Abstract
Hematopoietic stem cells (HSCs) reside in proximity to bone marrow endothelial cells (BM ECs) and maintenance of the HSC pool is dependent upon EC-mediated c-kit signaling. Here, we utilized genetic models to determine if radioprotection of BM ECs could facilitate hematopoietic regeneration following radiation-induced myelosuppression. We developed mice bearing deletion of the pro-apoptotic proteins, BAK and BAX, in Tie2+ endothelial cells (ECs) and HSCs (Tie2Bak/BaxFl/− mice) and compared their hematopoietic recovery following total body irradiation (TBI) with mice which retained Bax in Tie2+ cells. Mice bearing deletion of Bak and Bax in Tie2+ cells demonstrated protection of BM HSCs, preserved BM vasculature and 100% survival following lethal dose TBI. In contrast, mice that retained Bax expression in Tie2+ cells demonstrated depletion of BM HSCs, disrupted BM vasculature and 10% survival post-TBI. In a complementary study, VEcadherinBak/BaxFl/− mice, which lack Bak and Bax in VEcadherin+ ECs, also demonstrated increased recovery of BM stem/progenitor cells following TBI compared to mice which retained Bax in VEcadherin+ ECs. Importantly, chimeric mice which lacked Bak and Bax in HSCs but retained Bak and Bax in BM ECs displayed significantly decreased HSC content and survival following TBI compared to mice lacking Bak and Bax in both HSCs and BM ECs. These data suggest that the hematopoietic response to ionizing radiation is dependent upon HSC-autonomous responses but is regulated by BM EC-mediated mechanisms. Therefore, BM ECs may be therapeutically targeted as a means to augment hematopoietic reconstitution following myelosuppression.
INTRODUCTION
HSCs have been shown to reside in proximity to osteoblasts within the BM and activation of osteoblasts has been shown to promote the expansion of the HSC pool in vivo [1–3]. BM HSCs have also been found in close association with BM sinusoidal vessels and recent studies have suggested an essential role for BM endothelial cells (ECs) and perivascular CXCL12-abundant reticular cells (CARs) in maintaining the HSC pool in vivo [4–6]. Similarly, nestin+ mesenchymal stem cells (MSCs), as well as the sympathetic nervous system, have been shown to regulate the retention of HSCs in the BM and BM adipocytes have been shown to negatively regulate long-term HSC content within the BM [7–9]. Taken together, these studies suggest a dynamic regulation of the HSC pool during homeostasis via cells which comprise the BM microenvironment.
Whereas much is now known about the signaling mechanisms which regulate HSC homeostasis [10–12], the process of HSC regeneration following myelosuppressive injury is less well understood. We have shown that adult sources of human ECs elaborate soluble growth factors which promote the expansion of murine and human HSCs in vitro and the regeneration of murine and human HSCs in vitro following radiation exposure [13–17]. We have also demonstrated that systemic infusion of autologous or allogeneic ECs accelerates BM HSC reconstitution and hematologic recovery in mice following total body irradiation (TBI) [18, 19]. Conversely, systemic delivery of a neutralizing anti-VEcadherin antibody, which inhibits BM vasculogenesis, significantly delays hematologic recovery following myelosuppression [19, 20]. Recently, Ding et al. demonstrated a requirement for BM EC-mediated stem cell factor (SCF) signaling for the maintenance of the HSC pool during homeostasis [6] and Hooper et al. showed a requirement for VEGFR2+ sinusoidal ECs to allow for normal hematologic recovery following TBI [21]. While these studies have suggested an essential role for BM ECs in regulating HSC maintenance and regeneration in vivo, it remains to be determined whether augmentation of EC function can promote HSC expansion or regeneration in vivo. Here, we utilized the Cre;LoxP system to generate mice bearing a constitutive deletion of Bak1 and a conditional deletion of Bax in Tie2+ cells (Tie2Bak/BaxFl/− mice) or in VEcadherin+ cells (VEcadherinBak/BaxFl/− mice), along with littermate controls (Tie2Bak/BaxFl/+ mice and VEcadherinBak/BaxFl/+ mice), to determine whether deletion of these pro-apoptotic genes in Tie2+ or VEcadherin+ cells promotes HSC regeneration and improves survival following TBI. Using this approach, we show that the hematopoietic response to ionizing radiation is regulated by both HSC-autonomous responses and BM EC-mediated mechanisms.
MATERIALS AND METHODS
Animals
Tie2Cre;Bak1−/−;Bax+/− mice were bred with Bak1−/−;BaxFL/FL mice bearing a constitutive deletion of Bak1 and floxed Bax alleles to generate Tie2Bak/BaxFL/− experimental mice and Tie2Bak/BaxFL/+ littermate controls. In Tie2Cre mice, floxed alleles are recombined by Cre in Tie2-expressing cells and their progeny, which are referred to as Tie2+ cells. Tie2Bak/BaxFL/− and Tie2Bak/BaxFL/+ mice were generated as previously described [22]. To generate chimeric Tie2Bak/BaxFL/−;WT-EC mice, 4 × 106 BM cells from Tie2Cre;Bak1−/−;BaxFL/− (CD45.2+) mice were transplanted into B6.SJL mice (CD45.1+, Jackson Laboratory, Bar Harbor, ME) following 950 cGy TBI (Cs137 irradiator, dose rate 593 cGy/min). Control mice were generated using BM cells from Tie2Cre;Bak1−/−;BaxFL/+ mice transplanted into B6.SJL mice to create Tie2Bak/BaxFL/+;WT-EC mice. Full donor chimerism was verified by flow cytometric analysis at 12 weeks post-transplantation. VEcadherinCre mice (Jackson Laboratory, Bar Harbor, ME) were bred with Bak1−/−;Bax+/− mice to generate VEcadherinBak1−/−;Bax+/− mice. These progeny were crossed with Bak1−/−;BaxFL/FL mice to obtain VEcadherinBak/BaxFL/+ mice and VEcadherinBak/BaxFL/− mice. VEcadherinCre (−) mice, which lack Cre recombinase in VEcadherin+ cells, were also utilized as controls in survival studies. All animal studies described herein were approved by the Duke University Animal Care and Use Committee.
Isolation of BM ECs and Quantitative Real-Time PCR for Bax Expression
Mice femurs were collected and BM cells were flushed into PBS (Cellgro, Manassas, VA) with 10% fetal bovine serum (Hyclone, Logan, UT) and 1% penicillin-streptomycin (GIBCO, Grand Island, NY). Red blood cells were depleted using RBC lysis buffer (Sigma-Aldrich, St. Louis, MO). Cells were stained with anti-CD45 PE and anti-mouse endothelial cell antigen (MECA-32). Primary anti-MECA was washed and a secondary antibody Streptavidin AlexaFluor 488 (Invitrogen, Carlsbad, CA) was added. cDNA was generated from RNA using RNAeasy kit (Qiagen, Valencia, CA). Real time PCR was performed using Taqman probes for glyceraldehyde-3-phosphate (GAPDH) and Bax (Applied Biosystems, Foster City, CA) according to the manufacturer’s protocol. All primary antibodies were obtained from BD Biosciences (BD, San Jose, CA).
Hematopoietic Progenitor Cell Assays
Two hours or 7 days following ionizing radiation, C57Bl6 mice and transgenic mice were sacrificed and BM cells were collected into PBS with 10% serum and 1% penicillin/streptomycin as described above. Viable BM cells were quantified using trypan blue to exclude dead cells. Cells were then incubated with anti-c-kit, anti-Sca-1 and anti-lineage cocktail antibodies (BD) to measure ckit+sca-1+lin− (KSL) progenitor cells as previously described [18]. Anti-CD150 and anti-CD48/41 antibodies were utilized to analyze for CD150+CD48/41− KSL (SLAM+KSL) cells, which are enriched for HSCs [4]. CFU-S12 assays were also performed to measure functional hematopoietic stem/progenitor cell content. 1 × 105 cells were collected from donor mice and injected via tail vein into recipient C57Bl6 mice that had been given 950 cGy TBI. At day +12 post-injection, spleens from recipient mice were harvested and stained with Bouin’s fixative solution (Ricca Chemical Company, Arlington, TX), and colonies were counted as previously described [23]. Colony forming cell (CFC) assays for myeloid progenitor cells were performed following manufacturer’s guidelines (Stem Cell Technologies, Vancouver, CA).
