Abstract
Deficiencies in brain orexins and components of mitogen activated protein kinase (MAPK) signaling pathway have been reported in either human depression or animal model of depression. Brain administration of orexins affects behaviors toward improvement of depressive symptoms. However, the documentation of endogenous linkage between orexin receptor activation and MAPK signaling pathway remains to be insufficient. In this study, we report the effects of orexin 2 receptor (OX2R) activation on cell signaling in CHO cells over-expressing OX2R and in mouse hypothalamus cell line CLU172. Short-term extracellular signal-regulated kinase (ERK) phosphorylation and long-term cyclic adenosine monophosphate (cAMP) response element binding protein (CREB) phosphorylation were subsequently observed in CHO cells that over-express OX2R while 20 min of ERK phosphorylation was significantly detected in mouse adult hypothalamus neuron cell line CLU172. Orexin A, which can also activate OX2R, mediated ERK phosphorylation was as the same as orexin B in CHO cells. A MAPK inhibitor eliminated ERK phosphorylation but not CREB phosphorylation in CHO cells. Also, ERK and CREB phosphorylation was not mediated by protein kinase A (PKA) or calmodulin kinase (CaMK). However, inhibition of protein kinase C (PKC) by GF 109203X eliminated the phosphorylation of ERK and CREB in CHO cells. A significant decrease in ERK and CREB phosphorylation was observed with 1 μM GF 109203X pre-treatment indicating that the conventional and novel isoforms of PKC are responsible for CREB phosphorylation after OX2R activation. In contrast, ERK phosphorylation induced by orexin B in CLU172 cells cannot be inhibited by 1 μM of protein kinase C inhibitor.
From above observation we conclude that OX2R activation by orexin B induces ERK and CREB phosphorylation and orexin A played the same role as orexin B. Several isoforms of PKC may be involved in prolonged CREB phosphorylation. Orexin B induced ERK phosphorylation in mouse hypothalamus neuron cells differs from CHO cell line and cannot be inhibited by PKC inhibitor GF 109203X. And hypothalamus neuron cells may use different downsteam pathway for orexin B induced ERK phosphorylation. This result supports findings that orexins might have anti-depressive roles.
Keywords: Orexin, MAPK signaling, ERK, CREB, OX2R
Introduction
Orexins, including orexin A and orexin B (also called hypocretin 1 and hypocretin 2), are peptides found in the hypothalamus that contain 33 and 28 amino acids [1]. While the orexinergic neuronal system is important in wake/sleep regulation [2–4], eating behavior [5], energy metabolism [6,7], and the pathology of narcolepsy [8–11], evidence also implicates the orexinergic system in the pathology of depression. Our previous studies showed a significant reduction of orexins in a rat model of depression at the younger stage and an increase of orexins in mature rats [12]. Allard et al. reported that the number and size of orexin A-immunostained neurons were decreased in a genetic rat model of depression [13]. Orexin 2 receptor (OX2R) knockout mice displayed an increase in behavioral despair [14]. Furthermore, decreased levels of orexin were found in the cerebrospinal fluid of human patients diagnosed with depression [15,16]. Additionally, intracerebroventricular administration of orexin A induced an antidepressive effect in a rodent model [17]. Evidence that orexins promote wakefulness and that orexin levels are lower in human depression and animal models of depression provide biological support to the findings that sleep deprivation improves the symptoms of depression [18–23].
Orexins activate MAPK signaling pathway [24], which has been demonstrated as deficiency in suicide victim of human depression [25] and animal model of depression [26]. Both total extracellular signal-regulated kinases (ERK) and phosphorylated ERK (p-ERK) are significantly decreased in the frontal cortex and hippocampus in a rat model of depression compared to the controls [26]. In human studies, samples from victims of depression- related suicide had impaired signaling pathways, including significantly decreased phosphorylation of cyclic adenosine monophosphate (cAMP) response element binding protein (CREB) [27,28]. Also, antidepressive treatment increased the expression of CREB [29–31]. This evidence prompted interest in how orexins affect cell signaling.
Like most of GPCR signaling, orexin binds on orexin receptors to activate G proteins [32,33]. Orexin A and orexin B induces a phospholipase C (PLC)-mediated release of calcium from intracellular storages in different cell lines over expressing orexin 1 receptor (OX1R) and OX2R [32,34]. The OX1R linked to the influx of Ca2+ may go through receptor-operated calcium channels and conventional phospholipase C (PLC)-Ca2+ release-store-operated Ca2+ channel (SOC) pathways [35] and involves diacylglycerol-activated transient receptor potential canonical (TRPC) channels in neuronal cells [36]. Orexins had different effects on cAMP in different type of sources. Orexins may not stimulate cAMP accumulation [34] or inhibit the PACAP-induced increase in the cAMP level in PC12 cells [37]. Conversely, orexin significantly increased the cAMP level in human adrencortical cells [38]. Orexins induce a rapid, dose and time dependent increase in activation of ERK1/2 and p38 MAPK in variable type of cell lines and tissues [24,39]. This activation is through multiple G-proteins and different intracellular signaling pathways. ERK1/2 activation involves Gq/PLC/protein kinase C (PKC), but not PKA [40]. Inhibition of PKC and, in part, PKA prevents orexin induced ERK1/2 phosphorylation [41].
Although evidence has demonstrated that orexin induces ERK phosphorylation, how orexin induced ERK phosphorylation link to CREB phosphorylation is not known. Furthermore, systemic information regarding orexin induced cell signaling remains to be demonstrated. In the following, we report the result regarding orexins induced cell signaling in a CHO cell line over expressing OX2R and in mouse hypothalamus neuron cell line.
Material and methods
Materials
[Ala11, D-Leu15]-Orexin B (OBDL), orexin B and orexin A (Cat# 2142, 1457 and 1455) was purchased from TOCRIS Bioscience (Missouri, USA). The HCRTR2-Gα15-NFAT-bla CHO-K1 (CHO-OX2R) cell line was purchased from Invitrogen (Cat# K1246, California, USA). Mouse hypothalamus neuron cell line CLU172 was purchased from Cellutions Biosystems Inc. Antibodies were purchased from sources as shown in the figure legends. The thawing media used for cell restoration was 10% dialyzed FBS-DMEM supplemented with penicillin (100 U/mL), streptomycin (100 μg/mL), non-essential amino acids (0.1 mM), and HEPES buffer (25 mM). The cell growth medium was 10% dialyzed FBS-DMEM supplemented with penicillin (100 U/mL), streptomycin (100 μg/mL), zeocin (100 μg/mL), blasticidin (5 μg/mL), hygromycin (600 μg/mL), non-essential amino acids (0.1 mM), and HEPES buffer (25 mM). The assay medium was 1% dialyzed FBS-DMEM supplemented with penicillin (100 U/mL), streptomycin (100 μg/mL), non-essential amino acids (0.1 mM), and HEPES buffer (25 mM). The FACE assay kit was purchased from Active Motif (California, USA). SuperSignal West Femto reagent was purchased from Pierce Biotechnology Inc (Cat# 34096, Illinois, USA).
Cell culture
The CHO-OX2R cell line was restored with thawing medium for 2–3 days. Once the cells began growing, they were passaged with growth medium twice a week. For the Fast-Activation Cell-based ELISA (FACE) assay, 30,000 CHO-OX2R cells were seeded into 96-well plates. For Western blotting, 500,000 cells were seeded into 6-well plates for treatment with protein kinase inhibitors and orexin B as indicated. Mouse hypothalamus neuron cell line CLU172 was restored and cultured in DMEM medium with 10% FBS. 500,000 CLU172 cells were seeded into 6-well plates for orexin B treatment and protein kinase C inhibitor treatment.
