Highlights
► COMT gene can influence cognitive function in developmental stage specific manner. ► A large UK population-based sample with cognition measured at ages 8 and 15 years was used. ► Five functional COMT SNPs were tested for association with cognition. ► COMT rs737865 showed association with reading comprehension, verbal ability and global cognition at age 15 years in pubescent boys only. ► Further studies are necessary in order to make stronger conclusions.
Keywords: Dopamine, Birth cohort, Longitudinal study, Adolescent, Puberty
Abstract
Genetic variation in the catechol-O-methyltransferase gene (COMT) can influence cognitive function, and this effect may depend on developmental stage. Using a large representative British birth cohort, we investigated the effect of COMT on cognitive function (verbal and non-verbal) at ages 8 and 15 years taking into account the possible modifying effect of pubertal stage. Five functional COMT polymorphisms, rs6269, rs4818, rs4680, rs737865 and rs165599 were analysed. Associations between COMT polymorphisms and cognition were tested using regression and latent variable structural equation modelling (SEM). Before correction for multiple testing, COMT rs737865 showed association with reading comprehension, verbal ability and global cognition at age 15 years in pubescent boys only. Although there was some evidence for age- and sex-specific effects of the COMT rs737865 none remained significant after correction for multiple testing. Further studies are necessary in order to make firmer conclusions.
1. Introduction
Genetic variation in the catechol-O-methyltransferase (COMT) gene is likely to be particularly important for phenotypes associated with function of the prefrontal cortex (PFC), such as cognition (Dickinson and Elvevag, 2009). Neuroimaging studies confirm that the COMT Val158Met (rs4680) polymorphism affects human prefrontal cortical function, and as such is strongly associated with differences in neural process underlying cognitive output (Dennis et al., 2010; Mier et al., 2009). However, these findings do not necessarily imply any change in cognition. A recent meta-analysis observed no significant effect of the Val158Met single nucleotide polymorphism (SNP) on frontal cognitive tasks (Barnett et al., 2008). Nevertheless, some studies have indicated that this association might be specific to developmental stage (Barnett et al., 2007; Dumontheil et al., 2011; Raz et al., 2011). Thus further examination of COMT genetic variation is required for a better understanding of its role in a wider range of cognitive functions during development.
Relatively little is known about the role of COMT in cognition in children (Diamond et al., 2004; Dumontheil et al., 2011), and specifically in relation to developmental stages, such as puberty (Barnett et al., 2007). The cognitive effects attributable to COMT activity may depend on developmental stage because structural and functional changes occur in the PFC during adolescence (Casey et al., 2000; De Luca et al., 2003; Rubia et al., 2000; Supekar et al., 2010); cognitive functions performed by the PFC in adults may be governed by different or more diffuse circuits in children. If so, then COMT variation may have little effect on cognitive performance during childhood, but a stronger effect in adolescence. Moreover, increases in the level of reproductive hormones such as estrogen during puberty can down-regulate COMT transcription and lead to a sex difference in COMT activity, and therefore to a different effect of COMT on cognition in boys and girls (Tunbridge, 2010). Among 8-year-old children from the Avon Longitudinal Study of Parents and Children (ALSPAC) cohort Val158Met polymorphism was reported to have a larger effect on verbal IQ in pubertal children when compared with prepubertal children (Barnett et al., 2007). A recent study of 6–20-year-olds also suggests the role of development in the effect of COMT Val158Met polymorphism on working memory, specifically that visuospatial working memory capacity exhibited an age by genotype interaction, with a benefit of the Met allele (rs4680) emerging after 10 years of age (Dumontheil et al., 2011). However, these studies were cross-sectional in design, so did not investigate the genetic effect on cognition in the same children at different developmental stages.
Despite strong evidence for the biological importance of several COMT SNPs (Nackley et al., 2006) little is known about associations with cognition of any loci other than Val158Met. A functional three-SNP haplotype consists of Val158Met (rs4680) and two synonymous SNPs (rs6269 and rs4818): ValA/ValA, ValA/Met, ValA/ValB or Met/Met, ValB/Met, and ValB/ValB are diplotypes ranked from highest to lowest according to COMT enzyme activity. This haplotype exerts a major influence on the level of COMT expression and enzyme activity (Nackley et al., 2006) and has previously been shown to have a curvilinear association with measures of verbal inhibition and working memory (Barnett et al., 2009). Previous studies have also reported associations between rs165599 (located near the 3′UTR region) (Burdick et al., 2007; Chan et al., 2005), and rs737865 (located in intron 1) (Diaz-Asper et al., 2008; Liao et al., 2009) and cognitive function. These SNPs appear to be functional, with rs737865 G (=C) allele and rs165599 G (=C) allele being associated with lower expression of COMT mRNA in the human brain (Bray et al., 2003).
In the present study we include the Nackley's haplotype (rs6269–rs4818–rs4680) and the two functional SNPs, rs737865 and rs165599, to characterize better the combined effects of variation in the COMT gene on cognitive function. Using data from the British 1946 birth cohort we aimed: (1) to investigate the effect of the five COMT SNPs on cognitive function in the same boys and girls at two time-points (age 8 and 15 year follow-ups); (2) to test whether pubertal stage of cohort members established at the second assessment (age 15 years) modifies any observed associations.
