Abstract
The cornea is a tough transparent tissue admitting and focusing light in the eye. More than 90% of the cornea is stroma, a highly organized, transparent connective tissue maintained by keratocytes, quiescent mesenchymal cells of neural crest origin. A small population of cells in the mammalian stroma displays properties of mesenchymal stem cells, including clonal growth, multipotent differentiation, and expression of an array of stem cell-specific markers. Unlike keratocytes, the corneal stromal stem cells (CSSCs) undergo extensive expansion in vitro without loss of the ability to adopt a keratocyte phenotype. Several lines of evidence suggest CSSCs to be of neural crest line-age and not from bone marrow. CSSCs are localized in the anterior peripheral (limbal) stroma near to stem cells of the corneal epithelium. CSSCs may function to support potency of the epithelial stem cells in their unique limbal niche. On the other hand, little information is available documenting a role for CSSCs in vivo in stromal wound healing or regeneration. In vitro CSSCs reproduce the highly organized connective tissue of the stroma, demonstrating a potential use of these cells in tissue bioengineering. Direct introduction of CSSCs into the corneal stroma generated transparent tissue in a mouse model of corneal opacity. Human CSSCs injected into mice corneas did not elicit immune rejection over an extended period of time. The CSSCs therefore appear offer an opportunity to develop cell- and tissue-based therapies for irreversible corneal blindness, conditions affecting more than 10 million individuals worldwide.
Keywords: Stem cell, Neural crest, Cornea, Cell-based therapy, Bioengineering
The Corneal Stroma, a Unique Tissue Produced by Specialized Cells
The cornea is a tough connective tissue presenting a formidable barrier between the eye and the outside world. It is transparent to light, providing two-thirds of the refractive power required to focus light on the retina. Approximately 90% of the corneal volume is the stroma, a collagenous mesenchymal tissue composed of multiple lamellae of tightly packed parallel collagen fibrils. The small uniform diameter and orderly packing of stromal collagen is essential in rendering the tissue transparent. Stromal extracellular matrix also contains a group of tissue-specific keratan sulfate proteoglycans (KSPGs) that interact with stromal collagen, regulating the collagen fibril diameter and spacing required for transparency [1–5]. The stroma is populated by keratocytes, neural crest-derived mesenchymal cells occupying approximately 3% of the stromal volume. After birth, the number of dividing keratocytes decreases in rats, and by the time of eyelid opening, keratocytes have withdrawn from the cell cycle and become quiescent [6]. Keratocytes of most vertebrates remain quiescent throughout adult life, showing neither apoptotic nor mitotic figures in any significant numbers. Thus, unlike the self-renewing epithelia, the homeostasis of the corneal stroma is not based on a cycle of cell death and mitotic renewal. The stroma is contained on its anterior and posterior surfaces by the corneal epithelium and corneal endothelium, tissues regulating stromal hydration and providing a biological barrier to infection.
Scarring of the corneal stroma can result as a response to surgery, trauma, or by viral or bacterial infection (keratitis). Corneal scars are long lasting and disrupt vision for millions worldwide [7]. Currently, surgical replacement of the stroma is the only successful approach to restoration of vision in scarred corneas. The cells responsible for scar deposition are fibroblastic cells derived from stromal keratocytes [8]. Upon wounding, proximal keratocytes undergo apoptosis, and keratocytes distal to the wound become motile, mitotically active fibroblasts [9]. The fibroblasts express α-smooth muscle actin and an array fibrotic extracellular matrix [8, 10]. In rabbit corneal scars, secretion of fibrotic components is stable for months after healing [11]. In humans, corneal scars can remain for decades [12].
