Abstract
As the molecular composition of calcium-release activated calcium (CRAC) channels has been unknown for two decades, elucidation of selective inhibitors has been considerably hampered. By the identification of the two key components of CRAC channels, STIM1 and Orai1 have emerged as promising targets for CRAC blockers. The aim of this study was to thoroughly characterize the effects of two selective CRAC channel blockers on currents derived from STIM1/Orai heterologoulsy expressed in HEK293 cells. The novel compounds GSK-7975A and GSK-5503A were tested for effects on STIM1 mediated Orai1 or Orai3 currents by whole-cell patch-clamp recordings and for the effects on STIM1 oligomerisation or STIM1/Orai coupling by FRET microscopy. To investigate their site of action, inhibitory effects of these molecules were explored using Orai pore mutants. The GSK blockers inhibited Orai1 and Orai3 currents with an IC50 of approximately 4 μM and exhibited a substantially slower rate of onset than the typical pore blocker La3+, together with almost no current recovery upon wash-out over 4 min. For the less Ca2+-selective Orai1 E106D pore mutant, ICRAC inhibition was significantly reduced. FRET experiments indicated that neither STIM1–STIM1 oligomerization nor STIM1–Orai1 coupling was affected by these compounds.
These CRAC channel blockers are acting downstream of STIM1 oligomerization and STIM1/Orai1 interaction, potentially via an allosteric effect on the selectivity filter of Orai. The elucidation of these CRAC current blockers represents a significant step toward the identification of CRAC channel-selective drug compounds.
Keywords: Calcium-release activated calcium (CRAC) channel, GSK-7975A, ICRAC, Orai1, Orai3, Stromal interaction molecule 1 (STIM1)
1. Introduction
Store-operated channels (SOCs) represent a widespread route for Ca2+ entry into non-excitable cells [1] among which Ca2+ release-activated Ca2+ (CRAC) channels are the most highly characterized. Besides their involvement in a variety of processes such as muscle contraction, gene expression, proliferation, cell growth, and cell death [1], CRAC channels are vital for immunological reactions of T-cells and mast cells. CRAC channels therefore provide a promising means of modulating the immune system and represent attractive targets for asthma and allergic disorders.
The long-standing mystery of the molecular composition of CRAC channels has hampered the identification of specific inhibitors. Nevertheless a variety of compounds that strongly inhibit CRAC currents has been identified including divalent and trivalent cations such as La3+ and Gd3+, diverse imidazoles, 2-APB (2-aminoethoxydiphenylborate), capsaicin [2], NPPB (5-nitro-2-(3-phenylpropylamino)-benzoic acid) [3–5], BTP2 (a bistrifluoromethyl-pyrazole derivative) [6–8], DES (diethylstilbestrol) [9], BEL (bromenol lactone) [10], bile acids [11] and ML-9 (1-(5-chloronaphthalene-1-sulfonyl)homopiperazine) [12]. La3+ and Gd3+ represent general inhibitors of Ca2+-selective influx pathways comprising voltage-dependent Ca2+, TRPV5/6 and also CRAC channels [13,14]. Although 2-APB is widely used as CRAC channels blocker, its pharmacology is complex; at low concentrations of 2-APB (1–5 μM) CRAC currents are enhanced, whereas at concentrations higher than 10 μM they are completely blocked [15]. Furthermore 2-APB alters the kinetics of fast Ca2+-dependent inactivation of ICRAC, increasing its rate at low concentrations and completely blocking inactivation above 10 μM [15]. The immunosuppressive agent BTP2 potently blocks interleukin-2 (IL-2) production in lymphocytes [6], at least in part through the inhibition of CRAC channels. [7,8].
While these compounds have proven useful tools for exploring CRAC channel biology, their activity against other ion channels and signaling processes such as the transient receptor potential (TRP) channels limits their utility [7,8,15–34]. Identification of more selective CRAC channel blockers therefore represents a major step toward their utilization as therapeutics.
In 2006 a genome wide screen using RNA interference led to the identification of the stromal interaction molecule STIM1 and Orai1 as the limiting components of the CRAC pathway in human T-cells [35–37]. STIM1 functions as a calcium sensor located in the endoplasmic reticulum and Orai1 represents the CRAC channel pore. Together these proteins represent promising targets for the identification of novel and selective blockers targeting specific steps within the signaling cascade.
The widely used CRAC blocker 2-APB exhibits a similar bimodal effect on over-expressed STIM1 and Orai1 channels as compared with endogenous CRAC channels [28,38]. While low concentrations of 2-APB enhance Orai1 currents (5 μM) [39–41], higher concentrations (>40 μM) inhibit them [39–41]. Higher concentrations of 2-APB reversed cluster formation of STIM1, although this process was attenuated when Orai1 was co-expressed [42–44]. Therefore 2-APB-dependent inhibition of STIM1 and Orai1 mediated currents are expected to result at least in part from a reduction of STIM1 punctae formation. Moreover 2-APB represents a potent tool to distinguish between over-expressed Orai1, Orai2 and Orai3 channels. In contrast to the robust block of Orai1 by high 2-APB concentrations (50 μM), Orai2 is less sensitive, while Orai3 is robustly stimulated by 2-APB [38,40,42,43,45,46]. 2-APB stimulated Orai3 channels develop independently of STIM1 and exhibit altered ion selectivity [42,43,45,46] with an increase in pore diameter [45].
In this study we present selective CRAC blockers which inhibit STIM1 mediated Orai1 and Orai3 currents with significantly slower kinetics than the conventional blockers La3+, Gd3+ and 2-APB. We observed no interference of these drugs with STIM1/STIM1 oligomerization or STIM1/Orai1 interaction. Furthermore, they do not inhibit Orai pore mutants at concentrations that fully block the wild-type Orai channels suggesting a target site close or allosterically linked to the selectivity filter. The detailed characterization of these molecules strongly supports their use as agents for the wider investigation of CRAC channel biology.
2. Materials and methods
2.1. Cell culture
Experiments were performed on HEK293 cells cultured as described previously [28]. HEK293 cells are grown in an incubator (Forma Scientific) under a humidified (95%) atmosphere containing 8% CO2 and a temperature of 37 °C. RBL-2H3 cells were obtained from ATCC and cultured in RPMI 1640 supplemented with 10% FBS, 1% penicillin–streptomycin and 1% l-glutamine (all Gibco, UK) at 37 °C in a 5%CO2 humidified incubator.
2.2. Molecular cloning and mutagenesis
Human Orai1 (Orai1; accession number NM_032790) was kindly provided by A. Rao's Lab, Harvard Medical School, USA. C-terminally tagged pECFP-N1 and pEYFP-N1/Orai1 constructs were cloned using the XhoI and BamHI sites of the contemplated vectors. N-terminally tagged Orai1 constructs were cloned via SalI and SmaI restriction sites of pECFP-C1 and pEYFP-C1 expression vectors (Clontech). Human STIM1 (STIM1; accession number NM_003156) N-terminally ECFP- and EYFP-tagged was kindly provided by T. Meyer's Lab, Stanford University, USA. C-terminally EYFP-tagged STIM1 was purchased from GeneCopoeia™ (Catalog No.: EX-S0521-M02). The integrity of all resulting clones was confirmed by sequence analysis. The rat (r)TRPV6 construct (accession # AF160798, kindly provided by M. Hediger, University of Berne, Switzerland) was used. The coding region of rTRPV6 was cleaved from pTracer-CMV2 (Invitrogen, USA) and transferred to the plasmid of pEYFP-C1 (Clontech, Germany). For subcloning of rTRPV6 the coding region was cleaved with the restriction enzymes NaeI and XbaI and the purified fragment was ligated with SmaI and XbaI digested pEYFP-C1. This resulted in N-terminally tagged EYFP-constructs. pcDNA3-77 encoding for voltage-gated l-type Ca2+ channel α1C,77 subunit has been described previously [47]. pcDNA3-β2a and pcDNA3-α2-δ encoding subunits of the l-type ion channel are kindly provided by Franz Hofmann (Institute of Pharmacology, Munich).
2.3. Transfection
Transfection of HEK cells [28] was performed using TransFectin (Biorad, Germany) with the corresponding plasmids. Measurements were carried out 24 h following transfection.
2.4. CRAC channel blockers
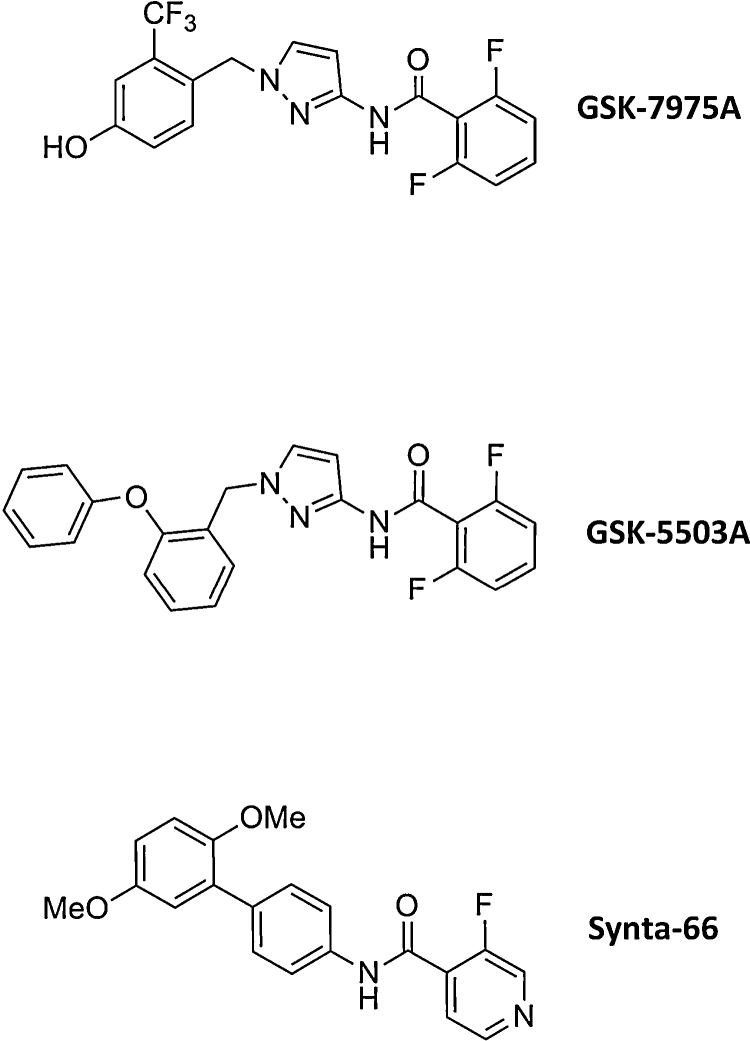
The CRAC channel blockers used in this study were obtained from GlaxoSmithKline, UK. Synta-66 (N-(2′,5′-dimethoxy-[1,1′-biphenyl]-4-yl)-3-fluoroisonicotinamide) is Example 66 from the patent WO2005/009954. GSK-5503A (2,6-difluoro-N-(1-(2-phenoxybenzyl)-1H-pyrazol-3-yl)benzamide) and GSK-7975A (2,6-difluoro-N-(1-(4-hydroxy-2-(trifluoromethyl)benzyl)-1H-pyrazol-3-yl)benzamide) are Examples 26 and 36, respectively, from the GSK patent WO2010/122089. The inhibitors were dissolved in DMSO to produce a 10 mM stock solution.
