Abstract
AIM
To investigate the role of Rho-associated protein kinase (ROCK) inhibitor, Y27632, in mediating the production of extracellular matrix (ECM) components including fibronectin, matrix metallo-proteinase-2 (MMP-2) and type I collagen as induced by connective tissue growth factor (CTGF) or transforming growth factor-β (TGF-β) in a human retinal pigment epithelial cell line, ARPE-19.
METHODS
The effect of Y27632 on the CTGF or TGF-β induced phenotype in ARPE-19 cells was measured with immunocytochemistry as the change in F-actin. ARPE-19 cells were treated with CTGF (1, 10, 100ng/mL) and TGF-β (10ng/mL) in serum free media, and analyzed for fibronectin, laminin, and MMP-2 and type I collagen by RT-qPCR and immunocytochemistry. Cells were also pretreated with an ROCK inhibitor, Y27632, to analyze the signaling contributing to ECM production.
RESULTS
Treatment of ARPE-19 cells in culture with TGF-β or CTGF induced an ECM change from a cobblestone morphology to a more elongated swirl pattern indicating a mesenchymal phenotype. RT-qPCR analysis and different gene expression analysis demonstrated an upregulation in expression of genes associated with cytoskeletal structure and motility. CTGF or TGF-β significantly increased expression of fibronectin mRNA (P=0.006, P=0.003 respectively), laminin mRNA (P=0.006, P=0.005), MMP-2 mRNA (P= 0.006, P= 0.001), COL1A1 mRNA (P=0.001, P=0.001), COL1A2 mRNA (P=0.001, P=0.001). Preincubation of ARPE-19 with Y27632 (10mmol/L) significantly prevented CTGF or TGF- β induced fibronectin (P=0.005, P=0.003 respectively), MMP-2 (P= 0.003, P=0.002), COL1A1 (P=0.006, P=0.003), and COL1A2 (P=0.006, P=0.004) gene expression, but not laminin (P=0.375, P=0.516)
CONCLUSION
Our study demonstrated that both TGF-β and CTGF upregulate the expression of ECM components including fibronectin, laminin, MMP-2 and type I collagen by activating the RhoA/ROCK signaling pathway. During this process, ARPE-19 cells were shown to change from an epithelial to a mesenchymal phenotype in vitro. Y27632, a ROCK inhibitor, inhibited the transcription of fibronectin, MMP-2 and type I collagen, but not laminin. The data from our work suggest a role for CTGF as a profibrotic mediator. Inhibiting the RhoA/ROCK pathway represents a potential target to prevent the fibrosis of RPE cells. This might lead to a novel therapeutic approach to preventing the onset of early PVR.
Keywords: Rho-associated protein kinase inhibitor, Connective tissue growth factor, transforming growth factor-β, proliferative vitreoretinopathy
INTRODUCTION
Dysfunction and death of the retinal pigment epithelium (RPE) constitute the final common pathway in proliferative vitreoretinopathy (PVR) [1], as well as age-related macular degeneration (AMD) [2], retinitis pigmentosa [3] and Stargardt's macular dystrophy [4]. RPE cells, as a monolayer of cuboidal cells, rest on the Bruch's membrane and play an important role in maintaining photoreceptor functions in vivo [5]. PVR is one of the most important causes of the failure of rhegmatogenous retinal detachment surgery. The pathogenesis of PVR includes a fibrotic reaction of RPE cells caused by connective tissue growth factor (CTGF) and transforming growth factor (TGF-β) [6],[7]. Under the influence of CTGF and TGF-β, RPE cells undergo transformation to fibroblast-like cells, proliferate and produce extracellular matrix (ECM).
TGF-β is a key mediator in the development of various fibrogenous diseases such as PVR. TGF-β appears to be a key mediator of the development of PVR as it is a strong inducer of ECM protein synthesis and accumulation. Moreover, TGF-β can induce the transformation of RPE cells into fibroblast-like cells in vitro [1], [8].
Connective tissue growth factor (CTGF, CCN2), a member of the CCN family of proteins, is a 38-KDa cysteine-rich polypeptide that plays an essential role in the formation of blood vessels, bone, and connective tissue [9]. CTGF is the main downstream mediator of TGF-β induced activation of fibroblasts, and its specific action on fibrotic tissue makes it a better therapeutic target than TGF-β [10]. As an angiogenic inducer, CTGF is structurally associated with secreted matrix cellular proteins, and function in cell adhesion, migration, proliferation and ECM synthesis [10]. CTGF has been shown to be a profibrogenic factor that stimulates fibroblast proliferation, cell adhesion, and extracellular matrix production. The potential role of CTGF in pathological fibrosis has been established [11], and CTGF has been suggested to be an attractive therapeutic target in some fibrotic diseases [12]-[14]. It has been shown that CTGF is upregulated in RPE cells when exposed to injury or oxidative stress [15].
Both TGF-β and CTGF can induce fibronectin and laminin mRNA and protein expressions [16]. Matrix metallo-proteinase-2 (MMP-2) is a known target of CTGF in other cell types, and has been identified as an important protease for regulating Bruch's membrane [17]. Type I collagen, a heterotrimer composed of two coordinately expressed α1 chains (COL1A1) and one α2 chain (COL1A2), is one of the major components of the ECM in PVR membranes [18]. COL1A1 and COL1A2 are encoded by distinct genes, and their expression is modulated by various cytokines [19].