2. Methods
2.1. Sample
The Medical Research Council (MRC) National Survey of Health and Development (NSHD) (also known as the British 1946 birth cohort) is a socially stratified birth cohort of 5362 individuals (2547 women and 2815 men), who have been followed up since their birth in 1946 with regular data collections (Wadsworth et al., 2003). Blood samples were collected from 2756 members at age 53 years. Every survey member with information on at least one cognitive test (phenotype indicator) at both age 8 and age 15 years and DNA genotyped for COMT SNPs was included in the descriptive analysis (n = 1029 boys and 1048 girls). Survey members with available DNA had higher cognitive scores on all cognitive tests at ages 8 and 15 years than those without genetic information; but were not different with respect to pubertal stage at age 15 years (p = 0.35) or social class of origin (p = 0.52). The results of comparing those with DNA and those without on cognitive tests measures are presented in Supplementary Table 1.
Ethical approval for this research was obtained from the North Thames Multi-Centre Research Ethics Committee, and from relevant local research ethics committees in the survey areas. Informed consent was given by all respondents.
2.2. Measures
2.2.1. Cognitive function
Children were assessed by teachers in a school setting at ages 8 and 15 years using tests devised by the National Foundation for Educational Research (Pigeon and Douglas, 1964; Pigeon et al., 1968). At age 8 years these were: (1) reading comprehension (selecting appropriate words to complete 35 sentences); (2) word reading (ability to read and pronounce 50 words); (3) vocabulary (ability to explain the meaning of the same 50 words); and (4) picture intelligence, consisting of a 60-item non-verbal reasoning test. At age 15 years these were: (1) Alice Heim Group Ability Test (AH4), a 130 item timed test, with separate verbal (analogies, comprehension, and numerical reasoning) and non-verbal (matching, spatial analysis, and non-verbal reasoning sections) tests; (2) The Watts-Vernon Reading Test, a test of reading comprehension requiring the participant to select appropriate words to complete 35 sentences; (3) A 47-item mathematics test, requiring the use of arithmetic, geometry, trigonometry, and algebra.
All scores reported in the results were standardised within the sample included in this analysis to a mean of 0 and a standard deviation of 1.
2.2.2. Pubertal status
At age 15 years, pubertal development in boys was classified at school by a physician based on the development of genitalia, presence of pubic hair, axillary hair and voice broken. Those with infantile genitalia or early adolescent genitalia, but no pubic or axillary hair and voice not broken were classified as prepubescent, all others as pubescent. Age at menarche was used as the marker of pubertal stage for girls, and was obtained from mothers’ reports in 1961. This information was used to construct a binary variable for pubertal status distinguishing between those who had menarche by age 15 (pubescent) and those who did not (prepubescent). The majority of the participants (89% of boys and 90% of girls) had reached pubertal status by age 15.
2.3. Genotyping
DNA was extracted and purified from whole blood using the Puregene DNA Isolation Kit (Flowgen, Leicestershire, UK) according to the manufacturer's protocol. The five SNPs, rs737865, rs6269, rs4818, rs4680 and rs165599, were typed by using the KASPar system by KBioscience, UK (www.kbioscience.co.uk). The integrity of the genotyping was checked by genotyping frequency, concordance of duplicates and Hardy–Weinberg equilibrium (HWE). The call rates for the genotyped SNPs were 97.8–99.2%, with >95% concordance between duplicate samples and there was no evidence of deviation from HWE (p > 0.05).
The programme PLINK v1.07 was used for haplotype analysis (Purcell et al., 2007). The haplotype frequencies (rs6269–rs4818–rs4680) were similar to those reported in the original paper (Nackley et al., 2006): GGG (=ValA)–40.8%, ACA (=Met)–50.8%, ACG (=ValB)–7.8%, all others–0.6%. The survey members were then assigned to one of six possible diplotypes (i.e., the pair of haplotypes) using a ‘phase’ option (Table 1): ValA/ValA, ValA/Met, ValA/ValB or Met/Met, ValB/Met, and ValB/ValB.
Table 1.
Descriptives for cognitive phenotypes and COMT genotypes/diplotypes by sex.
| Phenotype data | Boys |
Girls |
p | ||
|---|---|---|---|---|---|
| n total | Mean (SD) | n total | Mean (SD) | ||
| Cognition at age 8 | |||||
| Word reading | 1024 | −0.07 (1.04) | 1046 | 0.07 (0.96) | 0.002 |
| Vocabulary | 1024 | 0.04 (1.00) | 1046 | −0.04 (1.00) | 0.06 |
| Reading comprehension | 1024 | −0.07 (1.03) | 1046 | 0.07 (0.96) | 0.001 |
| Picture intelligence | 1029 | 0.01 (1.01) | 1046 | −0.01 (1.00) | 0.75 |
| Cognition at age 15 | |||||
| AH4 Verbal Ability | 1027 | 0.02 (1.01) | 1047 | −0.02 (0.98) | 0.26 |
| Reading Comprehension | 1029 | −0.06 (1.02) | 1047 | 0.06 (0.97) | 0.007 |
| Mathematics | 1028 | 0.18 (1.06) | 1047 | −0.17 (0.91) | <0.0001 |
| AH4 Non-verbal Ability | 1028 | 0.09 (1.00) | 1048 | −0.09 (1.00) | <0.0001 |
| Genotype data | n | Freq (%) | n | Freq (%) | p |
|---|---|---|---|---|---|
| COMT genotypes | |||||
| rs737865: CC/CT/TT | 995 | 9.8/38.3/51.9 | 1031 | 8.7/41.2/50.1 | 0.36 |
| rs6269: GG/AG/AA | 1016 | 17.6/48.2/34.2 | 1038 | 17.0/46.9/36.1 | 0.64 |
| rs4818: GG/CG/CC | 1020 | 17.8/47.8/34.4 | 1037 | 16.6/47.2/36.2 | 0.62 |
| rs4680: GG/AG/AA | 1020 | 24.5/50.8/24.7 | 1034 | 23.1/50.4/26.5 | 0.58 |
| rs165599: GG/AG/AA | 1008 | 9.8/40.7/49.5 | 1028 | 9.2/41.3/49.4 | 0.89 |
| COMT diplotypes* | |||||
| ValA/ValA | 181 | 17.8 | 175 | 16.9 | |
| ValA/Met | 428 | 42 | 426 | 41.1 | |
| ValA/ValB or Met/Met | 315 | 31 | 334 | 32.3 | |
| ValB/Met | 86 | 8.5 | 93 | 9 | |
| ValB/ValB | 7 | 0.7 | 7 | 0.7 | 0.94 |
Note: COMT diplotype = rs6269–rs4818–rs4680; reference groups are CC–for rs737865, GG–for 165599, ValA/ValA–for Nackley's diplotype.
