Abstract
AIM
To evaluate the efficacy of Seprafilm® transplantation following adhesiolysis for preventing postoperative readhesion and improving surgical outcomes.
METHODS
Primary blepharoplasty was carried out on both eyelids of 18 albino rabbits. After 2 weeks, a new skin incision was made, and adhesiolysis was performed on both eyelids. The rabbits were categorized into two groups, one with adhesiolysis alone in the left eyelid (control group), and the other with adhesiolysis with a Seprafilm® graft in the right eyelid (Seprafilm® group). The degrees of inflammation and fibrosis were examined with hematoxylin-eosin (HE) and Masson's trichrome stains. Expression of α-smooth muscle actin (α-SMA) was also immunohistochemically examined.
RESULTS
Eyelid examination immediately after the operation revealed mild swelling and hemorrhage in both groups, but these symptoms resolved after 1 week-2 weeks, and eyelid shape had recovered completely in both groups. Microscopic assessments demonstrated that the Seprafilm® group showed less inflammation and fibrosis than the control group. The Seprafilm® group also exhibited fewer α-SMA-positive cells than the control group.
CONCLUSION
Based on these findings, we conclude that Seprafilm® graft with adhesiolysis is an effective method for preventing postoperative readhesions after eyelid surgery.
Keywords: Seprafilm®, adhesion, adhesiolysis
Introduction
Adhesion after blepharoplasty is necessary in most cases, and is important when evaluated from functional and aesthetic aspects. While rare, inappropriate adhesion can cause a distorted eyelid shape, eyelid asymmetry, or triple eyelids [1],[2]. Inappropriate adhesion of the eyelid to the surrounding tissues after surgery is caused by poor surgical skills, repeated tissue damage by several surgeries, excessive bleeding, inflammatory tissue reactions to foreign objects (such as sutures), or postsurgical infection [3],[4]. These complications due to postoperative adhesions can be addressed by additional surgery, but a satisfactory surgical result cannot be achieved if reattachment occurs again. Thus, precise tissue dissection, reducing surgery time, and preventing bleeding and infection are important for minimizing adhesions [5]. Fat grafts (either free fat grafts or subcutaneous fat grafts) have been employed to prevent readhesions, but these methods leave a scar on the area from which the fat tissue is transplanted. It has also been reported that this technique does not significantly reduce inflammation [1],[2]. Preaponeurotic fat grafts are designed to improve these shortcomings; they do not require another incision and are known to be effective. However, the surgical technique is difficult. If not executed properly, it can damage the orbital septum and normal adipose tissue, which can cause eyelid furrows [5].
Studies in different fields have investigated various methods for preventing postoperative adhesions. Recently, obstetric and gynecological research on the use of a film-type antiadhesive agent that can prevent adhesions has been described [6]-[8]. The absorbent antiadhesive film is placed where adhesions may occur after surgery, creating a physical barrier that is also effective for wound healing. There is no need to remove the agent because it is absorbed over time. Based on animal experiments, it has been reported that these characteristics make the antiadhesive agent effective for preventing adhesions after ophthalmological surgeries for strabismus, glaucoma, and orbital fracture repair [9]-[13].
Our experimental study evaluated the antiadhesive effects of Seprafilm® (Genzyme Corporation, Cambridge, MA, USA), which is composed of a mixture of sodium hyaluronate (HA) and carboxymethylcellulose (CMC) and is used to prevent adhesions with surrounding tissue and to reduce inflammation after transplants, abdominal surgery, and other procedures. The present study is a first attempt to employ a film-formed product that prevents adhesion in ophthalmic plastic and reconstructive surgery.
Materials and Methods
The study was performed on 18 albino rabbits that were subjected to eyelid surgery and experimentally induced adhesions. Two weeks later, we performed adhesiolysis on both eyes. Appropriately sized Seprafilm® was transplanted onto an adhesiolysis area on the right eye of each rabbit in the Seprafilm® group. The rabbits' left eyes only received adhesiolysis and constituted the control group. Eyelid tissue samples were taken from the rabbits at postoperative week 1, 4, and 8. The pathological differences between the test and control groups were measured to compare the inflammation and fibrosis. All rabbits were treated in accordance with the ARVO Statement on the Use of Animals in Ophthalmic and Vision Research, and the experimental protocol was approved by the Animal Care and Use Committee of Dong-A University Medical School.