Competitive Repopulation Assay and Radiation Survival Studies
Donor B6.SJL mice and transgenic mice (all CD45.2+) were irradiated with 300 cGy TBI. Two hours later, BM cells were harvested from the donor mice and 1–3 × 105 BM cells were injected via tail vein into recipient C57Bl6 or B6.SJL mice (CD45.1+) that had been lethally irradiated with 950 cGy TBI. Host BM cells (1 × 105) were co-injected as competitor cells into recipient mice. Multilineage hematopoietic reconstitution was measured in the PB by flow cytometry at 4, 8, and 12 weeks post-transplant. For competitive repopulating assays using non-irradiated donor mice, a donor cell dose of 3 × 104 BM cells was utilized. For the high dose TBI survival studies, all mice were irradiated with 750 cGy TBI using an X-RAD 320 Biological Irradiator (Precision X-ray, Inc). Mice were treated at 72 cm from the radiation source (SSD) with a dose rate of 104 cGy/min with 320 kVp X-rays, using 12.5 mA and a filter consisting of 2.5 mm Aluminum and 0.1 mm Copper. Mice were evaluated daily to monitor for morbidity and survival through day +30.
Cell Survival and Proliferation Assays
BM ECs of the indicated genotypes were collected over a 70 micron filter (BD Falcon, Bedford, MA) and incubated with 0.25% trypsin-EDTA (GIBCO, Grand Island, NY) at 37oC for 5 minutes. Cells were bound with anti-CD45 microbeads (Miltenyi Biotech, Auburn, CA), then passed through LS MACS columns (Miltenyi Biotech, Auburn, CA) to enrich for ECs. BM ECs and hematopoietic cells were stained with anti-CD45 PE and anti-mouse endothelial cell antigen (MECA-32), and then a secondary antibody Streptavidin AlexaFluor 488 as noted above. Cells were washed and placed in 1X binding buffer, Annexin V-APC, and Propidium Iodide. For the VEcadherinBak/BaxFl/− and VEcadherinBak/BaxFl/+ cell survival studies, BM cells were passed through a lineage depletion column, then labeled with anti-c-kit FITC, anti-sca-1 PE-Cy7, Annexin V-APC, and Propidium Iodide. Cell apoptosis and necrosis were analyzed by flow cytometry according to manufacturer’s protocols (BD, San Jose, CA). Cell proliferation was measured in mice of the indicated genotypes following exposure to 300 cGy TBI and administered 5-bromo-2-deoxyridine (BrdU, BD, San Jose, CA) in drinking water from the day of irradiation until the day of collection. BM cells were labeled with anti-cKit PE, anti-sca1 PE-Cy7, anti-lineage APC, and anti-BrdU FITC. Incorporation of BrdU was analyzed by flow cytometry according to the manufacturer’s staining protocol (BD, San Jose, CA). All antibodies were obtained from BD Biosciences (BD, San Jose, CA).
BM Immunohistochemical Analyses
Femurs were decalcified and embedded in OCT media (Sakura Finetek, Torrance, CA) as previously described at day +10 following 750 cGy TBI [18, 19]. Ten micrometer sections were cut using the CryoJane tape system (Instrumedics Inc, Hackensack, NJ, USA). Femurs were stained with hematoxylin and anti-mouse endothelial cell antibody (MECA-32) as previously described [18, 24] to assess BM cellularity and vasculature after irradiation. Images were obtained using an Axiovert 200 microscope (Carl Zeiss, Thornwood, NY).
Cytokine Array Analysis
Whole BM was collected from adult, non-irradiated Tie2Bak/BaxFL/− mice and Tie2Bak/BaxFL/+ mice and C57Bl6 mice and at 6 hours following 750 cGy TBI. After centrifugation, BM supernatants were collected into IMDM and analyzed for cytokine concentrations using Quantibody mouse cytokine array 1000, according to manufacturer’s guidelines (RayBiotech, Inc., Norcross, GA).
Statistical Analyses
Data are shown as means ± SEM. We performed pair-wise comparisons with the Student’s t test (2 tailed distribution). Comparisons of mice survival after TBI were performed using a Fisher’s exact test and the Log rank test. Comparisons of cytokine concentrations in the different mice groups were made using a 1-tailed t test.
RESULTS
Deletion of Bak and Bax in Tie2+ Cells Protects BM HSCs from Radiation Injury
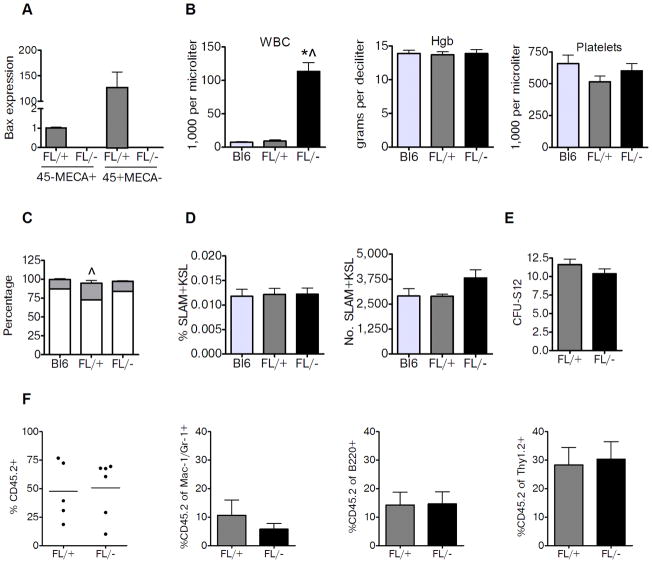
Mice expressing Cre under the control of the Tie2 promoter (Tie2Cre) were crossed with mice carrying floxed alleles for Bax in order to create mice bearing a targeted deletion of Bax in Tie2+ cells [25]. During development, BAK and BAX have overlapping functions in the vasculature [26], so we utilized mice bearing constitutive deletion of Bak1 [27] to generate Tie2Bak/BaxFL/− mice, which have Bak and Bax deletion in Tie2+ ECs and HSCs [22]. We compared these mice to Tie2Bak/BaxFL/+ littermate controls, which retain Bax expression in Tie2+ cells. We sought to assess whether deletion of Bak and Bax in Tie2+ cells could confer protection of the hematopoietic system following radiation exposure. Tie2Bak/BaxFL/− mice had no detectable levels of Bax expressed in BM ECs (CD45−, mouse endothelial cell antigen positive, MECA+) or CD45+MECA− hematopoietic cells, whereas Tie2Bak/BaxFL/+ mice had detectable Bax in both ECs and hematopoietic cells (Fig. 1a). We next examined the hematopoietic profile of non-irradiated Tie2Bak/BaxFL/− mice and Tie2Bak/BaxFL/+ mice to assess for baseline differences in these mice (Fig. 1b–f). Tie2Bak/BaxFL/− mice had elevated white blood cell (WBC) counts but a normal WBC differential and normal hemoglobin and platelet counts compared to Tie2Bak/BaxFL/+ mice and C57Bl6 mice (Fig. 1b,c). This phenotype is consistent with that described for mice bearing constitutive deletion of Bak and Bax [27]. However, we found no significant differences in the percentage or numbers of BM SLAM+KSL cells, which are highly enriched for HSCs [28], between Tie2Bak/BaxFL/− mice, Tie2Bak/BaxFL/+ mice and C57Bl6 mice (Fig. 1d). Furthermore, functional assays revealed no differences in BM CFU-S12 or HSC competitive repopulating capacity between Tie2Bak/BaxFL/− mice and Tie2Bak/BaxFL/+ mice (Fig. 1e,f). Taken together, these data demonstrated that Tie2Bak/BaxFL/− mice did not have increased BM HSC or progenitor content at baseline compared to Tie2Bak/BaxFL/+ mice or C57Bl6 mice.
Figure 1.
Deletion of Bak and Bax in Tie2+ cells does not alter baseline HSC content or function. (A) Endothelial cells (CD45−MECA+) and hematopoietic cells (CD45+MECA−) were isolated from the BM. Relative expression of Bax is shown in these cell populations from Tie2Bak/BaxFL/− mice (FL/−), Tie2Bak/BaxFL/+ mice (FL/+)(n=4–7/group). (B) Peripheral blood cells from 8 week old C57Bl6 (Bl6) mice, FL/+ mice and FL/− mice were analyzed for WBCs, hemoglobin (Hgb) concentration, and platelet counts. FL/− mice had significantly higher PB WBCs compared to control mice (n=5/group). *P<0.001 and ^P<0.001 versus FL/+ and Bl6 mice; means ± SEM. (C) Percentage of PB lymphocytes (gray) and neutrophils (white) is shown between non-irradiated Bl6 mice, FL/+ mice and FL/− mice (n=5/group). ^P=0.02 for lymphocyte percentage between FL/+ and FL/− mice. (D) Percentages of CD150+CD48−CD41−KSL cells (SLAM+KSL cells) and total numbers of SLAM+KSL cells per femur in the BM of non-irradiated Bl6, FL/+ and FL/− mice are shown (n=3–4/group). (E) Numbers of BM CFU-S12 in non-irradiated FL/+ and FL/− mice are shown (n=6–8/group). (F) Scatter plots show the donor CD45.2+ cell engraftment in recipient CD45.1+ mice at 12 weeks following competitive transplantation with 3 × 104 BM cells from non-irradiated FL/− or FL/+ mice with 1 × 105 competing B6.SJL (CD45.1+) BM cells (n=5–6/group). Mean levels of engraftment are represented by the horizontal lines (mean 50.8% vs. 47.5% CD45.2+ cells in PB at 12 weeks. The mean percentages of donor CD45.2+ cells within the myeloid (Mac-1/Gr-1), B cell (B220) and T cell (Thy 1.2) cell populations at 12 weeks are shown at right.