2.5. Electrophysiology
Electrophysiological recordings comparing characteristics of 2–3 constructs were carried out in paired comparison on the same day. Expression patterns and levels of the various constructs were carefully monitored by confocal fluorescence microscopy and were not significantly changed by the introduced mutations. Electrophysiological experiments were performed at 20–24 °C, using the patch-clamp technique in the whole-cell recording configuration. For STIM1/Orai current measurements voltage ramps were usually applied every 5 s from a holding potential of 0 mV, covering a range of −90 to +90 mV over 1 s. The internal pipette solution for passive store-depletion contained (in mM) 3.5 MgCl2, 145 cesium methane sulfonate, 8 NaCl, 10 HEPES, 20 EGTA, pH 7.2. Extracellular solution consisted of (in mM) 145 NaCl, 5 CsCl, 1 MgCl2, 10 HEPES, 10 glucose, 10 CaCl2, pH 7.4. Na+ divalent free (DVF) solution included 150 NaCl, 10 HEPES, 10 glucose and 10 EDTA. CRAC currents shown were leak-corrected either by subtraction of the initial trace or that following application of 10 μM La3+ at the end of the experiment.
2.6. Confocal Förster resonance energy transfer (FRET) fluorescence microscopy
Confocal FRET microscopy was performed as described previously [48]. In brief, a QLC100 Real-Time Confocal System (VisiTech Int., UK) was used for recording fluorescence images connected to two Photometrics CoolSNAPHQ monochrome cameras (Roper Scientific, USA) and a dual port adapter (dichroic: 505lp; cyan emission filter: 485/30; yellow emission filter: 535/50; Chroma Technology Corp., USA). This system was attached to an Axiovert 200M microscope (Zeiss, Germany) in conjunction with an argon ion multi-wavelength (457, 488, 514 nm) laser (Spectra Physics, USA). The wavelengths were selected by an Acousto Optical Tuneable Filter (VisiTech Int., UK). MetaMorph 5.0 software (Universal Imaging Corp.) was used to acquire images and to control the confocal system. Illumination times of about 900–1500 ms were typically used for CFP, FRET and YFP images that were consecutively recorded with a minimum delay. Prior to the calculation the images had to be corrected due to cross-talk as well as cross-excitation. For this, the appropriate crosstalk calibration factors were determined for each of the constructs on the day the FRET experiments were performed. The corrected FRET image (NFRET) was calculated on a pixel to pixel basis after background subtraction and threshold determination using custom-made software [49] integrated in MatLab 7.0.4 according to the published method [50]. The local ratio between CFP and YFP might vary due to different localisations of diverse protein constructs, which could lead to the calculation of false FRET values [51]. Accordingly, the analysis was limited to pixels with a CFP:YFP molar ratio between 1:10 and 10:1 to yield reliable results [51].
Statistics: Mean ± S.E.M. values were shown throughout the manuscript. Significance analysis was performed with the two-tailed Mann–Whitney test.
2.7. Calcium influx assay
24 h prior to experimentation cells were harvested, adjusted to a density of 0.2 × 106 cells/ml and 100 μl loaded into each well of a 96-well clear, flat bottomed, black sided plate (Costar, UK). The assay buffer contained (in mM) NaCl (145), KCl (2.5), HEPES (10), glucose (10), MgCl2 (1.2), CaCl2 (2.0), and probenecid (2.5) (all Sigma, UK). The loading buffer was assay buffer supplemented with 2 μM Fluo-4 AM (dissolved in 50:50 DMSO/pluronic acid, Invitrogen, UK).
Media was aspirated and replaced with loading buffer before being incubated at room temperature in the dark for 60 min. Loading buffer was aspirated and replaced with assay buffer. Loaded cells were incubated with GSK-7975A for 30 min at room temperature in the dark before being stimulated with 1 μM thapsigargin and returned to the dark for a further 5 min. The plate was read on a Tecan Sapphire fluorescence plate reader. Samples were read at excitation 488 nm and emission 520 nm with each data point being expressed as fold over basal using an unstimulated vehicle control.
3. Results
Fig. 1 depicts the chemical structure of the two GSK compounds (GSK-7975A and GSK-5503A) studied along with a previously described CRAC channel blocker Synta-66 [52].
Fig. 1.
Structures of GSK-CRAC channel inhibitors in comparison to Synta-66.
3.1. Both GSK compounds fully block Orai1 currents at 10 μM concentrations
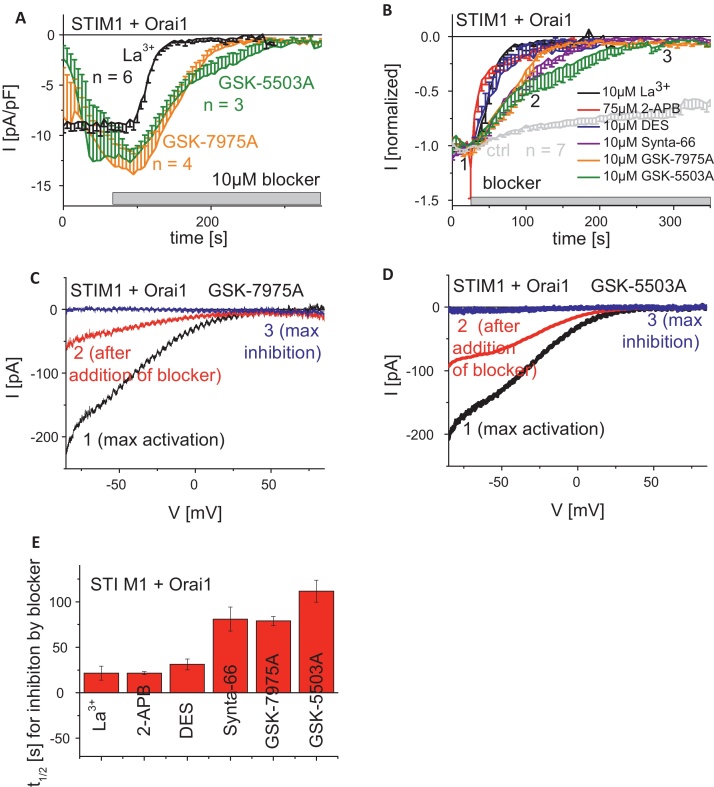
To evaluate the overall effect of the GSK compounds on STIM1-stimulated Orai currents, we employed the patch-clamp technique to investigate their effects on maximally activated STIM1-dependent Orai1/Orai3 currents in comparison with the conventional Ca2+ channel blocker La3+. HEK293 cells co-expressing STIM1 with either Orai1 or Orai3 were clamped in the whole-cell configuration employing 20 mM EGTA in the pipette solution for passive store-depletion. Following whole-cell formation, currents were measured in response to repetitive voltage-ramps from −90 mV to +90 mV applied from a holding potential of 0 mV, and the time-course of current inhibition during drug administration was determined at −74 mV (Fig. 2A and B) from each ramp (Fig. 2C and D). Time-axis in Fig. 2A and B were shifted to superimpose time-points of drug administration. Under our conditions the well-known complete inhibition of Orai1 currents by 10 μM La3+occurred with a t1/2 of ∼25 s (Fig. 2A and E). Application of the two novel compounds GSK-7975A and GSK-5503A at 10 μM concentrations also resulted in complete Orai1 current inhibition, but at a substantially slower rate with a t1/2 in the range 75–100 s (Fig. 2A and E). Similarly, 10 μM of the Synta-66 compound reduced Orai1 currents with a t1/2 of 75 s (Fig. 2B and E).
Fig. 2.
Inhibitory profiles of known and GSK-CRAC channel blockers on STIM1/Orai1 currents. (A) Time-course of whole cell inward currents at −74 mV maximally activated upon passive store-depletion of HEK293 cells co-expressing CFP-STIM1 with YFP-Orai1 upon perfusion of 10 μM La3+, 10 μM GSK-5503A and 10 μM GSK-7975A. Time-axes in (A and B) were shifted to superimpose time-points of drug administration. (B) Time-course of normalized whole cell inward currents at −74 mV, maximally activated upon passive store-depletion of HEK293 cells co-expressing CFP-STIM1 with YFP-Orai1 upon perfusion of 10 μM La3+, 10 μM GSK-5503A and 10 μM GSK-7975A in comparison to 75 μM 2-APB, 10 μM DES and 10 μM Synta-66 (t = 0 s was shifted to a time-point where currents had already reached their maximum). (C and D) Corresponding I/V relationships to (A, B: 1, 2, 3) of STIM1/Orai1 currents after maximal activation (1), after ∼half maximal block (2) as well as complete block (3) by 10 μM GSK-7975 (C) or 10 μM GSK-5503A (D). (E) Block diagram representing half-maximal inhibition time t1/2 of 10 μM La3+, 75 μM 2-APB, 10 μM DES, 10 μM Synta-66, 10 μM GSK-5503A and 10 μM GSK-7975A.