2.4. Statistical analysis
Of the five genotyped COMT SNPs, the central three, rs6269, rs4818 and rs4680, were in high linkage disequilibrium (LD): r2 = 0.72 for rs6269–rs4680 and rs4818–rs4680; and r2 = 0.97 for rs6269–rs4818. In contrast, the LD between the three SNPs in the haplotype block and the other two SNPs was low (all r2 < 0.38). We therefore chose to separately test associations between cognitive scores and rs737865 and rs165599 and with the three-SNP haplotype.
First, linear regression was used to test for associations between rs737865, rs165599 genotypes (under an additive model), the three-SNP haplotype and the cognitive measures. In addition, curvilinear regression was used to test for associations between the three-SNP haplotype and the cognitive measures. Analyses were performed separately for boys and girls, and the sex-by-genotype interaction term was fitted to test for sex differences. At age 15 years, analyses were also stratified by pubertal stage, and the puberty-by-genotype interaction term was fitted to test differences between pubertal and pre-pubertal groups.
The effect of COMT genotypes on global cognitive function in a longitudinal context was examined using structural equation modelling (SEM) (Schumacker and Lomax, 2004). Model estimation was performed with Mplus version 6 (Muthen and Muthen, 2007). The model fit was evaluated with recommended fit indices (Hu and Bentler, 1999): the Tucker–Lewis index (TLI), the root mean square error of approximation (RMSEA), and the comparative fit index (CFI).
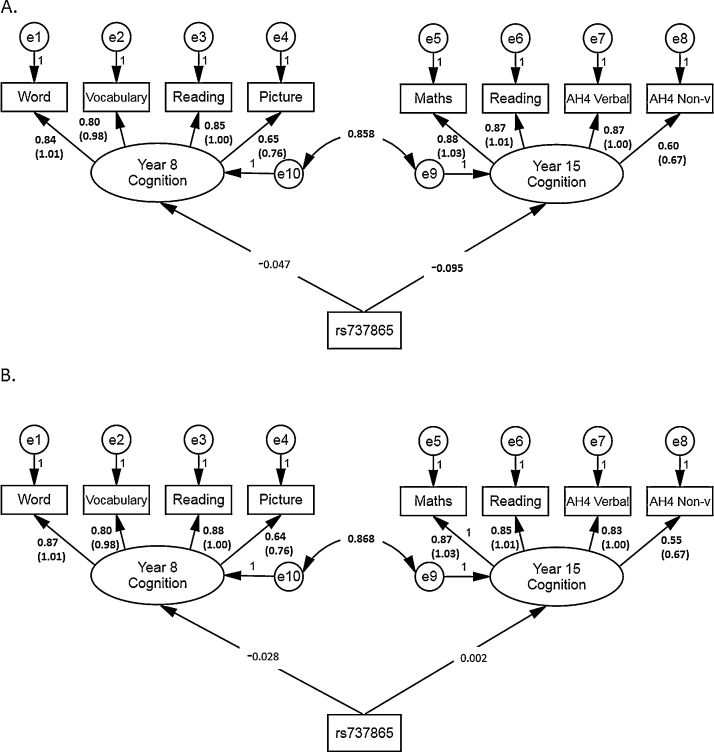
A graphical depiction of the model is shown in Fig. 2. The measurement part of the SEM model represents overall cognitive function at ages 8 and 15 years. The structural part includes direct paths from the COMT genotype to cognition at age 8 and age 15. We fitted the model using multiple group analysis (Byrne, 2004). In the final analytic model, the factor loadings of global cognition factors were constrained to be invariant across gender groups, whereas the path coefficients from COMT genotype to cognition were freely estimated.
Fig. 2.
SEM of longitudinal effect of COMT rs737865 on cognition at ages 8 and 15 years in boys (n = 1134, (a) and girls (n = 1157, (b); word = word reading, reading = reading comprehension, picture = picture intelligence, AH4 verbal = AH4 verbal ability, AH4 non-v = AH4 non-verbal ability; coefficients with p < 0.05 are in bold. For factor loadings, both standardised and unstandardised (in brackets) coefficients are presented.
