Abstract
Background:
Congenital pseudarthrosis is one of the greatest challenges of paediatric orthopaedic practice. Treatment options and literature addressing this condition are numerous, reflecting the difficulty in management. We aimed to study the effectiveness of free fibula transfer as a primary modality of treatment in children with this condition in terms of achieving length, union, and normal axis of the involved leg.
Materials and Methods:
During the period of 2001 to 2010, 26 children with congenital pseudarthrosis of tibia between age group of 2-8 years were evaluated and were reconstructed using contra-lateral free fibula graft, and then patients were followed up for 5 years subsequently. Patients were examined and time of bony union, weight bearing ability and walking without support were noted. Any complication directly or indirectly related to surgery with any complication seen in the due course of follow up of 5 years was also taken care of.
Results:
In our experience with treatment of congenital pseudarthrosis over a span of 10 years with free fibula transfer, the results have been gratifying with no flap loss. All our patients had UNEVENTFUL post-op recovery. Only 2 patients out of 26 had non-union, for which cancellous bone grafting was done (7.6%). Most of the patients were ambulatory with support by 3-6 months and continued to walk without support after a span of 18-24 months. The incidence of stress fracture in our study over the follow-up period was 4 out of 26 pts (15.3%). None of them had any deformity in the donor leg.
Conclusion:
When compared to other surgical modalities of treatment of the problem in question, vascularised free fibula transfer has emerged as a real saviour with good patient compliance and less complication rate in our study. It achieves the desired target with no residual deformities and near normal to normal gait with no stigma of bone removal from other leg.
KEY WORDS: Freefibula, pseudarthrosis, screwfixation
INTRODUCTION
The leg is a complex joint with functions of weight-bearing support, stability, and mobility. The osseous structure of the lower leg is composed of the tibia and the fibula. The tibia is most responsible for lower leg functions, while the fibula is a fairly expendable bone.[1]
Congenital pseudarthrosis of the tibia (CPT) is a rare malformation, occurring in 1 in 190,000 live births.[2] CPT is a complex condition with failure of normal bone formation and subsequent angulation leading to fracture.[3] There is dense cellular fibrous tissue with few vessels; normal callus does not form. There is thick periosteum at the site. Hamartamatous tissue at the fracture site does hamper the normal bony union. Masserman has classified CPT based on the status at birth into three types. Type I includes children who have no recognisable deformity at birth. Type II includes children with bowing at birth. Type III includes children with pseudarthrosis at birth.[2,4]
Pseudarthrosis is rare, always unilateral[5] and occurs equally in girls and boys.[6] Acute fractures often complicate the issue, which occurs during first 2 yrs of life, usually shortly after birth. Congenital pseudarthrosis rarely presents at birth but commonly develops during the first 18 months of life. Acquired pseudarthrosis manifests clinically as a chronically unstable bone union at the fracture site.[1]
Characteristically, pseudarthrosis is of 2 types[7]
-
(a)
Dysplastic type is characterized by narrowing, sclerosis, and obliteration of the medullary canal.
-
(b)
Cystic type shows no narrowing but instead has cyst-like areas, which resembles fibrous dysplasia microscopically; in this type, leg appears normal early in course, with fracture and pseudoarthrosis occurring after 5 years of age.
Crawford classification[8]
Type I: The medullary canal is preserved. Cortical thickening might be observed.
Type II: is defined by presence of thinned medullary canal, cortical thickening, and tabulation defect.
Type III: The dominant finding is a cystic lesion, which may be fractured.
Type IV: Pseudarthrosis is present with tibial and possibly fibular non-union [Diagram 1].
Diagram 1.
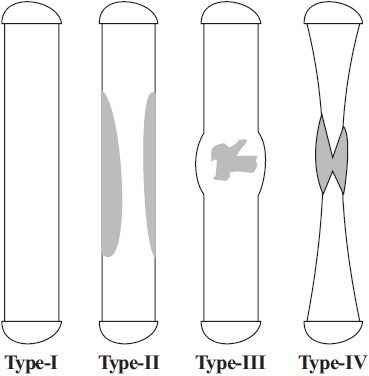
Crawford classification
Generally, the goal in the reconstruction of tibial defects is to restore the anatomy of the altered segment gaining a satisfactory functional recovery. Treatment modalities include non-operative and operative treatment. Non-operative treatment (for bowing without pseudoarthrosis or fracture) is done by bracing in total contact orthosis like patellar tendon-bearing cast, and operative treatment (for pseudarthrosis or fracture) techniques include bone grafting and surgical fixation with intra-medullary nailing or plating, vascularised free fibula transfer, limb lengthening by illizarov's external fixation.
MATERIALS AND METHODS
The study was conducted in our department of plastic surgery. During the period of 2001 to 2010, we performed microvascular-free transfer of vascularised fibula for 26 cases of congenital pseudarthrosis in children [Table 1]. The age at the time of surgery ranged from 2 years to 8 years (average age 6.2 yrs). In all cases, contra-lateral fibula was used as graft. Follow-up period of the patients averaged from 3 years to 5 years. Right-sided tibial pseudarthrosis was more common than left side in our series (18 Right, 8 Left).
Table 1.