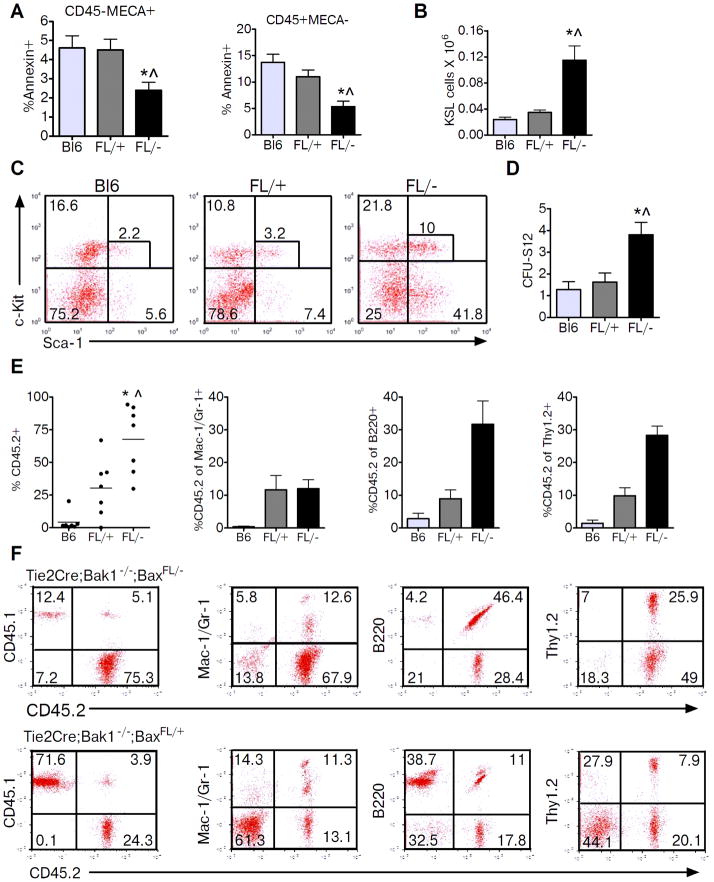
We next tested whether deletion of Bak and Bax in Tie2+ cells altered the response of the hematopoietic system to ionizing radiation injury. At 2 hours following 300 cGy TBI, BM ECs (CD45− MECA+) in Tie2Bak/BaxFL/− mice contained significantly less Annexin+ cells compared to BM ECs from Tie2Bak/BaxFL/+ mice (Fig. 2a). BM hematopoietic cells (CD45+MECA−) from Tie2Bak/BaxFL/− mice also displayed significantly decreased Annexin+ cells compared Tie2Bak/BaxFL/+ mice. To test whether deletion of Bak and Bax in Tie2+ cells protected BM HSCs from depletion following TBI, we compared BM HSC and progenitor cell content in Tie2Bak/BaxFL/− mice versus Tie2Bak/BaxFL/+ mice following 300 cGy TBI. At 2 hours after 300 cGy, Tie2Bak/BaxFL/− mice contained significantly increased numbers of BM KSL progenitor cells and BM CFU-S12 compared to irradiated Tie2Bak/BaxFL/+ controls (Fig. 2b–d). Importantly, recipient mice that were competitively transplanted with BM cells from irradiated Tie2Bak/BaxFL/− mice contained 2.5-fold and 18-fold increased donor hematopoietic cell engraftment at 12 weeks post-transplant compared to mice transplanted with an equal dose of BM cells from irradiated Tie2Bak/BaxFL/+ mice or C57Bl6 mice, respectively (Fig. 2e,f). Of note, B cell and T cell reconstitution was also significantly increased in recipient mice transplanted with BM cells from Tie2Bak/BaxFL/− mice compared to mice transplanted with HSCs from Tie2Bak/BaxFL/+ mice (Fig. 2e,f)
Figure 2.
Deletion of Bak and Bax in Tie2+ cells protects BM HSCs and progenitor cells from radiation injury. (A) Percentages of Annexin+ cells are shown for BM ECs (CD45−MECA+, left) and BM hematopoietic cells (CD45+MECA−, right) in BL6, FL/+ and FL/− mice at 2 hours after 300 cGy TBI (n=3–5/group). *P=0.04 and ^P=0.04 versus Bl6 and FL/+ for CD45−MECA+ cells, respectively. *P=0.009 and ^P=0.03 versus Bl6 and FL/+ for CD45+MECA− cells, respectively. (B) Bl6, FL/+ and FL/− mice were irradiated with 300 cGy TBI and BM cells were collected at +2 hours post-irradiation. BM KSL cells per femur were increased in FL/− mice compared to control mice (n=3–7/group). *P=0.006 and ^P=0.006 versus Bl6 and FL/+ mice, respectively. (C) Representative flow cytometric analysis of BM KSL cells in Bl6, FL/+, and FL/− mice following 300 cGy TBI. (D) BM CFU-S12 content was increased in FL/− mice compared to control mice. *P=0.003 and ^P=0.01 versus Bl6 and FL/+ mice, respectively (means ± SEM, n=6–8/group). (E) Scatter plots show the PB engraftment at 12 weeks post-transplant of donor BM CD45.2+ cells that were harvested from FL/− or FL/+ mice at +2 hours following 300 cGy and competitively transplanted at a dose of 3 × 105 BM cells into lethally irradiated CD45.1+ recipient mice with 1 × 105 non-irradiated host competitor BM cells. PB engraftment of the same dose of donor BM CD45.2+ cells harvested from irradiated Bl6 mice following transplant into lethally irradiated CD45.1+ recipient mice is shown at left. Each dot represents the engraftment of an individual mouse. Lines represent the mean levels of engraftment in each group (B6: 4.2%, FL/+: 30.4%, FL/−: 67.7%, n=7/group). The mean percentages of donor CD45.2+ cells within the myeloid, B cell and T cell populations at 12 weeks are shown at right. (F) Representative flow cytometric analysis of CD45.2+ donor cell engraftment at 12 weeks in recipients ofTie2Bak/BaxFL/− or Tie2Bak/BaxFL/+ BM cells.
