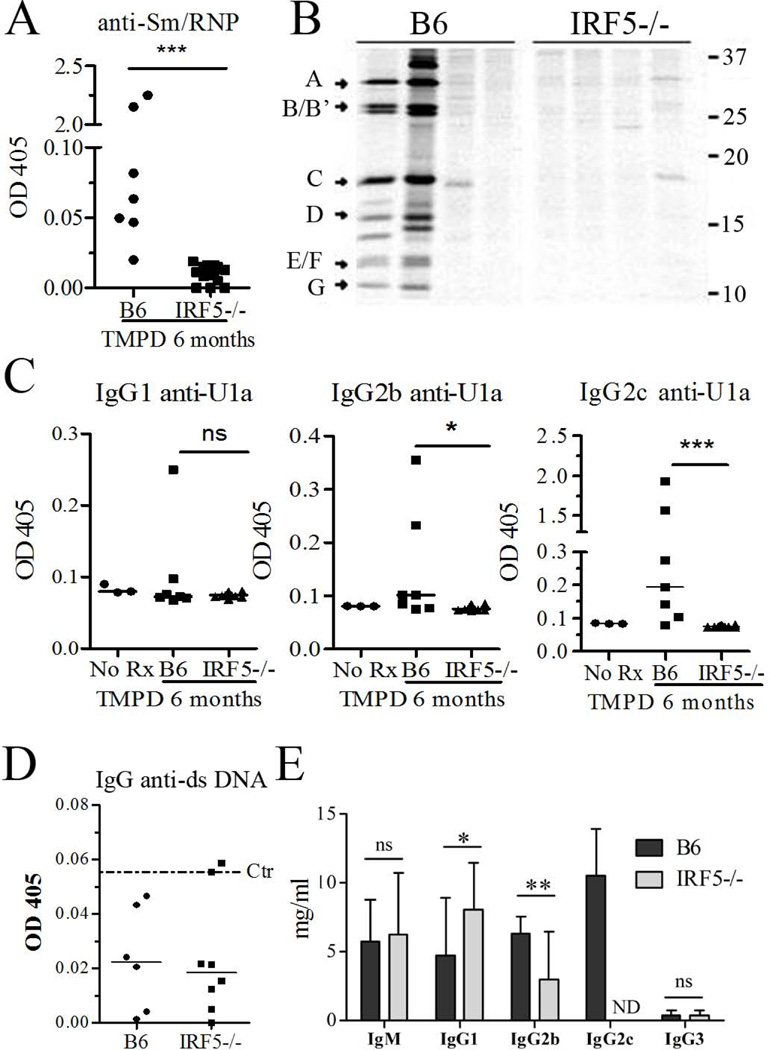
Pristane-treated B6 IRF5−/− mice (n = 14) did not produce anti-Sm/RNP autoantibodies detectable by ELISA and only one mouse was weakly positive by immunoprecipitation (Fig. 1A, B). In contrast, 2 out of 7 wild type B6 mice treated with pristane produced high levels of anti-Sm/RNP and another four displayed low levels of anti-Sm/RNP detectable by ELISA but not immunoprecipitation (Fig. 1A, B). The frequency of anti-Sm/RNP in wild type mice detectable by immunoprecipitation was consistent with our previous studies in which these autoantibodies were found in 24% pristane-treated female B6 mice (26). IgG autoantibodies specific for the U1A protein, a subset of anti-RNP, were absent in all IRF5−/− mice (n = 14). In contrast, 2/7 control mice produced high levels and several more produced lower levels of anti-U1A autoantibodies (Fig. 1C). As expected, in wild type mice there was a strong IgG2c anti-U1A response as well as a weaker IgG2b and IgG1 response (Fig. 1C). IgG2c, IgG2b and IgG1 anti-U1A antibodies were absent in IRF5−/− mice. IgG3 and IgM anti-U1A antibodies were not produced by either IRF5−/− or wild type mice (data not shown).
Figure 1. Absence of autoantibodies in IRF5−/− mice.
IRF5−/− and control B6 mice (B6 mice N=7, IRF5−/− mice N=14) were treated with pristane and 6 months later, serum autoantibody and immunoglobulin levels were evaluated. Sera collected from mice before pristane (tetramethylpentadecane, TMPD) injection served as a control (Ctr). A, Anti-Sm/RNP autoantibody levels (ELISA) in IRF5−/− and B6 control mice. B, immunoprecipitation of [35S]-radiolabeled protein components A–G of U1 small ribonucleoproteins by sera from 6 B6 and 6 IRF5−/− sera. Positions of molecular weight markers (kDa) are indicated on the right. C, Anti-U1A IgG1, IgG2b and IgG2c autoantibody levels (ELISA) in IRF5−/− and control mice (B6 mice N=7, IRF5−/− mice N=14). D, IgG anti-dsDNA autoantibody levels (ELISA) in IRF5−/− and control mice (B6 mice N=6, IRF5−/− mice N=8). Dotted line indicates the mean absorbance using sera from non-pristane-treated control mice. E, Serum levels of total IgM, IgG1, IgG2c, IgG2b, and IgG3 in pristane-treated IRF5−/− and B6 mice (B6 mice N=7, IRF5−/− mice N=14). ND: not detected. * P < 0.05; ** P <0.01; *** P <0.001 by Mann-Whitney test (Figure A, C, D, E).
Consistent with our previous observation that pristane does not induce IgG anti-dsDNA antibodies in B6 mice (26), little or no anti-dsDNA antibodies were detected in control or in IRF5−/− mice (Fig. 1D). To evaluate whether the absence of autoantibodies in IRF5−/− mice was related to defective isotype switching, we measured total IgM as well as IgG subclass levels (Fig. 1E). Although pristane treated IRF5−/− mice had very little serum IgG2c, they produced significant levels of IgG2b and actually had increased levels of IgG1. IgM levels were comparable in IRF5−/− and control mice, as reported previously (6). Nevertheless, IgG1, IgG2b, and IgG3 anti-U1A autoantibodies were not induced in IRF5−/− mice (Fig. 1C and data not shown). The low level of IgG2c (IFNγ dependent) and high level of IgG1 (IL-4 dependent) correlated well with changes in T helper cell subsets (see below).
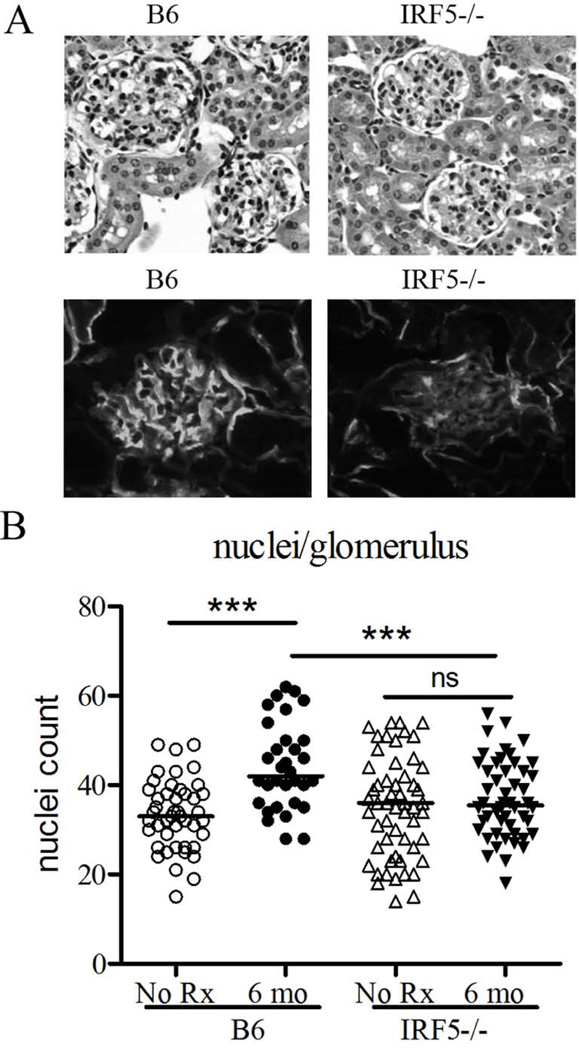
The relatively mild renal disease induced by pristane in B6 mice also was attenuated in IRF5−/− mice. H&E staining of the glomeruli revealed decreased cellularity in pristane-treated IRF5−/− mice vs. controls as determined by counting the numbers of nuclei per glomerulus (Fig. 2A upper panel, Fig. 2B). Similarly, direct immunofluorescence staining of glomerular C3 deposition was diminished in IRF5−/− mice indicating decreased immune complex deposition in glomeruli (Fig. 2A lower panel). Thus, IRF5−/− mice exhibited substantially decreased autoantibody production and attenuated renal pathology following pristane treatment in comparison with wild type mice.
