Abstract
Depression is associated with a deficiency of serotonergic neurons that have been found to suppress orexinergic neurons, which in turn activate these neurons in a feedback loop. This evidence suggests that orexins may be involved in the pathology of depression. Long Evans rats were treated with clomipramine (CLI) and saline (SAL) from postnatal days 8 through 21. One set of rats from both groups was sacrificed at 35 days of age for quantification of orexins in multiple brain regions. At 3–4 months of age a second set of rats was tested for immobility in a forced swim procedure, a common test for depressive signs in rats, and a third set was sacrificed for the quantification of orexins. Compared with the control rats, adult rats with neonatal CLI treatment had (1) increased forced swim immobility and (2) increased orexins A and B in the hypothalamus. However, both orexins A and B levels were decreased in multiple brain regions in the juvenile CLI rats compared with same-age controls. We concluded that although orexin levels were decreased in juvenile CLI rats, adult CLI rats with features of depression had significantly higher levels of hypothalamic orexins compared with adult controls. These results imply that orexins are likely to be involved in the pathological regulation of depression.
Keywords: clomipramine, depression, orexin, hypocretin, rat
Introduction
Clinical investigation has found that depression in humans is characterized by a set of behavioral and neurophysiological features. These include disturbances of normal wake–sleep patterns, loss of interest in pleasurable activities and alterations of 5-HT neurotransmission. Impairment of the 5-HTergic system is implied by several findings, including that depressed suicides have increased 5-HT1a receptors and decreased 5-HT transporters (Arango et al., 1990; Hrdina et al., 1993; Arango et al., 1995; Mann et al., 2000; Pandey et al., 2002; Boldrini et al., 2005). Anti-depressants such as selective 5-HT reuptake inhibitors and monoamine oxidase inhibitors target 5-HTergic alterations by enhancing the effect of this neurotransmitter (Wilson and Argyropoulos, 2005).
Recent studies have found that orexins, wake-promoting peptides synthesized in neurons of the perifornical region and lateral hypothalamus, activate 5-HT neurons and are associated with stress regulation (Winsky-Sommerer et al., 2004). We have recently found that the orexinergic system is altered in a prospective animal model of insomnia and stress-related behaviors (Feng et al., 2007). Orexins, including orexin A and B isolated from the hypothalamus (de Lecea et al., 1998; Sakurai et al., 1998), were originally studied as part of the hypothalamic network of energy homeostasis and were found to promote wakefulness and suppress sleep (Trivedi et al., 1998; Espana et al., 2001). Several studies have uncovered links between orexin and 5-HT. For example, both orexin A and B induce dose-dependent inward currents in most 5-HT neurons. At higher concentrations, orexins also increase spontaneous postsynaptic currents in 5-HT neurons. Orexins excite 5-HT neurons directly via TTX-insensitive, Na+/K+ nonselective cation currents (Brown et al., 2002; Liu et al., 2002). These facts suggest that the deficiency of 5-HT implicated in depression might be associated with a deficiency of orexinergic neurons and that animal models of depression might have altered orexin levels in the brain. However, studies of orexin in depression have been limited to CSF quantification in humans (Salomon et al., 2003) and immunohistochemical and morphological methods in rats (Allard et al., 2004). Thus, a better quantitative study of orexinergic alterations in animal models of depression is needed.
Several animal models have been established to better understand the neural mechanisms of depression (Nestler et al., 2002). An ideal model for this purpose would have well-defined and long-lasting behavioral and molecular features of unipolar depression. The model made by neonatal administration of clomipramine (CLI), a commonly prescribed anti-depressant known to inhibit reuptake of multiple aminergic neurotransmitters including 5-HT, norepinephrine and dopamine, has features that best fulfill the aforementioned requirement. This model (called the CLI rat) has been proposed as a preclinical model of endogenous depression (now called major depressive disorders with melancholic features) (Vogel and Vogel, 1982; Vogel et al., 1990b). The CLI rat exhibits diminished sexual activity (Mirmiran et al., 1981; Mirmiran et al., 1983; Neill et al., 1990; Velazquez-Moctezuma et al., 1993; Bonilla-Jaime et al., 1998; Feng et al., 2001), decreased pleasure-seeking behavior measured by intracerebral self-stimulation, (ICSS) (Vogel et al., 1990c), increased alcohol intake (Hilakivi et al., 1984; Hilakivi et al., 1988; Dwyer and Rosenwasser, 1998; Brower, 2003) and increased immobility in the forced swim test (Hilakivi et al., 1988; Velazquez-Moctezuma and Diaz Ruiz, 1992), which is commonly used by pharmaceutical companies to assess the efficacy of anti-depressants. Sleep disturbances are also a feature of the CLI rat. These include increased percentage of rapid eye movement (REM) sleep in total sleep (Mirmiran et al., 1981; Vogel et al., 1990a) and decreased REM latency (Mirmiran et al., 1981; Mirmiran et al., 1983; Vogel et al., 1990a). Such changes are consistent with sleep disturbances found in human depression, most notably increased REM sleep propensity (Salin-Pascual, 2002; Brower, 2003; Riemann and Voderholzer, 2003; Fava, 2004). The CLI rat also has decreased levels of 5-HT in the frontal cortex, hypothalamus, hippocampus and brain stem (Feenstra et al., 1996; Vijayakumar and Meti, 1999), reduced 5-HT dorsal raphé nucleus (DRN) neuronal firing rates (Kinney et al., 1997) and hyporeaction of 5-HT neurons to 5-HT reuptake blocker (Maudhuit et al., 1996). Additionally, this model also shows a reduction of ERK phosphorylation in the frontal cortex and hippocampus (Feng et al., 2003), which is comparable to findings from human suicide victims (Dwivedi et al., 2001).