Tie2+ BM ECs Regulate the Hematopoietic Response to Radiation Injury
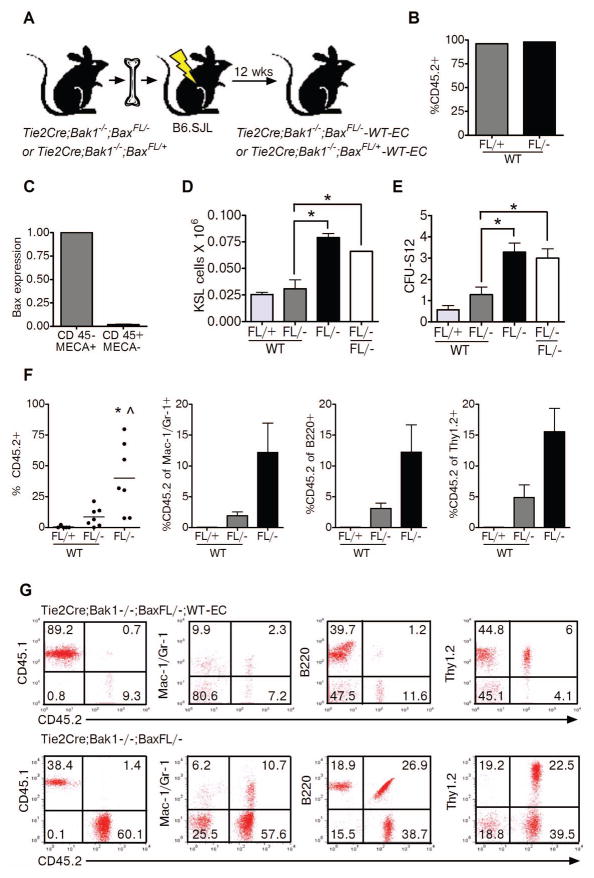
Tie2Cre mice express Cre recombinase in both ECs and a majority of hematopoietic cells. Therefore, it is not possible to determine the relative contributions of BM ECs versus HSC-autonomous mechanisms to the radiation protection of HSCs observed in Tie2Bak/BaxFL/− mice. In order to discriminate the specific contributions of Tie2+ BM ECs and Tie2+ HSCs in mediating the observed radioprotection in Tie2Bak/BaxFL/− mice, we generated chimeric mice. We transplanted 4 × 106 BM cells from Tie2Bak/BaxFL/− mice into lethally irradiated (950 cGy) B6.SJL mice such that, at 12 weeks post-transplant, the recipient mice were fully reconstituted with donor hematopoietic cells (mean 97.0 ± 2.0 donor CD45.2+ cells; Fig. 3a,b). These recipient mice demonstrated deletion of Bax in BM CD45+ hematopoietic cells while retaining expression of Bax in BM ECs (Tie2Bak/BaxFL/−; wild type ECs (WT-EC), Fig. 3c). We then exposed the chimeric Tie2Bak/BaxFL/−; WT-EC mice to 300 cGy TBI and compared their HSC and progenitor cell content to that of irradiated Tie2Bak/BaxFL/− mice. At 2 hours after 300 cGy TBI, Tie2Bak/BaxFL/−;WT-EC mice demonstrated significant depletion of BM KSL cells and CFU-S12 compared to irradiated Tie2Bak/BaxFL/− mice (Fig. 3d,e). As an additional control for the effects of TBI conditioning in the generation of the Tie2Bak/BaxFL/−;WT-EC mice, we also irradiated Tie2Bak/BaxFL/− mice with 950 cGy TBI and transplanted these mice with BM cells from Tie2Bak/BaxFL/− mice, to generate Tie2Bak/BaxFL/−;FL- mice. Following 300 cGy TBI, Tie2Bak/BaxFL/−;WT-EC mice also had significantly less BM KSL and CFU-S12 content than Tie2Bak/BaxFL/−;FL- mice (Fig. 3d,e), suggesting that the prior TBI conditioning did not account for the decreased BM progenitor cell content in Tie2Bak/BaxFL/−;WT-EC mice compared to Tie2Bak/BaxFL/− mice. Importantly, recipient mice that were transplanted competitively with BM cells from irradiated Tie2Bak/BaxFL/−;WT-EC mice displayed 5-fold lower donor CD45.2+ hematopoietic cell engraftment at 12 weeks compared to mice transplanted with the identical dose of BM from irradiated Tie2Bak/BaxFL/− mice (Fig. 3f,g). Taken together, these data suggest that Tie2+ BM ECs regulate the response of HSCs to radiation injury and that deletion of the intrinsic pathway of apoptosis in BM ECs contributes to the radioprotection of the HSC pool.
Figure 3.
(A) Schematic representation of the BM transplant model utilized to generate chimeric mice bearing deletion of Bak and Bax in Tie2+ hematopoietic cells while retaining Bak and Bax in BM ECs (Tie2Bak/BaxFL/−;WT-EC mice), along with control mice. (B) Mean levels ± SEM of CD45.2+ donor cell engraftment are shown in the PB of recipient CD45.1+ mice at 12 weeks following transplantation of BM cells from FL/+ and FL/− mice (n=4–5 mice/group). (C) Relative expression of Bax is shown in FACS-isolated CD45−MECA+ BM ECs and CD45+MECA− BM hematopoietic cells in recipient B6.SJL mice at 12 weeks following transplant with BM cells from Tie2Bak/BaxFL/− mice (means ± SEM, n=2). (D) BM KSL cells per femur were decreased in Tie2Bak/BaxFL/−;WT-EC mice (FL/−;WT) compared to Tie2Bak/BaxFL/− mice (FL/−) and Tie2Bak/BaxFL/−;FL-EC (FL/−;FL/−) mice following 300 cGy TBI. *P=0.007 and *P=0.046 for KSL cells in FL/−;WT-EC mice versus FL/− and FL/−;FL/− mice, respectively (means + SEM, n=2–3). (E) BM CFU-S12 were also significantly decreased in FL/−;WT mice compared to FL/− and FL/−;FL/− mice. *P=0.004 and *P=0.01 for CFU-S12 in FL/−;WT mice versus FL/− and FL/−;FL/− mice, respectively (means ± SEM, n=7). No significant differences were noted in BM KSL cells or CFU-S12 in FL/− mice compared to FL/−;FL/− mice. (F) The scatter plots show donor CD45.2+ cell engraftment in the PB at 12 weeks following competitive transplantation of 1 × 105 BM cells from irradiated FL/+;WT mice, FL/−;WT mice or FL/− mice into lethally irradiated CD45.1+ recipient mice. Each dot represents the engraftment of an individual mouse. Horizontal lines represent the mean levels of engraftment (FL/+;WT-EC: 0.1%, FL/−;WT-EC: 8.5%, FL/−: 40.0%, n=5–7/group). The mean percentages of donor CD45.2+ cells within the myeloid, B cell and T cell populations are shown at right. (G) Representative flow cytometric analysis of donor CD45.2+ cell engraftment and differentiation in recipient mice after transplantation with BM from irradiated FL/−;WT mice or FL/− mice.
Deletion of Bak and Bax in Tie2+ ECs Improves the Survival of Irradiated Mice
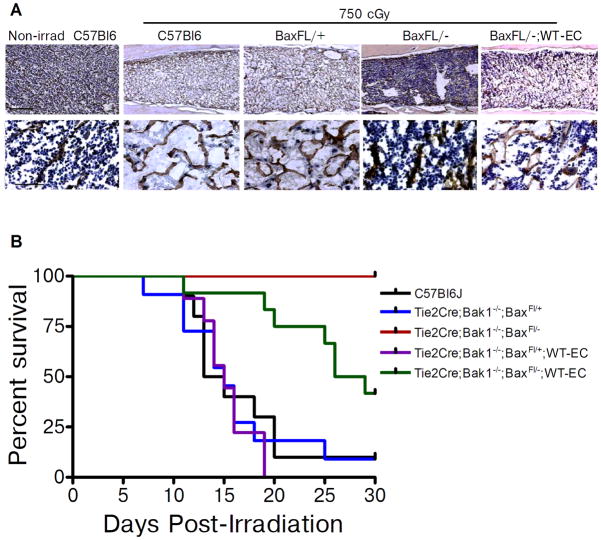
In order to determine if the radioprotection of BM HSCs observed in Tie2Bak/BaxFL/− mice was relevant at radiation dose levels that would affect survival, we examined the BM cellular response and survival of Tie2Bak/BaxFL/− mice, Tie2Bak/BaxFL/+ mice, Tie2Bak/BaxFL/−;WT-EC mice and C57Bl6 mice following a lethal dose of TBI (750 cGy). At day +10 following 750 cGy, C57Bl6 mice and Tie2Bak/BaxFL/+ mice demonstrated severe BM hypocellularity and disruption of the BM vasculature (Fig. 4a). In contrast, Tie2Bak/BaxFL/− mice demonstrated nearly normal BM cellularity and preserved sinusoidal vasculature at this time point. Tie2Bak/BaxFL/−;WT-EC mice demonstrated substantially decreased BM cellularity and increased disruption of the BM vasculature compared to Tie2Bak/BaxFL/− mice, confirming the contribution of Tie2+ BM ECs in regulating the hematopoietic response to radiation injury (Fig. 4a). However, Tie2Bak/BaxFL/−;WT-EC mice retained qualitatively increased BM cellularity compared to Tie2Bak/BaxFL/+ mice and C57Bl6 mice, suggesting that the HSC response to ionizing radiation was regulated in a cell-autonomous and an EC-dependent manner.
Figure 4.
Deletion of Bak and Bax in Tie2+ ECs preserves BM cellularity and improves survival following high dose TBI. (A) Representative cross sections of femurs from non-irradiated C57Bl6 mice and from C57Bl6 mice, Tie2Bak/BaxFL/+ mice (BaxFL/+), Tie2Bak/BaxFL/− mice (BaxFL/−) and Tie2Bak/BaxFL/−;WT-EC mice (BaxFL/−;WT-EC) at day +10 following 750 cGy TBI. Hematoxylin and mouse endothelial cell antigen (MECA) staining was performed. MECA-positive vessels are shown in brown (top, scale bar 250 microns; bottom, 50 microns), demonstrating preservation of BM sinusoidal vasculature in BaxFL/− mice and disruption of the vasculature in BaxFL/−;WT-EC mice, BaxFL/+ mice and C57Bl6 mice. The BaxFL/− mice also demonstrated substantially increased BM cellularity compared to BaxFL/−;WT-EC mice, C57Bl6 mice and BaxFL/+ mice. (B) Deletion of Bak and Bax in Tie2+ ECs improved the survival of mice exposed to lethal dose TBI. Adult C57Bl6 mice and mice bearing deletions of Bak and Bax (identified at top) were irradiated with 750 cGy TBI and subsequently followed for 30 days (n=9–12/group). Tie2Bak/BaxFL/− mice demonstrated 100% survival (10 of 10), compared to 10% survival (1 of 10) for C57Bl6 mice (P<0.0001) and 9% survival (1 of 11) for Tie2Bak/BaxFL/+ mice (P<0.0001). Tie2Bak/BaxFL/−;WT-EC mice (BaxFL/−;WT-EC) demonstrated significantly decreased survival (42%, 5 of 12) compared to Tie2Bak/BaxFL/− mice (*P=0.005), but significantly increased survival compared to Tie2Bak/BaxFL/+;WT-EC mice (BaxFL/+;WT EC) (0 of 9, P<0.0001). Log rank test was performed for all comparisons.
