Abstract
AIM
Excessive dissolve of corneal tissue induced by MMPs which were activated by cytokins and chemokines will lead to corneal ulcer. The molecular mechanism of Lipoxin A4 (LXA4) on corneal collagen degradation in three dimensions was investigated.
METHODS
Rabbit corneal fibroblasts were harvested and suspended in serum-free MEM. Type I collagen, DMEM, collagen reconstitution buffer and corneal fibroblast suspension were mixed on ice. The resultant mixture solidified in an incubator, after which test reagents and plasminogen was overlaid and the cultures were returned to the incubator. The supernatants from collagen gel incubations were collected and the amount of hydroxyproline in the hydrolysate was measured. Immunoblot analysis of MMP-1, -3 and TMMP-1,-2 was performed. MMP-2,-9 was detected by the method of Gelatin zymography. Cytotoxicity assay was measured.
RESULTS
LXA4 inhibited corneal collagen degradation in a dose and time manner. LXA4 inhibited the IL-1β induced increases in the pro-MMP-1, -2, -3, -9 and active MMP-1, -2, -3, -9 in a concentration dependent manner. LXA4 could also inhibit the IL-1β induced increases in TIMP-1, -2.
CONCLUSION
As a potent anti-inflammation reagent, LXA4 can inhibit corneal collagen degradation induced by IL-1β in corneal fibroblasts thus inhibiting corneal dissolving pathology process.
Keywords: lipoxin A4, IL-1β, cornea, collagen, dissolution
Introduction
Extracellular matrix proteins concerned with matrix metalloproteinase (MMPs) controlling ECM balance in vitro[1]-[3]. MMPs cleave cellular, extracellular and extracellular matrix substrates regulating tissue structure [4]. Excessive dissolve of corneal stroma will lead to corneal ulcer. IL-1β is a major proinflammatory factor [5].
Lipoxin A4 (LXA4), as the predominant endogenously generated lipoxin, was a high potency of anti-inflammatory lipid mediator. Its molecular formula was C20H32O5 [6]. LXA4 and its receptor (FPR2/ALX) was used as marker for resolving inflammation and tissue injury [7],[8]. LXA4 inhibited LPS-induced production of NO, IL-1β and TNF-α in a concentration-dependent manner via NF-κB, ERK, p38 MAPK and AP-1 signaling pathways in LPS-activated microglia [9]. LXA4 blocked IL-1β and TNF-α-mediated upregulation of E-selecting and intercellular cell adhesion molecule-1 (ICAM-1) and consequent binding of lymphocytes by activating its receptor [10]. LXA4-mediated reduction of MMP-1, -2, -3, -9 in different cell lines and are attributed to increased TIMP-1 expression [11]-[13].
Three dimensional culture of corneal fibroblasts in collagen gel mimics the in vivo situation [14]. Previously we showed that pseudomonas aeruginosa elastase and IL-1β can stimulate corneal collagen degradation by corneal fibroblasts [15]. We also showed that the female sex hormone inhibited IL-1β-induced collagen degradation by corneal fibroblasts [16],[17]. In this paper, we examined whether LXA4 inhibited collagen degradation by rabbit corneal fibroblasts in response to IL-1β. As LXA4 was a strong mediator of inflammation. We want to clarify the mechanism and effect of LXA4 on corneal collagen degradation by a three dimensional culture system. We will manifest whether LXA4 can be a new potent option in the treatment of corneal deliquescent pathology process.
Materials and Methods
Materials
Dulbecco's phosphate-buffered saline (DPBS), Minimum Essential Medium Eagle (MEM) and trypsin-EDTA were obtained from Weibo Chem company. Type 1 collagen (acid solubilized), 5×Dulbecco's modified Eagle's medium (DMEM) were from Nitta Gelatin Co., LTD. Fetal bovine serum (FBS) was from Shanghai Yantuo Biotecnology (Shanghai, China). Bovine plasminogen, protease inhibitor cocktail and LXA4 were from Chemicalbook (Shanghai, China). Recombinant human IL-1β was obtained from R&D Systems. Mouse monoclonal antibodies to rabbit MMP-1 and MMP-3 were obtained from antibodies-online (German). An enhanced chemiluminescence (ECL) kit was from GE Healthcare (Qfbio, Shanghai, China). Coomassie brilliant blue and gelatin were obtained from Bio-Rad (Seajet scitntic Inc, Beijing, China). A cytotoxicity assay (CytoTox 96Non-Radioactive) was from Promega (Beijing, China).