Immunofluorescence staining of monolayer cells
Phosphorylation of ERK and expression of OX2R were observed using immunofluorescence staining [42]. About 300,000 to 500,000 CHO-OX2R cells were seeded into 35-mm plates containing a cover slip and allowed to adhere overnight. After treatment, cells were fixed with 100% ice-cold methanol at room temperature for 15 min. The cells were rinsed twice with PBS for 5 min. For OX2R staining, the cells were blocked with 1% BSA-1% horse serum-PBS at room temperature for 1 h. For p-ERK staining, the cells were blocked with 5% non-fat milk-PBS at room temperature for 1 h. After blocking, the cells were rinsed twice for 5 min and incubated with primary antibody diluted in 1% BSA-PBS at 4 °C overnight. Secondary antibody incubation was performed at room temperature for 2 h after rinsing three times with 0.1% Triton X100-PBS for 5 min. Nuclei were counterstained with DAPI, and the cover slips were mounted with 50% glycerol in PBS.
FACE assay
A modified FACE assay [43] was used to measure the levels of total ERK and p-ERK in cells treated with OBDL. CHO-OX2R cells (30,000) were seeded in each well of a 96-well plate with 80 μL of serum-free medium for 24 h. Then, 20 μL of OBDL (0–1000 nM) in PBS was added into each well and incubated at 37 °C for 5 min. The cells were fixed with 100 μL of 4% cold formaldehyde in PBS for 20 min and washed three times with 200 μL of washing buffer (0.1% Triton X-100-PBS). The endogenous peroxidase activity was quenched by incubating with 100 μL of quenching buffer (washing buffer with 1% H2O2 and 0.1% sodium azide) at room temperature for 20 min. Then, the cells were rinsed twice with 200 μL of washing buffer for 5 min. The wells were blocked with 100 μL of antibody blocking buffer (5% non-fat milk in washing buffer) at room temperature for 1 h, rinsed twice with washing buffer for 5 min, and incubated with either rabbit anti-p-ERK (Cat# 9101S, Cell Signaling, Massachusetts, USA) monoclonal antibody (190 ng/mL) or rabbit anti-ERK (Cat# 9102, Cell Signaling, Massachusetts, USA) monoclonal antibody (38 ng/mL) in parallel experiments at 4 °C overnight. The cells were rinsed three times with washing buffer for 5 min and then incubated with a goat anti-rabbit secondary antibody coupled with peroxidase (5 ng/mL) in washing buffer at room temperature for 1 h with shaking. The cells were rinsed three times with washing buffer for 5 min and twice with PBS for 5 min. SuperSignal West Femto reagent (50 μL) was added to each well on ice. The plate was warmed to room temperature, and chemiluminescence was detected using a luminescence plate reader (FLUOstar OPTIMA, BMG Labtechnologies, Offenburg, Germany). The protein concentration was determined by staining cells with 100 μL of crystal violet solution. The cells were washed three times with PBS for 5 min and incubated with 1% SDS for 1 h. The 600 nm absorbance of crystal violet staining was measured.
Western blotting
p-ERK, p-CREB, ERK, and CREB were detected by Western blotting. CHO-OX2R cells (500,000) were seeded in each well of a 6-well plate overnight (about 90% confluence) and serum-starved with assay medium for 48 h. The cells were treated with different concentrations of OBDL (diluted in PBS) for various time periods. After treatment, the cells were rinsed once with PBS, and then 100 μL of lysis buffer (25 mM Tris–HCl, pH 7.6, 150 mM NaCl, 1% NP-40, 1% sodium deoxycholate, 0.1% SDS, protease inhibitor cocktail (Cat# S8820, Sigma-Aldrich, Missouri, USA), 1 mM sodium orthovanadate, 1 mM dithiothreitol) was added to each well. The cell lysate was collected and incubated on ice for 15–30 min. The cell lysates were centrifuged at 25,000g and 4 °C for 15 min. The supernatant was transferred into a new tube and stored at −20 °C for protein SDS-PAGE. Lysate protein (~5 μg) was loaded into each well of a 10% SDS-PAGE gel, and the gel was run at 150 V for about 1 h or until the phenol blue dye ran out of the gel. The gel transfer was performed at 100 V for about 1 h. The nitrocellulose membrane was rinsed once with 0.1% Triton X-100-PBS and blocked with 5% non-fat milk in 0.1% Triton X-100-PBS. Primary antibody was diluted with 5% non-fat milk in 0.1% Triton X-100-PBS and incubated with the membrane at 4 °C overnight with shaking. The membrane was rinsed three times with 0.1% Triton X-100-PBS for 10 min, and the secondary antibody coupled with peroxidase was diluted 1:2500 in PBS (Pierce Biotechnology, Illinois USA) and incubated with membrane with shaking. The membrane was rinsed three times with 0.1% Triton X-100-PBS for 10 min. Chemiluminescence substrate (2 mL SuperSignal West Femto) was applied to the membrane and incubated at room temperature for 1–2 min. Finally, the membrane was scanned using a ChemiDoc-It imaging system (UVP, California, USA).
Results
Over-expression of OX2R in CHO-OX2R cells
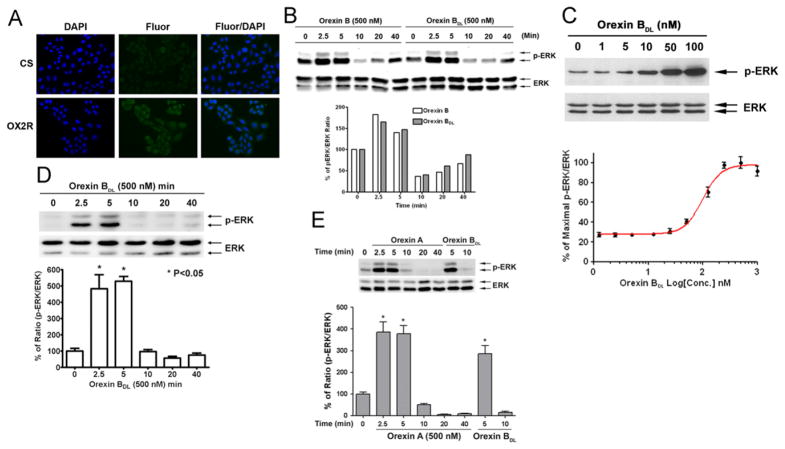
To confirm the over-expression of OX2R, we performed immunofluorescence staining for OX2R in CHO-OX2R cells using an antibody that recognizes the C-terminus of OX2R and DAPI counterstaining of the nuclei. Permeabilization by methanol fixation was used to expose the C-terminus of OX2R to the primary antibody. Compared to the goat serum control staining, the anti-OX2R fluorescence was much stronger and was localized to the cytoplasm. All of the OX2R over-expressing cells were positive for OX2R staining (Fig. 1A).
Fig. 1.
ERK phosphorylation induced by OBDL in OX2R-CHO cells. (A) Immunofluorescence staining of OX2R in CHO-OX2R cells. OX2R in methanol fixed CHO cells was stained with goat anti-OX2R antibody (SC-8074 Santa Cruz Biotech) and DNA was counterstained with DAPI. (B) Comparison of ERK phosphorylation induced by orexin B and OBDL. Time courses of ERK phosphorylation induced by 500 nM of orexin B and OBDL were used to compare the function difference of orexin B and OBDL. (C) Orexin B dose-dependent phosphorylation of ERK by Western blot and FACE assay. ERK phosphorylation was detected by anti-p-ERK and –ERK antibodies (9101 and 9102, Cell Signaling) at 1:1000 dilution. ERK phosphorylation at each orexin B concentration in FACE is the mean value of four repeat measurements. (D) Time course of ERK phosphorylation induced by orexin B. OBDL (500 nM) was used to treat cells for different times. p-ERK was normalized by total detected ERK. (E) Time course of ERK phosphorylation induced by orexin A. 500 nM of orexin A was used to treat CHO-OX2R cells for up to 40min. p-ERK was normalized by total ERK. The mean and P-value were calculated from three independent repeated experiments. Significance of p-value is less than 0.05.
