Abstract
Introduction
The present study is a pilot prospective safety evaluation of a new closed loop computerised protocol on ventilation and oxygenation in stable, spontaneously breathing children weighing more than 7 kg, during the weaning phase of mechanical ventilation.
Methods
Mechanically ventilated children ready to start the weaning process were ventilated for five periods of 60 minutes in the following order: pressure support ventilation, adaptive support ventilation (ASV), ASV plus a ventilation controller (ASV-CO2), ASV-CO2 plus an oxygenation controller (ASV-CO2-O2) and pressure support ventilation again. Based on breath-by-breath analysis, the percentage of time with normal ventilation as defined by a respiratory rate between 10 and 40 breaths/minute, tidal volume > 5 ml/kg predicted body weight and end-tidal CO2 between 25 and 55 mmHg was determined. The number of manipulations and changes on the ventilator were also recorded.
Results
Fifteen children, median aged 45 months, were investigated. No adverse event and no premature protocol termination were reported. ASV-CO2 and ASV-CO2-O2 kept the patients within normal ventilation for, respectively, 94% (91 to 96%) and 94% (87 to 96%) of the time. The tidal volume, respiratory rate, peak inspiratory airway pressure and minute ventilation were equivalent for all modalities, although there were more automatic setting changes in ASV-CO2 and ASV-CO2-O2. Positive end-expiratory pressure modifications by ASV-CO2-O2 require further investigation.
Conclusion
Over the short study period and in this specific population, ASV-CO2 and ASV-CO2-O2 were safe and kept the patient under normal ventilation most of the time. Further research is needed, especially for positive end-expiratory pressure modifications by ASV-CO2-O2.
Trial registration
ClinicalTrials.gov: NCT01095406
Introduction
The worldwide increase in patient complexity, more concern for quality and safety, and a shortage of resources are the challenging terms of the equation many physicians and respiratory therapists have to face nowadays [1-3]. Mechanical ventilation is one of the life-saving techniques used at the bedside that is also associated with complications, including ventilator-induced lung injury and ventilator-associated pneumonia [4,5]. To decrease the duration of mechanical ventilation, there is evidence suggesting that the use of a written protocol is helpful [6]. Writing and reading protocols are time consuming, resulting in fluctuation in protocol implementation and compliance; instructions cannot be explicit enough, resulting in variable interpretation of the protocol; and they are specific to one organisation, making transfer to another institution difficult. To overcome these limiting factors, protocols have been computerised and there is convincing evidence showing that computerised protocols may help to manage and wean adult patients [7,8] and children with severe lung diseases [9,10].
A new computerised protocol with closed loop control of ventilation and oxygenation (IntelliVent®; Hamilton Medical AG, Bonaduz, Switzerland) has recently become available and has been investigated in adult patients [11]. The present study is a pilot prospective evaluation of this new closed loop computerised protocol in stable mechanically ventilated children during the weaning phase.
Materials and methods
The study - which was approved by the Sainte Justine Hospital ethical committee (Montreal, Canada) and by Health Canada and was registered on clinicaltrials.gov (NCT01095406) - was realised between January 2010 and January 2011 in the paediatric ICU of the Sainte Justine Hospital, Montreal, Canada. Signed informed consent was obtained from legal representatives as well as consent for publication of this manuscript and accompanying images.
Patients
During the study period, all consecutive admitted critically ill children under mechanical ventilation for at least 12 hours, younger than 18 years old, having predicted body weight (PBW) ≥ 3 kg and body mass index < 40 kg/m2 were screened daily for the study 5 days a week (Monday to Friday). PBW was the weight at the 50th percentile for age and sex from the National Center of Health Statistics growth chart.
The study was in essence a pilot investigation and was designed primarily to evaluate the safety of the new computerised protocol. Children were therefore included if they fulfilled the following criteria: able to breathe spontaneously; ventilated with proximal airway plateau pressure ≤ 25 cmH2O, positive end-expiratory pressure (PEEP) ≤ 8 cmH2O, with fraction of inspired oxygen (FiO2) ≤ 60% to keep oxygen saturation from pulse oxymetry (SpO2) ≥ 95%, and arterial partial pressure of CO2 < 70 mmHg on the last arterial blood gas; endotracheal tube leakage < 20% of the inspired tidal volume (VT), because the new computerised protocol requires correct information on VT; arterial partial pressure of CO2 versus partial pressure in end-tidal CO2 (PEtCO2) difference ≤ 7 mmHg; or not receiving muscle relaxant, vasopressor or inotropic medication (except dopamine < 5 μg/kg/minute).
Children with a previous history of mechanical ventilation (more than 1 month) or tracheotomy, severe neuromuscular disease, cyanotic congenital heart disease and primary pulmonary hypertension were excluded, as well as brain-death children and children receiving palliative care.
An analysis was planned after the inclusion of five patients (run-in phase) because there was no clinical experience of this mode of ventilation. The analysis of the run-in phase showed that children with PBW < 7 kg had an increase in minute ventilation (MV) because of technical reasons (high apparatus dead space with the S1 device due to the proximal flow sensor + mainstream CO2 analyser in series). The investigators decided to only include children with PBW > 7 kg. The four patients under 7 kg PBW and already included in the run-in phase were not analysed, although they went through the study without adverse effects (see Safety evaluation below).
