Abstract
AIM
To investigate the expressions of type I collagen, α2 integrin and β1 integrin in the posterior sclera of guinea pigs with defocus myopia and whether basic fibroblast growth factor (bFGF) injection inhibits the formation and development of myopia by upregulating the expression of type I collagen, α2 integrin and β1 integrin.
METHODS
After 14 days of treatment, the refractive state and axial length were measured and the levels of type I collagen, α2 integrin and β1 integrin were assayed in the posterior sclerae of groups of guinea pigs that wore a monocular -7D polymethylmethacrylate (PMMA) lens or had -7D lens wear followed by the peribulbar injection of Phosphate Buffer Solution (PBS) or bFGF. The untreated fellow eye served as a control. Guinea pigs with no treatment served as normal group.
RESULTS
The results showed that 14 days of monocular defocus increased axial eye length and refraction, while bFGF delivery inhibited them markedly. Further, it was also found that the monocular -7D lens could decrease the levels of type I collagen, α2 integrin and β1 integrin expressions, while, unlike PBS, bFGF increased them significantly in comparison to contralateral control eyes and normal eyes.
CONCLUSION
bFGF can prevent the formation and development of defocus myopia by upregulating the expressions of type I collagen, α2 integrin and β1 integrin. Taken together, our results demonstrate that bFGF promotes sclera remodeling to prevent myopia in guinea pigs.
Keywords: defocus myopia, type I collagen, α2 integrin, β1 integrin, bFGF
Introduction
Myopia is the most common refractive error, eye sclera in determining the eyeball shape and size and further affecting the refractive power of the eye plays an important role. Many researchers believe that the occurrence of myopia is a consequence of scleral extracellular matrix (ECM) remodeling[1]-[5]. Namely, after ECM remodeling, human high myopia was characterized from axial growth, scleral thinning, and disorganized collagen fibers [6], [7]. Therefore, the sclera is a key target for the research and treatment of myopia. Previously, there were more studies for scleral proteoglycan, while less studies for the scleral extracellular matrix. ECM plays a key role in connective tissue remodeling and biochemical changes [8]. Collagens and collagen binding integrin receptor family are the most important components of extracellular matrix. Collagen accounts for 90% of scleral dry weight, and the majority of collagen are type I collagen [9]. Collagen binding integrin receptor family is the main adhesion molecules to cells and matrix. Recent researches [10]-[12] showed that there were the expressions of β1, β4, β5, β8, α1-6, α9-11 and αv integrin subtypes in normal tree shrews scleral tissue and scleral fibroblasts cultured, and these subtypes of β integrin can be combined to α integrin subtypes, which also prove that the mammal sclera has the ability to express all of 24 integrins identified. So it is considered that the researches for myopia treatments may target scleral ECM. The drugs, which can promote the synthesis of scleral extracellular matrix or inhibit its degradation, may prevent the formation of myopia by enhancing the function of the sclera. Basic fibroblast growth factor (bFGF) is an important member of fibroblast growth factor family, it plays roles mainly through high affinity receptors of cell membrane in promoting cell proliferation and differentiation, regulating cell apoptosis and extracellular matrix metabolism as well as increasing angiogenesis.
The experiment is to study the expressions of type I collagen, α2 integrin and β1 integrin in the posterior sclera of guinea pigs with defocus myopia and whether exogenous bFGF inhibit the formation and development of myopia by increasing the expressions of type I collagen, α2 integrin and β1 integrin.
