Abstract
Purpose
Acute epididymitis is considered to have an important role in children with scrotal pain. Recent reports have shown that urinalysis is not helpful for the diagnosis and treatment of acute epididymitis owing to negative microbiological findings. Therefore, we analyzed clinical and laboratory characteristics to examine the diagnostic yield of urinalysis in children.
Materials and Methods
We retrospectively reviewed the medical records of 139 patients who were diagnosed with acute epididymitis from 2005 to 2011. Diagnosis was based on symptoms, physical findings, and color Doppler ultrasonography (DUS). To investigate the characteristics of epididymitis in children, the patients were divided into 3 groups: group A (aged less than 18 years, 76 patients), group B (18 to 35 years old, 19 patients), and group C (older than 35 years, 44 patients).
Results
There were statistically significant differences in age, symptom duration, hospital stays, and lesion location in each group. White blood cell count and serum C-reactive protein levels, pyuria, and positive urine culture results were statistically higher in the older age group. The most common cause of acute epididymitis in children was idiopathic (96.1%).
Conclusions
In our group of children with epididymitis, 73 cases out of 76 (96.1%) resulted in negative pyuria in urinalysis. In addition, the most common cause of epididymitis was idiopathic. Because most urinalyses do not show pyuria, we believe that routine antibiotics may be not required in pediatric patients with epididymitis. If urinalysis shows pyuria with or without positive urine culture, antibiotics should be considered.
Keywords: Epididymitis, Pyuria, Urinalysis
INTRODUCTION
Whereas testicular appendix and testicular torsions are the most common causes of scrotal pain, acute epididymitis is considered to have an important role in children with scrotal pain [1,2]. Previous studies have demonstrated that enteric organisms are a common cause of epididymitis in older men with benign prostatic hyperplasia, and that epididymitis in adult males younger than 35 years of age is often due to sexually transmitted organisms [3]. However, the causes and management of epididymitis in children have not been fully elucidated. The etiologies include an ascending infection, hematogenously spread bacteria, viral causes, urinary reflux, and structural and functional anomalies of the urinary tract [1-5].
Because ascending infection is suspected as a cause of epididymitis, treatment of epididymitis in children has focused on antibiotic therapy [3]. To perform empirical or targeted antibiotic treatment, urinalysis with culture is generally recommended [3,6]. However, recent reports have indicated that the urine test is not helpful for the diagnosis and treatment of epididymitis in children owing to negative microbiological findings [6]. In addition, there are scant data to support a bacterial cause of epididymitis in children, and antibiotic therapy is not based on evidence [3].
Therefore, we analyzed clinical and laboratory characteristics to examine the diagnostic yield of urine testing in children with epididymitis. Additionally, we investigated whether differences exist in clinical data between children and adults.
MATERIALS AND METHODS
We retrospectively reviewed the medical records of 139 patients diagnosed with acute epididymitis from 2005 to 2011. All patients underwent physical examination, laboratory studies, and immediate color Doppler ultrasonography (DUS). Diagnosis was based on symptoms, physical findings, and color DUS. The diagnosis was made clinically and was confirmed by color DUS. The results of the color DUS were first read by the on-call radiologist and subsequently by a senior radiologist. The patients with other causes of acute scrotum, including torsion of testis and appendix testis, inguinal hernia, hydrocele, vasculitis, and recent urologic surgery, were excluded.
Clinical characteristics included age, symptom duration, hospital day, location of lesion, fever, dysuria, scrotal redness, swelling, and tenderness. Symptom duration was measured as time taken from onset of the symptoms to hospital arrival. Laboratory studies included white blood cell (WBC) count, serum C-reactive protein (CRP), and urine analysis. Microbiological study included urine cultures done by the clean catch method. Treatment of epididymitis consisted of systemic antibiotics and antiphlogistics treatment, bed rest, and local scrotal cooling. To investigate the characteristics of epididymitis in children, the patients were divided into 3 groups: group A (aged less than 18 years, 76 patients), group B (18 to 35 years old, 19 patients), and group C (older than 35 years, 44 patients).