Damage to the corneal epithelium not involving the corneal stroma can heal without scarring [13]. Such epithelial wounds cause keratocyte apoptosis subjacent to the basement membrane, and keratocytes peripheral to the injury migrate into the region and replicate. The mouse cornea, after epithelial debridement, regains expression of stromal matrix components within 12 weeks after wounding [14]. After human corneal transplants, stromal cells derived from the host have been identified in the grafted tissue indicating a potential of keratocytes to repopulate stromal tissue without scarring [15]. Such repopulation is slow, sometimes requiring decades. It is clear from these studies that keratocytes do not conform to the classic definition of “terminal differentiation,” and at least some cells in the stroma maintain the capability of replication and regeneration of transparent corneal tissue.
Identification of Keratocyte Progenitor Cells
As keratocytes replicate in vivo or in vitro, they become fibroblastic, losing their ability to secrete transparent corneal connective tissue [16]. Originally, it was thought that fibroblastic transformation was irreversible, but more recently it has become apparent that early passage stromal cells maintain a potential to re-express differentiated characteristics [17]. This property, however, is not equally distributed among all the cells of the stoma. Approximately 3% of freshly isolated adult bovine stromal cells grew clonally [18]. When these cloned cells were shifted to a reduced mitogen culture media, approximately 5% of the clones developed a dendritic morphology and upregulated expression of keratan sulfate, keratocan, and ALDH3A1, all products highly expressed by differentiated keratocytes [18]. This potential for keratocyte differentiation was maintained through greater than 50 population doublings indicating that the progenitor phenotype was a stable property of these cells [18]. The cells exhibited normal karyotype and reached replicative senescence after 70–80 population doublings indicating that the progenitors represent a population of nontransformed adult diploid cells. As the cells differentiate to keratocytes, mRNA for several gene products present in embryonic neural and/or neural crest cells was markedly downregulated. These genes included Six2, Six3, Notch1, and PAX6. These results demonstrate that corneal stromal cells are heterogeneous in their potential for regeneration. Many keratocytes appear to have exhausted regenerative potential, but it is maintained in a small number of cells expressing genes typical of neural crest precursors.
Stem Cells in the Human Corneal Stroma
In the past decade, small populations of stem cells have been identified in many mesenchymal tissues. Some phenotypic aspects of these mesenchymal stem cells (MSCs) vary from one tissue to the next, but they share several key properties: (a) they grow clonally, (b) they exhibit potential to differentiate into cells of multiple tissue lineages, and (c) they exhibit asymmetric division, producing both MSC and differentiated progeny. Isolation of bovine stromal progenitor cells was performed using the rationale that stem and progenitor cells exhibit clonal growth. Such clones were found to express a number of MSC markers including, Bmi1, CD90 (Thy1), CD73, CD166, ABCG2, Fhl1, stem cell factor (kit ligand), and Notch1 [18]. The only MSC property that was not confirmed in this report was that of multipotency.
The presence of multipotent cells in the stroma of other species, however, was demonstrated about the same time. Small numbers of cells from stroma of both mouse and rabbit could be expanded clonally in attachment-free cultures as floating aggregates, termed “neurospheres” [19–21]. Replating of the spheres on plastic substratum led to expression of keratocan as well as neural-specific proteins, such as β-III tubulin, and of α-smooth muscle. These experiments confirmed that adult stroma does contain multipotent clonal cells, thus meeting the definition of adult stem cells.
Identification of stem cells from human stroma was achieved by isolating a population of cells that efflux the DNA-binding dye Hoechst 33342 [22]. Effluxing such dyes reduces the fluorescence of the cells, allowing isolation of the population by flow cytometry as a “side population” (SP). The SP is observed in many mammalian tissues and has been extensively investigated as a source of adult stem cells [23]. SP cells were shown to be present in early passage cells from human corneal stroma at frequencies <1%. Dye efflux was blocked by verapamil, an inhibitor of the ABC cassette membrane transporter proteins reported to be responsible for the SP phenotype. Stromal SP cells collected by cell sorting could be expanded clonally, some clones showing properties of adult stem cells. These cells (termed corneal stromal stem cells [CSSCs]) could be expanded through 100 cumulative population doublings. When CSSCs were placed in a serum-free medium supplemented with insulin and ascorbate, they upregulated expression of keratocyte-specific markers [22]. Gene array analysis identified a panel of genes highly expressed in CSSCs that were weakly expressed in keratocytes, and vice versa. The CSSC-specific genes included MSC genes ABCG2, BMi1, CD166, cKIT, and Notch1 as well as genes present in early corneal development PAX6 and Six2. As they differentiated, the CSSC expressed high levels of keratocan, ALDH3A1, CXADR, PTDGS, and PDK4, all genes highly expressed in keratocytes.