For comparison, we extended our pharmacological investigations to compounds already known to inhibit CRAC currents [53] including the synthetic estrogen agonist diethylstilbestrol (DES) and 2-aminoethoxydiphenylborate (2-APB). In agreement with the effective block of CRAC/SOC currents in RBL cells by DES at concentrations of 30 μM, STIM1-activated Orai1 currents exhibited full and rapid blockade within ∼50 s (Fig. 2B). The CRAC channel blocker 2-APB displayed its unique dual effect on STIM1/Orai1 currents characterized by a transient stimulation followed by a complete inhibition of STIM1/Orai1 currents. Both DES and 2-APB rapidly inhibited STIM1/Orai1 currents with t1/2 of ∼25 s comparable with La3+. In summary, the two Orai1 current-blocking compounds GSK-7975A and GSK-5503A fully inhibited Orai1 currents at 10 μM with 2.5-fold slower kinetics than La3+.
3.2. Both GSK compounds effectively inhibit Orai1 and Orai3 currents
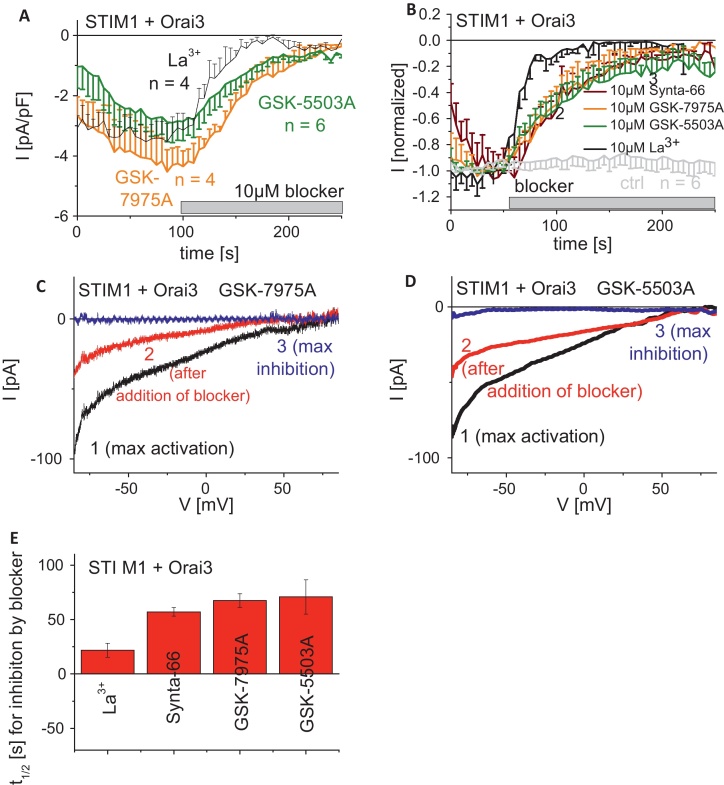
To evaluate specificity among the Orai protein family we further examined the effect of GSK-7975A and GSK-5503A on STIM1-activated Orai3 currents. Time-axis in Fig. 3A and B were shifted to superimpose time-points of drug administration. The two GSK compounds fully inhibited Orai3 currents at 10 μM (Fig. 3A, C, and D) with a comparable t1/2 of 75 s (Fig. 3E), indicating no obvious selectivity between Orai1 and Orai3. Similarly, Synta-66 (Fig. 3B and E) inhibited Orai3 currents at a similar rate to the GSK compounds. By contrast, 10 μM La3+ blocked Orai3 currents more rapidly with a t1/2 of ∼20 s (Fig. 3B and E) analogous to Orai1 current inhibition. The GSK compounds appeared to inhibit Orai3 currents slightly faster than those of Orai1. Overall these GSK compounds were equally effective at blocking Orai1 and Orai3, and inhibition occurred at a substantially slower rate than La3+.
Fig. 3.
Inhibitory profiles of known and GSK-CRAC channel blockers on STIM1/Orai3 currents. (A) Time-course of whole cell inward currents at −74 mV maximally activated upon passive store-depletion of HEK293 cells co-expressing CFP-STIM1 with YFP-Orai3 upon perfusion of 10 μM La3+, 10 μM GSK-5503A and 10 μM GSK-7975A. Time-axes in (A and B) were shifted to superimpose time-points of drug administration. (B) Time-course of normalized whole cell inward currents at −74 mV maximally activated upon passive store-depletion of HEK293 cells co-expressing CFP-STIM1 with YFP-Orai3 upon perfusion of 10 μM La3+, 10 μM GSK-5503A and 10 μM GSK-7975A in comparison to 10 μM Synta-66 (t = 0 s was shifted to a time-point where currents had already reached their maximum). (C and D) Corresponding I/V relationships to (A, B: 1, 2, 3) of STIM1/Orai3 currents after maximal activation (1), after ∼half maximal block (2) as well as complete block (3) by 10 μM GSK-7975 (C) or 10 μM GSK-5503A (D). (E) Block diagram representing half-maximal inhibition time t1/2 of 10 μM La3+, 10 μM Synta-66, 10 μM GSK-5503A and 10 μM GSK-7975A.
3.3. Inhibition of Orai currents by GSK compounds is not readily reversible
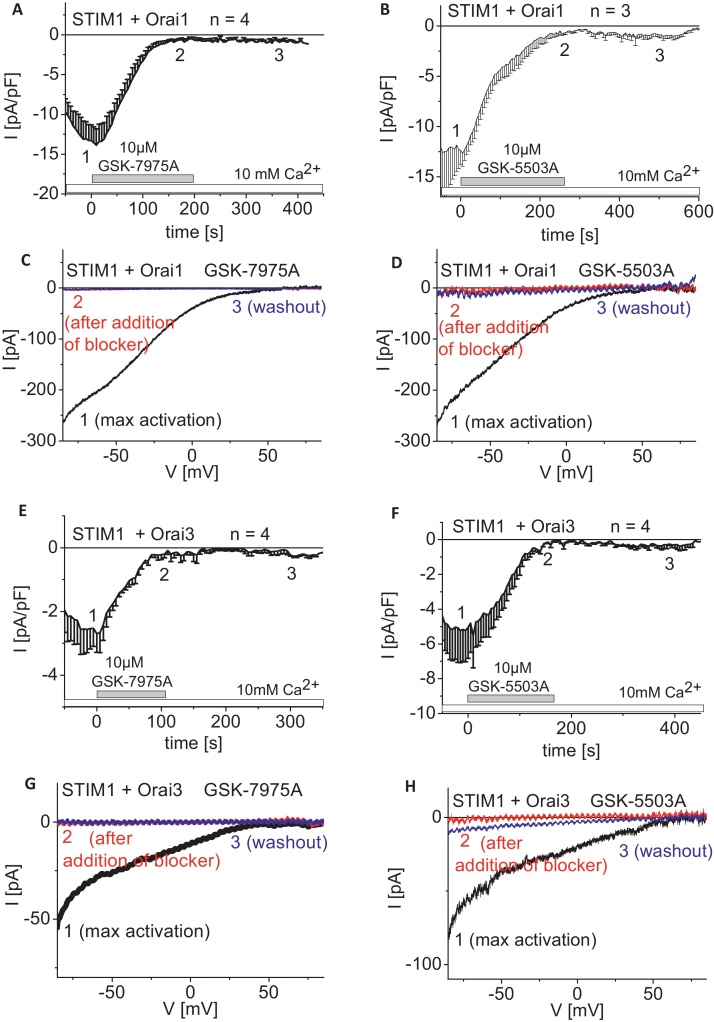
In a subsequent study we investigated recovery of Orai1/3 currents following maximal inhibition by GSK compounds. Wash-out of drugs was performed by re-perfusion of the 10 mM Ca2+ containing extracellular solution onto STIM1/Orai expressing HEK293 cells that exhibited fully blocked Orai1/Orai3 currents. Neither Orai1 (Fig. 4A–D) nor Orai3 (Fig. 4E–H) currents showed substantial recovery from block by GSK-7975A (Fig. 4A, C, E, and G) or GSK-5503A (Fig. 4B, D, F, and H) over a 4–5 min wash-out period. Hence, the blockade of STIM1-activated Orai1/3 currents by these compounds is not readily reversible within the time frame of this experiment.
Fig. 4.
Wash-out of GSK-CRAC channel blockers following inhibition of STIM1/Orai1 and STIM1/Orai3 currents. Time-course of whole cell inward currents at −74 mV maximally activated upon passive store-depletion of HEK293 cells co-expressing CFP-STIM1 with YFP-Orai1 (A and B) or YFP-Orai3 (E and F). Upon full blockade of STIM1/Orai currents by 10 μM GSK-7975A (A and E) or 10 μM GSK-5503A (B and F) 10 mM Ca2+ solution was perfused for wash-out (t = 0 s was shifted to a time-point where currents had already reached their maximum). Corresponding I/V relationships to (A, B, E, F: 1, 2, 3) of STIM1/Orai1 (C and D) and STIM1/Orai3 (G and H) currents upon maximal store-operated activation (1), upon complete inhibition (2) of 10 μM GSK-7975A (C and G) or 10 μM GSK-5503A (D and H) and after washout (3) of the respective blocker.
