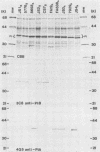
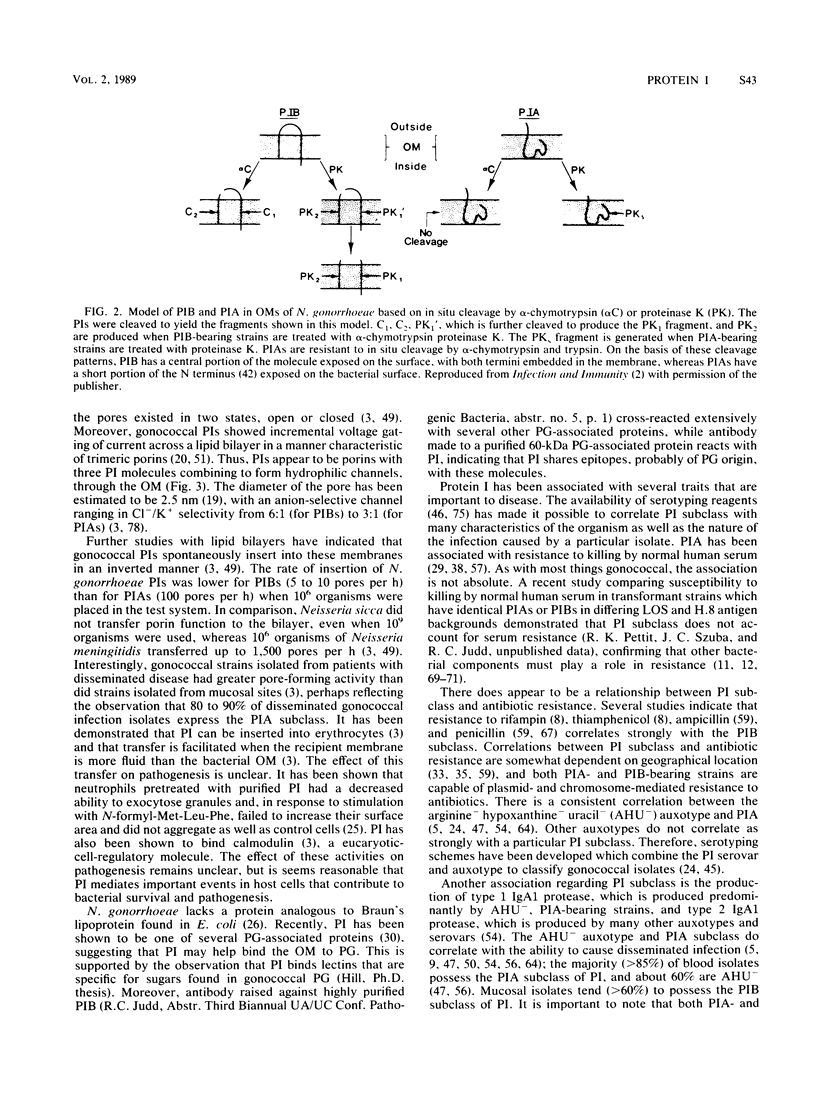
Full text
PDF

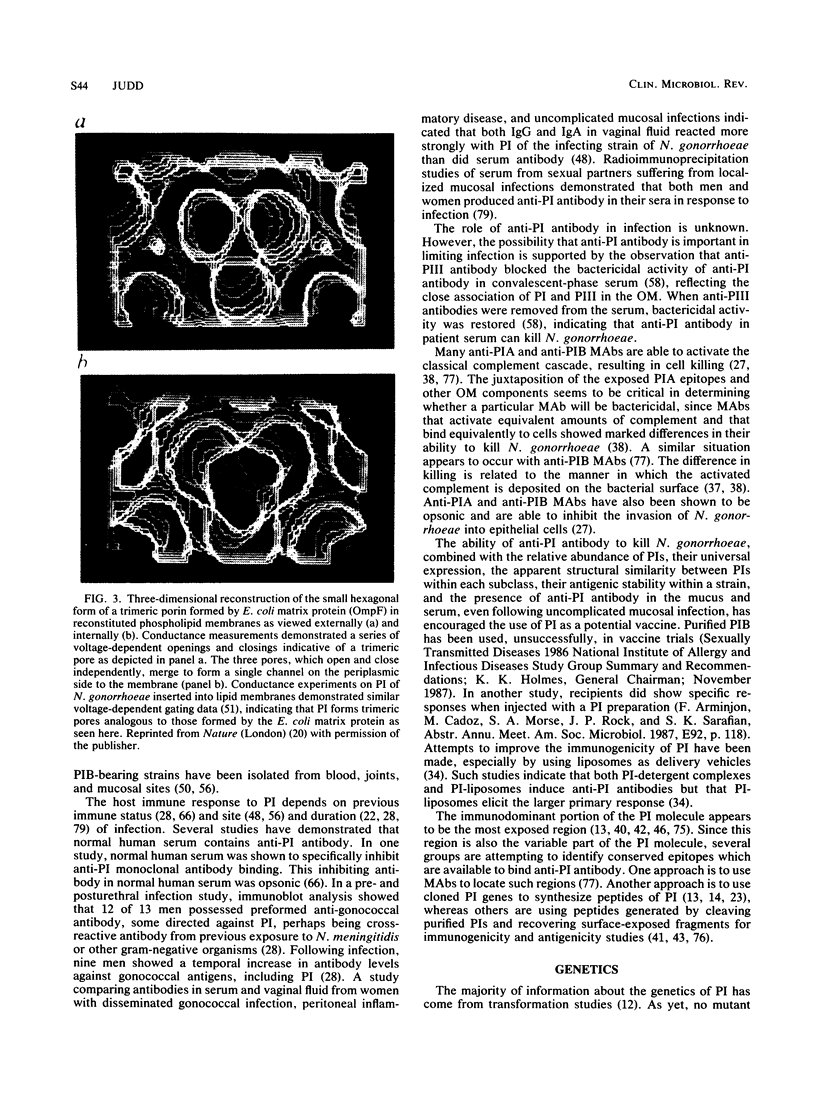
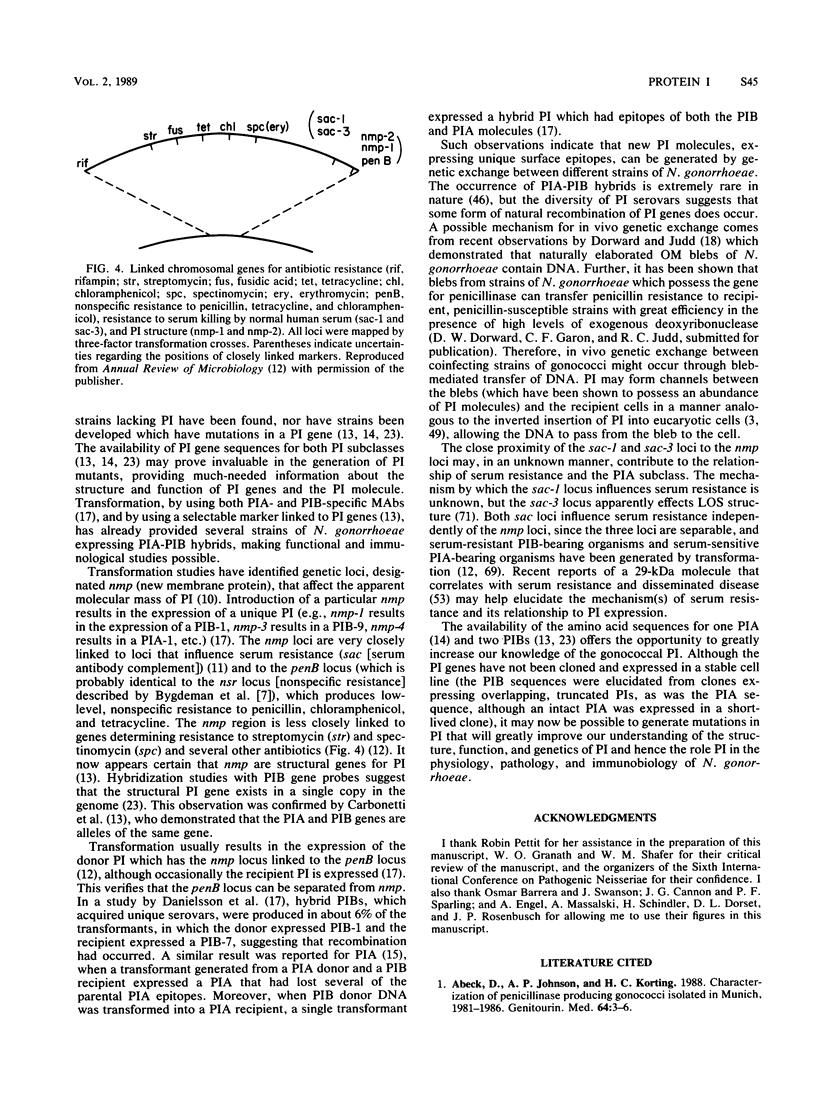



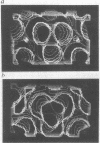
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abeck D., Johnson A. P., Korting H. C. Characterisation of penicillinase producing gonococci isolated in Munich, 1981-6. Genitourin Med. 1988 Feb;64(1):3–6. doi: 10.1136/sti.64.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrera O., Swanson J. Proteins IA and IB exhibit different surface exposures and orientations in the outer membranes of Neisseria gonorrhoeae. Infect Immun. 1984 Jun;44(3):565–568. doi: 10.1128/iai.44.3.565-568.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake M. S., Gotschlich E. C., Swanson J. Effects of proteolytic enzymes on the outer membrane proteins of Neisseria gonorrhoeae. Infect Immun. 1981 Jul;33(1):212–222. doi: 10.1128/iai.33.1.212-222.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunham R. C., Plummer F., Slaney L., Rand F., DeWitt W. Correlation of auxotype and protein I type with expression of disease due to Neisseria gonorrhoeae. J Infect Dis. 1985 Aug;152(2):339–343. doi: 10.1093/infdis/152.2.339. [DOI] [PubMed] [Google Scholar]
- Buchanan T. M., Hildebrandt J. F. Antigen-specific serotyping of Neisseria gonorrhoeae: characterization based upon principal outer membrane protein. Infect Immun. 1981 Jun;32(3):985–994. doi: 10.1128/iai.32.3.985-994.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bygdeman S. M., Mårdh P. A., Sandström E. G. Susceptibility of Neisseria gonorrhoeae to rifampicin and thiamphenicol: correlation with protein I antigenic determinants. Sex Transm Dis. 1984 Oct-Dec;11(4 Suppl):366–370. doi: 10.1097/00007435-198410001-00012. [DOI] [PubMed] [Google Scholar]
- Bygdeman S., Bäckman M., Danielsson D., Norgren M. Genetic linkage between serogroup specificity and antibiotic resistance in Neisseria gonorrhoeae. Acta Pathol Microbiol Immunol Scand B. 1982 Jun;90(3):243–250. doi: 10.1111/j.1699-0463.1982.tb00112.x. [DOI] [PubMed] [Google Scholar]
- Cannon J. G., Buchanan T. M., Sparling P. F. Confirmation of association of protein I serotype of Neisseria gonorrhoeae with ability to cause disseminated infection. Infect Immun. 1983 May;40(2):816–819. doi: 10.1128/iai.40.2.816-819.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon J. G., Klapper D. G., Blackman E. Y., Sparling P. F. Genetic locus (nmp-1) affecting the principal outer membrane protein of Neisseria gonorrhoeae. J Bacteriol. 1980 Aug;143(2):847–851. doi: 10.1128/jb.143.2.847-851.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon J. G., Lee T. J., Guymon L. F., Sparling P. F. Genetics of serum resistance in Neisseria gonorrhoeae: the sac-1 genetic locus. Infect Immun. 1981 May;32(2):547–552. doi: 10.1128/iai.32.2.547-552.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon J. G., Sparling P. F. The genetics of the gonococcus. Annu Rev Microbiol. 1984;38:111–133. doi: 10.1146/annurev.mi.38.100184.000551. [DOI] [PubMed] [Google Scholar]
