Abstract
Recent studies demonstrate that ketamine, a fast-acting antidepressant, rapidly activates the mammalian target of rapamycin (mTOR) and increases synaptogenesis in the prefrontal cortex (PFC). Because of the side effect and abuse potential of ketamine we are investigating alternative agents that produce similar effects. Here we demonstrate that a single dose of LY341495, an mGluR2/3 antagonist, produces ketamine-like biochemical and behavioral effects. LY341495 administration rapidly (1 hr) activates the mTOR pathway (mTOR, p70S6K, 4E-BP1) and subsequently (24 hrs) increases levels of synaptic proteins (PSD-95, GluR1 and Synapsin I) 24 hrs later, similar to the effects of ketamine. Finally, the antidepressant effects of LY341495 in the rat forced swim test are completely blocked by the mTOR inhibitor, rapamycin. The results indicate that the antidepressant actions of LY341495 are mediated by activation of mTOR and suggest that this and other mGluR2/3 antagonists could produce rapid antidepressant effects in depressed patients.
Keywords: Depression, synapse, glutamate, ketamine, behavior
Introduction
Depression is a debilitating psychiatric disorder that affects ~17% of the population (Kessler et al., 2003). All currently approved antidepressant treatments work through various mechanisms to modulate monoaminergic neurotransmitter systems and can take several weeks to months to produce a therapeutic response. Recently, efforts have focused on novel classes of drugs that work by modulating glutamatergic neurotransmission. Ketamine, a non-competitive N-methyl-D-aspartate (NMDA) receptor antagonist, has received the most attention. Ketamine produces rapid (within 2 hrs) and sustained (~1 week) antidepressant effects in depressed patients refractory to typical antidepressant treatment (Berman et al., 2000; Zarate et al., 2006). While the mechanisms underlying these surprisingly fast and robust effects remain somewhat unclear, we have found that ketamine treatment produces rapid activation of the mammalian target of rapamycin (mTOR) pathway and subsequent sustained increases in synaptic proteins and increased excitatory spine synapses in the prefrontal cortex (PFC) (Li et al., 2010; Li et al., 2011).
mTOR is a serine/threonine protein kinase that integrates signals from growth factors, such as brain-derived neurotrophic factor (BDNF) (Takei et al., 2001), as well and energy and nutrient levels to regulate protein synthesis (reviewed in Sengupta et al., 2010). Activation of mTOR signaling leads to induction of mRNA translation and new protein synthesis via phosphorylation of downstream substrates including p70S6 kinase and eukaryotic initiation factor 4E binding protein 1 (4E-BP1). We found that the phosphorylation of both of these proteins, as well as mTOR, is rapidly increased by ketamine (Li et al., 2010). In addition, ketamine-induction of mTOR signaling is followed by a sustained increase in levels of the synaptic proteins PSD-95, GluR1 and Synapsin I (Li et al., 2010).
While ketamine produces robust antidepressant effects, the deleterious side effects and abuse potential limit its widespread use for treatment of depression, as well as suicide (DiazGranados et al., 2010; Larkin and Beautrais, 2011). Therefore, there remains a clear unmet medical need for drugs that can produce ketamine-like antidepressant effects with a safer side effect profile and decreased abuse liability. In the present study, we sought to evaluate alternative ways to modulate the glutamatergic system that would result in ketamine-like effects.