Study design
All included patients were prospectively enrolled in a sequential nonrandomised study during which they received five consecutive 60-minute periods of ventilation (Figure 1): pressure support ventilation (PSV) using the ventilator already connected to the patient (Servo-i; Maquet Gmbh & Co. KG, Rastatt, Germany) with the level of inspiratory pressure (Pinsp) set by the attending intensivist prior to the inclusion (PSV_before); adaptive support ventilation (ASV) using a functional S1 prototype ventilator (Hamilton Medical AG); ASV with the CO2 controller activated (ASV-CO2); ASV with the CO2 and the O2 controller activated (ASV-CO2-O2); and PSV using the Servo-i ventilator at the initial level of support (PSV_after).
Figure 1.
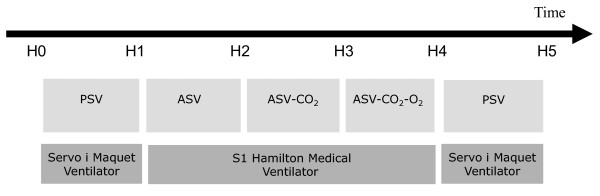
Study protocol. Included patients were prospectively enrolled in a sequential study during which they received five consecutive 1-hour periods of ventilation. PSV, pressure support mode; ASV, adaptive support ventilation mode; ASV-CO2, ASV and CO2 controller; ASV-CO2-O2, ASV-CO2 and oxygen controller.
Throughout the ventilation period with the S1 device and for safety reasons, a trained physician remained at the bedside.
The PSV mode was given with the servo-i ventilator because the S1 device had only the new computerised protocol and ASV inside. The ventilation modes were not randomised as we had to ventilate the children in PSV before and at the end of the study, and randomising the modes would require changing the ventilator in a randomised order as well, which was not allowed for safety reasons.
Ventilatory modalities
ASV is a ventilatory modality commonly used in adults [11,12] where, for a given MV set by the user according to the clinical conditions and arterial blood gases, the ventilator automatically selects an optimal combination of respiratory rate (RR) and VT based on the breath-by-breath estimation of the expiratory time constant. The level of support (mandatory rate and Pinsp) is automatically adjusted on a breath-by-breath basis to keep the patient as close as possible to the optimal RR and VT. If the patient breathes spontaneously, this mode works as a volume target-pressure regulated mode with a user-set MV guarantee. If not, the ASV mode works as a pressure control mode with a prescribed MV. In ASV, the MV is the parameter that is controlled and set by the user. To further control the VT (and more specifically to reduce the VT) or to limit Pinsp, the user must decrease the MV and the maximum Pinsp allowed (pressure limitation). At the bench, in doing so it has been recently shown that ASV was able to generate lower VT and Pinsp compared with the ARDSnetwork recommendations [13]. However, in some clinical studies when the maximum allowed pressure is permissive (60 cmH2O), high VT has been reported with ASV [14]. In our study, MV in ASV was set to match the MV measured during PSV_before. PEEP and FiO2 were set as during PSV_before. The pressure limitation was set to limit the Pinsp to 25 cmH2O.
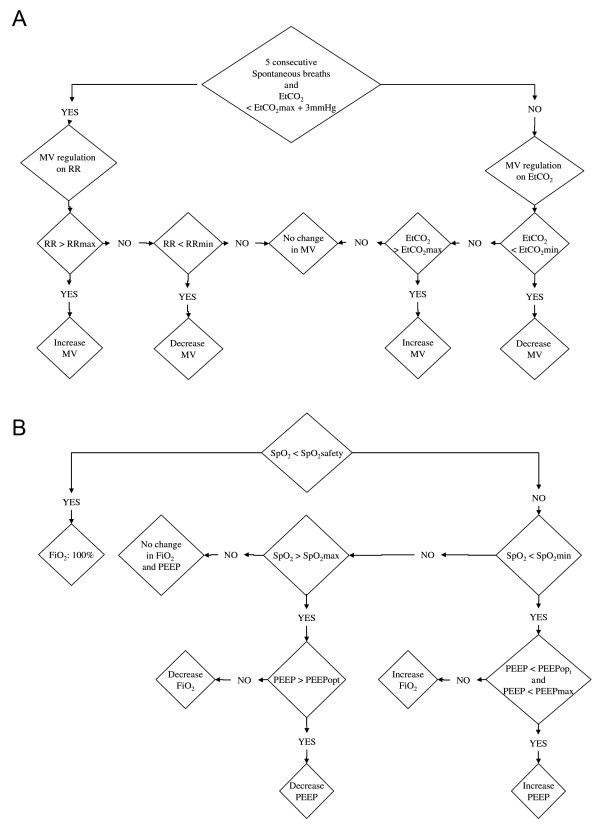
ASV-CO2 is an evolution of ASV where MV is not set by the user but is automatically adjusted to keep the patient within ranges of RR and PEtCO2, captured at the proximal airway using the S1 flow sensor (PN 279331, single-use flow sensor linear between -120 and 120 l/minute with ± 5% error of measure; Hamilton Medical AG) and a mainstream CO2 analyser (Capnostat 5, mainstream sensor with accuracy ± 5% for PEtCO2 between 41 and 70 mmHg, dead space 5 ml; Hamilton Medical AG). The target range of RR is defined by a lower limit based on the Otis concept of optimal RR to minimise the work of breathing [15], and an upper limit depending on the actual MV; the higher the MV, the higher the upper RR limit. Pinsp increases when the patient RR is above the upper RR limit; Pinsp decreases when the patient RR is below the lower RR limit. Filtered PEtCO2 is present in the background to keep the patient below the upper PEtCO2 acceptable value (Figure 2). Ventilation changes are made on a breath-by-breath basis.
Figure 2.