Methods
Cell isolation
Rabbit corneal fibroblasts were isolated and maintained as described previously [16]-[19]. In brief, the enucleated eye was washed with DPBS containing antibiotic-antimycotic mixture, the endothelial layer of the excised cornea was removed mechanically, and the remaining corneal tissue was incubated with dispase (2mg/mL, in MEM) for 1 hour at 37°C. After mechanical removal of the epithelial sheet, the remaining tissue was treated with collagenase (2mg/mL, in MEM) at 37°C until a single-cell suspension of corneal fibroblasts was obtained. The isolated corneal fibroblasts were cultured under a humidified atmosphere of 5% CO2 at 37°C in 60-mm culture dishes supplemented with 10% FBS. Proliferating cells were harvested for experiments at the subconfluent stage after four to seven passages in monolayer culture.
Three-dimensional culture system
Culture collagen gels were prepared as described [16]-[19]. In brief, corneal fibroblasts were harvested by exposure to Trypsin followed by centrifugation at 15 000g for 5 minutes, and they were then suspended in serum-free MEM. Acid-solubilized collagen type I (3mg/mL), 5×DMEM, collagen reconstitution buffer, and corneal fibroblast suspension (2.2×106/mL in MEM) were mixed on ice at a volume ratio of 7:2:1:1. The resultant mixture (0.5mL) was added to each well of a 24-well culture plate and allowed to solidify in an incubator containing 5% CO2 at 37°C, after which 0.5mL of serum-free MEM containing test reagents and plasminogen (60µg/mL) was overlaid and the cultures were returned to the incubator for 48 hours.
Assay of collagenolytic activity
Collagen degradation was measured as previously described [16]-[19]. In brief, the supernatants from collagen gel incubations were collected and native collagen fibrils with a molecular size of >100kDa were removed by ultrafiltration. The filtrate was subjected to hydrolysis with 6mol/L HCl for 24 hours at 110°C, and the amount of hydroxyproline in the hydrolysate was determined by measurement of absorbance at 558nm with a spectrophotometer.
Immunoblot analysis
Immunoblot analysis of MMP-1, -3 was performed as described previously [16]-[19].In brief, culture supernatants from collagen gel incubations were subjected to SDS-polyacrylamide gel electrophoresis on a 10% gel, and the separated proteins were transferred electrophoretically to a nitrocellulose membrane. Nonspecific sites of the membrane were blocked, and it was then incubated with antibodies to MMP-1, -3. Immune complexes were detected with the use of horseradish peroxidase-conjugated secondary antibodies and ECL reagents.
Gelatin zymography
Gelatin zymography was performed as described previously [16]-[19]. In brief, culture supernatants from collagen gel incubations were mixed with SDS sample buffer by the ratio of 2:1, and the resulting mixture were subjected to SDS-polyacrylamide gel electrophoresis in the dark at 4°C on a 10% gel containing 0.1% gelatin. The gel was then washed with 2.5% Triton X-100 for 1 hour before incubation for 18 hours at 37°C in a reaction mixture containing 50mmol/L Tris-HCl (pH 7.5), 5mmol/L CaCl2, and 1% Triton X-100. The gel was finally stained with Coomassie brilliant blue.
Cytotoxicity assay
LDH release was measured by Non-Radioactive Cytotoxicity Assay kit. In brief, 2×104] cells were cultured in 10% FBS in 96-well plates for 24 hours. After washing, the cells were secreted by the compounds in serum free for extra 24 hours. 0.1% Triton was taken as positive control. Supernatants and Substrate were mixed in assay buffer in a new plate (1:1 vol, 30 minutes, RT). Stop Solution was added and absorbance was recorded on spectrophotometer at 400nm.
Statistical Analysis
Data are presented as means±SEM and were analyzed with Dunnett's multiple comparison test. P value <0.0001 was considered statistically significant.