Figure 2. Attenuated renal disease in pristane-treated IRF5−/− mice.
A, Upper: kidneys from IRF5−/− mice and wild type B6 controls 6 months after pristane treatment were sectioned and stained with hematoxylin & eosin. Lower: Renal tissue was embedded in OCT and 4 µm sections were stained with FITC-conjugated anti-mouse complement component C3 monoclonal antibody. B, Glomerular cellularity was evaluated by counting the number of nuclei per glomerular cross-section (n=5 mice per group, 8 glomeruli per mouse). *** P <0.001, Student’s t-test.
Decreased inflammatory cytokine production in IRF5−/− mice
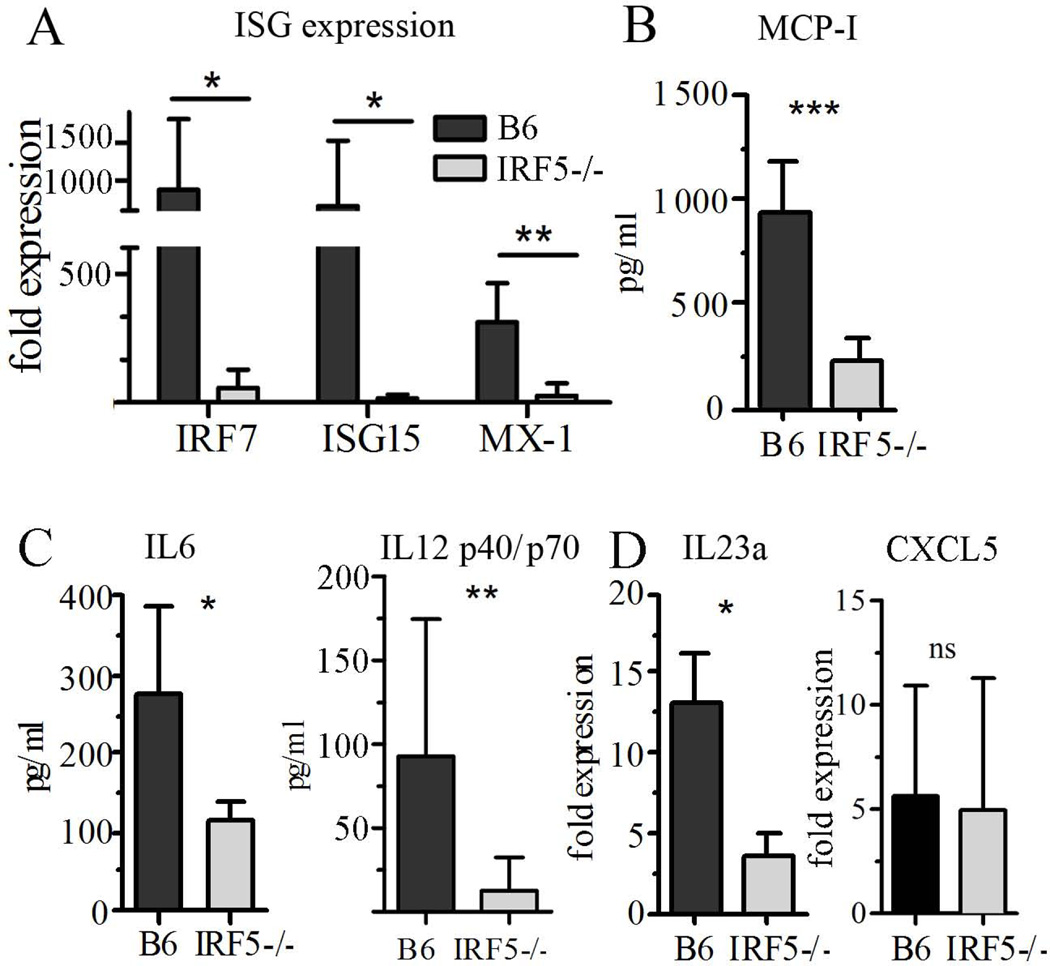
Pristane-induced lupus is abolished in the absence of signaling through the IFNAR (24). Consistent with the importance of IFN-I in the pathogenesis of this disease, peritoneal exudate cells from IRF5−/− mice expressed markedly lower levels of the interferon-inducible genes (ISGs) IRF7, ISG15, and Mx1 (Fig. 3A). Similarly, protein levels of the IFN-I inducible chemokine MCP-1 (CCL2) in peritoneal lavage fluid were significantly lower in IRF5−/− mice (Fig. 3B). The levels of other proinflammatory cytokines (IL-12 p40/p70 and IL-6) involved in the pathogenesis of pristane-lupus also were decreased in peritoneal lavage fluid (Fig. 3C) and expression of IL-23 p19 mRNA was decreased in pristane-treated IRF5−/− mice vs. controls (Fig. 3D). Taken together, these data suggest that deficiency of IRF5 attenuates lupus in pristane-treated mice by affecting the production of IFN-I and other key cytokines (IL-12, IL-23) that link innate immunity to the activation of TH1 and TH17 cells, respectively (27). Production of IL-4 (Fig. 5) and the chemokine CXCL5, which recruits neutrophils to peritoneal cavity (28), was similar in IRF5−/− mice and controls, suggesting that the effects of IRF5 deficiency on cytokine production were selective for TH1 cells and not due to an overall attenuation of the inflammatory response (Fig. 3D).
Figure 3. Decreased levels of inflammatory cytokines and chemokines in IRF5−/− mice.
IRF5−/− and B6 mice were injected with pristane and 2–3 weeks later, the peritoneum was lavaged and peritoneal exudate cells were collected by centrifugation for RNA isolation. The supernatant was used to assay cytokine/chemokine levels. A, Expression of the interferon-stimulated genes (ISGs) IRF7, ISG15, and Mx-1 in peritoneal exudate cells (Q-PCR). B, Level of MCP-1 (CCL2) in peritoneal lavage fluid (ELISA). C, Levels of IL-6 and IL-12 P40/P70 in peritoneal lavage fluid (ELISA). D, Expression of IL-23a (p19) and CXCL5 in peritoneal exudate cells (Q-PCR). * P <0.05; ** P <0.01; *** P <0.001 by Mann-Whitney test.
