Abstract
Acute kidney injury (AKI) occurring in patients admitted to the ICU may result in impaired renal function on long-term follow-up after ICU discharge. The damage induced by subclinical or manifest episodes of AKI may, in fact, produce an irreversible loss of a variable amount of renal mass with deleterious effects on overall renal function. This may be the case even though baseline glomerular filtration rate appears to return to normal but renal reserve is impaired. This may have an important effect on long-term outcomes, including progression to chronic kidney disease. Acute kidney insults should not be considered as isolated episodes but rather a sequence of progressive events that can lead to progressive deterioration of kidney tissue and eventual declines in renal function.
Acute kidney injury (AKI) is an independent risk factor for increased morbidity and mortality in critically ill patients. However, the effects of AKI extend beyond the incident hospital stay to include more long-lasting effects on patient outcome and progression of chronic kidney disease (CKD). A recent paper by Chun-Fu Lai and colleagues [1], despite the inherent limitations of a retrospective study, has great clinical importance in adding to our understanding of the lasting effects of AKI on patient outcomes.
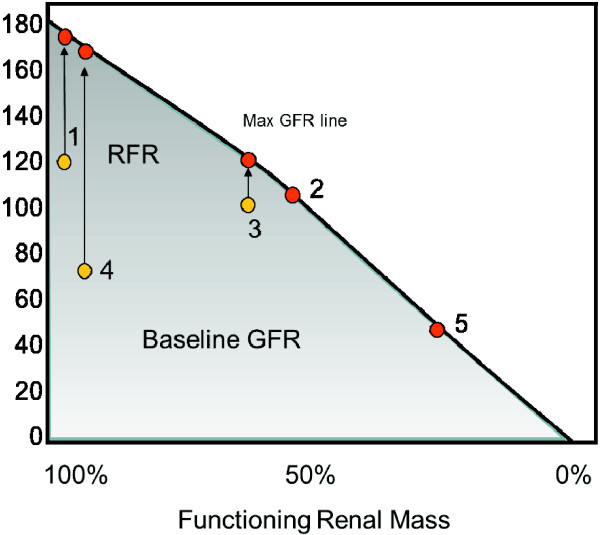
We are well aware that patients with pre-existing renal dysfunction are at high risk for AKI when admitted to the ICU [2,3]. However, how do we judge risk in those patients with an estimated glomerular filtration rate (GFR) or a measured GFR that is greater than 60 ml/minute? Within this group, there is likely much heterogeneity in functional renal mass, reserve and likely risk for AKI. For example, think of these following patients: patient 1, a 45-year-old male with a normal renal ultrasound and a GFR of 120 ml/minute; patient 2, a 45-year-old male with a history of a unilateral nephrectomy secondary to trauma at 6 years of age and a GFR of 110 ml/minute; patient 3, a 70-year-old male with a recent kidney biopsy showing that 30% of his glomeruli are sclerotic and a GFR of 97 ml/minute; patient 4, a 30-year-old female, vegetarian with a normal renal ultrasound and a GFR of 70 ml/minute; and patient 5, a 62-year-old female with documented CKD and a GFR of 42 ml/minute.
In the first three cases, all patients have a normal GFR despite differences in their functional renal mass, while a low GFR is observed in the fourth case in a young vegetarian female with no detectable renal disease. The fifth clearly has CKD.
Do the first three patients have the same renal function? The baseline GFR would suggest so, but this is unlikely to be true. In fact, if an increased functional demand were placed on the kidneys, it is likely that only patient 1 would be able to adequately increase GFR. This introduces the concept of renal functional reserve (RFR). It has been demonstrated that following stimulation with protein loading, either oral or intravenous, GFR can significantly increase to a value defined as GFRmax; the difference between baseline and GFRmax is defined as the RFR [4]. This is graphically represented in Figure 1. For patient 1, RFR is intact and a GFRmax can be reached in response to specific stimuli. This would be true irrespective of his baseline GFR. In patient 2 baseline GFR is normal but in the presence of stimulation the GFR does not change, showing there is no RFR. Patient 3 has only a limited RFR and, after stimulation, a small increase of GFR can be demonstrated with a lower GFRmax than seen in patient 1. Patient 4 has a low baseline GFR but after stimulation she can increase GFRmax to 170 ml/minute, showing an intact RFR. Patient 5 has no RFR and the low baseline GFR does not increase at all after stimulation.
Figure 1.
The GFR/renal mass domain map. Baseline and maximum glomerular filtration rate (max GFR) can be significantly different for renal mass greater than 50%. In this case renal functional reserve (RFR) is still present. For renal mass less than 50%, baseline and max GFR are often the same unless a very low protein diet is in place. See text for explanation; numbers refer to the cases discussed in the text.
