Abstract
Retinal degeneration is a leading cause of irreversible blindness in the developed world. Differentiation of retinal cells, including photoreceptors, from both mouse and human ES and iPS cells, potentially provide a renewable source of cells for retinal transplantation. Previously, we have shown both the functional integration of transplanted rod photoreceptor precursors, isolated from the postnatal retina, in the adult murine retina, and photoreceptor cell generation by stepwise treatment of ES cells with defined factors. In this study we assessed the extent to which this protocol recapitulates retinal development and also evaluated differentiation and integration of ES cell-derived retinal cells following transplantation using our established procedures. Optimized retinal differentiation via isolation of Rax.GFP retinal progenitors recreated a retinal niche and increased the yield of Crx+ and Rhodopsin+ photoreceptors. Rod birth peaked at day 20 of culture and expression of the early photoreceptor markers Crx and Nrl increased until day 28. Nrl levels were low in ES cell-derived populations compared with developing retinae. Transplantation of early stage retinal cultures produced large tumors, which were avoided by prolonged retinal differentiation (up to day 28) prior to transplantation. Integrated mature photoreceptors were not observed in the adult retina, even when more than 60% of transplanted ES cell-derived cells expressed Crx. We conclude that exclusion of proliferative cells from ES cell-derived cultures is essential for effective transplantation. Despite showing expression profiles characteristic of immature photoreceptors, the ES cell-derived precursors generated using this protocol did not display transplantation competence equivalent to precursors from the postnatal retina.
Keywords: Embryonic stem cells, Retina, Cell transplantation, Photoreceptor cells, Fluorescent protein reporter genes, Stem cell transplantation
Introduction
Photoreceptor degeneration, as a result of inherited or age related eye disease, is the leading cause of blindness. Once lost, photoreceptors are unable to regenerate and currently there are no effective treatments to restore sight. While strategies such as gene therapy may offer ways to slow the progression of disease, they are ineffective once photoreceptors have completely degenerated. Therefore, novel approaches to replace the light-sensitive cells of the eye represent an exciting therapeutic strategy that, if successful, would be applicable to a wide range of degenerative retinal diseases.
We have previously demonstrated the transplantation and integration of freshly isolated post mitotic photoreceptor precursors into the adult mouse retina. Cells isolated at the correct ontogenetic stage from the developing retina were able to integrate into the host retina, form functional synaptic connections and mediate light-stimulated behavior in mice with retinal degeneration (1, 2). However, the equivalent stage of development from which post mitotic photoreceptor precursors could be derived in humans are second trimester fetuses, an ethically and logistically challenging source. An alternative renewable cell source is embryonic stem (ES) cells, which have the potential to be differentiated into nearly all cell types (3). With the recent discovery of iPS cells, ES-like cells generated from reprogrammed adult somatic cells, it is hoped that they will eventually provide an ethical source of pluripotent cells for regenerative medicine (4, 5).
Several groups have reported the differentiation of different retinal cell types from both human and mouse ES cells, and more recently from iPS cells (6-12). We previously demonstrated for the first time, the generation of different retinal cell types by the stepwise treatment of mouse, primate and human ES cells with defined factors (9). This protocol used a serum-free embryoid body-like suspension culture, followed by an adherent culture in which differentiated cells migrate out from aggregated cell pellets. Retinal cell types had been differentiated previously from human ES cells only by the use of cocultures, conditioned media and Matrigel (6, 11). Since this first study, other defined conditions have been used to differentiate human ES cells towards retinal cell types (10). Lamba et al. recently reported the integration of human ES cell-derived photoreceptors following the transplantation of mixed ES cell-derived retinal cell populations to the subretinal space of immunosuppressed mice (13). These cells were differentiated using Matrigel, a solubilised basement membrane preparation derived from mouse sarcomas, which contains a variety of undefined growth factors and were labeled using a replication incompetent lentivirus expressing eGFP under a ubiquitous promoter (hEF1a) or an adenovirus driving eGFP under a CMV promoter (11, 13). The transplanted cells within the host outer nuclear layer (ONL) were identified by GFP fluorescence and expression of rod photoreceptor markers (13). The study found no evidence for the formation of teratomas, arising from the transplantation of undifferentiated human ES cells.
As yet, no studies have investigated the transplantation and integration of mouse ES cell-derived photoreceptors or ES cell-derived retinal cells differentiated in a defined manner, a more appropriate method for clinical application. There is limited evidence that ES cell-derived cells can mature into functional photoreceptors forming outer segments and synapses. Given that freshly dissociated early postnatal mouse photoreceptor precursors are able to integrate into the adult murine retina, we investigated whether mouse ES cell-derived photoreceptors could be cultured to an equivalent developmental stage using a defined protocol, and tested whether such cells could also integrate into the adult murine retina. Using a stepwise retinal differentiation protocol, we demonstrated the optimized generation of early retinal progenitors and differentiation of immature photoreceptors, and compared the timing and profile of ES-cell derived retinal differentiation with normal retinal development. We evaluated the transplantation competence of ES cell-derived retinal populations at different stages of development in the healthy and degenerating mouse retina, and demonstrated the critical importance of avoiding the transplantation of highly proliferative ES cell-derived cell populations that generate tumors.
Materials and Methods
ES cell lines and differentiation culture
An EB5 mouse ES cell line, Rax.GFP (kind gift of Y. Sasai) (14), was maintained and differentiated as previously described (8, 9). Details of the adapted protocol and the generation of the Rax.GFP/Nrl.RFP reporter line are described in the supplementary methods.
FACS analysis
Described in supplementary methods.
Real-time and RT-PCR analysis
Total RNA was extracted with RNeasy Micro or Mini Kit (QIAGEN), and Reverse transcription was performed using 1 μg of total RNA and QuantiTect Reverse Transcription Kit (Qiagen). The synthesized cDNA was amplified with gene-specific primers, shown in Supplementary Table 1. Real-time quantitative RT-PCR (qRT-PCR) is described in the supplementary methods.
Immunocytochemistry
Primary antibodies and working dilutions used are included in supplementary Table 2. Protocol details are described in supplementary methods.
Production of recombinant AAV2/9 CMV.GFP
Described in supplementary methods.
Transplantation of ES cell-derived cells
C57Bl/6J, Rho−/−, Gucy2e−/− and Gnat−/− mice were maintained in the animal facility at University College London. All experiments were conducted in accordance with the Policies on the Use of Animals and Humans in Neuroscience Research. Adult mice were 6-15 weeks old and kept on a standard 12hr light-dark cycle. Differentiated cells were dissociated and transplanted as previously described (1, 15-17), further details are provided in the supplementary methods.
Histology and Immunohistochemistry
Described in supplementary methods.
Microscopy, image acquisition and processing
Described in supplementary methods.
BrdU cell birth analysis and cell counts
For birth date experiments, day 10 Rax.GFP+ cells were plated in chamber slides and BrdU (Becton Dickinson) was added to the differentiation medium at various timepoints (see text), for 24 hrs at a final concentration of 10 μM. Double staining for BrdU and cell type specific markers was performed as described above and in supplementary methods.
Statistical Analysis
All means are presented ± SEM (standard error of the mean), unless otherwise stated; N, number of animals or independent experiments performed; n, number of eyes, sections or fields of view examined, where appropriate. The statistical program used for the analysis of all data was Prism 5 (GraphPad Software, Inc, La Jolla, USA); *P < 0.05, **P < 0.01, ***P < 0.001.