The Rho/ROCK (Rho-associated protein kinases) is a family of serine-threonine protein kinases that are activated by a number of extracellular stimuli. Downstream effects such as cellular proliferation, differentiation, and apoptosis are mediated by CTGF through activation of appropriate transcription factors. Y27632 is a Rho-kinase inhibitor, and has previously been shown to change the behavior of trabecular meshwork cells and reduce intraocular pressure by changing the behavior of trabecular meshwork cells [20], [21].
Some of the biological effects of CTGF are mediated by activation of the ROCK signaling pathway in certain cell types [22], [23]. However, the signaling pathway of CTGF in RPE cells is unknown. Since activation of the Rho kinase pathways is dependent in part on the cell type, we performed experiments to determine whether any of these pathways were involved in ECM regulation resulting from CTGF stimulation of ARPE-19 cells by inhibiting CTGF with Y27632, a Rho-kinase inhibitor, after CTGF stimulation, and evaluating the production of fibronectin and laminin as a functional outcome. In the present study, we also investigated the role of RhoA/Rho-kinase signaling in mediating the effects of CTGF synthesis by TGF-β in human retinal pigment epithelial cell line, ARPE-19.
MATERIALS AND METHODS
Cell culture and stimulation with recombinant CTGF
The human retinal pigment epithelial line ARPE-19 was used for experiments. ARPE-19 cells were seeded in 6-well plates and maintained in minimal essential medium (MEM; Sigma-Aldrich, Inc., St. Louis, MO, USA) supplemented with 10% heat-inactivated fetal bovine serum (FBS) in a humidified incubator at 37°C in 5% CO2. When the cultures achieved confluence, the medium was removed and replaced with serum-free MEM containing 1% bovine serum albumin (BSA). After 24 hours of serum starvation, various concentrations of CTGF (Cell Sciences, Canton, MA, USA), and the cultures were incubated for another 24 hours for RNA isolation. In the experiments using the Rho-kinase inhibitor, Y27632, we pre-incubated cells for 30 minutes before treatment with or without exogenous CTGF.
Real-time RT-qPCR
Total RNA was extracted using a purification kit (RNeasy Mini Kit; Qiagen Inc., Valencia, CA, USA) from ARPE-19 cells and reverse transcribed using a cDNA synthesis kit (SuperScript III; Invitrogen, Carlsbad, CA, USA).
RT-qPCR reactions were carried out in 96-well blocks with an Applied Biosystems 7 500 Real-time PCR System using the 2X SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA, USA) in a reaction volume of 20µL, which contains 9µl of the primary cDNA reaction mixture, and a primer pair. Primers (QuantiTect Primer Assay; Qiagen, Valencia, CA, USA) were used for fibronectin, laminin, MMP-2 and type 1 collagen (Table 1). The cDNA was denatured at 95°C for 15 minutes followed by PCR settings of 94°C for 15 seconds, Tm-60°C for 30 seconds, and 72°C for 30 seconds.
Table 1. Primers used in RT-qPCR.
| Exons | Primer sequence (forward/reverse) | Product size (bp) |
| COL1A1 | 5′-GATTCCCTGGACCTAAAGGTGC-3′ | 156 |
| 5′-AGCCTCTCCATCTTTGCCAGCA-3′ | ||
| COL1A2 | 5′-CCTGGTGCTAAAGGAGAAAGAGG-3′ | 159 |
| 5′-ATCACCACGACTTCCAGCAGGA-3′ | ||
| MMP2 | 5′-ATGACAGCTGCACCACTGAG-3′ | 149 |
| 5′-ATTTGTTGCCCAGGAAAGTG-3′ | ||
| FN | 5′-CTGGCCGAAAATACATTGTAAA-3′ | 187 |
| 5′-CCACAGTCGGGTCAGGAG-3′ | ||
| LAMININ | 5′-CTCCATCTCACTGGATAATGGTACTG -3′ | 119 |
| 5′-GACACTCATAAAGAGAAGTGTGGACC-3′ | ||
| CTGF | 5′-GCAGGCTAGAGAAGCAGA GC-3′ | 176 |
| 5′-ATGTCTTCATGCTGGTGCAG-3′ | ||
| GAPDH | 5′-CGACCACTTTGTCAAGCTCA-3′ | 123 |
| 5′-AGGGGTCTACATGGCAAC TG-3′ |
All RT-qPCR reactions were carried out in biological duplicates, each of which was used for RNA extraction followed by RT-qPCR in triplicate. The final threshold cycle (Ct) values were the mean of six values (biological duplicates, each with triplicate). The comparative ΔΔ Ct method was used to evaluate the relative quantities of each amplified product in the samples. The Ct was automatically determined for each reaction by the Applied Biosystems 7 500 Real-time PCR System set with default parameters.
Expression levels of all genes were normalized to GAPDH mRNA levels. The specificity of the RT-qPCR reactions was determined by melt curve analysis of the amplified products using the standard method installed in the system. The t-test was used to compare the differential gene expression. P<0.05 was considered significant.
Immunohistochemistry
ARPE-19 cells were fixed with 4% PFA for 10 minutes at room temperature, washed with PBS, permeabilized with 0.05% Triton X-100/PBS and stained with fluorescently-labeled phalloidin (Alexa Fluor 568 phalloidin,invitrogen).