Materials and methods
Materials
In order to observe the inhibitation of bFGF on defocus myopia in guinea pigs, the peribulbar injection method was used. The procedures adhered to the Association for Research in Vision and Ophthalmology Statement and were approved by the Institutional Animal Care and Use Committee of China Medical University. Prior to the experiment, 42 guinea pigs (3-week-old) were evaluated at baseline (without myopia and anisometropia) and then were randomly divided into 4 groups: 1) Normal group (n=6): both eyes of guinea pigs with no treatment;2) Optical defocus group (n=12): guinea pigs wore a monocular -7D lens in front of the randomly treated eye (polymethylmethacrylate contact lens is supported by a self-made adhesive nylon buckle) for 14 days to induce axial elongation and myopia; 3) Optical defocus plus phosphate buffer solution (PBS) group (n=12): during 14-day defocus, the peribulbar injection of 50µL PBS was performed every other day under ketamine anesthesia; 4) Optical defocus plus bFGF group (n=12): during 14-day defoucs, the peribulbar injection of 50µL bFGF (rhbFGF dissolved in PBS buffer at a concentration of 5ng/50µL, Sigma, USA) was executed every other day. All lens treatments were monocular, with the untreated fellow eye serving as a control. Numbers of right or left eye treatments were as balanced as possible within each group.
Methods
Measurements of refraction and axis
Before all experimental procedures, all guinea pigs were anesthetized by 1% tropicamide eye drops. Subsequently, streak retinoscopy and A-scan ultrasound (COMPUSCAN, USA) were used to measure respectively refractive state and axial length of both eyes to ensure that the treated and control eyes did not differ significantly at the start of the treatment period. All treated animals were maintained on 14 hours on/10 hours-off light/dark cycle and food and water were available ad libitum. At the end of the treatment, the lenses from the treated eyes of guinea pigs were removed and refractive and axial measurements of both eyes were made as reported previously [9].
Tissue preparation
On completion of the final refractive and axial measurements, all guinea pigs were sacrificed and both eyes were enucleated. Following enucleation, the posterior sclerae are taken at 2mm of the back of the corneoscleral junction as the measurement specimans. The inner surface of the sclera was scraped to remove retina and choroid, and the outer surface was scraped to remove adhering extraocular tissues. All samples were snap frozen in liquid nitrogen and then stored at -80°C until assayed.
Reverse transcription-polymerase chain peaction (RT-PCR)
Total ribose nucleic acid (RNA) was extracted from individual posterior sclerae using trizol reagent (Invitrogen, Carlsbad, CA, USA). RNA concentration and purity were determined by spectrophotometry at 260nm and 280nm. RNAs were reverse transcribed and RT-PCR reaction were performed with guinea pig-specific primers (Shenggong Biotechnology, China) for the α1 chain of type I collagen, α2 integrin and β1 integrin and β-actin (Table 1). The reactions contained 12µL master mix, 2.0µL of both forward and reverse primers, and cDNA corresponding to 4.0µL. After an initial denaturation of all primer sets at 94°C for 2 minutes, the same cycling parameters were used for type I collagen, α2 integrin and β1 integrin by 30 cycles of amplifification for denaturation at 94°C for 40 seconds and for extension 72°C for 60 seconds except that annealing temperatures were in order 61°C for 40 seconds, 68°C for 40 seconds, 63°C for 40 seconds, 58°C for 40 seconds. The 30 cycles of amplification were followed by final incubation for 10 minutes at 72°C. The expression of the target gene was normalized to that of β-actin.
Table 1. Primer sequences used for RT-PCR.