Results
Optimization of the generation of retinal progenitor cells from ES cells
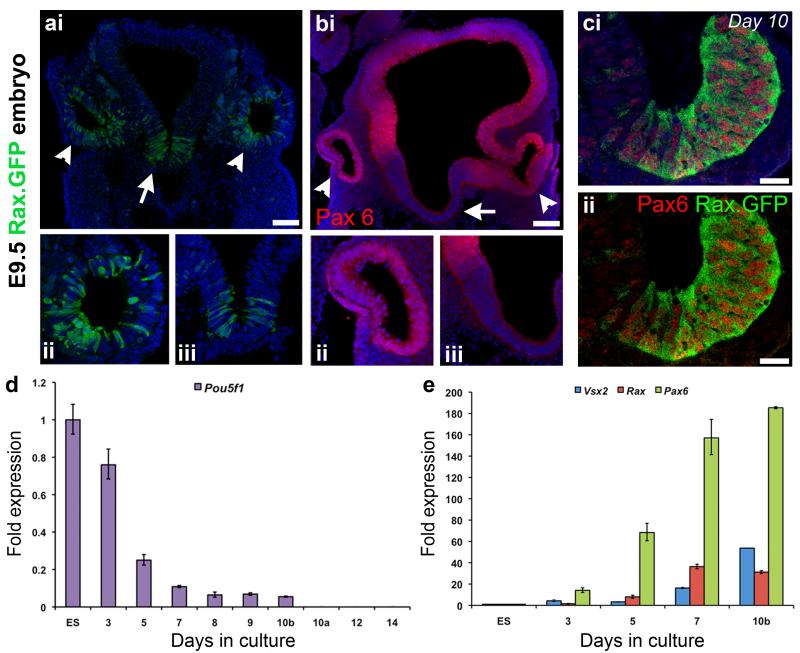
We previously showed the differentiation of retinal cells from mouse ES cells using a defined serum-free floating culture of embryoid body-like aggregates method (SFEB/DLFA culture, (8, 9)). We sought to further investigate this method of retinal differentiation, with the specific purpose of generating photoreceptor precursors for transplantation. Using the same EB5 mouse ES cell line, which contains the eGFP reporter inserted into the first exon of the Rax gene (kind gift of Y. Sasai) (14), we examined the stage-specific differentiation of early retinal progenitors. In chimeric embryos, the expression of Rax.GFP is restricted to the diencephalon, in particular to the eye field and subsequent optic vesicle (Figure 1ai,ii; white arrow heads; green) and the ventral floor plate cells of the hypothalamus (Figure 1ai,iii; white arrow; green) at E9.5. To distinguish retinal Rax.GFP progenitors from hypothalamic Rax.GFP progenitors the co-expression of the transcription factor Pax6 was utilized. Pax6 is widely expressed in the embryonic forebrain (Figure 1bi; red), including the optic vesicle (Figure 1bi,ii; white arrow heads; red), while the ventral floor plate cells of the hypothalamus do not express Pax6 (Figure 1bi,iii; white arrow). Using the SFEB/DLFA culture method, immunocytochemistry for Rax.GFP and Pax6, (Figure 1ci, ii) confirmed the in vitro generation of early retinal progenitors from the EB5 ES cell line. Quantitative transcriptional analysis of the cultures demonstrated a reduction in the pluripotency marker Oct3/4 (Pou5f1) over the first 10 days of differentiation, and a loss of expression after FAC-sorting (at day 10a) and further differentiation of the Rax.GFP+ cell population (Figure 1d). In contrast, the early retinal progenitor markers Rax, Pax6 and Vsx2 (Chx10) showed increased expression (Figure 1e). This expression profile is consistent with previous observations of neural differentiation using this protocol and the derivation of early retinal progenitors after 7-10 days of culture (8, 9, 14).
Figure 1. Differentiation of ES cell-derived retinal progenitor cells.
a,b, E9.5 mouse embryo showing Rax.GFP and Pax6. Rax expression was restricted to the optic vesicles (ai,ii; white arrow heads, green) and the ventral floor plate of the hypothalamus (ai,iii; white arrow, green). Pax6 is expressed the optic vesicles (bi,ii; white arrow heads; red), while the ventral floor plate cells of the hypothalamus do not express Pax6 (bi,iii; white arrow). ci,ii, Immunocytochemical analyses of a day 10 EB showing Pax6+/Rax.GFP+ retinal progenitors (red and green, respectively). d,e, Real time qPCR analysis showing the decreased expression of the pluripotency marker Pou5f1 (Oct3/4) (d) and the increased expression of the retinal progenitor markers Rax, Pax6, and Vsx2 (Chx10) (e) with days in culture (N = 3 independent experiments; b and a denote before and after FAC-sorting). Nuclei are stained with DAPI (blue). Scale bars, 100 μm (ai,bi) and 25 μm (ci,cii).
Since large numbers of differentiated retinal cells are required for transplantation studies we further optimized the percentage of Rax.GFP+ retinal progenitors achieved by day 10. We determined the optimal cell density for the generation of retinal progenitors by forming individual embryoid-like bodies (EBs) in 96 well plates and FAC-sorting the cells at day 10 to determine the percentage of Rax.GFP+ retinal progenitors present. The optimal EB size was 9,000-15,000 cells (Figure 2a; green Rax.GFP+ retinal progenitors; Figure 2b; 90-150 cells/μl of media), which was significantly more efficient than 3,000 or 6,000 cells/EB (Figure 2b; ANOVA, P<0.01). The loss of efficiency at the higher cell density of 27,000 cells/EB (270 cells/μl) is most likely due to cell starvation. Pax6 immunocytochemistry confirmed that the Rax.GFP+ cells were retinal progenitors and not hypothalamic progenitors at the various cell densities tested (Figure 2a). The larger EBs displayed neural epithelial-like organization of the Rax/Pax6+ cells (>9,000 cells/EB or >90 cells/μl) on days 7 and 10 of differentiation, reminiscent of the optic vesicle (Figure 2c). However, these regions did not progress to form optic cup-like structures when differentiated for longer (Figure 2c; days 12 and 14), as has been shown by others with the addition of extracellular matrix proteins (18). Therefore, the rest of the studies performed here used the lowest optimal cell density of 9,000 cells/EB to generate maximal yields of Rax.GFP retinal progenitors.
Figure 2. Optimization of the generation of Rax.GFP retinal progenitor cells.
a, Immunocytochemical analysis of day 10 EBs showing Pax6+/Rax.GFP+ cells (red and green, respectively) from different size ES cell starting populations (3k – 27k cells). b, A histogram showing the percentage of Rax.GFP+ retinal progenitors present by FACS analysis at day 10 of differentiation in different size EBs (mean ± SEM, ANOVA, P<0.01 ; n ≥ 3). c, Immunocytochemical analysis of EBs formed from 9k ES cells, showing Pax6+/Rax.GFP+ retinal epithelial structures at days 7-14 of differentiation. Nuclei are stained with DAPI (blue). Scale bars, 100 μm (a) and 50 μm (c).
We next examined the temporal transcriptional profiles of Rax.GFP+ retinal progenitors by FAC-sorting this population at progressive stages of differentiation. Rax.GFP+ cells were present from day 7 onwards, with significantly greater numbers of Rax.GFP cells present at days 9 and 10 of differentiation (Figure 3a; P<0.05, ANOVA). The decline in the number of Rax.GFP-expressing cells on days 12 and 14 is most likely due to the down-regulation of Rax expression in presumptive RPE progenitors (Figure 3a). Figure 3b shows a representative cumulative FACS plot and histogram for ES cell-derived cells following 10 days of differentiation culture. Sorted Rax.GFP+ retinal progenitors expressed the early eye field transcription factors Rax, Pax6, Six3, Six6, Lhx2, and Otx2 at all timepoints as examined by RT-PCR (Figure 3c). As the number of cells expressing Rax.GFP increases, but not the level of expression, the intensity of the RT-PCR bands remains constant in the sorted RAX.GFP-positive population at the various time points examined. The retinal progenitor markers Vsx2 (Chx10) and Sox9 were also present throughout the culture period, however Ikzf1 (Ikaros), an early retinal progenitor marker, demonstrated a decreasing expression profile with time suggesting that the cells were differentiating in a stage-dependent manner. The pluripotent marker Nanog, expressed highly in day 0 (D0) undifferentiated ES cells was not expressed in the Rax.GFP+ retinal progenitors from day 9 onwards (Figure 3c). As early retinal progenitor numbers were highest and no Nanog expression was observed at day 10, retinal progenitors were isolated from this stage of culture for all further retinal cell differentiation experiments.
