Abstract
Introduction
In granulomatosis with polyangiitis (GPA), a complex autoimmune small-vessel vasculitis frequently associated with chronic necrotizing inflammation of the nasal mucosa, elevated nasal Staphylococcus (S.) aureus carrier rates are a risk factor for relapse. As cytokines are primarily involved in the regulation of defense against potentially pathogenic microorganisms, the aim of this study was to compare healthy individuals and GPA patients with respect to their baseline cytokine expression of nasal epithelial cells (NEC), which form the first barrier against such triggers. The ability of S. aureus to influence the nasal microenvironment's cytokine secretion was assessed by exemplary stimulation experiments.
Methods
Baseline expression of 19 cytokines of primary NEC of GPA patients and normal controls (NC) was quantified by a multiplex cytokine assay. Stimulation experiments were performed with supernatants of S. aureus and expression of interleukin-8 was determined by ELISA.
Results
In GPA, an altered pattern of baseline cytokine expression with significantly up-regulated G-CSF and reduced interleukin (IL)-8 concentrations was observed. Both NEC of GPA patients and NC responded to stimulation with S. aureus, but GPA patients displayed a significantly lower IL-8 secretion and a diminished dynamic range of response towards the stimulus.
Conclusions
The data presented underline the hypothesis of a disturbed epithelial nasal barrier function in GPA. The dysregulated baseline expression of G-CSF and IL-8 and the reduced response to microbial stimulation may facilitate changes in the composition of the nasal flora and favour an imbalanced inflammatory response, which might be relevant for the disease course.
Introduction
Granulomatosis with polyangiitis (GPA) is characterized by chronic necrotizing granulomatous inflammation with a predilection of the upper and lower respiratory tract and proteinase 3 (PR3) specific cytoplasmic anti-neutrophil cytoplasmatic antibodies (C-ANCA) [1,2].
So far, the pathogenetic mechanisms resulting in inflammation and autoimmunity in GPA are poorly elucidated. A complex interaction between genetic susceptibility and environmental factors is discussed [3-6], whereas low familiar clustering [7] stresses the importance of the latter.
Epidemiological studies revealed higher Staphylococcus (S.) aureus colonisation rates in GPA-patients compared to healthy and diseased controls [8-10]. Moreover, nasal carriage was shown to be associated with significantly elevated relapse rates, endonasal disease activity and first manifestation in the upper respiratory tract [8,11,12]. In addition, treatment with trimethoprim/sulfamethoxazole reduces the annual number of respiratory infections and the incidence of relapses in GPA-patients in remission [10,13,14].
Various staphylococcal superantigens were demonstrated to exert strong stimulatory effects on immunocompetent cells [15]. Furthermore, T-cells were shown to exhibit irregular modes of differentiation and cytokine expression upon stimulation with S. aureus [16,17], and S. aureus specificity was found in T-cell clones generated from peripheral blood lymphocytes of GPA-patients [17].
Remarkably, recent findings suggest a dysbalanced microbiom of the nasal cavity rather than a distinct microbial trigger comparable to the dysbiosis in inflammatory bowel diseases like Morbus Crohn [18]. Kain et al. demonstrated that in ANCA-associated systemic vasculitides highly prevalent auto-antibodies to lysosomal membrane protein-2 can cross-react with adhesins of gram-negative pathogens [19].
Another hint at microbial triggering of GPA is given by Pendergraft et al. demonstrating that patients harbouring PR3-ANCA also produce auto-antibodies to complementary PR3 (cPR3), a peptide translated from the antisense DNA strand of PR3. Conversely, the presence of cPR3 leads to production of both anti-cPR3 and anti-PR3 auto-antibodies. Genetic sequences that could be translated to cPR3-peptides were identified in numerous microbial and fungal organisms, including S. aureus, Entamoeba histolytica and Ross-River virus [20].
Taken together, the evidence is increasingly pointing to an imbalanced inflammatory response to microbial challenge as a potentially relevant process in the pathogenesis of GPA or vice versa.
Cytokines are significantly involved in the regulation of immune and inflammatory processes [21]. They enable inter-cellular communication and initiate immune responses by recruiting and activating specific immune cells, thus playing a decisive role in successful local defense against microorganisms [22]. Although aberrant cytokine patterns in GPA-patients in serum or plasma [23-25], mononuclear cells [26,27], pulmonary lesions [28,29] and bronchoalveolar lavage fluid [30] are verified by numerous studies, so far no study has been performed to assess altered cytokine expression in nasal epithelial cells, which form the first barrier against potential exogenous triggers.
We hypothesized an alteration in the prevailing pattern of cytokine expression of nasal epithelial cells especially focusing on microbial defense. The main objectives of this study were, therefore, to determine: i) the basal expression levels of 19 cytokines closely linked to microbial defense mechanisms on protein level in GPA-patients and normal controls and, furthermore, ii) the ability of S. aureus to influence the nasal microenvironment's cytokine secretion by exemplary stimulation experiments.
Materials and methods
Patients and biopsies
For analysing baseline cytokine secretion, nasal mucosa biopsies were obtained from 20 patients with GPA and 19 normal controls (NC). For stimulation experiments, biopsies of 10 GPA-patients and 10 NC were generated. Biopsies were taken from nasal turbinates at sites that were visually free of disease activity and suspect alterations like granuloma, edema, bloody patches, purulent secretion or crusts.
The study protocol was approved by the ethics committee of the University of Kiel, Germany (AZ A101/07) and was in accordance with the principles of the Declaration of Helsinki (latest revision October 2008). All patients provided written informed consent prior to enrolment. Exclusion criteria included pregnancy, haemostatic disorder and age of less than 18 years.
GPA was diagnosed according to the American College of Rheumatology classification criteria and Chapel Hill definitions for GPA as recommended by the European League Against Rheumatism (EULAR) [31]. GPA-subgroups were determined according to the European Vasculitis Study Group definitions and recent EULAR recommendations [31]. Extent of the disease was assessed by the Disease Extent Index (DEI) [32], disease activity was classified using the Birmingham Vasculitis Activity Score (BVAS) [33]. The Vasculitis Damage Index (VDI) was applied to record organ damage as a consequence of granulomatous inflammation and vasculitis [34,35]. All patients underwent a standardized interdisciplinary evaluation [36] and were examined endoscopically by an ear, nose and throat - specialist to evaluate endonasal GPA activity [2].