PASW ver. 18.0 (IBM Co., Armonk, NY, USA) was used for all statistical analyses. One-way analysis of variance and chi-square tests were used to compare clinical and laboratory findings in each groups. A p-value <0.05 was considered statistically significant.
RESULTS
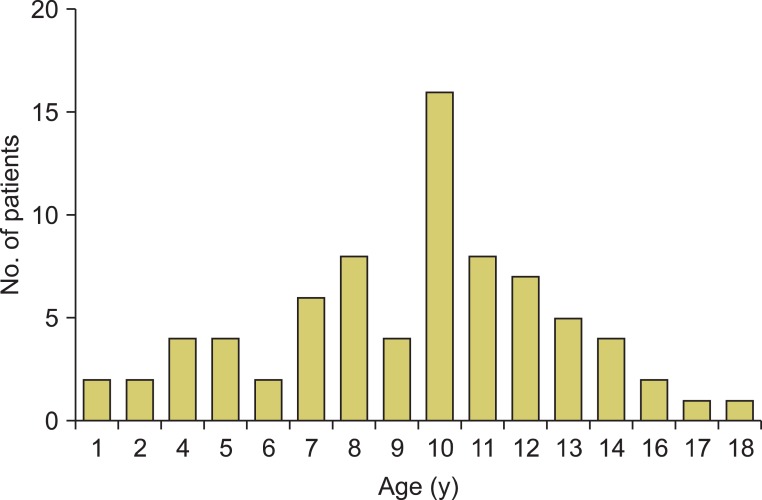
The mean ages in groups A, B, and C were 9.41 years (range, 3 to 17 years), 24.32 years (range, 18 to 35 years), and 59.27 years (range, 36 to 75 years), respectively. The prevalence of acute epididymitis in children peaked around 10 years of age (Fig. 1). The average symptom durations in groups A, B, and C were 58.10 hours (range, 1 to 84 hours), 88.32 hours (range, 1 to 150 hours), and 96.01 hours (range, 1 to 150), respectively. The average hospital stays in groups A, B, and C were 5.20 days (range, 3 to 7 days), 6.00 days (range, 3 to 10 days), and 8.52 days (range 4 to 11 days), respectively. In group A, 96.1% had a unilateral lesion and in 3.9% the lesion was bilateral. There was a significantly higher incidence of epididymitis on both sides in older ages. There were statistically significant differences in age, symptom duration, hospital stays, and lesion location (p<0.001, p=0.003, p<0.001, and p=0.005, respectively). The WBC count and serum CRP were significantly higher in the older age groups (p<0.001 and p<0.001, respectively). Pyuria and positive urine culture occurred more frequently in group C (p<0.001 and p=0.005, respectively). Compared with the adult group (15.8% and 50.0%), only 3 cases (3.9%) in the child group involved pyuria. A positive urine culture result for gram-positive cocci was reported in only 1 child (1.3%) with a posterior urethral valve (Table 1).
FIG. 1.
Age distribution of children with acute epididymitis.
TABLE 1.