| Gene | Primer sequence (bp) | Product size |
| Type I collagen | ||
| Forward | CTGGAAGAGCGGAGAGTA | 433 |
| Reverse | CACAAGGGTGCTGTAGGT | |
| Integrin α2 | ||
| Forward | TGTCACGATTCCCCTCATGA | 110 |
| Reverse | TGCAGTCATAGCCAACAGCA | |
| Integrin β1 | ||
| Forward | CCAAAGTAGAAAGCAGGGAG | 179 |
| Reverse | GATGATGTCGGGACCAGTAG | |
| β-actin | ||
| Forward | GACGAAGCCCAGAGCCA | 271 |
| Reverse | CAGAGGCATACAGGGACAG |
Western blotting
Sclera tissues were washed twice with ice-cold PBS, Then, they were homogenized in RIPA buffer (50mmol/L Tris pH8.0, 100mmol/L NaCl, 1% Triton X-100, 0.5%NP40, 50mmol/L EDTA, 1mmol/L PMSF, 10AM leupeptin, 0.2TIU/mL aprotinin, 2mmol/L sodium orthovanadate, 40mmol/L h-glycero-phosphate, 50mmol/L NaF and 100AM phenylarsine oxide) for 1 hour at 4°C, and lysate was centrifuged at 12 000r/min for 15 minutes. Protein lysates were separated by electrophoresis in 8% acrylamide/ bisacrylamide gels containing SDS, transferred to PVDF membranes. The blots were blocked for 2 hours at room temperature with 5% nonfat milk powder in TBS. The membranes were incubated overnight at 4°C with antibody against type I collagen, α2 integrin and β1 integrin (1:100, Santa Cruz Biotechnology, USA) and β-actin at 4°C overnight. Detection was performed using HRP-conjugated secondary antibodies (Santa Cruz Biotechnology, USA). Immunoreactivity was enchanced by chemiluminescence Kit. The intensities of the immunoblot band were quantified using the Quantity One software (Bio-Rad, USA).
Statistical Analysis
Data were expressed as means±standard deviation, and analyzed with SPSS13.0 software. Statistical analysis was performed by one-way ANOVA followed by Bonferroni multiple comparison test or by a paired two-tailed t test as indicated. P<0.05 was considered as statistically significant.
Results
Measurements of Refraction and Axis
Prior to defocus myopia construction, no statistical significance in refraction was found between both eyes of 3-week guinea pig (P >0.05), both eyes were under hypermetropia status (+3.0±0.15)D. Similar result was found for eye axial length before defocus myopia construction (7.33±0.02)mm, i.e. there was no significant difference between two eyes (P >0.05). After 14-day monocular defocus, the eyes of optical defocus group were produced a myopic shift in refractive state and axial elongation, and compared with contralateral control eyes and normal eyes, the refraction and axis length increased statistically in the defocus eyes (P<0.01). However, in eyes of optical defocus plus bFGF group, the refraction and axis length were decreased statistically compared with ones in optical defocus group (P<0.01), but PBS injection did not markedly inhibit the increase of refraction and axis length in defocus plus PBS group compared with optical defocus group. Measurements from contralateral control eyes were not significantly different from those obtained from normal animals (Table 2).
Table 2. Effects of bFGF on the refraction and axis length of guinea pigs induced myopia.
| Groups | n | Eye refraction (D) Untreated or Treated | Eye axis length (mm) |
||
| Control | Untreated or Treated | Control | |||
| Normal | 6 | 1.36±0.16 | 1.48±0.17 | 7.42±0.05 | 7.48±0.06 |
| Defocus | 12 | -3.52±0.35b,f | 1.22±0.16 | 8.15±0.1b,f | 7.52±0.09 |
| Defocus+PBS | 12 | -3.25±0.3b,f | 1.31±0.11 | 8.07±0.08b,f | 7.47±0.05 |
| Defocus+bFGF | 12 | -1.48±0.13d,f | 1.26±0.14 | 7.66±0.11d,f | 7.45±0.1 |
bP<0.01 vs normal eyes; dP<0.01 vs defocus eyes; fP<0.01 vs contralateral control eyes.
x ± s
RT-PCR Analysis
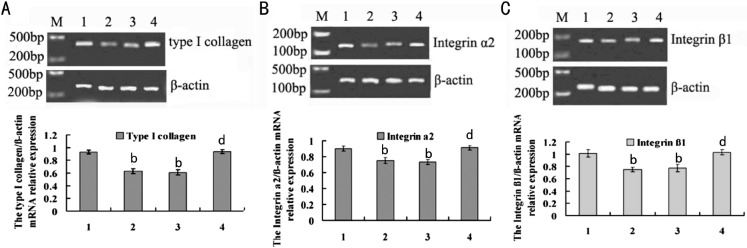
To identify the expressions of type I collagen, α2 integrin and β1 integrin mRNA in guinea pigs sclerae, RT-PCR was used to analyze them. The results showed that there were the amplification of type I collagen, α2 integrin and β1 integrin mRNA in all designed groups (Figure 1 A, B and C).