These examples demonstrate that baseline GFR does not necessarily tell the full story about the anatomical and functional conditions of the kidney and that a normal baseline GFR can be present despite significant renal impairment. Thus, when a critically ill patient has a normal baseline GFR, he could potentially be at increased risk of AKI due to a loss of RFR. Furthermore, when an episode of AKI is resolved and renal function recovery appears complete by measurement of GFR, this does not necessarily mean that a full restoration of renal mass and RFR has also occurred. For instance, if the patient has had an irreversible loss of 30% of the nephron mass, his baseline GFR may go back to normal but his RFR may be impaired. This will have consequences on future exposures to nephrotoxic insults and possibly on the progression of CKD. In some cases, episodes of AKI may occur and remain subclinical if only studied according to changes in creatinine and GFR [5]. In such conditions, even if creatinine does not increase during ICU stay, an episode of AKI may be documented by sensitive biomarkers that detect renal injury. These seemingly innocuous insults may result in worse long-term outcomes [6]. Finally, repeated episodes of AKI, even if dialysis is not required, may result in significant and progressive damage, progressively reducing RFR and leaving the patient with undiagnosed CKD. When the patient receives further insults or his clinical condition simply evolves due to comorbid conditions such as diabetes or hypertension, the CKD becomes manifest and can progressively accelerate. The lower the remnant kidney mass, the higher will be the stress imposed to residual nephrons, resulting in hyperfiltration and progression of kidney disease.
With these considerations, the paper by Chun-Fu Lai and colleagues becomes of significant importance for those caring for AKI in the ICU setting as well as those following the survivors of ICU admissions. The occurrence of subclinical or more significant episodes of AKI may have a deleterious effect on kidney function, even though baseline GFR seems to return to normal. This may have an important effect on long-term outcomes in such patients. As opposed to other organ systems, kidney dysfunction may evolve silently until it is too late. Acute kidney insults should not be considered as isolated episodes but rather a sequence of progressive events that can lead to progressive deterioration of kidney tissue and eventual declines in renal function.
Abbreviations
AKI: acute kidney injury; CKD: chronic kidney disease; GFR: glomerular filtration rate; RFR: renal functional reserve.
Competing interests
The authors declare that they have no competing interests.
See related research by Lai et al., http://ccforum.com/content/16/4/R123
Contributor Information
Claudio Ronco, Email: cronco@goldnet.it.
Mitchell H Rosner, Email: MHR9R@hscmail.mcc.virginia.edu.
References
- Lai CF, Wu VC, Huang TM, Yeh YC, Wang KC, Han YY, Lin YF, Jhuang YJ, Chao CT, Shiao CC, Tsai PR, Hu FC, Chou NK, Ko WJ, Wu KD, Group TN. on behalf of the NSARF group. Kidney function decline after a non-dialysis-requiring acute kidney injury is associated with higher long-term mortality in critically ill survivors. Crit Care. 2012;16:R123. doi: 10.1186/cc11419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellomo R, Kellum JA, Ronco C. Acute kidney injury. Lancet. 2012. [Epub ahead of print] [DOI] [PubMed]
- Hoste EA, Kellum JA, Katz NM, Rosner MH, Haase M, Ronco C. Epidemiology of acute kidney injury. Contrib Nephrol. 2010;165:1–8. doi: 10.1159/000313737. [DOI] [PubMed] [Google Scholar]
- Bosch JP, Saccaggi A, Lauer A, Ronco C, Belledonne M, Glabman S. Renal functional reserve in humans. Effect of protein intake on glomerular filtration rate. Am J Med. 1983;75:943–950. doi: 10.1016/0002-9343(83)90873-2. [DOI] [PubMed] [Google Scholar]
- Ronco C, Kellum JA, Haase M. Subclinical AKI is still AKI. Crit Care. 2012;16:313. doi: 10.1186/cc10920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haase M, Devarajan P, Haase-Fielitz A, Bellomo R, Cruz DN, Wagener G, Krawczeski CD, Koyner JL, Murray P, Zappitelli M, Goldstein SL, Makris K, Ronco C, Martensson J, Martling CR, Venge P, Siew E, Ware LB, Ikizler TA, Mertens PR. The outcome of neutrophil gelatinase-associated lipocalin-positive subclinical acute kidney injury: a multicenter pooled analysis of prospective studies. J Am Coll Cardiol. 2011;57:1752–1761. doi: 10.1016/j.jacc.2010.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]