- Carbonetti N. H., Simnad V. I., Seifert H. S., So M., Sparling P. F. Genetics of protein I of Neisseria gonorrhoeae: construction of hybrid porins. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6841–6845. doi: 10.1073/pnas.85.18.6841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbonetti N. H., Sparling P. F. Molecular cloning and characterization of the structural gene for protein I, the major outer membrane protein of Neisseria gonorrhoeae. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9084–9088. doi: 10.1073/pnas.84.24.9084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark V. L., Campbell L. A., Palermo D. A., Evans T. M., Klimpel K. W. Induction and repression of outer membrane proteins by anaerobic growth of Neisseria gonorrhoeae. Infect Immun. 1987 Jun;55(6):1359–1364. doi: 10.1128/iai.55.6.1359-1364.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielsson D., Faruki H., Dyer D., Sparling P. F. Recombination near the antibiotic resistance locus penB results in antigenic variation of gonococcal outer membrane protein I. Infect Immun. 1986 May;52(2):529–533. doi: 10.1128/iai.52.2.529-533.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel A., Massalski A., Schindler H., Dorset D. L., Rosenbusch J. P. Porin channel triplets merge into single outlets in Escherichia coli outer membranes. Nature. 1985 Oct 17;317(6038):643–645. doi: 10.1038/317643a0. [DOI] [PubMed] [Google Scholar]
- Fohn M. J., Mietzner T. A., Hubbard T. W., Morse S. A., Hook E. W., 3rd Human immunoglobulin G antibody response to the major gonococcal iron-regulated protein. Infect Immun. 1987 Dec;55(12):3065–3069. doi: 10.1128/iai.55.12.3065-3069.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotschlich E. C., Seiff M. E., Blake M. S., Koomey M. Porin protein of Neisseria gonorrhoeae: cloning and gene structure. Proc Natl Acad Sci U S A. 1987 Nov;84(22):8135–8139. doi: 10.1073/pnas.84.22.8135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gründer K., Baumgartner A., Kohl P. K., Tam M. R., Knapp J. S. Typisierung des Gonokokkus: Auxotypie und Serotypie mit monoklonalen Antikörpern. Z Hautkr. 1987 Jul 15;62(14):1086–1100. [PubMed] [Google Scholar]
- Haines K. A., Yeh L., Blake M. S., Cristello P., Korchak H., Weissmann G. Protein I, a translocatable ion channel from Neisseria gonorrhoeae, selectively inhibits exocytosis from human neutrophils without inhibiting O2- generation. J Biol Chem. 1988 Jan 15;263(2):945–951. [PubMed] [Google Scholar]