Tie2Bak/BaxFL/− mice also displayed significantly increased survival compared to control mice following lethal dose TBI. Following 750 cGy TBI, 90% of C57Bl6 mice and 91% of the Tie2Bak/BaxFL/+ mice died by day +30 (Fig. 4b). In contrast, 100% of the Tie2Bak/BaxFL/− mice remained alive and well at day +30 post-irradiation (P<0.001 vs. B6 and FL/+ mice). Interestingly, Tie2Bak/BaxFL/−;WT-EC mice demonstrated an intermediate survival of 42% compared to Tie2Bak/BaxFL/− mice (P=0.005), suggesting that retention of the intrinsic pathway of apoptosis in BM ECs significantly increased mortality after TBI (Fig. 4b). Of note, none of the Tie2Bak/BaxFL/+;WT-EC mice, which retained Bax in BM hematopoietic cells and BM ECs, survived past day +30. Therefore, these results indicate that deletion of the intrinsic pathway of apoptosis in BM HSCs is sufficient to protect a subset of mice from death due to hematopoietic failure after TBI, but significantly increased radioprotection is conferred by deletion of Bak and Bax in both ECs and HSCs.
Deletion of Bak and Bax in VEcadherin+ ECs Promotes Hematopoietic Recovery in Mice Following TBI
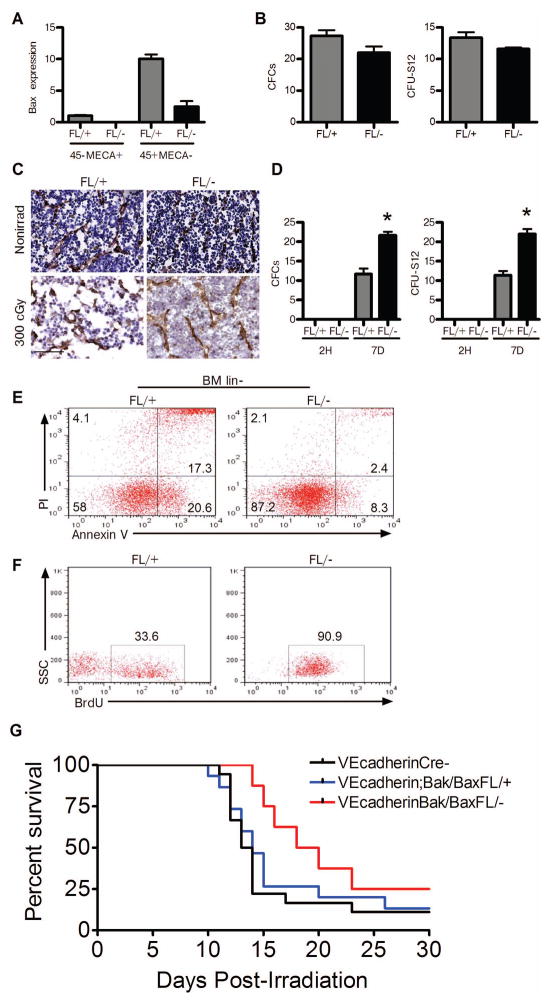
In order to test our hypothesis that BM ECs regulate the response of the HSC pool to ionizing radiation in a 2nd model, we utilized VEcadherinCre mice that express Cre in ECs and only a small minority of hematopoietic cells [29, 30]. We then generated VEcadherinBak/BaxFL/− mice in which Cre-mediated deletion of Bax occurs in VEcadherin+ cells and Bax is retained in CD45+MECA− hematopoietic cells (Fig. 5a). We compared functional hematopoietic progenitor content in non-irradiated VEcadherinBak/BaxFL/− mice and VEcadherinBak/BaxFL/+ mice and determined that there were no baseline differences in BM CFC and CFU-S12 content (Fig. 5b). Furthermore, these mice displayed comparable cellularity and vascular density at baseline (Fig. 5c). However, at 7 days following 300 cGy TBI, VEcadherinBak/BaxFL/+ mice displayed decreased BM cellularity and increased disruption of vascular integrity compared to VEcadherinBak/BaxFL/− mice (Fig. 5c). Interestingly, 300 cGy TBI caused significant depletion of BM CFCs and CFU-S12 in both VEcadherinBak/BaxFL/− mice and VEcadherinBak/BaxFL/+ mice at +2 hours post-TBI (Fig. 5d). However, at day +7 post-TBI, VEcadherinBak/BaxFL/− mice displayed significantly increased recovery of BM CFCs and CFUS-12 compared to VEcadherinBak/BaxFL/+ mice (Fig. 5d). At this same time point post-TBI, VEcadherinBak/BaxFL/− mice also displayed markedly increased survival of BM lin− hematopoietic cells and increased BrdU incorporation in BM KSL cells in vivo compared to VEcadherinBak/BaxFL/+ mice (Fig. 5e,f). These results suggest that BM ECs may not affect the immediate survival of HSCs following irradiation, but can promote the regeneration of HSCs and progenitor cells subsequent to radiation injury. While it is possible that the observed regeneration of HSCs and progenitors in VEcadherinBak/BaxFL/− mice is contributed to by a small number of hematopoietic cells with deletion of Bak and Bax, these results suggest a role for BM ECs in regulating HSC regeneration following radiation injury.
Figure 5.
Deletion of Bak and Bax in VEcadherin+ ECs promotes BM hematopoietic stem/progenitor cell regeneration in vivo. (A) Relative expression of Bax is shown in BM ECs (CD45−MECA+) and hematopoietic cells (CD45+MECA−) in VEcadherinBak/BaxFL/+ mice (FL/+) and VEcadherinBak/BaxFL/− mice (FL/−) (n=3/group). Bax was not detected in CD45−MECA+ ECs from FL/− mice but was detected in CD45+MECA− hematopoietic cells from FL/− mice. (B) Non-irradiated FL/+ and FL/− mice demonstrated comparable BM CFC and CFU-S12 content. (C) Representative cross sections of femurs are shown from non-irradiated VEcadherinBak/BaxFL/+ mice (FL/+) and VEcadherinBak/BaxFL/− mice (FL/−) and at day +7 following 300 cGy TBI. Mouse endothelial cell antigen (brown) and hematoxylin (blue) stained femurs showed similar BM vasculature and cellularity in non-irradiated mice. After 300 cGy, the FL/+ mice had significant disruption of the BM sinusoidal vasculature and decreased BM cellularity compared to FL/− mice. Scale bar is 50 microns. (D) FL/− and FL/+ mice were irradiated with 300 cGy TBI and BM cells were collected at 2 hours post-TBI and at day +7 for measurement of hematopoietic progenitor cell content. No differences were observed at 2 hours post-TBI in BM CFCs or CFU-S12 (n=3–5/group). At day +7 post TBI, mean numbers of BM CFCs and BM CFU-S12 were significantly increased in FL/− mice compared to FL/+ mice (n=3–5/group). *P=0.004 versus FL/+ for CFCs and *P=0.0002 versus FL/+ for CFU-S12. (E) Flow cytometric analysis demonstrated decreased apoptotic (Annexin+PI−) and necrotic (Annexin+PI+) BM lin− hematopoietic cells in FL/− mice at day +7 post TBI compared to FL/+ mice. (F) BrdU incorporation was increased in BM KSL progenitor cells in FL/− mice compared to FL/+ mice at day +7 following TBI. (G) Survival of VEcadherinBak/BaxFl/− mice (n=8), VEcadherinBak/BaxFl/+ mice (n=15) and VEcadherinCre (−) mice (n=18) following lethal dose TBI. Survival curves of VEcadherinBak/BaxFL/− mice (red line), VEcadherinBak/BaxFL/+ mice (blue line) and VEcadherinCre (−) mice (black line) are shown following 750 cGy TBI (15-day survival: P=0.04 and P=0.004 for VEcadherinBak/BaxFl/− mice vs. VEcadherinBak/BaxFl/+ mice and VEcadherinCre (−) mice, respectively, Fisher’s exact test; 30-day survival analysis: P=0.07 and P=0.06 for VEcadherinBak/BaxFl/− mice vs. VEcadherinBak/BaxFl/+ mice and VEcadherinCre (−) mice, respectively, Log Rank analysis.