Functional algorithm of the ventilation and oxygen controller. (A) Ventilation controller. The partial pressure in end-tidal CO2 (PEtCO2) values are given with the quality index and derived from the mainstream CO2 sensor with proprietary algorithms as a surrogate of arterial partial pressure of CO2. PEtCO2min and PEtCO2max are adjustable by the user and depend on the patient's severity estimated by the level of inspiratory pressure; that is, the higher the inspiratory pressure and the more permissive the PEtCO2 limits. As an example and by default for an inspiratory pressure of 10 cmH2O, PEtCO2min is 35 mmHg and PEtCO2max is 41 mmHg. (B) Oxygen controller. The oxygen saturation from pulse oxymetry (SpO2) limits (SpO2safety and SpO2min) are adjustable by the user and depending on the patient's severity estimated by the positive end-expiratory pressure (PEEP) level; that is, the higher the PEEP level and the more permissive the SpO2 limits. As an example and by default for a PEEP level of 5 cmH2O, SpO2safety is 88%, SpO2min is 93% and SPO2max is 98%. The PEEPopt is defined according to a PEEP-fraction of inspired oxygen (FiO2) table and PEEPmax set by the user. The patient SpO2 is provided with a quality index and is derived from the pulse oxymeter with proprietary algorithms for artefact and motion rejections. MV, minute ventilation; RR, respiratory rate.
ASV-CO2-O2 includes the above-described ASV-CO2 regulation plus an automatic adjustment of PEEP and FiO2 based on SpO2 monitoring included in the ventilator. SpO2 is obtained from a finger probe using standard technology, plus proprietary filtering and quality assessment before entering the algorithm: when SpO2 is below a user-adjustable value, FiO2 or PEEP is increased (Figure 2). These ventilation changes are made on a breath-by-breath basis. The choice between FiO2 and PEEP increase is based on the ARDSnetwork tables [16] with user inputs regarding maximal and minimal PEEP values. The FiO2 controllers and the PEEP controller can be activated or deactivated independently. In the present study both controllers were activated, but according to local policies regarding PEEP settings the minimal PEEP and maximal PEEP were respectively set at 5 and 10 cmH2O.
The investigators remained at the bedside during the entire duration of the study and were allowed to switch back to manual adjustment of the settings. As for any mode of ventilation, the user has to set alarms for VT, for airway pressure as well as for monitoring parameters such as SpO2 and PEtCO2.
Data collection
Patient characteristics including demographic data, diagnosis, severity scores, clinical data at inclusion and outcomes were collected from the charts. When connected to the S1 device, monitoring values (including SpO2, PEtCO2 and mechanical ventilation monitoring), settings and alarms were recorded on a breath-by-breath basis using a dedicated data-logging system attached to the ventilator. Minute-to-minute data from the Servo-i were collected using a compact flash reader connected to the device.
Breath-by-breath data were analysed ex vivo to calculate the number of normal breaths as defined by 10 breaths/minute < RR < 40 breaths/minute, VT > 5 ml/kg PBW and 25 mmHg < PEtCO2 < 55 mmHg. The primary end point of the study was the percentage of time spent with normal ventilation, defined as the number of normal breaths out of the total number of breaths collected. During PSV_before and PSV_after, PEtCO2 was dropped from the definition as it was not recorded with the Servo-i ventilator.
The number of ventilation setting changes was compared between the five consecutive 60-minute periods of ventilation. A ventilation setting change corresponded to a modification of PEEP or FiO2 in any ventilation mode, a change of Pinsp in PSV or a change of minute volume in ASV, ASV-CO2 or ASV-CO2-O2.
Safety evaluation
Safety was evaluated by recording adverse events as defined by ISO 14155 standards (ISO 14971:2007, Medical devices -- Application of risk management to medical devices) and by the number of switch back to PSV or to controlled ventilation by the intensivist present at the bedside, whatever the reason. The protocol was terminated and the child ventilated with the previous conventional ventilation if a sustained change was demonstrated in any of the following: decrease in SpO2 < 92% requiring increase in FIO2 > 60%; increase in PEtCO2 above the value before connection with the S1 device, requiring an increase of positive Pinsp > 25 cmH2O above PEEP; increase in heart rate > 180 beats/minute for 15 minutes; increase in RR > 60 breaths/minute for 15 minutes; or uncontrolled agitation.
Sample size
There were no previously existing data on the percentage of time spent with normal ventilation in children during mechanical ventilation. A similar study in adults observed that patients in PSV mode spent 66 ± 23% in the normal ventilation range compared with 93 ± 8% of patients ventilated with a closed loop computerised protocol [17]. From this study [17], we calculated that 16 patients would be required to find a statistical difference for such a difference with an α risk of 0.05 and a β risk of 0.9.
Statistical analysis
As the parameters studied did not have a normal distribution, data are shown as the median with 25% and 75% interquartile ranges. Medians were compared using a Kruskal-Wallis one-way analysis of variance on ranks and paired tests when P < 0.05 using Sigma Stats for Windows version 3.5 (Systat Software, Inc., Richmond, CA, US).
Results
During the study period, 196 patients were screened and 15 patients with PBW > 7 kg were included. These 15 patients were ventilated for a median of 55 hours before inclusion (Table 1). One patient (Patient 12, Table 1) did not undergo the ventilation periods with the S1 device due to the use of a neonatal flow sensor by mistake, which did not allow ventilating in the ASV mode, and was therefore excluded from analysis. The remaining 14 patients went through the study without side effects, without switching to PSV or controlled ventilation, or without early protocol termination when they were ventilated with the S1 ventilator.
Table 1.