Figure 3. Characterization of optimized Rax.GFP retinal progenitor differentiation.
a, Temporal FACS analysis of Rax.GFP+ retinal progenitors showing significantly greater numbers of Rax.GFP+ cells present at days 9 and 10 of differentiation (mean ± SEM, P<0.05, ANOVA; N ≥ 3). b, Representative FACS plot and histogram showing 34.40% of Rax.GFP+ cells (green) selected by flow cytometry c, RT-PCR analysis of FAC-sorted Rax.GFP+ cells after days 0 - 14 in culture (D0 - D14) showing the expression of early eye field transcription factors (EFTs), retinal progenitor cell (RPC) markers, and the absence of pluripotency marker, Nanog.
Temporal analysis of ES cell-derived retinal cell differentiation
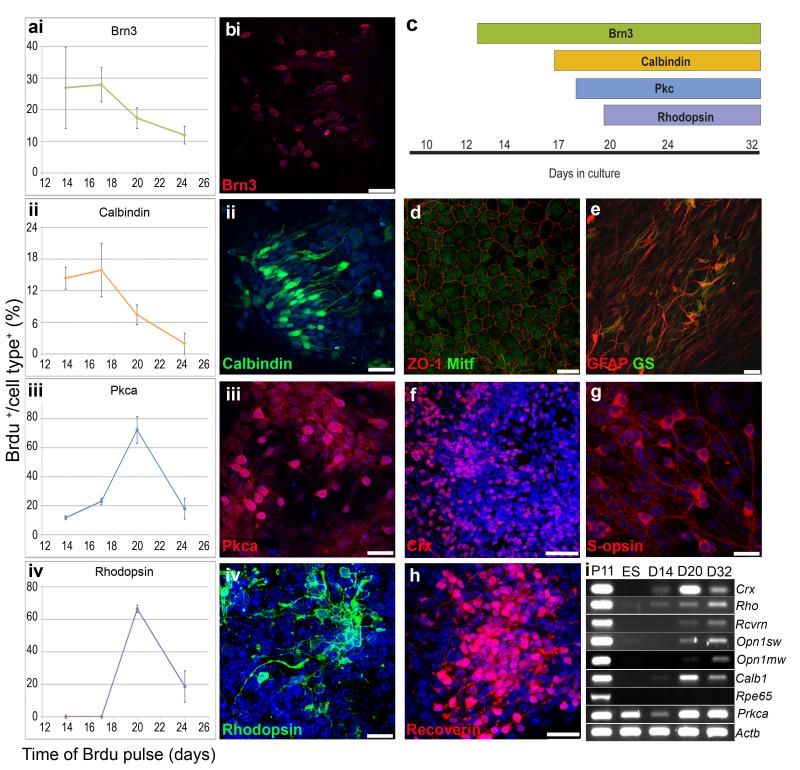
Isolated retinal progenitors (Rax.GFP+ cells) were differentiated as adherent cultures on poly-lysine, laminin and fibronectin coated plates for more than 20 days. The time and peak of birth in vitro for early and late born retinal cell types was investigated by BrdU pulse labeling at days 14, 17, 20 or 24 and immunocytochemical analysis of the cells at day 32 of culture (Figure 4a). Early born ganglion and horizontal cells were labeled with Brn3 and calbindin, respectively, and had higher percentages of cell birth at day 17 (Figure 4ai, ii and 4bi, ii). In contrast, the late born rod photoreceptor and bipolar cells labeled with rhodopsin and PKC, respectively, had the greatest percentage of cell birth at day 20 (Figure 4aiii, iv and 4biii, iv). A schematic demonstrating the presence of these cell types is shown in figure 3c. We also observed RPE cells labeled with Mitf and ZO-1 (Figure 4d), Müller cells labeled with GFAP and glutamine synthetase (Figure 4e), photoreceptor precursors labeled with Crx (Figure 4f), cone photoreceptors labeled with S-opsin (Figure 4g) and maturing rod and cone photoreceptors labeled with recoverin (Figure 4h). We confirmed the onset of expression of later photoreceptor markers rhodopsin, recoverin, S-opsin and M-opsin at day 32 of differentiation, by RT-PCR (Figure 4i), but the late RPE marker, RPE65, was not observed. PKC alpha expression was observed in ES cells. However, following FAC-sorting for Rax.GFP+ retinal progenitors, little PKC alpha expression was observed at day 14, but was readily detectable by days 20 and 32 (Figure 4i). Overall these data are consistent with the peak of rod and bipolar cell birth at day 20 of culture and the expression of mature photoreceptor cell markers at day 32, as previously described (9). Therefore, to ascertain the optimal stage of culture to derive post-mitotic photoreceptor precursors for transplantation, we focused on the day 20-28 culture period.
Figure 4. Temporal analysis of ES cell-derived retinal cell differentiation.
a,b, Birthdate analysis of neural retinal cell types by BrdU pulse labeling and immunocytochemistry. Cultures were pulse-labelled with BrdU for 24 hrs at days 14, 17, 20 and 24 of differentiation (ai-iv; x axis). Cultures were stopped at day 32 and the percentage of double positive cells for BrdU and the cell type markers Brn3 (ai,bi; ganglion cells; red), calbindin (aii,bii; horizontal cells; green), Pkc alpha (aiii,biii; bipolar cells; red) and rhodopsin (aiv,biv; rod photoreceptors; green) were quantified (ai-iv; y axis; mean ± SEM, N = 3 experiments, n > 9 fields of view). c, Schematic representation of retinal cell type markers present at various stages of ES cell differentiation culture. d-h, Other retinal cell types present following 32 days of ES cell differentiation culture, RPE cells (d; ZO-1 and Mitf; red and green respectively), Müller glia (e; GFAP and GS; red and green respectively) and photoreceptors (f,g,h; Crx, Recoverin and S-opsin; red). i, RT-PCR analysis of retinal gene expression with ES cell differentiation culture. Nuclei stained with DAPI (blue). GFAP, glial fibrillary acidic protein; GS, Glutamate Synthetase. Scale bars, 50 μm (f) and 25 μm.