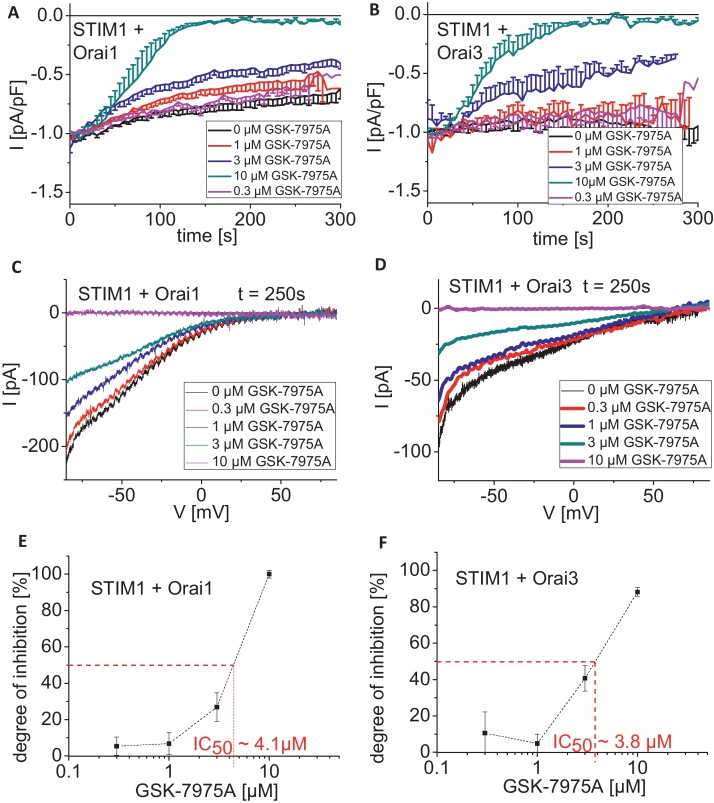
3.4. Inhibition of Orai1/3 currents by GSK-7975A occurs with a half maximal concentration of approximately 4 μM
As both GSK compounds demonstrated similar behavior toward Orai current inhibition, we focused on a more detailed characterization of GSK-7975A. Concentration–response curves for the inhibitory action of GSK-7975A on Orai1 and Orai3 currents were generated. Single concentrations (0.1, 0.3, 1, 3, 10 μM) were applied to maximally activated Orai currents in individual cells, which was required due to the slow rate of reaching steady-state inhibition (Fig. 5). The IC50 values of GSK-7975A were estimated as 4.1 μM and 3.8 μM for Orai1 (Fig. 5E) and Orai3 (Fig. 5F), respectively. The Hill coefficient for the inhibition of both Orai currents by GSK-7975A was calculated to be ∼1, suggesting a 1:1 molar interaction of GSK-7975A with the Orai channels. The similar IC50 of ∼4 μM for both Orai1 and Orai3 currents suggested conserved binding sites for GSK-7975A in both channels.
Fig. 5.
Dose-response relationships of the CRAC channel blocker GSK-7975A on STIM1/Orai1 and STIM1/Orai3 currents. (A and B) Time-course of whole cell inward currents at −74 mV maximally activated upon passive store-depletion of HEK293 cells co-expressing CFP-STIM1 with YFP-Orai1 (A) or YFP-Orai3 (B) upon perfusion of 0.3 μM, 1 μM, 3 μM, 10 μM GSK-7975A (t = 0 s was shifted to a time-point where currents had already reached their maximum). (C and D) Corresponding I/V relationships to (A and B) of STIM1/Orai1 (C) and STIM1/Orai3 (D) currents upon maximal inhibition by 0, 0.3, 1, 3, 10 μM GSK-7975A. (E and F) Concentration–response relationship depicting the inhibitory effect of GSK-7975A on CFP-STIM1-mediated YFP-Orai1 (E) and YFP-Orai3 (F) currents.
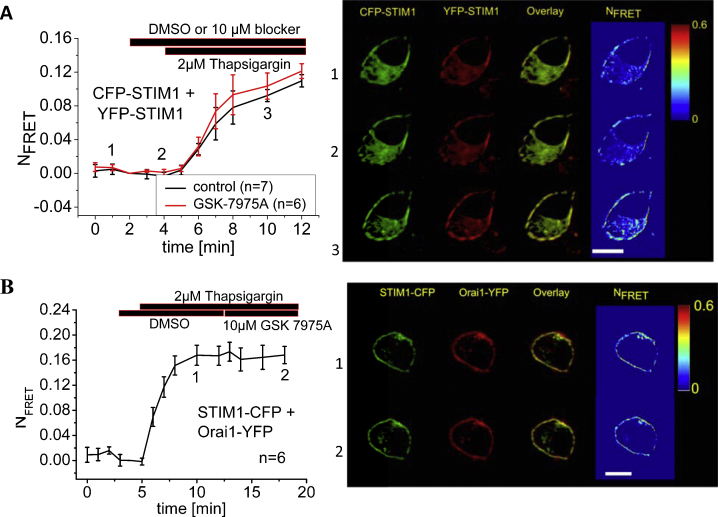
3.5. GSK-7975A affects neither STIM1 oligomerization nor STIM1/Orai1 interaction
The inhibitory effect upon Orai1 and Orai3 channels might evolve from a compound-induced impairment of the STIM1/Orai coupling processes or more classically from pore blockade. The former mechanism was approached by FRET measurements (Fig. 6) monitoring STIM1/STIM1 or STIM1/Orai1 interaction. We [54] and others [55] have recently reported that CFP- and YFP-labeled STIM1 proteins display an increase in FRET upon store-depletion reflecting store-operated oligomerization as a prerequisite for subsequent STIM1/Orai1 coupling. To approach the potential impairment of STIM1 oligomerization by GSK-7975A, we applied 10 μM GSK-7975A to CFP- and YFP-STIM1 expressing cells 5 min before store-depletion. Following the addition of 2 μM thapsigargin, FRET significantly increased and was unaffected by the presence of GSK-7975A (Fig. 6A). STIM1 oligomerization was therefore not affected by GSK-7975A.
Fig. 6.
GSK-7975A impaired neither STIM1 oligomerization nor STIM1/Orai1 coupling. (A) Left: time course of relative FRET between CFP-STIM1 and YFP-STIM1 with or without perfusion of GSK-7975A. Right: localization, overlay and calculated FRET life cell image series of YFP-STIM1 and CFP-STIM1 (1) in the absence and (2) presence of GSK-7975A and (3) following store-depletion in the presence of GSK-7975A. (B) Left: time course of relative FRET between STIM1-CFP and Orai1-YFP upon store-depletion and after application of GSK-7975A. Right: localization, overlay and calculated FRET life cell image series of STIM1-CFP and Orai1-YFP (1) after store-depletion and (2) after perfusion of GSK-7975A.
A potential effect on STIM1/Orai1 interaction was next investigated in HEK cells co-expressing STIM1-CFP and Orai1-YFP. Upon store-depletion these two proteins displayed a robust increase in FRET indicating their direct interaction. Application of 10 μM GSK-7975A did not significantly alter the FRET plateau representing STIM1/Orai1 interaction after store-depletion by thapsigargin (Fig. 6B). Hence GSK-7975A is unlikely to interfere with the interaction of STIM1 and Orai1. Taken together, these results imply that the inhibitory effect of GSK-7975A on CRAC currents does not result from interference with the overall processes that trigger the interaction of STIM1/Orai but rather involves a potential impairment of gating or pore blockade.
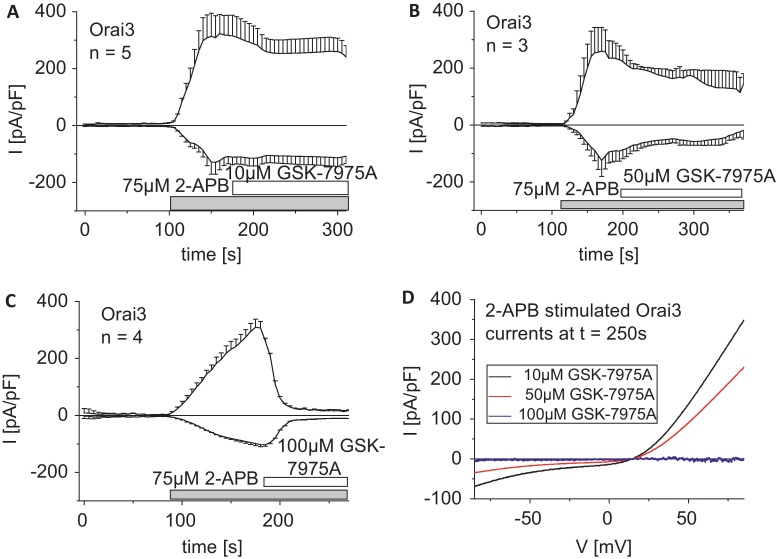
3.6. 2-APB stimulated Orai3 currents are less susceptible to GSK-7975A
Orai3, in contrast to STIM1-activated Orai1, exhibits robust current stimulation by 75 μM 2-APB [56–58]. These 2-APB evoked Orai3 currents are independent of STIM1, exhibit less Ca2+-selectivity and display a double rectifying current–voltage relationship that reflects the altered permeation properties linked to an increased pore size [57]. In a first approach we applied 10 μM GSK-7975A to maximally 2-APB stimulated Orai3 currents (Fig. 7A and D). Strikingly, 10 μM GSK-7975A was totally ineffective in inhibiting these Orai3 currents in contrast to those activated via STIM1. Upon application of increased concentrations of GSK-7975A to maximally 2-APB activated Orai3 currents we observed 50% inhibition by 50 μM GSK-7975A (Fig. 7B and D) and full inhibition by 100 μM GSK-7975A (Fig. 7C and D). Thus, the 2-APB elicited Orai3 currents were approximately 10-fold less sensitive to inhibition by GSK-7975A, which might result from the distinct mode of activation and/or the altered pore geometry as evident from the lower Ca2+-selectivity.
Fig. 7.
The less Ca2+-selective Orai3 current elicited by 2-APB is substantially less susceptible to GSK-7975A inhibition. (A–C) Time-courses of 2-APB-stimulated Orai3 currents, which were treated after maximal activation with 10 μM (A), 50 μM (B) and 75 μM (C) GSK-7975A. (D) I/V-relationship of 2-APB stimulated Orai3 currents upon addition of 10 μM, 50 μM or 100 μM GSK-7975A.