The ARPE-19 cells were given 3-minute rinses in PBS, fixed in 5% paraformaldehyde (PFA) for 30 minutes, and permeabilized with 0.2% Triton in PBS for 20 minutes. After a 1-hour blocking step with 1% BSA/PBS, the cells were stained with polyclonal antibodies against collagen type I (1:100 dilution in PBS, Southern Biotechnology, Birmingham, AL, USA), fibronectin (1:200 dilution in PBS, Biotech, USA), laminin (1:100 dilution in PBS, Biotech, USA), MMP-2 (1:300 dilution in PBS, anti-human, proteintech, Chicago, USA). Then the cells were incubated with Alexa Fluor555 or FITC-conjugated secondary antibodies. The cells were observed under a fluorescence microscope followed by nuclear 4′, 6-diamidino-2-phenylindole (DAPI) (Sigma) staining. Images were acquired using a Zeiss Axio Observer A1 Microscope.
RESULTS
Stimulation effect of CTGF in ARPE-19 cells
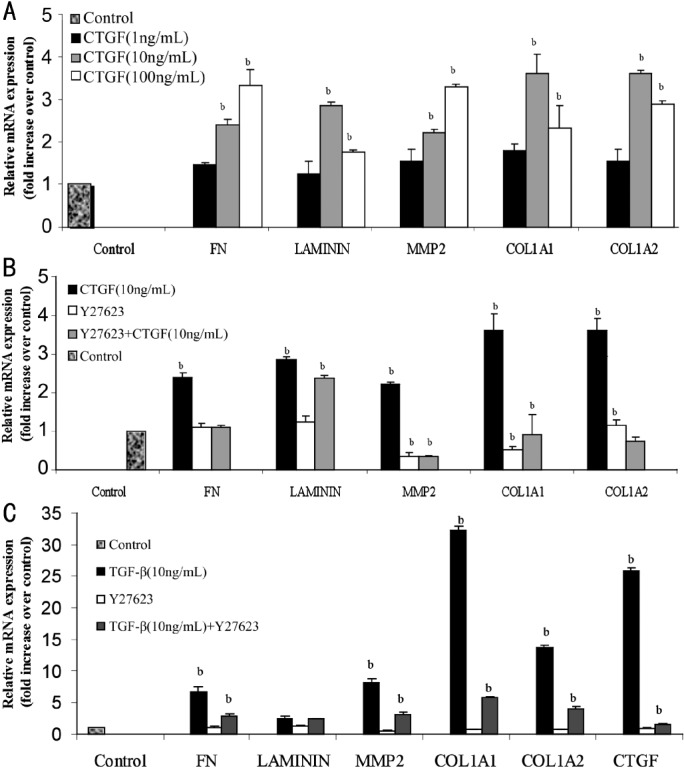
To determine whether CTGF activates fibronectin, laminin, MMP-2 and type I collagen, ARPE-19 cells were treated with various concentrations (1, 10, 100ng/mL) of CTGF for up to 24 hours. RT-qPCR was performed to analyze the production of fibronectin, laminin, MMP-2 and type I collagen. In our study, CTGF (10ng/mL) increased fibronectin mRNA expression by 2.3 fold (P=0.006), while laminin mRNA expression was increased by 2.8 fold (P=0.006) after 24 hours of exposure. We found that MMP-2 mRNA expression was significantly increased by 10ng/mL CTGF treatment (2.2 fold, P=0.006). The mRNA expressions of COL1A1 (3.6 fold, P=0.001) and COL1A2 (3.6 fold, P=0.001) were also increased in the 10ng/mL CTGF-treated cells by the 24-hour time point.
Effects of Rho-kinase inhibitor Y27632 on mRNA expression induced by CTGF
Quiescent ARPE-19 cells were pretreated with Y27632 (10mmol/L) and incubated in the presence or absence of CTGF (10ng/mL) for 24 hours, and mRNA expression levels of fibronectin, laminin, MMP-2 and type I collagen were evaluated by RT-qPCR. The Rho-kinase inhibitor treatments had very little effect on the basal levels of fibronectin and laminin mRNA expression. Figure 1 shows that at the 10ng/mL concentration of CTGF, targets of the inhibitor were specific. Pre-incubation of ARPE-19 cells with Y27632 significantly reduced the CTGF-induced expression of fibronectin mRNA level (P=0.005), type I collagen (COL1A1, P=0.006; COL1A2, P=0.006) and MMP-2 (P=0.003). However, treatment with Y27632 did not significantly reduce the laminin mRNA induced by CTGF (P=0.375).
Figure 1. A: ARPE-19 cells were treated with various concentrations (1, 10, 100ng/mL) of CTGF for up to 24 hours. RT-qPCR was performed to analyze the production of fibronectin (FN), laminin, MMP-2 and type I collagen (COL1A1 and COL1A2); B: Effects of Rho-kinase inhibitor Y27632 on CTGF-induced fibronectin, laminin, MMP-2 and type I collagen mRNA expression. Quiescent cells were pretreated with Y27632 (10mmol/L) and incubated in the presence or absence of CTGF (10ng/mL) for 24 hours; C: Effects of TGF-β (10ng/mL) were examined compare with the control. The relative levels of mRNA were normalized against GAPDH from the same cDNA preparation. Values represent the mean±SD of three independent experiments. bP<0.01 vs stimulation with the control group.