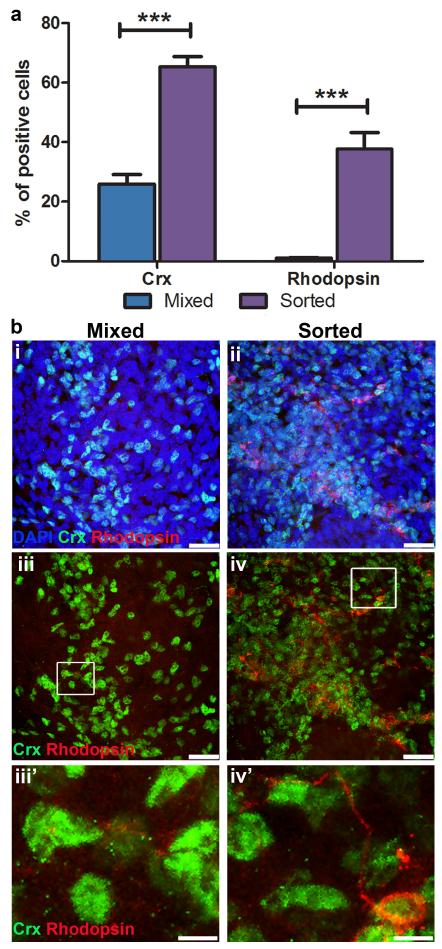
As large numbers of photoreceptor precursor cells are required for transplantation (200,000 cells per 1 μl injection) and FAC-sorting Rax.GFP differentiated cultures at day 10 limits the quantity of cells that can be differentiated at any one time, we investigated whether the presence of Rax.GFP− cells had any adverse effects on rod photoreceptor differentiation. Equal numbers of sorted Rax.GFP+ cells were re-aggregated (20,000 cells/ aggregate) and plated per well. One population was left as pure sorted RaxGFP+ and to the other additional Rax.GFP− cells were added so that the ratio of Rax.GFP+:RaxGFP− was 35:65% (this mixed population reflected the proportion of Rax.GFP+ cells per unsorted EB). The cells were further differentiated until day 32, when Crx+ and rhodopsin+ cells were quantified by immunocytochemistry. A significantly greater percentage of cells were Crx+ in the sorted compared with the mixed cell cultures (Figure 5a; 65.4 ± 9.6% and 25.9 ± 9.0%, respectively; P<0.001, 2way ANOVA, n = 9). The sorted population also contained significantly more rhodopsin+ cells (Figure 5a; 37.7 ± 15.7% versus 1.0 ± 0.7%; P<0.001, 2way ANOVA, n = 9) with very little rhodopsin staining present in the mixed cell population, as shown in figure 5b (i-iv; green Crx+ nuclei; red rhodopsin+ cells). We concluded that mixed neural cultures were not as useful for the efficient generation of rod photoreceptors. This suggests that, as in vivo, a retinal progenitor cell niche is required for efficient differentiation of photoreceptors, and that sorted Rax.GFP populations better simulate this requirement.
Figure 5. Efficiency of ES cell-derived photoreceptor differentiation.
a, A histogram demonstrating the percentage of crx+ and rhodopsin+ cells in mixed populations of neural cells versus sorted populations of pure retinal cells at day 32 of differentiation culture (mean ± SEM, ANOVA, P<0.001, N = 3 experiments, n = 9 fields of view). b, Immunocytochemistry for crx and rhodopsin positive photoreceptor cells (green and red, respectively) in mixed neural cell populations (bi,iii,iii’) and sorted retinal cell populations (bii,iv,iv’). Scale bars, 25 μm and 5 μm (inserts).
Comparative analysis of photoreceptor differentiation in vivo and in ES cell-derived cell cultures
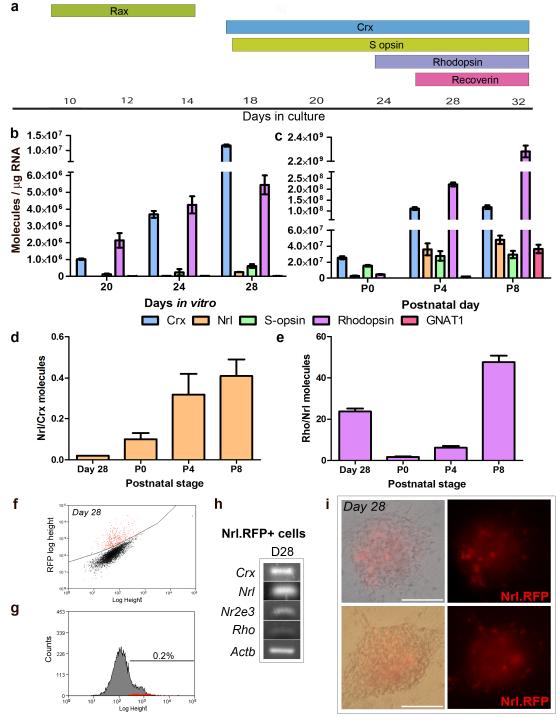
To determine optimal culture stages containing photoreceptor precursors for transplantation we examined a range of photoreceptor markers by immunocytochemistry over the retinal cell differentiation culture period and compared the profiles with that of the developing retina. (figure 5a). We observed early photoreceptor precursor markers Crx and S-opsin from around day 18-20 in culture and the later photoreceptor markers from days 24-26.
To establish the equivalent retinal developmental stage we performed qPCR to compare the number of molecules for relevant photoreceptor markers obtained from differentiated cultures at days 20, 24 and 28 of culture (Figure 5b) with the number of molecules obtained from P0, 4, and 8 postnatal retinae (Figure 5c). For the cultured cells, the number of Crx and Nrl molecules increased with time, whereas Rho and Opn1sw (rhodopsin and S-opsin, respectively) did not increase between days 24 and 28 of culture (Figure 5b). We observed very little Gnat1 (rod transducin) at any of the timepoints (Figure 5b). By contrast in the postnatal samples, Crx and Nrl increased until P4, with a reduction in Crx by P8 (Figure 5c). Rho increased greatly over the timecourse examined, while Gnat1 expression was readily detectable by P8 (Figure 5c). The highest levels of Crx and Nrl observed in the postnatal samples were 10 and 187 fold higher than the highest levels observed in the ES cell-derived cultures, respectively. In the cultures, the ratio of Nrl to Crx transcripts was much lower than that observed in the early postnatal retina (Figure 5d; 0.02 at day 28 vs. 0.1 at P0 and 0.32 at P4). The relatively high Crx expression was not matched by raised Opn1sw expression, as would be expected if the Crx+ cells were differentiating into cones instead of rods. By contrast, the ratio of Rho molecules to Nrl molecules at day 28 of culture was in the same range as the P4-8 retinas (Figure 5e; 23.7 at day 28 vs. 6.2 at P4 to 47.6 at P8) suggesting that the Nrl expressing cells were at a similar developmental stage. Hence, several notable differences in the expression profile of photoreceptor markers were found between in vivo and ES cell-derived populations. Most marked was the relatively low expression of the key rod differentiation factor, Nrl, in the ES cell-derived cultures compared with the high Crx expression.
To more directly investigate the production of Nrl+ cells in the ES cell cultures, we electroporated an Nrl promoter driving an RFP reporter into the EB5 Rax.GFP mouse ES cell line to generate double fluorescent Rax.GFP/Nrl.RFP reporter lines (n = 11). This promoter fragment has been previously shown to drive faithful expression of GFP in rod photoreceptors in transgenic mice (19). Using the new lines and following day 10 retinal progenitor differentiation and Rax.GFP FAC-sorting, we differentiated the cells to day 28 of culture before performing a second FACS analysis to determine the percentage of Nrl.RFP+ cells (Figure 5fi,ii). Only 0.2 ± 0.06% (N = 6) of the cells were positive for Nrl.RFP at day 28 in one line. RT-PCR analysis of these FAC-sorted cells confirmed Crx, Nrl, Nr2e3 and Rho expression (Figure 5g) consistent with expression of the Nrl.RFP transgene in rod photoreceptors. Although integration effects may influence transgene expression, these findings are consistent with the low level of Nrl expression detected by qPCR (Figure 5b). The low proportion of Nrl.RFP+ cells in culture (~0.2 %) made it impractical to FAC-sort these cells for transplantation.
Together these data support the conclusion that photoreceptor precursors are being generated in the ES cell cultures, but that rod differentiation may be delayed and that the usual close correspondence in the expression profiles of Crx and Nrl (20, 21) is not being recapitulated in vitro.
ES cell-derived retinal cells transplanted to the adult retina
Finally, we tested the behavior of the ES cell-derived retinal cells after transplantation into the adult retina, to determine whether they were able to complete photoreceptor differentiation in vivo and integrate into the host retina.
First we investigated the survival of ES cell-derived cultures and any potential harmful effects of transplantation, by transplanting sorted Rax.GFP+ retinal progenitors and unsorted mixed neural progenitors from day 10 of culture into the subretinal space of the adult retina. Both progenitor cell populations survived in the subretinal space and maintained Rax.GFP expression. No Rax.GFP+ cells integrated within the host retinal layers. 50% of the mixed neural progenitor transplants and 17% of the sorted retinal progenitor transplants resulted in either subretinal and/or scleral hyperplastic cell masses (referred to here as tumors) 4 weeks post transplantation (Figure 7a; single asterisk and double asterisk, respectively). The tumors displayed varied morphology but were negative for pluripotent markers Oct3/4 and Nanog and proliferative markers Ki67 and phospohistone 3 (pH3) (data not shown).