In the following study, we tested our hypothesis by quantifying orexin levels in the CLI model during both the juvenile period and the expression of the depressive phenotype in adulthood.
Materials and methods
Experimental design
Male Long Evans rats were used in this study. All procedures were approved by the Institutional Animal Care and Use Committee (IACUC) of Case Western Reserve University and the Louis Stokes Cleveland VA Medical Center. Neonatal rats were treated with either CLI or saline (SAL) to produce the rat model of depression and its control.
Neonatal treatment
Eighty-three male rat pups were acquired at the age of two days with companion mothers from Harlan Sprague Dawley, Inc (Indianapolis, IN). Pups were cross-fostered on the following day and assigned to either the CLI or SAL group. Consistent with our previous publication (Feng et al., 2003), rat pups in the CLI group were subcutaneously injected with CLI (20 mg/kg, twice daily) and those in the SAL group were similarly treated with equivolume saline. The treatment was conducted from postnatal day 8 through 21. During the treatment period, rat pups were separated from their mothers only while receiving injections. Rats were weaned at 24 days of age and thereafter group-housed in our animal facility under standard conditions.
Forced swim test
Eleven SAL and 14 CLI rats were tested for forced swimming behavior at four months of age using the method of West and Weiss (1998). A transparent plastic tank of height 71 cm and outside perimeter of 152 cm was filled halfway with 25°C water. Immediately prior to each test, a small piece of jumbo bubble wrap was secured to the back of the rat with a fabric hook-and-loop fastener strapped around the trunk of the body. A single rat was randomly selected and placed into the tank for 30 minutes, during which an observer scored its swimming behavior. Immobility was judged when the rat made only slight movements of its forelimbs required to keep its nose above the surface. A stopwatch was activated at the start of each immobile period and stopped during mobile periods. Coordinated movements of hindlimbs and forelimbs were considered indicators of mobility. At the conclusion of the test, the rat was removed from the tank and returned to its home cage and the cumulative immobile time was recorded. Each rat was identified only after completing the swim test. All tests were conducted between 9:00 am and 12:00 noon. All rats were subjected to the swim test twice over the course of two days.
Brain tissue collection and peptide extraction
In order to avoid possible effects from the behavioral test, rats that had undergone swim testing were not used in the molecular quantification. Eighteen SAL and 18 CLI rats were sacrificed at the age of 35 days, and 12 SAL and 10 CLI rats were sacrificed at the age of three to four months. Rats were sacrificed by decapitation between 9:00 and12:00 am. Brain samples were dissected from the frontal cortex, parietal cortex, hippocampus, hypothalamus, thalamus, midbrain, pons and medulla, according to definitions described in our previous publications (Feng et al., 2007). The tissues were weighed and processed in two different ways. One set of samples collected from juvenile SAL (n = 7) and CLI (n = 7) rats were processed according to an early commercial protocol (Phoenix Pharmaceuticals, Inc., Belmont, CA, USA). These samples were acidified with 1% trifluoroacetic acid (TFA) and loaded onto a C18 Sep-Column (Waters Corp., Milford, MA, USA). Peptides were eluted with 1% TFA/40% acetonitrile. The eluants were then dried and resuspended in radioimmunoassay (RIA) buffer before assay. This set of samples was used to quantify orexin B. The rest of the samples were processed according to a modified protocol from the same company. 0.5 Mol acetic acid with a volume equal to 10 times the tissue weight was added to each tube. The microtubes were then moved to a boiling water bath for 10 minutes. After removing the tissue blocks, the microtubes were centrifuged for 30 seconds at 5500 rpm. The remaining supernatants (containing total peptides) were air-dried under a hood at 60°C and subsequently stored at −80°C for future use.
Radioimmunoassay (RIA)
Both orexins A and B in juvenile rats and orexin B in adult rats were measured by RIA. RIA kits for detecting orexins A (#RK-003-30) and orexin B (#RK-003-32) were obtained from Phoenix Pharmaceuticals, Inc., and the standard protocol provided with the kits was followed. Orexins were detected by 125I-peptide. After completion of each assay, the radioactivity of each tube was determined by a gamma counter, Cobra II Auto-Gamma (Packard Instrument Company, Downers Grove, IL, USA). Sample values were compared to a standard curve and interpolated using GraphPad Prism software (San Diego, CA). Orexin levels were calculated as the value of pg/mg wet tissue.
Enzyme-linked immunoassay measurement of orexin A
Adult brain levels of orexin A were quantified by enzyme-linked immunoassay (ELISA) with a standard kit (#EK-003-30) obtained from Phoenix Pharmaceuticals, Inc. The choice of ELISA was intended to minimize the usage of radioactive materials and made because both ELISA and RIA methods used to quantify orexin have been evaluated by others (Lin et al., 2002). After following the standard protocol provided with the kit, optical densities of the 96-well microplates were read by an assay reader, FLUOstar (BMG LabTech, Germany). Peptide values were calculated as the value of pg/mg wet tissue.
Data analysis and Statistics
Two-way (region X treatment for brain levels of peptides and test X treatment for immobile time) ANOVAs were used for all statistical evaluations. In order to minimize the probability that the large variation in peptide levels between brain regions would mask differences due to treatment, brain regions that were found to have extremely low or high levels were analysed apart from the other regions. However, large variations remained between regions. To avoid overuse of t-tests, post hoc analyses were included with each ANOVA. All pairwise multiple comparison procedures (Bonferroni t-test) were used for further analysis of test-specific and region-specific differences. Data are presented as mean ± standard error.