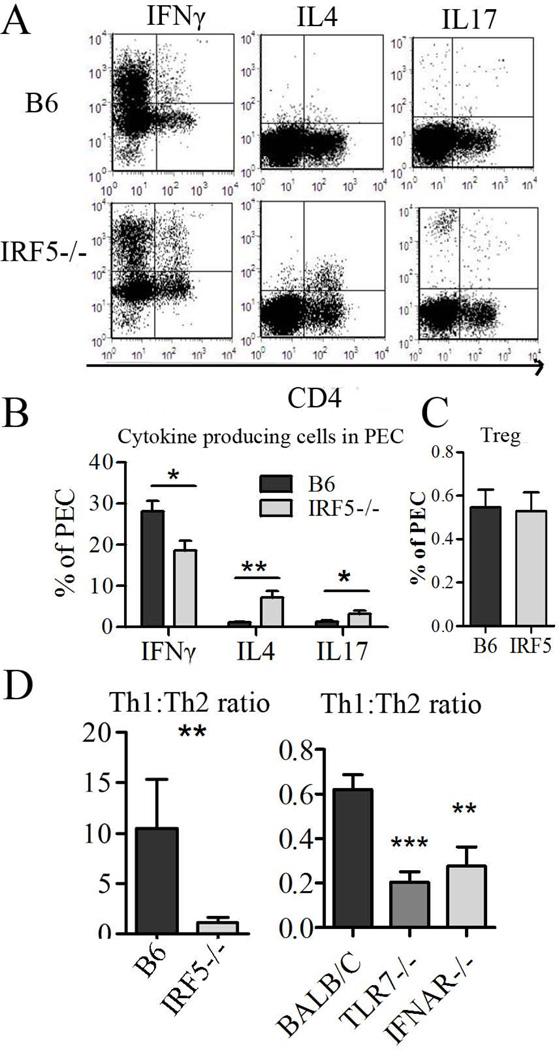
Figure 5. Inflammatory response to pristane is skewed toward TH2 cells IRF5−/− mice.
Two weeks after pristane injection, peritoneal lymphocytes were analyzed by flow cytometry. A, flow cytometry of IFNγ+, IL4+, and IL17+ cells in total peritoneal exudate cells from IRF5−/− vs. B6 control mice. B, Quantification of IFNγ, IL4, and IL17 producing peritoneal cells. C, Quantification of CD4+FoxP3+ (Treg-like) cells in IRF5−/− vs. B6 mice. D, TH1/TH2 ratio in peritoneal exudates of IRF5−/− vs. B6 mice and TLR7−/−, IFNAR−/−, and wild type BALB/c mice 2 weeks after pristane injection. Representative of two independent experiments, n = 6–14 mice per group, * P < 0.05; ** P <0.01; *** P <0.001 by Mann-Whitney test.
IRF5 deficiency alters the chronic inflammatory response to pristane
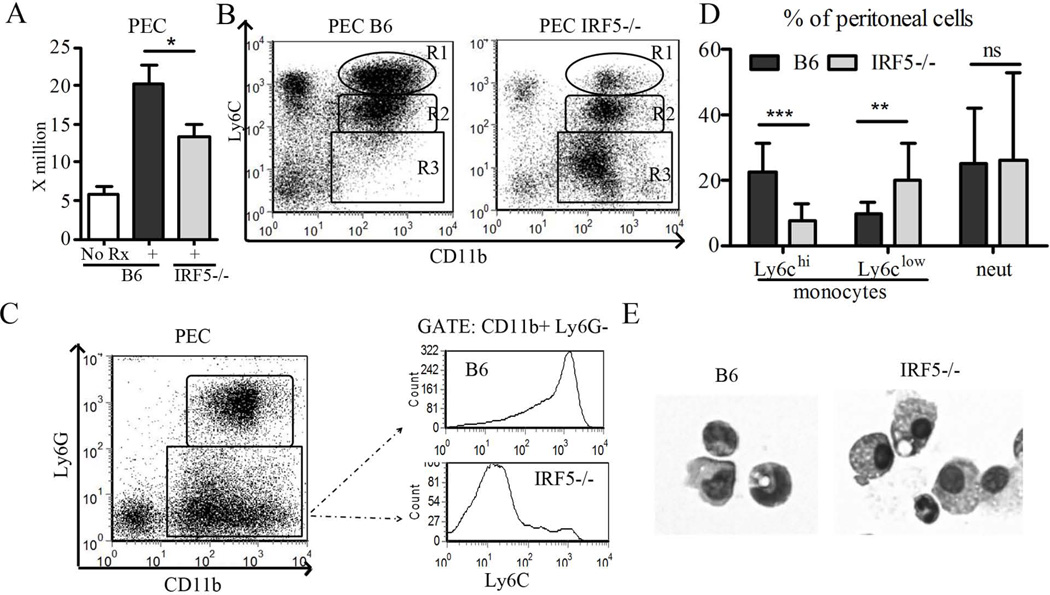
We have shown previously that a subset of inflammatory monocytes expressing CCL2 and high levels of the surface marker Ly6C is recruited to the peritoneum in response to MCP-1 and is a major source of IFN-I in pristane-treated mice (29). The total number of peritoneal cells in pristane-treated IRF5−/− mice was slightly lower than in wild type mice but much higher than in untreated mice indicating that pristane could induce an inflammatory response in IRF5−/− mice (Fig. 4A). The effect of IRF5 deficiency on the recruitment of Ly6Chi monocytes (CD11b+Ly6ChiLy6G−) also was examined (Fig. 4B–E). As shown previously in TLR7−/− and IFNAR−/− mice, peritoneal exudates from pristane-treated IRF5−/− mice contained few Ly6Chi monocytes in comparison with pristane-treated B6 controls (Fig. 4B, R1; Figs. 4C–D). In contrast, the peritoneal exudates from IRF5−/− mice contained many Ly6Clo monocytes (Fig. 4B, R3; Fig. 4C–D). Peritoneal neutrophils were largely unaffected (Fig. 4B, R2; Fig. 4D). Gating on all monocytes (CD11b+, Ly6G−) and analyzing fluorescence intensity of Ly6C staining underscored the dramatic difference in the profile of peritoneal monocyte populations in IRF5−/− mice vs. controls (Fig. 4C). In addition, monocytes in the peritoneal exudates had different morphological features: those in B6 mice had the appearance of immature monocytes, whereas those in IRF5−/− mice were larger and more granular, consistent with a more mature phenotype (Fig. 4E). Since Ly6Chi monocytes are a major source of IFN-I production in the inflamed peritoneum of pristane-treated mice (29), these results are consistent with the low IFN-I expression in peritoneal exudates cells from IRF5−/− mice (Fig. 3A). Interestingly, these differences in myeloid cell subsets (Ly6Chi and Ly6Clo monocytes) were not reflected in the circulation 2 weeks following pristane treatment (Fig. S1A).
Figure 4. Effect of IRF5 deficiency on the inflammatory response to pristane.
Two weeks after pristane injection, peritoneal cells were collected from IRF5−/− and wild type (B6) controls and analyzed by flow cytometry. A, Total peritoneal exudate cell count. B, Analysis of total peritoneal exudate cells by flow cytometry. Percentages of CD11b+Ly6Chi monocytes (R1), CD11b+Ly6Cint neutrophils (R2), and CD11b+Ly6Clo “mature” monocytes (R3) were assessed by flow cytometry as described previously (29). C, Fluorescence intensity of Ly6C on CD11b+Ly6G− monocytes in the peritoneal exudates was assessed by flow cytometry. D, percentages of Ly6Chi monocytes (CD11b+Ly6ChiLy6G−), Ly6Clo monocytes (CD11b+Ly6CloLy6G−), and neutrophils (CD11b+Ly6CintLy6G+) in the peritoneal exudates. Quantification was based on panel C. E, Morphology of peritoneal exudate monocytes from pristane-treated wild type (B6) vs. IRF5−/− mice. N = 14–22 per group, representative of two independent experiments. * P < 0.05; ** P <0.01; *** P <0.001 by Mann-Whitney test.