Clinical description of the patients at inclusion
| Patient | Age (months) | PBW (kg) | PIM2 (%) | PELOD | Main diagnosis | Data at inclusion | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| MV duration (hours) | Ppeak (cmH2O) | SpO2 (%) | FiO 2 | PEEP (cmH2O) | PaCO2 (mmHg) | ||||||
| 1 | 36 | 12 | 0 | 12 | Rhabdomyosarcoma | 21 | 15 | 100 | 0.25 | 5 | 36 |
| 2 | 195 | 57 | 0 | 2 | Scoliosis-encephalopathy | 26 | 13 | 98 | 0.25 | 5 | 34 |
| 3 | 93 | 27 | 3 | 2 | Polytraumatism | 240 | 15 | 98 | 0.30 | 5 | 40 |
| 4 | 28 | 14 | 2 | 3 | Liver transplantation | 72 | 25 | 98 | 0.40 | 7 | 46 |
| 5 | 59 | 17 | 0 | 1 | Cranio-facial surgery | 56 | 17 | 98 | 0.25 | 5 | 47 |
| 6 | 22 | 14 | 3 | 1 | Laryngitis | 44 | 15 | 96 | 0.35 | 5 | 38 |
| 7 | 187 | 38 | 0 | 2 | Congenital scoliosis | 55 | 21 | 99 | 0.25 | 5 | 39 |
| 8 | 29 | 10 | 3 | 2 | Septic shock | 203 | 26 | 95 | 0.40 | 5 | 48 |
| 9 | 32 | 14 | 1 | 3 | Encephalitis | 46 | 15 | 100 | 0.30 | 5 | 38 |
| 10 | 54 | 16 | 0 | 1 | Thyreoglosse cyst | 31 | 15 | 99 | 0.45 | 5 | 39 |
| 11 | 9 | 7 | 3 | 1 | Severe laryngitis | 41 | 18 | 98 | 0.35 | 5 | 41 |
| 12 | 62 | 18 | 1 | 11 | Pharyngeal abscess | 38 | 17 | 98 | 0.21 | 5 | 37 |
| 13 | 45 | 15 | 13 | 1 | Aspiration-encephalopathy | 235 | 11 | 98 | 0.30 | 5 | 59 |
| 14 | 16 | 10 | 3 | 14 | Encephalitis | 66 | 15 | 100 | 0.25 | 5 | 29 |
| 15 | 165 | 49 | 2 | 12 | Encephalopathy | 75 | 15 | 98 | 0.30 | 5 | 40 |
| Median | 45 | 15 | 2 | 2 | 55 | 15 | 98 | 0.30 | 5 | 39 | |
| IQR25 | 29 | 13 | 0 | 1 | 40 | 15 | 98 | 0.25 | 5 | 38 | |
| IQR75 | 78 | 23 | 3 | 7 | 73 | 18 | 99 | 0.35 | 5 | 43 | |
PBW, body weight; PIM2, Paediatric Index of Mortality (percentage of risk of death) [29]; PELOD, Paediatric Logistic Organ Dysfunction score [30]; MV, mechanical ventilation; Ppeak, peak pressure at the proximal airway; SpO2, pulse oxygen saturation; FiO2, inspired oxygen fraction; PEEP, positive end-expiratory pressure; PaCO2, arterial partial pressure of CO2; IQR25 and IQR75, 25% and 75% interquartile range.
Compared with PSV_before (93% (79 to 95%)) and PSV_after (96% (83 to 100%)), ASV, ASV-CO2 and ASV-CO2-O2 kept the patients within normal ventilation for a similar percentage of time (respectively: 93% (80 to 95%), 94% (91 to 96%) and 94% (87 to 96%)) (Table 2).
Table 2.
Time spent with normal ventilation, ventilation pattern, peak airway pressure, PEtCO2 and SpO2
| PSV_before | ASV | ASV-CO 2 | ASV-CO 2 -O 2 | PSV_after | P value | |
|---|---|---|---|---|---|---|
| Normal ventilation (% of the recording time) | 93 (82 to 95) | 94 (82 to 96) | 95 (92 to 96) | 95 (89 to 96) | 97 (85 to 100) | NS |
| Number of ventilation setting changes/patient | 0 (0 to 0) | 0 (0 to 0) | 78 (58 to 119) | 81 (35 to 250) | 0 (0 to 0) | < 0.001* |
| Respiratory rate (breaths/minute) | 17 (14 to 21) | 21 (18 to 24) | 23 (19 to 25) | 22 (18 to 27) | 20 (16 to 26) | NS |
| Tidal volume (ml/kg) | 8.3 (7.1 to 9.3) | 8.7 (7.7 to 9.7) | 8.9 (8.2 to 9.7) | 8.4 (7.8 to 9.7) | 7.9 (6.7 to 8.9) | NS |
| Paw-peak (cmH2O) | 16 (15 to 18) | 15 (14 to 22) | 16 (14 to 19) | 17 (15 to 21) | 15 (14 to 19) | NS |
| PEtCO2 (mmHg) | - | 43 (39 to 45) | 43 (40 to 45) | 43 (39 to 47) | - | NS |
| SpO2 (%) | - | 98 (95 to 100) | 99 (96 to 100) | 98 (95 to 100) | - | NS |
Results of time spent with normal ventilation, ventilation pattern, peak airway pressure, partial pressure of end-tidal CO2 (PEtCO2) and pulse oxygen saturation (SpO2) during the five 1-hour periods investigated. Results given as the median with 25% to 75% interquartile range. Normal ventilation is the percentage of time with normal breath as defined by 10 breaths/minute < respiratory rate < 40 breaths/minute, tidal volume > 5 ml/kg predicted body weight and 25 mmHg < PEtCO2 < 55 mmHg. PSV, pressure support ventilation; ASV, adaptive support ventilation, ASV-CO2, ASV plus the CO2 controller; ASV-CO2-O2, ASV-CO2 plus the oxygen controller; Paw-peak, peak proximal airway pressure; NS, P > 0.05. *P < 0.001 for ASV versus ASV-CO2 and for ASV versus ASV-CO2-O2.