The item intercepts were freely estimated in both groups because the main parameters of interest are path coefficients, hence invariance of factor loadings are sufficient (see (Gregorich, 2006), for a detailed discussion on level of measurement invariance). We performed the Wald χ2 test of parameter equalities for gender group differences in the structural regression paths.
Since SNPs associations were examined in multiple group SEM with cognition modelled as a latent variable, it was important to examine whether the measures of cognition were comparable across the gender groups (Byrne, 2004; Meredith, 1993). Two confirmatory factor analysis (CFA) models with different degrees of measurement parameter restrictions were specified in order to assess the extent to which the validity of the comparison of path coefficients across groups held. The baseline model tested configural invariance where latent global cognition variable had the same number of factor indicators, i.e. same number of items representing specific test domains in male and female groups. In this model, the factor loadings across gender groups were freely estimated. This model was a prerequisite for testing the next step, the metric invariance, where the factor loadings were constrained to be equal across groups. The measurement invariance in factor loadings ensures that the global cognition construct has the same substantive meaning across gender groups, thus warranting valid comparison of regression path coefficients in the SEM model. Then model fit indices of the two models were compared to evaluate the degree of measurement invariance of the loading parameters in the models.
We used the same range of the above-mentioned fit indices to investigate models of measurement invariance. The restrictive model is preferred if the fit indices are not significantly inferior compared to that of the less restrictive model. In terms of the RMSEA, the change should be less than .015 (Chen, 2007). For CFI, the change should be less than .01 in CFI (Chen, 2007; Cheung and Rensvold, 2001). We also presented the TLI and Chi-square as overall tests for goodness of fit (Marsh et al., 1998).
A Bonferroni correction was applied in an attempt to address the issue of multiple testing. The total number of independent tests was 15 (two individual SNPs plus one haplotype in two gender groups at two ages plus three tests [two SNPs and one haplotype] for pubertal status at age 15 in boys only). We did not treat each cognitive test as independent as they were highly inter-correlated (r = 0.6–0.9). This approach to inferences on independent tests required that the conservative α-level of 0.0033 to be used as the significance level for robust inferences.
3. Results
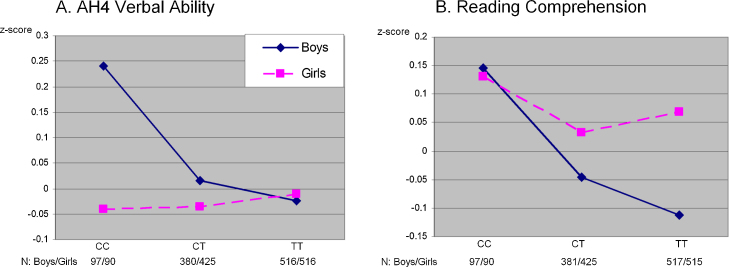
Descriptive statistics for the phenotype measures and genetic data, by sex, are presented in Table 1. The results of association analysis between the COMT SNPs and cognitive traits at age 8 and 15 years are presented separately for boys and girls in Table 2. There were no associations between rs737865, or rs165599 or diplotype and cognitive measures at age 8 years in either boys or girls. The regression analysis for cognition at age 15 years identified associations between rs737865 and AH4 verbal ability (β = −0.106, SE = 0.049, p = 0.031), and reading comprehension (β = −0.098, SE = 0.049, p = 0.044), but only in boys (Fig. 1). Boys with CC genotype had higher scores than those–carriers of T allele (both p's < 0.05). None of the findings survived p-value correction for multiple testing (α-level = 0.0033).
Table 2.
Results of linear regression analysis for the association between COMT and cognitive function at ages 8 years and 15 years in boys and girls.
| Cognitive measures | Boys |
Girls |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| rs737865 |
rs165599 |
Diplotypes* |
rs737865 |
rs165599 |
Diplotypes |
|||||||||||||
| n | β | p | n | β | p | n | β | p | n | β | p | n | β | p | n | β | p | |
| Age 8 | ||||||||||||||||||
| Word reading | 990 | −0.050 | 0.32 | 1003 | −0.033 | 0.50 | 1012 | −0.023 | 0.54 | 1029 | −0.068 | 0.14 | 1026 | −0.044 | 0.33 | 1033 | −0.012 | 0.71 |
| Vocabulary | 990 | −0.026 | 0.60 | 1003 | −0.005 | 0.91 | 1012 | −0.019 | 0.59 | 1029 | −0.024 | 0.61 | 1026 | −0.013 | 0.78 | 1033 | −0.016 | 0.65 |
| Reading comprehension | 990 | −0.083 | 0.09 | 1003 | −0.009 | 0.86 | 1012 | −0.017 | 0.64 | 1029 | −0.014 | 0.76 | 1026 | −0.013 | 0.78 | 1033 | −0.002 | 0.96 |
| Picture intelligence | 995 | 0.006 | 0.89 | 1008 | −0.003 | 0.96 | 1017 | 0.015 | 0.68 | 1029 | −0.037 | 0.43 | 1026 | −0.047 | 0.33 | 1033 | −0.007 | 0.83 |
| Age 15 | ||||||||||||||||||
| Reading Comprehension | 993 | −0.098 | 0.044 | 1006 | 0.019 | 0.70 | 1015 | −0.033 | 0.36 | 1031 | 0.019 | 0.70 | 1027 | −0.013 | 0.78 | 1034 | −0.030 | 0.39 |
| AH4 Verbal Ability | 995 | −0.106 | 0.031 | 1008 | −0.012 | 0.81 | 1017 | −0.044 | 0.22 | 1030 | −0.004 | 0.94 | 1027 | −0.018 | 0.7 | 1034 | 0.011 | 0.75 |
| AH4 Non-verbal Ability | 994 | −0.024 | 0.61 | 1007 | 0.042 | 0.38 | 1016 | −0.007 | 0.85 | 1031 | −0.023 | 0.63 | 1028 | −0.067 | 0.16 | 1035 | −0.039 | 0.26 |
| Mathematics | 994 | −0.086 | 0.09 | 1007 | −0.033 | 0.52 | 1016 | −0.018 | 0.64 | 1030 | −0.017 | 0.68 | 1027 | −0.083 | 0.06 | 1034 | −0.025 | 0.43 |
Note: *rs6269–rs4818–rs4680; reference groups are CC–for rs737865, GG–for 165599, ValA/ValA–for Nackley's diplotype. β based on standardized outcomes and unstandardized predictor.