Figure 1. mRNA expressions in the defocused and normal eyes of guinea pigs in groups with different treatments of defocus, defocus /PBS, defocus /bFGF or no treatment.
A: Type I collagen (α1 chain); B: α2 integrin; C: β1 integrin. M: Marker; 1: normal group; 2: defocus group; 3: defocus + PBS; 4: defocus +bFGF.
Type I collagen, α2 integrin and β1 integrin expression
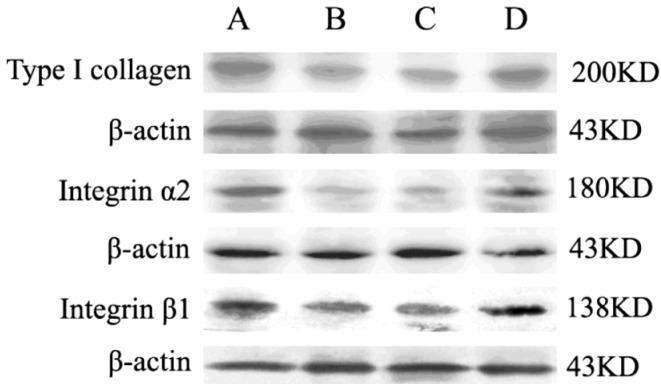
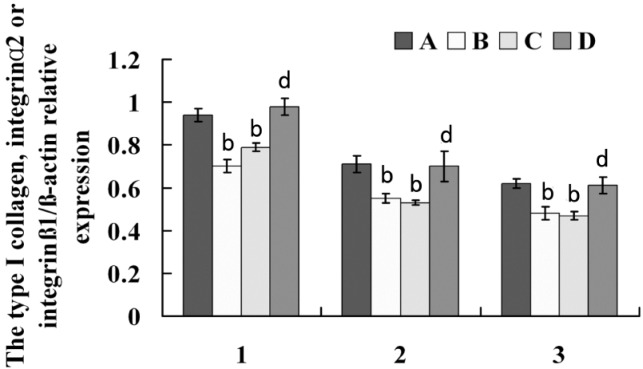
To further confirm the gene expression results, Western blot assays were carried out to investigate the protein level changes of type I collagen, α2 integrin and β1 integrin in experiment period. As showed in Figure 2 and 3, the expressions of type I collagen, α2 integrin and β1 integrin were significantly lowered in defocused-eyes compared with control eyes and normal eyes (Figure 2 A, B and 3 A, B). To identify whether bFGF can promote type I collagen, α2 integrin and β1 integrin expressions of defocused-eyes, the peribulbar injection of 50µL bFGF was executed every other day during the development of defocus myopia. The results showed that bFGF increased type I collagen, α2 integrin and β1 integrin expressions and there was a statistical significance compared with defocus group. In contrast, PBS had no effects on type I collagen, α2 integrin and β1 integrin expressions in the course of defocus (Figure 2 C, D and 3 C, D).
Figure 2. Western blot detection of type I collagen, α2 Integrin and β1 Integrin protein expression in each group.
A lane: normal group; B lane: defocus group; C lane: defocus + PBS group; D lane: defocus +bFGF group.
Figure 3. Immunoblot analysis of type I collagen, integrinα2 and β1 Integrin protein in each group.