Microvascular-free transfer of vascularised fibula for 26 cases of congenital pseudarthrosis in children
All the patients were examined and evaluated according to Crawford Classification, X rays were done, and a record chart was prepared, which showed all the details including sex, side, date of first consultation, date of surgery any special findings noteworthy during surgery and in post-op period. The same chart patient carried to us in the next visit and presence of union was noted. It was followed in the subsequent follow-ups and the surgical outcome, and its complications if present (deformity, limb length discrepancy and others) were noted. In view of all the options available for the treatment of congenital pseudarthrosis in children, vascularised free fibula transfer was chosen as the primary operative modality. None of the cases had any previous surgical exposure. None of them had undergone any other modality of treatment. In none of the patients, we did any form of MR or CT angiography. Three out of 26 patients had associated deformity of the foot (equinus), which was corrected at the time of surgery [Figures 1 and 2].
Figure 1.
Case- 1- Showing 7-year-old child presented to us with pseudoarthrosis of left leg
Figure 2.
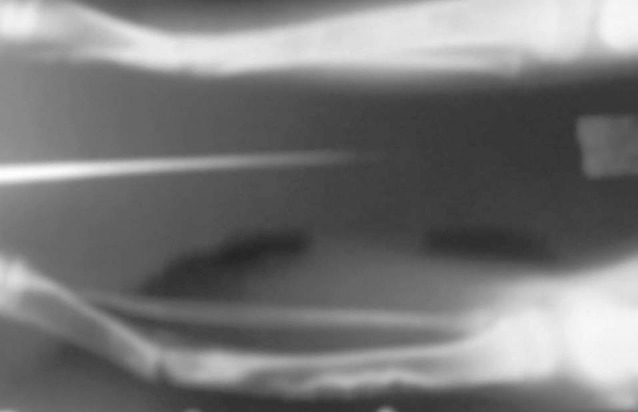
Case-1- X-ray showing pseudoarthrosed joint with diseased bone proximally
All patients were given general anaesthesia. Resection of the psedoarthrosis to healthy tissue was done, and defect was measured. Any soft tissue contracture, if present, was released adequately. Ipsilateral fibula was found to be involved in around 1/3rd of the cases but being a non-weight bearing bone, nothing was done in form of resection or release. Contra-lateral fibula was harvested from the normal side with 3-4 cms longer than the defect with the vascular pedicle. Lower end of fibula was denuded of periosteum for 2 cms and upper end for 1 cm [Figure 3].
Figure 3.
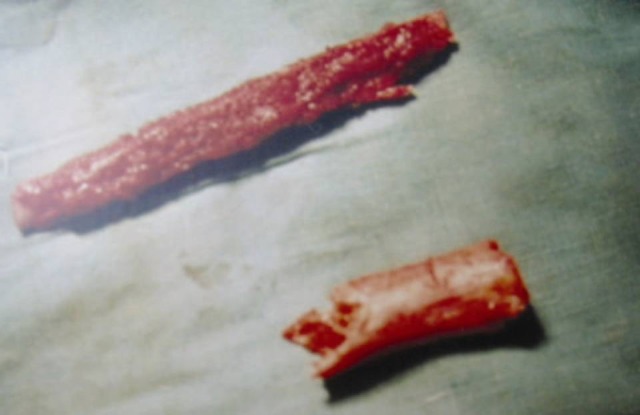
Case-1-(Above) harvested free fibula (below) diseased bone
Lower end of fibula (about 2 cms) was fixed by stroking in the tibia, and the upper end was fixed to tibia by cancellous screws after making slots in it [Figures 4–8]. Vascular anastamosis was done end to end with anterior tibial artery and venae-comitants under the operative microscope.
Figure 4.
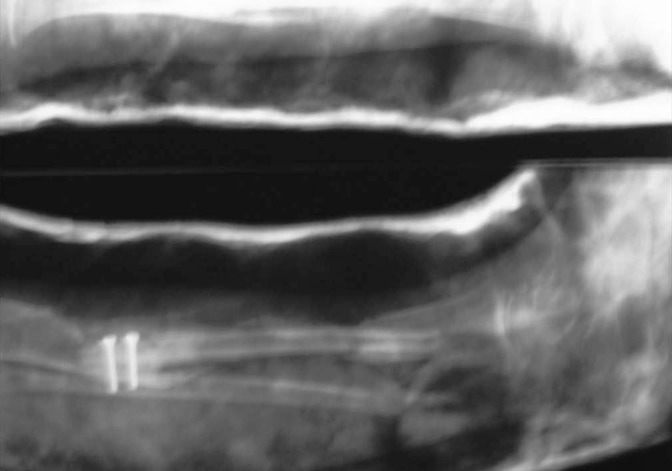
Case-1- Immediate post-op X-ray showing free fibula fixed with screws in tibia
Figure 8.