In order to assess the presence of systemic inflammation, white blood cell counts (WBC) as well as measurement of C-reactive protein (CRP) concentration and erythrocyte sedimentation rate (ESR) were performed. Besides slightly elevated ESR, as typical in GPA patients [37], all serologic parameters were within the normal range. In case of GPA, serologic titers of PR3-specific C-ANCA and myeloperoxidase-specific P-ANCA were determined according to Savige et al. [38].
In the normal control group, biopsies were taken while doctors were performing airway passage improving surgery. No individual of this group showed anamnestic or endoscopic signs of acute or chronic inflammatory or autoimmune alterations of the nasal mucosa or was under immunomodulating medication.
Detailed description of patient groups is given in Tables 1 and 2.
Table 1.
Patients' characteristics
| Baseline cytokine secretion | Stimulation | |||||||
|---|---|---|---|---|---|---|---|---|
| Normal control | Granulomatosis with polyangiitis | Normal control | Granulomatosis with polyangiitis | |||||
| (n = 19) | (n = 20) | (n = 10) | (n = 10) | |||||
| Age (yrs, mean and range) | 39.50 (18 to 76) | 51.55 (24 to 71) | 43.30 (23 to 56) | 50.80 (24 to 71) | ||||
| Sex | 11 male, 8 female | 12 male, 8 female | 7 male, 3 female | 7 male, 3 female | ||||
| First GPA manifestation till study entry (yrs, median and range) | 3.8 (19 to 0) | 3.3 (9 to 0) | ||||||
| First GPA diagnosis till study entry (yrs, median and range) | 1.6 (19 to 0) | 3.1 (9 to 0) | ||||||
| Serologic parameters | ||||||||
| mean value | SD | mean value | SD | mean value | SD | mean value | SD | |
| WBC (cells/nl) | 6.74 | 1.55 | 8.22 | 2.82 | 7.87 | 1.97 | 7.77 | 2.33 |
| ESR 1st h (mm) | 8.14 | 3.94 | 33.00 | 27.60 | 8.00 | 2.92 | 52.40 | 35.61 |
| median | range | median | range | median | range | median | range | |
| CRP (mg/dl) | 1.30 | 0.9 to 5.8 | 0.70 | 0 to 8.6 | 1.50 | 1.3 to 3.4 | 0.85 | 0.1 to 8.6 |
| C-ANCA-titre (1:) | 80 | 0 to 2560 | 640 | 0 to 2560 | ||||
| P-ANCA-titre (1:) | 0 | 0 to 640 | 0 | 0 to 61 | ||||
Table 2.
Characteristics of patients with GPA
| Baseline cytokine secretion | Stimulation | |||||
|---|---|---|---|---|---|---|
| n | % | n | % | |||
| Biopsy proof | GPA | 12 | 60 | 7 | 70 | |
| EULAR subgroups | generalized | 14 | 70 | 8 | 80 | |
| early systemic | 5 | 25 | 1 | 10 | ||
| local | 1 | 5 | 0 | 0 | ||
| severe | 0 | 0 | 1 | 10 | ||
| EULAR disease activity | remission | 6 | 30 | 2 | 20 | |
| response | 0 | 0 | 1 | 10 | ||
| relapse, minor | 8 | 40 | 2 | 20 | ||
| relapse, major | 4 | 20 | 3 | 30 | ||
| refractory | 1 | 5 | 0 | 0 | ||
| low-activity | 1 | 5 | 2 | 20 | ||
| Endoscopy: endonasal activity | none | 12 | 60 | 4 | 40 | |
| mild | 7 | 35 | 4 | 40 | ||
| moderate | 0 | 0 | 2 | 20 | ||
| not evaluated | 1 | 5 | 0 | 0 | ||
| Disease scores | median (range) | median (range) | ||||
| DEI | 2 (0 to 5) | 2 (0 to 5) | ||||
| BVAS-1 | 3 (0 to 13) | 1.5 (0 to 12) | ||||
| BVAS-2 | 0 (0 to 4) | 0 (0 to 4) | ||||
| VDI | 0.5 (0 to 3) | 2 (0 to 4) | ||||
| Immunomodulating therapy | n (%) | mean value (mg) | n (%) | mean value (mg) | ||
| prednisolone | 19 (95) | 12.89 | 9 (90) | 10.67 | ||
| methotrexate | 10 (50) | 22.25 | 2 (20) | 20 | ||
| cyclophosphamide regular | 3 (15) | 133.33 | 1 (10) | 100 | ||
| cyclophosphamide bolus | 2 (10) | 1000 | 2 (20) | 1000 | ||
| azathioprine | 2 (10) | 125 | 2 (20) | 125 | ||
| leflunomide | 2 (10) | 25 | 2 (20) | 25 | ||
| mycophenolate mofetil | 1 (5) | 2000 | 0 (0) | 0 | ||
| cummulative cyclophosphamide | 6 (30) | 45.17 (g) | 8 (80) | 14.88 (g) | ||
Primary cell culture
Nasal epithelial cells (NEC) were enzymatically isolated from biopsies using dispase (Invitrogen, Karlsruhe, Germany). The cells were grown in Airway Epithelial Cell Growth medium (Promocell, Heidelberg, Germany) in a 96-well plate until pre-confluence with approximately equal numbers of cells per well were obtained. After a mean cultivation time of 13 days, supernatants were collected and stored at -80°C.
Multiplex cytokine assay
The supernatants of NEC were analysed by performing a Bio-Plex™ Cytokine Assay according to the manufacturer's instructions (Bio-Rad Laboratories, Munich, Germany) allowing quantification of multiple cytokines with broad range standard curves in small-sample-sizes. Pro-inflammatory (interleukin (IL)-1α, IL-1β, IL-5, IL-6, IL-7, IL-8, IL-17, tumour necrosis factor-alpha (TNF-α)) and anti-inflammatory (IL-4, IL-10, IL-13) mediators, proteins mainly associated with adaptive immune responses (IL-2, IL-4, IL-12p70, IL-13, IFN-γ) or with recruitment of immune cells (IL-8, MCP-1, MIP-1), cytokines predominantly responsible for proliferation of inflammatory effector cells (G-CSF, GM-CSF) and control of apoptotic procedures associated with inflammation (TNF-β) were investigated.