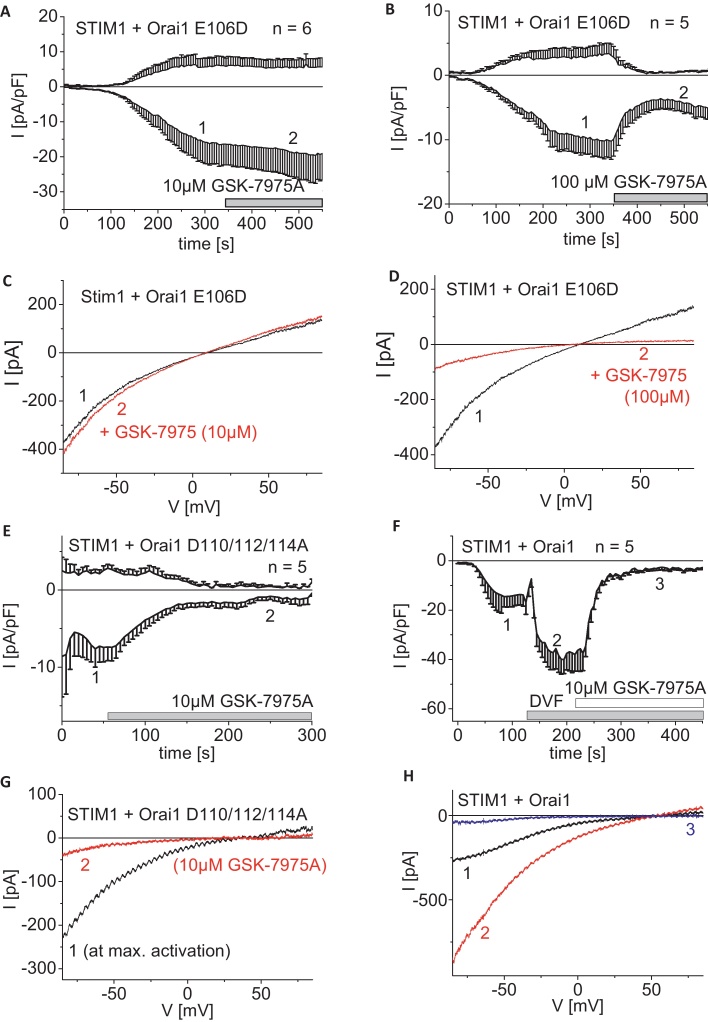
3.7. The Orai1 E106D pore mutant also lacks inhibition by 10 μM GSK-7975A
In an attempt to identify whether the altered pore geometry is linked to the less efficient inhibition of GSK-7975A, we utilized several Orai1 pore mutants which are still fully dependent on STIM1 for activation. The Orai1 E106D pore mutant displayed less Ca2+-selectivity together with a left-shifted reversal potential and an enhanced pore diameter [59–61]. STIM1-activated maximum Orai1 E106D currents exhibited no inhibition by application of 10 μM GSK-7975A similar to observations on 2-APB stimulated Orai3 currents (Fig. 8A and C). Nevertheless, increasing concentrations of GSK-7975A to 100 μM substantially but not fully restored the inhibitory effect on STIM1-mediated Orai1 E106D currents (Fig. 8B and D). For comparison, we examined Orai1 D110/112/114A with mutations in the outer vestibule of the pore that lead to a moderate left-shift of the reversal potential to ∼40 mV and an increased pore diameter to about 4.4 Ǻ [61]. Following STIM1-dependent maximum store-operated activation of this pore mutant, application of 10 μM GSK-7975A resulted in a substantial inhibition almost comparable to that of wild-type Orai1 (Fig. 8E and G), but distinctly different to the Orai1 E106D form. This observation suggested that the conformation of the selectivity filter rather than the outer vestibule of the Orai1 pore represented a molecular determinant for GSK-7975A blockade. Simple removal of Ca2+ ions from the extracellular solution as evaluated by a divalent free solution with sodium ions as main charge carriers led to the typical increases in inward currents, which were still fully blocked by 10 μM GSK-7975A (Fig. 8F and H). This further suggested that the presence of Ca2+ ions within the permeation pathway is not required for the inhibitory action of 10 μM GSK-7975A.
Fig. 8.
Inhibitory profile of GSK-7975A on less Ca2+-selective or monovalent Orai1 currents. (A and B) Time course of whole cell inward currents at −74 mV maximally activated upon passive store-depletion of HEK293 cells co-expressing CFP-STIM1 with YFP-Orai1 E106D upon perfusion of 10 μM (A) and 100 μM (B) GSK-7975A. (C and D) Corresponding I/V relationships to (A, B: 1, 2) of STIM1/Orai1 E106D currents after maximal store-operated activation (1) and upon addition (2) of 10 μM (C) or 100 μM (D) GSK-7975A. (E) Time course of whole cell inward currents at −74 mV maximally activated upon passive store-depletion of HEK293 cells co-expressing CFP-STIM1 with YFP-Orai1 D110/112/114A upon perfusion of 10 μM GSK-7975A (t = 0 s was shifted to a time-point where currents had already reached their maximum). (F) Time course of whole cell inward currents at −74 mV maximally activated upon passive store-depletion of HEK293 cells co-expressing CFP-STIM1 with YFP-Orai1 in 10 mM Ca2+ solution. Afterwards 10 mM Ca2+ solution was exchanged by a DVF solution and blocked by 10 μM GSK-7975A. (G) Corresponding I/V relationships to (E: 1, 2) of STIM1/Orai1 D110/112/114A currents after maximal store-operated activation (1) and upon addition (2) of 10 μM GSK-7975A. (H) Corresponding I/V relationships to (F: 1, 2, 3) of maximal activated STIM1/Orai1 current in 10mM Ca2+ containing solution (1), DVF solution (2) and upon addition (3) of 10 μM GSK-7975A in DVF solution.
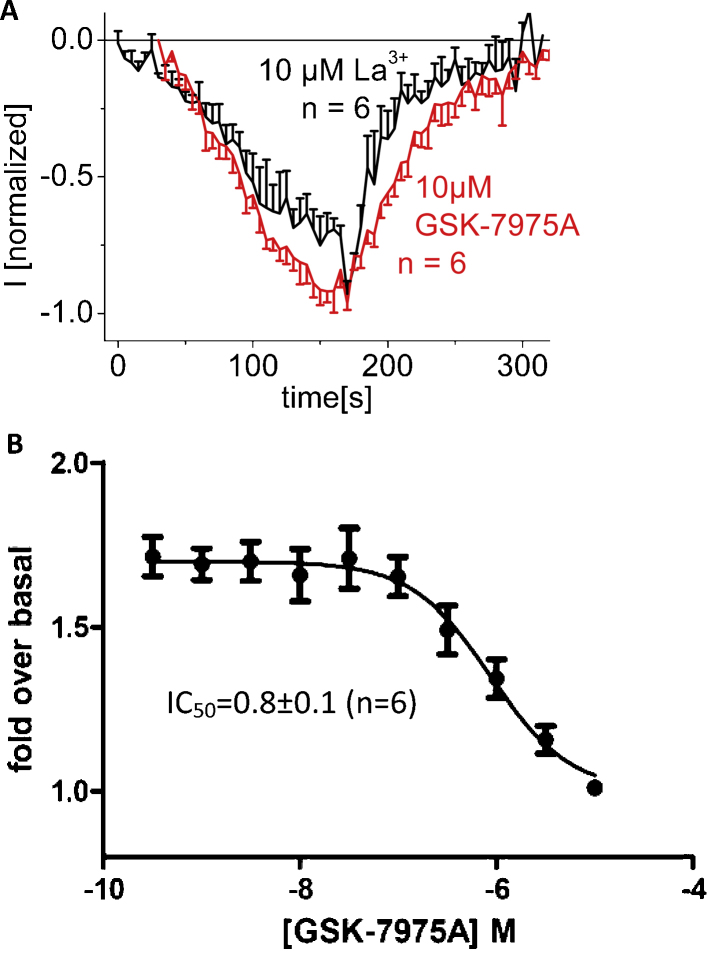
3.8. Endogenous CRAC channels of RBL mast cells are inhibited by GSK-7975A
For comparison with expressed Orai1 channels, we examined the inhibitory profile of GSK-7975A on rat basophilic leukemia (RBL-2H3) cells that are widely studied for their endogenous CRAC channels. 10 μM GSK-7975A fully blocked RBL CRAC currents at a slightly slower rate than 10 μM La3+ (Fig. 9A). A concentration–response curve obtained for the inhibition of thapsigargin-induced Ca2+ entry following a 30 min pre-incubation with increasing GSK-7975A concentrations yielded an IC50 of 0.8 ± 0.1 μM (Fig. 9B, n = 6). This value compares with the ∼4 μM estimated from the electrophysiological experiments on Orai1/STIM1-derived currents from HEK cells.
Fig. 9.
Inhibitory profile of GSK-7975A on endogenous CRAC currents and Ca2+ entry of RBL cells. (A) Time course of whole cell inward currents at −74 mV maximally activated upon passive store-depletion of RBL-2H3 cells upon perfusion of 10 μM GSK-7975A in comparison to 10 μM La3+. (B) Concentration-dependent inhibition of thapsigargin-evoked Ca2+ influx from Fura-4 loaded RBL cells expressed as fold over unstimulated cells.
3.9. Inhibitory profile of GSK-7975A on other ion channels
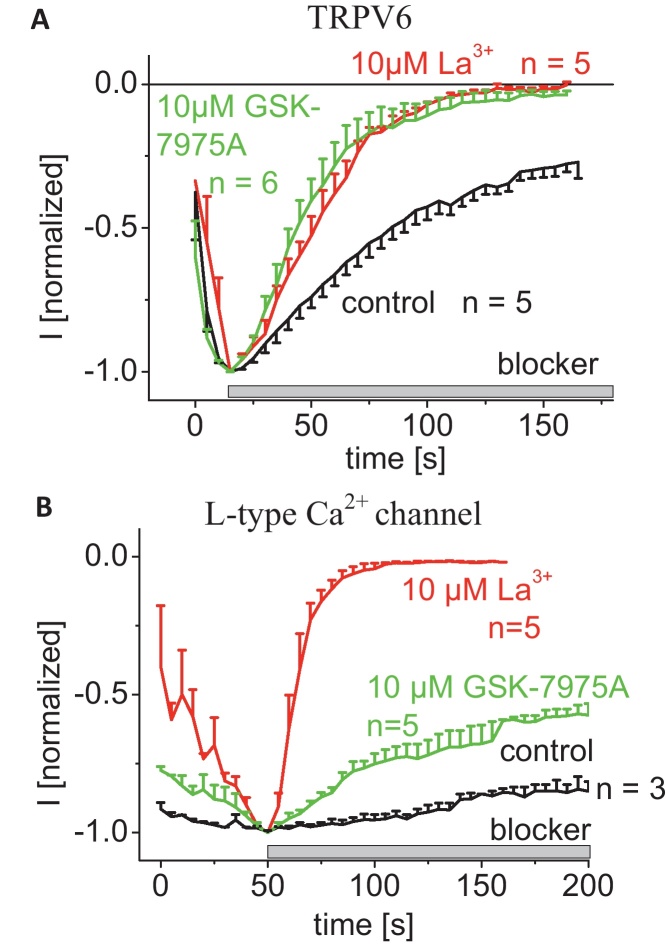
To explore selectivity against other ion channels, GSK-7975A was profiled against a variety of recombinantly expressed ion channel proteins including several members of the TRP channel family. Supplemental Table 1 lists the potential antagonistic (IC50) or agonistic (EC50) activity of GSK-7975A at concentrations up to 10 μM on sixteen ion channels revealing only a slight inhibitory effect on l-type (CaV1.2) Ca2+ channels.