Stimulation effect of TGF-β in ARPE-19 Cells
According to previous study, we examined the effects of TGF-β (10ng/mL) on ARPE-19 cells as control. After 24 hours of exposure to TGF-β, TGF-β increased the expressions of fibronectin mRNA by 6.6 fold (P=0.003), laminin mRNA by 2.3 fold (P=0.005), MMP-2 mRNA by 8.1 fold (P=0.001), and type I collagen mRNA (COL1A1, 32.3 fold, P =0.001; COL1A2, 13.3 fold, P=0.001).
Effects of Rho-kinase inhibitor on mRNA expression of ECM factors induced by TGF-β
The effects of 10mmol/L Y27632 on the mRNA expression levels of ECM factors in ARPE-19 cells with and without 10ng/mL TGF-β were evaluated by real-time RT-qPCR. Treatment with ROCK inhibitor had very little effect on the basal mRNA expression levels of fibronectin, laminin, MMP-2, type I collagen COL1A1 and COL1A2, and CTGF. Pre-incubation of ARPE-19 with Y27632, a Rho-kinase inhibitor, significantly reduced the TGF-β-induced mRNA expression levels of fibronectin (P=0.003), MMP-2 (P=0.002), type I collagen (COL1A1 P=0.003, COL1A2 P=0.003), and CTGF (P<0.01), but not laminin (P=0.516) (Figure 1).
Immunofluorescence observation of ARPE-19 treated with Y27632 in the presence of CTGF or TGF-β
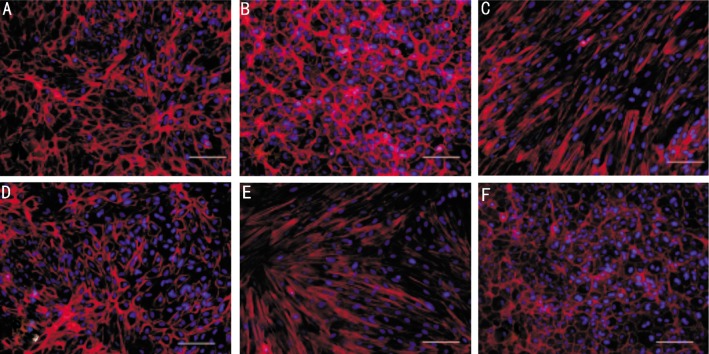
We studied the effects of CTGF or TGF-β on the cytoskeletal structure of ARPE-19 cells. CTGF (10ng/mL) or TGF-β (10ng/mL) was added when the ARPE-19 cells grew to 70% confluence, and the subsequent morphological changes were monitored by phase-contrast microscopy (Figure 2). In addition, the organization of the actin cytoskeleton was examined by phalloidin staining of F-actin (Figure 3). Vehicle-treated control cells remained tightly attached to each other and had a typical polygonal appearance (Figure 2A, 3A). In contrast, the TGF-β (Figure 2C, Figure 3C) or CTGF (Figure 2E, Figure 3E) treated cells became elongated in shape and many lost contact with their neighbors. Most of the cells exhibited a fibroblast-like shape. The ROCK inhibitor, Y27632, inhibited the morphological changes in the cells induced by TGF-β (Figure 2D, Figure 3D) and CTGF (Figure 2F, Figure 3F). The cells that were incubated with only Y27632 (Figure 2B, Figure 3B) retained their original shape. Both CTGF and TGF-β induced ARPE-19 cells to adopt a fibroblastic appearance, while ROCK inhibitor prevented this process in both CTGF- and TGF-β treated cells.
Figure 2. Phase-contrast photomicrographs of confluent cultures of ARPE-19 cells with or without Y27632 induced by CTGF (10ng/mL) or TGF-β (10ng/mL).
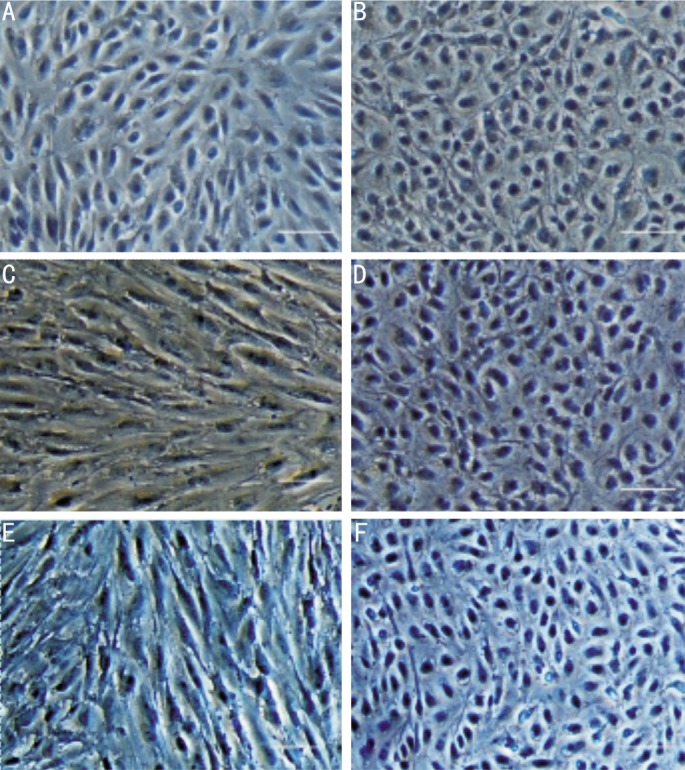
A: The vehicle-treated control cells remained tightly attached to each other and had a typical polygonal appearance; B: ARPE-19 cells incubated with the ROCK inhibitor, Y27632, retained their original shape; C: TGF-β treated cells became elongated in shape; D: Pre-incubation of ARPE-19 cells with Y27632 blocked the effect of TGF-β; E: CTGF-treated cells exhibited a fibroblast-like shape; F: Pre-incubation with Y27632 inhibited the morphological changes in ARP-19 cells induced by CTGF. Magnification, × 200. Bar, 50µm.