In order to determine if the deletion of Bak and Bax in VEcadherin+ ECs could also protect mice from lethal dose total body irradiation (TBI), we irradiated VEcadherinBak/BaxFL/− mice, VEcadherinBak/BaxFL/+ mice and VEcadherinCre (−) mice with 750 cGy TBI and compared the survival of each group. VEcadherinBak/BaxFL/− mice demonstrated a significant increase in 15-day survival compared to VEcadherinBak/BaxFl/+ mice and VEcadherinCre (−) mice following 750 cGy TBI (P=0.04 and P=0.004, respectively, Figure 5g). However, the differences in 30-day survival following lethal dose TBI between these groups did not reach statistical significance. Taken together, these data suggest that deletion of Bak and Bax in VEcadherin+ ECs alone can contribute to an earlier recovery of BM hematopoietic progenitor cells after sublethal irradiation and an early survival benefit following lethal dose TBI, but this effect is incomplete in the absence of protection of HSCs.
Deletion of Bak and Bax in Tie2+ Cells Alters Cytokine Concentrations in BM Serum
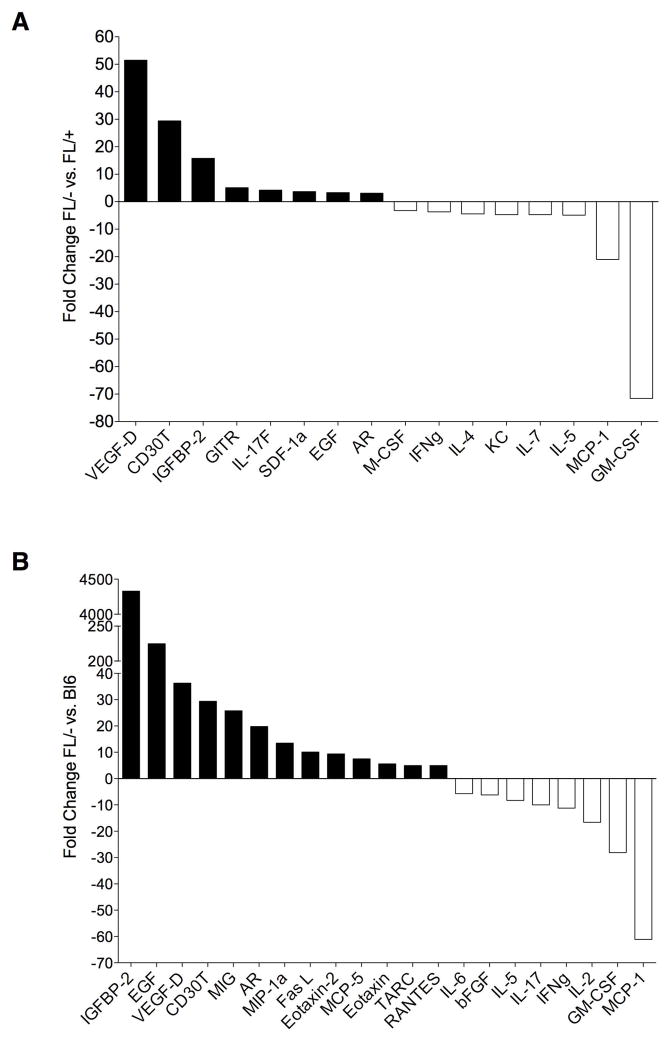
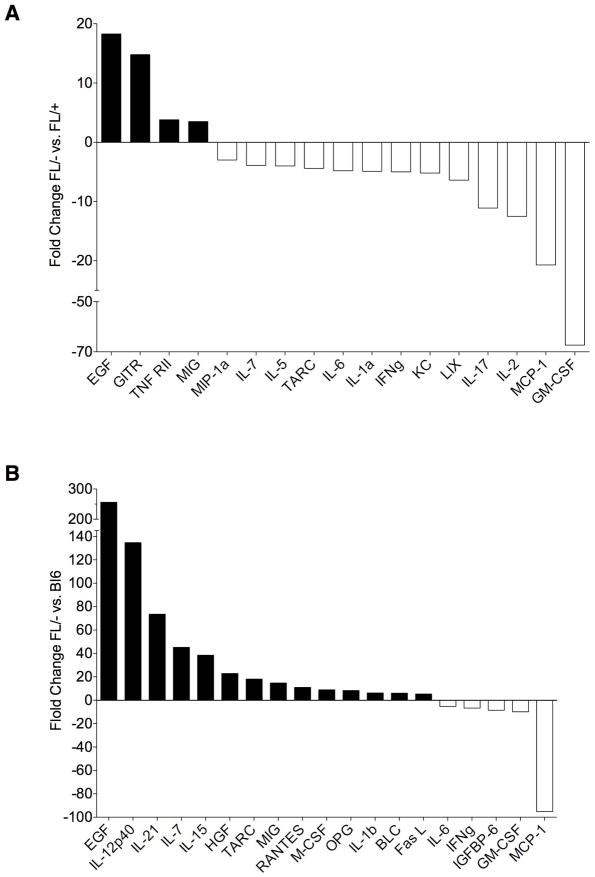
In order to begin to identify candidate mechanisms through which deletion of Bak and Bax in Tie2+ cells promotes the radioprotection of mice, we isolated BM serum from non-irradiated Tie2Bak/BaxFL/− mice, Tie2Bak/BaxFL/+ mice and C57Bl6 mice and at 6 hours following 750 cGy TBI and analyzed the concentrations of cytokines within the BM serum of the different groups of mice. Interestingly, numerous cytokines were substantially increased or decreased in Tie2Bak/BaxFL/− mice compared to Tie2Bak/BaxFL/+ mice and C57Bl6 mice at baseline and at 6 hours following 750 cGy TBI (Figures 6, 7 and Tables S1–S4). IGFBP2, epidermal growth factor (EGF), VEGF-D, and CD30T were upregulated more than 20-fold in the BM of non-irradiated Tie2Bak/BaxFL/− mice compared to C57Bl6 mice (P<0.05, Figure 6). VEGF-D, CD30T and IGFBP2 were also upregulated more than 10-fold in Tie2Bak/BaxFL/− mice compared to Tie2Bak/BaxFL/+ mice. Interestingly, granulocyte-monocyte colony stimulating factor (GMCSF) and monocyte chemotactic protein 1 (MCP1) were more than 10-fold downregulated in the BM of Tie2Bak/BaxFL/− mice compared to Tie2Bak/BaxFL/+ mice and C57Bl6 mice at baseline (Figure 6). At 6 hours following 750 cGy TBI, EGF, IL12p40, IL-21, IL-7, IL-15 and hepatocyte growth factor (HGF) were more than 20-fold increased in the BM of Tie2Bak/BaxFL/− mice compared to C57Bl6 mice (Figure 7). EGF and GITR were also significantly increased in the BM of Tie2Bak/BaxFL/− mice compared to Tie2Bak/BaxFL/+ mice, although the fold differences were smaller. As we observed in the analysis of non-irradiated mice, GMCSF and MCP1 were also the most significantly downregulated proteins in the BM of irradiated Tie2Bak/BaxFL/− mice compared to Tie2Bak/BaxFL/+ mice and C57Bl6 mice following 750 cGy. Taken together, these data identify candidate secreted proteins and signaling mechanisms that may contribute to the radioprotection of the hematopoietic system observed in Tie2Bak/BaxFL/− mice. Functional studies are now underway to determine if any of these proteins, alone or in combination, can mediate radiation protection or mitigation in vivo.
Figure 6.
Cytokine concentrations in the BM of non-irradiated Tie2Bak/BaxFl/− mice.Cytokines are shown which were upregulated (filled bars) or downregulated (unfilled bars) ≥ 3-fold in the BM of non-irradiated Tie2Bak/BaxFl/− mice (FL/−) versus Tie2Bak/BaxFl/+ mice (FL/+) (A) and ≥ 5-fold in non-irradiated FL/− versus C57Bl6 mice (B, P<0.05 for each cytokine). VEGF-D=vascular endothelial growth factor-D, IGFBP2=insulin like growth factor binding protein 2, GITR=glucocorticoid-induced TNFR-related protein, IL-17f=interleukin-17f, SDF1a=stromal derived factor 1a, AR=amphiregulin, IFNg=interferon gamma, KC=keratinocyte chemoattractant, MIG=migration inhibitory factor, MIP1a=macrophage inflammatory protein 1a, FasL=Fas ligand, TARC=thymus and activation regulated chemokine, RANTES=regulated and normal T cell expressed and secreted protein, bFGF=beta fibroblast growth factor
Figure 7.