Briefly, samples of 50 µl NEC supernatant were incubated with anti-cytokine antibody-coupled beads. After washing, the complexes were incubated with biotinylated anti-cytokine detection antibodies, and finally with streptavidin-phycoerythrin. Cytokine concentrations were measured using a Luminex 96-well plate reader (Bio-Plex™ 100-Multiplex-Suspension-Array-Reader; Bio-Rad Laboratories, Munich, Germany) and the Bio-Plex™ Manager Software 4.1.1 (Bio-Rad Laboratories, Munich, Germany). Human recombinant cytokines were used as standards.
Stimulation of human nasal epithelial cells with S. aureus
For stimulation of NEC, supernatants containing the bacterial secretory products of S. aureus strain T190-2 (kindly provided by B.M. Bröker, University of Greifswald, Germany), which has been described to predominate in nasal isolates in Western Europe, was chosen. The S. aureus supernatants were not analysed for virulence factors secreted into the growth medium. However, in PCR analysis the test strain was positive for toxic shock and enterotoxin genes [39]. Experiments were performed corresponding to Sachse et al. [40]. According to the results of preliminary experiments concerning time and dose dependency (8, 12, 16, 24 hours; data not shown), stimulation experiments were performed with S. aureus supernatants diluted 1:5 in a final volume of 300 µl for 24 hours. An impact of the bacterial growth medium tryptic soy broth on the stimulation was ruled out before and NEC incubated in fresh cell culture medium without bacterial supernatants served as controls. Cell viability greater than 95% was verified by trypan blue dye exclusion test, and vital cell morphology was controlled in phase contrast microscopy. Moreover, 10 µl of cell culture supernatants were incubated on Columbia blood agar to prove absence of bacterial contamination. After stimulation, cell culture supernatants were collected and stored at -80°C until analysis.
Quantification of IL-8 via ELISA
IL-8 concentrations in cell culture supernatants after stimulation were determined in duplicate by standard ELISA using the BD OptEIA human IL-8 set (BD Biosciences, San Diego, CA, USA).
Statistical analysis
All statistics were performed in an exploratory manner using SPSS statistical software for Windows, version 18 (SPSS Inc., Chicago, IL, USA). Based on the Shapiro-Wilk-Test, normal distribution could be assumed for neither the entire Bio-Plex™ data nor for the delta values (stimulated - basal) of the stimulation experiments. Therefore, differences between the two groups (GPA versus NC) were evaluated by the nonparametric Mann-Whitney-U-Test. Normally distributed stimulation data were analysed by means of a general linear model for repeated measurement procedures in order to test the effects of within-subject factors (effect of stimulation within each group) and of between-subject factors (comparison of GPA- and NC-group). A P-value of ≤0.05 was regarded as statistically significant.
Results
Altered pattern of baseline cytokine expression in GPA-patients
For 17 of the analysed 19 cytokines in the supernatants of NEC, no difference between GPA-patients and normal controls could be detected (see Table 3 for details).
Table 3.
Baseline cytokine expression of nasal epithelial cells (pg/ml)
| Normal control (NC) | Granulomatosis with polyangiitis (GPA) | NC vs. GPA | |||
|---|---|---|---|---|---|
| median | range | median | range | P-value | |
| IL- 1α | 71.74 | <1.51 to 319.24 | 92.38 | 7.90 to 477.11 | 0.322 |
| IL-1β | <2.67 | <2.67 to 8.67 | 2.70 | <2.67 to 12.74 | 0.224 |
| IL-2 | <1.42 | <1.42 to 6.46 | 3.60 | <1.42 to 6.00 | 0.070 |
| IL-4 | <0.32 | <0.32 to 0.80 | <0.32 | <0.32 to 0.72 | 0.283 |
| IL-5 | below detection limit (<2.51) | ||||
| IL-6 | 26.22 | 2.81 to 788.29 | 29.61 | 3.70 to 139.47 | 0.607 |
| IL-7 | below detection limit (<2.43) | ||||
| IL-8 | 1388.14 | <1.77 to 5188.14 | 319.16 | <1.77 to 3708.72 | 0.009 |
| IL-10 | below detection limit (<1.78) | ||||
| IL-12 | <2.55 | <2.55 to 3.75 | <2.55 | <2.55 to 4.03 | 0.283 |
| IL-13 | below detection limit (<2.68) | ||||
| IL-17 | 2.77 | <1.87 to 6.41 | 3.54 | <1.87 to 6.01 | 0.101 |
| G-CSF | 19.60 | <1.31 to 204.41 | 61.30 | 1.53 to 663.58 | 0.050 |
| GM-CSF | 10.50 | <0.74 to 28.70 | 12.50 | 7.74 to 21.56 | 0.084 |
| IFN-γ | 4.61 | <1.61 to 21.41 | 5.92 | 2.27 to 18.18 | 0.089 |
| MCP-1 | 3.66 | <1.81 to 8.21 | 4.12 | <1.81 to 7.90 | 0.667 |
| MIP-1β | <1.41 | <1.41 to 2.37 | 1.43 | <1.41 to 2.79 | 0.141 |
| TNF-α | <6.42 | <6.42 to 13.46 | <6.42 | <6.42 to 11.00 | 0.296 |
| TNF-β | 9.44 | <2.14 to 24.46 | 14.20 | 3.35 to 23.40 | 0.054 |
In contrast, NEC of GPA-patients showed significantly higher protein expression of granulocyte colony-stimulating factor (G-CSF, P = 0.050). Furthermore, concentrations of interleukin-8 (IL-8, CXCL8) were remarkably reduced in NEC of GPA-patients compared to NC (P = 0.009), as illustrated in Figure 1.
Figure 1.

Baseline cytokine expression. Significantly altered baseline cytokine expression of primary nasal epithelial cells concerning G-CSF (A) and IL-8 (B) of granulomatosis with polyangiitis (GPA) patients compared to normal controls (NC). Data are represented as box plots showing the median as a straight line; the boxes correspond to the 75th and 25th percentiles and the whiskers indicate normal variation limits.