IRF5 deficiency alters the T helper cell response to pristane
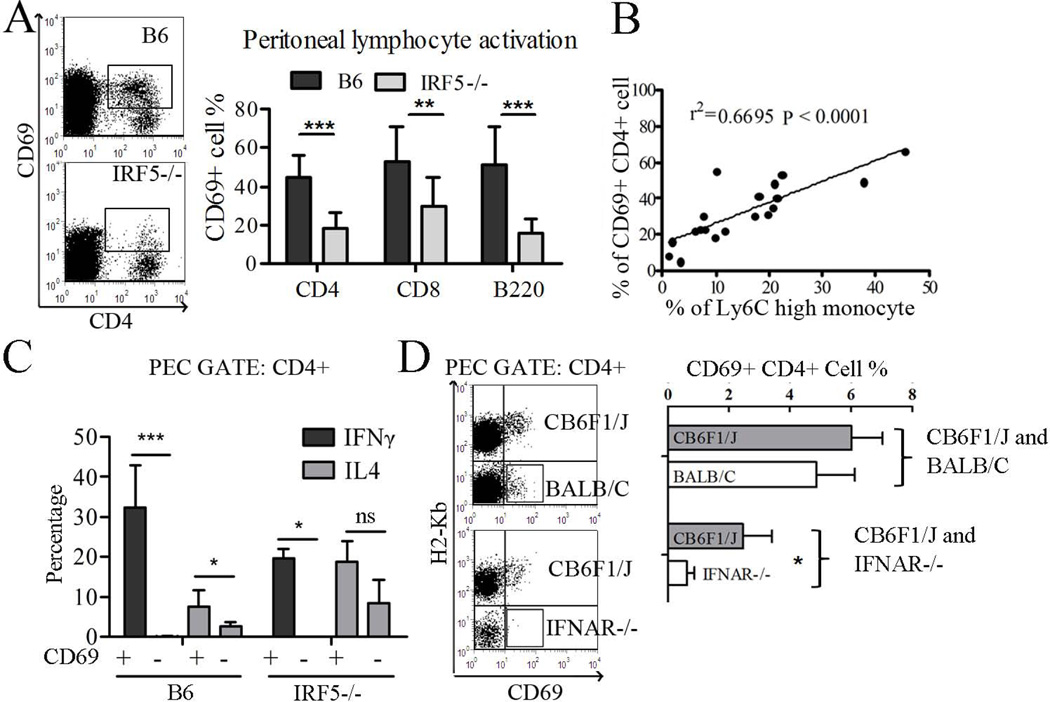
IRF-5 has a critical role in the development of TH1 responses to Leishmania donovani infection (30). Therefore we examined the T helper cell response to pristane. There were significant differences in the TH subsets present in the peritoneum 2 weeks after pristane injection. Intracellular cytokine staining revealed that IRF5−/− mice had fewer total peritoneal exudate cells producing IFNγ, whereas IL-4 and to a lesser degree IL-17 producing cells were increased (Fig. 5A,B). Although the IL-4 producing cells in IRF5−/− mice were primarily T lymphocytes, the cells producing IL-17 were CD4−CD8− (data not shown). To clarify whether the apparent TH cell skewing is due to the lack of IRF5, IFN-I, or other inflammatory cytokines such as IFNγ and IL12, we investigated T helper cell subsets in IFNAR−/− and TLR7−/− mice and found that both IFNγ and IL-4 (but not IL-17) producing cells were decreased in these strains (Fig. S1B). As in IRF5−/− mice, the ratio of TH1 to TH2 cells (defined as the ratio of IFNγ+ to IL4+ T cells) was lower in IFNAR−/− and TLR7−/− mice vs. their wild type controls (Fig. 5D). It should be noted that since B6 is a “TH1” strain whereas BALB/c is “TH2 prone” (31) the baseline ratios of IFNγ+ to IL4+ T cells in the two wild type control strains were quite different (Fig. 5D). Nevertheless, the cytokine pattern exhibited the same trend in both strains and both B6 and BALB/c mice are susceptible to pristane-lupus, although there are differences in autoantibody frequency and the severity of nephritis (19). Finally, it is of interest that the altered proportions of T helper cell subsets were seen only at the primary site of inflammation (the peritoneal cavity) and not in the spleen (Fig. S1C) and that the percentages of FoxP3+CD4+ (Treg-like) cells were comparable in IRF5−/− vs. B6 control mice in the peritoneum (Fig. 5C).
The recent observation that IRF5 promotes M1 macrophage polarization (32), along with the greatly reduced IL-12 (a major product of M1 macrophages) and IL-23 levels in peritoneal washings from IRF5−/− mice (Fig. 3) led us to hypothesize that deficiency of IRF5 may polarize monocyte/macrophage differentiation toward the M2 pathway in pristane-treated mice. We therefore examined the expression of Ym1, Ym2, and Fizz1, genes characteristically expressed by alternatively activated (M2) macrophages (Fig. S1D). We did not find a significant difference in the expression of these genes in peritoneal exudate cells from pristane-treated IRF5−/− mice vs. controls. Taken together, these data suggest the altered T helper cell ratio seen in IRF5−/− mice may reflect decreased IFN-I production and altered inflammatory cytokine production.
Decreased lymphocyte activation in IRF5−/− mice
The percentages and total cell counts of B220+, CD4+, and CD8+ lymphocytes in peritoneal exudates of pristane-treated mice were similar in IRF5−/− and wild type mice (not shown). However, the percentage of CD4+, CD8+, and B220+ peritoneal exudate cells expressing the activation marker CD69 was decreased in IRF5−/− mice vs. controls (Fig. 6A). Interestingly, the percentage of activated peritoneal CD69+CD4+ cells was highly correlated with the percentage of Ly6Chi monocytes (Fig. 6B), suggesting that IFN-I production, which also correlates strongly with numbers of Ly6Chi monocytes (29), might promote lymphocyte activation in pristane-treated mice. Although CD69 is an ISG (33), the presence of two distinct populations of CD4+ T cells, CD69+ and CD69− (Fig. 6A), argues against the possibility that increased expression of this surface marker in pristane-treated wild type mice was merely a reflection of IFN-I production and suggests that increased CD69 surface staining was due to T cell activation. This possibility also is supported by the observation that CD69+CD4+ T cells produced substantially more IFNγ and IL4 than CD69−CD4+ cells in B6 as well as IRF5−/− mice (Fig. 6C). Gated on the CD4+CD69+ population, ~30% of B6 T cells produced IFNγ (TH1) and ~8% produced IL4 (TH2) vs. ~20% cells producing IFNγ and IL4 respectively in IRF5−/− mice (Fig. 6C).
Figure 6. Role of IRF-5 in lymphocyte activation following pristane treatment.
Two weeks after pristane injection, peritoneal lymphocytes were analyzed by flow cytometry. A, Left: flow cytometry of CD4 and CD69 (gated on CD11b− cells). Right: The percentage of CD4+, CD8+, and B220+ cells that were positive for CD69 was determined. B, Correlation of CD69+ CD4+ T cells with the percentage of Ly6Chi monocytes. C, Intracellular staining of IFNγ and IL4 in B6 and IRF5−/− mice. Peritoneal lymphocytes were gated on the CD4+ population and then gated on the CD69+ and CD69− populations, respectively. Percentages of cells expressing IFNγ and IL4 are indicated (N=4 per group). ND, not detected. D, Left: peritoneal cells from bone marrow chimeric mice were gated on the CD11b−CD4+ population and CD69 expression was analyzed by flow cytometry in cells that were H2-Kb+ (CB6F1/J derived) or H2-Kb− (BALB/c or BALB/c IFNAR−/− derived). Right, quantification of CD69+CD4+ T cells as a percentage of total CD4+ T cells (N=4–5 per group). PEC, peritoneal exudate cells. Data are representative of two independent experiments. * P < 0.05; ** P <0.01; *** P <0.001 by Mann-Whitney test.
