Abstract
Purpose
Studies in non-gynecologic tumors indicate that metformin inhibits growth of cancer stem cells (CSC). Diabetic patients with ovarian cancer who are taking metformin have better outcomes than those not taking metformin. The purpose of this study was to directly address the impact of metformin on ovarian CSC.
Methods
The impact of metformin on ovarian cancer cell line growth and viability was assessed with trypan blue staining. Aldehyde dehydrogenase (ALDH) expressing CSC were quantified using FACS®. Tumor sphere assays were performed to determine the impact of metformin on cell line and primary human ovarian tumor CSC growth in vitro. In vivo therapeutic efficacy and the anti-CSC effects of metformin were confirmed using both tumor cell lines and ALDH(+) CSC tumor xenografts.
Results
Metformin significantly restricted the growth of ovarian cancer cell lines in vitro. This effect was additive with cisplatin. FACS analysis confirmed that metformin reduced ALDH(+) ovarian CSC. Consistent with this, metformin also inhibited the formation of CSC tumor spheres from both cell lines and patient tumors. In vivo, metformin significantly increased the ability of cisplatin to restrict whole tumor cell line xenografts. In addition, metformin significantly restricted the growth of ALDH(+) CSC xenografts. This was associated with a decrease in ALDH(+) CSC, cellular proliferation, and angiogenesis.
Conclusions
Metformin can restrict the growth and proliferation of ovarian cancer stem cells in vitro and in vivo. This was true in cell lines and in primary human CSC isolates. These results provide a rationale for using metformin to treat ovarian cancer patients.
Keywords: Ovarian cancer, Cancer stem cells, Metformin, Therapy, Angiogenesis
Introduction
Ovarian cancer is the deadliest gynecologic malignancy and the fifth most deadly malignancy of women in the United States [1]. Although multi-modality treatment with cytoreductive surgery and platinum-taxane based chemotherapy have prolonged survival, the overall cure rate of the disease has not changed dramatically. There have been few new developments in the treatment of ovarian cancer patients since the standardization of platinum and paclitaxel, which has been the mainstay of ovarian cancer adjuvant therapy for the last 20 years. Novel therapeutics are needed. One way forward is suggested by recent work implicating cancer stem cells (CSC) as the source of therapeutic resistance and disease recurrence.
The cancer stem cell hypothesis suggests that cancer stem cells are a rare population of inherently chemo-resistant cancer cells able to regenerate the various cell types within a tumor, thereby causing a disease relapse [2,3]. We have recently demonstrated that a population of ovarian CSC can be isolated based on aldehyde dehydrogenase (ALDH) activity [4]. ALDH(+) cells are inherently resistant to chemotherapy. Small numbers of ALDH(+) cells can initiate tumors in mice, while a 10–50 fold excess of ALDH(−) cells cannot. Interestingly, cells which express both ALDH and CD133 possess greater tumor initiation capacity [4].
Drugs that target cancer stem cells may offer a great promise. Metformin, a traditional type 2 diabetes medication, has also been associated with transcriptional repression of the epithelial–mesenchymal transition, a cellular phenotype associated with CSC [5]. Metformin has recently been shown to target CSC in breast cancer. Metformin inhibited cellular transformation and selectively killed breast cancer stem cells in vitro and in vivo [6]. Metformin selectively kills chemotherapy-resistant CSC in breast cancer cell lines [7]. Another study in breast cancer demonstrated that metformin synergistically interacts with trastuzumab, the anti-HER2 monoclonal antibody, to suppress the self-renewal and proliferation of CSC in HER-2 positive carcinomas [8]. Finally, the combination metformin and standard chemotherapy reduced tumor mass and prevented relapse in cell line xenograft mouse models of prostate and lung cancer [7]. All these data strongly implicate metformin as a CSC targeting agent.
Metformin may have similar activity in ovarian cancer. In vitro studies have demonstrated anti-proliferative and pro-apoptotic effects of metformin in ovarian cancer [9]. Metformin therapy has been associated with anti-proliferative effects through both AMPK-dependent and AMPK-independent pathways and increased tumor cell apoptosis and decreased metastasis [10,11]. In our study, we provide evidence that a major mechanism for metformin’s ability to inhibit the growth of ovarian cancer lies in its effect on ovarian cancer stem cells.