Clinical and laboratory characteristics of acute epididymitis in all patients
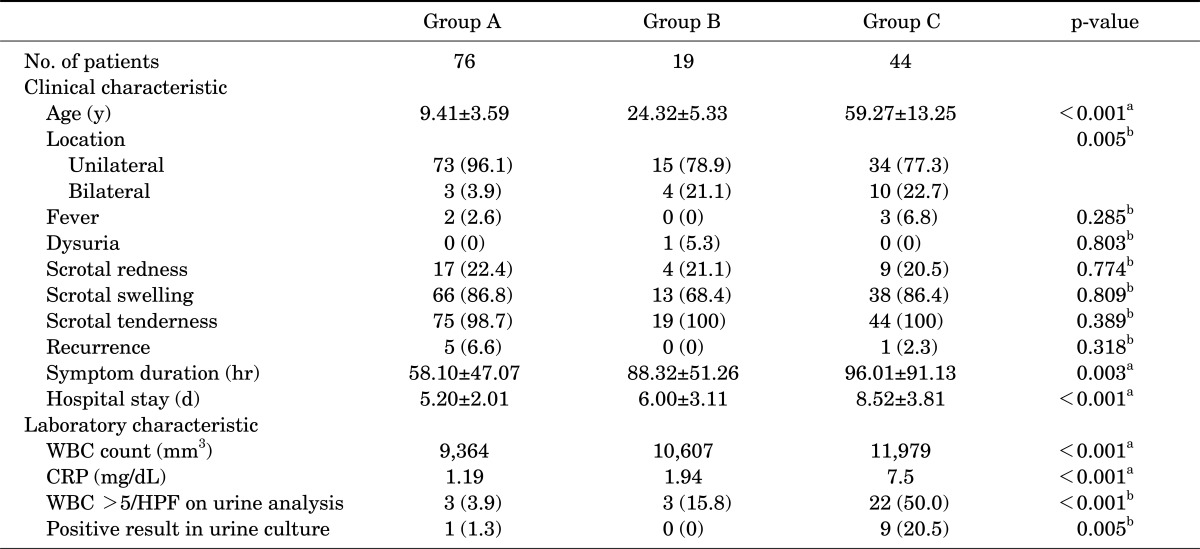
Values are presented as mean±standard deviation or number (%).
WBC, white blood cell; CRP, C reactive protein; HPF, high power field.
a:By one-way analysis of variance. b:Chi-square test.
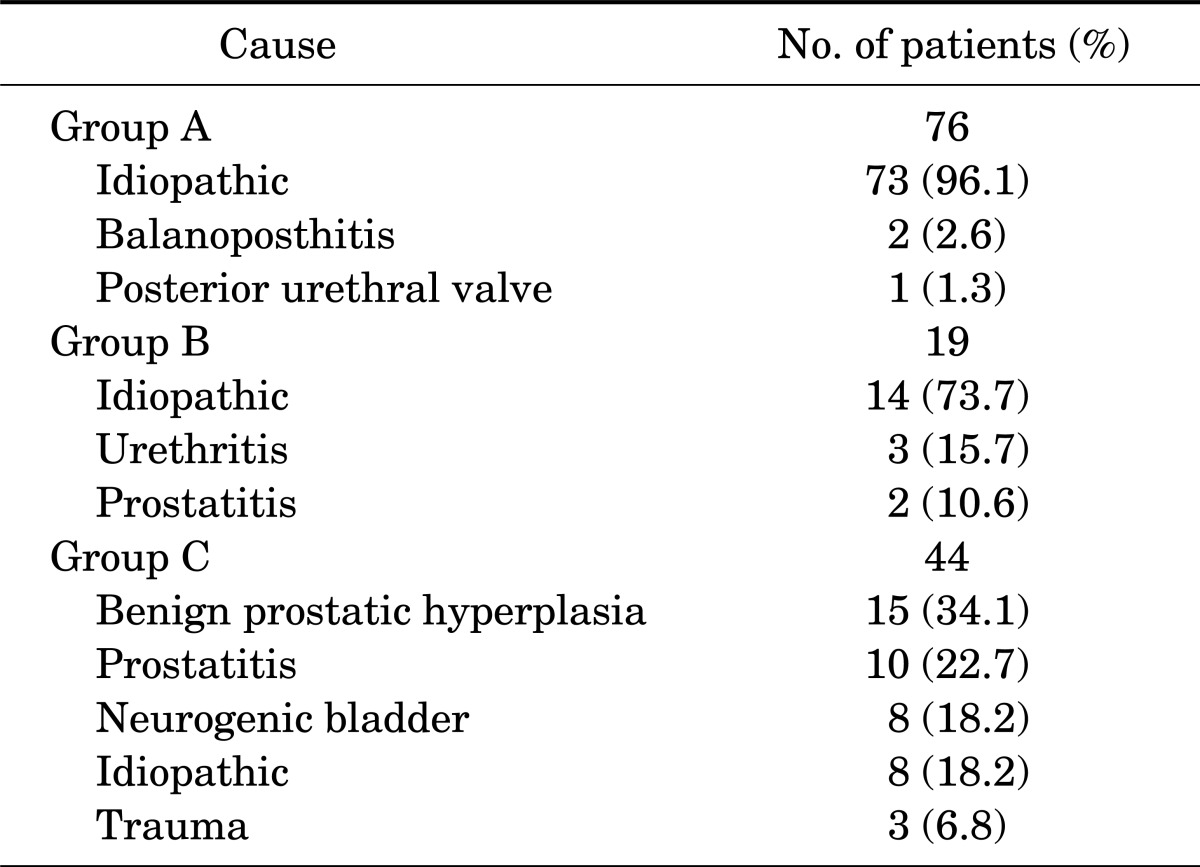
The underlying causes of acute epididymitis in the 3 groups are summarized in Table 2. The causes of acute epididymitis in the child group were categorized as 73 idiopathic cases (96.1%), 2 balanoposthitis (2.6%), and 1 posterior urethral valve (1.3%). In group B, urethritis and prostatitis were found in 3 cases (15.8%) and 2 cases (10.6%) , respectively. For group C, the categorizations were 15 benign prostatic hyperplasia (34.1%), 10 prostatitis (22.7%), 8 neurogenic bladder (12.7%), 8 idiopathic (12.7%), and 3 trauma (4.7%).
TABLE 2.
Underlying cause of acute epididymitis
DISCUSSION
Acute epididymitis in childhood is not a rare disease, and it is an important clinical syndrome because it must be diagnosed differentially from acute scrotum [6,7]. According to traditional theory, epididymitis is described as an ascending bacterial urinary infection via urinary reflux. Urinary reflux can be infectious or sterile (chemical epididymitis). Congenital lower genitourinary tract anomalies are also associated with retrograde urinary reflux: meatal stenosis in hypospadias, urethral stenosis, posterior urethral valves, ectopic ureter, vesicoureteral reflux, ureteral stenosis in double ureter anomaly, recto-urethral fistula in imperforate anus, and neurogenic voiding dysfunction. Hematological spread (viremia or bacteremia), postinfectious reaction, or posttraumatic epididymitis are also known to cause acute epididymitis. In addition, manipulations (e.g., urethrocystoscopy, clean intermittent self-catheterization, and surgery of the urethra or testis) can lead to epididymitis [4,5,8,9]. However, the exact etiology, incidence, and treatment of epididymitis have not been clearly defined in children.