Comparison of ERK phosphorylation induced by orexin B and modified orexin B
To confirm that ERK phosphorylation had a similar pattern as orexin B and modified orexin B (OBDL)-induced OX2R activation, OX2R-CHO cells were treated with 500 nM of either orexin B or OBDL for different time. p-ERK and ERK levels were determined by Western Blot. The band densities of p-ERK and ERK were converted into the ratio of p-ERK/ERK. p-ERK/ERK ratios increased from 2.5 to 5 min and declined after 10 min in both orexin B and OBDL treatments (Fig. 1B). A linear regression between orexin B- and OBDL- induced ERK phosphorylation time courses was run to determine the correlation of ERK phosphorylation induced by orexin B and OBDL. The r2 value was 0.9651, and the P-value was 0.0005. The slope of the linear regression (0.8327) revealed that orexin B induced ERK phosphorylation slightly more efficiently than OBDL. Thus, OBDL has the same function in OX2R activation and ERK phosphorylation as orexin B.
OBDL-induced ERK phosphorylation
To address whether orexin B induces ERK phosphorylation, CHO-OX2R cells were treated with different concentrations of OBDL for 5 min, and Western blotting was performed to quantify p-ERK levels. The level of p-ERK increased with 10 nM OBDL treatment and continued to increase linearly with OBDL concentration up to 100 nM (Fig. 1C). Total ERK remained unchanged with increasing OBDL concentrations. Also, fast-activation cell-based ELISA (FACE) assays were used to determine the dose response curve of ERK phosphorylation. CHO-OX2R cells were treated with different concentrations of OBDL (0–1000 nM) for 5 min. OBDL induced maximal ERK phosphorylation at the concentration of 250 nM, and the EC50 of OBDL was 98 nM (Fig. 1C). To determine how long ERK phosphorylation was maintained after OBDL treatment, cells were treated with 500 nM of OBDL from 2.5 to 40 min. ERK phosphorylation increased starting at 2.5 min, reached its maximal level at 5 min, and declined to the basal level at 10 min (Fig. 1D). Thus, OBDL induced transient ERK phosphorylation.
Orexin A induced ERK phosphorylation
Orexin A, as orexin B agonist, also binds on OX2R to activate G-protein [44]. To test whether orexin A induces ERK phosphorylation CHO-OX2R cells were treated with 500 nM of orexin A for different times (2.5 to 40 min). p-ERK and ERK were determined by Western blot. Relative p-ERK level (ratio of p-ERK/ERK) was induced by orexin A at 2.5 and 5 min then declined to basal level after 10 min like orexin B treatment (Fig. 1E). This result might reveal that orexin A played the same role as orexin B in OX2R activation mediated ERK phosphorylation.
OBDL-induced CREB phosphorylation
CREB is a transcription factor found in the central nervous system (CNS) [45,46]. To determine whether OBDL could induce CREB phosphorylation, CHO-OX2R cells were treated with different concentrations of OBDL (0–500 nM) for 5 min. OBDL-induced CREB phosphorylation was significantly increased at concentrations of 100 nM and 500 nM (Fig. 2A). Unlike ERK phosphorylation, lower concentrations of OBDL (10 and 50 nM) did not induce CREB phosphorylation. To determine the timing of CREB phosphorylation, OX2R cells were treated with 500 nM of OBDL from 2.5 to 40 min. CREB phosphorylation doubled at 2.5 min and maintained a high level at 40 min while total CREB remained constant (Fig. 2B).
Fig. 2.
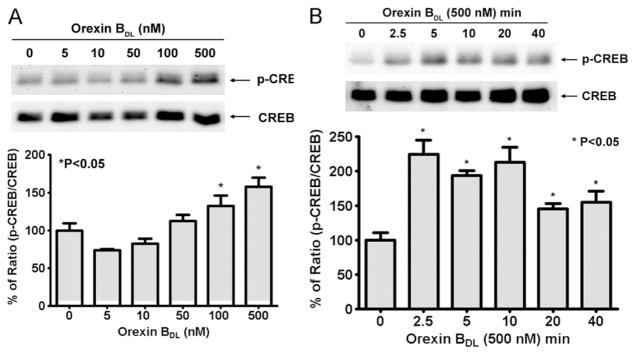
CREB phosphorylation induced by orexin B in OX2R-CHO cells. The level of p-CREB and total CREB were determined by Western blot with anti-p-CREB and CREB antibodies (9198 and 9197, Cell Signaling). The percentage of ratio p-CREB/CREB was used for the generation of an OBDL dose curve and time course response. (A) OBDL dose-dependent phosphorylation of CREB. The different concentrations of OBDL were used to treat OX2R cells for 5 min. The level of CREB phosphorylation was normalized by total CREB. (B) Time course of CREB phosphorylation induced by 500 nM OBDL. OX2R cells were stimulated by 500 nM OBDL for different times as indicated in the figure. The mean of p-CREB/CREB was calculated from three independent experiments and P-value was calculated by comparing with non-OBDL treatment. Significance of p-value is less than 0.05.
CREB phosphorylation is independent of the MAP kinase pathway
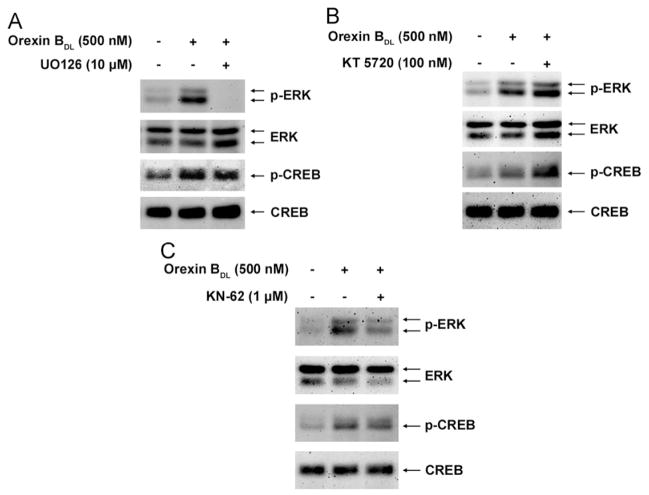
In earlier results, OBDL-induced ERK phosphorylation was transient but OBDL-induced CREB phosphorylation was extended. Since CREB phosphorylation is inhibited by MAP kinase inhibitors [47,48], we hypothesized that OBDL-induced CREB phosphorylation was mediated by MAP kinase. To suppress MAP kinase activity, 10 μM of UO126 (a MAP kinase inhibitor) or DMSO (a vehicle control) were used to treat the cells for 1 h. The cells were then stimulated with OBDL for 5 min. Levels of p-ERK and p-CREB were determined by Western blot and normalized by total ERK and CREB. ERK phosphorylation was completely abolished in cells treated with UO126, while CREB phosphorylation in treated cells remained unchanged (Fig. 3A). Thus, ERK and CREB are parallel downstream targets of orexin B-induced signal transduction but do not share MAP kinase as an upstream mediator.
Fig. 3.
Effects of inhibitors of MAP kinase, PKA, and CaM kinase on phosphorylation of ERK and CREB by Western blot. (A) UO126 (10 μM, U-400, Alamone Labs), a MAP kinase inhibitor, was used to treat the cells for 1 h before cells were treated with 500 nM OBDL for 5 min. (B) CaM kinase inhibitor (1 μM, 1277, Tocris), KN-62, was used to treat the cells for 1 h before cells were treated with 500 nM of OBDL for 5 min. (C) PKA inhibitor (0.1 μM, 1288, Tocris), KT 5720, was used to treat the cells for 1 h before cells were treated with 500 nM of OBDL for 5 min. Antibodies used in detecting p-ERK, ERK, p-CREB and CREB were used as previous experiments.