To further verify the role of IRF5-stimulated IFN-I in CD4+ T cell activation, we examined bone marrow chimeras. Bone marrow cells from wild type BALB/c X B6 F1 (CB6F1/J) mice (bearing both H2-Kb and H2-Dd) were mixed 1:1 with bone marrow cells from wild type BALB/c or BALB/c IFNAR−/− mice (H2-Dd single positive) and injected into lethally irradiated CB6F1/J recipients. Six weeks after reconstitution, pristane was administered to the reconstituted mice and 3 weeks later, peritoneal lymphocyte activation was examined (Fig. 6D). T cells derived from the CB6F1/J donor are H2-Kb+ while T cells derived from the BALB/C or IFNAR−/− donors are H2-Kb−. In the control group, there is a mixture of CB6F1/J and BALB/c donor cells and T cells from both donors became activated (CD69+) after pristane injection. In mice reconstituted with CB6F1/J and IFNAR−/− cells, only the CB6F1/J derived T cells were activated, whereas IFNAR−/− derived T cells remained CD69− (Fig. 6D, box). In contrast to the peritoneum, CD69 expression on CD4+ peripheral blood and splenic T cells was comparable in B6 and IRF5−/− mice (Fig. S1E). Together, these results suggest that 1) CD4+ T activation is both IFN-I and IRF5 dependent, 2) the T cells must express IFNAR endogenously, and 3) the exposure of T cells to IFN-I is local (peritoneal) and not systemic.
Plasmacytoid dendritic cell (pDC) recruitment is IRF5-dependent and IFN-I independent
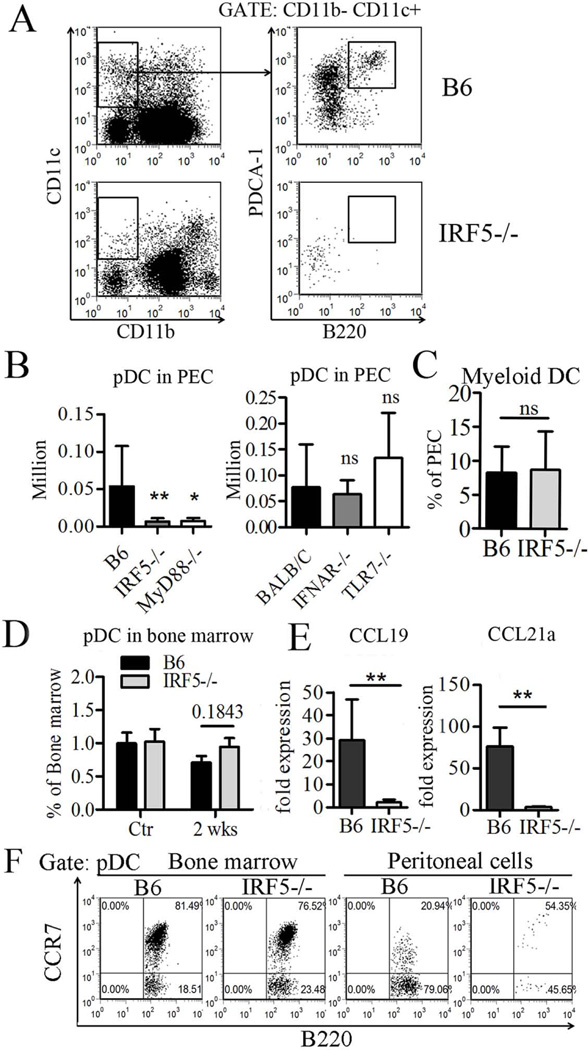
Unexpectedly, two weeks after pristane treatment, the number of CD11b−CD11c+B220+PDCA-1+ pDCs in the peritoneal cavity of IRF5−/− mice was strikingly reduced in comparison with wild type B6 mice (Fig. 7A). A similar reduction of pDCs in peritoneal exudates was seen in MyD88−/− mice, but not in IFNAR−/− or TLR7−/− mice (Fig. 7B). This discrepancy appeared to be limited to the pDC population, as myeloid dendritic cells (mDC, CD11b+CD11c+ CD8a−) were found at comparable levels in the peritoneal exudates of IRF5−/− and wild type mice (Fig. 7C). In contrast to the peritoneal exudates, the percentage of pDCs in the bone marrow was similar in IRF5−/− mice and wild type controls, both before pristane treatment and 2 weeks after treatment (Fig. 7D). CCR7 is expressed on T cells and dendritic cells and its ligands, CCL19 and CCL21, are essential for the homing of pDCs to lymph nodes (34). Therefore, we examined the expression of CCL19/CCL21 and found that both were decreased considerably in peritoneal exudate cells from IRF5−/− vs. control mice (Fig. 7E). Thus, defective pDC migration in IRF5 −/− mice is associated with decreased production of chemokines that mediate pDC homing. In view of the MyD88 dependence of pDC migration (Fig. 7B) and the ability of TLR ligands to enhance CCR7 expression (34), we examined CCR7 expression levels on bone marrow and peritoneal pDCs from IRF5−/− mice 2 weeks after pristane injection. The fluorescence intensity of B220+CD11b−CD11c+PDCA-1+ pDCs expressing CCR7 was similar in bone marrow from B6 and IRF5−/− mice (Fig. 7F). Unexpectedly the fluorescence intensity of CCR7 was lower in peritoneal pDCs from B6 mice than in B6 bone marrow pDCs, whereas IRF5−/− peritoneal pDCs (although few in number) exhibited similar fluorescence intensity to that in IRF5−/− bone marrow pDCs. Although the explanation is unclear, this could be due to CCL19/CCL21 blocking an epitope of the CCR7 receptor recognized by the monoclonal antibody in the B6 controls. These data suggest that MyD88 expression enhances pDC migration to the peritoneum by increasing IRF5-regulated CCL19/CCL21 and not by increasing CCR7 expression.
Figure 7. Decreased plasmacytoid dendritic cells (pDCs) in the peritoneum of IRF5−/−, but not TLR7−/− or IFNAR−/−, mice.
Two weeks after pristane injection, peritoneal exudate and bone marrow pDCs were quantified by flow cytometry. A, pDC staining. CD11b− CD11c+B220+PDCA-1+ cells were defined as pDC; this cell population was also Ly6C+ (not shown). B, Left, pDCs in peritoneal exudate cells (PEC) from IRF5−/−, MyD88−/−, and B6 control mice (N=5–12 per group). Right, pDC in TLR7−/−, IFNAR−/− and BALB/cJ PEC (N=6 per group). C, Peritoneal myeloid dendritic cells (CD11b+CD11c+CD8−) were quantified by flow cytometry (N=9–10 mice per group). D, pDCs in bone marrow of control B6 IRF5−/− and B6 mice before pristane injection (Ctr) and 2 weeks after pristane injection (N=3–5 per group). E, Expression of chemokines CCL19 and CCL21a in PEC by Q-PCR (N=4–6 per group). F, Expression of CCR7 on bone marrow and peritoneal pDCs (CD11b− CD11c+B220+PDCA-1+) was determined by flow cytometry (N=4 per group). Data are representative of two independent experiments. * P < 0.05; ** P <0.01; *** P <0.001 Mann-Whitney test.