Fig. 1.
Sex-specific associations between COMT rs737865 and cognitive function at age 15 years.
There were no associations between this SNP and level of cognitive functions in girls. Sex differences were tested by interaction terms but found not to be statistically significant at α-level of 0.0033: p for sex interaction = 0.13 and 0.09 for verbal ability and reading comprehension, respectively. There were no associations between diplotypes and any of the individual cognitive tests at age 15 years using linear (Table 2) or curvilinear (Supplementary Table 2) regression models.
The effect of pubertal stage on the associations between rs737865 and reading comprehension and verbal ability was then tested. In boys, we observed the similar effects of the SNP on verbal ability (β = −0.123, SE = 0.051, p = 0.016) and reading comprehension (β = −0.110, SE = 0.051, p = 0.031) in those who reached puberty by age 15 years. There were no associations between rs737865 and either verbal ability (β = 0.062, SE = 0.124, p = 0.62) or reading comprehension (β = −0.026, SE = 0.127, p = 0.84) in prepubescent boys. There was no statistically significant difference between prepubescent and pubescent groups: p-values for the puberty interaction tests were 0.18 and 0.17 for verbal ability and reading comprehension respectively.
We tested for the longitudinal effect of COMT rs737865, rs165599 and diplotype on cognition using SEM model for boys and girls (Fig. 2 for rs737865). Individual cognitive tests were modelled as components of global cognition at both ages. Results of modelling to test for measurement invariance of global cognitive measures showed that the less restrictive model demonstrated a good fit to the data (chi2 = 512.944, df = 34, CFI = 0.980, TLI = 0.968, RMSEA = 0.080). The second model with more restrictive equal factor loadings across gender groups showed even better fit indices due to model parsimony (chi2 = 560.089, df = 40, CFI = 0.979, TLI = 0.970, RMSEA = 0.076), supporting the invariance of factor loadings across gender groups. This provided a sufficient condition for comparison of the COMT SNP association with global cognitive measures across both genders. A multiple group SEM model was fitted with rs737865 as predictor of the global cognition (TLI = 0.971, CFI = 0.979, RMSEA = 0.065). The results of the SEM estimation suggested the effect of rs737865 on general cognition at age 15 in boys only (Table 3; Fig. 2), although the results of the Wald test for gender group differences in the structural regression paths were non-significant (χ2 = 2.19, p = 0.14).
Table 3.
Results of SEM of longitudinal effect of COMT on global cognition at ages 8 and 15 years.
| Boys |
Girls |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | Age 8 |
Age 15 |
n | Age 8 |
Age 15 |
|||||
| β | p | β | p | β | p | β | p | |||
| rs737865 | 1134 | −0.047 | 0.341 | −0.095 | 0.046 | 1157 | −0.028 | 0.568 | 0.002 | 0.971 |
| rs165599 | 1146 | 0.018 | 0.718 | 0.012 | 0.802 | 1156 | −0.037 | 0.449 | −0.06 | 0.22 |
| Diplotype* | 1156 | −0.01 | 0.792 | −0.033 | 0.362 | 1163 | −0.016 | 0.662 | −0.029 | 0.415 |
Note: Diplotype = rs6269–rs4818–rs4680; reference groups are CC–for rs737865, GG–for 165599, ValA/ValA–for Nackley's diplotype. β based on standardised outcomes and unstandardized predictor.
Stratified analysis showed that the association between rs737865 and global cognition at age 15 was significant in pubescent (β = −0.11, SE = 0.05, p = 0.037), but not in prepubescent (β = −0.01, SE = 0.16, p = 0.96) boys, although the Wald test revealed that the differences by developmental stage were not statistically significant (χ2 = 0.34, p = 0.56).
The results of the SEMs for rs165599 and diplotype provided no evidence for the association with cognitive function at age 8 or 15 years (Table 3).
4. Discussion
After correcting for multiple testing, the present study failed to demonstrate a significant effect of the five COMT SNPs on cognition in boys and girls at ages 8 and 15 years, providing little evidence that COMT variation can have an effect on cognitive abilities in childhood and adolescence.
However, several limitations should be taken into account when interpreting the present findings. Losses to follow-up and missing data are unavoidable in long running birth cohort studies such as the NSHD. There were differences in cognitive measures between those with DNA and those without DNA. This potentially could lead to underestimation of the effect of the COMT gene on cognition. However, that would be the case if the association operates differently in those with lower scores of cognitive abilities. We did not formally test for population stratification; however the 1946 birth cohort represents the general population of Britain of the middle of 20th century, which is of white Caucasian origin.