PKA and CaMK inhibitors do not inhibit CREB phosphorylation
PKA was reported to be an upstream kinase of CREB phosphorylation [49], and CaMKIV is involved in CREB phosphorylation [50]. To investigate the effects of PKA and CaMK on CREB phosphorylation, specific inhibitors of PKA and CaMKs (KT 5720 and KN-62, Toris, Missouri, USA) were used. After 1 h of inhibitor treatment, the CHO-OX2R cells were stimulated with 500 nM of OBDL for 5 min. CREB and ERK phosphorylation was determined by Western blotting with antibodies against p-CREB and p-ERK. The result showed that PKA and CaMK inhibitors did not suppress the phosphorylation of CREB or ERK (Fig. 3B and C). Therefore, PKA and CaMK appear not to be the upstream kinases.
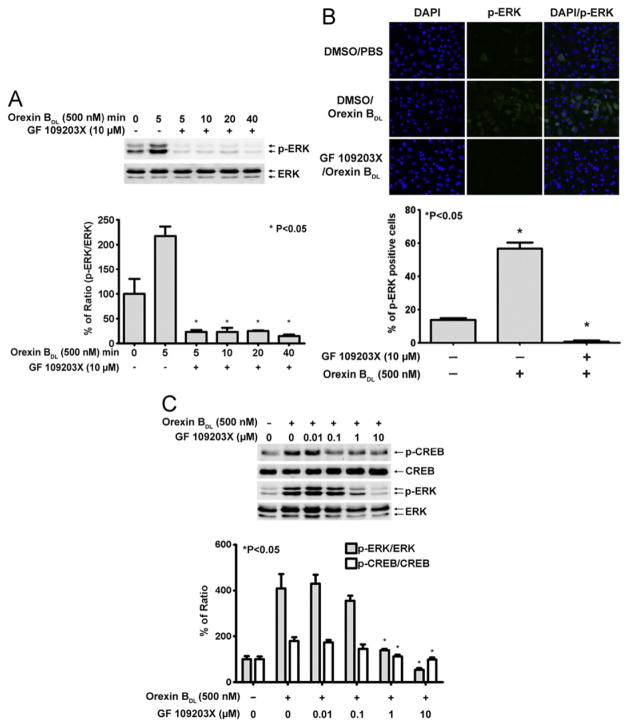
Phosphorylation of CREB and ERK is dependent on PKC in CHO cell line
To assess the effect of PKC on OX2R activation-induced ERK and CREB phosphorylation, cells were treated with 10 μM of the PKC inhibitor GF 109203X for 1 h followed by OBDL treatment for 5 to 40 min. As expected, OBDL stimulation in DMSO-treated control cells resulted in a 2.5-fold increase in relative p-ERK levels. In contrast, p-ERK levels in the PKC inhibitor-treated cells decreased below basal levels (Fig. 4A). Immunofluorescence staining also was used to observed ERK phosphorylation. p-ERK positive and negative cells were counted from 10 views, and 15% of cells were p-ERK positive without OBDL treatment. OBDL treatment (500 nM) resulted in transient ERK phosphorylation as indicated by 55% p-ERK positive cells. The transient ERK phosphorylation was attenuated by the PKC inhibitor, which also suppressed the basal level of ERK phosphorylation (Fig. 4B).
Fig. 4.
Inhibition of p-ERK and p-CREB by PKC inhibitor GF 109203X. (A) Inhibition of ERK phosphorylation. GF 109203X (10 μM), a PKC inhibitor, was used to treat the cells for 1 h before cells were stimulated with 500 nM OBDL for up to 40 min. ERK phosphorylation was determined by Western blotting and normalized by total ERK (9101 and 9102, Cell Signaling). Three independent experiments were used for calculating the mean, and a p-value was generated by comparing non-inhibitor treated and inhibitor treated samples using a t-test. Significance of p-value is less than 0.05. (B) Immunofluorescence staining of ERK phosphorylation. ERK phosphorylation was detected using the immunofluorescence staining protocol described in Methods. The cells were treated as shown in the figure. The fluorescence positive cells in 10 views of each treatment were counted. The different groups of cells indicated in the figure were compared using a t-test. Significance of p-value is less than 0.05. (C) PKC inhibitor completely inhibited orexin B-induced ERK and CREB phosphorylation. Different concentrations of GF 109203X, a PKC inhibitor, were used to treat the cells for 1 h before the 5 min OBDL treatment. Three independent experiments were used to calculate the mean value of pERK/ERK or pCREB/CREB. A p-value was generated by comparing PKC inhibitor treatment and non-PKC inhibitor treatment using a t-test. Significance of p-value is less than 0.05.
Orexin B-induced CREB phosphorylation has been reported but the upstream kinase has not been identified. PKC, a downstream molecule in GPCR signaling, has not been well-linked to CREB phosphorylation. GF 109203X is a broad-spectrum PKC inhibitor that inhibits different isoforms of PKC, MLCK, PKG, and PKA. Since the IC50 of GF 109203X was determined by calcium influx, the IC50 for ERK phosphorylation in living cells may vary. A broad range of concentrations (0.01, 0.1, 1, and 10 μM) of GF 109203X were used to treat cells for 1 h followed by 500 nM OBDL stimulation for 5 min. Inhibition of ERK and CREB phosphorylation was significant at 1 μM GF 109203F indicating that most conventional and novel isoforms of PKCs can be inhibited. Furthermore, since 0.01 μM of GF 109203F did not significantly eliminate CREB phosphorylation induced by OBDL, PKCα is not excluded as a putative upstream kinase. Thus, PKC conventional and novel isoforms α, β1, δ, and ε remain upstream candidate kinases in orexin B-induced ERK and CREB phosphorylations (Fig. 4C).
Orexin B induced ERK phosphorylation is prolonged and independent on PKC in hypothalamus neuron cell line CLU172
Due to functional difference of CHO-OX2R and neuron cells, neuron cells may have different orexin receptor and G-protein from CHO-OX2R cells. To investigate orexin B induced signal transduction in neuron cells mouse adult hypothalamus neuron cell line CLU172 was used to observe OBDL induced ERK phosphorylation. ERK phosphorylation was dramatically increased after 2.5 min treatment of OBDL like CHO-OX2R cells. The ERK phosphorylation induced by OBDL in CLU172 cells was significantly sustained up to 20 min (Fig. 5A) unlike CHO-OX2R cells. Since protein kinase C was major upstream kinase of ERK and CREB phosphorylation in CHO-OX2R cells, whether PKC is upstream kinase of ERK phosphorylation in CLU172 cells was observed with the PKC inhibitor GF109203X. The result showed that up to 5 μM of GF109203X could not significantly inhibit ERK phosphorylation in CLU172 cells unlike CHO-OX2R cells. 10 μM of GF 109203 partially inhibited ERK phosphorylation in CLU172 cells (Fig. 5B).
Fig. 5.
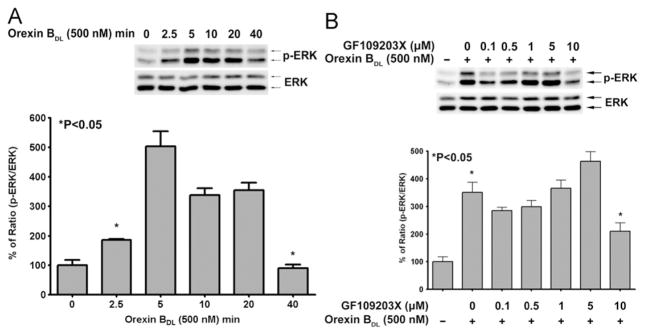
Orexin B induced ERK phosphorylation in adult mouse hypothalamus neuron cells and the inhibition of ERK phosphorylation by PKC inhibitor GF 109203X. (A) ERK phosphorylation was induced by 500 nM of OBDL in CLU172 cells for up to 40 min. p-ERK and ERK were detected by the antibodies (9101 and 9102, Cell Signaling). The mean of p-ERK/ERK (ERK phosphorylation) and p-value were calculated from three independent experiments by comparing with non OBDL treatment using a t-test. Significance of p-value is less than 0.05. (B) The inhibition of pretreatment with various doses of PKC inhibitor GF109203X on OBDL induced ERK phosphorylation in CLU172 cells. GF109203X was treated for 1 h before 5 min treatment of OBDL. The mean of ERK phosphorylation (p-ERK/ERK) was calculated from three independent experiment results. The different groups of cells indicated in the figure were compared using a t-test. Significance of p-value is less than 0.05.