Discussion
Genetic polymorphisms of IRF5 are strongly linked to susceptibility to SLE in humans (9, 10). Recent studies also link IRF5 to the pathogenesis of autoantibodies in both human SLE (35), pristane-induced lupus (6) and in MRL mice (17). Although it has been suggested that the primary effect of IRF5 in pristane-lupus may involve isotype switching of pathogenic autoantibodies (6), our data strongly suggest that the influence of IRF5 on the development of autoimmune disease in pristane-lupus is multifactorial. In the present studies, IRF5 not only affected autoantibody production, isotype switching, and the development of renal disease, but also modulated the polarization and activation of T helper cells as well as the differentiation and migration of inflammatory monocytes/macrophages and pDCs.
Effect of IRF5 deficiency on autoantibody production and renal disease
IRF5 promotes B cell maturation in part by modulating the expression of Blimp-1, a master regulator of plasma cell maturation (5). As they age, IRF5−/− mice have an increased number of CD19+B220− plasmablasts and decreased number of CD138hiB220hi plasma cells (5). The effects on serum immunoglobulin levels are complex: serum levels of IgG subclasses in young naïve IRF5−/− mice are similar to wild type, whereas in older mice, IgG1, IgG2a/IgG2c, and IgG2b are decreased. Following immunization with T cell dependent or independent antigens, IRF5−/− mice have a reduced antigen-specific IgG1 response, although IgG2a and IgG2b were not examined (5). In a previous study on pristane-induced lupus, the production of IgG2a/IgG2c and IgG2b anti-Sm and anti-dsDNA antibodies was abolished in IRF5−/− mice (6). Our data are generally consistent with these observations although we have not been able to detect anti-dsDNA antibodies in pristane-treated wild type B6 mice [Fig. 1 and (19)]. Strikingly, despite increased levels of total IgG1 in IRF5−/− mice, IgG1 anti-Sm/RNP autoantibodies were not produced, suggesting that IRF5 may affect on the regulation of tolerance to the Sm/RNP autoantigens.
Although wild type B6 mice are relatively resistant to the development of glomerulonephritis following pristane treatment (19), we confirmed that glomerular cellularity and renal immune complex deposits were decreased in IRF5−/− mice vs. wild type controls.
IRF5 is critical for inflammatory cytokine production and recruitment of Ly6Chi monocytes
Intraperitoneal injection of pristane induces the production of inflammatory cytokines, influx of immune cells, and a chronic inflammatory response, culminating in the development of a lupus-like disease (20). IRF5 deficiency had critical effects on the intraperitoneal production of multiple cytokines implicated in the pathogenesis of pristane-lupus, including IL-12, IL-23, and IL-6 (Fig. 3). Although this could be related to overall differences in cytokine production, it should be pointed out that the gene expression data reflect the presence of several cell types in the peritoneal exudates, so that differences in cytokine production also could be related to altered composition of the peritoneal exudate cells, as exemplified by the predominance of Ly6Chi monocytes in controls vs. Ly6Clo monocytes in IRF5−/− mice (Fig. 4A). IL-17 production by non-T cells also was affected by the absence of IRF5, but the present studies did not identify the peritoneal cell subset(s) producing IL-17 following pristane treatment. This will require further investigation.
Although the role of IRF5 in the induction of IFN-I is tissue specific and apparently of minor importance in some cases (1, 36), IRF5 appears to be essential for the expression of IFN-I regulated genes in the pristane model (Fig. 3A). IRF5−/− mice also failed to produce the IFN-I inducible chemokine CCL2 (MCP-I, Fig. 3B), which mediates the recruitment of Ly6Chi monocytes through CCR2 (21). Similar to the findings in IFNAR−/− mice, the small number of monocytes migrating to the peritoneal cavity of pristane-treated IRF5−/− mice rapidly acquired a Ly6Clo phenotype (Fig. 4) indicative of a more mature phenotype and enhanced phagocytic capacity. Taken together with our previous findings (21), IRF5 functions downstream of TLR7, MyD88, IL-1R-associated kinase (IRAK)-4, IRAK-1, and IRAK-2 to promote the production of IFN-I, MCP-I, and other proinflammatory chemokines and cytokines (21). On the other hand, granulocyte migration was unaffected by the absence of IRF5 (Fig. 4D), suggesting that IRF5 does not participate in the IL1α-dependent pathway of granulocyte recruitment in this model (28).
IRF5 deficiency alters T helper cell activation
TLR7-mediated IRF5 activation is necessary for protective TH1 responses to Leishmania donovani (30). Our data illustrate that TLR7 and IRF5 also contribute to the activation and polarization of TH1 cells in pristane-treated mice (Figs. 5–6). Peritoneal exudates from wild type mice contained two distinct subsets of CD4+ T cells: one CD69+ and the other CD69− (Fig. 5C). The CD69+ subset was largely absent in IFR5−/− mice. The effect of IRF5 on T cell activation could be either T cell intrinsic or mediated via its effects on IFN-I production. The IFNAR dependence in bone marrow chimera experiments (Fig. 6D) and the correlation between the number of CD69-expressing T cells and type-I interferon producing Ly6Chi monocytes (Fig. 6B) provide evidence that the effect of IRF5 on T cell activation by pristane is mediated, in part, by IFNAR signaling. Although CD69 is an IFN-I responsive gene (33), the absence of CD69+CD4+ T cells in pristane-treated IRF5−/− mice may reflect more than just the low levels IFN-I. In pristane-treated B6 mice, one population of CD4+ T cells expressed CD69, but another subset had no detectable CD69 staining, suggesting that CD69 expression may have a significance beyond being a marker of IFN-I exposure (Fig. 6A). The fact that intracellular IFNγ staining was preferentially seen in the CD69+CD4+ subset provides strong evidence that the CD69+ T cells were not only exposed to IFN-I but also activated (Fig. 6C). Thus, our data suggest that IRF5-induced IFN-I promotes T cell activation, consistent with previous observations that IFN-I also promotes the survival of activated T cells (37).
Pristane-induced lupus is augmented by a TH1 response, as both autoantibody production and renal disease are attenuated in pristane-treated IFNγ deficient mice whereas disease is exacerbated in IL-4−/− mice (38). The current study shows that in wild type mice, the predominance of a TH1 response occurs as early as 2 weeks after pristane injection (Fig. 5). This profile was limited to the inflamed peritoneal cavity, a site of IFN-I production, and it was not apparent in the spleen (Fig. S1). Interestingly, the TH1/TH2 ratio was strikingly altered in IRF5−/− mice, mainly due to increased numbers of IL-4 producing cells (Fig. 5). Similar findings were evident in IFNAR−/− and TLR7−/− mice (Figs. 6 and S1), suggesting that the shift toward a TH2 response in IRF5 −/− is due to impairment of IFN-I production rather than an intrinsic effect of IRF5 expression in T cells.
IRF-5 deficiency has an IFN-I independent effect on pDCs
An unexpected finding of this study was the near-absence of pDCs in the inflamed peritoneal cavity of IRF5−/− or MyD88−/− mice two weeks after TMPD injection (Fig. 7). In striking contrast, a normal number of pDCs were present in the peritoneum of pristane-treated TLR7−/− or IFNAR−/− mice, indicating that the lack of peritoneal pDCs was not a consequence of low IFN-I levels. The numbers of pDCs in the bone marrow of IRF5−/− mice were similar to controls, indicating that IRF5 deficiency does not cause decreased production of pDCs but instead results in defective recruitment of these cells to the inflamed peritoneum. The observation that CCL19 and CCL21 expression was abolished in the peritoneal cavity of IRF5−/− mice following pristane injection (Fig. 7E) strongly suggests that IRF5 regulates the expression of these chemokines. In contrast, the expression of CCR7 was similar or possibly increased in IRF5−/− mice (Fig. 7F), suggesting that IRF5 affects pDC migration mainly through regulation of CCL19 and CCL21, and not via the CCR7 receptor. The mechanism for chemokine modulation by IRF5 is unclear. This could be explained by either direct transcriptional regulation at the promoter region of CCL19/CCL21 or by indirect activation downstream of inflammatory mediators (other than IFN-I) regulated by IRF5 and MyD88. As CCL19 and CCL21 also play a role in the recruitment of naïve and central memory T cells, future studies to further characterize the T cell populations found in the peritoneal exudate and ectopic lymphoid tissue of pristane-treated mice are warranted.