The expression of CD34 in the corneal stroma has led to some confusion as to the source and function of the cells that express it. CD34 is a cell-surface glycoprotein highly expressed in hematopoietic stem cells in bone marrow and placenta. In human cornea, however, all or most keratocytes have been identified as CD34 positive [24–26]. Corneal fibroblasts and stromal cells in corneal pathologies, however, do not express CD34, nor do CSSCs in vitro [27]. Recent studies in our laboratory find that as CSSCs differentiate to keratocytes, CD34 is upregulated in the entire population (unpublished observation). CD34 was also reported to be expressed in human MSC as they differentiated to keratocytes after transplantation to the corneas of mice [28]. CD34 in human cornea, therefore, appears to provide a cell-surface marker of keratocyte phenotype not present on stem cells or fibroblasts. On the other hand, CD34 expression in rodent corneas was judged in one report to be associated with bone marrow-derived cells and not keratocytes, by virtue of coexpression of CD45 [24]. Other reports have suggested that CD34 expression by rabbit stromal cells represents an indication of their progenitor potential [29]. The disagreements as to the implication of CD34 expression serve as an object lesson that cell phenotype is most reliably assessed by multiple cell properties, and that single markers may not be reliable a priori predictors of a specific phenotype.
As with progenitors from rabbit and mouse, human CSSCs maintain a broad differentiation potential. Cloned CSSCs cultured in medium previously noted to produce chondrogenesis in adult stem cells underwent upregulation of mRNA and protein for a number of cartilage extracellular matrix molecules, including collagen II, aggrecan, and collagen oligomatrix protein [22]. The presence of these cartilage-specific proteins subsequently led to deposition of extracellular matrix staining with toluidine blue, a characteristic specific to cartilage due to the accumulation of proteoglycan. Similarly, when cultured in a neural induction culture medium including FGF2 and retinoic acid, CSSCs showed up-regulation of glial fibrillary acidic protein and neurofilament protein [22]. The ability to adopt a differentiation program clearly distinct from that of ocular mesenchymal tissues demonstrates the multipotent aspect of the CSSCs. Corneal fibroblasts do not exhibit a similar differentiation potential, further supporting the hypothesis that the CSSC population is distinct from that of most cells of the corneal stroma.
Localization of Stromal Stem Cells
Stromal cells staining for ABCG2 and PAX6 proteins were observed largely in the transitional zone between cornea and sclera known as the limbus. These cells were in the anterior stroma subjacent to the epithelial basement membrane, in regions where the basement membrane has ripples and folds (Fig. 1). These anatomical features, termed the Palisades of Vogt, are thought to provide a niche for limbal epithelial stem cells (LESCs) [30]. The corneal epithelium is a self-renewing tissue maintained by LESCs, a population of slow-cycling stem cells. Loss of corneal LESCs results in migration of conjunctival epithelium across the cornea obscuring vision and leading to irreversible blindness. Clinical trials are in place for transplanting corneal LESCs either from contralateral eyes or from other individuals. Restoration of corneal transparency in individuals with LESC deficiency has been achieved in a number of cases [31–33].
Figure 1.