mGluR2/3 receptors are metabotropic glutamate receptors distributed in regions of the brain implicated in depression, including cortical and limbic regions (Ohishi et al., 1994; Ohishi et al., 1993a). mGluR2 receptors are primarily located in the preterminal portion of neurons (Shigemoto et al., 1997) acting as autoreceptors or heteroceptors whereas mGluR3 receptors are located post-synaptically and on glia (Ohishi et al., 1993b; Tanabe et al., 1993). Both subtypes are Gi-coupled receptors that negatively regulate adenylyl cyclase and decrease glutamatergic and monoaminergic neurotransmission. Antagonism of these receptors increases extracellular glutamate in limbic structures, including the PFC (Hascup et al., 2010; Xi et al., 2002). Since ketamine has also been shown to increase glutamatergic outflow (Moghaddam et al., 1997) and the behavioral and biochemical effects of ketamine are dependent on α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor activation (Li et al., 2010; Maeng et al., 2008), we hypothesized that mGluR2/3 blockade would have the potential to produce ketamine-like biochemical and behavioral effects. In fact, evidence demonstrates that the antidepressant effects of mGluR2/3 antagonists are also blocked by AMPA receptor blockade (Karasawa et al., 2005). In the present study we demonstrate that the selective mGluR2/3 antagonist, LY341495 (Kingston et al., 1998) produces the same biochemical effects as ketamine and that the behavioral effects of this drug are dependent on mTOR signaling.
Materials and Methods
Animals, Drug Administration and Surgical Procedures
Adult male Sprague Dawley rats (Charles River Laboratories) weighing ~150–250 g were pair housed in a 12 hr light:dark cycle with ad libitum access to food and water. All procedures were done in accordance with the NIH guidelines for the care and use of laboratory animals and the Yale University Institutional Animal Care and Use Committee (IACUC). Rats received a single injection of LY341495 (3 or 10 mg/kg, i.p.) prepared in 2% Tween 80 in dH2O or ketamine (10 mg/kg) as a positive control for synaptic protein expression studies and were tested or sacrificed at the indicated time points (1 hr or 24 hrs later). For intracerebroventricular (ICV) drug administration, rats were anesthetized with 50 mg/kg of i.p. pentobarbital and a guide cannula (22G) was implanted using the following stereotaxic coordinates: From bregma: −0.9 anterior/posterior (AP), −1.5 medial/lateral (ML), −3.5 dorsal/ventral (DV). After 1 week of recovery rats were infused with 0.2 nmol rapamycin (2 μL volume) at a rate of 0.25 μL/min. 30 min after rapamycin treatment, rats were injected with LY341495 (3 mg/kg, i.p.). An n=3–4 rats were used for biochemical studies and n=5–7 was used for behavioral testing.
Synaptosome Preparation and Western Blotting
Crude synaptosomes were prepared as previously described (Li et al., 2010). Briefly, the PFC of rats was dissected on ice and homogenized in 0.32 M sucrose, 20 mM HEPES (pH 7.4), 1 mM EDTA, 1× protease inhibitor cocktail, 5 mM NaF, and 1 mM NaVO3. Homogenates were centrifuged at 2,800 rpm and supernatants were then centrifuged at 12,000 rpm to obtain a pellet enriched in crude synaptosomes. Pellets were sonicated in protein lysis buffer containing: 50 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1% Triton X-100, 0.1% SDS, 2 mM EDTA, 1 mM NaVO3, 5 mM NaF and 1× protease inhibitor cocktail and protein concentrations were measured using a BCA kit (Thermo Scientific, Rockford, IL). Proteins were separated by SDS-PAGE and then transferred to nitrocellulose membranes and blocked for 1 hr in 2% BSA in PBS + 0.1% Tween 20 (PBS-T). Primary antibodies for pmTOR (Ser2448), mTOR, pp70S6K (Thr389), p70S6K, p4E-BP1 (Thr37/46), pERK (Thr202/Tyr204), ERK (all from Cell Signaling, Boston, MA), GluR1 (Abcam, Cambridge, MA), PSD-95 (Invitrogen, Camarillo, CA), Synapsin I (BD Biosciences, San Jose, CA) and GAPDH (Advanced Immunochemical, Long Beach, CA) were used. After overnight or 1 hr incubation with primary antibodies, membranes were washed in PBS-T (3×, 10 min) and incubated with peroxidase labeled secondary antibodies for 1 hr. Membranes were then washed again and protein bands were detected using enhanced chemiluminescence. Membranes were then incubated in stripping buffer (Thermo Scientific) blocked in 5% Milk in PBS-T and incubated with primary antibodies directed against their respective non phosphorylated or total protein or GAPDH. Bands were quantified using densiotometric analysis with Image J software (NIH). Data were analyzed by normalizing phospho-protein levels to total protein levels or GAPDH and analyzed using a one-way ANOVA with LSD post-hoc tests as appropriate. Data are expressed as fold change versus control levels.