- Hebeler B. H., Young F. E. Chemical composition and turnover of peptidoglycan in Neisseria gonorrhoeae. J Bacteriol. 1976 Jun;126(3):1180–1185. doi: 10.1128/jb.126.3.1180-1185.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckels J. E., Virji M., Zak K., Fletcher J. N. Immunobiology of gonococcal outer membrane protein I. Antonie Van Leeuwenhoek. 1987;53(6):461–464. doi: 10.1007/BF00415503. [DOI] [PubMed] [Google Scholar]
- Hicks C. B., Boslego J. W., Brandt B. Evidence of serum antibodies to Neisseria gonorrhoeae before gonococcal infection. J Infect Dis. 1987 Jun;155(6):1276–1281. doi: 10.1093/infdis/155.6.1276. [DOI] [PubMed] [Google Scholar]
- Hildebrandt J. F., Mayer L. W., Wang S. P., Buchanan T. M. Neisseria gonorrhoeae acquire a new principal outer-membrane protein when transformed to resistance to serum bactericidal activity. Infect Immun. 1978 Apr;20(1):267–272. doi: 10.1128/iai.20.1.267-272.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitchcock P. J. Analyses of gonococcal lipopolysaccharide in whole-cell lysates by sodium dodecyl sulfate-polyacrylamide gel electrophoresis: stable association of lipopolysaccharide with the major outer membrane protein (protein I) of Neisseria gonorrhoeae. Infect Immun. 1984 Oct;46(1):202–212. doi: 10.1128/iai.46.1.202-212.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitchcock P. J., Hayes S. F., Mayer L. W., Shafer W. M., Tessier S. L. Analyses of gonococcal H8 antigen. Surface location, inter- and intrastrain electrophoretic heterogeneity, and unusual two-dimensional electrophoretic characteristics. J Exp Med. 1985 Dec 1;162(6):2017–2034. doi: 10.1084/jem.162.6.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hook E. W., 3rd, Judson F. N., Handsfield H. H., Ehret J. M., Holmes K. K., Knapp J. S. Auxotype/serovar diversity and antimicrobial resistance of Neisseria gonorrhoeae in two mid-sized American cities. Sex Transm Dis. 1987 Jul-Sep;14(3):141–146. doi: 10.1097/00007435-198707000-00004. [DOI] [PubMed] [Google Scholar]
- Jiskoot W., Teerlink T., Van Hoof M. M., Bartels K., Kanhai V., Crommelin D. J., Beuvery E. C. Immunogenic activity of gonococcal protein I in mice with three different lipoidal adjuvants delivered in liposomes and in complexes. Infect Immun. 1986 Nov;54(2):333–338. doi: 10.1128/iai.54.2.333-338.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson A. P., Abeck D., Wall R. A., Mabey D. C., Taylor-Robinson D. Plasmid content, auxotype and protein-I serovar of gonococci isolated in the Gambia. Epidemiol Infect. 1987 Dec;99(3):669–674. doi: 10.1017/s0950268800066528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston K. H., Holmes K. K., Gotschlich E. C. The serological classification of Neisseria gonorrhoeae. I. Isolation of the outer membrane complex responsible for serotypic specificity. J Exp Med. 1976 Apr 1;143(4):741–758. doi: 10.1084/jem.143.4.