Seprafilm® preparation
Seprafilm® is a translucent, film-type, synthetic, absorbable antiadhesion agent. It is a mixture of sodium HA and CMC at a 2:1 ratio by weight. It obtains a hydrated gel state within 24-48 hours after being applied, remains in the applied area for approximately 7 days, and is excreted within 28 days. It was stored at room temperature and hydrated with normal saline to obtain the gel state immediately before application.
First operation-blepharoplasty
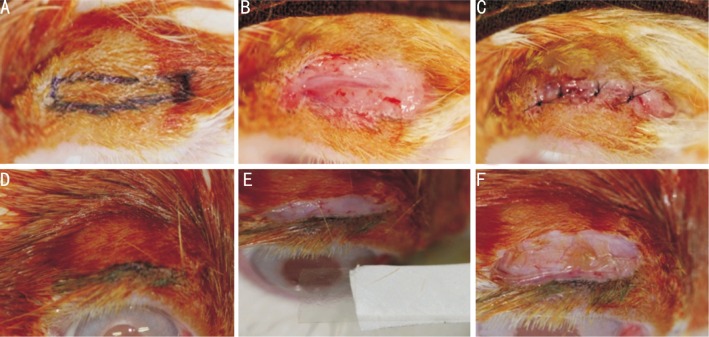
We examined the condition of the rabbits' upper eyelids before surgery and photographed any abnormal findings. We used 18 albino rabbits weighing approximately 3kg. A 50-mg dose (2 mL) of ketamine hydrochloride and xylazine hydrochloride was used to anesthetize the animals. We shaved both upper eyelids to expose the skin, injected lidocaine, and made a 15mm×2 mm skin excision 4mm from the lid margin with a number 15 blade. After simulating eyelid surgery by dissecting the subcutaneous tissue and exposing the tarsal plate, we peeled off the surrounding tissue and sutured the skin using 6-0 nylon. After surgery, we applied tobramycin eye ointment and gentamicin (80mg) to the eyelids. The tobramycin eye ointment was used once a day thereafter (Figure 1A-C).
Figure 1. Primary blepharoplasty and secondary adhesiolysis with Seprafilm® transplantation.
A: The design for the blepharoplasty with marker pen (4mm from the eyelid margin, 15-mm long, and 2-mm wide); B: The skin was excised, and the tarsal plate was exposed; C: The skin was closed with 6-0 nylon interrupted sutures; D: After 2 weeks, a new skin incision line was made 2mm from the eyelid margin; E: Dissection and adhesiolysis were performed. The Seprafilm® was sized for transplantation (15-mm long and 6-mm wide); F: Seprafilm® was appropriately positioned on the wound.
Second operation-adhesiolysis and Seprafilm® transplantation
Two weeks after the first operation, ketamine hydrochloride and xylazine hydrochloride were again administered to all the rabbits, and lidocaine was applied to the upper eyelids. A 15-mm skin incision was made 2mm from the lid margin, and sufficient dissection was performed on the adhesion area from the previous eyelid surgery. After complete dissection, a 15mm×4mm piece of Seprafilm® was folded in half and placed where adhesiolysis had been performed on the right eyelids. Sutures were not necessary because Seprafilm® creates strong biological adhesion. We performed adhesiolysis without transplantation on the left eyes. Later, skin sutures were placed on both eyes using 6-0 nylon (Figure 1D-F).
Macroscopic examination
At the 1st, 4th, and 8th weeks after the second operation, we grossly observed the upper-eyelid surgery areas to compare redness, swelling, and extent of skin adhesion between the two groups.
Histopathological and immunohistochemical findings
At the 1st, 4th and 8th postoperative weeks, the rabbits were sacrificed by injecting phenobarbital into their ear veins. Both upper eyelids were removed en bloc, and formalin was used to produce histological specimens that were stained with hematoxylin-eosin (HE) and Masson's trichrome to assess inflammation, collagen fibers, and scar formation. One pathologist examined all of the specimens in a blinded fashion. Inflammation and fibrosis were graded using the modified method described by Ozkan et al [9].