Materials and methods
Cell lines and cytotoxic assays
A2780 cells were obtained from Dr. Susan Murphy (Duke University, Durham, NC). SKOV3 cells were obtained from Dr. Rebecca Liu (University of Michigan, Ann Arbor, MI). In order to achieve ~60% confluency on the culture plate, 1.8×105 SKOV3 cells and 2.5×105 A2780 cells were plated in replicate in RPMI-10 (10% fetal bovine serum and 1% penicillin/streptomycin (Invitrogen)) and rested for 24 h. Cells were then treated with cisplatin (APP Pharmaceuticals) 1.5 μg/mL for SKOV3 cells or 0.5 μg/mL for A2780 cells with increasing doses of metformin (Sigma-Aldrich) (100 μM, 300 μM, 1 mM). Cells were evaluated for viability with trypan blue and analyzed using flow cytometry, as described below.
Flow cytometry and fluorescence-activated cell sorting (FACS®)
Cells from primary ovarian tumors, human ascites or human ovarian cancer cell lines (SKOV3 and A2780) were counted in single cell suspensions. ALDH enzymatic activity was assessed using the ALDEFLUOR kit per manufacturer’s protocol (Stem Cell Technologies, Vancouver, BC, Canada). For each sample an aliquot of cells was treated with ALDH inhibitor diethylaminobenzaldehyde (DEAB-50 mM/L). A2780 cells were stained with anti-CD133/2-APC (Miltenyi Biotec) before the ALDEFLUOR assay. Gating was established using propidium iodide exclusion for viability; ALDEFLUOR-stained cells treated with DEAB defined negative gates. Flow cytometric analysis was performed using either the BD FACSAria or FACSDiva instruments (Becton Dickinson, San Diego, CA, USA). Results were analyzed using FlowJo (Tree Star Inc.).
Tumor processing
Patients consented for tissue donation in accordance with a protocol approved by the University of Michigan’s Institutional Review Board. Freshly resected tumor samples were immediately processed into taken single cell suspensions as previously described [12]. Briefly, a portion of each specimen was mechanically dissected and filtered, and red cells were lysed with ACK buffer (Lonza Walkersville Inc.). Cells were isolated using a ficol gradient per manufacturer protocol (Histopaque—Sigma, St. Louis, MO, USA). All tumors were stage III or IV epithelial ovarian, fallopian tube or primary peritoneal cancer, serous histology.
Tumor sphere assays
Tumor associated ascites cells were isolated by centrifugation, treated with ACK lysis buffer (Fischer Scientific) and then 4000 cells plated in triplicate in ultra-low-adherence plates (Corning, Acton, MA, USA) in 2 mL of supplemented MEBM (Lonza) [12]. Alternatively ALDH(+) and ALDH(−) cells were FACS isolated and plated in a parallel manner. After 24 h, cells were treated with either metformin 1 mM or media alone. Media with or without metformin was changed every 2 to 3 days. Tumor sphere counts were performed after 2 weeks. Spheres were photographed using Olympus Microsuite Biological Suite software.
Animal studies
Nude mice were obtained from Charles River Laboratories. All animals were performed with the approval of the University Committee on Use and Care of Animals of the University of Michigan. To generate tumors, 1×105 SKOV3 cells were combined with 100 μL of DPBS and 200 μL of Matrigel (BD Biosciences), and then implanted subcutaneously into the axillae of female mice. Tumors were allowed to grow for 7 days and then mice received: (i) no treatment, (ii) cisplatin 250 μg/kg intra-peritoneal (i.p.) for 3 days, or (iii) cisplatin 250 μg/kg i.p. plus metformin 150 mg/kg i.p. daily (n=8 animals per treatment group). Tumor growth was measured using calipers, and volumes were calculated based on the modified ellipsoid formula (L×W×W/2), where L represents length and W represents width. The mice were euthanized when tumors reached 1.5 to 2 cm3 or animal safety euthanasia guidelines were met.
For CSC specific tumors, 1000 FACS isolated ALDH(+) or ALDH(−) tumor cells were suspended in 100 μL of DPBS and 200 μL of Matrigel (BD Biosciences) and then implanted subcutaneously into the axillae of NOD/Shi-scid/IL-2Rγnull (NOG) mice. Tumors were allowed to grow for 7 days and then mice were treated and tumors assessed as above.