Diminished response to stimulation with S. aureus culture supernatants in GPA-patients
Based on the results of the multiplex cytokine assay, a more detailed functional study of IL-8 expression of NEC upon stimulation with secretory products containing S. aureus-culture-supernatants was performed.
While both groups (GPA and NC) showed a statistically significant response to the stimulus (NC: P = 0.005, GPA: P = 0.005), baseline IL-8 expression (mean value 1,247.9, SD 881.6, maximum 2,735.3, minimum 379.0 pg/ml) as well as IL-8 expression after stimulation (mean value 2,753.0, SD 1,938.2, maximum 6,744.4, minimum 776.5 pg/ml) was significantly lower (P = 0.006) in GPA-patients compared to NC (basal: mean value 2,876.1, SD 1,498.3, maximum 4,935.5, minimum 500.0 pg/ml; stimulated: mean value 5,647.4, SD 2,407.9, maximum 9,516.1, minimum 1,521.9 pg/ml), as demonstrated in Figure 2A. Remarkably, also the dynamic range of the reaction (stimulated minus basal) was considerably more restricted in NEC of GPA-patients (P = 0.029, Figure 2B).
Figure 2.
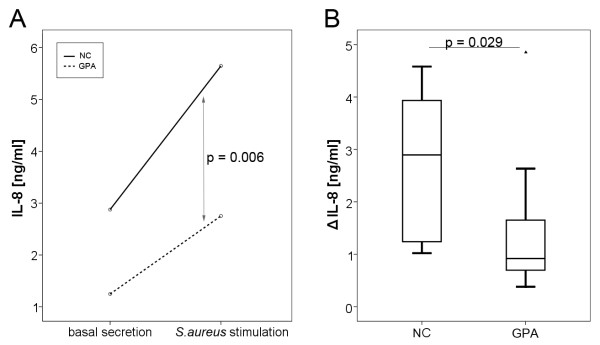
Effect of stimulation with S. aureus. Significant differences in IL-8 expression of primary nasal epithelial cells (NEC) comparing granulomatosis with polyangiitis (GPA) patients and normal controls (NC) before and after stimulation with S. aureus supernatants (A, P = 0.006). Furthermore, the dynamic range (Δ IL-8, secretion after stimulation minus basal secretion) is significantly reduced in GPA-patients (B, P = 0.029).
No correlation between nasal S. aureus-colonisation, endonasal GPA-activity or prednisolone dosages and these results could be detected (data not shown).
Discussion
Cytokines' elaborate interplay enables inter-cellular communication and constitutes a crucial part in the regulation of immune and inflammatory processes - two important components in the pathogenesis of GPA - thus allowing an adequate response to microbial challenges.
Apart from Balding et al., who described an altered Th2-cytokine milieu in nasal biopsies [41], no comprehensive analysis of baseline cytokine expression in the nasal epithelium of GPA-patients has been performed so far.
For GPA, alterations in cytokine levels in pulmonary lesions have been described [28,29], but none of the 19 cytokines relevant for microbial defense included in this study were examined. Results of Richter et al., who reported elevated concentrations of the pro-inflammatory cytokines IL-1α, IL-1β and IL-6 in bronchoalveolar lavage fluid of GPA-patients [30], are difficult to compare to our findings obtained from a distinct compartment but hint at an altered cytokine spectrum.
Once induced by an inflammatory stimulus, almost all tissues within the human body are capable of producing G-CSF, thus leading to an increase in number and activation of neutrophils [42]. G-CSF serum levels are markedly elevated in response to infection and usually fall in parallel with the recovery process [43], whereas they remain elevated and correlate with disease activity in chronic inflammatory conditions, such as rheumatoid arthritis and Behçet disease [44,45]. Locally elevated G-CSF concentrations have also been observed in inflammatory bowel disease [46]. These findings match our results of locally elevated concentrations in NEC reasonably.
A prolonged life time of neutrophils resulting from a delayed apoptosis induced by G-CSF [47] increases the possibility of being primed and expressing PR3 on the cell surface [23,48]. Further activation through binding of ANCA can result in damage and lysis of endothelial cells [49]. In endothelial cells, G-CSF has been shown to down-regulate lipopolysaccharide-induced IL-8 expression [50], which would be conceivable for epithelial cells as well. Besides, the possible ability of G-CSF to reduce neutrophil killing of S. aureus [51] could have tremendous negative impacts on an effective immune defense.
IL-8 chemoattracts polymorphonuclear neutrophils (PMN) and monocytes and further promotes their activation [52], thus creating and maintaining an inflammatory microenvironment at the site of infection.
Lamprecht et al. suggested a down-regulation of monocytic production of IL-8 during the course of GPA [26], which is in line with our findings. Stimulation of PMN with IL-8, especially after previous treatment with TNF-α, leads to PR3 translocation to the cell surface [53], thus providing the prerequisite for interactions with PR3-ANCA, which directly activates diverse inflammatory reactions in PMN [54]. Variation of IL-8 expression levels is fine-tuned by graduated activation of at least three signaling pathways: NF-κB, JNK (JUN-N terminal protein kinase) and p38-MPK (mitogen-activated protein kinase) [55]. In order to evaluate whether this complex interplay is operating effectively in GPA-patients, we stimulated NEC with culture supernatants of S. aureus, which apart from its particular role in the pathogenesis of GPA has been shown to be a potent inducer of IL-8 expression in nasal epithelial cells [56,57]. The imbalance of IL-8 with a reduced baseline expression and a diminished response to bacterial stimulus of GPA-NEC could reasonably lead to a shift in the microbial flora towards an overbalance of facultative pathogenic microorganisms.
These data, in addition to the observation of a severely impaired ciliar beat frequency [58] and a reduced production of the antimicrobial peptide human β-defensin 3 of NEC upon stimulation with S. aureus [59], as well as an imbalanced regulation of genes involved in epithelial barrier function [18], fortify the hypothesis of considering GPA a disease with a disturbed epithelial barrier function as was also discussed for other chronic inflammatory diseases with nasal involvement [60].