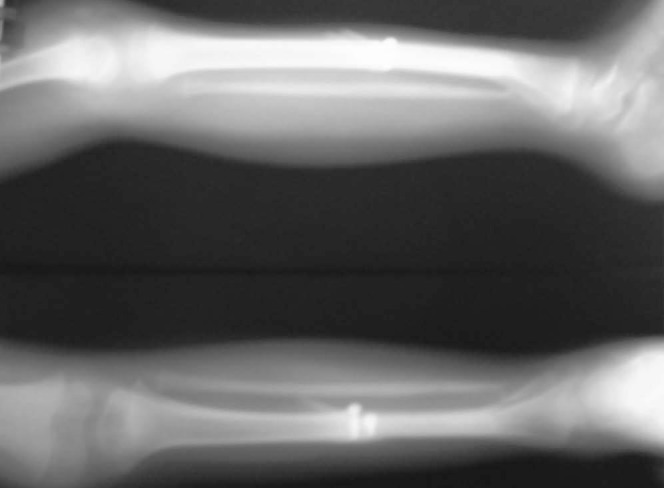
Case-2- X-ray of the patient showing free fibula fixed with screws in tibia
Area was cleaned, and drain was inserted. POP slab was used to immobilize the limb. Drain was removed on third post-op day. Sutures were removed after 10 days, and above knee POP cast was given [Figure 4]. Patient was evaluated again after 3 months and assessed for radiological evidence of union, and if present, POP cast was removed, and above knee PVC CAST was applied [Figure 5]. Partial weight-bearing was started after putting the leg in PVC cast using crutches after 3 months. Patient was asked to walk with support with above knee PVC cast [Figure 9]. Patient was advised to remove the cast at the time of taking bath but do need to put it regularly even at night. This was done for about 1 year. Within 9 months to 1 year, signs of fibular hyperplasia normally start appearing radiologically [Figures 6, 7, 10 and 11]. Once the signs of fibular hyperplasia appear, above knee PVC cast was changed to below knee cast and Patient was asked to walk without support. Patient was advised not to run and do exercises. They were again evaluated at 1½ years and if significant hypertrophy of bone was noted, cast was removed, and patient was advised to walk unaided and free [Figures 12 and 13].
Figure 5.
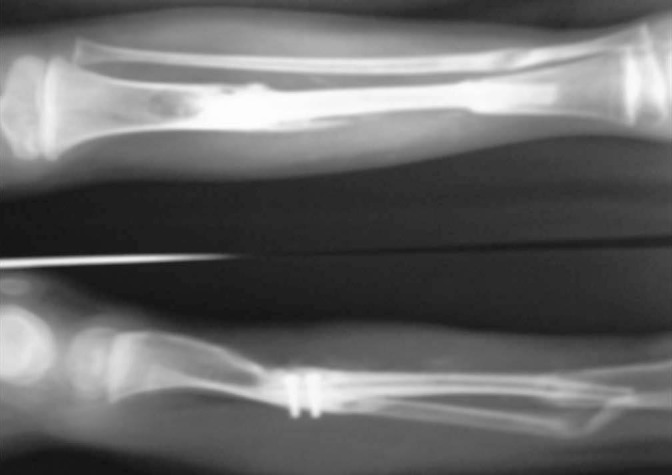
Case-1- 3 months post-op X-ray showing union of the free fibula
Figure 9.
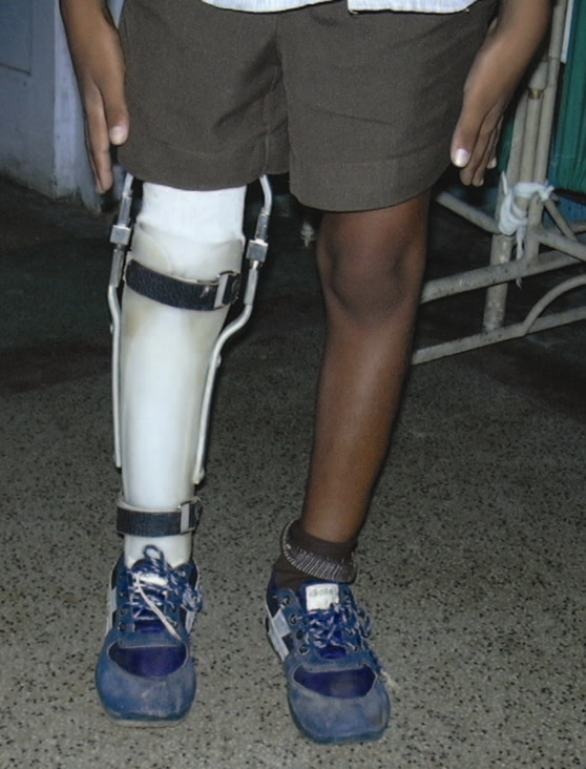
Case 2- Child mobilized with brace
Figure 6.
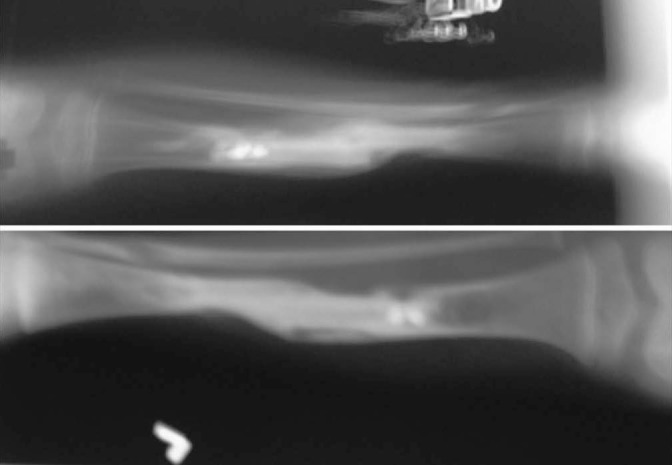
Case-1- 5 months post-op X-ray showing stress fracture of the fibula, which was managed in the cast (above) and 1 year post-op picture with signs of fibular hyperplasia (below)
Figure 7.