Results
Inhibition effect of LXA4 on IL-1β induced collagen degradation by corneal fibroblasts
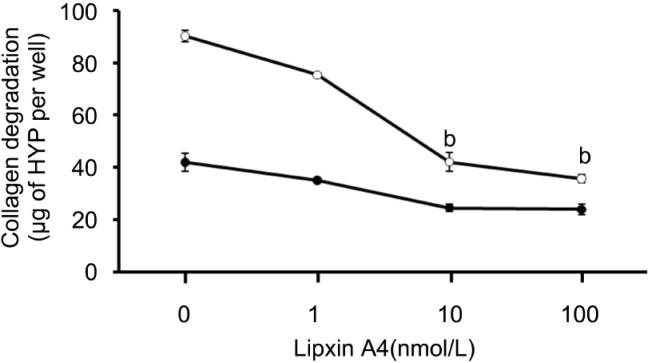
IL-1β can markedly increases the extent of collagen degradation by cultured corneal fibroblasts as before [16]-[19]. To investigate and analysis the inhibition effect of LXA4 on collagen degradation resulting from IL-1β stimulation in three dimensional cultures of rabbit corneal fibroblasts, the cells incubated for 48 hours with LXA4 (1µmol/L-100µmol/L) resulted in a concentration dependent inhibition of collagen degradation in the presence of IL-1β (0.1ng/mL, Figure 1).
Figure 1. Dose-depended inhibition effect by LXA4 of IL-1β induced collagen degradation by corneal fibroblasts.
Rabbit fibroblasts were cultured in the absence (closed symbols) or presence (open symbols) of IL-1β (0.1ng/mL) and in the presence of the indicated concentrations of LXA4. After incubation of the cells for 48 hours, the amount of degraded collagen in the culture supernatants was determined. Data are expressed as micrograms of hydroxyproline (HYP) per well and are means±SEM of values from an experiment that was repeated three times with similar results. bP<0.001(Dunnett's test) versus the value for cells cultured with IL-1β in the absence of LXA4.
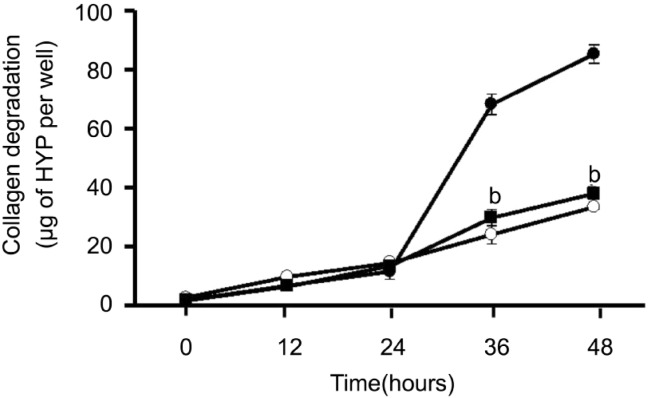
Except the results above, we carried out the time course of collagen degradation by corneal fibroblasts in the absence or presence of IL-1β (0.1ng/mL) or 10nmol/L LXA4. In different time points, the amount of degraded collagen increased gradually. Compared to the amount of collagen degradation by plasminogen, IL-1β increased the amount of degraded collagen dramatically at 36 and 48 hours. This effect was inhibited by 10nmol/L LXA4 at 36 and 48 hours (Figure 2).
Figure 2. Time-depended inhibition effect of LXA4 on IL-1β induced collagen degradation by corneal fibroblasts.
Cells were cultured in collagen gels for the indicated times in the presence of 60µg/mL plasminogen and in the absence (open symbols) or presence of 0.1ng/mL IL-1β (close symbols), in the absence (circles) or presence (squares) of 10nmol/L LXA4, after which the amount of degraded collagen was determined. Data are mean±SEM of values from three experiments. bP< 0.001 (Dunnett test) versus the corresponding value for cells cultured with IL-1β and plasminogen.