Dose justification
A typical human treatment dose of metformin is 1000 to 2500 mg, usually given twice daily. Metformin steady state plasma concentrations (typically <1 μg/mL (6 mM)) are reached in 24 to 48 h (http://www.fda.gov/ohrms/dockets/dailys/02/May02/053102/800471e6.pdf) [13]. This concentration is above the doses used in this study.
In murine tumor models we used a metformin dose of 150 mg/kg. This can be translated to the human equivalent dose by using the well-established Reagan-Shaw method [14]. According to the formula, the human equivalent dose (mg/kg)=animal dose (mg/kg) × animal Km/human Km. The Km values are based on body surface area. Km for a 60 kg human adult is 37 and for a 20 g mouse is 3. Thus, the human equivalent of the murine dose of 150 mg/kg in a mouse is 730 mg in an average-sized woman of 60 kg. This is less than half the maximum metformin dose of 2550 mg/day recommended by the Food and Drug Administration [13]. Thus all dosing in this study is within a therapeutic range safely obtainable in humans.
Immunohistochemistry
Fresh murine tumors were fixed in 10% formalin (Fischer Scientific) overnight and then placed in 70% ethanol (Ricca Chemical Company). Tumors were then paraffin embedded and stained at the histology core at the University of Michigan, using EDTA-based antigen retrieval and mouse anti-CD31 antibody, or anti-Ki67 antibody (Abcam no. 15580, 1:2000). For stain quantification, 9 sections from each of the 3 tumors per treatment group were analyzed. CD31 + blood vessels and Ki67 + cells were counted from 4 high-power (100×) fields per section.
Statistical analysis
p values of less than 0.05 were considered statistically significant. All counts were compared using a 2-sided Student’s t test and Microsoft Excel software (Redmond, WA). GraphPad Prism version 5.00 for Windows, GraphPad Software, (San Diego, CA, USA, www.graphpad.com) was used to generate Kaplan–Meier curves and curves were compared using the Log-rank (Mantel Cox) test.
Results
Metformin as an anti-neoplastic in ovarian cancer
Some in vitro studies of metformin’s impact on ovarian cancer cell lines used supra-physiologic doses (50 mM) of metformin [9]. We performed dose-titration studies examining the effects of physiologic doses of metformin on ovarian cancer cell lines. Metformin therapy inhibited the proliferation of SKOV3 cells at doses of 1 mM and higher (Fig. 1A and data not shown). Metformin inhibited the growth of A2780 cells in doses of 300 μM and higher. We estimated an IC50 of 1–3 mM in two cell lines; a dose easily achieved in patients with standard dosing of metformin (Fig. 1A and data not shown). We next assessed metformin’s effectiveness in combination with cisplatin chemotherapy. With a fixed dose of cisplatin, for both cisplatin sensitive A2780 ovarian cancer cells and cisplatin-resistant SKOV3 ovarian cancer cells, we observed an increase in cyto-toxicity with the increasing doses of metformin (Fig. 1B). For A2780 cells, the combination index with cisplatin was 0.6 (IC50–metformin 3 mm, IC50 cisplatin 0.5 μg, combined dosing to achieve IC50–0.25 μg cisplatin and 300 μM metformin) suggesting synergy of the two drugs [15].
Fig. 1.