Forced Swim Test
Rats were tested in a forced swim test as previously described (Li et al., 2010). Briefly, rats were subjected to a 15 min pre-swim in 25° C water in a clear Plexiglas cylinder (65 cm height, 30 cm diameter). After the pre-swim, rats were removed and dried with a clean cloth. 24 hours later, rats received an ICV infusion of rapamycin 30 min prior to a systemic injection (i.p.) with LY341495. 24 hours following treatment, rats were again placed in the swimming cylinders for a 10 min test swim. All sessions were video recorded and data were analyzed by scoring total immobility time (making only movements necessary to keep afloat) between minutes 2–6 (5 min total) by an experimenter blind to the treatment groups. After the swim, brains were removed and placement of the ICV cannula was verified histologically. Rats with incorrect placement of the injection cannula were not included in the data analysis. Immobility values were analyzed using a one-way ANOVA with LSD post-hoc tests as appropriate. Significance was determined at P<0.05 and data were plotted as total seconds immobile.
Results
mGluR2/3 blockade rapidly increases mTOR signaling in the PFC
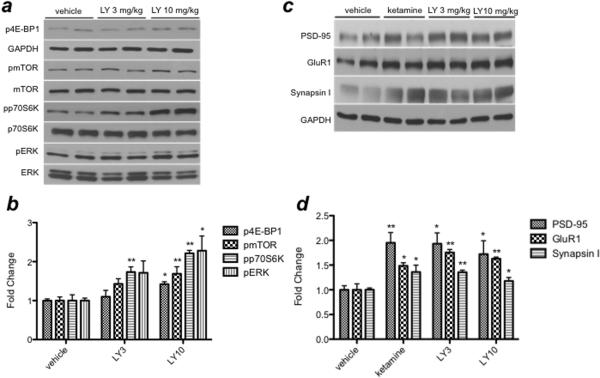
Recent evidence indicates that signaling through the mTOR pathway is rapidly (30–60 min) increased by ketamine administration (Li et al., 2010). Additionally, post-mortem analysis of the PFC of subjects with major depressive disorder revealed deficits in mTOR signaling (Jernigan et al., 2011). To determine whether blockade of mGluR2/3 receptors increases mTOR signaling in rodents, we examined the influence of acute LY341495 (3 or 10 mg/kg, 1 hr) on the phosphorylated forms of mTOR, p70S6 kinase, and 4E-BP1, as well as ERK, an upstream regulator of mTOR signaling. Western blotting analysis revealed significant, dose-dependent increases in the phosphorylated forms of mTOR [F(2,11)=6.182; P<0.05], p70S6 kinase [F(2,11)=24.850; P<0.0001], 4E-BP1 [F(2,11)=4.471; P<0.05], and ERK [F(2,11)=5.118; P<0.05] after LY341495 treatment (Fig. 1a and 1b). Studies of hippocampus revealed much smaller increases, withLY341495 only producing a significant xx% increase phospho-ERK at 10 mg/kg of LY341495 [F(2,11)=4.772; P<0.05], with a trend for a xx% increase of phospho-p70S6K [F(2,11)=2.861; P=0.109], a modest, non-significant 20% increase in phospho-mTOR activation [F(2,11)=0.933; P=0.428] and no increase in phospho-4E-BP1 levels [F(2,11)=0.301; P=0.747. The lower dose of LY341495 (3 mg/kg) had no effects on these mTOR signaling proteins in the hippocampus. Taken together, these data indicate that mGluR2/3 blockade activates the mTOR signaling pathway in the PFC and that there are regional differences that confer altered response to LY341495 in the hippocampus.