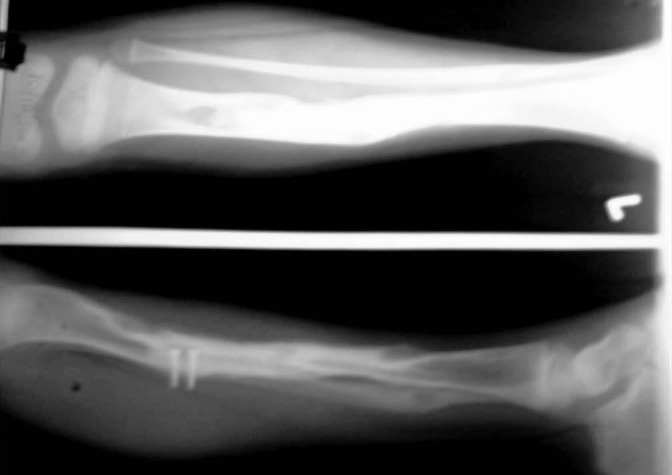
Case-1- X-ray showing hypertrophied fibula in regular follow-up
Figure 10.
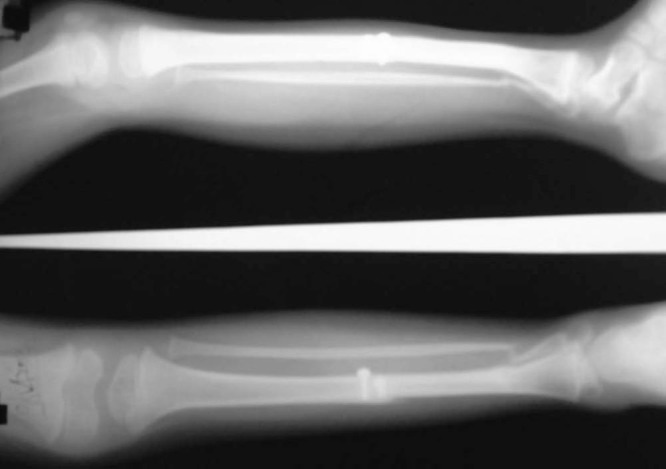
Case-2- 2 year follow-up showing hypertrophied fibula
Figure 11.
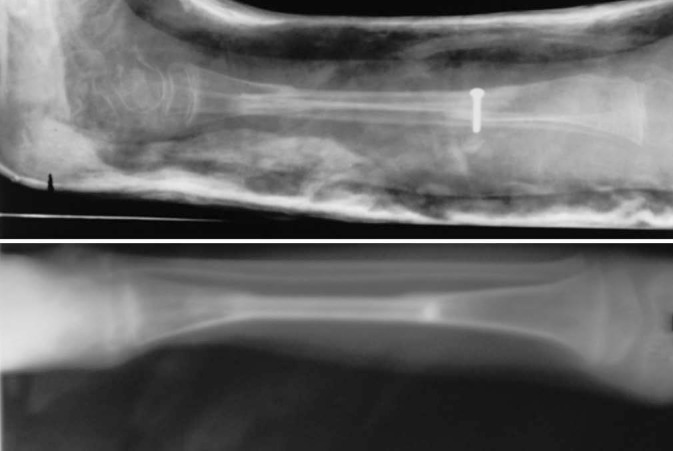
Case-3-Showing pseudarthrosed bone excision with free fibula bone grafting (above) hypertrophied fibula seen in regular follow-up (below)
Figure 12.
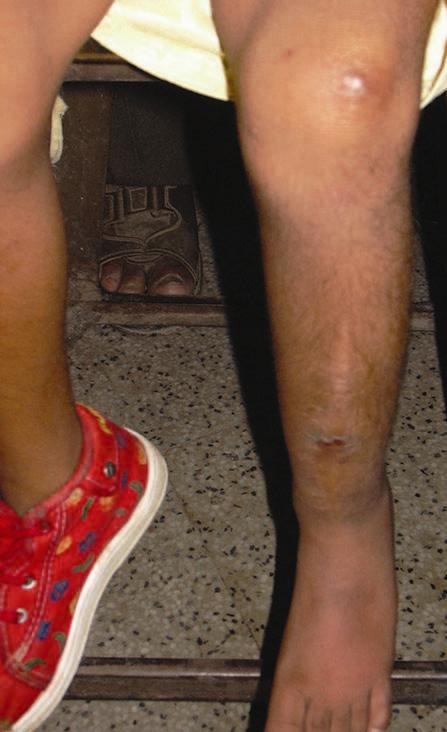
Case 1 – Long-term follow-up of the case
Figure 13.
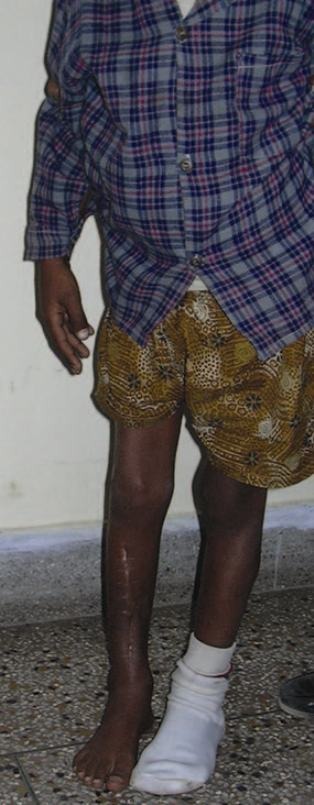
10 year follow-up of the case
Patients were then evaluated regularly at 6 months duration for 3 years and then yearly till 5 years.