The VT, RR, peak inspiratory airway pressure (Paw-peak) and MV were equivalent with all modalities. PEtCO2 and SpO2 were also equivalent between ASV, ASV-CO2 and ASV-CO2-O2 (Table 2). The standard deviation to estimate the variability of Paw-peak was much higher with ASV, ASV-CO2 and ASV-CO2-O2 as compared with PSV (Figure 3). As compared with ASV, there were significantly more ventilator setting adjustments (automatic) in ASV-CO2 and ASV-CO2-O2 (77 (57 to 120) per patient and 80 (32 to 272) per patient, respectively) (Table 2).
Figure 3.
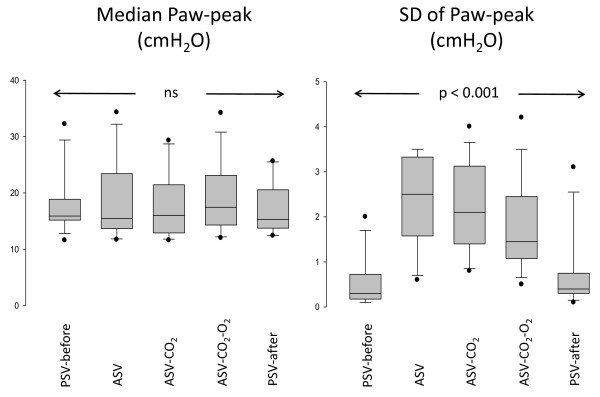
Peak airway pressure of children during the 1-hour periods of mechanical ventilation. Peak inspiratory airway pressure (Paw-peak; median and standard deviation (SD)) of the 14 children included during the five 1-hour periods of mechanical ventilation. The median values were not statistically different, but the SDs of individual breath-by-breath values (right panel) were significantly higher, suggesting more variability in adaptive support ventilation (ASV), ASV and CO2 controller (ASV-CO2) and ASV-CO2 and oxygen controller (ASV-CO2-O2) as compared with pressure support ventilation (PSV).
In four patients, during the 60-minute period with ASV-CO2-O2, an automatic increase of PEEP from 5 cmH2O to 7 to 9 cmH2O was observed due to a transient decrease in SpO2. The physician manually returned the PEEP to 5 cmH2O because it was not in his practice to increase PEEP in this situation.
The median total duration on the ventilator was 97 hours (55 to 207 hours) and the length of stay in the ICU was 5 days (4 to 13 days). At 28 days after the inclusion, all patients were alive, none was on mechanical ventilation and five patients remained in the hospital.
Discussion
ASV alone or combined with the automatic adjustment of minute volume was safe and provided a similar breath pattern as compared with PSV. This was achieved, however, with more ventilator automatic adjustments and more variability in Paw-peak as compared with ASV and PSV. The apparatus dead space with the S1 device due to the proximal flow sensor + mainstream CO2 analyser in series restrained the ventilation to children with body weight > 7 kg. As the patients were in a stable situation and not hypoxemic, little information was obtained regarding the oxygenation controller. In four patients, however, an automatic increase in PEEP was observed that was not considered usual practice by the physician in charge.
The present pilot study has several limitations that need to be addressed before raising some conclusions. First, comparing ASV, ASV-CO2 and ASV-CO2-O2 from the S1 device with PSV given with another ventilator is arguable. Even if the aim of the study was not to compare the performance of each mode in supporting the patient, this may indeed impact on the primary endpoint - that is, the time spent with normal ventilation, which was defined based on the RR, VT and PEtCO2. Furthermore, the data were acquired differently with the S1 device (breath by breath) as compared with the Maquet ventilator (minute by minute, equivalent to a filtering). PEtCO2 was also not part of the definition of normal breathing in PSV, and PEtCO2 is known to show high fluctuation on both sides in spontaneously breathing patients [18]. Whether minute-by-minute filtering and not having PEtCO2 in PSV are overestimating the time in normal ventilation during PSV is questionable. As the S1 ventilator did not have the PSV mode, however, the study had to be carried out with the methodology described above.
Second, the different modes of ventilation were given sequentially and without randomisation. The total duration of the study was 5 hours and very obviously the patient's conditions may change over such a long period. However, the patient's respiratory conditions in PSV_after as compared with PSV_before were not different (Table 2).
Third, the study was finally underpowered (14 patients analysed as compared with the 16 patients required by the power calculation). This was because of four patients weighing < 7 kg and not being analysed. In one patient the new computerised protocol was also unable to work because of the wrong flow sensor (neonatal flow sensor) being on the device. However, an increased number of patients would probably not achieve statistical significance as the observed time spent in normal ventilation during PSV mode was higher than expected (93% instead of 66% as expected from a previous adult study [17]).
Fourth, the different modes were investigated over a short period of time (60 minutes) and in a very specific subpopulation of stable patients during the weaning phase. It is indeed impossible to estimate the safety of such a closed loop computerised protocol in more critically ill patients. The clinical impact of such a mode of ventilation is also not addressed by the present study. These studies will be the next steps.