Effects of LXA4 on the expressions of MMP-1, -3
MMP-1, -3 expressions were detected using the methods of immunoblot analysis. Corneal fibroblasts were cultured in collagen gels for 48 hours in the presence of IL-1β and in the presence of LXA4 (1nmol/L-100nmol/L). Coincident with our previous result[9],[10],[19],[20], immunoblot analysis with antibodies to human biotinylated MMP-1 revealed that the culture supernatants of cells incubated with IL-1β contained large amounts of 61-kDa and 57-kDa immunoreactive proteins corresponding to pro-MMP-1. 49-kDa and 45-kDa immunoreactive proteins were in correspondence to active MMP-1. LXA4 can inhibit the IL-1β induced increases in pro-MMP-1 and active MMP-1 in a dose dependent manner (Figure 3).
Figure 3. Effects of LXA4 on the expressions of pro-MMP-1 and MMP-1 by corneal fibroblasts.
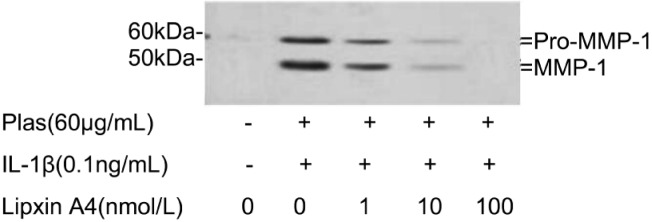
Cells were cultured in collagen gels for 48 hours in the absence or presence of IL-1β (0.1ng/mL), and in the presence of the indicated concentrations of LXA4. The culture supernatants were then subjected to immunoblot analysis with antibodies to MMP-1. Data are representative of three times. The positions of bands corresponding to the pro-MMP-1 and MMP-1 are indicated on the right, and those of molecular size are shown on the left.
Immunoblot analysis with antibodies to MMP-3 detects 57-kDa and 45-kDa immunoreactive proteins corresponding to pro-MMP-3 and active MMP-3 in the culture supernatants of cells incubated with IL-1β. LXA4 inhibited the IL-1β-induced increases in the pro-MMP-3 and active MMP-3 in a concentration-dependent manner (Figure 4).
Figure 4. Effects of LXA4 on the expressions of pro-MMP-3 and MMP-3 by corneal fibroblasts.
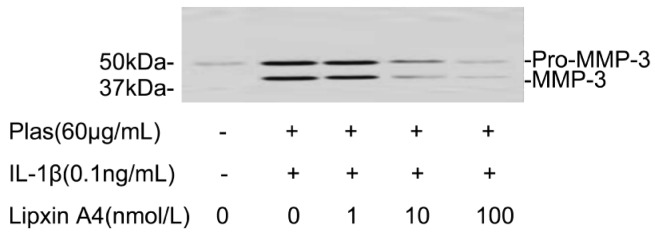
Cells were cultured in collagen gels for 48 hours in the absence or presence of IL-1β (0.1ng/mL), and in the presence of the indicated concentrations of LXA4. The culture supernatants were then subjected either to immunoblot analysis with antibodies to MMP-3. Data are representative of three times. The positions of bands corresponding to the pro-MMP-3 and MMP-3 are indicated on the right, and those of molecular size are shown on the left.
Effects of LXA4 on the expressions of MMP-2, 9
The expressions of MMP-2, -9 were detected by gelatin zymography. Cells cultured in the presence of IL-1β (0.1ng/mL) resulted in an increase in the intensity of the band corresponding to active MMP-2 and the appearance of bands corresponding to active MMP-9 respectively. LXA4 inhibited the IL-1β-induced increases in the amounts of the active MMP-9 and active MMP-2 in a concentration-dependent manner (Figure 5).
Figure 5. Effects of LXA4 on the expressions of MMP-2, -9 by corneal fibroblasts.