RESULTS
In our experience with congenital pseudarthrosis, we encountered 26 cases, out of them, 11 were female child, and 15 were males. Most of them belonged to good socio-economic strata. None of the patients had defect of less than 3 cms (ruling out cancellous bone grafting). All the defects were of type-III and type-IV. Most of the defects ranged from 6-12 cms, for which we opted for free fibula transfer. The defects were in middle and lower 1/3 of leg, sparing the upper leg in most of the cases. There were no associated features suggestive of neurofibromatosis in any of the cases. None of the children had received any form of treatment before for the same condition.
The follow-up period of the patients ranged from 2 years to 5 years, except in last 2 cases which were recently done who are in follow-up period.
Primary union was achieved in 24 CASES (92.6%). Two patients had non-union at lower end, for which cancellous bone grafting was done.
Radiological union was evident at 3 months for 20 patients (76.9%). Three patients showed union at 5 months and 1 at 6 months. The other 2 cases mentioned above did not achieve union at their respective upper and lower ends.
All the patients were kept on plaster of Paris cast till corticalization of the transferred fibula was evident, which on an average was 3-6 months. The graft was further protected by brace for 1½ year.
Full unsupported weight-bearing was allowed from 1½ year. It was seen in all the 22 cases, which achieved primary union. The 2 cases which had non-union had delayed full unsupported weight-bearing (>2 years). Two cases recently done who are in follow-up period have still to achieve that [Figure 8].
Hypertrophy of fibula was evident in 1 year, and the hypertrophied fibula was difficult to distinguish from normal tibia [Figure 10].
Stress fracture of the fibula was seen in 4 cases (15.4%). As compared to worldwide study data of stress fracture of fibula, which is approximately around 18-33%, our rate was quite less. Once the fracture ensues, it was managed in a cast conservatively for about 2 months. We also saw in our patients that once the fracture fibula occurred, it led to rapid hypertrophy of the bone subsequently. The stress fractures occurred mostly late in the course of treatment (after 1 year)–1 at 1.2 years and other 2 at 1.5 years, but all healed well without any residual deformity. Only one patient had stress fracture fibula early in the course of treatment i.e., 5 months [Figure 6].
There was no complication of angulation or growth disturbance seen during the follow-up period in the transferred fibula. This could be attributed to the adequate soft tissue release and generous resection of diseased bone done at the time of surgery till healthy margins were achieved clinically, which in post-op and growth period did not hamper the normal axial development of the transplanted fibula. We used only screws for the fixation of transplanted fibula to tibia avoiding other methods of rigid fixation, which can lead to reduction of blood supply and growth plate disruption. The methods of fixation used for fibula like nail/plates can de-vascularise a long segment of bone in children. This technique of screw fixation has an ease to be fixed to fibula in children along with no risks mentioned above. They can be left in body unlike rigid plates and nails, which are removed later. This does not lead to any growth disturbances in the fibula [Figures 8 and 10]. Once bone union is achieved and weight-bearing started, good quality of bone regenerate and hypertrophied fibula lowers down the possibility of fracture developing later in life.
Residual shortening i.e., limb length discrepancy noted maximum was 2-2.5 cms, for which nothing was done, and it usually passed unnoticed. It was seen in 5 patients (19.2%).
Gait was normal in most of our patients. There was no deformity of the ankle seen in the donor leg. The harvested free fibula was of osseous type without any skin paddle.
There was no failure seen in our series as acute failure in terms of micro-vascular anastamosis or in terms of not achieving the desired goal of union and wt-bearing, for which amputation was advised in other studies.[11]
DISCUSSION
Treatment of congenital pseudarthrosis depends on the age of the patient and the severity of the disease. The treatment ranges from illizarov's technique to bone grafting and finally to amputation.
Bracing of the affected limb was also suggested as one of the treatment options, but was seen that it was found to be ineffective[9] for patients who have pseudarthrosis or fracture. Spontaneous remodelling is not expected with this treatment.
The other extreme was amputation, which was normally considered as a final modality after numerous failed surgeries. But, considering this as a primary option for patients is not acceptable.[10,11] It is indicated with 3 failed surgical attempts and when limb length inequality is greater than 5 cm and/or severe foot deformity is present.[5,13] Presence of pseudarthrosis by itself is not an indication for amputation. Amputation through pseudarthrosis segment may leave scars over amputation stumps, and over growth phenomena may end up requiring additional revision amputations. Syme's amputation is often superior to BKA due to atrophic and scarred calf muscle in these patients.[13] Weber has reported amputation of the calf in 9-14% of the patients.[12]
History suggests few other modalities of treatment like electrical stimulation to increase production of callus.[14,15] Boyd's onlay graft which consists of grafts of cortical bone on either side of the pseudarthrosis and of cancellous bone between them[16] and Mac Farland's bypass graft technique in which long bone graft is inserted medially under compression and fixed to tibia, graft acting as a strut to prevent motion.[17] But, all these techniques resulted in low rates of bone union.
Treatment of congenital pseudarthrosis requires the ability to regenerate bone in vivo. Ilizarov, through his experiments in the 1950s and 1960s, summarized this in his ‘Principle of Tension—Stress’ that living tissue, when subjected to slow steady traction, becomes metabolically active in both biosynthetic and proliferative pathways, a phenomenon dependent on vascularity and functional use.