1: Type I collagen; 2: α2 Integrin; 3: β1 Integrin. A: normal group; B: defocus group; C: defocus + PBS group; D: defocus +bFGF group. All protein levels are expressed as mean±SD. Defocused eyes and defocused plus PBS eyes are significantly different from normal eyes (bP<0.01); defocused plus bFGF eyes are markly different from defocused eyes and defocused plus PBS eyes (dP <0.01).
DISCUSSION
It has been well-known that formed deprivation and visual defocus can induce axial myopia [13]. The former animal can not get a clear retinal image, and there is no visual feedback and adjustment mechanism. But the optical defocus may cause a series of local and central eyeball feedback which make eyeball grow toward focus, and ultimately get a clear image, this directional growth results in axial elongation, further forms myopia [14],[15]. As mammals, tissue features of guinea pig eyes are similar to that of human eyes. In the present study, we chose 3-week-old guinea pigs to induce myopia by being worn a monocular -7D lens. Our results showed that -7D concave lens could induce a myopic shift in refractive state and axial elongation, and compared with contralateral control eyes and normal eyes, the refraction and axis length increased statistically in the defocus eyes (P<0.01). However, bFGF decreased statistically the refraction and axis length optical defocus eyes. The success of defocus myopia model lies in the continuity of defocus. Zhu et al [16] demonstrated that the retina and choroid could inhibit myopia by self-changes after 30 minutes of defoucs recovery. Therefore, guinea pigs whose goggles fell off were not included in the study.
In the development of myopia, some components of the sclera undergo the process of abnormal activity adjustment. Integrin receptors are heterodimers comprised of α and β integrin subunit. β1 Integrin is the important β subunit in integrin molecule family, which can bind to the different α subunit to constitute the majority of ECM receptors, these ECM receptors are not only indispensable adhesion molecules between cells and ECM, but also play an important role in cytomechanics signal transduction. α2, β1 integrin is the major receptor type I collagen, which can recognize aspartic acid-glycine-alanine (Asp-Gly-Glu-Ala, DGEA) sequence of α1 chain of type I collagen and combine with it to induce a series of biological effects, for example, regulating collagen synthesis [17],[18].
In view of integrin roles on eye growth being known very little. The expressions of type I collagen, α2 integrin and β1 integrin were identified in the posterior sclera of guinea pig with experimental myopia by RT-PCR and western blot methods. It was found that type I collagen, α2 integrin and β1 integrin were expressed in all designed groups, but compared with specimens in normal guinea pig sclera (type I collagen: 0.94±0.03; α2 integrin: 0.71±0.04; β1 integrin: 0.62±0.02), the protein expressions of type I collagen was lowered in the concave lens-induced myopia ones (0.7±0.03), and consistent with the decreases of α2 integrin and β1 integrin protein (α2 integrin: 0.55±0.02; β1 integrin:0.48±0.03), indicating that the decreased expression of integrin receptors is closely correlated with lowered collagen synthesis, they were cooperatively involved in the pathogenesis of myopic axial elongation.
As mentioned above, type I collagen, α2 integrin and β1 integrin reductions play an important role in axial elongation of myopia. Thus, it is necessary to find ways to inhibit their decreased expressions. Rohrer [19] by subconjunctival and intravitreal delivery observed the effects of bFGF on formed deprivation myopia (FDM), as a result, the bFGF-treated occluded eyes was not found to suffer from myopia. Namely, bFGF inhibited the formation and development of FDM. In this study, we investigated the roles of bFGF on the expressions of type I collagen, α2 integrin and β1 integrin in the sclera of defocused-myopia by peribulbar injection method. This research confirmed bFGF is a positive regulator of type I collagen, α2 integrin and β1 integrin decreased during defocused-myopia (type I collagen: 0.98±0.04; α2 integrin: 0.7±0.07; β1 integrin: 0.61±0.04) and may play an important role in scleral remodeling.