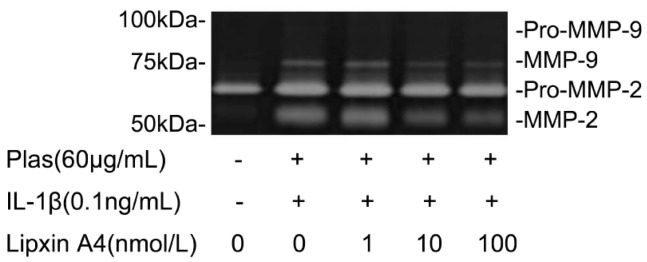
Cells were cultured in the absence or presence of IL-1β (0.1ng/mL) and the indicated concentrations of LXA4. Culture supernatants were then subjected to gelatin zymography. The positions of bands corresponding to pro-MMP-2 and pro-MMP-9 and active MMP-2, -9 are indicated on the right, and those of molecular size are shown on the left. Data are representative of three times.
Effects of LXA4 on the expression of TIMP-1, -2
Further analyses emphasized the effects of LXA4 on the expressions of TIMPs secreted by corneal fibroblasts. Immunoblot analysis with antibodies to TIMP-1 revealed that TIMP-1 protein levels increased in the presence of IL-1β (0.1ng/mL) and plasminogen. The increased secretion of TIMP-1 can be inhibited by LXA4 in a dose-dependent manner (Figure 6). LXA4 can also inhibit the expression of TIMP-2 in a dose-depended manner, which corresponding to 21-kDa immunoreactive protein (Figure 7).
Figure 6. Effects of LXA4 on the expression of TIMP-1 by corneal fibroblasts.
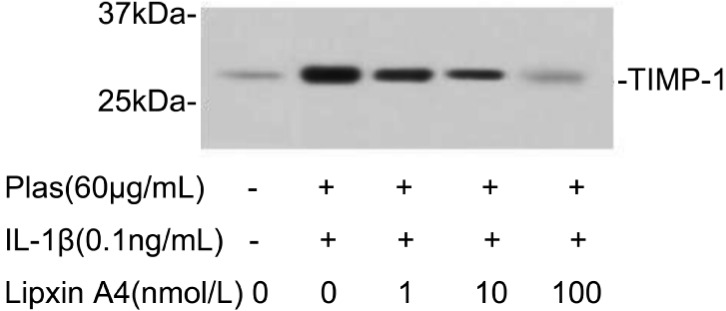
Cells were cultured in the absence or presence of IL-1β (0.1ng/mL), and in the presence of the indicated concentrations of LXA4. The culture supernatants were then subjected to immunoblot analysis with antibodies to TIMP-1, Data are representative of three times. The positions of bands corresponding to the TIMP-1 are indicated on the right, and those of molecular size are shown on the left.
Figure 7. Effects of LXA4 on the expression of TIMP-2 by corneal fibroblasts.
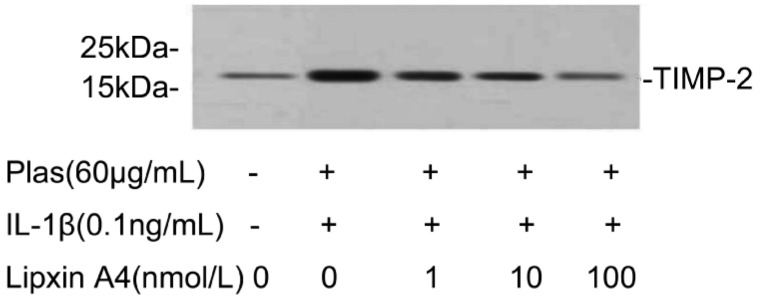
Cells were cultured in the absence or presence of IL-1β (0.1ng/mL), and in the presence of the indicated concentrations of LXA4. The culture supernatants were then subjected to immunoblot analysis with antibodies to TIMP-2, Data are representative of three independent experiments. The positions of bands corresponding to the TIMP-2 are indicated on the right, and those of molecular size are shown on the left.
LDH detection
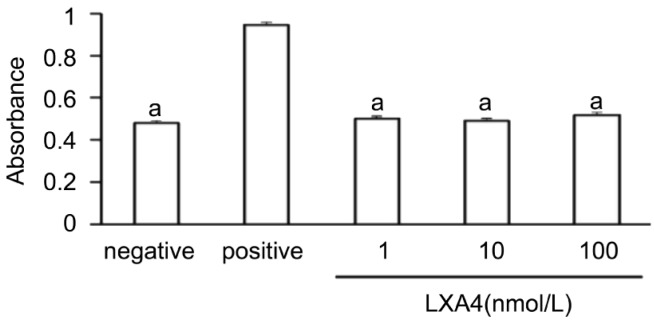
Measurement of LDH release revealed that LXA4 at 1nmol/L, 10nmol/L, 100nmol/L had no cytotoxic effect on corneal fibroblasts (Figure 8).