The strength of the study is its representative large sample. Yet, given the small number of prepubescent adolescents, there might still not be enough power to detect the small effect of the COMT individual SNPs or diplotypes in the groups stratified by pubertal status at age 15 years. In light of the possibility of the Type II error, the present results should be interpreted with caution. Indeed, the effect sizes of the original regressions were small (<1% of the common variance), and the results did not withstand correction for multiple testing.
Another strength of the study is the longitudinal analysis of the COMT gene in cognitive function using the SEM approach. The SEM approach allows for greater precision in phenotype measurement due to correction for measurement error in the cognitive outcomes, which may otherwise have reduced the statistical power to detect any robust associations with these genes (van der Sluis et al., 2012)
Before applying Bonferroni correction for multiple testing, only one COMT SNP, rs737865, was associated with verbal cognition in pubescent boys at age 15. Therefore, our finding did not confirm the results in the ALSPAC cohort showing a significant effect of COMT rs4680 on verbal IQ in pubescent boys (Barnett et al., 2007). The results of the analysis of COMT diplotypes on cognitive functions in children were not statistically significant and did not confirm the previous findings of curvilinear association between COMT diplotypes and cognition in ALSPAC cohort (Barnett et al., 2008).
We believe that testing for the effect of the COMT gene on various cognitive abilities is important for several reasons. It has been demonstrated that COMT protein has the strongest effect on the dopamine neurotransmission in the PFC, the brain region that plays an important role in a wide variety of cognitive functions, including cognitive control and IQ (Green et al., 2012). Moreover, performances on diverse tests of cognitive function tend to correlate; this underlying covariance represents general cognitive ability (‘g’). It is therefore logical that COMT could affect general intelligence as well as specific executive tasks (Duncan et al., 2000)
In our study, we were able to test the effect of the COMT gene on cognitive abilities at different developmental stages. It has been reported, that the heritability of general cognitive ability increases significantly and linearly from childhood through young adulthood (Haworth et al., 2010). In line with this observation, a recent study of 6–20-year-olds showed that visuospatial working memory capacity exhibited an age by genotype interaction with a benefit of the Met allele emerges during adolescence (Dumontheil et al., 2011).
The level of a reproductive hormone oestrogen, which down-regulates COMT transcription (Xie et al., 1999), increases during puberty in girls. This suggests that adolescence is an important developmental period, when the sex difference in COMT activity emerges (Xie et al., 1999), and the effect of COMT on cognitive abilities between boys and girls differentiates. Therefore, adolescence can be an important period when the sex difference in COMT activity emerges, and the effect of COMT on cognition between boys and girls differentiates. However, our study was not able to confirm the effect of COMT variation in adolescent girls or boys at age 15. It remains unclear whether the previously reported age-specific effect may be due to puberty. The puberty-by-gene interaction effects were not significant in boys, and there is a lack of power in our study since the group of prepubescent boys is small. On the other hand, many developmental changes may occur between ages 8 and 15 years, and young people are exposed to influences from many environmental factors. Therefore, we cannot exclude possible genotype-environment interaction on cognition. Recently, epigenetic mechanism for the interaction of the COMT with lifetime stress has been discovered: the greater stress led to lower methylation of the Val158 that was related to reduced cortical efficiency during a cognitive task (Ursini et al., 2011). Future studies will need to address these issues by employing longitudinal designs, using repeated measures of cognitive functions, and exploring the effects of specific DNA variants in interaction with environmental factors on trajectory of changes across cognitive development.
In conclusion, the present longitudinal study provides some evidence that COMT variation may affect cognitive function in a sex or developmental stage-specific manner. Further studies are necessary in order to make stronger conclusions.
Acknowledgements
We wish to thank all the survey members for their participation. This work was supported by the Wellcome Trust [to M.R., P.B.J., D.G, and T.J.C.], Medical Research Council [to D.G., M.R.], and the Department of Health (NIHR) [Career Scientist Award to T.J.C.]. Dr. Barnett is an employee of Cambridge Cognition, Ltd.; and she is also a co-inventor on patent PCT/GB2005/003279 (methods for assessing psychotic disorders). Prof. Jones has received research grant support from GlaxoSmithKline; he has received a speaker's honorarium from Eli Lilly; and he is a co-inventor on patent PCT/GB2005/003279 (methods for assessing psychotic disorders). Drs. Gaysina, Xu, Croudace, Wong and Richards report no competing interests.
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.biopsycho.2012.11.007.