Given the IC50 of ∼8 μM for l-type Ca2+ channels we went on to investigate by electrophysiology the inhibition of both l-type and TRPV6 channels, the most Ca2+-selective channels and compared the effects of GSK-7975A to the typical Ca2+ channel blocker La3+. While La3+ fully blocked both Ca2+ channels, 10 μM GSK-7975A completely inhibited rat-TRPV6 channels at a similar rate as La3+ (Fig. 10A), but only exhibited moderate inhibition of l-type Ca2+ channels (Fig. 10B, Suppl. Table 1).
Fig. 10.
Inhibitory profile of GSK-7975A on other Ca2+-selective channels. (A) Time course of whole cell inward currents at −74 mV of TRPV6 expressing HEK293 cells upon perfusion of 10 μM GSK-7975A in comparison to 10 μM La3+. (B) Time course of whole cell inward currents at −74 mV of l-type Ca2+-channel expressing HEK293 cells upon perfusion of 10 μM GSK-7975A in comparison to 10 μM La3+.
TRPV6 and l-type calcium channels have almost no primary sequence homology so in some ways it is surprising that GSK-7975A inhibits both of these channels. This might be explained by architectural similarities in the selectivity filters of these channels, with both l-type channels and TRPV6 containing a similar ring of negative charges [62]. While the selectivity filters appear structurally similar, it should be noted that the pore diameter at its narrowest point is slightly larger for l-type channels (∼6.2 Å) than TRPV6 (∼5.4 Å) [62]. By contrast, the selectivity filter of Orai1 channels has been determined to be much smaller at just 3.6 Å [61].
4. Discussion
CRAC channels are important in the physiology and pathophysiology of particular immune cell types and underlie several disease states including a severe combined immunodeficiency syndrome [63]. Their molecular components STIM1 and Orai1 have emerged as potential targets for drug discovery [64,65]. To date, compounds described in the literature as CRAC channel inhibitors have been found to lack selectivity by blocking other signaling pathways and/or have poorly characterized sites of action on CRAC channels [64,66].
Here we characterized for the first time CRAC channel inhibitors which exert their action downstream of the STIM1–Orai coupling machinery, since they leave STIM1 oligomerization and STIM1–Orai1 interaction unaffected. CRAC currents derived from HEK cells expressing STIM1/Orai1 were inhibited with an IC50 of ∼4 μM and a Hill coefficient of ∼1. A similar inhibitory profile has been reported for Synta-66 [67,68] on RBL mast cells exhibiting an IC50 of ∼1-3 μM and a Hill coefficient of 1.1. The detailed mechanism of the Synta-66 compound is unknown, though it has been found in smooth muscle cells that it does not interfere with STIM1 clustering [69] consistent with our findings for GSK-7975A. The action of GSK-7975A is apparently determined by the geometry of the selectivity filter of the Orai pore, since the Orai1 E106D pore mutant required at least 10-fold higher concentrations for inhibition compared to wild-type Orai1. This apparent loss in affinity might be caused by the altered pore geometry, reflected in the lower Ca2+ selectivity, and increased pore diameter of 5.3 Å compared to 3.8 Å for the wild-type form. Alternatively, GSK-7975A might act as a gating modifier strengthening Ca2+-induced inactivation [61] to promote channel closure. As Ca2+-induced fast inactivation is almost eliminated in the Orai1 E106D mutant [61], this could conceivably have interfered with the action of GSK-7975A. However, application of 3 μM GSK-7975A, a submaximal inhibitory concentration for Orai1 block, did not significantly increase inactivation (Suppl. Fig. 1) rendering an altered Ca2+-induced fast inactivation as the mechanism of inhibition by GSK-7975A less likely. As the onset of action was much slower than that of the widely used Ca2+ channel blocker La3+, the GSK compounds may have restricted access to their site of action. The inhibitory fast action of La3+ on Orai currents has been attributed to an interaction with the negatively charged residues within the first extracellular loop, but not with the E106 at the selectivity filter [70,71]. Neutralization of the negatively charged residues in the first extracellular loop of Orai1 did not affect overall characteristics of inhibition by the CRAC blocker GSK-7975A rendering this site an unlikely target, consistent with its slow action compared to La3+. Thus it is tempting to speculate that these GSK compounds interfere with the permeation through the Orai1 pore by binding to a site that is close or allosterically linked to the selectivity filter compatible with the shift in affinity observed when increasing the pore size via mutation (Orai1 E106D) or 2-APB (Orai3). While it may be the case that access to the binding site occurs via the narrow channel pore, we cannot exclude the possibility that these compounds reach their site of action by diffusion through the cellular membrane given their moderate lipophilicity (clogP 3.4 for GSK-7975A) and high artificial membrane permeability (360 nm/s for GSK-7975A). Intracellular application of 10 μM GSK-7975A led only to a slight inhibition of CRAC currents probably due to the compound leaking out of the cell (data not shown). Further identification of the binding site using fluorescently labeled compounds has so far proven unsuccessful due to loss of affinity of the labeled compounds.
The GSK CRAC channel blockers did not differentiate between Orai1 and Orai3 channels consistent with the conserved pore geometry and selectivity filter among the Orai isoforms. Hence, the GSK blockers characterized in this study interfere with the permeation through the Orai pore by slow access to a site which is affected by alterations in the selectivity filter.
The native CRAC current of RBL mast cells was also inhibited by 10 μM GSK-7975A consistent with a recent report on its action in human lung mast cells where it significantly reduced Ca2+ influx and release of inflammatory mediators at a concentration of 3 μM [72]. Furthermore, endogenous, store-operated Ca2+ entry of HEK293 cells was substantially inhibited by pre-incubation with 10 μM GSK-7975A. However, the amount of Ca2+ released from ER stores via stimulation by either carbachol (CCH) or BHQ was not markedly altered pointing to a minor effect of GSK-7975A on pathways affecting the ER Ca2+ content (Suppl. Fig. 2).
Extension of the characterization of GSK-7975A to other ion channels revealed a high degree of selectivity (Supplemental Table 1). Only two ion channels were affected by GSK-7975A, and while inhibition of l-type Ca2+ channels occurred only to a weak extent, TRPV6 channels displayed complete block at a rate similar to the inhibition obtained with La3+. Hence, it might be supposed that TRPV6 and Orai proteins share some structural similarities in their target site for the GSK blocker, although the pore diameter [73] of TRPV6 is much larger (5.4 Å) and is of similar size to that of the Orai1 E106D mutant [61].
In summary, the two GSK CRAC channel blockers described herein target Orai channels and will function as lead compounds to facilitate the discovery of highly selective inhibitors. Furthermore these compounds will help validate the role of CRAC channels in asthma and allergic diseases.
Conflict of interest
The authors declared that there is no conflict of interest.
Acknowledgements
Isabella Derler (T466) is a Hertha-Firnberg scholarship holder. This work was in part supported by GlaxoSmithKline and by the Austrian Science Foundation (FWF): project P22747 to R.S. and project P22565 to C.R.