Supplementary Material
Acknowledgements
We are grateful to Dr. Katherine Fitzgerald (University of Massachusetts, MA) for providing B6 IRF5−/− mice and Dr. Joan Durbin (Nationwide Children’s Hospital, Ohio State University, Columbus OH) for providing BALB/c IFNAR−/− mice. We thank Dr. Eric Sobel (University of Florida) for advice on the generation of bone marrow chimeric mice. This paper is dedicated to the memory of Mr. Michael J. Keefer, President and CEO of the Lupus Foundation of Florida.
This work was supported by research grant R01-AR44731 from the US Public Health Service and a grant from the Lupus Research Institute. HZ was an NIH T32 trainee (AR007603).
References
- 1.Takaoka A, Yanai H, Kondo S, Duncan G, Negishi H, Mizutani T, Kano S, Honda K, Ohba Y, Mak TW, Taniguchi T. Integral role of IRF-5 in the gene induction programme activated by Toll-like receptors. Nature. 2005;434:243–249. doi: 10.1038/nature03308. [DOI] [PubMed] [Google Scholar]
- 2.Barnes BJ, Moore PA, Pitha PM. Virus-specific activation of a novel interferon regulatory factor, IRF-5, results in the induction of distinct interferon alpha genes. J Biol Chem. 2001;276:23382–23390. doi: 10.1074/jbc.M101216200. [DOI] [PubMed] [Google Scholar]
- 3.Schoenemeyer A, Barnes BJ, Mancl ME, Latz E, Goutagny N, Pitha PM, Fitzgerald KA, Golenbock DT. The interferon regulatory factor, IRF5, is a central mediator of toll-like receptor 7 signaling. J Biol Chem. 2005;280:17005–17012. doi: 10.1074/jbc.M412584200. [DOI] [PubMed] [Google Scholar]
- 4.Couzinet A, Tamura K, Chen HM, Nishimura K, Wang Z, Morishita Y, Takeda K, Yagita H, Yanai H, Taniguchi T, Tamura T. A cell-type-specific requirement for IFN regulatory factor 5 (IRF5) in Fas-induced apoptosis. Proc Natl Acad Sci U S A. 2008;105:2556–2561. doi: 10.1073/pnas.0712295105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lien C, Fang CM, Huso D, Livak F, Lu R, Pitha PM. Critical role of IRF-5 in regulation of B-cell differentiation. Proc Natl Acad Sci U S A. 2010;107:4664–4668. doi: 10.1073/pnas.0911193107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Savitsky DA, Yanai H, Tamura T, Taniguchi T, Honda K. Contribution of IRF5 in B cells to the development of murine SLE-like disease through its transcriptional control of the IgG2a locus. Proc Natl Acad Sci U S A. 2010;107:10154–10159. doi: 10.1073/pnas.1005599107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Graham RR, Kyogoku C, Sigurdsson S, Vlasova IA, Davies LR, Baechler EC, Plenge RM, Koeuth T, Ortmann WA, Hom G, Bauer JW, Gillett C, Burtt N, Cunninghame Graham DS, Onofrio R, Petri M, Gunnarsson I, Svenungsson E, Ronnblom L, Nordmark G, Gregersen PK, Moser K, Gaffney PM, Criswell LA, Vyse TJ, Syvanen AC, Bohjanen PR, Daly MJ, Behrens TW, Altshuler D. Three functional variants of IFN regulatory factor 5 (IRF5) define risk and protective haplotypes for human lupus. Proc Natl Acad Sci U S A. 2007;104:6758–6763. doi: 10.1073/pnas.0701266104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mancl ME, Hu G, Sangster-Guity N, Olshalsky SL, Hoops K, Fitzgerald-Bocarsly P, Pitha PM, Pinder K, Barnes BJ. Two discrete promoters regulate the alternatively spliced human interferon regulatory factor-5 isoforms. Multiple isoforms with distinct cell type-specific expression, localization, regulation, and function. J Biol Chem. 2005;280:21078–21090. doi: 10.1074/jbc.M500543200. [DOI] [PubMed] [Google Scholar]
- 9.Feng D, Stone RC, Eloranta ML, Sangster-Guity N, Nordmark G, Sigurdsson S, Wang C, Alm G, Syvanen AC, Ronnblom L, Barnes BJ. Genetic variants and disease-associated factors contribute to enhanced interferon regulatory factor 5 expression in blood cells of patients with systemic lupus erythematosus. Arthritis Rheum. 2010;62:562–573. doi: 10.1002/art.27223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Niewold TB, Kelly JA, Flesch MH, Espinoza LR, Harley JB, Crow MK. Association of the IRF5 risk haplotype with high serum interferon-alpha activity in systemic lupus erythematosus patients. Arthritis Rheum. 2008;58:2481–2487. doi: 10.1002/art.23613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paun A, Reinert JT, Jiang Z, Medin C, Balkhi MY, Fitzgerald KA, Pitha PM. Functional characterization of murine interferon regulatory factor 5 (IRF-5) and its role in the innate antiviral response. J Biol Chem. 2008;283:14295–14308. doi: 10.1074/jbc.M800501200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Graham RR, Kozyrev SV, Baechler EC, Reddy MV, Plenge RM, Bauer JW, Ortmann WA, Koeuth T, Gonzalez Escribano MF, Pons-Estel B, Petri M, Daly M, Gregersen PK, Martin J, Altshuler D, Behrens TW, Alarcon-Riquelme ME. A common haplotype of interferon regulatory factor 5 (IRF5) regulates splicing and expression and is associated with increased risk of systemic lupus erythematosus. Nat Genet. 2006;38:550–555. doi: 10.1038/ng1782. [DOI] [PubMed] [Google Scholar]
- 13.Sigurdsson S, Nordmark G, Goring HH, Lindroos K, Wiman AC, Sturfelt G, Jonsen A, Rantapaa-Dahlqvist S, Moller B, Kere J, Koskenmies S, Widen E, Eloranta ML, Julkunen H, Kristjansdottir H, Steinsson K, Alm G, Ronnblom L, Syvanen AC. Polymorphisms in the tyrosine kinase 2 and interferon regulatory factor 5 genes are associated with systemic lupus erythematosus. Am J Hum Genet. 2005;76:528–537. doi: 10.1086/428480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harley IT, Kaufman KM, Langefeld CD, Harley JB, Kelly JA. Genetic susceptibility to SLE: new insights from fine mapping and genome-wide association studies. Nat Rev Genet. 2009;10:285–290. doi: 10.1038/nrg2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rullo OJ, Woo JM, Wu H, Hoftman AD, Maranian P, Brahn BA, McCurdy D, Cantor RM, Tsao BP. Association of IRF5 polymorphisms with activation of the interferon alpha pathway. Ann Rheum Dis. 2010;69:611–617. doi: 10.1136/ard.2009.118315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Richez C, Yasuda K, Bonegio RG, Watkins AA, Aprahamian T, Busto P, Richards RJ, Liu CL, Cheung R, Utz PJ, Marshak-Rothstein A, Rifkin IR. IFN regulatory factor 5 is required for disease development in the FcgammaRIIB−/−Yaa and FcgammaRIIB−/− mouse models of systemic lupus erythematosus. J Immunol. 2010;184:796–806. doi: 10.4049/jimmunol.0901748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tada Y, Kondo S, Aoki S, Koarada S, Inoue H, Suematsu R, Ohta A, Mak TW, Nagasawa K. Interferon regulatory factor 5 is critical for the development of lupus in MRL/lpr mice. Arthritis Rheum. 2011;63:738–748. doi: 10.1002/art.30183. [DOI] [PubMed] [Google Scholar]