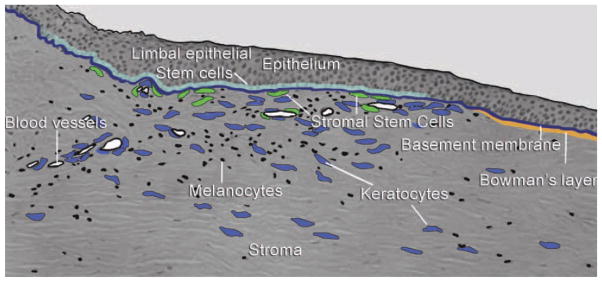
Anatomical and cellular features of the stromal stem cell niche. The section shows the anterior region of the transition zone between cornea and sclera known as the limbus. Epithelium is thickened over regions of undulations in the epithelial basement membrane know as the Palisades of Vogt. Limbal epithelial stem cells (LESCs) are localized in limbal basal epithelium. Unlike the central cornea, limbal stroma is vascularized, containing melanocytes (black) and mesenchymal keratocytes (blue). Corneal stromal stem cells (green) are located subjacent to the basement membrane near LESCs [18].
The limbal stroma near the Palisades has several specialized features. A blood supply is present, as are melanocytes (Fig. 1). It is hypothesized that mesenchymal cells in this region (termed “niche cells”) help maintain the stem phenotype of the LESCs [34]. Studies comparing fibroblasts as feeder layers for LESCs in vitro found cells from the limbal stroma to be superior in this function [35]. Surgical isolation of the limbal epithelium with small amounts of limbal stroma found that LESCs are more readily expanded in vitro, presumably due to the presence of the associated mesenchymal cells in the cultures [36]. The stromal cells associated with LESCs express a number of genes in common with MSC, including CD105, CD106, CD54, CD166, CD90, CD29, and CD71 [34]. These cells also express PAX6, a homeodomain gene present in early development known to be a master controller of eye formation. Although keratocytes have lost PAX6 expression, CSSCs maintain this eye-specific protein, and this expression allows identification of CSSCs in the stroma [18, 22]. The expression of PAX6 by MSC-like niche cells from the limbal stroma suggests that these cells are identical with the CSSCs. Studies are currently underway in our laboratories to compare gene expression patterns and differentiation potential between CSSCs and the mesenchymal niche cells coisolated with LESCs from Palisades explants.
Embryonic Origin of CSSCs
Corneal epithelium is a derivative of embryonic ectoderm, but stromal and endothelial tissues are derived from neural crest. The expression of PAX6 in CSSCs as well as several genes expressed by descendants of the neural ectoderm, Six2, Six3, and Notch1, suggests a neural crest lineage for these CSSCs. Bone marrow-derived cells have been identified in the corneal stroma, and some studies have proposed a bone marrow origin for all adult connective tissue stem cells [24]. Another popular hypothesis presents the idea that adult MSCs throughout the body derive from perivascular cells (pericytes) in each tissue [37]. The phenotypic plasticity of stem cells makes this hypothesis difficult to test in human CSSCs. Mouse, however, also has a population of multipotent progenitor cells in the stromal limbus analogous to the human CSSCs. As with human CSSCs, these cells are CD45(−) suggesting that they are not bone marrow-derived cells [21]. In lethally irradiated mice rescued by transplantation of green fluorescent protein (GFP)-bone marrow cells, some green cells were observed in the corneal stoma, but clonal spheres formed by the stromal progenitor cells did not contain bone marrow-derived cells based on the absence of green cells in clonal spheres [21]. Furthermore, spheres from transgenic mice encoding P0-Cre/ Floxed-EGFP as well as Wnt1-Cre/Floxed-EGFP were GFP(+), indicating an ocular and neural crest embryonic lineage of the stromal progenitor cells [21]. These results were confirmed by the expression of the embryonic neural crest markers Twist, Snail, Slug, and Sox9. The similar character of CSSCs and mouse stromal progenitor cells supports the idea that these stromal stem cells are ocular in embryonic origin and are not derived from pericytes or other bone marrow cells.