Cytokine concentrations in the BM of irradiated Tie2Bak/BaxFl/− mice. Cytokines which were upregulated or downregulated ≥ 3-fold at 6 hours following 750 cGy in the BM of FL/−-versus FL/+ mice (A) and ≥ 5-fold in FL/− versus C57Bl6 mice (B) are also shown. TNF RII=tumor necrosis factor receptor II, LIX=lipopolysaccharide-induced CXC chemokine, HGF=hepatocyte growth factor, OPG=osteoprotegerin, BLC=B lymphocyte chemoattractant
DISCUSSION
Recent studies have suggested that BM ECs play an essential role in the maintenance of the HSC pool during homeostasis and perhaps in regulating hematologic recovery following myelosuppression [6, 19–21]. However, the precise contribution of BM ECs, either independently or in concert with HSC-autonomous effects, toward regeneration of the HSC pool or the hematopoietic system has yet to be delineated. Here, using a combination of the Cre-LoxP system and BM transplant models, we show that targeted deletion of Bak and Bax in Tie2+ cells protects BM HSCs from radiation-induced depletion compared to mice that retained Bax expression in Tie2+ cells. Moreover, chimeric Tie2Bak/BaxFl/−;WT-EC mice, bearing deletion of Bak and Bax in BM HSCs and retention of Bak and Bax in BM ECs, displayed a significant depletion of BM HSCs following TBI compared to Tie2Bak/BaxFl/− mice in which Bak and Bax were deleted in both BM ECs and HSCs. These results suggest that BM ECs regulate the response of the HSC pool to radiation. In order to test our hypothesis further using a different model, we also examined the hematopoietic response to ionizing radiation in VEcadherinBak/BaxFL/− mice, which have deletion of Bak and Bax in VEcadherin+ ECs. VEcadherinCre mice, unlike Tie2Cre mice, demonstrate Cre activation in only a small subset of hematopoietic cells in the adult mice, so it provides a more EC-specific deletion of target genes [29][30]. Deletion of Bak and Bax in VEcadherin+ ECs did not protect BM HSCs from radiation-induced depletion immediately (+ 2 hrs) after exposure, but HSC and progenitor cell recovery was accelerated in VEcadherinBak/BaxFl/− mice at day +7 following 300 cGy irradiation compared to VEcadherinBak/BaxFl/+ control mice. Taken together, these results suggest an important contribution from BM ECs in regulating the regeneration of hematopoietic stem/progenitor cells following ionizing radiation.
In order to determine whether radioprotection of BM ECs could also improve the survival of mice following lethal dose TBI, we examined the survival of Tie2Bak/BaxFl/− mice, Tie2Bak/BaxFl/−;WT-EC mice and control mice following 750 cGy TBI. The results of these studies suggested that protection of the BM vasculature may improve survival following TBI, since mice bearing deletion of Bak and Bax in BM HSCs and BM ECs had 100% survival following TBI, whereas only 42% of mice which retained Bak and Bax in BM ECs survived, despite deletion of Bak and Bax in BM HSCs. When VEcadherinBak/BaxFL/− mice were exposed to lethal dose TBI, these mice demonstrated a significant increase in 15-day survival compared to VEcadherinBak/BaxFl/+ mice and VEcadherinCre (−) mice, but the effect was not durable and the magnitude of this effect was less than that observed in Tie2Bak/BaxFL/−;WT-EC mice. These results highlight several interesting findings. First, deletion of Bak and Bax in both Tie2+ HSCs and BM ECs provides 100% protection from lethal dose TBI. Second, deletion of Bak and Bax solely in BM HSCs, in the absence of protection of BM ECs, provides incomplete radioprotection which confers significantly lower survival following lethal dose TBI compared to mice with deletion of Bak and Bax in both HSCs and ECs. Third, deletion of Bak and Bax primarily in BM ECs, in the absence of protection of BM HSCs, provides early but incomplete radioprotection of mice following lethal dose TBI. Taken together, these data demonstrate that the hematopoietic response to ionizing radiation is dependent upon HSC-autonomous responses, but is regulated by BM EC-mediated mechanisms.
The results presented here provide important new information about the role of BAK and BAX in regulating radiation-induced injury to HSCs and ECs. Our data also suggest that targeted inhibition of BAK- and BAX-mediated apoptosis in BM ECs has therapeutic potential as a means to promote hematopoietic reconstitution following radiation injury. Since Tie2Bak/BaxFL/+ mice displayed comparable radiosensitivity of the hematopoietic system to C57Bl6 mice, and increased radiosensitivity compared to Tie2Bak/BaxFL/− mice, this suggests that BAK and BAX play a redundant role within the hematopoietic system in initiating the intrinsic pathway of apoptosis. Our findings relating to bone marrow ECs complement studies in other systems which have suggested redundant roles for BAK and BAX in mediating the intrinsic apoptotic pathway [22, 31], but do not support conclusions from a prior study which suggested that BAK and BAX do not exhibit functional redundancy in radiation-induced EC apoptosis in the intestinal mucosa [32]. One possible explanation for these divergent observations is that BAK and BAX may fill overlapping or non-overlapping functions depending upon the organ system. However, in the present study, we provide compelling genetic data that in bone marrow ECs, deletion of both BAK and BAX are required to block radiation-induced apoptosis.
Our observation that deletion of Bak and Bax in Tie2+ cells confers radioprotection on HSCs is consistent with prior studies which demonstrated that deletion of PUMA (p53 upregulated mediator of apoptosis), a member of the BH3 family of pro-apoptotic molecules, protected HSCs from radiation-induced apoptosis [33, 34]. PUMA, along with other BH3 family members (BID and BIM), promotes apoptosis via activation of BAK and BAX or inhibition of anti-apoptotic BCL-2/BCL-XL/MCL-1 proteins [35]. The unique finding here is the demonstration that genetic deletion of Bak and Bax expression in BM ECs modulated BM hematopoietic stem and progenitor cell regeneration following TBI. Therefore, targeted inhibition of pro-apoptotic proteins in BM ECs may have therapeutic potential as a means to augment the regeneration of the hematopoietic system after radiation-induced failure.
In recent years, it has become increasingly evident that cells within the BM microenvironment regulate HSC homeostasis in vivo [1, 3–9]. A role for BM ECs and osteoblasts in regulating hematopoietic regeneration following myelosuppression has also been suggested [2, 21]. However, the precise mechanisms through which BM microenvironmental cells regulate HSC regeneration and hematopoietic reconstitution remain unclear. Nonetheless, the translational potential of targeting niche elements as a means to augment hematopoiesis and hematopoietic reconstitution has been demonstrated [1, 2, 14]. Calvi et al. [1] showed that systemic administration of parathyroid hormone (PTH), which activates the PTH receptor on BM osteoblasts, to wild type mice for 5 weeks caused a significant increase in BM HSCs in vivo. Adams et al. [2] showed that PTH-mediated augmentation of BM osteoblast function amplified GCSF-induced mobilization of BM hematopoietic progenitors into the PB [2], and protected the BM progenitor cell compartment from the toxic effects of recurrent exposure to cyclophosphamide treatment [2]. Therefore, pharmacologic targeting of the HSC niche represents a promising approach for augmentation of the HSC pool and hematopoietic regeneration in vivo. Here, we demonstrate via a genetic approach that BM ECs modulate HSC regeneration, hematopoietic reconstitution and overall survival in mice following TBI. Utilizing cytokine array analysis of BM supernatants from Tie2Bak/BaxFL/− mice and Tie2Bak/BaxFL/+ mice and C57Bl6 mice, we have identified several soluble proteins and candidate signaling pathways which can now be interrogated to determine their specific role in regulating the hematopoietic response to ionizing radiation. We anticipate that these studies will reveal novel mechanisms through which BM ECs regulate HSC regeneration after injury.
Supplementary Material
Acknowledgments
The authors wish to acknowledge Dr. Tannishtha Reya for critical comments on the manuscript. This work was supported in part by NHLBI grant HL-086998-01 (JPC) and NIAID grant AI-067798-06 (JPC) and a pilot project from the NIAID Centers for Medical Countermeasures grant AI-067798-01 (DGK). P.L.D. was supported by NIH training grant, T32 HL0070757-33 and the Barton Haynes Award (Duke University).
Footnotes
AUTHOR CONTRIBUTIONS
Phuong L. Doan: Collection and assembly of data, Data analysis and interpretation, Manuscript writing
J. Lauren Russell: Collection and/or assembly of data, Data analysis and interpretation
Heather A. Himburg: Collection and/or assembly of data
Katherine Helms: Collection and/or assembly of data
Jeffrey Harris: Collection and/or assembly of data
Joseph Lucas: Data analysis and interpretation
Kirsten Holshausen: Collection and/or assembly of data
Sarah K. Meadows: Collection and/or assembly of data
Laura B. Jeffords: Provision of study materials, Collection and/or assembly of data
Nelson J. Chao: Data analysis and interpretation, Manuscript writing, Final approval of manuscript
David G. Kirsch: Data analysis and interpretation, Manuscript writing, Final approval of manuscript
John P. Chute: Conception and design, Data analysis and interpretation, Manuscript writing, Final approval of manuscript
DISCLOSURE OF POTENTIAL CONFLICTS OF INTEREST: Dr. John Chute is a scientific advisor to Becton Dickinson (Durham, NC).