Fig. 1.
(a, b) LY341495 increases mTOR signaling in the PFC. Phosphorylation of 4E-BP1, mTOR, p70S6K and ERK is increased 1 hr after a single i.p. injection of LY341495 (3 and 10 mg/kg). Levels of phosphorylated proteins were normalized to either GAPDH (4E-BP1) or levels of total protein (p70S6K, mTOR, and ERK). (c, d) LY341495 increases synaptic protein expression in the PFC. PSD-95, GluR1 and Synapsin I expression is increased 24 hrs after a single i.p. injection of LY341495 (3 or 10 mg/kg) or ketamine (10 mg/kg). Levels of synaptic proteins were normalized to GAPDH. Results are expressed as fold change compared to vehicle control, and are the mean ± SEM (n=3–4, *P<0.05, **P<0.01 compared to vehicle; ANOVA)
mGluR2/3 blockade increases synaptic protein expression in the PFC
A role for mTOR-dependent formation of new synapses – and thus, expression of synaptic proteins – in mediating the antidepressant response to ketamine has recently been identified (Li et al., 2010). To evaluate whether mGluR2/3 blockade increases synaptic proteins, we measured levels of synaptic proteins in the PFC 24 hrs after a single dose of LY341495. We observed significant increases in post-synaptic markers PSD-95 [F(2,11)=5.589; P<0.05] and GluR1 [F(2,11)=25.778; P<0.0001] as well as the pre-synaptic marker Synapsin I [F(2,10)=14.983; P<0.01] (Fig. 1c and 1d). Of note, the magnitude of increase at the 3 and 10 mg/kg dose of LY341495 is nearly identical to the increase produced by ketamine. Analysis of hippocampus revealed small but significant increases in PSD-95 (xx%) [F(2,11)=6.098; P<0.05] and GluR1 (xx%) [F(2,11)=6.864; P<0.05] but only at 10 mg/kg of LY341495 (no effects at 3 mg/kg).
mTOR signaling is required for the antidepressant effects of mGluR2/3 blockade
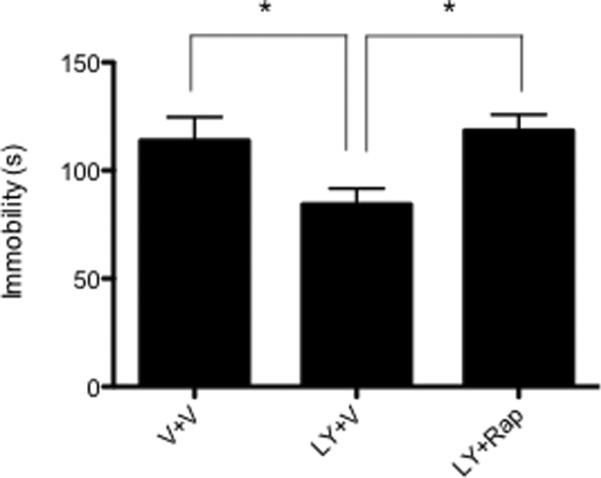
To determine whether the antidepressant response produced by mGluR2/3 blockade depends on mTOR signaling, we pretreated rats with rapamycin (ICV), a specific inhibitor of mTOR (Brown et al., 1994), 30 min prior to LY341495 treatment and tested rats 24 hrs later for behavioral despair in the forced swim test. Based on our data showing increased expression of synaptic proteins 24 hrs after a single 3 mg/kg dose of LY341495, we selected this time point and dose for behavioral testing. LY341495 produced a significant decrease in immobility time compared to vehicle-treated rats and pretreatment with rapamycin completely blocked this effect [F(2,17)=3.911; P<0.05] (Fig. 2). These data indicate that mTOR signaling is required for the antidepressant effects of mGluR2/3 blockade in the forced swim test.