In past studies, most reported cases in children were idiopathic [6]. Likewise, this study found that the most common cause of epididymitis in children was idiopathic (96.1%). In idiopathic epididymitis, other systemic diseases such as serositis (familial Mediterranean fever, sarcoidosis, and Kawasaki disease) and vasculitis (Schönlein-Henoch purpura and polyarteritisnodosa) must be ruled out [1]. In addition, epididymitis was reportedly more likely to be related to genitourinary abnormalities in children than in older males. In adult patients under 35 years of age, the most common cause of epididymitis is sexually transmitted pathogens associated with urethritis, including Neisseria gonorrhoeae and Chlamydia trachomatis [10]. However, Takahashi et al. [11] reported that chlamydia trachomatis was detected in 29.3% of asymptomatic males who did not have pyuria. C. trachomatis may reach the epididymis through the urethra and cause acute epididymitis without prominent inflammation of the urethra [12]. In our cases, a major cause of epididymitis was idiopathic, with the exception of 5 patients (3 with urethritis and 2 with prostatitis). We believe that some patients with idiopathic causes may have sexually mediated "silent" chlamydial infection. In men older than 35 years of age, epididymitis is most commonly caused by gram-negative bacilli responsible for urinary tract infection. More than half of the patients had underlying urinary pathology (e.g., benign prostatic hyperplasia, transurethral resection of the prostate, chronic prostatitis, indwelling urethral catheters, and neurogenic bladder) [10]. In our study, benign prostatic hypertrophy, prostatitis, and neurogenic bladder were the majority of the causes.