CSSCs Differentiate to Functional Keratocytes
When cultured in low-mitogen, ascorbate-containing media, CSSCs express an array of genes characteristic of keratocytes [22]. When the cells are removed from substratum and cultured as a pellet, a more complete keratocyte gene expression pattern was observed [38]. When cultured on substratum of parallel aligned polymeric nanofibers, CSSCs produced layers of highly parallel collagen fibers with packing and fibril diameter indistinguishable from that of the human stromal lamellae [39]. The ability of CSSCs to adopt keratocyte function was most striking in vivo. When injected into mouse corneal stroma, human CSSCs express keratocyte mRNA and protein, replacing mouse extracellular matrix with human matrix components [40]. These injected cells remained viable for many months, apparently having permanently become quiescent keratocytes [40]. These results suggest that keratocyte represents the default lineage for the CSSCs; the implication of such is that some aspect of their limbal microenvironment maintains the stem/progenitor character of CSSCs in vivo, but if they enter the stroma, the CSSCs become keratocytes. The proximity of CSSCs and LESCs in vivo suggests the possibility that each of these populations provides symbiotic support for maintenance of the stem cell phenotype of the other. Beyond these observations, however, little is known about participation of CSSCs in normal stromal homeostasis or in tissue healing and/or regeneration in response to trauma or pathology.
Immune Privilege of Stromal Stem Cells
In addition to exhibiting characteristics of adult stem cells, the CSSCs display another interesting attribute. Injection of human CSSCs into mouse corneal stroma in vivo resulted in no T-cell-mediated immune rejection of these cells. A transient inflammatory response similar in magnitude only to that produced by sham injections occurred but subsided within a week. Flow cytometric analysis showed the inflammatory cells to be largely neutrophils [40]. Injection of human corneal fibroblasts produced a similar transient response, followed by a marked increase in CD45+ cells after 1 week. Immunostaining of the injected tissue at 2 weeks showed CD3 T cells associated with the injected human fibroblasts, but no T cells were observed in tissue injected with CSSCs. The fibroblast- injected eyes exhibited visible haze increasing after 2 weeks, but the CSSC-injected eyes remained clear. Finally, chimeric mice rescued with GFP-bone marrow cells exhibited only a transient influx of green cells after CSSC injection. Conversely, injected human corneal fibroblasts elicited a strong influx of green cells into the cornea after 10 days. All these data point to an immunomodulatory function for CSSCs. Immune suppression of allogenic and xenogenic T-cell-mediated responses has been characterized for adult stem cells from several other sources [40]. The cornea exhibits a widely known immune privilege by which it avoids much of the immune rejection typical of most allogenic tissue transplants. The ability of cells in the stromal limbus to suppress T-cell-mediated tissue rejection may well aid this immune privilege. Such a feature of these cells, maintained in vitro, could well provide a therapeutic tool for mediating inflammatory response and tissue rejection in transplants or other situations. It is therefore important to characterize the mechanism of the immunomodulatory function of CSSCs in greater detail.
Therapeutic Potential of Stromal Stem Cells
The ability of the CSSCs to maintain a corneal phenotype over a very high number of population doublings and, importantly, to modulate immune response of the host makes them an excellent candidate for generation of bioengineered corneal stromal constructs. Such constructs could be used to replace scarred stroma using partial thickness transplantation methodology currently in practice. Development of such constructs could provide an off-the-shelf material free of the complications that limit distribution of cadaveric donor tissue in many parts of the world. Development of such stromal replacement constructs with CSSCs is therefore a high priority in the therapeutic application of CSSCs.
In addition to utility in bioengineering stromal tissue, CSSCs may have the potential to provide direct cell-based therapy for corneal scarring. A model of cell-based therapy was demonstrated using the Lum−/− mouse, a genotype lacking one of the stromal KSPGs. This mouse has thin corneas with distinct haze and a disruption of stromal collagen organization. CSSCs injected into the Lum−/− mouse stroma spread throughout the stroma. The CSSCs remained viable and did not fuse with mouse cells. Human KSPGs were deposited in the mouse stroma, but no immune rejection of the cells was observed. After 3 months, corneal clarity of the mice was assessed using confocal microscopy. Both transparency and thickness of the mutant corneas were found to be restored to that of wild-type mice [40]. Electron microscopic analysis of the collagen of the injected corneas showed a rescue of the collagen fibril organization as well [40]. These results support the idea that delivery of CSSCs to scarred human stromas may provide alleviation of corneal scars without requiring surgery.