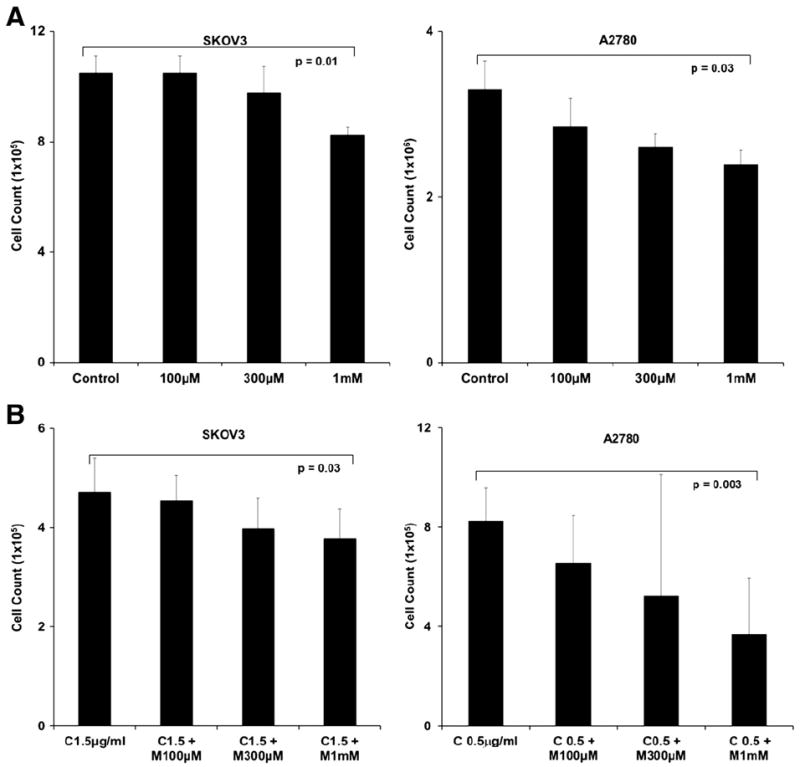
Anti-tumor effects of metformin in vitro. (A) Dose-dependent toxicity of metformin. Cell counts of SKOV3 and A2780 ovarian cancer cells treated with increasing doses of metformin for 3 days. (B) Dose dependent toxicity of metformin when combined with chemotherapy. Cell counts of SKOV3 and A2780 ovarian cancer cells treated with 1.5 μg/mL cisplatin or 0.5 μg/mL of cisplatin, respectively, and metformin (M) 100 μM, 300 μM, and 1 mM for 3 days. For both experiments, cell viability was determined using trypan blue staining. Experiments are representative of 4 independent experiments. All samples were analyzed in triplicate. p values are as indicated.
Metformin targets ovarian cancer stem cells
Given previous studies implicating metformin as a CSC targeting agent in breast cancer, we investigated the impact of metformin on ovarian CSC. We have demonstrated that ALDH is an excellent marker of ovarian CSC in both patient tumor specimens and in tumor cell lines [4]. We therefore assessed the percentage of ALDH(+) cells in SKOV3 and A2780 cells treated with cisplatin, metformin 1 mM, or both, for 3 days. As previously observed cisplatin therapy induced an increase in the percentage of ALDH(+) CSC. However, treatment with metformin, either alone or in the presence of cisplatin, reduced the CSC population below control levels (Fig. 2A[1] and A[2]).
Fig. 2.

Metformin impacts ovarian CSC. (A1 and 2) FACS analysis of ALDH(+) cells in SKOV3 (1) and A2780 cells (2) after treatment with cisplatin (1.5 μg/mL), metformin (1 mM), or both. DEAB was used as a negative control. Numbers represent the percentage of ALDH(+) cells. (A3 and 4) Average percentage of ALDH(+) cells in untreated controls and cisplatin, metformin, or cisplatin+metformin for SKOV3 (3) and A2780 (4). The results are averages of 4 independent experiments. (B1 and 2) Representative images (200×) of tumor spheres formed from 4000 FACS isolated ALDH(+) SKOV3 cells in the presence or absence of 1 mM metformin and (B2) average sphere numbers. Cells were plated in triplicate and spheres were counted after 14 days. p values are as indicated.
CSC are characterized by their ability to form tumor spheres in suspension in serum-free media. We therefore performed tumor-sphere assays with FACS sorted ALDH(+) SKOV3 cells grown in the presence or absence of metformin. Metformin therapy resulted in a 5-fold decrease in ALDH+ cell tumor sphere formation (Fig. 2B[1] and B[2]). As ALDH may not identify all CSC populations, these experiments were then repeated using unsorted primary human tumor or ascites cells from 3 separate (chemo-naive) patients in the presence and absence of metformin (Fig. 3A and B). In the presence of metformin, we observed an average 2-fold decrease in the number of tumor spheres (Fig. 3B). Finally, we FACS sorted primary ovarian tumor tissue from two different patient samples into ALDH(+) and ALDH(−) cells and treated them with or without metformin 1 mM. As we previously reported [4] the ALDH(−) cells generated few if any spheres, regardless of metformin treatment (Fig. 3A). With metformin treatment, however, we observed an average 3-fold decrease in the number of tumor spheres formed from ALDH(+) cells (Fig. 3A and C). Taken together these studies suggest that metformin exerts anti-tumor effects on ALDH(+) ovarian CSC.
Fig. 3.

Metformin inhibits the formation of tumor spheres in patient ovarian tumor cells. (A) Representative images of patient tumor spheres treated with media or metformin for 14 days. (B) Average number of spheres formed from 4000 ovarian cancer patient ascites cells grown with and without metformin. (C) Average number of spheres formed from 4000 FACS isolated ALDH(+) patient ascites cells grown with and without metformin. All samples were plated in triplicate. p values are as indicated.