Drugs for treating experimental myopia are mostly administrated by intravitreal delivery. However, by this delivery, drugs can not be across the blood-retinal barrier to reach the sclera, but indirectly acted on the sclera through regulating the biochemical substances (termed: secondary messenger) of the retinal pigment epithelium and choroidal melanocytes. This mode of administration in the treatment of myopia lacks practical significance for further clinical application. So it should be found effective treatment approaches to change secondary messengers in sclera and play a role by administration of eye drops [20].
In this study, we used peribulbar injection method to deliver bFGF. bFGF was able to reach the sclera by local spreading to work on, avoiding the impact of drugs on the cornea by subconjunctival delivery. Tree shrews and human scleral fibroblasts express bFGF specific receptor FGFR-1 mRNA, indicating that bFGF is an important cytokine in scleral remodeling of experimental myopia. The binding of exogenous bFGF with its specific receptor in the scleral fibroblasts may regulate matrix synthesis, degradation and proliferation of scleral fibroblasts [21],[22].
This study found that bFGF promoted the expression of scleral type I collagen, α2 integrin and β1 integrin during the formation of experimental myopia, indicating that exogenously delivered bFGF may increase the flexibility and toughness of sclera by increasing the expressions of scleral type I collagen, α2 integrin and β1 integrin, further inhibit the formation of experimental myopia. Sclera is an important target for exogenous bFGF, peribulbar injection of bFGF can play a role via reaching the sclera. At present, recombinant bFGF has been gradually used in clinical treatment of various diseases, bFGF constituted-eye drops have also been applied in ophthalmology. However, the mechanisms for bFGF preventing myopia still need more researches.
References
- 1.Christensen AM, Wallman J. Evidence that increased scleral growth underlies visual deprivation myopia in chicks. Invest Ophthalmol Vis Sci. 1991;32(7):2143–2150. [PubMed] [Google Scholar]
- 2.McBrien NA, Gentle A. Role of the sclera in the development and pathological complications of myopia. Prog Retin Eye Res. 2003;22(3):307–338. doi: 10.1016/s1350-9462(02)00063-0. [DOI] [PubMed] [Google Scholar]
- 3.Wallman J, Winawer J. Homeostasis of eye growth and the question of myopia. Neuron. 2004;43(4):447–468. doi: 10.1016/j.neuron.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 4.McBrien NA, Jobling AI, Gentle A. Biomechanics of the sclera in myopia: extracellular and cellular factors. Optom Vis Sci. 2009;86(1):E23–30. doi: 10.1097/OPX.0b013e3181940669. [DOI] [PubMed] [Google Scholar]
- 5.Frost MR, Norton TT. Alterations in protein expression in tree shrew sclera during development of lens-induced myopia and recovery. Invest Ophthalmol Vis Sci. 2012;53(1):322–336. doi: 10.1167/iovs.11-8354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rada JA, Achen VR, Penugonda S, Schmidt RW, Mount BA. Proteoglycan composition in the human sclera during growth and aging. Invest Ophthalmol Vis Sci. 2000;41(7):1639–1648. [PubMed] [Google Scholar]
- 7.Rada JA, Nickla DL, Troilo D. Decreased proteoglycan synthesis associated with form deprivation myopia in mature primate eyes. Invest Ophthalmol Vis Sci. 2000;41(8):2050–2058. [PubMed] [Google Scholar]
- 8.Schwartz MA, DeSimone DW. Cell adhesion receptors in mechanotransduction. Curr Opin Cell Biol. 2008;20(5):551–556. doi: 10.1016/j.ceb.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zorn N, Hernandez MR, Norton TT, Yang J, Ye HO. Collagen gene expression in the developing tree shrew sclera. Invest Ophthalmol Vis Sci. 1992;33(Suppl.):1053. [Google Scholar]