Figure 7. ES cell-derived retinal cell transplantation.
a,b, Retinal cryosections demonstrating tumor formation following day 10 (a; Rax.GFP+ cells; green) and day 20 (b; CMV.GFP+ cells; green) ES cell-derived cell transplantation to the subretinal space. c, Table showing the incidence of tumors following ES cell-derived cell transplantation from different stages of culture in both day 10 sorted retinal cell populations and unselected mixed neural cell populations. d,e,f, Immunohistochemistry for the presence of apoptotic cells (d; activated caspase 3; red), increased gliosis (e; GFAP; red) and macrophages (f; F4/80; red) following day 28 ES cell-derived cell transplantation (d,e,f; CMV.GFP+ cells; green). GFAP, glial fibrillary acidic protein. Scale bars, 100 μm (a,b) and 25 μm.
Next, we assessed whether further differentiation of the progenitor cells in vitro prior to transplantation, reduced the prevalence of tumors in vivo. To ensure ready identification of the transplanted cells, we labeled the retinal progenitors in vitro with an adeno-associated viral vector (pseudotype 2/9) carrying a GFP reporter driven by a CMV promoter (AAV2/9.CMV.GFP). Cells were transfected on days 14-18 and 20-24 for day 20 and 28 transplants, respectively. AAV2/9 transduces many fetal and neonatal neuronal cell types (22) as well as the majority of retinal cell types including photoreceptors (23). We confirmed this in vitro and found at day 20 and day 28, an average 45% of the cells, including many neuronal cells, were labeled with GFP (Supplementary figure 1).
We analysed two differentiation timepoints, day 20 and day 28 in transplantation experiments, as the peak of rod cell birth was around day 20, equivalent to P0 stage in vivo, whereas Crx transcript numbers at day 28 were similar to the early postnatal retina (P0-4). Transplantation of day 20 retinal cells differentiated from day 10 mixed neural progenitor cell populations resulted in tumors in 55% of transplants, whereas cells differentiated from day 10 Rax.GFP sorted retinal progenitor cell populations resulted in tumors in 17% of transplants (Figure 7b,c). Oct3/4, Nanog, Ki67 and phospohistone 3 (pH3) were not observed in the tumors and the GFP labeling was not observed in the majority of cells (Figure 7b). Varied cell morphologies were present in the tumors resulting from unsorted day 20 cells, whereas neural morphology and rosettes were observed in the tumors derived from sorted day 20 cell populations (Supplementary figure 2). These results suggest that application of the retinal cell differentiation protocol up to day 20, did not eliminate the tumorogenic risk presented by proliferative (ES-like or progenitor) cells within the culture.
In contrast to cells transplanted from days 10 and 20 of culture, cell populations from day 28 of the differentiation protocol did not give rise to any tumors in either the day 10 Rax.GFP sorted retinal cell populations or the mixed neural cell populations (Figure 7c). Examination of proliferative markers in vitro demonstrated increased numbers of proliferative cells in day 20 and day 24 cell cultures compared with day 28 of culture (Supplementary figure 3), consistent with our observations following transplantation. These data indicate that greater differentiation, even of unsorted ES cell-derived populations, reduces the risk of tumorogenesis following transplantation.
Finally, we examined the morphology, location and immunohistochemical character of ES cell-derived (AAV2/9 CMV.GFP transduced) transplanted cells that migrated into the host retinae and those in the sub retinal space. Small GFP+ cell masses survived in the subretinal space at 2-3 weeks post transplantation. No immunostaining was detected for Crx, rhodopsin and recoverin in these cells (data not shown). A few GFP+ integrated photoreceptors (~0-5 per eye) were observed in the ONL of the host retina for some (33%, n = 54 eyes) of the day 20 and day 28 transplants. However, on no occasion were GFP+ cells detected that co-expressed the relevant photoreceptor markers absent in knock out models (Supplementary figure 4; RetGC−/−, Rho−/−, Gnat−/− respectively). We therefore suppose that these are virally transduced host cells rather than integrated transplanted cells (Supplementary Figure 4). Transduced host cells were not observed in the culture medium injected controls (n = 6 eyes), and the virally transfected cells were washed extensively and FAC-sorted prior to transplantation, but we cannot rule out the possibility that a few viral particles have been shuttled with the cells, as has been shown previously for lentiviral vectors (24).
To assess whether increased cell death in the transplanted cell population contributed to the low integration of cells we examined the transplanted cell mass for active caspase 3+ cells or a lack of GFP+ cells in the subretinal space. Very few active caspase 3+ cells were observed in the subretinal space or the host retina (Figure 7d) and around 60% of the subretinal cell masses contained GFP+ cells suggesting that these cells were viable. We examined the recipient retinas for the presence of increased gliosis by GFAP immunohistochemistry as we have previously shown that the induction of GFAP labeled glial processes along the outer edge of the host retina can inhibit photoreceptor precursor cell integration into the ONL (15, 25). Although we found increased GFAP staining in Müller glial processes spanning the retina, as expected following a subretinal transplantation, there was no indication of gliosis (Figure 7e). We also performed immunohistochemistry for the macrophage marker F4/80, as we have previously shown reduced integration with increased infiltration of activated macrophages to the host retina and subretinal space (17). Few activated macrophages were present within the recipient retinas and the level of inflammatory response was similar to that observed in the majority of freshly dissociated retinal cell transplants suggesting that the ES cell-derived transplants did not promote an increased immune response (Figure 7f).
In summary, no robust integration and maturation of photoreceptors was observed in any of the wildtype (n = 34) or degenerate (n = 19) recipient retinas following ES cell-derived retinal cell transplantation. Our data suggests that the transplanted population of ES cell-derived photoreceptor precursors lack some essential properties of photoreceptor precursors isolated from the developing retina that are required for integration into the adult retina.
Discussion
We have previously used a defined SFEB/DLFA method of ES cell differentiation to successfully differentiate all retinal cell types (9). Our present study used this protocol to determine whether mouse ES cell-derived photoreceptor precursors have the ability to migrate and integrate into the adult retina as efficiently as freshly dissociated retinal cells derived from an equivalent developmental stage in vivo. We showed the efficient generation of early retinal progenitors and the correct birth order of retinal cell types in accordance with in vivo retinal neurogenesis, using an optimized version of this ES cell differentiation protocol. We generated populations in which all the cell aggregates contained Crx positive cells (≤ 65 % of cells Crx+) by day 24 as shown by immunocytochemistry and increased numbers of Crx transcripts by day 28, as demonstrated by qPCR, with similar numbers of transcripts compared with the early postnatal retina (P0). However, following the transplantation of this retinal cell population no integrated ES cell-derived photoreceptors were observed.
Following the scale up of the ES cell differentiation protocol to isolate populations for transplantation we found very few Nrl expressing cells. This may be due to several reasons including increased concentrations of inhibitory factors derived from other retinal cell types, such as ciliary neurotrophic factor (CNTF) produced by astrocytes and glial cells (26-29), and previously shown to inhibit rod differentiation (30, 31) and to block Nrl and Crx expression (32). In vivo retinal development involves gradients of secreted signaling molecules, such as FGF2 and Shh (33) that may not be present in the adherent ES cell-derived cultures. The lack of correct cell-cell contact, neural epithelial organization and polarity that are present in the in vivo retina are not simulated in the 2D adherent culture environment, and may account for the reduced differentiation observed (34, 35). It was not possible to determine whether further time in culture increases the number of Nrl+ precursors as the survival of the cultured cells was compromised from day 32 onwards, with morphological deterioration by day 34. However, the majority of rod photoreceptors were born at day 20 of culture and mature photoreceptor markers were starting to be expressed at day 28 with maintained expression until day 32 and there was little indication of increased Nrl expression at this later stage.