The inflammatory reaction involves lymphocytes, plasma cells, histiocytes, and polymorphonuclear leukocytes. The grading was as follows: 0 = no inflammation; 1 = a few lymphocytes and plasma cells; 2 = mild inflammatory infiltrate composed of lymphocytes, plasma cells, and polymorphonuclear leukocytes; 3 = grade 2 plus neutrophils; and 4 = high concentrations (collections) of lymphocytes, plasma cells, polymorphonuclear leukocytes, histiocytes, and ulceration.
A scale of 0 to 4 for fibrosis was based on the amount of collagen formation as follows: 0 = no fibrosis; 1 = mild fibrotic reaction around wound (stained collagen was detectable only in thin bands); 2 = easily detected thick bands; 3 = well-developed, dense collagen bands; and 4 = a severe fibrotic response replacing large areas.
To examine the proliferative activity of cells adjacent to the surgical wounds, we immunohistochemically measured α-SMA expression, which was graded using the method described by Jeung et al [14].
A scale of 1 to 4 for α-SMA expression was based on the stained area (high-resolution microscopic view, ×400), and the grading was as follows: 1= 0-10%, 2= 10%-30%, 3=30%-50%; 4=50%-100% stained area.
Statistical Analysis
Data are expressed as the mean±standard deviation (SD). Statistical analysis was performed using Mann Whitney U tests, and P<0.05 was considered statistically significant.
Results
We included 18 rabbits (36 eyes) in the experiment. Six rabbits were sacrificed at the 1st, 4th, and 8th postoperative weeks, and the bilateral eyelid tissues were removed and pathologically examined.
Macroscopic Findings
Preoperatively, most of the rabbit corneas were exposed. Although rabbits have smaller eyelids than humans, they are similarly shaped, and the epidermal and dermal thicknesses are comparable.
There was slight eyelid edema and hemorrhage immediately after the surgery, but swelling and bleeding showed improvement from the immediate postoperative period compared to 1 week-2 weeks later. There were no complications, such as infection, inflammation, or wound opening. We removed the stitches from all rabbits in the first week after the second operation. There were no significant complications found after 8 weeks (Figure 2).
Figure 2. Gross eyelid examination findings (A, C, and E: Seprafilm® group; B, D, and F: control group).
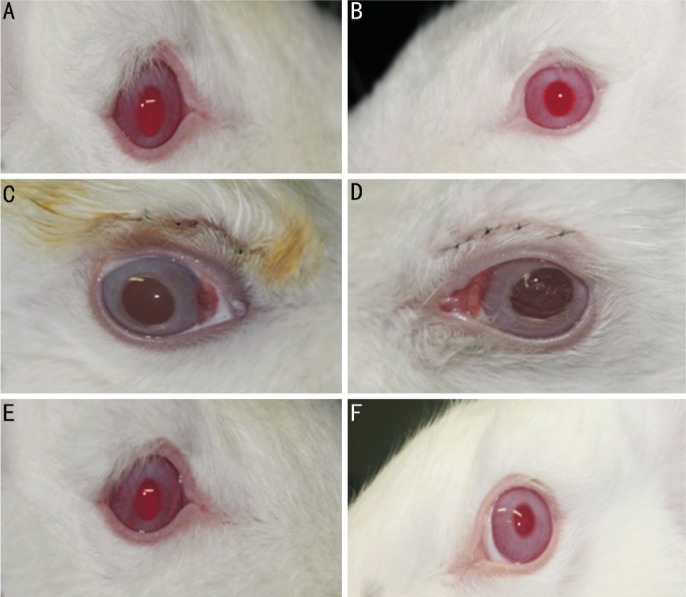
A and B: Preoperative state (right and left eyelid), C and D: Postoperative state (primary operation-blepharoplasty); E and F: Postoperative 8 weeks (secondary operation-adhesiolysis).