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joiner K. A., Warren K. A., Brown E. J., Swanson J., Frank M. M. Studies on the mechanism of bacterial resistance to complement-mediated killing. IV. C5b-9 forms high molecular weight complexes with bacterial outer membrane constituents on serum-resistant but not on serum-sensitive Neisseria gonorrhoeae. J Immunol. 1983 Sep;131(3):1443–1451. [PubMed] [Google Scholar]
- Joiner K. A., Warren K. A., Tam M., Frank M. M. Monoclonal antibodies directed against gonococcal protein I vary in bactericidal activity. J Immunol. 1985 May;134(5):3411–3419. [PubMed] [Google Scholar]
- Judd R. C. 125I-peptide mapping of protein III isolated from four strains of Neisseria gonorrhoeae. Infect Immun. 1982 Aug;37(2):622–631. doi: 10.1128/iai.37.2.622-631.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judd R. C. Evidence for N-terminal exposure of the protein IA subclass of Neisseria gonorrhoeae protein I. Infect Immun. 1986 Nov;54(2):408–414. doi: 10.1128/iai.54.2.408-414.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judd R. C. Radioiodination and 125I-labeled peptide mapping of proteins on nitrocellulose membranes. Anal Biochem. 1987 Feb 1;160(2):306–315. doi: 10.1016/0003-2697(87)90052-2. [DOI] [PubMed] [Google Scholar]
- Judd R. C. Surface peptide mapping of protein I and protein III of four strains of Neisseria gonorrhoeae. Infect Immun. 1982 Aug;37(2):632–641. doi: 10.1128/iai.37.2.632-641.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judd R. C., Tam M., Joiner K. Characterization of protein I from serum-sensitive and serum-resistant transformants of Neisseria gonorrhoeae. Infect Immun. 1987 Jan;55(1):273–276. doi: 10.1128/iai.55.1.273-276.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp J. S., Tam M. R., Nowinski R. C., Holmes K. K., Sandström E. G. Serological classification of Neisseria gonorrhoeae with use of monoclonal antibodies to gonococcal outer membrane protein I. J Infect Dis. 1984 Jul;150(1):44–48. doi: 10.1093/infdis/150.1.44. [DOI] [PubMed] [Google Scholar]
- Kohl P. K., Knapp J. S., Hofmann H., Gruender K., Petzoldt D., Tams M. R., Holmes K. K. Epidemiological analysis of Neisseria gonorrhoeae in the Federal Republic of Germany by auxotyping and serological classification using monoclonal antibodies. Genitourin Med. 1986 Jun;62(3):145–150. doi: 10.1136/sti.62.3.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammel C. J., Sweet R. L., Rice P. A., Knapp J. S., Schoolnik G. K., Heilbron D. C., Brooks G. F. Antibody-antigen specificity in the immune response to infection with Neisseria gonorrhoeae. J Infect Dis. 1985 Nov;152(5):990–1001. doi: 10.1093/infdis/152.5.990. [DOI] [PubMed] [Google Scholar]