Discussion
OX2R activation may induce multiple changes in gene expression. Major findings in this study include the short time course of ERK phosphorylation and prolonged CREB phosphorylation in CHO-OX2R cells. Additionally, PKC was identified as an upstream kinase of ERK and CREB phosphorylation in OX2R signal transduction in CHO-OX2R cells. Unlike most studies, performed in CHO cells, on orexin-induced ERK phosphorylation that last 60 min, we observed that this phosphorylation was sustained for less than 10 min in CHO-OX2R cells. Our novel finding was that OX2R activation induced CREB phosphorylation. The OBDL-induced dose response and time course of CREB phosphorylation were tested in the CHO-OX2R cell line. In contrast to ERK phosphorylation, a much higher concentration of OBDL was required to induce CREB phosphorylation. The CREB phosphorylation induced by OX2R activation persisted up to 40 min, which was very different from the time course of ERK phosphorylation. Inhibition of PKC, but not PKA, CaMK, or MAPK, suppressed OX2R-induced CREB phosphorylation. Using mouse adult hypothalamus neuron cells (CLU172) we found that OBDL induced ERK phosphorylatioin was extended to 20 min and the phosphorylation was not affected by protein kinase C inhibitor GF 109203X.
ERK phosphorylation induced by both OX2R and OX1R activation has been investigated in several studies [24,40,41,51]. Early studies also reported that ERK phosphorylation induced by orexin A or B was sustained up to 60 min [24,40]. Our results show that ERK phosphorylation induced by OX2R activation was transient and lasted for less than 10 min in CHO-OX2R cells. This discrepancy could be resulted from the difference in host cell line or ligand–receptor interaction. However, in a study using a related cell line (CHO cells overexpressing OX1R), ERK phosphorylation was seen for up to 60 min [24]. Therefore, the effect of cell line on the time period of ERK phosphorylation can be excluded. Another possibility for the transient ERK phosphorylation found in our study could be the modified orexin B, OBDL, which is more specific for OX2R activation than endogenous orexin B [52]. Comparing ERK phosphorylation time courses induced by orexin B and OBDL, both types of orexin B function very similarly (Fig. 1B). Meanwhile, we also found that the neuron cells (CLU172) had a prolonged ERK phosphorylation followed orexin B treatment. Therefore, orexin B induced ERK phosphorylation varies upon cell types.
MAP kinase pathway signaling plays an important role in the CNS [53], a rat model of depression, and human depression subjects [25,54–56]. Using isolated neuronal cells, Ammoun et al. reported that ERK phosphorylation induced by OX1R activation may be protective from cell death [39]. Our previous study also found that levels of ERK and p-ERK were reduced in the frontal cortex in a rat model of depression [26]. Our current results on OX2R-induced ERK phosphorylation imply that transient ERK phosphorylation is a potential downstream signal transduction target and orexin B induced ERK phosphorylation was sustained for longer period in neuron cells, which further supports hypotheses that orexin may play a beneficial role in depression.
CREB, a cAMP-activated transcription factor, has been studied for its important role in neurogenesis and anti-depressive function [57,58]. CREB phosphorylation, induced by orexin A, in neurons from brain tissue has been reported [51]. Since orexin A binds both OX1R and OX2R, orexin A-induced CREB phosphorylation could occur via either type of orexin receptors. In this study, CREB phosphorylation was systematically investigated in CHO-OX2R cells using modified orexin B. OX2R-mediated CREB phosphorylation lasted up to 40 min. In addition, OX2R-mediated CREB phosphorylation required a higher concentration of orexin B than was required to induce ERK phosphorylation. As a transcription factor, CREB regulates many gene expression processes, such as brain-derived neurotrophic factor (BDNF) [59], corticotropin-release factor (CRF) [60], and several K+ channels [61], which all regulate mood, stress, and anxiety. The level of CREB was reduced in brain tissue from human patients with depression [62–65]. CREB was upregulated by chronic antidepressant treatment, and increased CREB levels in rodent models resulted in antidepressant-like behaviors [30]. Taking our results, along with the evidence of decreased CREB expression in tissues from human major depression patients, OX2R activation-induced CREB phosphorylation seems to be beneficial for depression treatment.
Orexin induced signal transduction involves phospholipase C (PLC) [66,67], PKC [67–69], and PKA [38,70,71]. Orexin induces Gq activation, and then Gq activates PLC. PLC, via PKC, leads to ERK phosphorylation [40]. In our study, inhibition of PKC, but not PKA or CaMK, suppressed OX2R-induced ERK and CREB phosphorylation in CHO-OX2R cells. Furthermore, MAP kinase inhibitors did not suppress CREB phosphorylation, which suggests that MAP kinase is not upstream of CREB phosphorylation. Thus, CREB phosphorylation is mediated by kinases other than MAPK, but both ERK and CREB use PKC as an upstream kinase in CHO-OX2R cells. In adult mouse hypothalamus neuron cells, we observed only high concentration of PKC inhibitor (10 μM) can partially inhibit ERK phosphorylation induced by orexin B. This result requires further experiments by using different kinase inhibitors to identify the upstream kinases of orexin B induced ERK phosphorylation in neuron cells.
PKC is an upstream kinase of ERK phosphorylation in orexin A- and B-stimulated cells [40,41]. Using different concentrations of the PKC inhibitor, GF 109203X, we found that CREB and ERK phosphorylation was significantly suppressed by 1 μM but not 0.01 μM GF 109203X in CHO-OX2R cells. Earlier studies showed that GF 109203X had similar IC50 values for the conventional isoforms of PKC tested in a cell-free system [72]. Since novel isoforms of PKC are also inhibited by GF 109203X [73], both conventional and novel isoforms of PKC may still be the upstream kinases of ERK and CREB phosphorylation in CHO-OX2R cells. Since the neuron cells used in our experiment was not sensitive to PKC inhibitor GF 109203X in orexin B induced ERK phosphorylation, other PKC inhibitors will be used in a further study.
Conclusion
In conclusion, OX2R activation induces brief ERK phosphorylation and relatively long-lasting CREB phosphorylation in CHO-OX2R cells. PKC isoforms β1, δ, and ε are candidate upstream kinases in CHO-OX2R cells. Neuron cells had a long lasting ERK phosphorylation. The finding that ERK and CREB are downregulated in depression models and human major depression patients highlights the potential importance of ERK and CREB phosphorylation. Our results provide evidence that orexin B, a wakefulness regulator, may also benefit depression therapy by activating ERK and CREB phosphorylation.
Acknowledgments
This work was supported by the VA Merit Award and the Medical Service of the Louis Stokes Cleveland VA Medical Center.