Figure 3. Immunofluorescent localization of fluorescently-labeled phalloidin F-actin staining in ARPE-19 cells. The nucleus was counter-stained with DAPI (blue), as determined by fluorescence microscopy.
A: Vehicle-treated control cells remained tightly attached to each other; B: ARPE-19 cells were incubated in the presence of ROCK inhibitor Y27632 (10ng/mL); C: ARPE-19 cells were incubated with TGF-β (10ng/mL) for 48 hours; D: Y27362 pretreatment inhibited the morphological changes in ARPE-19 cells as induced by TGF-β. E: ARPE-19 cells were incubated with CTGF (10ng/mL) for 48 hours; F: Serum-starved ARPE-19 cells were pretreated with Y27632 and subsequently treated with CTGF (10ng/mL). Magnification, ×200. Bar, 50µm.
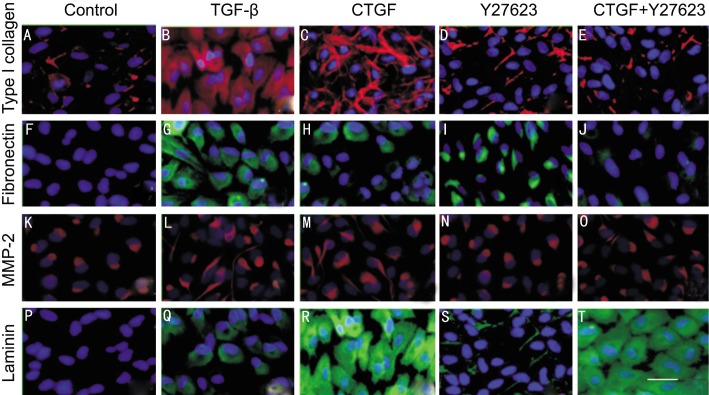
Vehicle-treated control cells show the expressing of type I collegan (4A) and MMP2 (4K). The expression of fibronectin (4F) and laminin (4P) were not observed in Vehicle-treated control ARPE-19 cells. The expression of type I collegan (4B, 4C), fibronectin (4G, 4H) MMP2 (4L, 4M) increased when cells were cultured in both TGF-β and CTGF. The protein level of laminin in CTGF (4R) was higher than in TGF-β (4Q). The expression of type I collegan (4E), fibronectin (4J) and MMP2 (4O) decreased but not laminin (4T) when ARPE-19 cells were pretreated with Y27632 and then incubated in the presence of CTGF.
Figure 4. Immunofluorescence staining for fibronectin laminin MMP2 and type I collagen in ARPE-19 cells treated by TGF-β CTGF or CTGF+ROCK inhibitor.
Vehicle-treated control cells show the expressing of type I collegan (A) and MMP2 (K). The expression of fibronectin (F) and laminin (P) were not observed in Vehicle-treated control ARPE-19 cells. The expression of type I collegan (B, C), fibronectin (G, H) MMP-2 (L, M) increased when cells were cultured in both TGF-β and CTGF. The protein level of laminin in CTGF (R) was higher than in TGF-β(Q). The expression of type I collegan (E), fibronectin (J) and MMP-2 (O) decreased but not laminin (T) when ARPE-19 cells were pretreated with Y27632 and then incubated in the presence of CTGF. Immunofluorescence staining with DAPI nuclear staining: Magnification, × 400. Bar, 100µm.
DISCUSSION
RPE cells, as the main cell type found in the epiretinal membranes, play a crucial role in the pathogenesis of PVR [24]. However, the mechanisms by which RPE cells induce extracellular matrix protein synthesis and the fibrosis reaction that are observed in PVR remain incompletely understood. It has been previously established that both CTGF and TGF-β play a role in regulating the production of ECM proteins. In this study, we hypothesized that both CTGF and TGF-β are involved in the pathophysiology of PVR by promoting fibroblastic-type changes in RPE cells. Utilizing a human RPE cell line (ARPE-19), we investigated the response of RPE cells to CTGF or TGF-β stimulation and compared their effects on the production of components of ECM deposits, including fibronectin, laminin, MMP-2 and type I collagen. In addition, using the ROCK inhibitor Y27632, we investigated the role of the RhoA/Rho-kinase signaling pathway in mediating the effects of CTGF and TGF-β on RPE cells' production of ECM proteins.