Results
Immobility measured in the forced swimming test
The forced swim test has been recognized as an effective method for screening anti-depressants and, thus, a reasonable method for evaluating depressive behavior in animal studies (Nestler et al., 2002). Eleven SAL and 14 CLI rats were each tested twice for immobility. The mean immobile times were 15.93 ± 0.98 versus 19.97 ± 0.63 min for SAL and CLI in test 1, and 15.72 ± 1.18 versus 20.31 ± 0.75 min in test 2 (Figure 1). Compared with the mean immobile times of the SAL group, the CLI group had an increase of 25.34% in the first test and 29.18% in the second test. A two-way ANOVA confirmed a statistically significant difference (F = 24.375, P < 0.001). All pairwise multiple comparison procedures (Bonferroni t-test) found that differences were significant in both tests 1 (t= 3.268, P = 0.002) and test 2 (t = 3.714, P < 0.001). These results are consistent with literature, which reports that neonatal treatment with CLI produces depressive behaviors in adulthood (Vogel et al., 1990b; Velazquez-Moctezuma and Diaz Ruiz, 1992).
Figure 1.

Mean immobile time calculated during a 30-minute forced swim test. The CLI group had a significantly longer immobile time than the control (SAL) group in both the first and the second test. **P = 0.002, ***P < 0.001.
Orexin levels in juvenile CLI and SAL rats
To determine whether changes of orexin levels occurred prior to the emergence of depressive pathology in adulthood, orexins were quantified in juvenile (35 days old) SAL and CLI rats. Both orexins A and B were quantified by RIA because this method is more sensitive than ELISA and is better able to detect the lower levels of peptides observed in juvenile rats.
Orexin A levels were found to be considerably higher in the hypothalamus and the regions within the brain stem, including the medulla, pons and midbrain. A two-way ANOVA found that the differences between treatment groups (SAL n = 10 and CLI n = 11) approached significance in these regions (P = 0.083). However, pairwise multiple comparison procedures (Bonferroni t-test) found significant region-specific differences between groups (t = 3.039, P = 0.004). Specifically, the mean level of orexin A in the CLI group was significantly lower than the SAL group in the pons (t= 2.914, P = 0.005) and hypothalamus (t = 3.348, P = 0.001) (Figure 2a). Orexin A levels in the other measured regions were generally much lower. Of the four cortical areas (frontal cortex, parietal cortex, temporal cortex and occipital cortex), the level of orexin A in the CLI group was significantly lower in the frontal cortex (t = 2.344, P = 0.021) (Figure 2b). Thus, the juvenile CLI rats had significantly lower levels of orexin A in the pons, hypothalamus and frontal cortex than the SAL rats.
Figure 2.
Brain levels of orexin A in juvenile rats (35 days old) neonatally treated with either CLI or SAL. In the pons and hypothalamus, orexin A was significantly decreased in the CLI group (a). Among the cortical areas, thalamus and hippocampus, orexin A levels in the CLI group were significantly decreased only in the frontal cortex (b). **P < 0.005; ***P < 0.001.
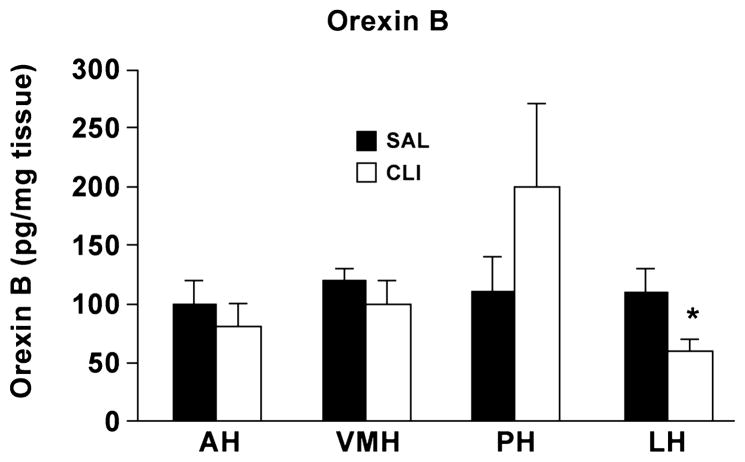
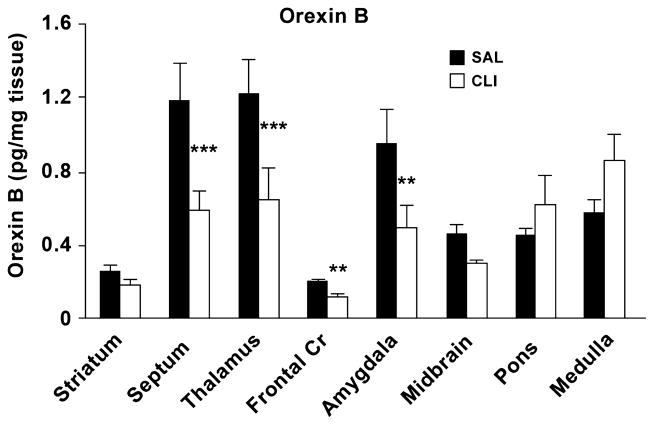
To determine orexin B levels in juvenile SAL and CLI rats, the hypothalamus was further dissected into the anterior hypothalamic (AH) area; ventromedial hypothalamic (VMH) nucleus; posterior hypothalamic (PH) area and lateral hypothalamic (LH) area. The levels of orexin B in the CLI group were lower in the PH and LH areas, but significantly so only in the LH area (P < 0.05), compared with the SAL group (Figure 3). We then evaluated eight other brain regions including the medulla, pons, midbrain, thalamus, septum, striatum and amygdala and separately evaluated the frontal cortex with a t-test. The two-way ANOVA found a statically significant difference between the eight regions (Treatment x Region, F = 3.921, P < 0.001). Pairwise multiple comparison procedures (Bonferroni t-test) found that orexin B levels in the CLI group were significantly lower than the SAL group in the septum (t = 3.672, P < 0.001), thalamus (t = 3.553, P < 0.001) and amygdala (t =2.640, P= 0.01). However, considerable mean differences in the medulla were not significantly different (t = 1.731, P = 0.087) (Figure 4). Evaluation of frontal cortex showed that the CLI group had a significant reduction of orexin B compared with the SAL group (t = 1.796, P = 0.004).