References
- 1.de Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, Fukuhara C, Battenberg EL, Gautvik VT, Bartlett FS, 2nd, Frankel WN, van den Pol AN, Bloom FE, Gautvik KM, Sutcliffe JG. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci USA. 1998;95:322–327. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Selbach O, Haas HL. Hypocretins: the timing of sleep and waking. Chronobiol Int. 2006;23:63–70. doi: 10.1080/07420520500545961. [DOI] [PubMed] [Google Scholar]
- 3.Bonnavion P, de Lecea L. Hypocretins in the control of sleep and wakefulness. Curr Neurol Neurosci Rep. 2010;10:174–179. doi: 10.1007/s11910-010-0101-y. [DOI] [PubMed] [Google Scholar]
- 4.Nunez A, Rodrigo-Angulo ML, Andres ID, Garzon M. Hypocretin/ Orexin neuropeptides: participation in the control of sleep-wakefulness cycle and energy homeostasis. Curr Neuropharmacol. 2009;7:50–59. doi: 10.2174/157015909787602797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Willie JT, Chemelli RM, Sinton CM, Yanagisawa M. To eat or to sleep? Orexin in the regulation of feeding and wakefulness. Annu Rev Neurosci. 2001;24:429–458. doi: 10.1146/annurev.neuro.24.1.429. [DOI] [PubMed] [Google Scholar]
- 6.Tsuneki H, Wada T, Sasaoka T. Role of orexin in the regulation of glucose homeostasis. Acta Physiol (Oxf) 2010;198:335–348. doi: 10.1111/j.1748-1716.2009.02008.x. [DOI] [PubMed] [Google Scholar]
- 7.Tsujino N, Sakurai T. Orexin/hypocretin: a neuropeptide at the interface of sleep, energy homeostasis, and reward system. Pharmacol Rev. 2009;61:162–176. doi: 10.1124/pr.109.001321. [DOI] [PubMed] [Google Scholar]
- 8.Kondo H, Kanbayashi T, Shimizu T. Orexin nervous system and narcolepsy. Nihon Naika Gakkai Zasshi. 2006;95:748–755. doi: 10.2169/naika.95.748. [DOI] [PubMed] [Google Scholar]
- 9.Wurtman RJ. Narcolepsy and the hypocretins. Metabolism. 2006;55:S36–39. doi: 10.1016/j.metabol.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 10.Berezinska M, Zawilska JB. Hypocretins: involvement in the regulation of sleep-wakefulness cycle and pathogenesis of narcolepsy. Postepy Hig Med Dosw (Online) 2007;61:1–12. [PubMed] [Google Scholar]
- 11.Ritchie C, Okuro M, Kanbayashi T, Nishino S. Hypocretin ligand deficiency in narcolepsy: recent basic and clinical insights. Curr Neurol Neurosci Rep. 2010;10:180–189. doi: 10.1007/s11910-010-0100-z. [DOI] [PubMed] [Google Scholar]
- 12.Feng P, Vurbic D, Wu Z, Hu Y, Strohl KP. Changes in brain orexin levels in a rat model of depression induced by neonatal administration of clomipramine. J Psychopharmacol. 2008;22:784–791. doi: 10.1177/0269881106082899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Allard JS, Tizabi Y, Shaffery JP, Trouth CO, Manaye K. Stereological analysis of the hypothalamic hypocretin/orexin neurons in an animal model of depression. Neuropeptides. 2004;38:311–315. doi: 10.1016/j.npep.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 14.Scott MM, Marcus JN, Pettersen A, Birnbaum SG, Mochizuki T, Scammell TE, Nestler EJ, Elmquist JK, Lutter M. Hcrtr1 and 2 signaling differentially regulates depression-like behaviors. Behav Brain Res. 2011;222:289–294. doi: 10.1016/j.bbr.2011.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salomon RM, Ripley B, Kennedy JS, Johnson B, Schmidt D, Zeitzer JM, Nishino S, Mignot E. Diurnal variation of cerebrospinal fluid hypocretin-1 (Orexin-A) levels in control and depressed subjects. Biol Psychiatry. 2003;54:96–104. doi: 10.1016/s0006-3223(02)01740-7. [DOI] [PubMed] [Google Scholar]
- 16.Brundin L, Bjorkqvist M, Petersen A, Traskman-Bendz L. Reduced orexin levels in the cerebrospinal fluid of suicidal patients with major depressive disorder. Eur Neuropsychopharmacol. 2007;17:573–579. doi: 10.1016/j.euroneuro.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 17.Ito N, Yabe T, Gamo Y, Nagai T, Oikawa T, Yamada H, Hanawa T. I.c.v. administration of orexin-A induces an antidepressive- like effect through hippocampal cell proliferation. Neuroscience. 2008;157:720–732. doi: 10.1016/j.neuroscience.2008.09.042. [DOI] [PubMed] [Google Scholar]
- 18.Giedke H, Schwarzler F. Therapeutic use of sleep deprivation in depression. Sleep Med Rev. 2002;6:361–377. [PubMed] [Google Scholar]
- 19.Wu J, Buchsbaum MS, Gillin JC, Tang C, Cadwell S, Wiegand M, Najafi A, Klein E, Hazen K, Bunney WE, Jr, Fallon JH, Keator D. Prediction of antidepressant effects of sleep deprivation by metabolic rates in the ventral anterior cingulate and medial prefrontal cortex. Am J Psychiatry. 1999;156:1149–1158. doi: 10.1176/ajp.156.8.1149. [DOI] [PubMed] [Google Scholar]
- 20.Vogel GW. A review of REM sleep deprivation. Arch Gen Psychiatry. 1975;32:749–761. doi: 10.1001/archpsyc.1975.01760240077006. [DOI] [PubMed] [Google Scholar]
- 21.Berger M, Vollmann J, Hohagen F, Konig A, Lohner H, Voderholzer U, Riemann D. Sleep deprivation combined with consecutive sleep phase advance as a fast-acting therapy in depression: an open pilot trial in medicated and unmedicated patients. Am J Psychiatry. 1997;154:870–872. doi: 10.1176/ajp.154.6.870. [DOI] [PubMed] [Google Scholar]
- 22.Demet EM, Chicz-Demet A, Fallon JH, Sokolski KN. Sleep deprivation therapy in depressive illness and Parkinson’s disease. Prog Neuropsychopharmacol Biol Psychiatry. 1999;23:753–784. doi: 10.1016/s0278-5846(99)00039-1. [DOI] [PubMed] [Google Scholar]
- 23.Adrien J. Neurobiological bases for the relation between sleep and depression. Sleep Med Rev. 2002;6:341–351. [PubMed] [Google Scholar]
- 24.Ammoun S, Johansson L, Ekholm ME, Holmqvist T, Danis AS, Korhonen L, Sergeeva OA, Haas HL, Akerman KE, Kukkonen JP. OX1 orexin receptors activate extracellular signal-regulated kinase in Chinese hamster ovary cells via multiple mechanisms: the role of Ca2+ influx in OX1 receptor signaling. Mol Endocrinol. 2006;20:80–99. doi: 10.1210/me.2004-0389. [DOI] [PubMed] [Google Scholar]
- 25.Dwivedi Y, Rizavi HS, Roberts RC, Conley RC, Tamminga CA, Pandey GN. Reduced activation and expression of ERK1/2 MAP kinase in the post-mortem brain of depressed suicide subjects. J Neurochem. 2001;77:916–928. doi: 10.1046/j.1471-4159.2001.00300.x. [DOI] [PubMed] [Google Scholar]
- 26.Feng P, Guan Z, Yang X, Fang J. Impairments of ERK signal transduction in the brain in a rat model of depression induced by neonatal exposure of clomipramine. Brain Res. 2003;991:195–205. doi: 10.1016/j.brainres.2003.08.018. [DOI] [PubMed] [Google Scholar]