Fig. 2.
Rapamycin blocks the antidepressant effects of LY341495. Rats received an ICV infusion of rapamycin (0.2 nmol) 30 min before a single i.p. injection of LY341495 (3 mg/kg). 24 hrs after treatment, LY341495 significantly decreased immobility time and rapamycin pretreatment completely blocked this effect. Results are mean ± SEM (n=6–8, *P<0.05; ANOVA)
Discussion
Previously, our lab has demonstrated that the NMDA receptor antagonist ketamine rapidly increases mTOR signaling (Li et al., 2010), and here, we demonstrate similar findings in response to mGluR2/3 blockade. LY341495 increased signaling through the mTOR pathway in the PFC, including increased levels of phosphorylated mTOR, p70S6 kinase, and 4E-BP1, at the same doses that produce antidepressant effects in rodent models (Bespalov et al., 2008). We also found that LY341495 increased levels of phospho-ERK, an upstream regulator of mTOR. Interestingly, we failed to see the same magnitude of mTOR activation in the hippocampus, with only modest increases at 10 mg/kg of LY341495. As the mTOR pathway is involved in protein translation and since ketamine increases synaptic protein levels that are dependent on mTOR signaling, we measured increases in synaptic protein markers after mGluR2/3 blockade. We observed significant increases in levels of PSD-95, GluR1 and Synapsin I in the PFC, again similar to the effects of ketamine. In the hippocampus, smaller but significant increases in synaptic proteins were observed at 10 mg/kg, indicating that only modest increases in mTOR signaling are required to increase synaptic protein expression. Furthermore, the results suggest a more prominent role for the PFC than the hippocampus in mediating the antidepressant effects of mGluR2/3 blockade since 3 mg/kg of LY341495 produced antidepressant effects in the FST.
Previous studies failed to observe effects of typical antidepressants (acute or chronic administration) or electroconvulsive shock on mTOR signaling and synaptic proteins (Li et al., 2010). The current findings demonstrating that mGluR2/3 antagonism produces effects similar to ketamine indicate that mGluR blockade is a particularly promising strategy for the treatment of depression that until now has seemed to be unique to ketamine.
We next asked what role mTOR signaling plays in the behavioral antidepressant effects of mGluR2/3 blockade in the forced swim test, a widely used animal model of depression. In our recent study, we report that the antidepressant effects of ketamine require mTOR signaling (Li et al., 2010). Here we show that a single dose of LY341495 produces an antidepressant response in the forced swim test measured 24 hr after treatment, and that this effect is completely blocked by pretreatment with the mTOR inhibitor rapamycin. While the current manuscript was under review, there was another report that the sustained (24 hr) antidepressant effects of mGluR2/3 antagonism in the tail suspension test is blocked by rapamycin (Koike et al., 2011), supporting the results presented here.
Taken together, the results demonstrate that mGluR2/3 blockade produces molecular changes that are similar to ketamine, suggesting that common signaling mechanisms underlie the antidepressant actions of these agents. Given the unique and surprising rapid acting antidepressant effects of ketamine, these data highlight mGluR2/3 as a compelling target for the treatment of depression. Future studies will be required to determine whether the effects of acute mGluR2/3 antagonism are long lasting like ketamine (~1 week) (Li et al., 2010; Zarate et al., 2006), and to examine the influence of chronic LY341495 treatment on mTOR signaling and synaptic proteins. Finally, these studies provide strong support for future studies to determine the ability of mGluR2/3 blockade to produce rapid and efficacious antidepressant actions in humans.
Acknowledgements
The authors would like to thank Lundbeck Pharmaceuticals for generously providing LY341495.
Funding Support: This work is supported by US Public Health Service grants MH093897 (RSD), the Connecticut Mental Health Center, and a National Science Foundation Graduate Research Fellowship (JMD).