- 10.McBrien NA, Metlapally R, Jobling A I, Gentle A. Expression of collagen-binding integrin receptors in the mammalian sclera and their regulation during the development of myopia. Invest Ophthalmol Vis Sci. 2006;47(11):4674–4682. doi: 10.1167/iovs.05-1150. [DOI] [PubMed] [Google Scholar]
- 11.Metlapally R, Jobling AI, Gentle A, McBrien NA. Characterization of the integrin receptor subunit profile in the mammalian sclera. Mol Vis. 2006;12:725–734. [PubMed] [Google Scholar]
- 12.Hu S, Cui D, Yang X, Hu J, Wan W, Zeng J. The crucial role of collagen-binding integrins in maintaining the mechanical properties of human scleral fibroblasts-seeded collagen matrix. Mol Vis. 2011;17(5):1334–420. [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou X, Lu F, Xie R, Jiang L, Wen J, Li Y, Shi J, He T, Qu J. Recovery from axial myopia induced by a monocularly deprived facemask in adolescent (7-week-old) guinea pigs. Vision Res. 2007;47(8):1103–1111. doi: 10.1016/j.visres.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 14.Schmid KL, Wildsoet CF. Effects on the compensatory responses to positive and negative lenses of intermittent lens wear and ciliary nerve section in chicks. Vision Res. 1996;36(7):1023–1036. doi: 10.1016/0042-6989(95)00191-3. [DOI] [PubMed] [Google Scholar]
- 15.Fujikado T, Kawasaki Y, Suzuki A, Ohmi G, Tano Y. Retinal function with lens-induced myopia compared with form-deprivation myopia in chicks. Graefes Arch Clin Exp Ophthalmol. 1997;235(5):320–324. doi: 10.1007/BF01739642. [DOI] [PubMed] [Google Scholar]
- 16.Zhu XY, Winawer JA, Wallman J. Potency of myopic defocus in spectacle lens compensation. Invest Ophthalmol Vis Sci. 2003;44(7):2818–2827. doi: 10.1167/iovs.02-0606. [DOI] [PubMed] [Google Scholar]
- 17.Friedrichs J, Manninen A, Muller DJ, Helenius J. Galectin-3 regulates integrin alpha2, beta1- mediated adhesion to collagen-I and -IV. J Biol Chem. 2008;283(47):32264–32272. doi: 10.1074/jbc.M803634200. [DOI] [PubMed] [Google Scholar]
- 18.Taubenberger A, Cisneros DA, Friedrichs J, Puech PH, Muller DJ, Franz CM. Revealing early steps of alpha2beta1 integrin-mediated adhesion to collagen type I by using single-cell force spectroscopy. Mol Biol Cell. 2007;18(5):1634–44. doi: 10.1091/mbc.E06-09-0777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rohrer B, Stell W K. Basic fibroblast growth factor (bFGF) and transforming growth factor beta (TGF-beta) act as stop and go signals to modulate postnatal ocular growth in the chick. Exp Eye Res. 1994;58(5):553–561. doi: 10.1006/exer.1994.1049. [DOI] [PubMed] [Google Scholar]
- 20.Hu DN, Mc-Cormick SA. Thorn F, Troilo D, Gwiazda J, editors. Role of RPE-choroid axis on production, binding and response to various growth factors modulate the occurrence of myopia. 2000. pp. 213–216. Myopia 2000: Proceedings of the VIII International Conference on Myopia. Boston: Conference on Myopia 2000, Inc.
- 21.Rohrer B, Iuvone PM, Stell WK. Stimulation of dopaminergic amacrine cells by stroboscopic illumination or fibroblast growth factor (bFGF, FGF-2) injections: possible roles in prevention of form-deprivation myopia in the chick. Brain Res. 1995;686(2):169–81. doi: 10.1016/0006-8993(95)00370-6. [DOI] [PubMed] [Google Scholar]
- 22.Gental A, McBrien NA. Retinoscleral control of scleral remodeling in refractive development: a role for endogeneous FGF-2. Cytokine. 2002;18(6):344–348. doi: 10.1006/cyto.2002.1046. [DOI] [PubMed] [Google Scholar]