- 27.Dowlatshahi D, MacQueen GM, Wang JF, Reiach JS, Young LTG. Protein-coupled cyclic AMP signaling in postmortem brain of subjects with mood disorders: effects of diagnosis, suicide, and treatment at the time of death. J Neurochem. 1999;73:1121–1126. doi: 10.1046/j.1471-4159.1999.0731121.x. [DOI] [PubMed] [Google Scholar]
- 28.Yamada S, Yamamoto M, Ozawa H, Riederer P, Saito T. Reduced phosphorylation of cyclic AMP-responsive element binding protein in the postmortem orbitofrontal cortex of patients with major depressive disorder. J Neural Transm. 2003;110:671–680. doi: 10.1007/s00702-002-0810-8. [DOI] [PubMed] [Google Scholar]
- 29.Koch JM, Hinze-Selch D, Stingele K, Huchzermeier C, Goder R, Seeck-Hirschner M, Aldenhoff JB. Changes in CREB phosphorylation and BDNF plasma levels during psychotherapy of depression. Psychother Psychosom. 2009;78:187–192. doi: 10.1159/000209350. [DOI] [PubMed] [Google Scholar]
- 30.Blendy JA. The role of CREB in depression and antidepressant treatment. Biol Psychiatry. 2006;59:1144–1150. doi: 10.1016/j.biopsych.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 31.Qi X, Lin W, Li J, Li H, Wang W, Wang D, Sun M. Fluoxetine increases the activity of the ERK-CREB signal system and alleviates the depressive-like behavior in rats exposed to chronic forced swim stress. Neurobiol Dis. 2008;31:278–285. doi: 10.1016/j.nbd.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 32.Smart D, Jerman JC, Brough SJ, Rushton SL, Murdock PR, Jewitt F, Elshourbagy NA, Ellis CE, Middlemiss DN, Brown F. Characterization of recombinant human orexin receptor pharmacology in a Chinese hamster ovary cell-line using FLIPR. Br J Pharmacol. 1999;128:1–3. doi: 10.1038/sj.bjp.0702780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Darker JG, Porter RA, Eggleston DS, Smart D, Brough SJ, Sabido-David C, Jerman JC. Structure-activity analysis of truncated orexin-A analogues at the orexin-1 receptor. Bioorg Med Chem Lett. 2001;11:737–740. doi: 10.1016/s0960-894x(01)00043-9. [DOI] [PubMed] [Google Scholar]
- 34.Harris DM, Go VL, Reeve JR, Jr, Wu SV. Stimulation of amylase release by Orexin is mediated by Orexin 2 receptor in AR42J cells. Pancreas. 2002;25:405–410. doi: 10.1097/00006676-200211000-00014. [DOI] [PubMed] [Google Scholar]
- 35.Johansson L, Ekholm ME, Kukkonen JP. Regulation of OX1 orexin/hypocretin receptor-coupling to phospholipase C by Ca2+ influx. Br J Pharmacol. 2007;150:97–104. doi: 10.1038/sj.bjp.0706959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nasman J, Bart G, Larsson K, Louhivuori L, Peltonen H, Akerman KE. The orexin OX1 receptor regulates Ca2+ entry via diacylglycerol-activated channels in differentiated neuroblastoma cells. J Neurosci. 2006;26:10658–10666. doi: 10.1523/JNEUROSCI.2609-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nanmoku T, Isobe K, Sakurai T, Yamanaka A, Takekoshi K, Kawakami Y, Ishii K, Goto K, Nakai T. Orexins suppress catecholamine synthesis and secretion in cultured PC12 cells. Biochem Biophys Res Commun. 2000;274:310–315. doi: 10.1006/bbrc.2000.3137. [DOI] [PubMed] [Google Scholar]
- 38.Malendowicz LK, Tortorella C, Nussdorfer GG. Orexins stimulate corticosterone secretion of rat adrenocortical cells, through the activation of the adenylate cyclase-dependent signaling cascade. J Steroid Biochem Mol Biol. 1999;70:185–188. doi: 10.1016/s0960-0760(99)00110-7. [DOI] [PubMed] [Google Scholar]
- 39.Ammoun S, Lindholm D, Wootz H, Akerman KE, Kukkonen JP. G-protein-coupled OX1 orexin/hcrtr-1 hypocretin receptors induce caspase-dependent and -independent cell death through p38 mitogen-/stress-activated protein kinase. J Biol Chem. 2006;281:834–842. doi: 10.1074/jbc.M508603200. [DOI] [PubMed] [Google Scholar]
- 40.Tang J, Chen J, Ramanjaneya M, Punn A, Conner AC, Randeva HS. The signalling profile of recombinant human orexin-2 receptor. Cell Signal. 2008;20:1651–1661. doi: 10.1016/j.cellsig.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 41.Wenzel J, Grabinski N, Knopp CA, Dendorfer A, Ramanjaneya M, Randeva HS, Ehrhart-Bornstein M, Dominiak P, Johren O. Hypocretin/orexin increases the expression of steroidogenic enzymes in human adrenocortical NCI H295R cells. Am J Physiol Regul Integr Comp Physiol. 2009;297:R1601–1609. doi: 10.1152/ajpregu.91034.2008. [DOI] [PubMed] [Google Scholar]
- 42.Hitomi M, Yang K, Guo Y, Fretthold J, Harwalkar J, Stacey DW. p27Kip1 and cyclin dependent kinase 2 regulate passage through the restriction point. Cell Cycle. 2006;5:2281–2289. doi: 10.4161/cc.5.19.3318. [DOI] [PubMed] [Google Scholar]
- 43.Shoemaker JL, Ruckle MB, Mayeux PR, Prather PL. Agonist-directed trafficking of response by endocannabinoids acting at CB2 receptors. J Pharmacol Exp Ther. 2005;315:828–838. doi: 10.1124/jpet.105.089474. [DOI] [PubMed] [Google Scholar]
- 44.Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richardson JA, Kozlowski GP, Wilson S, Arch JR, Buckingham RE, Haynes AC, Carr SA, Annan RS, McNulty DE, Liu WS, Terrett JA, Elshourbagy NA, Bergsma DJ, Yanagisawa M. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–585. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- 45.Sato-Bigbee C, Chan EL, Yu RK. Oligodendroglial cyclic AMP response element-binding protein: a member of the CREB family of transcription factors. J Neurosci Res. 1994;38:621–628. doi: 10.1002/jnr.490380604. [DOI] [PubMed] [Google Scholar]
- 46.Dash PK, Moore AN. Characterization and phosphorylation of CREB-like proteins in Aplysia central nervous system. Brain Res Mol Brain Res. 1996;39:43–51. doi: 10.1016/0169-328x(95)00350-2. [DOI] [PubMed] [Google Scholar]
- 47.Hisaoka K, Maeda N, Tsuchioka M, Takebayashi M. Antidepressants induce acute CREB phosphorylation and CRE-mediated gene expression in glial cells: a possible contribution to GDNF production. Brain Res. 2008;1196:53–58. doi: 10.1016/j.brainres.2007.12.019. [DOI] [PubMed] [Google Scholar]
- 48.Rosethorne EM, Nahorski SR, Challiss RA. Regulation of cyclic AMP response-element binding-protein (CREB) by Gq/11-protein- coupled receptors in human SH-SY5Y neuroblastoma cells. Biochem Pharmacol. 2008;75:942–955. doi: 10.1016/j.bcp.2007.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shaywitz AJ, Greenberg ME. CREB: a stimulus-induced transcription factor activated by a diverse array of extracellular signals. Annu Rev Biochem. 1999;68:821–861. doi: 10.1146/annurev.biochem.68.1.821. [DOI] [PubMed] [Google Scholar]
- 50.Deisseroth K, Bito H, Tsien RW. Signaling from synapse to nucleus: postsynaptic CREB phosphorylation during multiple forms of hippocampal synaptic plasticity. Neuron. 1996;16:89–101. doi: 10.1016/s0896-6273(00)80026-4. [DOI] [PubMed] [Google Scholar]