Figure 8. Lack of a cytotoxic effect of LXA4 on corneal fibroblasts.
Cells were incubated for 24 hours in MEM and in the absence (negative control) or presence of 1nmol/L, 10nmol/L or 100nmol/L LXA4, after which the culture supernatants were assayed for LDH activity with a colorimetric assay. The amount of LDH released from cells by 0.1% Triton was determined as a positive control. Data are means±SEM from three times. aP<0.05 vs 0.1% Triton (Dunnett's test).
Discussion
We manifested that LXA4 can inhibit the amount of collagen degradation of rabbit corneal fibroblasts induced by IL-1β in dose and time dependent manner. LXA4 can suppress the expressions of MMP-1, -2, -3, -9 in corneal fibroblasts exposed to IL-1β in a dose dependent manner.
Fibroblast cells in affected area triggering cytokine and chemokine production which recruitment leukocyte and activate MMPs thus destruct inflammation tissue [20]. The anti-MMP treatment may provide a therapeutic tool for the pathological destruction of ECM and chronic inflammatory disease [21].
IL-1β is an important marker of inflammation in many diseases [22]-[24]. We proved that IL-1β can induce collagen degradation by corneal fibroblasts and activate MMPs expression as before. LXA4 can inhibit MMPs expression induced by IL-1β thus inhibiting the corneal collagen degradation.
As an endogenous, anti-inflammatory mediator, LXA4 was involved in the resolution of inflammation. LXA4 can inhibit the expressions of MMPs and stimulate the expressions of TIMPs in many pathology processes. LXA4 can be a negative feedback loop opposing inflammatory cytokine induced inflammation [11]-[13],[25]-[26].
We found that LXA4 can inhibit the expressions of Tissue inhibitors of MMPs (TIMPs) not as anticipated. TIMP-1, -2 are produced in relation to urokinase (uPA) and MMP-1, -9 in cornea injury. There is an imbalance between the expression of this proteolytic enzyme and its inhibitors, which may contribute to changes in the wound-healing process and ultimately lead to corneal ulcer development [27].
LXA4 can inhibit corneal collagen degradation induced by IL-1β, this effect was the consequence of the reduction of MMP-1, -2, -3, -9 and TIMP-1, -2. LXA4 showed no evident cytotoxicity on corneal fibroblasts. Therefore, LXA4 may have therapeutic potential for corneal dissolved collagen degradation induced by cytokine thus can be a potent drug for corneal inflammation disease.
Footnotes
Foundation items: Jilin University Basic Scientific Research Operating Expenses Fund, China (Research Fund of the Bethune B Plan of Jilin University, 2012; No. 2012230); Research Fund of Jilin Provincial Science and Technology Department, China (international cooperation item, No.20120726)
References
- 1.Vollberg TM, Sr, George MD, Jetten AM. Induction of extracellular matrix gene expression in normal human keratinocytes by transforming growth factor beta is altered by cellular differentiation. Exp Cell Res. 1991;193(1):93–100. doi: 10.1016/0014-4827(91)90542-3. [DOI] [PubMed] [Google Scholar]
- 2.Kim HS, Shang T, Chen Z, Pflugfelder SC, Li DQ. TGF-beta1 stimulates production of gelatinase (MMP-9), collagenases (MMP-1, -13) and stromelysins (MMP-3, -10, -11) by human corneal epithelial cells. Exp Eye Res. 2004;79(2):263–274. doi: 10.1016/j.exer.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 3.Lijnen HR. Plasmin and matrix metalloproteinases in vascular remodeling. Thromb Haemost. 2001;86(1):324–333. [PubMed] [Google Scholar]