However, this study has its limitations. Patients with GPA received immunomodulating therapy, for which negative impact on cytokine expression has been described [61,62]. Differential expression of the investigated cytokines (most of them without differences between NC and GPA) argues for successful prevention of such effects by cultivating NEC for an average of 13 days prior to the investigation.
The fact that the pattern of cytokine expression of NEC can be influenced by external stimuli paves the way for local pharmacological interference. Shifting the basal cytokine balance towards a higher IL-8 level, for example, by applying recombinant human IL-8 [63] or non-pathogenic bacteria components [64] could be a future therapeutic option.
Conclusions
Taken together, our data suggest a specifically altered pattern of baseline cytokine expression of the nasal epithelium in patients with GPA compared to NC. This can facilitate changes in the composition of microbial colonisation and favour an imbalanced inflammatory response to microbial challenge and thus disease exacerbation. Our findings of an aberrant response to S. aureus stimulation in patients with GPA further underline this hypothesis. It remains to be investigated whether our results exemplify a general alteration in the reactive cytokine response to external stimuli. Taken into account the results of all 19 examined cytokines, we assume pathophysiological relevance of IL-8 and G-CSF in GPA, which could have potential therapeutic implications.
Abbreviations
BVAS: Birmingham Vasculitis Activity Score; C-ANCA: cytoplasmic anti-neutrophil cytoplasmatic antibodies; cPR3: complementary PR3; CRP: C-reactive protein; DEI: Disease Extent Index; ELISA: enzyme-linked immunosorbent assay; ESR: erythrocyte sedimentation rate; EULAR: European League Against Rheumatism; G-CSF: granulocyte colony-stimulating factor; GM-CSF: granulocyte macrophage colony-stimulating factor; GPA: Granulomatosis with polyangiitis; IFN-γ: interferon-γ; IL: interleukin; JNK: JUN-N terminal protein kinase; MAPK: mitogen-activated protein kinase; MCP-1: monocyte chemotactic protein-1; MIP-1: macrophage inflammatory protein-1; NC: normal controls; NEC: nasal epithelial cells; P-ANCA: perinuclear anti-neutrophil cytoplasmatic antibodies; PMN: polymorphonuclear neutrophils; PR3: proteinase 3; S. aureus: Staphylococcus aureus; TNF: tumor necrosis factor; VDI: Vasculitis Damage Index; WBC: white blood cell count.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
ML, RP, JH and PA designed and coordinated the study. JW carried out the experiments. ML, JW, KB and PL were involved in data acquisition and interpretation. JH, JW and ML performed the statistical analysis. JW, KB, PL, RP, JH, PA and ML drafted and revised the manuscript. All authors provided final approval of the submitted manuscript.
Contributor Information
Janet Wohlers, Email: janet.wohlers@gmx.de.
Katrin Breucker, Email: kati.breucker@t-online.de.
Rainer Podschun, Email: podschun@infmed.uni-kiel.de.
Jürgen Hedderich, Email: hedderich@medinfo.uni-kiel.de.
Peter Lamprecht, Email: peter.lamprecht@uk-sh.de.
Petra Ambrosch, Email: ambrosch@hno.uni-kiel.de.
Martin Laudien, Email: laudien@hno.uni-kiel.de.
Acknowledgements
This study was supported by the German Research Foundation (DFG) funded Clinical Research Unit (KFO 170). We thank A. Hölzgen, U. Kreutz, T. Görögh and A.-M. Röen for their expert technical assistance. We gratefully acknowledge participation of all patients and healthy volunteers who took part in this study.
References
- Bacon PA. The spectrum of Wegener's granulomatosis and disease relapse. N Engl J Med. 2005;352:330–332. doi: 10.1056/NEJMp048338. [DOI] [PubMed] [Google Scholar]
- Paulsen JI, Rudert H. Manifestations of primary vasculitis in the ENT region. Z Rheumatol. 2001;60:219–225. doi: 10.1007/s003930170047. [DOI] [PubMed] [Google Scholar]
- Hamidou MA, Audrain M, Ninin E, Robillard N, Muller JY, Bonneville M. Staphylococcus aureus, T-cell repertoire, and Wegener's granulomatosis. Joint Bone Spine. 2001;68:373–377. doi: 10.1016/s1297-319x(01)00293-7. [DOI] [PubMed] [Google Scholar]
- Jagiello P, Gross WL, Epplen JT. Complex genetics of Wegener granulomatosis. Autoimmun Rev. 2005;4:42–47. doi: 10.1016/j.autrev.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Laudien M, Ambrosch P, Till A, Podschun R, Lamprecht P. [Diagnosis, therapy and current research aspects of selected chronic inflammatory diseases with head and neck involvement] Z Rheumatol. 2008;67:397–406. doi: 10.1007/s00393-008-0324-3. [DOI] [PubMed] [Google Scholar]
- Tervaert JW, Popa ER, Bos NA. The role of superantigens in vasculitis. Curr Opin Rheumatol. 1999;11:24–33. doi: 10.1097/00002281-199901000-00005. [DOI] [PubMed] [Google Scholar]
- Wieczorek S, Holle JU, Epplen JT. Recent progress in the genetics of Wegener's granulomatosis and Churg-Strauss syndrome. Curr Opin Rheumatol. 2010;22:8–14. doi: 10.1097/BOR.0b013e3283331151. [DOI] [PubMed] [Google Scholar]
- Laudien M, Gadola SD, Podschun R, Hedderich J, Paulsen J, Reinhold-Keller E, Csernok E, Ambrosch P, Hellmich B, Moosig F, Gross WL, Sahly H, Lamprecht P. Nasal carriage of Staphylococcus aureus and endonasal activity in Wegener s granulomatosis as compared to rheumatoid arthritis and chronic rhinosinusitis with nasal polyps. Clin Exp Rheumatol. 2010;28:51–55. [PubMed] [Google Scholar]