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.ceca.2012.11.005.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- 1.Berridge M.J., Bootman M.D., Roderick H.L. Calcium signalling: dynamics, homeostasis and remodelling. Nature Reviews Molecular Cell Biology. 2003;4(7):517–529. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- 2.Fischer B.S., Qin D., Kim K., McDonald T.V. Capsaicin inhibits Jurkat T-cell activation by blocking calcium entry current I(CRAC) Journal of Pharmacology and Experimental Therapeutics. 2001;299(1):238–246. [PubMed] [Google Scholar]
- 3.Gericke M., Oike M., Droogmans G., Nilius B. Inhibition of capacitative Ca2+ entry by a Cl− channel blocker in human endothelial cells. European Journal of Pharmacology. 1994;269(3):381–384. doi: 10.1016/0922-4106(94)90046-9. [DOI] [PubMed] [Google Scholar]
- 4.Li J.H., Spence K.T., Dargis P.G., Christian E.P. Properties of Ca(2+) release-activated Ca(2+) channel block by 5-nitro-2-(3-phenylpropylamino)-benzoic acid in Jurkat cells. European Journal of Pharmacology. 2000;394(2–3):171–179. doi: 10.1016/s0014-2999(00)00144-8. [DOI] [PubMed] [Google Scholar]
- 5.Reinsprecht M., Rohn M.H., Spadinger R.J., Pecht I., Schindler H., Romanin C. Blockade of capacitive Ca2+ influx by Cl- channel blockers inhibits secretion from rat mucosal-type mast cells. Molecular Pharmacology. 1995;47(5):1014–1020. [PubMed] [Google Scholar]
- 6.Ishikawa J., Ohga K., Yoshino T., Takezawa R., Ichikawa A., Kubota H. A pyrazole derivative, YM-58483, potently inhibits store-operated sustained Ca2+ influx and IL-2 production in T lymphocytes. Journal of Immunology. 2003;170(9):4441–4449. doi: 10.4049/jimmunol.170.9.4441. [DOI] [PubMed] [Google Scholar]
- 7.Takezawa R., Cheng H., Beck A., Ishikawa J., Launay P., Kubota H. A pyrazole derivative potently inhibits lymphocyte Ca2+ influx and cytokine production by facilitating transient receptor potential melastatin 4 channel activity. Molecular Pharmacology. 2006;69(4):1413–1420. doi: 10.1124/mol.105.021154. [DOI] [PubMed] [Google Scholar]
- 8.Zitt C., Strauss B., Schwarz E.C., Spaeth N., Rast G., Hatzelmann A. Potent inhibition of Ca2+ release-activated Ca2+ channels and T-lymphocyte activation by the pyrazole derivative BTP2. Journal of Biological Chemistry. 2004;279(13):12427–12437. doi: 10.1074/jbc.M309297200. [DOI] [PubMed] [Google Scholar]
- 9.Zakharov S.I., Smani T., Dobrydneva Y., Monje F., Fichandler C., Blackmore P.F. Diethylstilbestrol is a potent inhibitor of store-operated channels and capacitative Ca(2+) influx. Molecular Pharmacology. 2004;66(3):702–707. doi: 10.1124/mol.66.3.. [DOI] [PubMed] [Google Scholar]
- 10.Winstead M.V., Balsinde J., Dennis E.A. Calcium-independent phospholipase A(2): structure and function. Biochimica et Biophysica Acta. 2000;1488(1–2):28–39. doi: 10.1016/s1388-1981(00)00107-4. [DOI] [PubMed] [Google Scholar]
- 11.Aromataris E.C., Castro J., Rychkov G.Y., Barritt G.J. Store-operated Ca(2+) channels and stromal interaction molecule 1 (STIM1) are targets for the actions of bile acids on liver cells. Biochimica et Biophysica Acta. 2008;1783(5):874–885. doi: 10.1016/j.bbamcr.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 12.Tran Q.K., Watanabe H., Le H.Y., Pan L., Seto M., Takeuchi K. Myosin light chain kinase regulates capacitative ca(2+) entry in human monocytes/macrophages. Arteriosclerosis, Thrombosis, and Vascular Biology. 2001;21(4):509–515. doi: 10.1161/01.atv.21.4.509. [DOI] [PubMed] [Google Scholar]
- 13.Hoth M., Penner R. Calcium release-activated calcium current in rat mast cells. Journal of Physiology. 1993;465:359–386. doi: 10.1113/jphysiol.1993.sp019681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ross P.E., Cahalan M.D. Ca2+ influx pathways mediated by swelling or stores depletion in mouse thymocytes. Journal of General Physiology. 1995;106(3):415–444. doi: 10.1085/jgp.106.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prakriya M., Lewis R.S. Potentiation and inhibition of Ca(2+) release-activated Ca(2+) channels by 2-aminoethyldiphenyl borate (2-APB) occurs independently of IP(3) receptors. Journal of Physiology. 2001;536(Pt 1):3–19. doi: 10.1111/j.1469-7793.2001.t01-1-00003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chandy K.G., Wulff H., Beeton C., Pennington M., Gutman G.A., Cahalan M.D. K+ channels as targets for specific immunomodulation. Trends in Pharmacological Sciences. 2004;25(5):280–289. doi: 10.1016/j.tips.2004.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chung M.K., Lee H., Mizuno A., Suzuki M., Caterina M.J. 2-Aminoethoxydiphenyl borate activates and sensitizes the heat-gated ion channel TRPV3. Journal of Neuroscience. 2004;24(22):5177–5182. doi: 10.1523/JNEUROSCI.0934-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cui J., Bian J.S., Kagan A., McDonald T.V. CaT1 contributes to the stores-operated calcium current in Jurkat T-lymphocytes. Journal of Biological Chemistry. 2002;277(49):47175–47183. doi: 10.1074/jbc.M205870200. [DOI] [PubMed] [Google Scholar]
- 19.Delmas P. Assembly and gating of TRPC channels in signalling microdomains. Novartis Foundation Symposium. 2004;258:75–89. discussion 89–102, 283–266. [PubMed] [Google Scholar]
- 20.Diver J.M., Sage S.O., Rosado J.A. The inositol trisphosphate receptor antagonist 2-aminoethoxydiphenylborate (2-APB) blocks Ca2+ entry channels in human platelets: cautions for its use in studying Ca2+ influx. Cell Calcium. 2001;30(5):323–329. doi: 10.1054/ceca.2001.0239. [DOI] [PubMed] [Google Scholar]
- 21.He L.P., Hewavitharana T., Soboloff J., Spassova M.A., Gill D.L. A functional link between store-operated and TRPC channels revealed by the 3,5-bis(trifluoromethyl)pyrazole derivative, BTP2. Journal of Biological Chemistry. 2005;280(12):10997–11006. doi: 10.1074/jbc.M411797200. [DOI] [PubMed] [Google Scholar]
- 22.Hermosura M.C., Monteilh-Zoller M.K., Scharenberg A.M., Penner R., Fleig A. Dissociation of the store-operated calcium current I(CRAC) and the Mg-nucleotide-regulated metal ion current MagNuM. Journal of Physiology. 2002;539(Pt 2):445–458. doi: 10.1113/jphysiol.2001.013361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hinman A., Chuang H.H., Bautista D.M., Julius D. TRP channel activation by reversible covalent modification. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(51):19564–19568. doi: 10.1073/pnas.0609598103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim D., Cavanaugh E.J. Requirement of a soluble intracellular factor for activation of transient receptor potential A1 by pungent chemicals: role of inorganic polyphosphates. Journal of Neuroscience. 2007;27(24):6500–6509. doi: 10.1523/JNEUROSCI.0623-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lievremont J.P., Bird G.S., Putney J.W., Jr. Mechanism of inhibition of TRPC cation channels by 2-aminoethoxydiphenylborane. Molecular Pharmacology. 2005;68(3):758–762. doi: 10.1124/mol.105.012856. [DOI] [PubMed] [Google Scholar]
- 26.Missiaen L., Van Acker K., Van Baelen K., Raeymaekers L., Wuytack F., Parys J.B. Calcium release from the Golgi apparatus and the endoplasmic reticulum in HeLa cells stably expressing targeted aequorin to these compartments. Cell Calcium. 2004;36(6):479–487. doi: 10.1016/j.ceca.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 27.Nilius B., Prenen J., Vennekens R., Hoenderop J.G., Bindels R.J., Droogmans G. Pharmacological modulation of monovalent cation currents through the epithelial Ca2+ channel ECaC1. British Journal of Pharmacology. 2001;134(3):453–462. doi: 10.1038/sj.bjp.0704272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schindl R., Kahr H., Graz I., Groschner K., Romanin C. Store depletion-activated CaT1 currents in rat basophilic leukemia mast cells are inhibited by 2-aminoethoxydiphenyl borate. Evidence for a regulatory component that controls activation of both CaT1 and CRAC (Ca(2+) release-activated Ca(2+) channel) channels. Journal of Biological Chemistry. 2002;277(30):26950–26958. doi: 10.1074/jbc.M203700200. [DOI] [PubMed] [Google Scholar]
- 29.Trebak M., Bird G.S., McKay R.R., Putney J.W., Jr. Comparison of human TRPC3 channels in receptor-activated and store-operated modes. Differential sensitivity to channel blockers suggests fundamental differences in channel composition. Journal of Biological Chemistry. 2002;277(24):21617–21623. doi: 10.1074/jbc.M202549200. [DOI] [PubMed] [Google Scholar]
- 30.Turner H., Fleig A., Stokes A., Kinet J.P., Penner R. Discrimination of intracellular calcium store subcompartments using TRPV1 (transient receptor potential channel, vanilloid subfamily member 1) release channel activity. Biochemical Journal. 2003;371(Pt 2):341–350. doi: 10.1042/BJ20021381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Voets T., Prenen J., Fleig A., Vennekens R., Watanabe H., Hoenderop J.G. CaT1 and the calcium release-activated calcium channel manifest distinct pore properties. Journal of Biological Chemistry. 2001;276(51):47767–47770. doi: 10.1074/jbc.C100607200. [DOI] [PubMed] [Google Scholar]
- 32.Wang Y., Deshpande M., Payne R. 2-Aminoethoxydiphenyl borate inhibits phototransduction and blocks voltage-gated potassium channels in Limulus ventral photoreceptors. Cell Calcium. 2002;32(4):209–216. doi: 10.1016/s0143416002001562. [DOI] [PubMed] [Google Scholar]
- 33.Xu S.Z., Zeng F., Boulay G., Grimm C., Harteneck C., Beech D.J. Block of TRPC5 channels by 2-aminoethoxydiphenyl borate: a differential, extracellular and voltage-dependent effect. British Journal of Pharmacology. 2005;145(4):405–414. doi: 10.1038/sj.bjp.0706197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yoshino T., Ishikawa J., Ohga K., Morokata T., Takezawa R., Morio H. YM-58483, a selective CRAC channel inhibitor, prevents antigen-induced airway eosinophilia and late phase asthmatic responses via Th2 cytokine inhibition in animal models. European Journal of Pharmacology. 2007;560(2–3):225–233. doi: 10.1016/j.ejphar.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 35.Liou J., Kim M.L., Heo W.D., Jones J.T., Myers J.W., Ferrell J.E., Jr. STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Current Biology. 2005;15(13):1235–1241. doi: 10.1016/j.cub.2005.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peinelt C., Vig M., Koomoa D.L., Beck A., Nadler M.J., Koblan-Huberson M. Amplification of CRAC current by STIM1 and CRACM1 (Orai1) Nature Cell Biology. 2006;8(7):771–773. doi: 10.1038/ncb1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Soboloff J., Spassova M.A., Tang X.D., Hewavitharana T., Xu W., Gill D.L. Orai1 and STIM reconstitute store-operated calcium channel function. Journal of Biological Chemistry. 2006;281(30):20661–20665. doi: 10.1074/jbc.C600126200. [DOI] [PubMed] [Google Scholar]