- 4.Lijnen HR. Matrix metalloproteinases and cellular fibrinolytic activity. Biochemistry (Mosc) 2002;67(1):92–98. doi: 10.1023/a:1013908332232. [DOI] [PubMed] [Google Scholar]
- 5.Thakur A, Barrett RP, McClellan S, Hazlett LD. Regulation of Pseudomonas aeruginosa corneal infection in IL-1 beta converting enzyme (ICE, caspase-1) deficient mice. Curr Eye Res. 2004;29(4-5):225–233. doi: 10.1080/02713680490516710. [DOI] [PubMed] [Google Scholar]
- 6.Börgeson E, McGillicuddy FC, Harford KA, Corrigan N, Higgins DF, Maderna P, Roche HM, Godson C. Lipoxin A4 attenuates adipose inflammation. FASEB J. 2012 doi: 10.1096/fj.12-208249. [DOI] [PubMed] [Google Scholar]
- 7.Dakin SG, Werling D, Hibbert A, Abayasekara DR, Young NJ, Smith RK, Dudhia J. Macrophage sub-populations and the Lipoxin A4 receptor implicate active inflammation during equine tendon repair. PLoS One. 2012;7(2):e32333. doi: 10.1371/journal.pone.0032333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Serhan CN. Resolution phase of inflammation: novel endogenous anti-inflammatory and proresolving lipid mediators and pathways. Annu Rev Immunol. 2007;25:101–137. doi: 10.1146/annurev.immunol.25.022106.141647. [DOI] [PubMed] [Google Scholar]
- 9.Wang YP, Wu Y, Li LY, Zheng J, Liu RG, Zhou JP, Yuan SY, Shang Y, Yao SL. Aspirin-triggered Lipoxin A4 attenuates LPS-induced pro-inflammatory responses by inhibiting activation of NF-κB and MAPKs in BV-2 microglial cells. J Neuroinflammation. 2011;8:95. doi: 10.1186/1742-2094-8-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chinthamani S, Odusanwo O, Mondal N, Nelson J, Neelamegham S, Baker OJ. Lipoxin A4 inhibits immune cell binding to salivary epithelium and vascular endothelium. Am J Physiol Cell Physiol. 2012;302(7):C968–978. doi: 10.1152/ajpcell.00259.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu Y, Wang YP, Guo P, Ye XH, Wang J, Yuan SY, Yao SL, Shang Y. A Lipoxin A4 analog ameliorates blood-brain barrier dysfunction and reduces MMP-9 expression in a rat model of focal cerebral ischemia-reperfusion injury. J Mol Neurosci. 2012;46(3):483–491. doi: 10.1007/s12031-011-9620-5. [DOI] [PubMed] [Google Scholar]
- 12.Chen QH, Zhou WD, Pu DM, Huang QS, Li T, Chen QX. 15-Epi-lipoxin A(4) inhibits the progression of endometriosis in a murine model. Fertil Steril. 2010;93(5):1440–1447. doi: 10.1016/j.fertnstert.2009.01.107. [DOI] [PubMed] [Google Scholar]
- 13.Sodin-Semrl S, Spagnolo A, Barbaro B, Varga J, Fiore S. Lipoxin A4 counteracts synergistic activation of human fibroblast-like synoviocytes. Int J Immunopathol Pharmacol. 2004;17(1):15–25. doi: 10.1177/039463200401700103. [DOI] [PubMed] [Google Scholar]
- 14.Nishida T, Ueda A, Fukuda M, Mishima H, Yasumoto K, Otori T. Interactions of extracellular collagen and corneal fibroblasts: morphologic and biochemical changes of rabbit corneal cells cultured in a collagen matrix. In Vitro Cell Dev Biol. 1988;24(10):1009–1014. doi: 10.1007/BF02620874. [DOI] [PubMed] [Google Scholar]
- 15.Nagano T, Hao JL, Nakamura M, Kumagai N, Abe M, Nakazawa T, Nishida T. Stimulatory effect of pseudomonal elastase on collagen degradation by cultured keratocytes. Invest Ophthalmol Vis Sci. 2001;42(6):1247–1253. [PubMed] [Google Scholar]