- Stegeman CA, Tervaert JW, Sluiter WJ, Manson WL, De Jong PE, Kallenberg CG. Association of chronic nasal carriage of Staphylococcus aureus and higher relapse rates in Wegener granulomatosis. Ann Intern Med. 1994;120:12–17. doi: 10.7326/0003-4819-120-1-199401010-00003. [DOI] [PubMed] [Google Scholar]
- Stegeman CA, Tervaert JW, De Jong PE, Kallenberg CG. Trimethoprim-sulfamethoxazole (co-trimoxazole) for the prevention of relapses of Wegener's granulomatosis. Dutch Co-Trimoxazole Wegener Study Group. N Engl J Med. 1996;335:16–20. doi: 10.1056/NEJM199607043350103. [DOI] [PubMed] [Google Scholar]
- Popa ER, Stegeman CA, Abdulahad WH, van der Meer B, Arends J, Manson WM, Bos NA, Kallenberg CG, Cohen Tervaert JW. Staphylococcal toxic-shock-syndrome-toxin-1 as a risk factor for disease relapse in Wegener's granulomatosis. Rheumatology (Oxford) 2007;46:1029–1033. doi: 10.1093/rheumatology/kem022. [DOI] [PubMed] [Google Scholar]
- Zycinska K, Wardyn KA, Zielonka TM, Demkow U, Traburzynski MS. Chronic crusting, nasal carriage of Staphylococcus aureus and relapse rate in pulmonary Wegener's granulomatosis. J Physiol Pharmacol. 2008;59(Suppl 6):825–831. [PubMed] [Google Scholar]
- De Groot K, Reinhold-Keller E, Tatsis E, Paulsen J, Heller M, Nolle B, Gross WL. Therapy for the maintenance of remission in sixty-five patients with generalized Wegener's granulomatosis. Methotrexate versus trimethoprim/sulfamethoxazole. Arthritis Rheum. 1996;39:2052–2061. doi: 10.1002/art.1780391215. [DOI] [PubMed] [Google Scholar]
- Reinhold-Keller E, de Groot K, Rudert H, Nölle B, Heller M, Gross WL. Response to trimethoprim/sulfamethoxazole in Wegener's granulomatosis depends on the phase of disease. QJM. 1996;89:15–23. doi: 10.1093/oxfordjournals.qjmed.a030133. [DOI] [PubMed] [Google Scholar]
- Popa ER, Stegeman CA, Kallenberg CG, Tervaert JW. Staphylococcus aureus and Wegener's granulomatosis. Arthritis Res. 2002;4:77–79. doi: 10.1186/ar392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdulahad WH, Stegeman CA, Limburg PC, Kallenberg CG. CD4-positive effector memory T cells participate in disease expression in ANCA-associated vasculitis. Ann N Y Acad Sci. 2007;1107:22–31. doi: 10.1196/annals.1381.003. [DOI] [PubMed] [Google Scholar]
- Mayet WJ, Marker-Hermann E, Schlaak J, Meyer Zum Büschenfelde KH. Irregular cytokine pattern of CD4+ T lymphocytes in response to Staphylococcus aureus in patients with Wegener's granulomatosis. Scand J Immunol. 1999;49:585–594. doi: 10.1046/j.1365-3083.1999.00544.x. [DOI] [PubMed] [Google Scholar]
- Laudien M, Häsler R, Wohlers J, Böck J, Lipinski S, Bremer L, Podschun R, Ambrosch P, Lamprecht P, Rosenstiel P, Till A. Molecular signatures of a disturbed nasal barrier function in the primary tissue of Wegener's granulomatosis. Mucosal Immunol. 2011;4:564–573. doi: 10.1038/mi.2011.9. [DOI] [PubMed] [Google Scholar]
- Kain R, Exner M, Brandes R, Ziebermayr R, Cunningham D, Alderson CA, Davidovits A, Raab I, Jahn R, Ashour O, Spitzauer S, Sunder-Plassmann G, Fukuda M, Klemm P, Rees AJ, Kerjaschki D. Molecular mimicry in pauci-immune focal necrotizing glomerulonephritis. Nat Med. 2008;14:1088–1096. doi: 10.1038/nm.1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pendergraft WF III, Preston GA, Shah RR, Tropsha A, Carter CW Jr, Jennette JC, Falk RJ. Autoimmunity is triggered by cPR-3(105-201), a protein complementary to human autoantigen proteinase-3. Nat Med. 2004;10:72–79. doi: 10.1038/nm968. [DOI] [PubMed] [Google Scholar]
- Tosi MF. Innate immune responses to infection. J Allergy Clin Immunol. 2005;116:241–249. doi: 10.1016/j.jaci.2005.05.036. [DOI] [PubMed] [Google Scholar]
- Medzhitov R, Janeway C Jr. Innate immunity. N Engl J Med. 2000;343:338–344. doi: 10.1056/NEJM200008033430506. [DOI] [PubMed] [Google Scholar]
- Csernok E, Szymkowiak CH, Mistry N, Daha MR, Gross WL, Kekow J. Transforming growth factor-beta (TGF-beta) expression and interaction with proteinase 3 (PR3) in anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis. Clin Exp Immunol. 1996;105:104–111. doi: 10.1046/j.1365-2249.1996.d01-715.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krumbholz M, Specks U, Wick M, Kalled SL, Jenne D, Meinl E. BAFF is elevated in serum of patients with Wegener's granulomatosis. J Autoimmun. 2005;25:298–302. doi: 10.1016/j.jaut.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Torheim EA, Yndestad A, Bjerkeli V, Halvorsen B, Aukrust P, Froland SS. Increased expression of chemokines in patients with Wegener's granulomatosis - modulating effects of methylprednisolone in vitro. Clin Exp Immunol. 2005;140:376–383. doi: 10.1111/j.1365-2249.2005.02770.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamprecht P, Kumanovics G, Mueller A, Csernok E, Komocsi A, Trabandt A, Gross WL, Schnabel A. Elevated monocytic IL-12 and TNF-alpha production in Wegener's granulomatosis is normalized by cyclophosphamide and corticosteroid therapy. Clin Exp Immunol. 2002;128:181–186. doi: 10.1046/j.1365-2249.2002.01801.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludviksson BR, Sneller MC, Chua KS, Talar-Williams C, Langford CA, Ehrhardt RO, Fauci AS, Strober W. Active Wegener's granulomatosis is associated with HLA-DR+ CD4+ T cells exhibiting an unbalanced Th1-type T cell cytokine pattern: reversal with IL-10. J Immunol. 1998;160:3602–3609. [PubMed] [Google Scholar]