The presence of tumors following ES cell and ES cell-derived transplantation to the eye has been shown previously in several other studies (36-39). In transplantations to the brain, selection of Sox1.GFP+ cells and culture prior to transplantation attenuated tumor formation (40). Here we found that despite selecting Rax.GFP+ retinal progenitors prior to transplantation there was still a risk of tumor formation from ES cell-derived cultures. However, following selection and extended retinal differentiation time, the percentage of transplants that presented tumors was greatly reduced. Further differentiation partially negated the need for FAC-sorting as any remaining progenitor/stem cells differentiated in vitro prior to transplantation. This is important if mixed populations are to be transplanted. However, the most efficient method for the transplantation of freshly dissociated photoreceptors is by FAC-sorting the relevant Nrl+ cell population prior to transplantation. This is also likely to be the case for ES cell-derived retinal cells and the future selection of photoreceptor precursors by reporter expression or surface marker combinations may enable the improved quantification and isolation of this population within the culture (41).
When assessing whether transplantation of ES cell-derived retinal cells resulted in the integration of new photoreceptors, we observed a small number of false positive results that we conclude were due to AAV2/9 CMV.GFP transduction of host photoreceptors. Like the host cells in the recipient knockout models, these cells did not express photoreceptor markers. It is important to note that even following several changes of media in the days preceding transplantation, extensive washing prior to dissociation and FAC-sorting of the cell population it still may be possible that some residual infective virus particles may be shuttled with the cells. Using ES and iPS cell lines that contain endogenous reporters would avoid the problem of false positives due to viral vector. Increased cell death in the transplanted cell population, increased gliosis of the host retinas or increased innate immune responses in the recipients were all excluded as possible explanations for the lack of integration of the ES cell-derived retinal cell populations. We propose that the most likely explanation for the lack of integrated cells was the presence of low numbers of optimally staged ES cell-derived precursors in the cultures. We have previously shown that Crx.GFP expressing embryonic stage cells from the developing retina show at least a ten-fold lower integration efficiency than those from the postnatal retina (42), so the Crx+/ Nrl− ES cell-derived populations may show similarly low transplantation competence.
Our results differ from those of Lamba et al. who have reported the integration of human ES cell-derived photoreceptors into the neo-natal and adult mouse retina (13). This disparity may be due to inherent differences between the efficiency of retinal differentiation of human and mouse ES cells in vitro. In the study by Lamba et al., 15 % of human ES cell-derived cells were reported to be Nrl+ following 3 weeks of retinal differentiation culture, and therefore the transplanted cell population may have contained greater numbers of integration-competent photoreceptor precursors (13). However, one similarity with our study is the use of viral vectors to label the transplanted cell population, which we demonstrated could lead to false-positive results. In these circumstances, as shown in our study, it is essential to use additional, unambiguous markers to identify transplanted cells.
Our study indicates that further investigations are required to demonstrate convincingly that ES cell-derived cell cultures can give rise to new mature functional photoreceptors. We conclude that the defined SFEB/DLFA method of ES cell differentiation which relies on a 2D culture method for retinal cell differentiation could not be scaled up sufficiently to produce large numbers of transplantation competent photoreceptor precursors. These data suggest that although this culture method generated Crx-expressing photoreceptor precursors, the majority of Crx+ cells were impaired/delayed in their differentiation pathway and failed to activate the Nrl driven rod differentiation pathway. After transplantation into the adult retinal environment we found no evidence that the ES cell-derived cells were able to further differentiate and exhibit outer segments. New methods such as the 3D differentiation method recently published by Eiraku et al. may enable transplantation competent photoreceptor precursor cells to be generated in vitro to better facilitate transplantation studies (18). This recent work has shown the generation of optic cup-like structures that give rise to layered retinal structures containing high numbers of photoreceptors. This new method of in vitro differentiation may greatly improve the transplantation potential of ES cell-derived cells as they differentiate in a tissue niche, which may help to promote full differentiation to the photoreceptor precursor stage equivalent to early postnatal development.
Supplementary Material
Supplementary Figure 1. AAV2/9 CMV.GFP viral transduction of ES cell-derived cultures.
a, Representative FACS histogram showing 44.0% of CMV.GFP+ labeled cells (green) selected by flow cytometry at day 28 of differentiation culture. b, Light and fluorescent images of ES cell differentiation showing AAV2/9 CMV.GFP+ labeled cells (bi,ii; green) at day 28 of culture. c, Immunocytochemistry for neural markers (ci; β-tubulin; red), proliferative markers (cii; Ki67; red), glial markers (ciii; GFAP; red), epithelial markers (civ; ZO-1; red) and photoreceptor markers (cv; crx; red) with AAV2/9 CMV.GFP labeled cells (ci-v; green) at day 20 and 28 of differentiation culture. AAV2/9 CMV.GFP virus showed increased transduction of neural as opposed to epithelial and glial cell types as shown by morphology and immunocyctochemistry in the differentiated cultures. GFAP, glial fibrillary acidic protein. Scale bars: 100 μm (bi-ii) and 25 μm (ci-v).
Supplementary Figure 2. Morphological examination of tumors from ES cell-derived differentiation cultures.
a, b, Retinal cryosections stained with haemotoxylin and eosin showing the morphology of tumors formed from day 20 ES cell-derived differentiation cultures following transplantation to the subretinal space. a, Subretinal and scleral tumors were observed (ai; single asterisk, double asterisk, respectively). Varied cell morphologies were observed in tumors derived from unsorted day 20 retinal differentiation cultures (aii-iv; white arrow heads). b, Neural rosettes were observed in a tumor derived from sorted day 20 retinal differentiation culture (bi,ii; dashed outline). Scale bars, 200 μm (ai), 100 μm (aii,bi) and 50 μm (aiii-iv,bii).
Supplementary Figure 3. Proliferative cells in ES cell-derived differentiation cultures.
a, b, Immunocytochemistry for the incorporation of BrdU following a 24 hr pulse at day 20 (ai,ii; green) and 24 (bi,ii; green) of ES cell differentiation culture. c,d, Immunocytochemistry for proliferative cells (c,d; Ki67; red) at day 20 (ci,ii) and 28 (di,ii) of ES cell differentiation culture. Scale bars, 100 μm.
Supplementary Figure 4. AAV2/9 CMV.GFP viral transduction of host photoreceptors.
a,b,c Retinal sections of ES cell-derived cell transplantation to the subretinal space demonstrating CMV.GFP labeled photoreceptors in the host ONL. a, CMV.GFP labeled cells transplanted into the Gucy2e−/− retina did not co-stain for retGC (a; red), the protein lacking in this model. b, CMV.GFP labeled cells transplanted into the Gnat1−/− retina did not co-stain for rod transducin (b; red), the protein lacking in this model. c, Non-specific secondary antibody staining of immune cells (c; red) in the Rho−/− retina do not co-localise with CMV.GFP+ labeled cells. Scale bars, 25 μm and 10μm (inserts).
Figure 6. Comparative analysis of in vitro and in vivo photoreceptor differentiation.
a, Schematic representation of rod and cone photoreceptor markers present at various stages of ES cell differentiation culture. b,c, Absolute qPCR analysis of photoreceptor gene expression at day 20, 24, and 28 of ES cell differentiation culture (b; mean ± SD, N = 3) and postnatal days 0, 4 and 8 of the developing retina (c; mean ± SD, N = 3). d,e, Histograms demonstrating the ratio of Nrl to Crx molecules (d; mean ± SD) and rhodopsin to Nrl molecules (e; mean ± SD) in the day 28 ES cell differentiation cultures compared with the postnatal retina. f, Representative FACS plot and histogram showing 0.18% of Nrl.RFP+ cells (red) selected by flow cytometry g, RT-PCR analysis of FAC-sorted Nrl.RFP+ cells showing the expression of photoreceptor specific genes. h, Light and fluorescent images of ES cell differentiation showing Nrl.RFP+ cells (red) at day 28 of culture. Scale bars, 100 μm.