Metformin as an anti-neoplastic in ovarian cancer in a xenograft mouse
We next evaluated the therapeutic efficacy of metformin in vivo using human ovarian cancer cell line murine tumor xenografts. Cisplatin resistant SKOV3 tumor xenografts were allowed to grow for 7 days prior to initiation of treatment. The mice were divided into 4 treatment groups: (i) no treatment, (ii) metformin daily, (iii) cisplatin×3 days or (iv) cisplatin×3 days+metformin daily. When compared to untreated controls, both cisplatin treatment alone and metformin treatment alone demonstrated similarly non-significant decreases in tumor growth. In contrast, the combination of cisplatin and metformin significantly restricted tumor growth compared to both untreated and cisplatin treated controls (Fig. 4A). Indeed, in the cisplatin+metformin treatment group, 3 of the 8 mice had complete resolution of their tumors. Kaplan Meier survival curves were plotted for the mice in the 4 treatment groups (Fig. 4B). After just over 60 days all the untreated mice met criteria for euthanization. At 90 days, mice treated with either cisplatin or metformin demonstrated similar survival. Significantly more mice from the cisplatin+metformin group were still alive compared to the other three groups. This experiment was repeated 3 separate times with similar results.
Fig. 4.

Metformin restricts ovarian tumor growth in vivo. (A) Tumor growth curves of SKOV3 tumors in mice (i) untreated, or treated (ii) with metformin 150 mg/kg i.p daily, (iii) cisplatin 250 μg/kg i.p for 3 days starting on day 7, or (iv) cisplatin 250 μg/kg i.p. for 3 days starting on day 7+metformin daily starting on day 7 (n=8 animals per treatment group). (B) Kaplan Meier survival curves were calculated for the four treatment groups indicated above. p values are as indicated.
Metformin in combination with chemotherapy reduces tumor proliferation, angiogenesis, and CSC
Our previous studies demonstrated that ALDH(+) ovarian CSC are highly angiogenic. We therefore analyzed the impact of metformin therapy on tumor angiogenesis. Immunohistochemical analysis of murine CD31 staining showed significantly reduced microvessel density in the cisplatin+metformin treated tumors, as compared to cisplatin treatment alone and untreated controls (Fig. 5A and B). We also evaluated tumor cellular proliferation rates as detected by Ki67 stain [16]. Immunohistochemical analysis of Ki67 in the four treatment groups demonstrated that both metformin alone and cisplatin alone reduced Ki67 stains relative to untreated controls (Fig. 5A and B2). The combination of cisplatin and metformin compared to both untreated controls and the monotherapy treatment groups, showed a statistically significant reduction in the number of proliferating cells (Fig. 5B2).
Fig. 5.

Metformin in combination with chemotherapy inhibits proliferation and angiogenesis. (A) Immunohistochemical stain of SKOV3 tumor for CD31 and Ki67 in the indicated treatment groups. (B and C) Quantification of CD31 and Ki67 staining in the four indicated treatment groups. (D) Average percentage of ALDH(+) cells from 4 tumors from each treatment group. p values are as indicated.
Next, three representative tumors from each treatment group were FACS analyzed for ALDH(+) CSC. While cisplatin had no significant impact on the number of ALDH(+) CSC, metformin therapy alone and combined therapy with cisplatin and metformin led to a significant reduction in ALDH(+) CSC compared to both untreated controls and cisplatin treated tumors (Fig. 5C).
Metformin restricts the growth of ovarian CSC in vivo
Finally, in order to directly test the effects of metformin on ovarian CSC, we generated tumor xenografts in bilateral axillae with 1000 FACS isolated ALDH(−) and ALDH(+) SKOV3 cells. The tumors were allowed to engraft for 7 days and then treatment was initiated with metformin, cisplatin, or metformin+cisplatin as in the previous study. Tumor volumes were measured weekly. After 11 weeks, as anticipated, the ALDH(−) cells generated tumor in only 2 of 8 mice. No ALDH(−) cell initiated tumors were observed in the metformin treated animals (data not shown). All ALDH(+) cell xenografts treated with monotherapy initiated tumors. However, compared to untreated controls, there was a similar statistically significant reduction in tumor growth for both cisplatin and metformin treatment groups (Fig. 6A). Only 2 of 8 tumors treated with both cisplatin and metformin initiated tumors. Combination therapy led to an even greater reduction in tumor growth (Fig. 6A). FACS analysis of these tumors demonstrated two-fold reduction in ALDH(+) cells in metformin treated tumors compared to controls (Fig. 6B). The addition of metformin to cisplatin led to a 4.4 fold reduction in ALDH(+) cells. These data support that metformin can specifically impact the growth of ovarian CSC.