Histopathological Findings
The mean inflammation scores (HE) in the Seprafilm® group were 1.83±0.41, 1.67±0.52, and 1.17±0.41 at 1 week, 4, and 8 weeks postoperatively, respectively. In the control group, these same values were 2.67±0.52, 2.50±0.55, and 1.33±0.52, respectively. Postoperative inflammation was significantly lower in the Seprafilm® group at 1 week and 4 weeks (Table 1). Mean fibrosis scores (Masson's trichrome stains) in the Seprafilm® group were 1.33±1.03, 2.00±0.63, and 2.50±0.55 at 1 week, 4, and 8 weeks postoperatively, respectively. In the control group, these values were 1.17±0.41, 2.83±0.41, and 3.33±0.52, respectively. Postoperative fibrosis was significantly lower in the Seprafilm® group at 4 and 8 weeks (Table 2). Mean α-SMA grading scores in the Seprafilm® group were 1.50±0.55, 1.83±0.41, and 2.17±0.41 at 1 week, 4, and 8 weeks, respectively, compared to 1.67±0.52, 2.50±0.55, and 2.83±0.41 in the control group. Postoperative α-SMA staining in the Seprafilm® group was significantly lower than that in the control group at 4 and 8 weeks (Table 3).
Table 1. Inflammation scores (HE stains).
| Postoperative |
|||
| 1 week | 4 weeks | 8 weeks | |
| Seprafilm® | 1.83±0.41 | 1.67±0.52 | 1.17±0.41 |
| Control | 2.67±0.52 | 2.50±0.55 | 1.33±0.52 |
| 1P | 0.018 | 0.030 | 0.523 |
1Mann Whitney U test.
x ± s
Table 2. Fibrosis scores (Masson's trichrome stains).
| Postoperative |
|||
| 1 week | 4 weeks | 8 weeks | |
| Seprafilm® | 1.33±1.03 | 2.00±0.63 | 2.50±0.55 |
| Control | 1.17±0.41 | 2.83±0.41 | 3.33±0.52 |
| 1P | 0.849 | 0.026 | 0.030 |
1Mann Whitney U test.
x ± s
Table 3. α-SMA staining.
| Postoperative |
|||
| 1 week | 4 weeks | 8 weeks | |
| Seprafilm® | 1.50±0.55 | 1.83±0.41 | 2.17±0.41 |
| Control | 1.67±0.52 | 2.50±0.55 | 2.83±0.41 |
| 1P | 0.575 | 0.043 | 0.027 |
1Mann Whitney U test.
x ± s
Figure 3 shows the histopathological findings of HE staining from the eyelids (Figure 3). At 1 week after the second surgery, both groups demonstrated early-stage inflammation, as evidenced by polymorphonuclear cell infiltration. However, the Seprafilm® group demonstrated reduced inflammatory cell infiltration in the deeper layers of the eyelid within the epidermis and at the surgical site.
Figure 3. HE staining 1 week and 4 weeks after adhesiolysis.