Pseudarthrosis may be treated with compression for 2 to 3 weeks, which creates stability and crushes fibrous and fibro-cartilaginous tissues between the bone ends, followed by gradual distraction. It simultaneously attends to various aspects of this condition, including resection of the pseudarthrosis, deformity correction, shortening defect, infections, articular function and weight-bearing. The disadvantages of this technique are the duration of treatment, its relative complexity, pin tract infections, ankle valgus and refractures.[18,19] If in the due course of treatment stability is less, the bones fail to ossify and pass through cartilaginous stage instead of osseous stage.
Paley and his colleagues[19] were the first Western surgeons to report results of circular external fixation for CPT. Their series consisted of 16 cases with a union rate of 94% with one treatment. Boero[20] reported results of 21 patients treated with the Ilizarov device. Primary consolidation was achieved in 17 of 21 patients in an average of 13.7 months (80%). Refractures, deformity and re-operation were frequent sequalae. Grill, in a multicentre study, analyzed the different therapeutic methods used by the European Paediatric Orthopaedic Society.[21] The treatment data of 340 patients who underwent 1287 procedures for CPT were analysed and found out that this method achieved the highest rate of union (75.5%). This technique has its own disadvantages, because of which it is not so appealing in primary cases. Moreover, the rate of primary union achieved with this technique in all the studies described was same or less than free fibula transfer. The Ilizarov technique is useful in many cases of CPT, in which union failed to occur despite of many previous surgeries. The use of this method does not preclude the use of other procedures. The Ilizarov method takes considerable time and effort to obtain good results.[22]
Intra-medullary nailing with bone grafting was considered as an option, but was found to have more complication rate with low target rate.[23]
Generally, in patients with congenital pathologies who present with a well-vascularised bed, bone defects smaller than 6 cm can be repaired easily with autologous transplantation of de-vascularised cancellous bone harvested from the iliac crest and stabilized with external fixation. With this technique, nearly 90% of patients with a defect no larger than 2.5 cm achieve bone union, with low morbidity at the donor site. Currently, no unequivocal reports exist regarding the percentage of bone unions for gaps of 2.5-6 cm.
The autologous transplantation of cancellous bone usually results in poor results, often complicated by stress fractures. The primary limitations of the use of cancellous bone are (a) the quantity of bone tissue available (defects >6 cm are difficult to fill) and (b) the prolonged immobilisation time required before complete functional recovery and full-load stability. In these patients, the more favourable local vascular and soft tissue conditions make reconstruction easier; nevertheless, bone grafts still yield high complication rates when gaps greater than 6 cm must be restored.
Bone defects larger than 6 cm are treated with the transplantation of cortical bone. Enneking has successfully treated skeletal defects as wide as 25 cm with single or double de-vascularised fibula grafting, reporting a union success rate of 67% for gaps of 7.5-12.5 cm, with an incidence rate of stress fractures of 17%.[24] A similar success rate of 68% was reported for gaps of 12.5-25 cm, with a higher incidence of stress fractures (58%). The chances of union in cancellous bone graft is less than that with free vascularised bone transfer.[25]
Cortical bone auto-transplants have few limitations. A long period for re-vascularisation is required. A long incorporating and remodelling time, undergoing creeping substitution, is necessary. Spontaneous stress fractures may occur late (reported to occur even 3 y after surgery). Finally, late stress fractures cause pseudarthrosis at the fracture site in approximately 33% of these patients. Nevertheless, the success rate reported encourages the use of this technique for the reconstruction of long bone defects.
The free fibula transfer is not an innovative procedure in the orthopaedic field but can be considered the evolution of an old intuition by orthopaedic surgeons, such as Putti, who were transferring the fibula as a non-vascularised graft to reconstruct congenital absence of the tibia. It is also the result of the recent biological advances in tissue vascularisation and bone microcirculation of fractures treated with autologous bone transplantation.
Treatment of this problem with vascularised fibula is not a new concept. It was done first by CHEN in 1979.[26] Vascularised free fibula is highly successful in achieving union, but cannot solve all problems, the facility which is offered by ring fixator.[27,28] It is also not risk-free, leading to complications like insufficient consolidation, stress fractures and sometimes limb length discrepancy is not corrected with donor site immobility.[29]
But ilizarov has its own disadvantage like difficulty of maintenance of the apparatus in child, not a single-stage procedure, and long time is needed for the bone to consolidate as compared to vascularised free fibula transfer.
Furthermore, fibula is not essential for load-bearing and ambulation, but it can be used to reconstruct the tibia when the main support of lower leg is no longer able to fulfil its function. In young patients, transplanting fibular bone, with its epiphyseal growing nucleus, makes it possible to reconstruct contra-lateral bone and restore limb growth. Gilbert reported 23 cases of congenital pseudarthrosis treated with free fibula protibia, some of whom were operated on several times and observed for up to 5 years, with a final success rate of 85%.[30]
Korompilias V in his study over vascularised free fibula transfer in 9 cases of CPT also found out it to be best modality of treatment for it. In his study, stability after reconstruction was maintained with external fixation, intra-medullary pins and internal fixation, with which he achieved union in 7 cases. He performed bone grafting for the cases which had non-union of fibula. He had stress fracture in 1 out of 9 patients who subsequently underwent multiple procedures to achieve union.[31]
Weight-bearing after the bone transfer should be prolonged, but for how long is still a matter of debate. Chen allowed patients to walk freely in less than 1 year. Gilbert believed that weight-bearing should be avoided for 2 or even 3 years.[30] In a study of 19 pts by Andrew J. Weiland , most of their patients wear a long brace on the recipient side until they reach skeletal maturity.[32] We preferred to ambulate our patients within 18-24 months.