Figure 3.
Hypothalamic orexin B levels in juvenile rats (35 days old) neonatally treated with either CLI or SAL. AH, anterior hypothalamic area; VMH, ventromedial hypothalamic nucleus; PH, posterior hypothalamic area; LH: lateral hypothalamic area. *p < 0.05.
Figure 4.
Brain levels of orexin B in juvenile rats (35 days old) neonatally treated with either CLI or saline (SAL). Orexin B was significantly decreased in multiple brain regions including the frontal cortex, septum, thalamus and amygdala. **p ≤ 0.01; ***p < 0.001.
Orexin levels in adult CLI and SAL rats
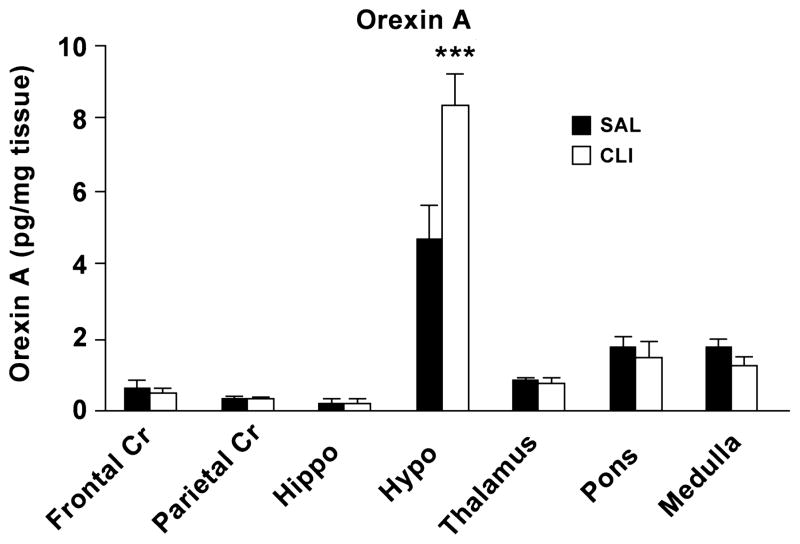
Orexin A levels in adult SAL (n = 10) and CLI (n = 8) rats were measured by ELISA. Overall, hypothalamic levels of orexin A were considerably higher than all other measured regions in both groups. A two-way ANOVA found that the overall difference in the mean values between treatments was not significant. However, there was a statistically significant interaction between region and treatment (F = 4.245, P < 0.001), which may be a result of differences between the hypothalamus and the other brain regions. All pairwise multiple comparison procedures (Bonferroni t-test) found that the difference between treatments within hypothalamus was significant (t = 5.179, P < 0.001). The mean level of hypothalamic orexin A was 75.32% higher in the CLI group (8.32 ± 1.52 pg/mg) than the SAL group (4.74 ± 0.84 pg/mg) (Figure 5).
Figure 5.
Brain levels of orexin A in adult rats neonatally treated with CLI or SAL. Orexin A was significantly increased in the hypothalamus but not other measured brain regions. ***P < 0.001.
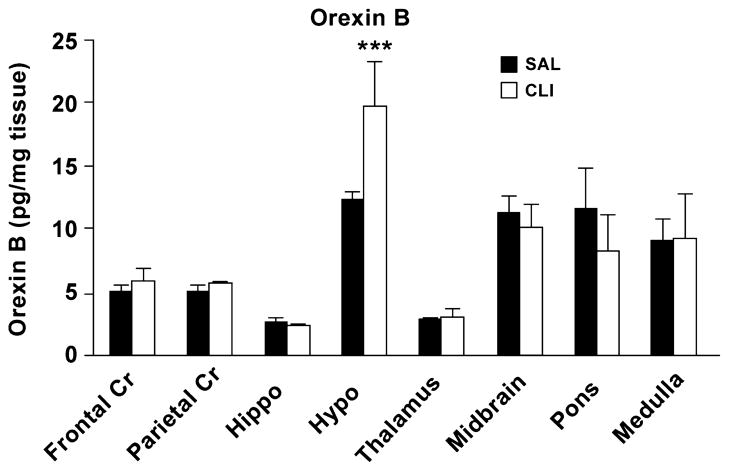
Orexin B levels in adult SAL (n = 12) and CLI (n = 10) rats were quantified using RIA because no ELISA kit for the measurement of orexin B was available. A two-way (Treatment X region) ANOVA found that the overall difference in the mean values between treatments was significant (F = 2.005, P = 0.042). Pairwise multiple comparison procedures (Bonferroni t-test) found that the difference between treatments within the hypothalamus was significant (t = 4.073, P < 0.001). That is, the mean level of orexin B in the hypothalamus was significantly higher in the CLI group (19.77 ± 3.56 pg/mg) than the SAL group (12.30 ± 0.82 pg/mg) (Figure 6). Orexin B was increased by 60.79% compared with that of the SAL group.
Figure 6.
Brain levels of orexin B in adult rats neonatally treated with CLI and SAL rats. Hypothalamic levels of orexin B was significantly increased in the CLI rat compared with the SAL rat. *** p < 0.001.