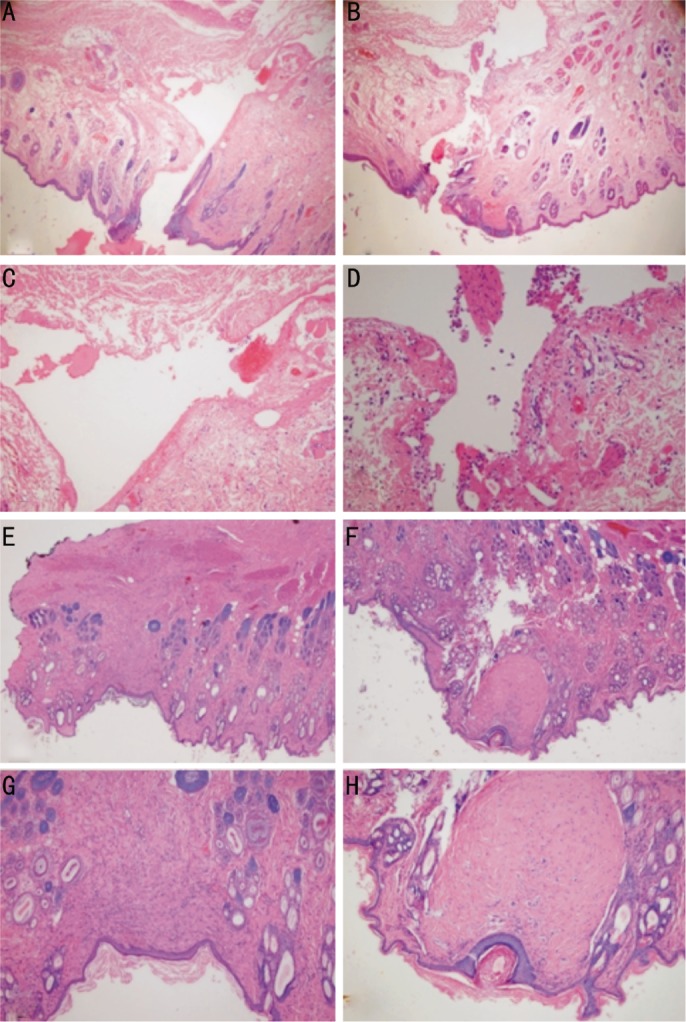
A and C: Operation site 1 week after adhesiolysis and Seprafilm® transplantation (Seprafilm® group), B and D: Operation site 1 week after simple adhesiolysis (control group), E and G: Operation site 4 weeks after adhesiolysis and Seprafilm® transplantation (Seprafilm® group); F and H: Operation site 4 weeks after simple adhesiolysis (control group). Less inflammatory cell inflammation is observed in the Seprafilm® group at both time points, and dense fibrosis and collagen deposition was observed in the control group at 4 weeks (F and H).
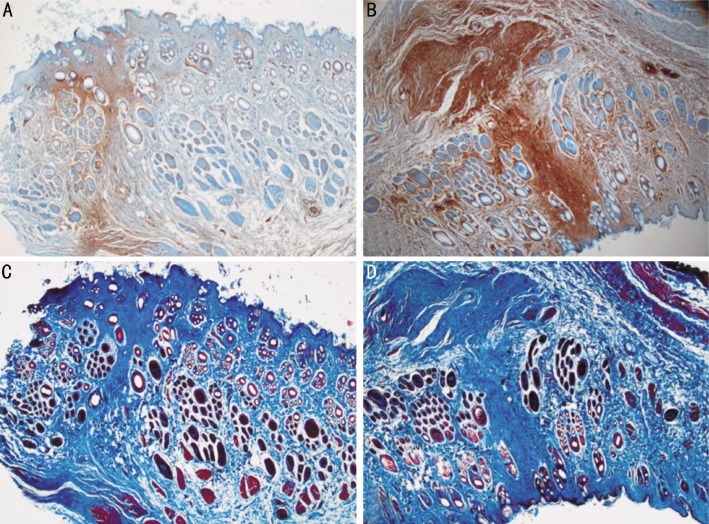
α-SMA positive cells around the wound and α-SMA-positive myofibroblasts were found in both groups, but the density of positive cells was lower in the Seprafilm® group compared to the control group (Figure 4A-B). Masson's trichrome staining revealed that the control group demonstrated more extensive fibrosis and increased collagen deposition compared to the Seprafilm® group (Figure 4C-D).
Figure 4. α-SMA immunoreactivity and Masson's trichrome staining 4 weeks after adhesiolysis.
A and B: postoperative α-SMA expression. A: Adhesiolysis and Seprafilm® transplantation. B: Simple adhesiolysis (control group). In the Seprafilm® group (A), fewer myofibroblasts are present compared to control group (B). C and D: postoperative fibrosis expression. C: Adhesiolysis and Seprafilm® transplantation. D: Simple adhesiolysis (control group). Fibrosis is less severe in the Seprafilm® group (C) compared to the control group (D).