Appendix A. Supplementary data
The following are the supplementary data to this article:
References
- Barnett J.H., Heron J., Goldman D., Jones P.B., Xu K. Effects of catechol-O-methyltransferase on normal variation in the cognitive function of children. American Journal of Psychiatry. 2009;166:909–916. doi: 10.1176/appi.ajp.2009.08081251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett J.H., Heron J., Ring S.M., Golding J., Goldman D., Xu K., Jones P.B. Gender-specific effects of the catechol-O-methyltransferase Val108/158Met polymorphism on cognitive function in children. American Journal of Psychiatry. 2007;164:142–149. doi: 10.1176/ajp.2007.164.1.142. [DOI] [PubMed] [Google Scholar]
- Barnett J.H., Scoriels L., Munafo M.R. Meta-analysis of the cognitive effects of the catechol-O-methyltransferase gene Val158/108Met polymorphism. Biological Psychiatry. 2008;64:137–144. doi: 10.1016/j.biopsych.2008.01.005. [DOI] [PubMed] [Google Scholar]
- Bray N.J., Buckland P.R., Williams N.M., Williams H.J., Norton N., Owen M.J., O’Donovan M.C. A haplotype implicated in schizophrenia susceptibility is associated with reduced COMT expression in human brain. American Journal of Human Genetics. 2003;73:152–161. doi: 10.1086/376578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdick K.E., Funke B., Goldberg J.F., Bates J.A., Jaeger J., Kucherlapati R., Malhotra A.K. COMT genotype increases risk for bipolar I disorder and influences neurocognitive performance. Bipolar Disorder. 2007;9:370–376. doi: 10.1111/j.1399-5618.2007.00384.x. [DOI] [PubMed] [Google Scholar]
- Byrne B.M. Testing for multigroup invariance using AMOS graphics: a road less traveled. Structural Equation Modeling: A Multidisciplinary Journal. 2004;11:272–300. [Google Scholar]
- Casey B.J., Giedd J.N., Thomas K.M. Structural and functional brain development and its relation to cognitive development. Biological Psychology. 2000;54:241–257. doi: 10.1016/s0301-0511(00)00058-2. [DOI] [PubMed] [Google Scholar]
- Chan R.C., Chen R.Y., Chen E.Y., Hui T.C., Cheung E.F., Cheung H.K., Sham P., Li T., Collier D. The differential clinical and neurocognitive profiles of COMT SNP rs165599 genotypes in schizophrenia. Journal of the International Neuropsychological Society. 2005;11:202–204. doi: 10.1017/s1355617705050241. [DOI] [PubMed] [Google Scholar]
- Chen F.F. Sensitivity of goodness of fit indexes to lack of measurement invariance. Structural Equation Modeling. 2007;14:464–504. [Google Scholar]
- Cheung G.W., Rensvold R.B. The effects of model parsimony and sampling error on the fit of structural equation models. Organizational Research Methods. 2001;4:236–264. [Google Scholar]
- De Luca C.R., Wood S.J., Anderson V., Buchanan J.A., Proffitt T.M., Mahony K., Pantelis C. Normative data from the CANTAB I: development of executive function over the lifespan. Journal of Clinical and Experimental Neuropsychology. 2003;25:242–254. doi: 10.1076/jcen.25.2.242.13639. [DOI] [PubMed] [Google Scholar]
- Dennis N.A., Need A.C., LaBar K.S., Waters-Metenier S., Cirulli E.T., Kragel J., Goldstein D.B., Cabeza R. COMT val108/158 met genotype affects neural but not cognitive processing in healthy individuals. Cerebral Cortex. 2010;20:672. doi: 10.1093/cercor/bhp132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond A., Briand L., Fossella J., Gehlbach L. Genetic and neurochemical modulation of prefrontal cognitive functions in children. The American Journal of Psychiatry. 2004;161:125–132. doi: 10.1176/appi.ajp.161.1.125. [DOI] [PubMed] [Google Scholar]
- Diaz-Asper C.M., Goldberg T.E., Kolachana B.S., Straub R.E., Egan M.F., Weinberger D.R. Genetic variation in catechol-O-methyltransferase: effects on working memory in schizophrenic patients, their siblings, and healthy controls. Biological Psychiatry. 2008;63:72–79. doi: 10.1016/j.biopsych.2007.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson D., Elvevag B. Genes, cognition and brain through a COMT lens. Neuroscience. 2009;164:72–87. doi: 10.1016/j.neuroscience.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumontheil I., Roggeman C., Ziermans T., Peyrard-Janvid M., Matsson H., Kere J., Klingberg T. Influence of the COMT genotype on working memory and brain activity changes during development. Biological Psychiatry. 2011;70:222–229. doi: 10.1016/j.biopsych.2011.02.027. [DOI] [PubMed] [Google Scholar]
- Duncan J., Seitz R.J., Kolodny J., Bor D., Herzog H., Ahmed A., Newell F.N., Emslie H. A neural basis for general intelligence. Science. 2000;289:457–460. doi: 10.1126/science.289.5478.457. [DOI] [PubMed] [Google Scholar]
- Green A.E., Kraemer D.J., Deyoung C.G., Fossella J.A., Gray J.R. A gene-brain-cognition pathway: prefrontal activity mediates the effect of COMT on cognitive control and IQ. Cerebral Cortex. 2012 doi: 10.1093/cercor/bhs035. Published online February 24, http://dx.doi.org/10.1093/cercor/bhs035. [DOI] [PubMed] [Google Scholar]