Acknowledgements
For their kind gifts we would like to thank Y. Sasai for the Rax.GFP EB5 mouse ES cell line, C. Gregory-Evans for the Crx antibody and K. Palczewski for the RetGC antibody. We would also like to thank N. Gent, and Y. Duran for technical assistance, A. Eddaoudi and A. Angheluta at the UCL Institute of Child Health Flow Cytometry facility, M. Signore at the UCL Institute of Child Health Embryonic stem cells / Chimera Production facility and the Department of Genetics and the Developmental Biology Unit for helpful discussions on the work.
Grants: This work was supported by grants from the Wellcome Trust (082217), the Medical Research Council UK (G03000341 and G0901550) and the Japanese Ministry of Education, Sports and Culture of Japan (MEXT, Japan; Leading Project for the Realization of Regenerative Medicine). R.A.P is a Royal Society University Research Fellow; A.G.C is a Wellcome Trust PhD student; C.H is supported by the British Retinitis Pigmentosa Society (GR566); J.C.S is supported by Great Ormond Street Hospital Children’s Charity. R.R.A was partially funded by the Department of Health’s National Institute for Health Research Biomedical Research Centre at Moorfields Eye Hospital. Funding to pay the Open Access Charge was provided by the Wellcome Trust.
Footnotes
The authors indicate no potential conflicts of interest.
Reference List
- 1.MacLaren RE, Pearson RA, MacNeil A, et al. Retinal repair by transplantation of photoreceptor precursors. NATURE. 2006;444(7116):203–207. doi: 10.1038/nature05161. [DOI] [PubMed] [Google Scholar]
- 2.Pearson RA, Barber AC, Rizzi M, et al. Restoration of vision following transplantation of photoreceptors. NATURE. 2012 doi: 10.1038/nature10997. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. NATURE. 1981;292(5819):154–156. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- 4.Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. CELL. 2007;131(5):861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 5.Park IH, Zhao R, West JA, et al. Reprogramming of human somatic cells to pluripotency with defined factors. NATURE. 2008;451(7175):141–146. doi: 10.1038/nature06534. [DOI] [PubMed] [Google Scholar]
- 6.Zhao X, Liu J, Ahmad I. Differentiation of embryonic stem cells into retinal neurons. BIOCHEM. BIOPHYS. RES. COMMUN. 2002;297(2):177–184. doi: 10.1016/s0006-291x(02)02126-5. [DOI] [PubMed] [Google Scholar]
- 7.Hirami Y, Osakada F, Takahashi K, et al. Generation of retinal cells from mouse and human induced pluripotent stem cells. NEUROSCI. LETT. 2009;458(3):126–131. doi: 10.1016/j.neulet.2009.04.035. [DOI] [PubMed] [Google Scholar]
- 8.Ikeda H, Osakada F, Watanabe K, et al. Generation of Rx+/Pax6+ neural retinal precursors from embryonic stem cells. PROC. NATL. ACAD. SCI. U. S. A. 2005;102(32):11331–11336. doi: 10.1073/pnas.0500010102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Osakada F, Ikeda H, Mandai M, et al. Toward the generation of rod and cone photoreceptors from mouse, monkey and human embryonic stem cells. NAT. BIOTECHNOL. 2008;26(2):215–224. doi: 10.1038/nbt1384. [DOI] [PubMed] [Google Scholar]
- 10.Meyer JS, Shearer RL, Capowski EE, et al. Modeling early retinal development with human embryonic and induced pluripotent stem cells. PROC. NATL. ACAD. SCI. U. S. A. 2009;106(39):16698–16703. doi: 10.1073/pnas.0905245106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lamba DA, Karl MO, Ware CB, et al. Efficient generation of retinal progenitor cells from human embryonic stem cells. PROC. NATL. ACAD. SCI. U. S. A. 2006;103(34):12769–12774. doi: 10.1073/pnas.0601990103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lamba DA, McUsic A, Hirata RK, et al. Generation, purification and transplantation of photoreceptors derived from human induced pluripotent stem cells. PLOS. ONE. 2010;5(1):e8763. doi: 10.1371/journal.pone.0008763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lamba DA, Gust J, Reh TA. Transplantation of human embryonic stem cell-derived photoreceptors restores some visual function in Crx-deficient mice. CELL STEM CELL. 2009;4(1):73–79. doi: 10.1016/j.stem.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wataya T, Ando S, Muguruma K, et al. Minimization of exogenous signals in ES cell culture induces rostral hypothalamic differentiation. PROC. NATL. ACAD. SCI. U. S. A. 2008;105(33):11796–11801. doi: 10.1073/pnas.0803078105. %19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pearson RA, Barber AC, West EL, et al. Targeted disruption of outer limiting membrane junctional proteins (Crb1 and ZO-1) increases integration of transplanted photoreceptor precursors into the adult wild-type and degenerating retina. CELL TRANSPLANT. 2010;19(4):487–503. doi: 10.3727/096368909X486057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.West EL, Pearson RA, Tschernutter M, et al. Pharmacological disruption of the outer limiting membrane leads to increased retinal integration of transplanted photoreceptor precursors. EXP. EYE RES. 2008;86(4):601–611. doi: 10.1016/j.exer.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.West EL, Pearson RA, Barker SE, et al. Long-term survival of photoreceptors transplanted into the adult murine neural retina requires immune modulation. STEM CELLS. 2010;28(11):1997–2007. doi: 10.1002/stem.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eiraku M, Takata N, Ishibashi H, et al. Self-organizing optic-cup morphogenesis in three-dimensional culture. NATURE. 2011;472(7341):51–56. doi: 10.1038/nature09941. [DOI] [PubMed] [Google Scholar]
- 19.Akimoto M, Cheng H, Zhu D, et al. Targeting of GFP to newborn rods by Nrl promoter and temporal expression profiling of flow-sorted photoreceptors. PROC. NATL. ACAD. SCI. U. S. A. 2006;103(10):3890–3895. doi: 10.1073/pnas.0508214103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blackshaw S, Harpavat S, Trimarchi J, et al. Genomic analysis of mouse retinal development. PLOS. BIOL. 2004;2(9):E247. doi: 10.1371/journal.pbio.0020247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hennig AK, Peng GH, Chen S. Regulation of photoreceptor gene expression by Crx-associated transcription factor network. BRAIN RES. 2008;1192:114–133. doi: 10.1016/j.brainres.2007.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rahim AA, Wong AM, Hoefer K, et al. Intravenous administration of AAV2/9 to the fetal and neonatal mouse leads to differential targeting of CNS cell types and extensive transduction of the nervous system. FASEB J. 2011;25(10):3505–3518. doi: 10.1096/fj.11-182311. [DOI] [PubMed] [Google Scholar]
- 23.Lei B, Zhang K, Yue Y, et al. Adeno-associated virus serotype-9 efficiently transduces the retinal outer plexiform layer. MOL. VIS. 2009;15:1374–82. 1374–1382. [PMC free article] [PubMed] [Google Scholar]
- 24.Blomer U, Gruh I, Witschel H, et al. Shuttle of lentiviral vectors via transplanted cells in vivo. GENE THER. 2005;12(1):67–74. doi: 10.1038/sj.gt.3302384. [DOI] [PubMed] [Google Scholar]