Discussion
Consistent with previous findings from studies utilizing this model, the CLI group had significantly longer mean immobile times than the SAL group (Vogel et al., 1990b). This result confirms that we were able to produce animals that expressed depressive phenotypes.
The major finding of this study was that administration of the anti-depressant CLI during the neonatal period had paradoxical effects on orexins at 35 days of age and adulthood. The juvenile CLI rats were found to have significantly lower brain levels of both orexins A and B, whereas the adults did not. In fact, adult levels of orexins were actually increased in the CLI rats. This effect was found at three to four months of age, which corresponds chronologically to the time that depressive phenotypes are often found in humans. This is the first time that brain levels of orexins are reported in any model of depression. These data suggest that alterations of the orexinergic system may play a role in the development of depression.
Orexin alterations in juvenile rats
An important finding is that both orexins A and B were significantly decreased in juvenile CLI rats, but this pattern was reversed in the adults. In acute studies, orexins have been reduced by monoaminergic neurotransmitters, such as those produced by 5-HTergic (Muraki et al., 2004), dopaminergic and noradrenergic neurons (Li and van den Pol, 2005), and increased by forced motor (swim) activity (Martins et al., 2004), fasting and insulin-induced hypoglycemia (Lopez et al., 2000; Cai et al., 2001; Karteris et al., 2005) and/or CRH (Winsky-Sommerer et al., 2004). Motor activity is a strong activator of orexinergic neurons (Torterolo et al., 2003). These facts suggest that alterations of brain orexins at the age of 35 days were likely extensions of an acute effect, even though orexin measurement occurred 14 days after the last injection. This possibility is further supported by recent results obtained in our laboratory which show that three doses of CLI administered over 24 hours induced a significant reduction of orexin B in the hypothalamus (in preparation).
Adult changes in orexins
Another major finding in this study is that both orexins A and B levels in the hypothalamus were increased in the adult CLI rat. These results do not support our hypothesis that orexin levels are decreased in depression, but partially match the findings of increased hypothalamic levels of orexin A in a rat model of chronic stress (Feng et al., 2007). These surprising results may be explained by the orexinergic–monoaminergic feedback loop, which appears to be a closed circuit. Orexins have been found to excite monoaminergic neurons (Brown et al., 2002; Liu et al., 2002), whereas monoamines suppress orexinergic neurons (Muraki et al., 2004; Li and van den Pol, 2005). Consistent with the findings from human studies, monoamines are suppressed in the CLI model. These rats have been found to have reduced brain levels of monoamines in the frontal cortex, hypothalamus, hippocampus and brainstem (Feenstra et al., 1996; Vijayakumar and Meti, 1999), hyporeaction of 5-HT neurons to 5-HT reuptake blocker (Maudhuit et al., 1996) and decreased 5-HT DRN firing rate (Kinney et al., 1997). It is plausible that the increased brain levels of orexins may be a result of disinhibition from defective aminergic neurons.
Interestingly, the increased brain levels of orexins A and B in adult CLI rats are compatible with many of the sleep features associated with depression, particularly chronic insomnia (Riemann et al., 2002; Tsuno et al., 2005; Becker, 2006; Lam, 2006) because orexins promote wakefulness and suppress sleep (Espana et al., 2001). Depressed patients have also been found to benefit from sleep deprivation even though they regularly experience sleep disturbances (Vogel et al., 1968; Vogel, 1975). Additionally, most anti-depressants up-regulate the 5-HT system and deprive sleep. These facts are noteworthy in light of the known effects of orexins on wake–sleep regulation and the 5-HT system and support a possible role for orexin in adult depression, and the sleep disturbances that often accompany the disorder. The results are also surprising, however, as they show significant increases of orexins, whereas previous studies with this model have found only longer mean and maximum duration of wakefulness without dramatic reduction of total sleep (Vogel et al., 1990a). These seemingly controversial data may lead to a new understanding of how orexins work together.
An important question raised by these findings is specifically how the reduced orexin levels during the juvenile period were upturned to produce depressive symptoms in adulthood. Unfortunately, current evidence is insufficient to adequately explain this mechanism. Several possibilities exist, including that increases in adulthood represent a compensatory process in response to early decreases. However, the data indirectly suggest that blockage of the orexin-5-HT feedback loop may play a role in this patho-development. We postulate that the early reduction of orexins leads to decreased excitation of 5-HTergic neurons, effectively withdrawing feedback inhibition of orexinergic neurons. This hypothesis requires further testing.
Significance of orexin changes in the CLI rat
Our findings of increased brain levels of orexins in the adult CLI rat suggest that orexin changes are likely involved in depression. Furthermore, this involvement may be associated with sleep disturbances commonly found in depression, as these symptoms are consistent with the known effects of orexin. However, such speculation is only preliminary. These results also provide new insights for further investigation of the neurobiological regulation of sleep disturbances in depression, particularly chronic insomnia. Despite the benefits of these findings, however, further study of the involvement of orexin in human depression is greatly needed.
Acknowledgments
We graciously thank Drs Ling Lin and Emmanuel Mignot (Stanford University) for their efforts in the quantification of orexin B levels in juvenile CLI and SAL rats. This work is supported by the NIMH grant MH 069854 and Research of Louis Stokes Cleveland VA Medical.