Conclusions
CSSCs are a population of neural crest-derived MSC localized in the limbal stroma, subjacent to the epithelial basement membrane. These cells are clearly distinct from corneal epithelial stem cells, but the proximity of the two populations in vivo suggests their interactivity. The CSSCs maintain an enhanced potential to become functional keratocytes after multiple rounds of expansion compared to the majority of the cells in the stroma. The CSSCs may therefore be the source of keratocytes replacement, which migrates into the stroma in response to corneal trauma. The ability of the CSSCs to grow through multiple rounds of replication in vitro without loss of differentiation potential makes them excellent candidates for use in corneal bioengineering applications. The ability to regenerate a normal stroma in a mouse scar model and the apparent immunomodulatory properties of these cells present exciting prospects for their use in direct cell-based therapy for human corneal scarring.
Acknowledgments
This work was supported by NIH Grants EY016415 and P30-EY008098, The Eye and Ear Foundation (Pittsburgh, PA), and Research to Prevent Blindness Inc. The authors acknowledge the contribution of Kira Lathrop for the illustration.
Footnotes
Disclosure of Potential Conflicts of Interest
The authors declare no potential conflicts of interest.
Author contributions: P.N.: conception and design and manuscript writing; J.L.F.: editing, final approval of manuscript, and financial support.
References
- 1.Parfitt GJ, Pinali C, Young RD, et al. Three-dimensional reconstruction of collagen-proteoglycan interactions in the mouse corneal stroma by electron tomography. J Struct Biol. 2010;170:392–397. doi: 10.1016/j.jsb.2010.01.019. [DOI] [PubMed] [Google Scholar]
- 2.Lewis PN, Pinali C, Young RD, et al. Structural interactions between collagen and proteoglycans are elucidated by three-dimensional electron tomography of bovine cornea. Structure. 2010;18:239–245. doi: 10.1016/j.str.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 3.Hassell JR, Birk DE. The molecular basis of corneal transparency. Exp Eye Res. 2010;91:326–335. doi: 10.1016/j.exer.2010.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kao WW, Liu CY. Roles of lumican and keratocan on corneal transparency. Glycoconj J. 2002;19:275–285. doi: 10.1023/A:1025396316169. [DOI] [PubMed] [Google Scholar]
- 5.Funderburgh JL. Keratan sulfate: Structure, biosynthesis, and function. Glycobiology. 2000;10:951–958. doi: 10.1093/glycob/10.10.951. [DOI] [PubMed] [Google Scholar]
- 6.Zieske JD. Corneal development associated with eyelid opening. Int J Dev Biol. 2004;48:903–911. doi: 10.1387/ijdb.041860jz. [DOI] [PubMed] [Google Scholar]
- 7.Johnson GJ. Vision 2020: The right to sight: Report on the Sixth General Assembly of the International Agency for the Prevention of Blindness (IAPB) Community Eye Health. 1999;12:59–60. [PMC free article] [PubMed] [Google Scholar]
- 8.Funderburgh JL, Mann MM, Funderburgh ML. Keratocyte phenotype mediates proteoglycan structure: A role for fibroblasts in corneal fibrosis. J Biol Chem. 2003;278:45629–45637. doi: 10.1074/jbc.M303292200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilson SE. Analysis of the keratocyte apoptosis, keratocyte proliferation, and myofibroblast transformation responses after photorefractive keratectomy and laser in situ keratomileusis. Trans Am Ophthalmol Soc. 2002;100:411–433. [PMC free article] [PubMed] [Google Scholar]