Using RT-PCR, our studies showed that in ARPE-19 cells, both TGF-β and CTGF significantly increased the expression of fibronectin by activating the Rho/ROCK signaling pathway. Cells expressed a basal level of fibronectin, and when treated with exogenous CTGF, fibronectin mRNA expression increased markedly in ARPE-19 cells as previously described [25]. Similarly, CTGF and TGF-β significantly increased the expression of laminin, MMP-2, and type I collagen. In addition, TGF-β also significantly increased the expression of CTGF.
The immunochemistry results demonstrate that the expression of fibronectin, laminin, MMP-2 and type I collagen were different when cells were exposed to TGF-β CTGF or Y27632. CTGF and TGF-β significantly increased the expression of fibronectin, MMP-2, and type I collagen. The level of laminin was significantly increased by CTGF but not TGF-β. This might due to that CTGF is the main downstream mediator of TGF-β which is more specific. RhoA/Rho-kinase pathway might not be the only way to control the production of laminin.
Furthermore, we investigated the relationships between CTGF, TGF-β and the Rho/ROCK pathway using Y27632, a pharmacologic inhibitor of ROCK activity. Our experiments using RT-PCR and immunocytochemistry supported a role of the Rho-kinase signaling pathway in mediating the effects of TGF-β and CTGF on ECM protein synthesis. We found that treating ARPE-19 cells with Y27632 prevented CTGF- and TGF-β –mediated upregulation of fibronectin, MMP-2, and type I collagen. A decrease in laminin production was observed but the effect was not statistically significant. These observations confirmed that the Rho/ROCK pathway controls CTGF expression in ARPE-19 cells. Moreover, the uncoupled expression of TGF-β and CTGF at an early time point suggests that TGF-β does not upregulate CTGF expression when the Rho/ROCK pathway is inhibited.
It was known that CTGF can activate RhoA-related signals in ARPE-19 as well as in some other cell types [12]-[14]. Generally, similar to other small GTPases, RhoA acts as a molecular switch that transmits cellular signals through a group of effector proteins [25]. Although a specific CTGF receptor has not yet been identified, CTGF appears to perform many of its functions through the ROCK pathway. In this study, we found that CTGF can mimic the effects of TGF-β on intercellular matrix production in ARPE-19 cells. Furthermore, we found that TGF-β mediated the synthesis of CTGF and that inhibition of Rho/ROCK pathway prevented TGF-β's effect on the production of CTGF and ECM proteins.
Early studies of cells in culture media with serum showed that CTGF is produced in response to TGF-β as an immediate early gene product [26],[27]. In addition, CTGF is highly expressed in human PVR membranes and partially colocalized with cytokeratin-positive RPE cells [11]. This demonstrated that the increased CTGF has functional consequences related to the RPE cells' ECM production. The RPE is believed to play a primary role in the formation of basal deposits. It is reported that the earliest basal deposits consist mainly of normal basement membrane proteins such as laminin and fibronectin [28]. Furthermore, oxidative stress to the RPE increases the production of extracellular matrix components [29]. These suggest that the increased ECM deposition might result from increased matrix protein synthesis or faulty degradation.
CTGF was significantly suppressed by the disruption of RhoA mediated cytoskeletal tension [30]. Studies revealed that the RhoA/ROCK signaling pathway regulates the tension in the actin cytoskeleton which is a key player in many cellular processes including proliferation, differentiation, stabilization of cell-matrix adhesion and modulation of gene expression [25]. In adipose-derived stromal cells, CTGF regulates the cytoskeletal tension associated with RhoA mediated cytoskeletal tension. The molecular connection between the RhoA mediated actin cytoskeletal tension and CTGF expression was revealed by examining the expression of CTGF in cells after the treatment of cytochalasin D.
MMP-2 is a known target of CTGF in cultured mesangial cells and renal interstitial fibroblasts [17] and has been identified as very important for regulating Bruch membrane [16].We found that mRNA of MMP-2 increased 2 fold and 8 fold after incubating with CTGF or TGF-β in ARPE-19 cells, respectively. The effect of TGF-β on MMP-2 expression is more than three times that of CTGF. This difference is likely due to earlier evidence that suggested the presence of many other pathways by which TGF-β controls the expression of MMP-2 in ARPE-19 cells, such as Smad pathway and p38 MAPK pathway [25].
In the present study, we demonstrated by RT-PCR the increase in expression of type I collagen genes (COL1A1, COL1A2) by TGF-β and CTGF. Frazier et al [12] previously showed that CTGF significantly increased the transcripts encoding type I collagen, integrin, and fibronectin in normal rat kidney fibroblasts. It has been shown that TGF-β induced the expression of type I collagen through Smad and p38 MAPK [16]. In this study, we demonstrated that both TGF-β and CTGF induced the mRNA expression of type I collagen by activating the RhoA/Rho-kinase pathway in a cultured human retinal pigment epithelial cell line, ARPE-19. This was supported by our study using Y27632 which reduced the potent upregulatory effects of TGF-β on COL1A1 and COL1A2 [25].
We did not find a significant inhibition of laminin production by blocking RhoA/Rho-kinase pathway. This suggests that though the RhoA/ROCK pathway may have a role in the synthesis of laminin, other cellular mechanisms exist in the production of laminin.