Footnotes
Conflict of interest statement
None
Contributor Information
P. Feng, Division of Pulmonary, Critical Care and Sleep Medicine, Department of Medicine, Case Western Reserve University, Department of Psychiatry, Case Western Reserve University; Louis Stokes VA Medical Center, Cleveland, OH, USA
D. Vurbic, Division of Pulmonary, Critical Care and Sleep Medicine, Department of Medicine, Case Western Reserve University; Louis Stokes VA Medical Center, Cleveland, OH, USA
Z. Wu, Division of Pulmonary, Critical Care and Sleep Medicine, Department of Medicine, Case Western Reserve University; Louis Stokes VA Medical Center, Cleveland, OH, USA
Y. Hu, Division of Pulmonary, Critical Care and Sleep Medicine, Department of Medicine, Case Western Reserve University; Louis Stokes VA Medical Center, Cleveland, OH, USA
KP. Strohl, Division of Pulmonary, Critical Care and Sleep Medicine, Department of Medicine, Case Western Reserve University; Louis Stokes VA Medical Center, Cleveland, OH, USA
References
- Allard JS, Tizabi Y, Shaffery JP, Trouth CO, Manaye K. Stereological analysis of the hypothalamic hypocretin/orexin neurons in an animal model of depression. Neuropeptides. 2004;38:311–315. doi: 10.1016/j.npep.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Arango V, Underwood MD, Gubbi AV, Mann JJ. Localized alterations in pre- and postsynaptic serotonin binding sites in the ventrolateral prefrontal cortex of suicide victims. Brain Res. 1995;688:121–133. doi: 10.1016/0006-8993(95)00523-s. [DOI] [PubMed] [Google Scholar]
- Arango V, Ernsberger P, Marzuk PM, Chen JS, Tierney H, Stanley M, Reis DJ, Mann JJ. Autoradiographic demonstration of increased serotonin 5-HT2 and beta-adrenergic receptor binding sites in the brain of suicide victims. Arch Gen Psychiatry. 1990;47:1038–1047. doi: 10.1001/archpsyc.1990.01810230054009. [DOI] [PubMed] [Google Scholar]
- Becker PM. Treatment of sleep dysfunction and psychiatric disorders. Curr Treat Options Neurol. 2006;8:367–375. doi: 10.1007/s11940-006-0026-6. [DOI] [PubMed] [Google Scholar]
- Boldrini M, Underwood MD, Mann JJ, Arango V. More tryptophan hydroxylase in the brainstem dorsal raphe nucleus in depressed suicides. Brain Res. 2005;1041:19–28. doi: 10.1016/j.brainres.2005.01.083. [DOI] [PubMed] [Google Scholar]
- Bonilla-Jaime H, Retana-Marquez S, Velazquez-Moctezuma J. Pharmacological features of masculine sexual behavior in an animal model of depression. Pharmacol Biochem Behav. 1998;60:39–45. doi: 10.1016/s0091-3057(97)00484-x. [DOI] [PubMed] [Google Scholar]
- Brower KJ. Insomnia, alcoholism and relapse. Sleep Med Rev. 2003;7:523–539. doi: 10.1016/s1087-0792(03)90005-0. [DOI] [PubMed] [Google Scholar]
- Brown RE, Sergeeva OA, Eriksson KS, Haas HL. Convergent excitation of dorsal raphe serotonin neurons by multiple arousal systems (orexin/hypocretin, histamine and noradrenaline) J Neurosci. 2002;22:8850–8859. doi: 10.1523/JNEUROSCI.22-20-08850.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, Fukuhara C, Battenberg EL, Gautvik VT, Bartlett FS, 2nd, Frankel WN, van den Pol AN, Bloom FE, Gautvik KM, Sutcliffe JG. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci USA. 1998;95:322–327. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwivedi Y, Rizavi HS, Roberts RC, Conley RC, Tamminga CA, Pandey GN. Reduced activation and expression of ERK1/2 MAP kinase in the post-mortem brain of depressed suicide subjects. J Neurochem. 2001;77:916–928. doi: 10.1046/j.1471-4159.2001.00300.x. [DOI] [PubMed] [Google Scholar]
- Dwyer SM, Rosenwasser AM. Neonatal clomipramine treatment, alcohol intake and circadian rhythms in rats. Psychopharmacology (Berl) 1998;138:176–183. doi: 10.1007/s002130050660. [DOI] [PubMed] [Google Scholar]
- Espana RA, Baldo BA, Kelley AE, Berridge CW. Wake-promoting and sleep-suppressing actions of hypocretin (orexin): basal forebrain sites of action. Neuroscience. 2001;106:699–715. doi: 10.1016/s0306-4522(01)00319-0. [DOI] [PubMed] [Google Scholar]
- Fava M. Daytime sleepiness and insomnia as correlates of depression. J Clin Psychiatry. 2004;65(Suppl 16):27–32. [PubMed] [Google Scholar]
- Feenstra MG, van Galen H, Te Riele PJ, Botterblom MH, Mirmiran M. Decreased hypothalamic serotonin levels in adult rats treated neonatally with clomipramine. Pharmacol Biochem Behav. 1996;55:647–652. doi: 10.1016/s0091-3057(96)00276-6. [DOI] [PubMed] [Google Scholar]
- Feng P, Ma Y, Vogel GW. The critical window of brain development from susceptive to insusceptive. Effects of clomipramine neonatal treatment on sexual behavior. Brain Res Dev Brain Res. 2001;129:107–110. doi: 10.1016/s0165-3806(01)00158-4. [DOI] [PubMed] [Google Scholar]
- Feng P, Guan Z, Yang X, Fang J. Impairments of ERK signal transduction in the brain in a rat model of depression induced by neonatal exposure of clomipramine. Brain Res. 2003;991:195–205. doi: 10.1016/j.brainres.2003.08.018. [DOI] [PubMed] [Google Scholar]