In the same study with vascularised free fibula transfer in CPT, Weiland had 95% union rates (over follow-up period of 6.3 years). Fourteen patients achieved union within 6 months, and 5 patients had non-union. He did multiple bone grafting procedures in the cases with non-union, which later healed. He did amputation for 1 patient. Two of his patients had stress fractures, who later underwent bone grafting. He did 15 secondary bone grafting procedures, 1 amputation, 6 nail/plate removal in the recipient leg and 3 osteotomies to correct the angulation deformity in the healed tibia. The donor leg had no problem except in 1 case, which had non-displaced fracture of tibia. Residual misalignment was a troublesome problem in his study. Eleven patients had a valgus deformity of the involved leg, and 4 had anterior bowing of tibia. No intra-operative release of soft tissue was done in any of his cases, which he thought was the problem causing this.
The choice of the soft tissue reconstructive procedure mainly depends on the location and extent of the soft tissue defect. Usually, soft tissue reconstruction is mandatory for open fractures and for resection related to osteomyelitis, but it is questionable for closed fractures and for resection related to pseudoarthrosis. In our study, we did not encounter any soft tissue defect, so there was no need for it to be reconstructed.
CONCLUSION
Achieving union is the prime problem in management of congenital pseudarthrosis of tibia, and the previous studies have documented high rate of amputation in such patients with other modalities and even with free vascularised fibula transfer. We, in our experience with the disease in question, have noted better results with free fibula transfer with not a single acute and even long-term failure. It is a very effective and safe method, and gives superior results as compared to any of the other described modalities, including Ilizarov technique. Even though it is documented that it leads to stress fractures, angulation deformities and growth disturbances sometimes, we didn’t encounter 2 of the latter deformities at all with 15% incidence of stress fracture in long-term period. It is a single-stage procedure, and patient compliance is relatively good. The bony union achieved is relatively stronger than after any other procedures. The angulation deformity produced as cited by previous articles was not found in our series, which we attribute to the good soft tissue release and adequate bone debridement till healthy margin is achieved. Minimal hardware should be used for fixation of the transplanted fibula, which otherwise can lead to growth disturbances.
Surgery should be advised to the parents at their first visit and should be told in detail about the necessities and complications if they don’t undergo the same at an early age (secondary deformities like growth abnormalities, abnormal physeal inclination, posterior bowing of the proximal tibia and foot deformities). We believe that if union is achieved early, all these secondary problems can be prevented.
Although the procedure can be done even in case of multiple failed procedures, it gives best results when done primarily. Critical thing is anastomosis; operating in the unscarred field gives 100% good results. We recommend free vascularised fibula as primary treatment for congenital pseudoarthrosis of tibia as it acts as a bone substitute of adequate length, customized to the defect, covered by vascularised muscle and is stabilized in the anatomic position of tibia, which is compatible with maximal functional return.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Santanelli F, Grippaudo FR, Paolini G, Renzi LF. Lower Extremity Reconstruction, Tibia. [Last updated on 2008 June 28]. Available from: http://www.emedicine.com .
- 2.Herring JA. 3rd ed. Philadelphia: WB Saunders; 2001. Tachdjians's Pediatric Orthopaedics. [Google Scholar]
- 3.Boyd HB. Pathology and natural history of congenital pseudarthrosis of the tibia. Clin Orthop. 1982;166:5–13. [PubMed] [Google Scholar]
- 4.McCarthy RE. Amputation for congenital pseudarthrosis of the tibia. Clin Orthop. 1982;166:58–61. [PubMed] [Google Scholar]
- 5.Berkshire SB, Jr, Maxwell EN, Sams BF. Bilateral symmetrical pseudarthrosis in a newborn. Radiology. 1970;97:389–90. doi: 10.1148/97.2.389. [DOI] [PubMed] [Google Scholar]
- 6.Roach JW, Shindell R, Green NE. Late-onset pseudarthrosis of the dysplastic tibia. J Bone Joint Surg Am. 1993;75:1593–601. doi: 10.2106/00004623-199311000-00003. [DOI] [PubMed] [Google Scholar]
- 7.Clifford R, Wheelers CR., 2nd . North Carolina: Data trace internet publishing, LLC; Wheelers Textbook of Orthopaedics. Copyright 1996-2011. [Google Scholar]
- 8.Crawford AH. Neurofibromatosis in children. Acta Orthop Scand. 1986;218:1–60. [PubMed] [Google Scholar]