In summary, our study demonstrated that both TGF-β and CTGF upregulate the expression of fibronectin, laminin, MMP-2 and type I collagen. These factors are shown to be important contributors to the fibrosis process of RPE cells. We demonstrated that the ROCK inhibitor, Y27632, inhibited the transcription of fibronectin, MMP-2 and type I collagen, but not laminin. The data from our work suggest a role for CTGF as a profibrotic mediator by activating the Rho/ROCK signaling pathway which induces the production of ECM proteins. Further work to clarify the exact role of ROCK inhibitor in matrix expansion might lead to a novel therapeutic approach to preventing the onset of early PVR.
REFERENCE
- 1.Lee J, Ko M, Joo CK. Rho plays a key role in TGF-β1 induced cytoskeletal rearrangement in human retinal pigment epithelium. J Cell Physiol. 2008;216(2):520–526. doi: 10.1002/jcp.21424. [DOI] [PubMed] [Google Scholar]
- 2.Binder S, Stanzel BV, Krebs I, Glittenberg C. Transplantation of the RPE in AMD. Prog Retin Eye Res. 2007;26(5):516–554. doi: 10.1016/j.preteyeres.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 3.Bovolenta P, Cisneros E. Retinitis pigmentosa: cone photoreceptors starving to death. Nat Neurosci. 2009;12(1):5–6. doi: 10.1038/nn0109-5. [DOI] [PubMed] [Google Scholar]
- 4.Walia S, Fishman GA. Natural history of phenotypic changes in Stargardt macular dystrophy. Ophthalmic Genet. 2009;30(2):63–68. doi: 10.1080/13816810802695550. [DOI] [PubMed] [Google Scholar]
- 5.Strauss O. The retinal pigment epithelium in visual function. Physiol Rev. 2005;85(3):845–881. doi: 10.1152/physrev.00021.2004. [DOI] [PubMed] [Google Scholar]
- 6.Connor TB, Jr, Roberts AB, Sporn M, Danielpour D, Dart LL, Michels RG, de Bustros S, Enger C, Kato H, Lansing M. Correlation of fibrosis and transforming growth factor-beta type 2 levels in the eye. J Clin Invest. 1989;83(5):1661. doi: 10.1172/JCI114065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Setten G, Berglin L, Blalock TD, Schultz G. Detection of connective tissue growth factor in subretinal fluid following retinal detachment: possible contribution to subretinal scar formation, preliminary results. Ophthalmic Res. 2005;37(6):289–292. doi: 10.1159/000087698. [DOI] [PubMed] [Google Scholar]
- 8.Lee SC, Kwon OW, Seong GJ, Kim SH, Ahn JE, Kay ED. Epitheliomesenchymal transdifferentiation of cultured RPE cells. Ophthalmic Res. 2001;33(2):80–86. doi: 10.1159/000055648. [DOI] [PubMed] [Google Scholar]
- 9.Chaqour B, Goppelt-Struebe M. Mechanical regulation of the Cyr61/CCN1 and CTGF/CCN2 proteins. FEBS J. 2006;273(16):3639–3649. doi: 10.1111/j.1742-4658.2006.05360.x. [DOI] [PubMed] [Google Scholar]
- 10.Brigstock DR. The CCN family: a new stimulus package. J Endocrinol. 2003;178(2):169–175. doi: 10.1677/joe.0.1780169. [DOI] [PubMed] [Google Scholar]
- 11.He S, Chen Y, Khankan R, Barron E, Burton R, Zhu DH, Ryan SJ, Oliver N, Hinton DR. Connective tissue growth factor as a mediator of intraocular fibrosis. Invest Ophthalmol Vis Sci. 2008;49(9):4078–4088. doi: 10.1167/iovs.07-1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frazier K, Williams S, Kothapalli D, Klapper H, Grotendorst GR. Stimulation of fibroblast cell growth, matrix production, and granulation tissue formation by connective tissue growth factor. Invest Ophthalmol Vis Sci. 1996;107(3):404–411. doi: 10.1111/1523-1747.ep12363389. [DOI] [PubMed] [Google Scholar]
- 13.Kireeva ML, Latinkic BV, Kolesnikova TV, Chen CC, Yang GP, Abler AS, Lau LF. Cyr61 and Fisp12 are both ECM-associated signaling molecules: activities, metabolism, and localization during development. Exp Cell Res. 1997;233(1):63–77. doi: 10.1006/excr.1997.3548. [DOI] [PubMed] [Google Scholar]
- 14.Leask A. Transcriptional profiling of the scleroderma fibroblast reveals a potential role for connective tissue growth factor (CTGF) in pathological fibrosis. Keio J Med. 2004;53(2):74–77. doi: 10.2302/kjm.53.74. [DOI] [PubMed] [Google Scholar]
- 15.Matsuda S, Gomi F, Katayama T, Koyama Y, Tohyama M, Tano Y. Induction of connective tissue growth factor in retinal pigment epithelium cells by oxidative stress. Jpn J Ophthalmol. 2006;50(3):229–234. doi: 10.1007/s10384-005-0317-6. [DOI] [PubMed] [Google Scholar]