References
- 1.Calvi LM, Adams GB, Weibrecht KW, et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425:841–846. doi: 10.1038/nature02040. [DOI] [PubMed] [Google Scholar]
- 2.Adams GB, Martin RP, Alley IR, et al. Therapeutic targeting of a stem cell niche. Nat Biotechnol. 2007;25:238–243. doi: 10.1038/nbt1281. [DOI] [PubMed] [Google Scholar]
- 3.Arai F, Hirao A, Ohmura M, et al. Tie2/angiopoietin-1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell. 2004;118:149–161. doi: 10.1016/j.cell.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 4.Kiel MJ, Yilmaz OH, Iwashita T, et al. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121:1109–1121. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 5.Sugiyama T, Kohara H, Noda M, et al. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity. 2006;25:977–988. doi: 10.1016/j.immuni.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 6.Ding L, Saunders TL, Enikolopov G, et al. Endothelial and perivascular cells maintain haematopoietic stem cells. Nature. 2012;481:457–462. doi: 10.1038/nature10783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mendez-Ferrer S, Michurina TV, Ferraro F, et al. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466:829–834. doi: 10.1038/nature09262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Katayama Y, Battista M, Kao WM, et al. Signals from the sympathetic nervous system regulate hematopoietic stem cell egress from bone marrow. Cell. 2006;124:407–421. doi: 10.1016/j.cell.2005.10.041. [DOI] [PubMed] [Google Scholar]
- 9.Naveiras O, Nardi V, Wenzel PL, et al. Bone-marrow adipocytes as negative regulators of the haematopoietic microenvironment. Nature. 2009;460:259–263. doi: 10.1038/nature08099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blank U, Karlsson G, Karlsson S. Signaling pathways governing stem-cell fate. Blood. 2008;111:492–503. doi: 10.1182/blood-2007-07-075168. [DOI] [PubMed] [Google Scholar]
- 11.Zon LI. Intrinsic and extrinsic control of haematopoietic stem-cell self-renewal. Nature. 2008;453:306–313. doi: 10.1038/nature07038. [DOI] [PubMed] [Google Scholar]
- 12.Dahlberg A, Delaney C, Bernstein ID. Ex vivo expansion of human hematopoietic stem and progenitor cells. Blood. 2011;117:6083–6090. doi: 10.1182/blood-2011-01-283606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chute JP, Saini AA, Chute DJ, et al. Ex vivo culture with human brain endothelial cells increases the SCID-repopulating capacity of adult human bone marrow. Blood. 2002;100:4433–4439. doi: 10.1182/blood-2002-04-1238. [DOI] [PubMed] [Google Scholar]
- 14.Chute JP, Muramoto GG, Fung J, et al. Soluble factors elaborated by human brain endothelial cells induce the concomitant expansion of purified human BM CD34+CD38− cells and SCID-repopulating cells. Blood. 2005;105:576–583. doi: 10.1182/blood-2004-04-1467. [DOI] [PubMed] [Google Scholar]
- 15.Himburg HA, Muramoto GG, Daher P, et al. Pleiotrophin regulates the expansion and regeneration of hematopoietic stem cells. NAT MED. 2010;16:475–482. doi: 10.1038/nm.2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chute JP, Fung J, Muramoto G, et al. Ex vivo culture rescues hematopoietic stem cells with long-term repopulating capacity following harvest from lethally irradiated mice. Exp Hematol. 2004;32:308–317. doi: 10.1016/j.exphem.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 17.Muramoto GG, Chen B, Cui X, et al. Vascular endothelial cells produce soluble factors that mediate the recovery of human hematopoietic stem cells after radiation injury. Biol Blood Marrow Transplant. 2006;12:530–540. doi: 10.1016/j.bbmt.2005.12.039. [DOI] [PubMed] [Google Scholar]
- 18.Chute JP, Muramoto GG, Salter AB, et al. Transplantation of vascular endothelial cells mediates the hematopoietic recovery and survival of lethally irradiated mice. Blood. 2007;109:2365–2372. doi: 10.1182/blood-2006-05-022640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Salter AB, Meadows SK, Muramoto GG, et al. Endothelial progenitor cell infusion induces hematopoietic stem cell reconstitution in vivo. Blood. 2009;113:2104–2107. doi: 10.1182/blood-2008-06-162941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Butler JM, Nolan DJ, Vertes EL, et al. Endothelial cells are essential for the self-renewal and repopulation of Notch-dependent hematopoietic stem cells. Cell Stem Cell. 2010;6:251–264. doi: 10.1016/j.stem.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hooper AT, Butler JM, Nolan DJ, et al. Engraftment and reconstitution of hematopoiesis is dependent on VEGFR2-mediated regeneration of sinusoidal endothelial cells. Cell Stem Cell. 2009;4:263–274. doi: 10.1016/j.stem.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kirsch DG, Santiago PM, di Tomaso E, et al. p53 controls radiation-induced gastrointestinal syndrome in mice independent of apoptosis. Science. 327:593–596. doi: 10.1126/science.1166202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Purton LE, Scadden DT. Limiting factors in murine hematopoietic stem cell assays. Cell Stem Cell. 2007;1:263–270. doi: 10.1016/j.stem.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 24.Kopp HG, Avecilla ST, Hooper AT, et al. Tie2 activation contributes to hemangiogenic regeneration after myelosuppression. Blood. 2005;106:505–513. doi: 10.1182/blood-2004-11-4269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Branda CS, Dymecki SM. Talking about a revolution: The impact of site-specific recombinases on genetic analyses in mice. Dev Cell. 2004;6:7–28. doi: 10.1016/s1534-5807(03)00399-x. [DOI] [PubMed] [Google Scholar]
- 26.Hahn P, Lindsten T, Tolentino M, et al. Persistent fetal ocular vasculature in mice deficient in bax and bak. Arch Ophthalmol. 2005;123:797–802. doi: 10.1001/archopht.123.6.797. [DOI] [PubMed] [Google Scholar]
- 27.Lindsten T, Ross AJ, King A, et al. The combined functions of proapoptotic Bcl-2 family members bak and bax are essential for normal development of multiple tissues. Molecular Cell. 2000;6:1389–1399. doi: 10.1016/s1097-2765(00)00136-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yilmaz OH, Kiel MJ, Morrison SJ. SLAM family markers are conserved among hematopoietic stem cells from old and reconstituted mice and markedly increase their purity. Blood. 2006;107:924–930. doi: 10.1182/blood-2005-05-2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alva JA, Zovein AC, Monvoisin A, et al. VE-Cadherin-Cre-recombinase transgenic mouse: a tool for lineage analysis and gene deletion in endothelial cells. Dev Dyn. 2006;235:759–767. doi: 10.1002/dvdy.20643. [DOI] [PubMed] [Google Scholar]
- 30.Lee CL, Moding EJ, Cuneo KC, et al. p53 Functions in Endothelial Cells to Prevent Radiation-Induced Myocardial Injury in Mice. Sci Signal. 2012;5:ra52. doi: 10.1126/scisignal.2002918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kandasamy K, Srinivasula SM, Alnemri ES, et al. Involvement of proapoptotic molecules Bax and Bak in tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced mitochondrial disruption and apoptosis: differential regulation of cytochrome c and Smac/DIABLO release. Cancer Res. 2003;63:1712–1721. [PubMed] [Google Scholar]
- 32.Rotolo JA, Maj JG, Feldman R, et al. Bax and Bak do not exhibit functional redundancy in mediating radiation-induced endothelial apoptosis in the intestinal mucosa. Int J Radiat Oncol Biol Phys. 2008;70:804–815. doi: 10.1016/j.ijrobp.2007.11.043. [DOI] [PubMed] [Google Scholar]
- 33.Yu H, Shen H, Yuan Y, et al. Deletion of Puma protects hematopoietic stem cells and confers long-term survival in response to high-dose gamma-irradiation. Blood. 2010;115:3472–3480. doi: 10.1182/blood-2009-10-248278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shao L, Sun Y, Zhang Z, et al. Deletion of proapoptotic Puma selectively protects hematopoietic stem and progenitor cells against high-dose radiation. Blood. 2010;115:4707–4714. doi: 10.1182/blood-2009-10-248872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim H, Tu HC, Ren D, et al. Stepwise activation of BAX and BAK by tBID, BIM, and PUMA initiates mitochondrial apoptosis. Mol Cell. 2009;36:487–499. doi: 10.1016/j.molcel.2009.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.