Finally, the PEEP controller - which is probably the most critical in terms of safety - was set to very conservative working conditions (minimal PEEP 5 cmH2O and maximal PEEP 10 cmH2O). In four patients an increase of PEEP from 5 cmH2O to 7 to 9 cmH2O was observed due to a transient decrease in SpO2. The physician manually returned the PEEP to 5 cmH2O because it was not in his practice to increase PEEP in this situation. The present study is therefore not able to raise any conclusions regarding the PEEP closed loop computerised protocol, and further data are needed on the optimal PEEP during the weaning phase.
The present pilot study is therefore exploratory and can only indicate that in this stable population, over a short period of time (60 minutes) and within the experimental conditions (PEEP limited and manually controlled), ASV-CO2 and ASV-CO2-O2 were safe. Additional studies are definitely required before raising any conclusion on safety or effectiveness during longer period of ventilation and in patients with more critical conditions.
Interesting observations can be raised, however, from the present study. First, in babies with body weight < 7 kg, the apparatus dead space (proximal flow sensor in series with the PEtCO2 sensor) was relatively high, inducing an increase in MV as compared with PSV. As already shown in adult patients, the dead-space-related increase in MV and subsequent changes in the patient's optimal RR and VT combination is hardly predictable [19]. As most of the children admitted to the paediatric ICU weigh < 7 kg [20], it is critical to reduce the apparatus dead space as much as possible before using such a computerised protocol.
Second, more ventilator automatic adjustments and more variability in Paw-peak were observed in ASV-CO2 and ASV-CO2-O2 as compared with ASV and PSV with ultimately equivalent PEtCO2. This is most probably due to the patients' subclinical changing conditions that are taken into account by ASV-CO2 and not with ASV or PSV. More variable ventilation may have an impact on lung recruitment, as suggested in atelectatic animal models [21,22], and may have an impact on patient comfort, as suggested by studies on variable modes using pressure support [23,24].
Third, although designed for children and adult patients, and already on the market for 10 years, publications are lacking on ASV in children. In the very first publication from Laubscher and coworkers in 1994 [25], some paediatric patients were included but ASV was applied only for a couple of minutes to test the start-up procedure in selecting the VT and RR. To our best knowledge, ASV has never been compared with PSV in the paediatric population. Bearing in mind the abovementioned limitations and publications from adult populations reporting higher VT with ASV when the pressure limitation is high [14], it is worth noting that breath patterns with both modes were not drastically different. Once again, this would deserve additional investigation.
Finally, although not really challenged by the population enrolled in the study, an automatic control of FiO2 based on SpO2 has been already investigated in neonates [26,27] and in adults [28], with increased time spent with adequate SpO2 when compared with usual care. The present study is in line with these previously published results.
Conclusion
The present pilot prospective study shows the feasibility of using a closed loop computerised protocol on ventilation and oxygenation over a short time period in stable, spontaneously breathing paediatric patients starting the weaning process. Over the study period, children with body weight > 7 kg were kept with normal ventilation most of the time and no safety issues were reported. Future studies should be designed to address safety and effectiveness of such a closed loop computerised protocol as compared with conventional ventilation in children with more challenging respiratory conditions and over a longer period of time.
Key messages
• A closed loop computerised protocol seems feasible in paediatric ventilated patients during the weaning phase.
• Compared with PSV, the closed loop computerised protocol may keep patients within normal ventilation for a similar percentage of time.
• Compared with PSV, the closed loop computerised protocol seems to generate more airway pressure variability.
• Compared with PSV, the closed loop computerised protocol may adjust the ventilator more often.
Abbreviations
ASV: adaptive support ventilation; ASV-CO2: adaptive support ventilation with computerised protocol on ventilation; ASV-CO2-O2: adaptive support ventilation with computerised protocol on oxygenation; CO2: carbon dioxide; FiO2: fraction of inspired oxygen; MV: minute ventilation; O2: oxygen; Paw-peak: peak inspiratory airway pressure; PBW: predicted body weight; PEEP: positive end-expiratory pressure; PEtCO2: partial pressure in end-tidal CO2; Pinsp: inspiratory airway pressure; PSV: pressure support ventilation; RR: respiratory rate; SpO2: oxygen saturation from pulse oxymetry; VT: tidal volume.
Competing interests
Intellivent® and an S1 respirator were provided for research purposes by Hamilton Medical. PJ was invited twice to present the results of the clinical research on IntelliVent® at international meetings organised by Hamilton Medical without any honoraria. PJ also has a respirator Servo-i and an Evita XL, provided for research purposes by Maquet Medical (Solna, Sweden) and Drager Medical (Lübeck, Germany), respectively. PJ receives a research salary from the Fonds de Recherche du Québec - Santé in respiratory critical care. PJ's research on clinical decision support systems is funded by a public grant from the Natural Sciences and Engineering Research Council of Canada. MW was the director of the Research and Development department of Hamilton Medical until the end of 2011. MW co-shares a patent on IntelliVent® (WO/2007/085110 and WO/2007/085108). RLG is a medical research engineer employed by Hamilton Medical. The other authors declare they have no competing interests. These declarations do not mean that the interpretation of data and presentation of information was influenced by these relationships. This is fair research in collaboration with industry to obtain a final product helpful for children.