- 18.Hron JD, Peng SL. Type I IFN protects against murine lupus. J Immunol. 2004;173:2134–2142. doi: 10.4049/jimmunol.173.3.2134. [DOI] [PubMed] [Google Scholar]
- 19.Reeves WH, Lee PY, Weinstein JS, Satoh M, Lu L. Induction of autoimmunity by pristane and other naturally occurring hydrocarbons. Trends Immunol. 2009;30:455–464. doi: 10.1016/j.it.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee PY, Kumagai Y, Li Y, Takeuchi O, Yoshida H, Weinstein J, Kellner ES, Nacionales D, Barker T, Kelly-Scumpia K, van Rooijen N, Kumar H, Kawai T, Satoh M, Akira S, Reeves WH. TLR7-dependent and FcgammaR-independent production of type I interferon in experimental mouse lupus. J Exp Med. 2008;205:2995–3006. doi: 10.1084/jem.20080462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee PY, Li Y, Kumagai Y, Xu Y, Weinstein JS, Kellner ES, Nacionales DC, Butfiloski EJ, van Rooijen N, Akira S, Sobel ES, Satoh M, Reeves WH. Type I interferon modulates monocyte recruitment and maturation in chronic inflammation. Am J Pathol. 2009;175:2023–2033. doi: 10.2353/ajpath.2009.090328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Richards HB, Satoh M, Shaw M, Libert C, Poli V, Reeves WH. Interleukin 6 dependence of anti-DNA antibody production: evidence for two pathways of autoantibody formation in pristane-induced lupus. J Exp Med. 1998;188:985–990. doi: 10.1084/jem.188.5.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nacionales DC, Weinstein JS, Yan XJ, Albesiano E, Lee PY, Kelly-Scumpia KM, Lyons R, Satoh M, Chiorazzi N, Reeves WH. B cell proliferation, somatic hypermutation, class switch recombination, and autoantibody production in ectopic lymphoid tissue in murine lupus. J Immunol. 2009;182:4226–4236. doi: 10.4049/jimmunol.0800771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nacionales DC, Kelly-Scumpia KM, Lee PY, Weinstein JS, Lyons R, Sobel E, Satoh M, Reeves WH. Deficiency of the type I interferon receptor protects mice from experimental lupus. Arthritis Rheum. 2007;56:3770–3783. doi: 10.1002/art.23023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sobel ES, Kakkanaiah VN, Kakkanaiah M, Cheek RL, Cohen PL, Eisenberg RA. T-B collaboration for autoantibody production in lpr mice is cognate and MHC-restricted. J Immunol. 1994;152:6011–6016. [PubMed] [Google Scholar]
- 26.Satoh M, Richards HB, Shaheen VM, Yoshida H, Shaw M, Naim JO, Wooley PH, Reeves WH. Widespread susceptibility among inbred mouse strains to the induction of lupus autoantibodies by pristane. Clin Exp Immunol. 2000;121:399–405. doi: 10.1046/j.1365-2249.2000.01276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hunter CA. New IL-12-family members: IL-23 and IL-27, cytokines with divergent functions. Nat Rev Immunol. 2005;5:521–531. doi: 10.1038/nri1648. [DOI] [PubMed] [Google Scholar]
- 28.Lee PY, Kumagai Y, Xu Y, Li Y, Barker T, Liu C, Sobel ES, Takeuchi O, Akira S, Satoh M, Reeves WH. IL-1alpha modulates neutrophil recruitment in chronic inflammation induced by hydrocarbon oil. J Immunol. 2011;186:1747–1754. doi: 10.4049/jimmunol.1001328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee PY, Weinstein JS, Nacionales DC, Scumpia PO, Li Y, Butfiloski E, van Rooijen N, Moldawer L, Satoh M, Reeves WH. A novel type I IFN-producing cell subset in murine lupus. J Immunol. 2008;180:5101–5108. doi: 10.4049/jimmunol.180.7.5101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paun A, Bankoti R, Joshi T, Pitha PM, Stager S. Critical role of IRF-5 in the development of T helper 1 responses to Leishmania donovani infection. PLoS Pathog. 2011;7 doi: 10.1371/journal.ppat.1001246. e1001246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sacks D, Noben-Trauth N. The immunology of susceptibility and resistance to Leishmania major in mice. Nat Rev Immunol. 2002;2:845–858. doi: 10.1038/nri933. [DOI] [PubMed] [Google Scholar]
- 32.Krausgruber T, Blazek K, Smallie T, Alzabin S, Lockstone H, Sahgal N, Hussell T, Feldmann M, Udalova IA. IRF5 promotes inflammatory macrophage polarization and TH1-TH17 responses. Nat Immunol. 2011;12:231–238. doi: 10.1038/ni.1990. [DOI] [PubMed] [Google Scholar]
- 33.Shiow LR, Rosen DB, Brdickova N, Xu Y, An J, Lanier LL, Cyster JG, Matloubian M. CD69 acts downstream of interferon-alpha/beta to inhibit S1P1 and lymphocyte egress from lymphoid organs. Nature. 2006;440:540–544. doi: 10.1038/nature04606. [DOI] [PubMed] [Google Scholar]
- 34.Seth S, Oberdorfer L, Hyde R, Hoff K, Thies V, Worbs T, Schmitz S, Forster R. CCR7 essentially contributes to the homing of plasmacytoid dendritic cells to lymph nodes under steady-state as well as inflammatory conditions. J Immunol. 2011;186:3364–3372. doi: 10.4049/jimmunol.1002598. [DOI] [PubMed] [Google Scholar]
- 35.Niewold TB, Kelly JA, Kariuki SN, Franek BS, Kumar AA, Kaufman KM, Thomas K, Walker D, Kamp S, Frost JM, Wong AK, Merrill JT, Alarcon-Riquelme ME, Tikly M, Ramsey-Goldman R, Reveille JD, Petri MA, Edberg JC, Kimberly RP, Alarcon GS, Kamen DL, Gilkeson GS, Vyse TJ, James JA, Gaffney PM, Moser KL, Crow MK, Harley JB. IRF5 haplotypes demonstrate diverse serological associations which predict serum interferon alpha activity and explain the majority of the genetic association with systemic lupus erythematosus. Ann Rheum Dis. 2011 doi: 10.1136/annrheumdis-2011-200463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Honda K, Taniguchi T. IRFs: master regulators of signalling by Toll-like receptors and cytosolic pattern-recognition receptors. Nat Rev Immunol. 2006;6:644–658. doi: 10.1038/nri1900. [DOI] [PubMed] [Google Scholar]
- 37.Marrack P, Kappler J, Mitchell T. Type I interferons keep activated T cells alive. J Exp Med. 1999;189:521–530. doi: 10.1084/jem.189.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Richards HB, Satoh M, Jennette JC, Croker BP, Yoshida H, Reeves WH. Interferon-gamma is required for lupus nephritis in mice treated with the hydrocarbon oil pristane. Kidney Int. 2001;60:2173–2180. doi: 10.1046/j.1523-1755.2001.00045.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.