Discussion
Eyelid surgery is commonly performed for aesthetic plastic surgery, and it is also important for reconstruction following facial trauma. Inappropriate tissue adhesions can result in several unfavorable outcomes, including distorted eyelid shape, eyelid asymmetry, or triple eyelids. A number of methods for preventing these complications or reducing their severity have been examined in various studies and are currently employed during surgery [1],[2]. Free dermal fat grafting, subcutaneous fat grafting, free fat grafts, and fascia fat grafts have been used to control readhesions around surgical sites after initial or secondary eyelid surgery. However, the transplanted grafts remain in the area, making it hard to predict postoperative eyelid thickness. To compensate for these shortcomings and prevent readhesions, Kim et al [5] successfully transplanted preaponeurotic fat in 72 patients. Unfortunately, this procedure has a significant risk of complications, such as damage to the orbital septum and bleeding.
Demirel et al [15] and Kwon et al [16] performed amniotic membrane transplantation after eyelid surgery in albino rabbits, and reported that it effectively prevented adhesions after eyelid or strabismus surgery.
Adhesions can create cellulose-rich blood clots because vasoactive factors are released, resulting in inflammatory cell accumulation and stromal cell stimulation. Fibroblasts, macrophages, and giant cells infiltrate into the area and are replaced by vascular-granulation tissue and collagen. Eventually, connective tissue is organized, and adhesions are formed [17]. The techniques based on apoptotic mechanisms include the application of a mixture of HA and CMC (HACMC) on areas prone to developing postsurgical adhesions.
HA is a high-molecular-weight hydrophilic polymer comprised of mucopolysaccharide glycosaminoglycans. It has an amplified-coil structure, which provides considerable binding capacity for water molecules (approximately 1 000 times). In vivo, it exists in connective tissue, body fluids, and the vitreous humor, and is widely used in cataract surgery. Because it has high viscosity and absorption, it has recently been used as an antiadhesive agent during eye and other types of surgery. However, the half-life of HA is only 1 day-3 days because it is easily degraded by endogenous hyaluronidase [18]. Mixing CMC, a low-molecular-weight cellulose derivative, with HA extends the half-life because there are no enzymes that degrade CMC found in the body [19]. CMC is mainly used as a food or cosmetic filler; it is harmless to the human body and is an effective postsurgical antiadhesive agent [20]-[22]. The HACMC mixture does not degrade readily, making a useful physical barrier [19].
One study has examined the use of the HACMC gels Guardix-sol® and Healon® for preventing postsurgical readhesions. Yaacobi et al [20] reported that adhesions after strabismus surgery can be reduced by using HA (Healon®), and Kwon et al [10] performed animal experiments and found that using an HACMC mixture (Guardix-sol®) on surgical areas effectively reduced postsurgical adhesions. However, Kwon et al [10] also mentioned that more research is necessary to study how the gel-state matter is maintained on the surgical area and low long it can be used as a physical barrier.
Seprafilm® is a translucent film form of an HACMC mixture (2:1 ratio), and serves as a physical barrier that prevents adhesion. It reaches a hydrated gel state within 24-48 hours of application, remains on the applied area for about 7 days, and is degraded within 28 days. Because it is composed of absorbent biocompatible materials, it has a low risk of pathogenic response and strongly adheres to wet tissue, which eliminates the requirement for sutures.
Beck et al [6] reported that a film type of HACMC (i.e., Seprafilm®) could reduce postsurgical adhesive obstructions during abdominal surgery. Kelekci et al [7] also discussed adhesion prevention during surgery. In ophthalmology, Ozkan et al [9] described the effects of Seprafilm® during experimental strabotomy in an animal model. Tsurumaru et al [11] and Takeuchi et al [12] conducted experiments using Seprafilm® for glaucoma surgery in albino rabbits, and demonstrated that it prevents adhesions between the conjunctiva and the sclera.
There has not yet been a study assessing the use of an absorbable antiadhesive agent during eyelid surgery. Here, we used HA and CMC film in albino rabbits to determine how well it prevents adhesions after eyelid surgery. We observed less inflammation in the Seprafilm® group at 1 week and 4 weeks after surgery, and fibrosis and myofibroblasts were also reduced in the Seprafilm® group at 4 and 8 weeks. These findings indicate that wound healing may be improved by Seprafilm® use, and they support findings from previous studies on inflammation and adhesion formation [9]-[13].