Authors' contributions
PJ was the principal investigator of the study, designed the study, was at the bedside for data collection, analysed the results, performed the statistics and wrote the manuscript. AE, VP and AB were co-investigators, screening the patients for inclusion and collecting the data at the bedside. GE was a co-investigator, discussing the study design and reviewing the manuscript. RLG was research engineer for Hamilton Medical and in charge of the logistics of the study, designed and realised the macro for breath-by-breath analysis, and was in charge of storing and securing the data. MW was head of medical research when the study started, was involved in the research plan and in discussing the data and the results of the study, and was involved in finalising the manuscript before the submission. All authors read and approved the final manuscript.
Contributor Information
Philippe Jouvet, Email: philippe.jouvet@umontreal.ca.
Allen Eddington, Email: allen_eddington@hotmail.com.
Valérie Payen, Email: payen.v@gmail.com.
Alice Bordessoule, Email: alicebordessoule@gmail.com.
Guillaume Emeriaud, Email: guillaume.emeriaud@gmail.com.
Ricardo Lopez Gasco, Email: rlopezgasco@yahoo.es.
Marc Wysocki, Email: marcwysocki@gmail.com.
References
- Mathews SC, Pronovost PJ. The need for systems integration in health care. JAMA. 2011;305:934–935. doi: 10.1001/jama.2011.237. [DOI] [PubMed] [Google Scholar]
- Higgins AM, Pettila V, Bellomo R, Harris AH, Nichol AD, Morrison SS. Expensive care - a rationale for economic evaluations in intensive care. Crit Care Resusc. 2010;12:62–66. [PubMed] [Google Scholar]
- Hill AD, Fan E, Stewart TE, Sibbald WJ, Nauenberg E, Lawless B, Bennett J, Martin CM. Critical care services in Ontario: a survey-based assessment of current and future resource needs. Can J Anaesth. 2009;56:291–297. doi: 10.1007/s12630-009-9055-4. [DOI] [PubMed] [Google Scholar]
- Ricard JD, Dreyfuss D, Saumon G. Ventilator-induced lung injury. Curr Opin Crit Care. 2002;8:12–20. doi: 10.1097/00075198-200202000-00003. [DOI] [PubMed] [Google Scholar]
- Chastre J, Fagon JY. Ventilator-associated pneumonia. Am J Respir Crit Care Med. 2002;165:867–903. doi: 10.1164/ajrccm.165.7.2105078. [DOI] [PubMed] [Google Scholar]
- Ely EW, Baker AM, Dunagan DP, Burke HL, Smith AC, Kelly PT, Johnson MM, Browder RW, Bowton DL, Haponik EF. Effect on the duration of mechanical ventilation of identifying patients capable of breathing spontaneously. N Engl J Med. 1996;335:1864–1869. doi: 10.1056/NEJM199612193352502. [DOI] [PubMed] [Google Scholar]
- Lellouche F, Mancebo J, Jolliet P, Roeseler J, Schortgen F, Dojat M, Cabello B, Bouadma L, Rodriguez P, Maggiore S, Reynaert M, Mersmann S, Brochard L. A multicenter randomized trial of computer-driven protocolized weaning from mechanical ventilation. Am J Respir Crit Care Med. 2006;174:894–900. doi: 10.1164/rccm.200511-1780OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- East TD, Heermann LK, Bradshaw RL, Lugo A, Sailors RM, Ershler L, Wallace CJ, Morris AH, McKinley B, Marquez A, Tonnesen A, Parmley L, Shoemaker W, Meade P, Thaut P, Hill T, Young M, Baughman J, Olterman M, Gooder V, Quinn B, Summer W, Valentine V, Carlson J, Steinberg K. Efficacy of computerized decision support for mechanical ventilation: results of a prospective multi-center randomized trial. Proc AMIA Symp. 1999. pp. 251–255. [PMC free article] [PubMed]
- Jouvet P, Farges C, Hatzakis G, Monir A, Lesage F, Dupic L, Brochard L, Hubert P. Weaning children from mechanical ventilation with a computer-driven system (closed-loop protocol): a pilot study. Pediatr Crit Care Med. 2007;8:425–432. doi: 10.1097/01.PCC.0000282157.77811.F9. [DOI] [PubMed] [Google Scholar]
- Jouvet P, Hernert P, Wysocki M. Development and implementation of explicit computerized protocols for mechanical ventilation in children. Ann Intensive Care. 2011;1:51–60. doi: 10.1186/2110-5820-1-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnal JM, Wysocki M, Novotni D, Demory D, Lopez R, Donati S, Granier I, Corno G, Durand-Gasselin J. Safety and efficacy of a fully closed-loop control ventilation (IntelliVent-ASV®) in sedated ICU patients with acute respiratory failure: a prospective randomized crossover study. Intensive Care Med. 2012;38:781–787. doi: 10.1007/s00134-012-2548-6. [DOI] [PubMed] [Google Scholar]
- Arnal JM, Wysocki M, Nafati C, Donati S, Granier I, Corno G, Durand-Gasselin J. Automatic selection of breathing pattern using adaptive support ventilation. Intensive Care Med. 2008;34:75–81. doi: 10.1007/s00134-007-0847-0. [DOI] [PubMed] [Google Scholar]
- Sulemanji D, Marchese A, Garbarini P, Wysocki M, Kacmarek RM. Adaptive support ventilation: an appropriate mechanical ventilation strategy for acute respiratory distress syndrome? Anesthesiology. 2009;111:863–870. doi: 10.1097/ALN.0b013e3181b55f8f. [DOI] [PubMed] [Google Scholar]