Previous studies reported no significant differences in location, symptom duration, or hospital stay between the child and adult groups [13-15]. However, in this study, the adult group had a greater number of cases of bilateral acute epididymitis, longer symptom duration, and more protracted hospital stay. We suggest that concurrent urological disease, such as neurogenic bladder, may have affected disease severity and the longer hospital stay in the adult group.
Santillanes et al. [3] reported on 140 pediatric patients with epididymitis who underwent urinalysis, of whom 5 (4%) had a pyuria. Positive urine cultures in children with epididymitis are a rare finding [1,5-7]. In any case with a positive urine culture, we should consider congenital anomalies [10]. Urinary tract ultrasonography (US) and voiding cystourethrography are recommended in patients with positive urine cultures, recurrent epididymitis, or underlying anomalies that may be associated with urologic malformation (i.e., imperforated anus) [16]. In our study, there were only 3 cases (3.9%) with pyuria and 1 case (1.3%) with a positive urine culture. The latter patient had an involvement in the posterior urethral valve. Kim et al. [17] found that pyuria was present in 16.7% of adult patients with acute epididymitis. The finding of the current study of pyuria in 15.7% (3 of 19) of patients is consistent with the results of previous studies. Several studies observed increases in serum CRP and WBC count in epididymitis patients [14,17]. Our result revealed statistically high CRP and WBC in the adult group, which may have reflected the severity of the disease. However, simply comparing the laboratory values of pediatric patients with those of adults may present some limitations.
Management of acute epididymitis depends on the most likely cause or organism [18]. However, recent literature has voiced increasing doubts about this practice in children. Most cases of epididymitis in children are idiopathic without bacteriuria [19]. Some studies have suggested that antibiotic therapy can be reserved for young infants with pyuria or positive urine culture results. Prophylactic antibiotics may not be necessary to treat idiopathic and single-episode epididymitis in prepubertal boys without urological tract anomaly or pyuria [3]. Although we use antibiotics in children with epididymitis empirically, this use should be reconsidered for patients with negative urinalysis. Other treatments consist of analgesics, non-steroidal anti-inflammatory drugs, bed rest, and scrotal cooling [18]. In addition, when obvious causes of urinary tract infection (e.g., vesicoureteral reflux, benign prostatic hyperplasia, and neurogenic bladder) are present, correction may be needed.
Our study had the limitations of being a retrospective data review and having a small sample size for comparison. Prospective randomized clinical trials with a larger number of cases are needed to test the role of urine analysis in child epididymitis.
CONCLUSIONS
In our group of children with epididymitis, 73 cases out of 76 (96.1%) resulted in negative pyuria in urinalysis. In addition, the most common cause of epididymitis was idiopathic. Because most urinalysis results do not show pyuria, we believe that routine antibiotics may be not required in pediatric patients with epididymitis. If urinalysis shows pyuria with or without positive urine culture, antibiotics should be considered.
Footnotes
The authors have nothing to disclose.