- 25.West EL, Pearson RA, Duran Y, et al. Manipulation of the recipient retinal environment by ectopic expression of neurotrophic growth factors can improve transplanted photoreceptor integration and survival. CELL TRANSPLANT. 2012 doi: 10.3727/096368911X623871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cao W, Li F, Steinberg RH, et al. Development of normal and injury-induced gene expression of aFGF, bFGF, CNTF, BDNF, GFAP and IGF-I in the rat retina. EXP. EYE RES. 2001;72(5):591–604. doi: 10.1006/exer.2001.0990. [DOI] [PubMed] [Google Scholar]
- 27.Wen R, Song Y, Cheng T, et al. Injury-induced upregulation of bFGF and CNTF mRNAS in the rat retina. J. NEUROSCI. 1995;15(11):7377–7385. doi: 10.1523/JNEUROSCI.15-11-07377.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Honjo M, Tanihara H, Kido N, et al. Expression of ciliary neurotrophic factor activated by retinal Muller cells in eyes with. INVEST OPHTHALMOL. VIS. SCI. 2000;41(2):552–560. [PubMed] [Google Scholar]
- 29.Walsh N, Valter K, Stone J. Cellular and subcellular patterns of expression of bFGF and CNTF in the normal and light stressed adult rat retina. EXP. EYE RES. 2001;72(5):495–501. doi: 10.1006/exer.2000.0984. [DOI] [PubMed] [Google Scholar]
- 30.Ezzeddine ZD, Yang X, DeChiara T, et al. Postmitotic cells fated to become rod photoreceptors can be respecified by CNTF treatment of the retina. DEVELOPMENT. 1997;124(5):1055–1067. doi: 10.1242/dev.124.5.1055. [DOI] [PubMed] [Google Scholar]
- 31.Zhang SS, Wei J, Qin H, et al. STAT3-mediated signaling in the determination of rod photoreceptor cell fate in mouse retina. INVEST OPHTHALMOL. VIS. SCI. 2004;45(7):2407–2412. doi: 10.1167/iovs.04-0003. [DOI] [PubMed] [Google Scholar]
- 32.Graham DR, Overbeek PA, Ash JD. Leukemia inhibitory factor blocks expression of Crx and Nrl transcription factors to inhibit photoreceptor differentiation. INVEST OPHTHALMOL. VIS. SCI. 2005;46(7):2601–2610. doi: 10.1167/iovs.05-0129. [DOI] [PubMed] [Google Scholar]
- 33.Yang XJ. Roles of cell-extrinsic growth factors in vertebrate eye pattern formation and retinogenesis. SEMIN. CELL DEV. BIOL. 2004;15(1):91–103. doi: 10.1016/j.semcdb.2003.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Birgersdotter A, Sandberg R, Ernberg I. Gene expression perturbation in vitro--a growing case for three-dimensional (3D) culture systems. SEMIN. CANCER BIOL. 2005;15(5):405–412. doi: 10.1016/j.semcancer.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 35.Phillips MJ, Otteson DC. Differential expression of neuronal genes in Muller glia in two- and three-dimensional cultures. INVEST OPHTHALMOL. VIS. SCI. 2011;52(3):1439–1449. doi: 10.1167/iovs.10-6400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hara A, Niwa M, Kumada M, et al. Intraocular injection of folate antagonist methotrexate induces neuronal differentiation of embryonic stem cells transplanted in the adult mouse retina. BRAIN RES. 2006;1085(1):33–42. doi: 10.1016/j.brainres.2006.02.079. [DOI] [PubMed] [Google Scholar]
- 37.Chaudhry GR, Fecek CM, Lia M, et al. Fate of ESC Derivatives Implanted into the Vitreous of a Slow Retinal Degenerative Mouse Model. STEM CELLS DEV. 2008 doi: 10.1089/scd.2008.0057. [DOI] [PubMed] [Google Scholar]
- 38.Arnhold S, Klein H, Semkova I, et al. Neurally selected embryonic stem cells induce tumor formation after long-term survival following engraftment into the subretinal space. INVEST OPHTHALMOL. VIS. SCI. 2004;45(12):4251–4255. doi: 10.1167/iovs.03-1108. [DOI] [PubMed] [Google Scholar]
- 39.Li Y, Zhong X, Yan J, et al. Pluripotent embryonic stem cells developed into medulloepithelioma in nude mice eyes. YAN. KE. XUE. BAO. 2002;18(1):37–44. [PubMed] [Google Scholar]
- 40.Chung S, Shin BS, Hedlund E, et al. Genetic selection of sox1GFP-expressing neural precursors removes residual tumorigenic pluripotent stem cells and attenuates tumor formation after transplantation. J. NEUROCHEM. 2006;97(5):1467–1480. doi: 10.1111/j.1471-4159.2006.03841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lakowski J, Han YT, Pearson RA, et al. Effective transplantation of photoreceptor precursor cells selected via cell surface antigen expression. STEM CELLS. 2011;29(9):1391–1404. doi: 10.1002/stem.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lakowski J, Baron M, Bainbridge J, et al. Cone and rod photoreceptor transplantation in models of the childhood retinopathy Leber congenital amaurosis using flow-sorted Crx-positive donor cells. HUM. MOL. GENET. 2010;19(23):4545–4559. doi: 10.1093/hmg/ddq378. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. AAV2/9 CMV.GFP viral transduction of ES cell-derived cultures.
a, Representative FACS histogram showing 44.0% of CMV.GFP+ labeled cells (green) selected by flow cytometry at day 28 of differentiation culture. b, Light and fluorescent images of ES cell differentiation showing AAV2/9 CMV.GFP+ labeled cells (bi,ii; green) at day 28 of culture. c, Immunocytochemistry for neural markers (ci; β-tubulin; red), proliferative markers (cii; Ki67; red), glial markers (ciii; GFAP; red), epithelial markers (civ; ZO-1; red) and photoreceptor markers (cv; crx; red) with AAV2/9 CMV.GFP labeled cells (ci-v; green) at day 20 and 28 of differentiation culture. AAV2/9 CMV.GFP virus showed increased transduction of neural as opposed to epithelial and glial cell types as shown by morphology and immunocyctochemistry in the differentiated cultures. GFAP, glial fibrillary acidic protein. Scale bars: 100 μm (bi-ii) and 25 μm (ci-v).
Supplementary Figure 2. Morphological examination of tumors from ES cell-derived differentiation cultures.
a, b, Retinal cryosections stained with haemotoxylin and eosin showing the morphology of tumors formed from day 20 ES cell-derived differentiation cultures following transplantation to the subretinal space. a, Subretinal and scleral tumors were observed (ai; single asterisk, double asterisk, respectively). Varied cell morphologies were observed in tumors derived from unsorted day 20 retinal differentiation cultures (aii-iv; white arrow heads). b, Neural rosettes were observed in a tumor derived from sorted day 20 retinal differentiation culture (bi,ii; dashed outline). Scale bars, 200 μm (ai), 100 μm (aii,bi) and 50 μm (aiii-iv,bii).
Supplementary Figure 3. Proliferative cells in ES cell-derived differentiation cultures.
a, b, Immunocytochemistry for the incorporation of BrdU following a 24 hr pulse at day 20 (ai,ii; green) and 24 (bi,ii; green) of ES cell differentiation culture. c,d, Immunocytochemistry for proliferative cells (c,d; Ki67; red) at day 20 (ci,ii) and 28 (di,ii) of ES cell differentiation culture. Scale bars, 100 μm.
Supplementary Figure 4. AAV2/9 CMV.GFP viral transduction of host photoreceptors.
a,b,c Retinal sections of ES cell-derived cell transplantation to the subretinal space demonstrating CMV.GFP labeled photoreceptors in the host ONL. a, CMV.GFP labeled cells transplanted into the Gucy2e−/− retina did not co-stain for retGC (a; red), the protein lacking in this model. b, CMV.GFP labeled cells transplanted into the Gnat1−/− retina did not co-stain for rod transducin (b; red), the protein lacking in this model. c, Non-specific secondary antibody staining of immune cells (c; red) in the Rho−/− retina do not co-localise with CMV.GFP+ labeled cells. Scale bars, 25 μm and 10μm (inserts).