In addition to reducing complications due to inappropriate postsurgical adhesions, Seprafilm® has several advantages: it is easier to apply than the gel type of the HACMC mixture (Guardix-sol®), and it can function as a physical barrier for a longer period of time. Furthermore, Seprafilm® prevents adhesions without the need for additional procedures, such as a fat grafting. There was also less rejection between grafted material and tissue than when using amniotic membrane, which leads us to believe that Seprafilm® may be a safer antiadhesive agent.
Although we observed histopathological differences, there were no differences between the Seprafilm® and control groups by macroscopic observation. Unlike the human eyelid, the rabbit eyelid is strongly resilient and has a short recovery time. While this is considered a limitation of the study, it also serves as motivation to develop more relevant animal disease models for adhesion inhibitors and to perform additional clinical studies aimed at detecting adhesion-preventing effects that can be observed macroscopically.
In conclusion, Seprafilm® graft with adhesiolysis may reduce inflammation, speed wound recovery, and prevent readhesions in patients at high risk for developing complications caused by inappropriate adhesion after eyelid surgery. This method may be an effective alternative for cases in which other techniques (tissue reshaping and fat grafts) cannot be used. Our results in an animal model suggest that this method should be tested in clinical trials.
Footnotes
Foundation item: Supported by the Dong-A University Research Fund
References
- 1.Hin LC. Oriental blepharoplasty: a critical review of technique and potential hazards. Ann Plast Surg. 1981;7(5):362–374. doi: 10.1097/00000637-198111000-00005. [DOI] [PubMed] [Google Scholar]
- 2.Shin KS, Chung S, Cho IC. Treatment of complicated Oriental blepharoplasty. Korean J Plat Reconstr Surg. 1996;1:75. [Google Scholar]
- 3.Dunlap EA. Surgery of muscle adhesions and effects of multiple operations. Br J Ophthalmol. 1974;58(3):307–312. doi: 10.1136/bjo.58.3.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gomel V, Urman B, Gurgan T. Pathophysiology of adhesion formation and strategies for prevention. J Reprod Med. 1996;41(1):35–41. [PubMed] [Google Scholar]
- 5.Kim YW, Park HJ, Kim S. Secondary correction of unsatisfactory blepharoplasty: removing multilaminated septal structures and grafting of preaponeurotic fat. Plast Reconstr Surg. 2000;106(6):1399–1404. doi: 10.1097/00006534-200011000-00030. [DOI] [PubMed] [Google Scholar]
- 6.Beck DE, Cohen Z, Fleshman JW, Kaufman HS, van Goor H, Wolff BG. A prospective, randomized, multicenter, controlled study of the safety of Seprafilm adhesion barrier in abdominopelvic surgery of the intestine. Dis Colon Rectum. 2003;46(10):1310–1319. doi: 10.1007/s10350-004-6739-2. [DOI] [PubMed] [Google Scholar]
- 7.Kelekci S, Uygur D, Yilmaz B, Sut N, Yesildaglar N. Comparison of human amniotic membrane and hyaluronate/carboxymethylcellulose membrane for prevention of adhesion formation in rats. Arch Gynecol Obstet. 2007;276(4):355–359. doi: 10.1007/s00404-007-0376-7. [DOI] [PubMed] [Google Scholar]
- 8.Naito Y, Shin'oka T, Hibino N, Matsumura G, Kurosawa H. A novel method to reduce pericardial adhesion: a combination technique with hyaluronic acid biocompatible membrane. J Thorac Cardiovasc Surg. 2008;135(4):850–856. doi: 10.1016/j.jtcvs.2007.10.062. [DOI] [PubMed] [Google Scholar]