References
- 1.Somekh E, Gorenstein A, Serour F. Acute epididymitis in boys: evidence of a post-infectious etiology. J Urol. 2004;171:391–394. doi: 10.1097/01.ju.0000102160.55494.1f. [DOI] [PubMed] [Google Scholar]
- 2.McAndrew HF, Pemberton R, Kikiros CS, Gollow I. The incidence and investigation of acute scrotal problems in children. Pediatr Surg Int. 2002;18:435–437. doi: 10.1007/s00383-002-0806-3. [DOI] [PubMed] [Google Scholar]
- 3.Santillanes G, Gausche-Hill M, Lewis RJ. Are antibiotics necessary for pediatric epididymitis? Pediatr Emerg Care. 2011;27:174–178. doi: 10.1097/PEC.0b013e31820d647a. [DOI] [PubMed] [Google Scholar]
- 4.Bukowski TP, Lewis AG, Reeves D, Wacksman J, Sheldon CA. Epididymitis in older boys: dysfunctional voiding as an etiology. J Urol. 1995;154(2 Pt 2):762–765. [PubMed] [Google Scholar]
- 5.Likitnukul S, McCracken GH, Jr, Nelson JD, Votteler TP. Epididymitis in children and adolescents: a 20-year retrospective study. Am J Dis Child. 1987;141:41–44. doi: 10.1001/archpedi.1987.04460010041019. [DOI] [PubMed] [Google Scholar]
- 6.Gislason T, Noronha RF, Gregory JG. Acute epididymitis in boys: a 5-year retrospective study. J Urol. 1980;124:533–534. doi: 10.1016/s0022-5347(17)55528-7. [DOI] [PubMed] [Google Scholar]
- 7.Sakellaris GS, Charissis GC. Acute epididymitis in Greek children: a 3-year retrospective study. Eur J Pediatr. 2008;167:765–769. doi: 10.1007/s00431-007-0584-y. [DOI] [PubMed] [Google Scholar]
- 8.Hutcheson J, Peters CA, Diamond DA. Amiodarone induced epididymitis in children. J Urol. 1998;160:515–517. [PubMed] [Google Scholar]
- 9.Kiviat MD, Shurtleff D, Ansell JS. Urinary reflux via the vas deferens: unusual cause of epididymitis in infancy. J Pediatr. 1972;80:476–479. doi: 10.1016/s0022-3476(72)80511-0. [DOI] [PubMed] [Google Scholar]
- 10.Berger RE. Acute epididymitis. Sex Transm Dis. 1981;8:286–289. [PubMed] [Google Scholar]
- 11.Takahashi S, Kurimura Y, Hashimoto J, Sunaoshi K, Takeda K, Suzuki N, et al. Management for males whose female partners are diagnosed with genital chlamydial infection. J Infect Chemother. 2011;17:76–79. doi: 10.1007/s10156-010-0083-3. [DOI] [PubMed] [Google Scholar]
- 12.Ito S, Tsuchiya T, Yasuda M, Yokoi S, Nakano M, Deguchi T. Prevalence of genital mycoplasmas and ureaplasmas in men younger than 40 years-of-age with acute epididymitis. Int J Urol. 2012;19:234–238. doi: 10.1111/j.1442-2042.2011.02917.x. [DOI] [PubMed] [Google Scholar]
- 13.Haecker FM, Hauri-Hohl A, von Schweinitz D. Acute epididymitis in children: a 4-year retrospective study. Eur J Pediatr Surg. 2005;15:180–186. doi: 10.1055/s-2004-830355. [DOI] [PubMed] [Google Scholar]
- 14.Park CH, Im JK, Kim KK, Park HW. Clinical characteristics of acute epididymitis in children and adults. Korean J Urol. 1999;40:674–676. [Google Scholar]
- 15.van Gool JD, Hjalmas K, Tamminen-Mobius T, Olbing H. Historical clues to the complex of dysfunctional voiding, urinary tract infection and vesicoureteral reflux. The International Reflux Study in Children. J Urol. 1992;148(5 Pt 2):1699–1702. doi: 10.1016/s0022-5347(17)37006-4. [DOI] [PubMed] [Google Scholar]
- 16.Al-Taheini KM, Pike J, Leonard M. Acute epididymitis in children: the role of radiologic studies. Urology. 2008;71:826–829. doi: 10.1016/j.urology.2007.10.071. [DOI] [PubMed] [Google Scholar]
- 17.Kim JM, Lee CY, Lee SD. Acute epididymitis in children; 10-year retrospective study of single center. Korean J Urogenit Tract Infect Inflamm. 2007;2:173–178. [Google Scholar]
- 18.Tracy CR, Steers WD, Costabile R. Diagnosis and management of epididymitis. Urol Clin North Am. 2008;35:101–108. doi: 10.1016/j.ucl.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 19.Graumann LA, Dietz HG, Stehr M. Urinalysis in children with epididymitis. Eur J Pediatr Surg. 2010;20:247–249. doi: 10.1055/s-0030-1253356. [DOI] [PubMed] [Google Scholar]