- 10.Fini ME. Keratocyte and fibroblast phenotypes in the repairing cornea. Prog Retin Eye Res. 1999;18:529–551. doi: 10.1016/s1350-9462(98)00033-0. [DOI] [PubMed] [Google Scholar]
- 11.Cintron C, Kublin CL. Regeneration of corneal tissue. Dev Biol. 1977;61:346–357. doi: 10.1016/0012-1606(77)90304-9. [DOI] [PubMed] [Google Scholar]
- 12.Whitcher JP, Srinivasan M, Upadhyay MP. Corneal blindness: A global perspective. Bull World Health Organ. 2001;79:214–221. [PMC free article] [PubMed] [Google Scholar]
- 13.Zieske JD, Guimaraes SR, Hutcheon AE. Kinetics of keratocyte proliferation in response to epithelial debridement. Exp Eye Res. 2001;72:33–39. doi: 10.1006/exer.2000.0926. [DOI] [PubMed] [Google Scholar]
- 14.Carlson EC, Wang IJ, Liu CY, et al. Altered KSPG expression by keratocytes following corneal injury. Mol Vis. 2003;9:615–623. [PubMed] [Google Scholar]
- 15.Wollensak G, Green WR. Analysis of sex-mismatched human corneal transplants by fluorescence in situ hybridization of the sex-chromosomes. Exp Eye Res. 1999;68:341–346. doi: 10.1006/exer.1998.0611. [DOI] [PubMed] [Google Scholar]
- 16.Long CJ, Roth MR, Tasheva ES, et al. Fibroblast growth factor-2 promotes keratan sulfate proteoglycan expression by keratocytes in vitro. J Biol Chem. 2000;275:13918–13923. doi: 10.1074/jbc.275.18.13918. [DOI] [PubMed] [Google Scholar]
- 17.Ren R, Hutcheon AE, Guo XQ, et al. Human primary corneal fibroblasts synthesize and deposit proteoglycans in long-term 3-D cultures. Dev Dyn. 2008;237:2705–2715. doi: 10.1002/dvdy.21606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Funderburgh ML, Du Y, Mann MM, et al. PAX6 expression identifies progenitor cells for corneal keratocytes. FASEB J. 2005;19:1371–1373. doi: 10.1096/fj.04-2770fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Amano S, Yamagami S, Mimura T, et al. Corneal stromal and endothelial cell precursors. Cornea. 2006;25:S73–S77. doi: 10.1097/01.ico.0000247218.10672.7e. [DOI] [PubMed] [Google Scholar]
- 20.Yoshida S, Shimmura S, Shimazaki J, et al. Serum-free spheroid culture of mouse corneal keratocytes. Invest Ophthalmol Vis Sci. 2005;46:1653–1658. doi: 10.1167/iovs.04-1405. [DOI] [PubMed] [Google Scholar]
- 21.Yoshida S, Shimmura S, Nagoshi N, et al. Isolation of multipotent neural crest-derived stem cells from the adult mouse cornea. Stem Cells. 2006;24:2714–2722. doi: 10.1634/stemcells.2006-0156. [DOI] [PubMed] [Google Scholar]
- 22.Du Y, Funderburgh ML, Mann MM, et al. Multipotent stem cells in human corneal stroma. Stem Cells. 2005;23:1266–1275. doi: 10.1634/stemcells.2004-0256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Golebiewska A, Brons NH, Bjerkvig R, et al. Critical appraisal of the side population assay in stem cell and cancer stem cell research. Cell Stem Cell. 2011;8:136–147. doi: 10.1016/j.stem.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 24.Sosnova M, Bradl M, Forrester JV. CD34+ corneal stromal cells are bone marrow-derived and express hemopoietic stem cell markers. Stem Cells. 2005;23:507–515. doi: 10.1634/stemcells.2004-0291. [DOI] [PubMed] [Google Scholar]
- 25.Joseph A, Hossain P, Jham S, et al. Expression of CD34 and L-selectin on human corneal keratocytes. Invest Ophthalmol Vis Sci. 2003;44:4689–4692. doi: 10.1167/iovs.02-0999. [DOI] [PubMed] [Google Scholar]