- Feng P, Vurbic D, Wu Z, Strohl KP. Brain orexins and wake regulation in rats exposed to maternal deprivation. Brain Res. 2007;1154C:163–172. doi: 10.1016/j.brainres.2007.03.077. [DOI] [PubMed] [Google Scholar]
- Hilakivi LA, Sinclair JD, Hilakivi IT. Effects of neonatal treatment with clomipramine on adult ethanol related behavior in the rat. Brain Res. 1984;317:129–132. doi: 10.1016/0165-3806(84)90148-2. [DOI] [PubMed] [Google Scholar]
- Hilakivi LA, Taira T, Hilakivi I, Loikas P. Neonatal treatment with monoamine uptake inhibitors alters later response in behavioural ‘despair’ test to beta and GABA-B receptor agonists. Pharmacol Toxicol. 1988;63:57–61. doi: 10.1111/j.1600-0773.1988.tb00910.x. [DOI] [PubMed] [Google Scholar]
- Hrdina PD, Demeter E, Vu TB, Sotonyi P, Palkovits M. 5-HT uptake sites and 5-HT2 receptors in brain of antidepressant-free suicide victims/depressives: increase in 5-HT2 sites in cortex and amygdala. Brain Res. 1993;614:37–44. doi: 10.1016/0006-8993(93)91015-k. [DOI] [PubMed] [Google Scholar]
- Kinney GG, Vogel GW, Feng P. Decreased dorsal raphe nucleus neuronal activity in adult chloral hydrate anesthetized rats following neonatal clomipramine treatment: implications for endogenous depression. Brain Res. 1997;756:68–75. doi: 10.1016/s0006-8993(97)00119-4. [DOI] [PubMed] [Google Scholar]
- Lam RW. Sleep disturbances and depression: a challenge for antidepressants. Int Clin Psychopharmacol. 2006;21(Suppl 1):S25–S29. doi: 10.1097/01.yic.0000195658.91524.61. [DOI] [PubMed] [Google Scholar]
- Li Y, van den Pol AN. Direct and indirect inhibition by catecholamines of hypocretin/orexin neurons. J Neurosci. 2005;25:173–183. doi: 10.1523/JNEUROSCI.4015-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L, Wisor J, Shiba T, Taheri S, Yanai K, Wurts S, Lin X, Vitaterna M, Takahashi J, Lovenberg TW, Koehl M, Uhl G, Nishino S, Mignot E. Measurement of hypocretin/orexin content in the mouse brain using an enzyme immunoassay: the effect of circadian time, age and genetic background. Peptides. 2002;23:2203–2211. doi: 10.1016/s0196-9781(02)00251-6. [DOI] [PubMed] [Google Scholar]
- Liu RJ, van den Pol AN, Aghajanian GK. Hypocretins (orexins) regulate serotonin neurons in the dorsal raphe nucleus by excitatory direct and inhibitory indirect actions. J Neurosci. 2002;22:9453–9464. doi: 10.1523/JNEUROSCI.22-21-09453.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann JJ, Huang YY, Underwood MD, Kassir SA, Oppenheim S, Kelly TM, Dwork AJ, Arango V. A serotonin transporter gene promoter polymorphism (5-HTTLPR) and prefrontal cortical binding in major depression and suicide. Arch Gen Psychiatry. 2000;57:729–738. doi: 10.1001/archpsyc.57.8.729. [DOI] [PubMed] [Google Scholar]
- Martins PJ, D’Almeida V, Pedrazzoli M, Lin L, Mignot E, Tufik S. Increased hypocretin-1 (orexin-a) levels in cerebrospinal fluid of rats after short-term forced activity. Regul Pept. 2004;117:155–158. doi: 10.1016/j.regpep.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Maudhuit C, Hamon M, Adrien J. Effects of chronic treatment with zimelidine and REM sleep deprivation on the regulation of raphe neuronal activity in a rat model of depression. Psychopharmacology (Berl) 1996;124:267–274. doi: 10.1007/BF02246667. [DOI] [PubMed] [Google Scholar]
- Mirmiran M, van de Poll NE, Corner MA, van Oyen HG, Bour HL. Suppression of active sleep by chronic treatment with chlorimipramine during early postnatal development: effects upon adult sleep and behavior in the rat. Brain Res. 1981;204:129–146. doi: 10.1016/0006-8993(81)90657-0. [DOI] [PubMed] [Google Scholar]
- Mirmiran M, Scholtens J, van de Poll NE, Uylings HB, van der Gugten J, Boer GJ. Effects of experimental suppression of active (REM) sleep during early development upon adult brain and behavior in the rat. Brain Res. 1983;283:277–286. doi: 10.1016/0165-3806(83)90184-0. [DOI] [PubMed] [Google Scholar]
- Muraki Y, Yamanaka A, Tsujino N, Kilduff TS, Goto K, Sakurai T. Serotonergic regulation of the orexin/hypocretin neurons through the 5-HT1A receptor. J Neurosci. 2004;24:7159–7166. doi: 10.1523/JNEUROSCI.1027-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neill D, Vogel G, Hagler M, Kors D, Hennessey A. Diminished sexual activity in a new animal model of endogenous depression. Neurosci Biobehav Rev. 1990;14:73–76. doi: 10.1016/s0149-7634(05)80162-9. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Gould E, Manji H, Buncan M, Duman RS, Greshenfeld HK, Hen R, Koester S, Lederhendler I, Meaney M, Robbins T, Winsky L, Zalcman S. Preclinical models: status of basic research in depression. Biol Psychiatry. 2002;52:503–528. doi: 10.1016/s0006-3223(02)01405-1. [DOI] [PubMed] [Google Scholar]