- Lynch E. C., Blake M. S., Gotschlich E. C., Mauro A. Studies of Porins: Spontaneously Transferred from Whole Cells and Reconstituted from Purified Proteins of Neisseria gonorrhoeae and Neisseria meningitidis. Biophys J. 1984 Jan;45(1):104–107. doi: 10.1016/S0006-3495(84)84127-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin D., Turgeon P. L., Mathieu L. G. Approximate molecular weight of envelope protein 1 and colony opacity of Neisseria gonorrhoeae strains isolated from patients with disseminated or localized infection. Sex Transm Dis. 1986 Apr-Jun;13(2):71–75. doi: 10.1097/00007435-198604000-00003. [DOI] [PubMed] [Google Scholar]
- Mauro A., Blake M., Labarca P. Voltage gating of conductance in lipid bilayers induced by porin from outer membrane of Neisseria gonorrhoeae. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1071–1075. doi: 10.1073/pnas.85.4.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDade R. L., Jr, Johnston K. H. Characterization of serologically dominant outer membrane proteins of Neisseria gonorrhoeae. J Bacteriol. 1980 Mar;141(3):1183–1191. doi: 10.1128/jb.141.3.1183-1191.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McShan W. M., Williams R. P., Hull R. A. A recombinant molecule from a disseminating strain of Neisseria gonorrhoeae that confers serum bactericidal resistance. Infect Immun. 1987 Dec;55(12):3017–3022. doi: 10.1128/iai.55.12.3017-3022.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulks M. H., Knapp J. S. Immunoglobulin A1 protease types of Neisseria gonorrhoeae and their relationship to auxotype and serovar. Infect Immun. 1987 Apr;55(4):931–936. doi: 10.1128/iai.55.4.931-936.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newhall W. J., Sawyer W. D., Haak R. A. Cross-linking analysis of the outer membrane proteins of Neisseria gonorrhoeae. Infect Immun. 1980 Jun;28(3):785–791. doi: 10.1128/iai.28.3.785-791.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien J. P., Goldenberg D. L., Rice P. A. Disseminated gonococcal infection: a prospective analysis of 49 patients and a review of pathophysiology and immune mechanisms. Medicine (Baltimore) 1983 Nov;62(6):395–406. [PubMed] [Google Scholar]
- Odum L., Buchanan T. M., Knapp J. S. Protein I serotype of serum-resistant versus serum-sensitive Neisseria gonorrhoeae strains. Acta Pathol Microbiol Immunol Scand B. 1987 Feb;95(1):1–4. doi: 10.1111/j.1699-0463.1987.tb03079.x. [DOI] [PubMed] [Google Scholar]