Fig. 6.
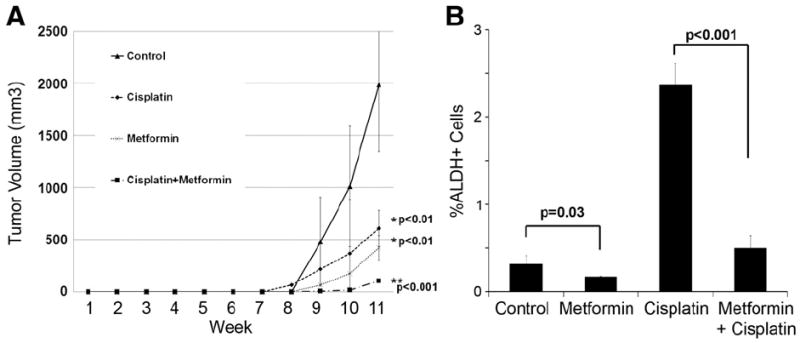
Metformin restricts the in vivo growth of FACS isolated ALDH(+) ovarian CSC. (A) Tumor growth curves of tumors initiated by FACS isolated ALDH(+) cells and either (i) untreated, or treated with (ii) metformin 150 mg/kg i.p daily, (iii) cisplatin 250 μg/kg i.p for 3 days starting on day 7, or (iv) cisplatin 250 μg/kg i.p. for 3 days starting on day 7+metformin daily (n=8 animals per treatment group). (B) Average percentage of CSC in ALDH(+) cells in representative xenografts from the four treatment groups. p values are indicated.
Discussion
Metformin has been shown to be active against ovarian cancer cells in vitro and in vivo. We demonstrate here that metformin acts on ovarian cancer stem cells, reducing the percentage of ALDH(+) CSC in vitro and in vivo, and inhibiting the growth of ovarian tumor spheres. Metformin was active against primary human CSC in vitro and, metformin therapy alone slows the growth of ovarian CSC in vivo.
Our results are most consistent with metformin having anti-proliferative rather than cytotoxic effects. This is consistent with the results of others suggesting that metformin can induce cell cycle arrest at G1 [17] and restrict cellular proliferation in AMPK dependent and independent manners [18]. While we did not observe significant cytotoxicity with metformin, metformin has been reported to induce apoptosis in ovarian cancer cells [10]. Apoptosis was enhanced in the presence of cisplatin therapy.
Our results suggesting that metformin reduces tumor microvascular density are also consistent with previous studies which reported reduced VEGF levels and reduced microvascular density with metformin therapy [11,18]. Interestingly, CSC and tumor vasculature appear to be mutually dependent on one another [19]. While angiogenesis is essential for tumor growth and tumor endothelial cells provide important growth factors for CSC [20], CSC are in turn highly angiogenic [4,21]. Our studies raise the possibility that the anti-angiogenic effects of metformin are due to the actions of metformin on CSC and that the reduction in CSC subsequently leads to a reduction in angiogenesis.
Our data adds to the growing number of laboratory studies (i) indicating anti-CSC effects of metformin and (ii) supporting metformin therapy for ovarian cancer. Metformin has been demonstrated to have anti-CSC activity on breast, prostate, and lung cancer cell lines [5,7]. One of the earlier studies indicating a role for metformin in cancer therapy demonstrated that metformin acted primarily on tumors with p53 mutations [22]. Importantly, sequencing of approximately 500 ovarian tumors as part of the Tumor Genome Atlas Project recently revealed that greater than 95% of ovarian cancers carry p53 mutations [23]. Thus if the anti-neoplastic effects of metformin are dependent on p53 mutations, ovarian cancer represents an ideal target for use of metformin.