- 16.Zhou H, Kimura K, Orita T, Nishida T, Sonoda KH. Inhibition by medroxyprogesterone acetate of interleukin-1β-induced collagen degradation by corneal fibroblasts. Invest Ophthalmol Vis Sci. 2012;53(7):4213–4219. doi: 10.1167/iovs.11-8822. [DOI] [PubMed] [Google Scholar]
- 17.Zhou H, Kimura K, Orita T, Nishida T, Sonoda KH. Inhibition by female sex hormones of collagen degradation by corneal fibroblasts. Mol Vis. 2011;17:3415–3422. [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang WS, Hao JL, Wang S, Bi MM, Zhang H, Zhou HY. Molecular mechanism of the inhibition effect of Celecoxib on corneal collagen degradation in three dimensions. Int J Ophthalmol. 2012;5(4):434–439. doi: 10.3980/j.issn.2222-3959.2012.04.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hao JL, Nagano T, Nakamura M, Kumagai N, Mishima H, Nishida T. Galardin inhibits collagen degradation by rabbit keratocytes by inhibiting the activation of pro-matrix metalloproteinases. Exp Eye Res. 1999;68(5):565–572. doi: 10.1006/exer.1998.0637. [DOI] [PubMed] [Google Scholar]
- 20.Galligan CL, Fish EN. Circulating fibrocytes contribute to the pathogenesis of arthritis. Arthritis Rheum. 2012 doi: 10.1002/art.34589. [DOI] [PubMed] [Google Scholar]
- 21.Mannello F, Medda V, Ligi D, Raffetto JD. Glycosaminoglycan sulodexide inhibition of MMP-9 gelatinase secretion and activity: possible pharmacological role against collagen degradation in vascular chronic diseases. Curr Vasc Pharmacol. 2012 doi: 10.2174/1570161111311030010. [DOI] [PubMed] [Google Scholar]
- 22.Lee SH, Kim DW, Eom SA, Jun SY, Park M, Kim DS, Kwon HJ, Kwon HY, Han KH, Park J, Hwang HS, Eum WS, Choi SY. Suppression of 12-O-tetradecanoylphorbol-13-acetate (TPA)-induced skin inflammation in mice by transduced Tat-Annexin protein. BMB Rep. 2012;45(6):354–359. doi: 10.5483/bmbrep.2012.45.6.036. [DOI] [PubMed] [Google Scholar]
- 23.Pineda MA, McGrath MA, Smith PC, Al-Riyami L, Rzepecka J, Gracie JA, Harnett W, Harnett MM. The parasitic helminth product ES-62 suppresses pathogenesis in CIA by targeting of the IL-17-producing cellular network at multiple sites. Arthritis Rheum. 2012 doi: 10.1002/art.34581. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 24.Efrati S, Berman S, Abu Hamad R, Nakib RE, Chanimov M, Siman-Tov Y, Weissgarten J. Hyperglycemia, inflammation, RAS activation: three culprits to blame for acute kidney injury emerging in healthy rats during general anaesthesia. Nephrology (Carlton) 2012 Jun 22; doi: 10.1111/j.1440-1797.2012.01638.x. [DOI] [PubMed] [Google Scholar]
- 25.Cezar-de-Mello PF, Vieira AM, Nascimento-Silva V, Villela CG, Barja-Fidalgo C, Fierro IM. ATL-1, an analogue of aspirin-triggered lipoxin A4, is a potent inhibitor of several steps in angiogenesis induced by vascular endothelial growth factor. Br J Pharmacol. 2008;153(5):956–965. doi: 10.1038/sj.bjp.0707650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Titos E, Clària J, Planagumà A, López-Parra M, González-Périz A, Gaya J, Miquel R, Arroyo V, Rodés J. Inhibition of 5-lipoxygenase-activating protein abrogates experimental liver injury: role of Kupffer cells. J Leukoc Biol. 2005;78(4):871–878. doi: 10.1189/jlb.1204747. [DOI] [PubMed] [Google Scholar]
- 27.Kim JH, Huh JE, Baek YH, Lee JD, Choi DY, Park DS. Effect of phellodendron amurense in protecting human osteoarthritic cartilage and chondrocytes. J Ethnopharmacol. 2011;134(2):234–242. doi: 10.1016/j.jep.2010.12.005. [DOI] [PubMed] [Google Scholar]