- 9.Ozkan SB, Kir E, Culhaci N, Dayanir V. The effect of Seprafilm on adhesions in strabismus surgery-an experimental study. J AAPOS. 2004;8(1):46–49. doi: 10.1016/S1091853103002714. [DOI] [PubMed] [Google Scholar]
- 10.Kwon SW, Seo YW, Cho YA. Antiadhesive effect of the mixed solution of hyaluronate and sodium carboxymethylcellulose after strabismus surgery. J Korean Ophthalmol Soc. 2009;50(1):145–150. [Google Scholar]
- 11.Tsurumaru N, Arai M, Teruya K, Sueda J, Yamakawa R. Seprafilm as a new antifibrotic agent following trabeculectomy in rabbit eyes. Jpn J Ophthalmol. 2009;53(2):164–170. doi: 10.1007/s10384-008-0638-3. [DOI] [PubMed] [Google Scholar]
- 12.Takeuchi K, Nakazawa M, Yamazaki H, Miyakawa Y, Ishikawa F, Metoki T. Solid hyaluronic acid film and the prevention of postoperative fibrous scar formation in experimental animal eyes. Arch Ophthalmol. 2009;127(4):460–464. doi: 10.1001/archophthalmol.2009.70. [DOI] [PubMed] [Google Scholar]
- 13.Taban M, Nakra T, Mancini R, Douglas RS, Goldberg RA. Orbital wall fracture repair using Seprafilm. Ophthal Plast Reconstr Surg. 2009;25(3):211–214. doi: 10.1097/IOP.0b013e3181a2fd1e. [DOI] [PubMed] [Google Scholar]
- 14.Jeung WJ, Ha SJ, Park WC, Yoo KW. The effects of amniotic membrane for prevention of adhesion in strabismus surgery in rabbits. J Korean Ophthalmol Soc. 2002;43(2):402–410. [Google Scholar]
- 15.Demirel S, Atilla H, Okcu Heper A, Erkam N. Effects of amniotic membrane on wound healing and adhesions in experimental strabismus surgery. Eur J Ophthalmol. 2009;19(6):899–904. doi: 10.1177/112067210901900601. [DOI] [PubMed] [Google Scholar]
- 16.Kwon YH, Park JM, Rho MS, Ahn HB. The efficacy of amniotic membrane graft with adhesiolysis in experimentally induced lid adhesion in rabbits. J Korean Ophthalmol Soc. 2006;47(8):1323–1331. [Google Scholar]
- 17.Hellebrekers BW, Trimbos-Kemper TC, Trimbos JB, Emeis JJ, Kooistra T. Use of fibrinolytic agents in the prevention of postoperative adhesion formation. Fertil Steril. 2000;74(2):203–212. doi: 10.1016/s0015-0282(00)00656-7. [DOI] [PubMed] [Google Scholar]
- 18.Johns DB, Keyport GM, Hoehler F, diZerega GS. Reduction of postsurgical adhesions with Intergel adhesion prevention solution: a multicenter study of safety and efficacy after conservative gynecologic surgery. Fertil Steril. 2001;76(3):595–604. doi: 10.1016/s0015-0282(01)01954-9. [DOI] [PubMed] [Google Scholar]
- 19.Kim JH, Lee JH, Yoon JH, Chang JH, Bae JH, Kim KS. Antiadhesive effect of the mixed solution of sodium hyaluronate and sodium carboxymethylcellulose after endoscopic sinus surgery. Am J Rhynol. 2007;21(1):95–99. doi: 10.2500/ajr.2007.21.2911. [DOI] [PubMed] [Google Scholar]
- 20.Yaacobi Y, Hamed LM, Kaul KS, Fanous MM. Reduction of postoperative adhesions secondary to strabismus surgery in rabbits. Ophthalmic Surg. 1992;23(2):123–128. [PubMed] [Google Scholar]
- 21.Gago LA, Saed GM, Chauhan S, Elhammady EF, Diamond MP. Seprafilm (modified hyaluronic acid and carboxymethylcellulose) acts as a physical barrier. Fertil Steril. 2003;80(3):612–616. doi: 10.1016/s0015-0282(03)00767-2. [DOI] [PubMed] [Google Scholar]
- 22.Oncel M, Remzi FH, Senagore AJ, Connor JT, Fazio VW. Comparison of a novel liquid (adcon-P®) and sodium hyaluronate and carboxymethylcellulose membrane (Seprafilm™) in postsurgical adhesion formation in a murine model. Dis Colon Rectum. 2003;46(2):187–191. doi: 10.1007/s10350-004-6523-3. [DOI] [PubMed] [Google Scholar]