As noted above, epidemiologic data also supports the thesis that metformin is active against ovarian cancer. We recently demonstrated that type 2 diabetes has a negative impact on the survival of ovarian cancer patients [24]. Interestingly, diabetic patients were more likely to have a poorly differentiated tumor histology [24]. Poor tumor differentiation could be consistent with increased tumor “stemness.” However, ovarian cancer patients with type 2 diabetes treated with metformin have better outcomes than those treated with other anti-glycemic agents, supporting a role for metformin as a therapeutic in ovarian cancer. Furthermore, recent studies found that the long-term use of metformin, but not of sulfonylureas, was associated with a decreased risk of ovarian cancer [25]. Thus metformin use may actually prevent or delay ovarian cancer development.
Taken together, these data strongly indicate that metformin is an active therapeutic in ovarian cancer which impacts ovarian CSC growth. These important results shed light on how metformin works and provide critical preclinical rationale for the use of metformin in phase 2 clinical trials for ovarian cancer patients. We believe that metformin co-treatment with chemotherapy may prevent ovarian cancer recurrence and improve long-term survival.
HIGHLIGHTS.
-
►
Metformin has anti-ovarian cancer activity in vitro and in vivo.
-
►
Metformin restricts the growth of primary human ovarian cancer stem cells.
-
►
Clinical trials to assess the impact of metformin on ovarian cancer outcomes are indicated.
Acknowledgments
This work was supported by a grant from the Michigan Institute for Clinical Health Research and the National Institutes of Health New Innovator Directors Award grant #00440377.
Footnotes
Conflict of interest statement
None of the authors have a conflict of interest.
References
- 1.Edwards BK, Brown ML, Wingo PA, Howe HL, Ward E, Ries LA, et al. Annual report to the nation on the status of cancer, 1975–2002, featuring population-based trends in cancer treatment. J Natl Cancer Inst. 2005;97:1407–27. doi: 10.1093/jnci/dji289. [DOI] [PubMed] [Google Scholar]
- 2.Boman BM, Wicha MS. Cancer stem cells: a step toward the cure. J Clin Oncol. 2008;26:2795–9. doi: 10.1200/JCO.2008.17.7436. [DOI] [PubMed] [Google Scholar]
- 3.Burgos-Ojeda D, Rueda BR, Buckanovich RJ. Ovarian cancer stem cell markers: prognostic and therapeutic implications. Cancer Lett. 2012;322:1–7. doi: 10.1016/j.canlet.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Silva IA, Bai S, McLean K, Yang K, Griffith K, Thomas D, et al. Aldehyde dehydrogenase in combination with CD133 defines angiogenic ovarian cancer stem cells that portend poor patient survival. Cancer Res. 2011;71:3991–4001. doi: 10.1158/0008-5472.CAN-10-3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vazquez-Martin A, Oliveras-Ferraros C, Cufi S, Del Barco S, Martin-Castillo B, Menendez JA. Metformin regulates breast cancer stem cell ontogeny by transcriptional regulation of the epithelial–mesenchymal transition (EMT) status. Cell Cycle. 2010;9:3807–14. [PubMed] [Google Scholar]
- 6.Hirsch HA, Iliopoulos D, Tsichlis PN, Struhl K. Metformin selectively targets cancer stem cells, and acts together with chemotherapy to block tumor growth and prolong remission. Cancer Res. 2009;69:7507–11. doi: 10.1158/0008-5472.CAN-09-2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iliopoulos D, Hirsch HA, Struhl K. Metformin decreases the dose of chemotherapy for prolonging tumor remission in mouse xenografts involving multiple cancer cell types. Cancer Res. 2011;71:3196–201. doi: 10.1158/0008-5472.CAN-10-3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vazquez-Martin A, Oliveras-Ferraros C, Barco SD, Martin-Castillo B, Menendez JA. The anti-diabetic drug metformin suppresses self-renewal and proliferation of trastuzumab-resistant tumor-initiating breast cancer stem cells. Breast Cancer Res Treat. 2011;126:355–64. doi: 10.1007/s10549-010-0924-x. [DOI] [PubMed] [Google Scholar]