- 38.Lis A., Peinelt C., Beck A., Parvez S., Monteilh-Zoller M., Fleig A. CRACM1, CRACM2, and CRACM3 are store-operated Ca2+ channels with distinct functional properties. Current Biology. 2007;17(9):794–800. doi: 10.1016/j.cub.2007.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Feske S., Gwack Y., Prakriya M., Srikanth S., Puppel S.H., Tanasa B. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature. 2006;441(7090):179–185. doi: 10.1038/nature04702. [DOI] [PubMed] [Google Scholar]
- 40.Mercer J.C., Dehaven W.I., Smyth J.T., Wedel B., Boyles R.R., Bird G.S. Large store-operated calcium selective currents due to co-expression of Orai1 or Orai2 with the intracellular calcium sensor Stim1. Journal of Biological Chemistry. 2006;281(34):24979–24990. doi: 10.1074/jbc.M604589200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Spassova M.A., Soboloff J., He L.P., Xu W., Dziadek M.A., Gill D.L. STIM1 has a plasma membrane role in the activation of store-operated Ca2+ channels. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(11):4040–4045. doi: 10.1073/pnas.0510050103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dehaven W.I., Smyth J.T., Boyles R.R., Bird G.S., Putney J.W., Jr. Complex actions of 2-aminoethyldiphenyl borate (2-APB) on store-operated calcium entry. Journal of Biological Chemistry. 2008;283(28):19265–19273. doi: 10.1074/jbc.M801535200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peinelt C., Lis A., Beck A., Fleig A., Penner R. 2-APB directly facilitates and indirectly inhibits STIM1-dependent gating of CRAC channels. Journal of Physiology. 2008;586(13):3061–3073. doi: 10.1113/jphysiol.2008.151365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tamarina N.A., Kuznetsov A., Philipson L.H. Reversible translocation of EYFP-tagged STIM1 is coupled to calcium influx in insulin secreting beta-cells. Cell Calcium. 2008;44(6):533–544. doi: 10.1016/j.ceca.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 45.Schindl R., Bergsmann J., Frischauf I., Derler I., Fahrner M., Muik M. 2-Aminoethoxydiphenyl borate alters selectivity of Orai3 channels by increasing their pore size. Journal of Biological Chemistry. 2008;283(29):20261–20267. doi: 10.1074/jbc.M803101200. [DOI] [PubMed] [Google Scholar]
- 46.Zhang S.L., Kozak J.A., Jiang W., Yeromin A.V., Chen J., Yu Y. Store-dependent and -independent modes regulating CRAC channel activity of human Orai1 and Orai3. Journal of Biological Chemistry. 2008;283(25):17662–17674. doi: 10.1074/jbc.M801536200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Romanin C., Gamsjaeger R., Kahr H., Schaufler D., Carlson O., Abernethy D.R. Ca(2+) sensors of l-type Ca(2+) channel. FEBS Letters. 2000;487(2):301–306. doi: 10.1016/s0014-5793(00)02361-9. [DOI] [PubMed] [Google Scholar]
- 48.Singh A., Hamedinger D., Hoda J.C., Gebhart M., Koschak A., Romanin C. C-terminal modulator controls Ca2+-dependent gating of Ca(v)1.4 l-type Ca2+ channels. Nature Neuroscience. 2006;9(9):1108–1116. doi: 10.1038/nn1751. [DOI] [PubMed] [Google Scholar]
- 49.Derler I., Hofbauer M., Kahr H., Fritsch R., Muik M., Kepplinger K. Dynamic but not constitutive association of calmodulin with rat TRPV6 channels enables fine tuning of Ca2+-dependent inactivation. Journal of Physiology. 2006;577(Pt 1):31–44. doi: 10.1113/jphysiol.2006.118661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xia Z., Liu Y. Reliable and global measurement of fluorescence resonance energy transfer using fluorescence microscopes. Biophysical Journal. 2001;81(4):2395–2402. doi: 10.1016/S0006-3495(01)75886-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Berney C., Danuser G. FRET or no FRET: a quantitative comparison. Biophysical Journal. 2003;84(6):3992–4010. doi: 10.1016/S0006-3495(03)75126-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Beech D.J. Orai1 calcium channels in the vasculature. Pflügers Archiv: European Journal of Physiology. 2012;463(5):635–647. doi: 10.1007/s00424-012-1090-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sweeney Z.K., Minatti A., Button D.C., Patrick S. Small-molecule inhibitors of store-operated calcium entry. ChemMedChem. 2009;4(5):706–718. doi: 10.1002/cmdc.200800452. [DOI] [PubMed] [Google Scholar]
- 54.Muik M., Frischauf I., Derler I., Fahrner M., Bergsmann J., Eder P. Dynamic coupling of the putative coiled-coil domain of ORAI1 with STIM1 mediates ORAI1 channel activation. Journal of Biological Chemistry. 2008;283(12):8014–8022. doi: 10.1074/jbc.M708898200. [DOI] [PubMed] [Google Scholar]
- 55.Navarro-Borelly L., Somasundaram A., Yamashita M., Ren D., Miller R.J., Prakriya M. STIM1–Orai1 interactions and Orai1 conformational changes revealed by live-cell FRET microscopy. Journal of Physiology. 2008;586(Pt 22):5383–5401. doi: 10.1113/jphysiol.2008.162503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.DeHaven W.I., Smyth J.T., Boyles R.R., Bird G.S., Putney J.W., Jr. Complex actions of 2-aminoethyldiphenyl borate on store-operated calcium entry. Journal of Biological Chemistry. 2008;283(28):19265–19273. doi: 10.1074/jbc.M801535200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schindl R., Bergsmann J., Frischauf I., Derler I., Fahrner M., Muik M. 2-Aminoethoxydiphenyl borate alters selectivity of Orai3 channels by increasing their pore size. Journal of Biological Chemistry. 2008;283(29):20261–20267. doi: 10.1074/jbc.M803101200. [DOI] [PubMed] [Google Scholar]
- 58.Zhang S.L., Kozak J.A., Jiang W., Yeromin A.V., Chen J., Yu Y. Store-dependent and -independent modes regulating Ca2+ release-activated Ca2+ channel activity of human Orai1 and Orai3. Journal of Biological Chemistry. 2008;283(25):17662–17671. doi: 10.1074/jbc.M801536200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Prakriya M., Feske S., Gwack Y., Srikanth S., Rao A., Hogan P.G. Orai1 is an essential pore subunit of the CRAC channel. Nature. 2006;443(7108):230–233. doi: 10.1038/nature05122. [DOI] [PubMed] [Google Scholar]
- 60.Vig M., Beck A., Billingsley J.M., Lis A., Parvez S., Peinelt C. CRACM1 multimers form the ion-selective pore of the CRAC channel. Current Biology. 2006;16(20):2073–2079. doi: 10.1016/j.cub.2006.08.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yamashita M., Navarro-Borelly L., McNally B.A., Prakriya M. Orai1 mutations alter ion permeation and Ca2+-dependent fast inactivation of CRAC channels: evidence for coupling of permeation and gating. Journal of General Physiology. 2007;130(5):525–540. doi: 10.1085/jgp.200709872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Owsianik G., Talavera K., Voets T., Nilius B. Permeation and selectivity of trp channels. Annual Review of Physiology. 2006;68:685–717. doi: 10.1146/annurev.physiol.68.040204.101406. [DOI] [PubMed] [Google Scholar]
- 63.Feske S. CRAC channelopathies. Pflügers Archiv: European Journal of Physiology. 2010;462(2):417–435. doi: 10.1007/s00424-009-0777-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Derler I., Schindl F.R., Romanin C R. CRAC channels: inhibitors and potential applications. Expert Opinion in Drug Discovery. 2008;3:787–800. doi: 10.1517/17460441.3.7.787. [DOI] [PubMed] [Google Scholar]
- 65.Derler I., Fritsch R., Schindl R., Romanin C. CRAC inhibitors: identification and potential. Expert Opinion on Drug Discovery. 2008;3(7):787–800. doi: 10.1517/17460441.3.7.787. [DOI] [PubMed] [Google Scholar]
- 66.Putney J.W. Pharmacology of store-operated calcium channels. Molecular Interventions. 2010;10(4):209–218. doi: 10.1124/mi.10.4.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Di Sabatino A., Rovedatti L., Kaur R., Spencer J.P., Brown J.T., Morisset V.D. Targeting gut T cell Ca2+ release-activated Ca2+ channels inhibits T cell cytokine production and T-box transcription factor T-bet in inflammatory bowel disease. Journal of Immunology. 2009;183(5):3454–3462. doi: 10.4049/jimmunol.0802887. [DOI] [PubMed] [Google Scholar]
- 68.Ng S.W., di Capite J., Singaravelu K., Parekh A.B. Sustained activation of the tyrosine kinase Syk by antigen in mast cells requires local Ca2+ influx through Ca2+ release-activated Ca2+ channels. Journal of Biological Chemistry. 2008;283(46):31348–31355. doi: 10.1074/jbc.M804942200. [DOI] [PubMed] [Google Scholar]
- 69.Li J., McKeown L., Ojelabi O., Stacey M., Foster R., O’Regan D. Nanomolar potency and selectivity of a Ca(2+) release-activated Ca(2+) channel inhibitor against store-operated Ca(2+) entry and migration of vascular smooth muscle cells. British Journal of Pharmacology. 2011;164(2):382–393. doi: 10.1111/j.1476-5381.2011.01368.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.McNally B.A., Yamashita M., Engh A., Prakriya M. Structural determinants of ion permeation in CRAC channels. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(52):22516–22521. doi: 10.1073/pnas.0909574106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yeromin A.V., Zhang S.L., Jiang W., Yu Y., Safrina O., Cahalan M.D. Molecular identification of the CRAC channel by altered ion selectivity in a mutant of Orai. Nature. 2006;443(7108):226–229. doi: 10.1038/nature05108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ashmole I., Duffy S.M., Leyland M.L., Morrison V.S., Begg M., Bradding P. CRACM/Orai ion channel expression and function in human lung mast cells. Journal of Allergy and Clinical Immunology. 2012;129(6):1628–1635. doi: 10.1016/j.jaci.2012.01.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Owsianik G., D’Hoedt D., Voets T., Nilius B. Structure–function relationship of the TRP channel superfamily. Reviews of Physiology Biochemistry and Pharmacology. 2006;156:61–90. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.