- Dongelmans DA, Paulus F, Veelo DP, Binnekade JM, Vroom MB, Schultz MJ. Adaptive support ventilation may deliver unwanted respiratory rate-tidal volume combinations in patients with acute lung injury ventilated according to an open lung concept. Anesthesiology. 2011;114:1138–1143. doi: 10.1097/ALN.0b013e31820d8676. [DOI] [PubMed] [Google Scholar]
- Otis AB, Fenn WO, Rahn H. Mechanics of breathing in man. J Appl Physiol. 1950;2:592–607. doi: 10.1152/jappl.1950.2.11.592. [DOI] [PubMed] [Google Scholar]
- Brower RG, Lanken PN, MacIntyre N, Matthay MA, Morris A, Ancukiewicz M, Schoenfeld D, Thompson BT. National Heart, Blood Institute ARDS Clinical Trials Network. Higher versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome. N Engl J Med. 2004;351:327–336. doi: 10.1056/NEJMoa032193. [DOI] [PubMed] [Google Scholar]
- Dojat M, Harf A, Touchard D, Lemaire F, Brochard L. Clinical evaluation of a computer-controlled pressure support mode. Am J Respir Crit Care Med. 2000;161:1161–1166. doi: 10.1164/ajrccm.161.4.9904064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galia F, Brimioulle S, Bonnier F, Vandenbergen N, Dojat M, Vincent JL, Brochard LJ. Use of maximum end-tidal CO2 values to improve end-tidal CO2 monitoring accuracy. Respir Care. 2011;56:278–283. doi: 10.4187/respcare.00837. [DOI] [PubMed] [Google Scholar]
- Jaber S, Sebbane M, Verzilli D, Matecki S, Wysocki M, Eledjam JJ, Brochard L. Adaptive support and pressure support ventilation behavior in response to increased ventilatory demand. Anesthesiology. 2009;110:620–627. doi: 10.1097/ALN.0b013e31819793fb. [DOI] [PubMed] [Google Scholar]
- Payen V, Jouvet P, Lacroix J, Ducruet T, Gauvin F. Risk factors associated with increased length of mechanical ventilation in children. Pediatr Crit Care Med. 2012;13:152–157. doi: 10.1097/PCC.0b013e3182257a24. [DOI] [PubMed] [Google Scholar]
- Suki B, Alencar AM, Sujeer MK, Lutchen KR, Collins JJ, Andrade JS Jr, Ingenito EP, Zapperi S, Stanley HE. Life-support system benefits from noise. Nature. 1998;393:127–128. doi: 10.1038/30130. [DOI] [PubMed] [Google Scholar]
- Ruth Graham M, Goertzen AL, Girling LG, Friedman T, Pauls RJ, Dickson T, Espenell AE, Mutch WA. Quantitative computed tomography in porcine lung injury with variable versus conventional ventilation: recruitment and surfactant replacement. Crit Care Med. 2011;39:1721–1730. doi: 10.1097/CCM.0b013e3182186d09. [DOI] [PubMed] [Google Scholar]
- Wysocki M, Richard JC, Meshaka P. Noninvasive proportional assist ventilation compared with noninvasive pressure support ventilation in hypercapnic acute respiratory failure. Crit Care Med. 2002;30:323–329. doi: 10.1097/00003246-200202000-00010. [DOI] [PubMed] [Google Scholar]
- Liet JM, Dejode JM, Joram N, Gaillard-Le Roux B, Betremieux P, Roze JC. Respiratory support by neurally adjusted ventilatory assist (NAVA) in severe RSV-related bronchiolitis: a case series report. BMC Pediatr. 2011;11:92–100. doi: 10.1186/1471-2431-11-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laubscher TP, Frutiger A, Fanconi S, Jutzi H, Brunner JX. Automatic selection of tidal volume, respiratory frequency and minute ventilation in intubated ICU patients as start up procedure for closed-loop controlled ventilation. Int J Clin Monitor Comp. 1994;11:19–30. doi: 10.1007/BF01132840. [DOI] [PubMed] [Google Scholar]
- Urschitz MS, Horn W, Seyfang A, Hallenberger A, Herberts T, Miksch S, Popow C, Müller-Hansen I, Poets CF. Automatic control of the inspired oxygen fraction in preterm infants: a randomized crossover trial. Am J Respir Crit Care Med. 2004;170:1095–1100. doi: 10.1164/rccm.200407-929OC. [DOI] [PubMed] [Google Scholar]
- Claure N, Bancalari E, D'Ugard C, Nelin L, Stein M, Ramanathan R, Hernandez R, Donn SM, Becker M, Bachman T. Multicenter crossover study of automated control of inspired oxygen in ventilated preterm infants. Pediatrics. 2011;127:e76–e83. doi: 10.1542/peds.2010-0939. [DOI] [PubMed] [Google Scholar]
- Johannigman JA, Branson RD, Edwards MG. Closed loop control of inspired oxygen concentration in trauma patients. J Am Coll Surg. 2009;208:763–768. doi: 10.1016/j.jamcollsurg.2009.01.033. [DOI] [PubMed] [Google Scholar]
- Slater A, Shann F, Pearson G. PIM2: a revised version of the Paediatric Index of Mortality. Intensive Care Med. 2003;29:278–285. doi: 10.1007/s00134-002-1601-2. [DOI] [PubMed] [Google Scholar]
- Leteurtre S, Martinot A, Duhamel A, Proulx F, Grandbastien B, Cotting J, Gottesman R, Joffe A, Pfenninger J, Hubert P, Lacroix J, Leclerc F. Validation of the paediatric logistic organ dysfunction (PELOD) score: prospective, observational, multicentre study. Lancet. 2003;362:192–197. doi: 10.1016/S0140-6736(03)13908-6. [DOI] [PubMed] [Google Scholar]