- 9.Gotlieb WH, Saumet J, Beauchamp MC, Gu J, Lau S, Pollak MN, et al. In vitro metformin anti-neoplastic activity in epithelial ovarian cancer. Gynecol Oncol. 2008;110:246–50. doi: 10.1016/j.ygyno.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 10.Yasmeen A, Beauchamp MC, Piura E, Segal E, Pollak M, Gotlieb WH. Induction of apoptosis by metformin in epithelial ovarian cancer: involvement of the Bcl-2 family proteins. Gynecol Oncol. 2011;121:492–8. doi: 10.1016/j.ygyno.2011.02.021. [DOI] [PubMed] [Google Scholar]
- 11.Rattan R, Graham RP, Maguire JL, Giri S, Shridhar V. Metformin suppresses ovarian cancer growth and metastasis with enhancement of cisplatin cytotoxicity in vivo. Neoplasia. 2011;13:483–91. doi: 10.1593/neo.11148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pulaski HL, Spahlinger G, Silva IA, McLean K, Kueck AS, Reynolds RK, et al. Identifying alemtuzumab as an anti-myeloid cell antiangiogenic therapy for the treatment of ovarian cancer. J Transl Med. 2009;7:49. doi: 10.1186/1479-5876-7-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zydus Pharmaceuticals (USA) Inc. Metformin: Official FDA information, side effects and uses. 2011 [Google Scholar]
- 14.Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2008;22:659–61. doi: 10.1096/fj.07-9574LSF. [DOI] [PubMed] [Google Scholar]
- 15.Zhao L, Wientjes MG, Au JL. Evaluation of combination chemotherapy: integration of nonlinear regression, curve shift, isobologram, and combination index analyses. Clin Cancer Res. 2004;10:7994–8004. doi: 10.1158/1078-0432.CCR-04-1087. [DOI] [PubMed] [Google Scholar]
- 16.McLean K, Gong Y, Choi Y, Deng N, Yang K, Bai S, et al. Human ovarian carcinoma-associated mesenchymal stem cells regulate cancer stem cells and tumorigenesis via altered BMP production. J Clin Invest. 2011;121:3206–19. doi: 10.1172/JCI45273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li C, Liu VW, Chan DW, Yao KM, Ngan HY. LY294002 and metformin cooperatively enhance the inhibition of growth and the induction of apoptosis of ovarian cancer cells. Int J Gynecol Cancer. 2012;22:15–22. doi: 10.1097/IGC.0b013e3182322834. [DOI] [PubMed] [Google Scholar]
- 18.Rattan R, Giri S, Hartmann LC, Shridhar V. Metformin attenuates ovarian cancer cell growth in an AMP-kinase dispensable manner. J Cell Mol Med. 2011;15:166–78. doi: 10.1111/j.1582-4934.2009.00954.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Calabrese C, Poppleton H, Kocak M, Hogg TL, Fuller C, Hamner B, et al. A perivascular niche for brain tumor stem cells. Cancer Cell. 2007;11:69–82. doi: 10.1016/j.ccr.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 20.Shen Q, Goderie SK, Jin L, Karanth N, Sun Y, Abramova N, et al. Endothelial cells stimulate self-renewal and expand neurogenesis of neural stem cells. Science. 2004;304:1338–40. doi: 10.1126/science.1095505. [DOI] [PubMed] [Google Scholar]
- 21.Bao S, Wu Q, Sathornsumetee S, Hao Y, Li Z, Hjelmeland AB, et al. Stem cell-like glioma cells promote tumor angiogenesis through vascular endothelial growth factor. Cancer Res. 2006;66:7843–8. doi: 10.1158/0008-5472.CAN-06-1010. [DOI] [PubMed] [Google Scholar]
- 22.Buzzai M, Jones RG, Amaravadi RK, Lum JJ, DeBerardinis RJ, Zhao F, et al. Systemic treatment with the antidiabetic drug metformin selectively impairs p53-deficient tumor cell growth. Cancer Res. 2007;67:6745–52. doi: 10.1158/0008-5472.CAN-06-4447. [DOI] [PubMed] [Google Scholar]
- 23.Cancer Genome Atlas Research Network. Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474:609–15. doi: 10.1038/nature10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bakhru A, Buckanovich RJ, Griggs JJ. The impact of diabetes on survival in women with ovarian cancer. Gynecol Oncol. 2011;121:106–11. doi: 10.1016/j.ygyno.2010.12.329. [DOI] [PubMed] [Google Scholar]
- 25.Bodmer M, Becker C, Meier C, Jick SS, Meier CR. Use of metformin and the risk of ovarian cancer: a case–control analysis. Gynecol Oncol. 2011;123:200–4. doi: 10.1016/j.ygyno.2011.06.038. [DOI] [PubMed] [Google Scholar]