- Rice P. A., Vayo H. E., Tam M. R., Blake M. S. Immunoglobulin G antibodies directed against protein III block killing of serum-resistant Neisseria gonorrhoeae by immune serum. J Exp Med. 1986 Nov 1;164(5):1735–1748. doi: 10.1084/jem.164.5.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice R. J., Biddle J. W., JeanLouis Y. A., DeWitt W. E., Blount J. H., Morse S. A. Chromosomally mediated resistance in Neisseria gonorrhoeae in the United States: results of surveillance and reporting, 1983-1984. J Infect Dis. 1986 Feb;153(2):340–345. doi: 10.1093/infdis/153.2.340. [DOI] [PubMed] [Google Scholar]
- Robinson E. N., Jr, McGee Z. A., Buchanan T. M., Blake M. S., Hitchcock P. J. Probing the surface of Neisseria gonorrhoeae: simultaneous localization of protein I and H.8 antigens. Infect Immun. 1987 May;55(5):1190–1197. doi: 10.1128/iai.55.5.1190-1197.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandstrom E. G., Chen K. C., Buchanan T. M. Serology of Neisseria gonorrhoeae: coagglutination serogroups WI and WII/III correspond to different outer membrane protein I molecules. Infect Immun. 1982 Nov;38(2):462–470. doi: 10.1128/iai.38.2.462-470.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandstrom E. G., Knapp J. S., Buchanan T. B. Serology of Neisseria gonorrhoeae: W-antigen serogrouping by coagglutination and protein I serotyping by enzyme-linked immunosorbent assay both detect protein I antigens. Infect Immun. 1982 Jan;35(1):229–239. doi: 10.1128/iai.35.1.229-239.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandström E. G., Knapp J. S., Reller L. B., Thompson S. E., Hook E. W., 3rd, Holmes K. K. Serogrouping of Neisseria gonorrhoeae: correlation of serogroup with disseminated gonococcal infection. Sex Transm Dis. 1984 Apr-Jun;11(2):77–80. doi: 10.1097/00007435-198404000-00005. [DOI] [PubMed] [Google Scholar]
- Sandström E., Danielsson D. Serology of Neisseria gonorrhoeae. Classification by co-agglutination. Acta Pathol Microbiol Scand B. 1980 Feb;88(1):27–38. doi: 10.1111/j.1699-0463.1980.tb02600.x. [DOI] [PubMed] [Google Scholar]
- Sarafian S. K., Tam M. R., Morse S. A. Gonococcal protein I-specific opsonic IgG in normal human serum. J Infect Dis. 1983 Dec;148(6):1025–1032. doi: 10.1093/infdis/148.6.1025. [DOI] [PubMed] [Google Scholar]
- Schalla W. O., Rice R. J., Biddle J. W., Jeanlouis Y., Larsen S. A., Whittington W. L. Lectin characterization of gonococci from an outbreak caused by penicillin-resistant Neisseria gonorrhoeae. J Clin Microbiol. 1985 Oct;22(4):481–483. doi: 10.1128/jcm.22.4.481-483.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt S., Layh G., Buchanan T. B. Surface-exposed antigenic cleavage fragments of Neisseria gonorrhoeae proteins 1A and IB. Infect Immun. 1986 Dec;54(3):841–845. doi: 10.1128/iai.54.3.841-845.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafer W. M., Joiner K., Guymon L. F., Cohen M. S., Sparling P. F. Serum sensitivity of Neisseria gonorrhoeae: the role of lipopolysaccharide. J Infect Dis. 1984 Feb;149(2):175–183. doi: 10.1093/infdis/149.2.175. [DOI] [PubMed] [Google Scholar]