- Gregorich S.E. Do self-report instruments allow meaningful comparisons across diverse population groups? Testing measurement invariance using the confirmatory factor analysis framework. Medical Care. 2006;44:S78–S94. doi: 10.1097/01.mlr.0000245454.12228.8f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haworth C.M., Wright M.J., Luciano M., Martin N.G., de Geus E.J., van Beijsterveldt C.E., Bartels M., Posthuma D., Boomsma D.I., Davis O.S., Kovas Y., Corley R.P., Defries J.C., Hewitt J.K., Olson R.K., Rhea S.A., Wadsworth S.J., Iacono W.G., McGue M., Thompson L.A., Hart S.A., Petrill S.A., Lubinski D., Plomin R. The heritability of general cognitive ability increases linearly from childhood to young adulthood. Molecular Psychiatry. 2010;15:1112–1120. doi: 10.1038/mp.2009.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu L., Bentler P.M. Cutoff criteria for fit indexes in covariance structure analysis: conventional criteria versus new alternatives. Structural Equation Modeling: A Multidisciplinary Journal. 1999;6:1–55. [Google Scholar]
- Liao S.Y., Lin S.H., Liu C.M., Hsieh M.H., Hwang T.J., Liu S.K., Guo S.C., Hwu H.G., Chen W.J. Genetic variants in COMT and neurocognitive impairment in families of patients with schizophrenia. Genes, Brain and Behavior. 2009;8:228–237. doi: 10.1111/j.1601-183X.2008.00467.x. [DOI] [PubMed] [Google Scholar]
- Marsh H.W., Hau K.T., Balla J.R., Grayson D. Is more ever too much? The number of indicators per factor in confirmatory factor analysis. Multivariate Behavioral Research. 1998;33:181–220. doi: 10.1207/s15327906mbr3302_1. [DOI] [PubMed] [Google Scholar]
- Meredith W. Measurement invariance, factor analysis and factorial invariance. Psychometrika. 1993;58:525–543. [Google Scholar]
- Mier D., Kirsch P., Meyer-Lindenberg A. Neural substrates of pleiotropic action of genetic variation in COMT: a meta-analysis. Molecular Psychiatry. 2009;15:918–927. doi: 10.1038/mp.2009.36. [DOI] [PubMed] [Google Scholar]
- Muthen L., Muthen B. Muthen & Muthen; Los Angeles: 2007. Mplus Users's Guide. [Google Scholar]
- Nackley A.G., Shabalina S.A., Tchivileva I.E., Satterfield K., Korchynskyi O., Makarov S.S., Maixner W., Diatchenko L. Human catechol-O-methyltransferase haplotypes modulate protein expression by altering mRNA secondary structure. Science. 2006;314:1930–1933. doi: 10.1126/science.1131262. [DOI] [PubMed] [Google Scholar]
- Pigeon D.A., Douglas J.W.B. Appendix 1, The home and the School. London; Macgibbon and Kee: 1964. Tests used in the 1954 and 1957 surveys. [Google Scholar]
- Pigeon D.A., Douglas J.W.B., Ross J.M., Simpson H.R. Appendix 1, All our Future. Davies; London: 1968. Details of the fifteen years tests. [Google Scholar]
- Purcell S., Neale B., Todd-Brown K., Thomas L., Ferreira M.A., Bender D., Maller J., Sklar P., de Bakker P.I., Daly M.J., Sham P.C. PLINK: a tool set for whole-genome association and population-based linkage analyses. American Journal of Human Genetics. 2007;81:559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N., Dahle C.L., Rodrigue K.M., Kennedy K.M., Land S. Effects of age, genes, and pulse pressure on executive functions in healthy adults. Neurobiology of Aging. 2011;32:1124–1137. doi: 10.1016/j.neurobiolaging.2009.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubia K., Overmeyer S., Taylor E., Brammer M., Williams S.C., Simmons A., Andrew C., Bullmore E.T. Functional frontalisation with age: mapping neurodevelopmental trajectories with fMRI. Neuroscience and Biobehavioral Reviews. 2000;24:13–19. doi: 10.1016/s0149-7634(99)00055-x. [DOI] [PubMed] [Google Scholar]
- Schumacker R.E., Lomax R.G. Lawrence Erlbaum Associates Inc.; New Jersey: 2004. A Beginner's Guide to Structural Equation Modeling. [Google Scholar]
- Supekar K., Uddin L.Q., Prater K., Amin H., Greicius M.D., Menon V. Development of functional and structural connectivity within the default mode network in young children. Neuroimage. 2010;52:290–301. doi: 10.1016/j.neuroimage.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunbridge E.M. The catechol-O-methyltransferase gene: its regulation and polymorphisms. Basic Aspects of Catechol-O-Methyltransferase and the Clinical Applications of its Inhibitors. 2010;95:7. doi: 10.1016/B978-0-12-381326-8.00002-8. [DOI] [PubMed] [Google Scholar]
- Ursini G., Bollati V., Fazio L., Porcelli A., Iacovelli L., Catalani A., Sinibaldi L., Gelao B., Romano R., Rampino A., Taurisano P., Mancini M., Di Giorgio A., Popolizio T., Baccarelli A., De Blasi A., Blasi G., Bertolino A. Stress-related methylation of the catechol-O-methyltransferase Val 158 allele predicts human prefrontal cognition and activity. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2011;31:6692–6698. doi: 10.1523/JNEUROSCI.6631-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Sluis S., Verhage M., Posthuma D., Dolan C.V. Phenotypic complexity, measurement bias, and poor phenotypic resolution contribute to the missing heritability problem in genetic association studies. PLoS One. 2012;5(11):e13929. doi: 10.1371/journal.pone.0013929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadsworth M.E., Butterworth S.L., Hardy R.J., Kuh D.J., Richards M., Langenberg C., Hilder W.S., Connor M. The life course prospective design: an example of benefits and problems associated with study longevity. Social Science and Medicine. 2003;57:2193–2205. doi: 10.1016/s0277-9536(03)00083-2. [DOI] [PubMed] [Google Scholar]
- Xie T., Ho S.L., Ramsden D. Characterization and implications of estrogenic down-regulation of human catechol-O-methyltransferase gene transcription. Molecular Pharmacology. 1999;56:31–38. doi: 10.1124/mol.56.1.31. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.