- Pandey GN, Dwivedi Y, Rizavi HS, Ren X, Pandey SC, Pesold C, Roberts RC, Conley RR, Tamminga CA. Higher expression of serotonin 5-HT(2A) receptors in the postmortem brains of teenage suicide victims. Am J Psychiatry. 2002;159:419–429. doi: 10.1176/appi.ajp.159.3.419. [DOI] [PubMed] [Google Scholar]
- Riemann D, Voderholzer U. Primary insomnia: a risk factor to develop depression? J Affect Disord. 2003;76:255–259. doi: 10.1016/s0165-0327(02)00072-1. [DOI] [PubMed] [Google Scholar]
- Riemann D, Voderholzer U, Berger M. Sleep and sleep-wake manipulations in bipolar depression. Neuropsychobiology. 2002;45(Suppl 1):7–12. doi: 10.1159/000049255. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richarson JA, Kozlowski GP, Wilson S, Arch JR, Buckingham RE, Haynes AC, Carr SA, Annan RS, McNulty DE, Liu WS, Terrett JA, Elshourbagy NA, Bergsma DJ, Yanagisawa M. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:1–696. doi: 10.1016/s0092-8674(02)09256-5. [DOI] [PubMed] [Google Scholar]
- Salin-Pascual RJ. Relationship between mood improvement and sleep changes with acute nicotine administration in non-smoking major depressed patients. Rev Invest Clin. 2002;54:36–40. [PubMed] [Google Scholar]
- Salomon RM, Ripley B, Kennedy JS, Johnson B, Schmidt D, Zeitzer JM, Nishino S, Mignot E. Diurnal variation of cerebrospinal fluid hypocretin-1 (Orexin-A) levels in control and depressed subjects. Biol Psychiatry. 2003;54:96–104. doi: 10.1016/s0006-3223(02)01740-7. [DOI] [PubMed] [Google Scholar]
- Torterolo P, Yamuy J, Sampogna S, Morales FR, Chase MH. Hypocretinergic neurons are primarily involved in activation of the somatomotor system. Sleep. 2003;26:25–28. [PubMed] [Google Scholar]
- Trivedi P, Yu H, MacNeil DJ, Van der Ploeg LH, Guan XM. Distribution of orexin receptor mRNA in the rat brain. FEBS Lett. 1998;438:71–75. doi: 10.1016/s0014-5793(98)01266-6. [DOI] [PubMed] [Google Scholar]
- Tsuno N, Besset A, Ritchie K. Sleep and depression. J Clin Psychiatry. 2005;66:1254–1269. doi: 10.4088/jcp.v66n1008. [DOI] [PubMed] [Google Scholar]
- Velazquez-Moctezuma J, Diaz Ruiz O. Neonatal treatment with clomipramine increased immobility in the forced swim test: an attribute of animal models of depression. Pharmacol Biochem Behav. 1992;42:737–739. doi: 10.1016/0091-3057(92)90022-8. [DOI] [PubMed] [Google Scholar]
- Velazquez-Moctezuma J, Aguilar-Garcia A, Diaz-Ruiz O. Behavioral effects of neonatal treatment with clomipramine, scopolamine, and idazoxan in male rats. Pharmacol Biochem Behav. 1993;46:215–217. doi: 10.1016/0091-3057(93)90343-r. [DOI] [PubMed] [Google Scholar]
- Vijayakumar M, Meti BL. Alterations in the levels of monoamines in discrete brain regions of clomipramine-induced animal model of endogenous depression. Neurochem Res. 1999;24:345–349. doi: 10.1023/a:1020992314534. [DOI] [PubMed] [Google Scholar]
- Vogel GW. A review of REM sleep deprivation. Arch Gen Psychiatry. 1975;32:749–761. doi: 10.1001/archpsyc.1975.01760240077006. [DOI] [PubMed] [Google Scholar]
- Vogel GW, Vogel FA. A new animal model of human endogenous depression. Sleep Research. 1982;11:222. [Google Scholar]
- Vogel GW, Traub AC, Ben-Horin P, Meyers GM. REM deprivation. II. The effects on depressed patients. Arch Gen Psychiatry. 1968;18:301–311. doi: 10.1001/archpsyc.1968.01740030045006. [DOI] [PubMed] [Google Scholar]
- Vogel G, Neill D, Kors D, Hagler M. REM sleep abnormalities in a new animal model of endogenous depression. Neurosci Biobehav Rev. 1990a;14:77–83. doi: 10.1016/s0149-7634(05)80163-0. [DOI] [PubMed] [Google Scholar]
- Vogel G, Neill D, Hagler M, Kors D. A new animal model of endogenous depression: a summary of present findings. Neurosci Biobehav Rev. 1990b;14:85–91. doi: 10.1016/s0149-7634(05)80164-2. [DOI] [PubMed] [Google Scholar]
- Vogel G, Neill D, Hagler M, Kors D, Hartley P. Decreased intracranial self-stimulation in a new animal model of endogenous depression. Neurosci Biobehav Rev. 1990c;14:65–68. doi: 10.1016/s0149-7634(05)80160-5. [DOI] [PubMed] [Google Scholar]
- West CH, Weiss JM. Effects of antidepressant drugs on rats bred for low activity in the swim test. Pharmacol Biochem Behav. 1998;61:67–79. doi: 10.1016/s0091-3057(98)00076-8. [DOI] [PubMed] [Google Scholar]
- Wilson S, Argyropoulos S. Antidepressants and sleep: a qualitative review of the literature. Drugs. 2005;65:927–947. doi: 10.2165/00003495-200565070-00003. [DOI] [PubMed] [Google Scholar]
- Winsky-Sommerer R, Yamanaka A, Diano S, Borok E, Roberts AJ, Sakurai T, Kilduff TS, Horvath TL, de Lecea L. Interaction between the corticotropin-releasing factor system and hypocretins (orexins): a novel circuit mediating stress response. J Neurosci. 2004;24:11439–11448. doi: 10.1523/JNEUROSCI.3459-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]