- 51.Shin HS, Cho HS, Sung KW, Yoon BJ. Orexin-A increases cell surface expression of AMPA receptors in the striatum. Biochem Biophys Res Commun. 2009;378:409–413. doi: 10.1016/j.bbrc.2008.11.051. [DOI] [PubMed] [Google Scholar]
- 52.Asahi S, Egashira S, Matsuda M, Iwaasa H, Kanatani A, Ohkubo M, Ihara M, Morishima H. Development of an orexin-2 receptor selective agonist, [Ala(11), D-Leu(15)]orexin-B. Bioorg Med Chem Lett. 2003;13:111–113. doi: 10.1016/s0960-894x(02)00851-x. [DOI] [PubMed] [Google Scholar]
- 53.Cruz CD, Cruz F. The ERK 1 and 2 pathway in the nervous system: from basic aspects to possible clinical applications in pain and visceral dysfunction. Curr Neuropharmacol. 2007;5:244–252. doi: 10.2174/157015907782793630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Qi X, Lin W, Li J, Pan Y, Wang W. The depressive-like behaviors are correlated with decreased phosphorylation of mitogen-activated protein kinases in rat brain following chronic forced swim stress. Behav Brain Res. 2006;175:233–240. doi: 10.1016/j.bbr.2006.08.035. [DOI] [PubMed] [Google Scholar]
- 55.Dwivedi Y, Rizavi HS, Zhang H, Roberts RC, Conley RR, Pandey GN. Aberrant extracellular signal-regulated kinase (ERK)1/2 signalling in suicide brain: role of ERK kinase 1 (MEK1) Int J Neuropsychopharmacol. 2009;12:1337–1354. doi: 10.1017/S1461145709990575. [DOI] [PubMed] [Google Scholar]
- 56.Gourley SL, Wu FJ, Kiraly DD, Ploski JE, Kedves AT, Duman RS, Taylor JR. Regionally specific regulation of ERK MAP kinase in a model of antidepressant-sensitive chronic depression. Biol Psychiatry. 2008;63:353–359. doi: 10.1016/j.biopsych.2007.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gass P, Riva MA. CREB, neurogenesis and depression. Bioessays. 2007;29:957–961. doi: 10.1002/bies.20658. [DOI] [PubMed] [Google Scholar]
- 58.Vaidya VA, Duman RS. Depresssion–emerging insights from neurobiology. Br Med Bull. 2001;57:61–79. doi: 10.1093/bmb/57.1.61. [DOI] [PubMed] [Google Scholar]
- 59.Nair SG, Golden SA, Shaham Y. Differential effects of the hypocretin 1 receptor antagonist SB 334867 on high-fat food self-administration and reinstatement of food seeking in rats. Br J Pharmacol. 2008;154:406–416. doi: 10.1038/bjp.2008.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kageyama K, Akimoto K, Suda T. Corticotrophin-releasing factor gene transcription is directly activated after deprivation of glucocorticoids in hypothalamic cells. J Neuroendocrinol. 2010;22:971–978. doi: 10.1111/j.1365-2826.2010.02048.x. [DOI] [PubMed] [Google Scholar]
- 61.Wallace DL, Han MH, Graham DL, Green TA, Vialou V, Iniguez SD, Cao JL, Kirk A, Chakravarty S, Kumar A, Krishnan V, Neve RL, Cooper DC, Bolanos CA, Barrot M, McClung CA, Nestler EJ. CREB regulation of nucleus accumbens excitability mediates social isolation-induced behavioral deficits. Nat Neurosci. 2009;12:200–209. doi: 10.1038/nn.2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pandey GN, Dwivedi Y, Ren X, Rizavi HS, Roberts RC, Conley RR. Cyclic AMP response element-binding protein in postmortem brain of teenage suicide victims: specific decrease in the prefrontal cortex but not the hippocampus. Int J Neuropsychopharmacol. 2007;10:621–629. doi: 10.1017/S1461145706007231. [DOI] [PubMed] [Google Scholar]
- 63.Ciafaloni E, Mignot E, Sansone V, Hilbert JE, Lin L, Lin X, Liu LC, Pigeon WR, Perlis ML, Thornton CA. The hypocretin neurotransmission system in myotonic dystrophy type 1. Neurology. 2008;70:226–230. doi: 10.1212/01.wnl.0000296827.20167.98. [DOI] [PubMed] [Google Scholar]
- 64.Yamada M, Tanabe K, Wada K, Shimoke K, Ishikawa Y, Ikeuchi T, Koizumi S, Hatanaka H. Differences in survival-promoting effects and intracellular signaling properties of BDNF and IGF-1 in cultured cerebral cortical neurons. J Neurochem. 2001;78:940–951. doi: 10.1046/j.1471-4159.2001.00497.x. [DOI] [PubMed] [Google Scholar]
- 65.Dwivedi Y, Rao JS, Rizavi HS, Kotowski J, Conley RR, Roberts RC, Tamminga CA, Pandey GN. Abnormal expression and functional characteristics of cyclic adenosine monophosphate response element binding protein in postmortem brain of suicide subjects. Arch Gen Psychiatry. 2003;60:273–282. doi: 10.1001/archpsyc.60.3.273. [DOI] [PubMed] [Google Scholar]
- 66.Hill SM, Frasch T, Xiang S, Yuan L, Duplessis T, Mao L. Molecular mechanisms of melatonin anticancer effects. Integr Cancer Ther. 2009;8:337–346. doi: 10.1177/1534735409353332. [DOI] [PubMed] [Google Scholar]
- 67.Uramura K, Funahashi H, Muroya S, Shioda S, Takigawa M, Yada T. Orexin-a activates phospholipase C- and protein kinase C-mediated Ca2+ signaling in dopamine neurons of the ventral tegmental area. Neuroreport. 2001;12:1885–1889. doi: 10.1097/00001756-200107030-00024. [DOI] [PubMed] [Google Scholar]
- 68.Ozcan M, Ayar A, Serhatlioglu I, Alcin E, Sahin Z, Kelestimur H. Orexins activates protein kinase C-mediated Ca(2+) signaling in isolated rat primary sensory neurons. Physiol Res. 2010;59:255–262. doi: 10.33549/physiolres.931739. [DOI] [PubMed] [Google Scholar]
- 69.Xu R, Roh SG, Gong C, Hernandez M, Ueta Y, Chen C. Orexin-B augments voltage-gated L-type Ca(2+) current via protein kinase C-mediated signalling pathway in ovine somatotropes. Neuroendocrinology. 2003;77:141–152. doi: 10.1159/000069507. [DOI] [PubMed] [Google Scholar]
- 70.Ziolkowska A, Spinazzi R, Albertin G, Nowak M, Malendowicz LK, Tortorella C, Nussdorfer GG. Orexins stimulate glucocorticoid secretion from cultured rat and human adrenocortical cells, exclusively acting via the OX1 receptor. J Steroid Biochem Mol Biol. 2005;96:423–429. doi: 10.1016/j.jsbmb.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 71.Nakamura Y, Miura S, Yoshida T, Kim J, Sasaki K. Cytosolic calcium elevation induced by orexin/hypocretin in granule cell domain cells of the rat cochlear nucleus in vitro. Peptides. 2010;31:1579–1588. doi: 10.1016/j.peptides.2010.04.029. [DOI] [PubMed] [Google Scholar]
- 72.Toullec D, Pianetti P, Coste H, Bellevergue P, Grand-Perret T, Ajakane M, Baudet V, Boissin P, Boursier E, Loriolle F, Duhamelll L, Charon D, Kirilovsky J. The bisindolylmaleimide GF 109203X is a potent and selective inhibitor of protein kinase C. J Biol Chem. 1991;266:15771–15781. [PubMed] [Google Scholar]
- 73.Le Panse R, Coulomb B, Mitev V, Bouchard B, Lebreton C, Dubertret L. Differential modulation of human fibroblast and keratinocyte growth by the protein kinase C inhibitor GF 109203X. Mol Pharmacol. 1994;46:445–451. [PubMed] [Google Scholar]