- Spratt S. K., Jones F., Shockley T. E., Jackson J. H. Cotransformation of a serum resistance phenotype with genes for arginine biosynthesis in Neisseria gonorrhoeae. Infect Immun. 1980 Jul;29(1):287–289. doi: 10.1128/iai.29.1.287-289.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens D. S., Shafer W. M. Evidence that the serum resistance genetic locus sac-3 of Neisseria gonorrhoeae is involved in lipopolysaccharide structure. J Gen Microbiol. 1987 Sep;133(9):2671–2678. doi: 10.1099/00221287-133-9-2671. [DOI] [PubMed] [Google Scholar]
- Swanson J., Mayer L. W., Tam M. R. Antigenicity of Neisseria gonorrhoeae outer membrane protein(s) III detected by immunoprecipitation and Western blot transfer with a monoclonal antibody. Infect Immun. 1982 Nov;38(2):668–672. doi: 10.1128/iai.38.2.668-672.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J. Studies on gonococcus infection. XVIII. 125I-labeled peptide mapping of the major protein of the gonococcal cell wall outer membrane. Infect Immun. 1979 Mar;23(3):799–810. doi: 10.1128/iai.23.3.799-810.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam M. R., Buchanan T. M., Sandström E. G., Holmes K. K., Knapp J. S., Siadak A. W., Nowinski R. C. Serological classification of Neisseria gonorrhoeae with monoclonal antibodies. Infect Immun. 1982 Jun;36(3):1042–1053. doi: 10.1128/iai.36.3.1042-1053.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virji M., Zak K., Heckels J. E. Monoclonal antibodies to gonococcal outer membrane protein IB: use in investigation of the potential protective effect of antibodies directed against conserved and type-specific epitopes. J Gen Microbiol. 1986 Jun;132(6):1621–1629. doi: 10.1099/00221287-132-6-1621. [DOI] [PubMed] [Google Scholar]
- Virji M., Zak K., Heckels J. E. Monoclonal antibodies to gonococcal outer membrane protein IB: use in investigation of the potential protective effect of antibodies directed against conserved and type-specific epitopes. J Gen Microbiol. 1986 Jun;132(6):1621–1629. doi: 10.1099/00221287-132-6-1621. [DOI] [PubMed] [Google Scholar]
- Young J. D., Blake M., Mauro A., Cohn Z. A. Properties of the major outer membrane protein from Neisseria gonorrhoeae incorporated into model lipid membranes. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3831–3835. doi: 10.1073/pnas.80.12.3831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zak K., Diaz J. L., Jackson D., Heckels J. E. Antigenic variation during infection with Neisseria gonorrhoeae: detection of antibodies to surface proteins in sera of patients with gonorrhea. J Infect Dis. 1984 Feb;149(2):166–174. doi: 10.1093/infdis/149.2.166. [DOI] [PubMed] [Google Scholar]