- Coulomb-L'Hermine A, Capron F, Zou W, Piard F, Galateau F, Laurent P, Crevon MC, Galanaud P, Emilie D. Expression of the chemokine RANTES in pulmonary Wegener's granulomatosis. Hum Pathol. 2001;32:320–326. doi: 10.1053/hupa.2001.22757. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Huang D, Farver C, Hoffman GS. Relative importance of CCR5 and antineutrophil cytoplasmic antibodies in patients with Wegener's granulomatosis. J Rheumatol. 2003;30:1541–1547. [PubMed] [Google Scholar]
- Richter AG, Stockley RA, Harper L, Thickett DR. Pulmonary infection in Wegener granulomatosis and idiopathic pulmonary fibrosis. Thorax. 2009;64:692–697. doi: 10.1136/thx.2008.110445. [DOI] [PubMed] [Google Scholar]
- Hellmich B, Flossmann O, Gross WL, Bacon P, Cohen-Tervaert JW, Guillevin L, Jayne D, Mahr A, Merkel PA, Raspe H, Scott DG, Witter J, Yazici H, Luqmani RA. EULAR recommendations for conducting clinical studies and/or clinical trials in systemic vasculitis: focus on anti-neutrophil cytoplasm antibody-associated vasculitis. Ann Rheum Dis. 2007;66:605–617. doi: 10.1136/ard.2006.062711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhold-Keller E, Kekow J, Schnabel A, Schmitt WH, Heller M, Beigel A, Duncker G, Gross WL. Influence of disease manifestation and antineutrophil cytoplasmic antibody titer on the response to pulse cyclophosphamide therapy in patients with Wegener's granulomatosis. Arthritis Rheum. 1994;37:919–924. doi: 10.1002/art.1780370622. [DOI] [PubMed] [Google Scholar]
- Luqmani RA, Bacon PA, Moots RJ, Janssen BA, Pall A, Emery P, Savage C, Adu D. Birmingham Vasculitis Activity Score (BVAS) in systemic necrotizing vasculitis. QJM. 1994;87:671–678. [PubMed] [Google Scholar]
- Exley AR, Bacon PA, Luqmani RA, Kitas GD, Gordon C, Savage CO, Adu D. Development and initial validation of the Vasculitis Damage Index for the standardized clinical assessment of damage in the systemic vasculitides. Arthritis Rheum. 1997;40:371–380. doi: 10.1002/art.1780400222. [DOI] [PubMed] [Google Scholar]
- Luqmani RA. Assessing disease activity in the systemic vasculitides. Curr Opin Rheumatol. 2002;14:23–28. doi: 10.1097/00002281-200201000-00005. [DOI] [PubMed] [Google Scholar]
- Reinhold-Keller E, Beuge N, Latza U, De Groot K, Rudert H, Nolle B, Heller M, Gross WL. An interdisciplinary approach to the care of patients with Wegener's granulomatosis: long-term outcome in 155 patients. Arthritis Rheum. 2000;43:1021–1032. doi: 10.1002/1529-0131(200005)43:5<1021::AID-ANR10>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Fauci AS, Haynes BF, Katz P, Wolff SM. Wegener's granulomatosis: prospective clinical and therapeutic experience with 85 patients for 21 years. Ann Intern Med. 1983;98:76–85. doi: 10.7326/0003-4819-98-1-76. [DOI] [PubMed] [Google Scholar]
- Savige J, Gillis D, Benson E, Davies D, Esnault V, Falk RJ, Hagen EC, Jayne D, Jennette JC, Paspaliaris B, Pollock W, Pusey C, Savage CO, Silvestrini R, van der Woude F, Wieslander J, Wiik A. International Consensus Statement on Testing and Reporting of Antineutrophil Cytoplasmic Antibodies (ANCA) Am J Clin Pathol. 1999;111:507–513. doi: 10.1093/ajcp/111.4.507. [DOI] [PubMed] [Google Scholar]
- Holtfreter S, Grumann D, Schmudde M, Nguyen HT, Eichler P, Strommenger B, Kopron K, Kolata J, Giedrys-Kalemba S, Steinmetz I, Witte W, Broker BM. Clonal distribution of superantigen genes in clinical Staphylococcus aureus isolates. J Clin Microbiol. 2007;45:2669–2680. doi: 10.1128/JCM.00204-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachse F, von Eiff C, Stoll W, Becker K, Rudack C. Induction of CXC chemokines in A549 airway epithelial cells by trypsin and staphylococcal proteases - a possible route for neutrophilic inflammation in chronic rhinosinusitis. Clin Exp Immunol. 2006;144:534–542. doi: 10.1111/j.1365-2249.2006.03089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balding CE, Howie AJ, Drake-Lee AB, Savage CO. Th2 dominance in nasal mucosa in patients with Wegener's granulomatosis. Clin Exp Immunol. 2001;125:332–339. doi: 10.1046/j.1365-2249.2001.125002332.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyles JL, Roberts AW, Metcalf D, Wicks IP. Granulocyte colony-stimulating factor and neutrophils--forgotten mediators of inflammatory disease. Nat Clin Pract Rheumatol. 2006;2:500–510. doi: 10.1038/ncprheum0291. [DOI] [PubMed] [Google Scholar]
- Kawakami M, Tsutsumi H, Kumakawa T, Abe H, Hirai M, Kurosawa S, Mori M, Fukushima M. Levels of serum granulocyte colony-stimulating factor in patients with infections. Blood. 1990;76:1962–1964. [PubMed] [Google Scholar]
- Kawakami T, Ohashi S, Kawa Y, Takahama H, Ito M, Soma Y, Mizoguchi M. Elevated serum granulocyte colony-stimulating factor levels in patients with active phase of sweet syndrome and patients with active behcet disease: implication in neutrophil apoptosis dysfunction. Arch Dermatol. 2004;140:570–574. doi: 10.1001/archderm.140.5.570. [DOI] [PubMed] [Google Scholar]