- 26.Toti P, Tosi GM, Traversi C, et al. CD-34 stromal expression pattern in normal and altered human corneas. Ophthalmology. 2002;109:1167–1171. doi: 10.1016/s0161-6420(02)01042-4. [DOI] [PubMed] [Google Scholar]
- 27.Barbaro V, Di Iorio E, Ferrari S, et al. Expression of VSX1 in human corneal keratocytes during differentiation into myofibroblasts in response to wound healing. Invest Ophthalmol Vis Sci. 2006;47:5243–5250. doi: 10.1167/iovs.06-0185. [DOI] [PubMed] [Google Scholar]
- 28.Liu H, Zhang J, Liu CY, et al. Cell therapy of congenital corneal diseases with umbilical mesenchymal stem cells: Lumican null mice. PLoS One. 2010;5:e10707. doi: 10.1371/journal.pone.0010707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mimura T, Amano S, Yokoo S, et al. Isolation and distribution of rabbit keratocyte precursors. Mol Vis. 2008;14:197–203. [PMC free article] [PubMed] [Google Scholar]
- 30.Shortt AJ, Secker GA, Munro PM, et al. Characterization of the limbal epithelial stem cell niche: Novel imaging techniques permit in vivo observation and targeted biopsy of limbal epithelial stem cells. Stem Cells. 2007;25:1402–1409. doi: 10.1634/stemcells.2006-0580. [DOI] [PubMed] [Google Scholar]
- 31.Shortt AJ, Tuft SJ, Daniels JT. Corneal stem cells in the eye clinic. Br Med Bull. 2011;100:209–225. doi: 10.1093/bmb/ldr041. [DOI] [PubMed] [Google Scholar]
- 32.Rama P, Matuska S, Paganoni G, et al. Limbal stem-cell therapy and long-term corneal regeneration. N Engl J Med. 2010;363:147–155. doi: 10.1056/NEJMoa0905955. [DOI] [PubMed] [Google Scholar]
- 33.Sangwan VS, Basu S, Vemuganti GK, et al. Clinical outcomes of xeno-free autologous cultivated limbal epithelial transplantation: A 10-year study. Br J Ophthalmol. 2011;95:1525–1529. doi: 10.1136/bjophthalmol-2011-300352. [DOI] [PubMed] [Google Scholar]
- 34.Polisetty N, Fatima A, Madhira SL, et al. Mesenchymal cells from limbal stroma of human eye. Mol Vis. 2008;14:431–442. [PMC free article] [PubMed] [Google Scholar]
- 35.Ainscough SL, Linn ML, Barnard Z, et al. Effects of fibroblast origin and phenotype on the proliferative potential of limbal epithelial progenitor cells. Exp Eye Res. 2011;92:10–19. doi: 10.1016/j.exer.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 36.Mariappan I, Maddileti S, Savy S, et al. In vitro culture and expansion of human limbal epithelial cells. Nat Protoc. 2010;5:1470–1479. doi: 10.1038/nprot.2010.115. [DOI] [PubMed] [Google Scholar]
- 37.Corselli M, Chen CW, Crisan M, et al. Perivascular ancestors of adult multipotent stem cells. Arterioscler Thromb Vasc Biol. 2010;30:1104–1109. doi: 10.1161/ATVBAHA.109.191643. [DOI] [PubMed] [Google Scholar]
- 38.Du Y, Sundarraj N, Funderburgh ML, et al. Secretion and organization of a cornea-like tissue in vitro by stem cells from human corneal stroma. Invest Ophthalmol Vis Sci. 2007;48:5038–5045. doi: 10.1167/iovs.07-0587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu J, Du Y, Watkins SC, et al. The engineering of organized human corneal tissue through the spatial guidance of corneal stromal stem cells. Biomaterials. 2011;33:1343–1352. doi: 10.1016/j.biomaterials.2011.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Du Y, Carlson EC, Funderburgh ML, et al. Stem cell therapy restores transparency to defective murine corneas. Stem Cells. 2009;27:1635–1642. doi: 10.1002/stem.91. [DOI] [PMC free article] [PubMed] [Google Scholar]