- 16.Nagai N, Klimava A, Lee WH, Izumi-Nagai K, Handa JT. CTGF is increased in basal deposits and regulates matrix production through the ERK (p42/p44mapk) MAPK and the p38 MAPK signaling pathways. Invest Ophthalmol Vis Sci. 2009;50(4):1903–1910. doi: 10.1167/iovs.08-2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang M, Huang H, Li J, Huang W, Wang H. Connective tissue growth factor increases matrix metalloproteinase-2 and suppresses tissue inhibitor of matrix metalloproteinase-2 production by cultured renal interstitial fibroblasts. Wound Repair Regen. 2007;15(6):817–824. doi: 10.1111/j.1524-475X.2007.00284.x. [DOI] [PubMed] [Google Scholar]
- 18.Scheiffarth O, Kampik A, Günther H, Mark K. Proteins of the extracellular matrix in vitreoretinal membranes. Graefes Arch Clin Exp Ophthalmol. 1988;226(4):357–361. doi: 10.1007/BF02172967. [DOI] [PubMed] [Google Scholar]
- 19.Slack JL, Liska DJ, Bornstein P. Regulation of expression of the type I collagen genes. Am J Med Genet. 2005;45(2):140–151. doi: 10.1002/ajmg.1320450203. [DOI] [PubMed] [Google Scholar]
- 20.Koga T, Awai M, Tsutsui J, Yue BYJT, Tanihara H. Rho-associated protein kinase inhibitor, Y-27632, induces alterations in adhesion, contraction and motility in cultured human trabecular meshwork cells. Exp Eye Res. 2006;82(3):362–370. doi: 10.1016/j.exer.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 21.Nakamura Y, Hirano S, Suzuki K, Seki K, Sagara T, Nishida T. Signaling mechanism of TGF-beta1-induced collagen contraction mediated by bovine trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2002;43(11):3465–3472. [PubMed] [Google Scholar]
- 22.Haydont V, Mathe D, Bourgier C, Abdelali J, Aigueperse J, Bourhis J, Vozenin-Brotons MC. Induction of CTGF by TGF-beta1 in normal and radiation enteritis human smooth muscle cells: Smad/Rho balance and therapeutic perspectives. Radiother Oncol. 2005;76(2):219–225. doi: 10.1016/j.radonc.2005.06.029. [DOI] [PubMed] [Google Scholar]
- 23.Rodrigues-Diez R, Carvajal-Gonzalez G, Sanchez-Lopez E, Rodriguez-Vita J, Rodrigues Diez R, Selgas R, Ortiz A, Egido J, Mezzano S, Ruiz-Ortega M. Pharmacological modulation of epithelial mesenchymal transition caused by angiotensin II. Role of ROCK and MAPK pathways. Pharm Res. 2008;25(10):2447–2461. doi: 10.1007/s11095-008-9636-x. [DOI] [PubMed] [Google Scholar]
- 24.Saika S, Kono-Saika S, Tanaka T, Yamanaka O, Ohnishi Y, Sato M, Muragaki Y, Ooshima A, Yoo J, Flanders KC. Smad3 is required for dedifferentiation of retinal pigment epithelium following retinal detachment in mice. Lab Invest. 2004;84(10):1245–1258. doi: 10.1038/labinvest.3700156. [DOI] [PubMed] [Google Scholar]
- 25.Itoh Y, Kimoto K, Imaizumi M, Nakatsuka K. Inhibition of RhoA/Rho-kinase pathway suppresses the expression of type I collagen induced by TGF-[beta] 2 in human retinal pigment epithelial cells. Exp Eye Res. 2007;84(3):464–472. doi: 10.1016/j.exer.2006.10.017. [DOI] [PubMed] [Google Scholar]
- 26.Folger PA, Zekaria D, Grotendorst G, Masur SK. Transforming growth factor-beta-stimulated connective tissue growth factor expression during corneal myofibroblast differentiation. Invest Ophthalmol Vis Sci. 2001;42(11):2534–2541. [PubMed] [Google Scholar]
- 27.Grotendorst GR, Okochi H, Hayashi N. A novel transforming growth factor beta response element controls the expression of the connective tissue growth factor gene. Cell Growth Differ. 1996;7(4):469–480. [PubMed] [Google Scholar]
- 28.Schaft TL, Mooy CM, Bruijn WC, Bosman FT, Jong PTVM. Immunohistochemical light and electron microscopy of basal laminar deposit. Graefes Arch Clin Exp Ophthalmol. 1994;232(1):40–46. doi: 10.1007/BF00176436. [DOI] [PubMed] [Google Scholar]
- 29.Espinosa-Heidmann DG, Suner IJ, Catanuto P, Hernandez EP, Marin-Castano ME, Cousins SW. Cigarette smoke–related oxidants and the development of sub-RPE deposits in an experimental animal model of dry AMD. Invest Ophthalmol Vis Sci. 2006;47(2):729–737. doi: 10.1167/iovs.05-0719. [DOI] [PubMed] [Google Scholar]
- 30.Cicha I, Goppelt-Struebe M, Muehlich S, Yilmaz A, Raaz D, Daniel WG, Garlichs CD. Pharmacological inhibition of RhoA signaling prevents connective tissue growth factor induction in endothelial cells exposed to non-uniform shear stress. Atherosclerosis. 2008;196(1):136–145. doi: 10.1016/j.atherosclerosis.2007.03.016. [DOI] [PubMed] [Google Scholar]