- Nakamura H, Ueki Y, Sakito S, Matsumoto K, Yano M, Miyake S, Tominaga T, Tominaga M, Eguchi K. High serum and synovial fluid granulocyte colony stimulating factor (G-CSF) concentrations in patients with rheumatoid arthritis. Clin Exp Rheumatol. 2000;18:713–718. [PubMed] [Google Scholar]
- Ina K, Kusugami K, Hosokawa T, Imada A, Shimizu T, Yamaguchi T, Ohsuga M, Kyokane K, Sakai T, Nishio Y, Yokoyama Y, Ando T. Increased mucosal production of granulocyte colony-stimulating factor is related to a delay in neutrophil apoptosis in inflammatory bowel disease. J Gastroenterol Hepatol. 1999;14:46–53. doi: 10.1046/j.1440-1746.1999.01807.x. [DOI] [PubMed] [Google Scholar]
- Maianski NA, Mul FP, van Buul JD, Roos D, Kuijpers TW. Granulocyte colony-stimulating factor inhibits the mitochondria-dependent activation of caspase-3 in neutrophils. Blood. 2002;99:672–679. doi: 10.1182/blood.v99.2.672. [DOI] [PubMed] [Google Scholar]
- Hellmich B, Csernok E, Trabandt A, Gross WL, Ernst M. Granulocyte-macrophage colony-stimulating factor (GM-CSF) but not granulocyte colony-stimulating factor (G-CSF) induces plasma membrane expression of proteinase 3 (PR3) on neutrophils in vitro. Clin Exp Immunol. 2000;120:392–398. doi: 10.1046/j.1365-2249.2000.01205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallenberg CG, Stegeman CA, Heeringa P. Autoantibodies vex the vasculature. Nat Med. 2008;14:1018–1019. doi: 10.1038/nm1008-1018. [DOI] [PubMed] [Google Scholar]
- Schneider EM, Lorenz I, Ma X, Weiss M. G-CSF modulates LPS-induced apoptosis and IL-8 in human microvascular endothelial cells: involvement of calcium signaling. Ann N Y Acad Sci. 2003;1010:78–85. doi: 10.1196/annals.1299.012. [DOI] [PubMed] [Google Scholar]
- Leavey PJ, Sellins KS, Thurman G, Elzi D, Hiester A, Silliman CC, Zerbe G, Cohen JJ, Ambruso DR. In vivo treatment with granulocyte colony-stimulating factor results in divergent effects on neutrophil functions measured in vitro. Blood. 1998;92:4366–4374. [PubMed] [Google Scholar]
- Apostolakis S, Vogiatzi K, Amanatidou V, Spandidos DA. Interleukin 8 and cardiovascular disease. Cardiovasc Res. 2009;84:353–360. doi: 10.1093/cvr/cvp241. [DOI] [PubMed] [Google Scholar]
- Csernok E, Ernst M, Schmitt W, Bainton DF, Gross WL. Activated neutrophils express proteinase 3 on their plasma membrane in vitro and in vivo. Clin Exp Immunol. 1994;95:244–250. doi: 10.1111/j.1365-2249.1994.tb06518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouwer E, Huitema MG, Mulder AH, Heeringa P, van Goor H, Tervaert JW, Weening JJ, Kallenberg CG. Neutrophil activation in vitro and in vivo in Wegener's granulomatosis. Kidney Int. 1994;45:1120–1131. doi: 10.1038/ki.1994.149. [DOI] [PubMed] [Google Scholar]
- Hoffmann E, Dittrich-Breiholz O, Holtmann H, Kracht M. Multiple control of interleukin-8 gene expression. J Leukoc Biol. 2002;72:847–855. [PubMed] [Google Scholar]
- Damm M, Quante G, Rosenbohm J, Rieckmann R. Proinflammatory effects of Staphylococcus aureus exotoxin B on nasal epithelial cells. Otolaryngol Head Neck Surg. 2006;134:245–249. doi: 10.1016/j.otohns.2005.11.016. [DOI] [PubMed] [Google Scholar]
- Rudack C, Sachse F, Albert N, Becker K, von Eiff C. Immunomodulation of nasal epithelial cells by Staphylococcus aureus-derived serine proteases. J Immunol. 2009;183:7592–7601. doi: 10.4049/jimmunol.0803902. [DOI] [PubMed] [Google Scholar]
- Ullrich S, Gustke H, Lamprecht P, Gross WL, Schumacher U, Ambrosch P, Laudien M. Severely impaired respiratory ciliar function in Wegener's granulomatosis. Ann Rheum Dis. 2008;68:1067–1071. doi: 10.1136/ard.2008.096974. [DOI] [PubMed] [Google Scholar]
- Hui Y, Wohlers J, Podschun R, Hedderich J, Lamprecht P, Ambrosch P, Laudien M. Antimicrobial peptides in nasal secretion and mucosa with respect to S. aureus colonization in Wegener's granulomatosis. Clin Exp Rheumatol. 2011;29:49–56. [PubMed] [Google Scholar]
- Tan BK, Schleimer RP, Kern RC. Perspectives on the etiology of chronic rhinosinusitis. Curr Opin Otolaryngol Head Neck Surg. 2010;18:21–26. doi: 10.1097/MOO.0b013e3283350053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachse F, von Eiff C, Becker K, Rudack C. Anti-inflammatory effects of ciprofloxacin in S. aureus Newman induced nasal inflammation in vitro. J Inflamm (Lond) 2008;5:11. doi: 10.1186/1476-9255-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szekanecz Z, Vegvari A, Szabo Z, Koch AE. Chemokines and chemokine receptors in arthritis. Front Biosci (Schol Ed) 2010;2:153–167. doi: 10.2741/s53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwierzina H, Holzinger I, Gaggl S, Wolf H, Schollenberger S, Lam C, Bammer T, Geissler D, Lindley I. Recombinant human interleukin-8 restores function in neutrophils from patients with myelodysplastic syndromes without stimulating myeloid progenitor cells. Scand J Immunol. 1993;37:322–328. doi: 10.1111/j.1365-3083.1993.tb02560.x. [DOI] [PubMed] [Google Scholar]
- Iwase T, Uehara Y, Shinji H, Tajima A, Seo H, Takada K, Agata T, Mizunoe Y. Staphylococcus epidermidis Esp inhibits Staphylococcus aureus biofilm formation and nasal colonization. Nature. 2010;465:346–349. doi: 10.1038/nature09074. [DOI] [PubMed] [Google Scholar]