- 9.Van Nes CP. Congenital Pseudarthrosis of the Leg. J Bone Joint Surg. 1966;48-A:1467–83. [PubMed] [Google Scholar]
- 10.Jacobsen ST, Crawford AH, Millar EA, Steel HH. Syme Amputation in Patients with Congenital Pseudarthrosis of theTibia. J Bone Joint Surg. 1983;65:533–7. [PubMed] [Google Scholar]
- 11.Aitken GT. Amputation as a treatment for certain lower-extremity congenital abnormalities. J Bone Joint Surg. 1959;41:1267–85. [PubMed] [Google Scholar]
- 12.Weber M. Neurovascular calaneo–cutaneous pedicled graft for stump capping in congenital pseudoarthrosis of tibia: Preliminary report of a new technique. J Pediatr Orthop. 2002;11:47–52. doi: 10.1097/00009957-200201000-00008. [DOI] [PubMed] [Google Scholar]
- 13.Guille JT, Kumar SJ, Shah A. Spontaneous union of a congenital pseudarthrosis of the tibia after Syme amputation. Clin Orthop Relat Res. 1998;351:180–5. [PubMed] [Google Scholar]
- 14.Bassett CA, Caulo N, Kort J. Congenital pseudarthroses of the Tibia: Treatment with pulsing electromagnetic fields. Clin Orthop Relat Res. 1981;154:136–48. [PubMed] [Google Scholar]
- 15.Brighton CT, Friedenberg ZB, Zemsky LM, Pollis PR. Direct-current stimulation of non-union and congenital pseudarthrosis-exploration of its clinical application. J Bone Joint Surg. 1975;57:368–77. [PubMed] [Google Scholar]
- 16.Boyd HB. Congenital pseudarthrosis-treatment by dual bone grafts. J Bone Joint Surg. 1941;23:497–515. [Google Scholar]
- 17.Mcfarland B. Pseudarthrosis of the tibia in childhood. J Bone Joint Surg. 1952:3336–46. doi: 10.1302/0301-620X.33B1.36. [DOI] [PubMed] [Google Scholar]
- 18.Guidera KJ, Raney EM, Ganayt, Albani W, Pugh L, Ogden JA. Ilizarov treatment of congenital pseudarthrosis of the tibia. J Pediatr Orthop. 1997;17:668–74. doi: 10.1097/00004694-199709000-00018. [DOI] [PubMed] [Google Scholar]
- 19.Paley D, Catagni M, Argnani F, Prevot J, Bell D, Armstrong P. Treatment of congenital pseudoarthrosis of the tibia using the Ilizarov technique. Clin Orthop Relat Res. 1992;280:81–93. [PubMed] [Google Scholar]
- 20.Boero S, Catagni M, Donzelli O, Facchini R, Frediani PV. Congenital pseudarthrosis of the tibia associated with neurofibromatosis-1: Treatment with Ilizarovs device. J Pediatr Orthop. 1997;17:675–84. doi: 10.1097/00004694-199709000-00019. [DOI] [PubMed] [Google Scholar]
- 21.Grill F, Bollini G, Dungi P, Fixsen J, Hefti, Ippolito E. Treatment approaches for congenital pseudarthrosis of the tibia: Result of the EPOS multicentre study. J Pediatr Orthop. 2000;9:75–89. doi: 10.1097/01202412-200004000-00002. [DOI] [PubMed] [Google Scholar]
- 22.Rose RE, Wright DE. Treatment of congenital pseudarthrosis of the tibia with the Ilizarov technique- A case report. West Indian Med J. 2007;56:294–9. doi: 10.1590/s0043-31442007000300022. [DOI] [PubMed] [Google Scholar]
- 23.Charnley J. Congenital pseudarthrosis of the tibia treated by the intramedullary nail. J Bone Joint Surg. 1956;38:283–90. [PubMed] [Google Scholar]
- 24.Enneking WF, Eady JL, Burchardt H. Autogenous cortical bone grafts in the reconstruction of segmental skeletal defects. J Bone Joint Surg Am. 1980;62:1039–58. [PubMed] [Google Scholar]
- 25.Ostrup LT, Fredrickson JM. Distant transfer of a free, living bone graft by microvascular anastomoses- An experimental study. Plast Reconstr Surg. 1974;54:274–85. doi: 10.1097/00006534-197409000-00004. [DOI] [PubMed] [Google Scholar]
- 26.Chen CW, Yu ZJ, Wang Y. A new method of treatment of congenital pseudarthrosis of the tibia using free vascularised fibula graft- A preliminary report. Ann Acad Med Singapore. 1979;8:465–73. [PubMed] [Google Scholar]
- 27.Coleman SS, Coleman DA. Congenital pseudoarthrosis of the tibia: Treatment by transfer of ipsilateral fibula with vascular pedicle. J Pediatr Orthop. 1994;14:156–60. doi: 10.1097/01241398-199403000-00005. [DOI] [PubMed] [Google Scholar]
- 28.Ghanem I, Damsim JP, Carlioz H. Ilizarov's technique in treatment of congenital pseudarthrosis of the tibia. J Pediatr Orthop. 1997;17:685–90. doi: 10.1097/00004694-199709000-00020. [DOI] [PubMed] [Google Scholar]
- 29.Poul J, Vesely J, Gal P. Treatment of congenital pseudarthrosis of the tibia using vascularised fibula graft. Acta Chir Orthop Trauma. 2006;73:10–7. [PubMed] [Google Scholar]
- 30.Gilbert A. Vascularized fibular transfer for treatment of congenital pseudarthrosis. Read at the Annual meeting of the American Academy of Orthopaedic Surgeons, Anaheim, California. 1983 Mar [Google Scholar]
- 31.Korompilias AV, Lykissas MG, Soucacos PN, Kostas I, Beris AE. Vascularized free fibular bone graft in the management of congenital tibial pseudarthrosis. Microsurgery. 2009;29:346–52. doi: 10.1002/micr.20649. [DOI] [PubMed] [Google Scholar]
- 32.Weiland AJ, Weiss AP, Moore JR, Tolo VT. Vascularized fibular grafts in the treatment of congenital pseudarthrosis of the tibia. J Bone Joint Surg. 1990;72:654–62. [PubMed] [Google Scholar]