Footnotes
Statement of Interest The authors declare no conflicts of interest.
References
- Berman RM, Cappiello A, Anand A, Oren DA, et al. Antidepressant effects of ketamine in depressed patients. Biological psychiatry. 2000;47(4):351–354. doi: 10.1016/s0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- Bespalov AY, van Gaalen MM, Sukhotina IA, Wicke K, et al. Behavioral characterization of the mGlu group II/III receptor antagonist, LY-341495, in animal models of anxiety and depression. European journal of pharmacology. 2008;592(1–3):96–102. doi: 10.1016/j.ejphar.2008.06.089. [DOI] [PubMed] [Google Scholar]
- Brown EJ, Albers MW, Shin TB, Ichikawa K, et al. A mammalian protein targeted by G1-arresting rapamycin-receptor complex. Nature. 1994;369(6483):756–758. doi: 10.1038/369756a0. [DOI] [PubMed] [Google Scholar]
- DiazGranados N, Ibrahim LA, Brutsche NE, Ameli R, et al. Rapid resolution of suicidal ideation after a single infusion of an N-methyl-D-aspartate antagonist in patients with treatment-resistant major depressive disorder. The Journal of clinical psychiatry. 2010;71(12):1605–1611. doi: 10.4088/JCP.09m05327blu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hascup ER, Hascup KN, Stephens M, Pomerleau F, et al. Rapid microelectrode measurements and the origin and regulation of extracellular glutamate in rat prefrontal cortex. Journal of neurochemistry. 2010;115(6):1608–1620. doi: 10.1111/j.1471-4159.2010.07066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jernigan CS, Goswami DB, Austin MC, Iyo AH, et al. The mTOR signaling pathway in the prefrontal cortex is compromised in major depressive disorder. Progress in neuro-psychopharmacology & biological psychiatry. 2011 doi: 10.1016/j.pnpbp.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karasawa J, Shimazaki T, Kawashima N, Chaki S. AMPA receptor stimulation mediates the antidepressant-like effect of a group II metabotropic glutamate receptor antagonist. Brain research. 2005;1042(1):92–98. doi: 10.1016/j.brainres.2005.02.032. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, et al. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R) JAMA: the journal of the American Medical Association. 2003;289(23):3095–3105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- Kingston AE, Ornstein PL, Wright RA, Johnson BG, et al. LY341495 is a nanomolar potent and selective antagonist of group II metabotropic glutamate receptors. Neuropharmacology. 1998;37(1):1–12. doi: 10.1016/s0028-3908(97)00191-3. [DOI] [PubMed] [Google Scholar]
- Koike H, Iijima M, Chaki S. Involvement of the mammalian target of rapamycin signaling in the antidepressant-like effect of group II metabotropic glutamate receptor antagonists. Neuropharmacology. 2011 doi: 10.1016/j.neuropharm.2011.08.034. [DOI] [PubMed] [Google Scholar]
- Larkin GL, Beautrais AL. A preliminary naturalistic study of low-dose ketamine for depression and suicide ideation in the emergency department. The international journal of neuropsychopharmacology / official scientific journal of the Collegium Internationale Neuropsychopharmacologicum. 2011;14(8):1127–1131. doi: 10.1017/S1461145711000629. [DOI] [PubMed] [Google Scholar]
- Li N, Lee B, Liu RJ, Banasr M, et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329(5994):959–964. doi: 10.1126/science.1190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Liu RJ, Dwyer JM, Banasr M, et al. Glutamate N-methyl-D-aspartate receptor antagonists rapidly reverse behavioral and synaptic deficits caused by chronic stress exposure. Biological psychiatry. 2011;69(8):754–761. doi: 10.1016/j.biopsych.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeng S, Zarate CA, Jr., Du J, Schloesser RJ, et al. Cellular mechanisms underlying the antidepressant effects of ketamine: role of alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors. Biological psychiatry. 2008;63(4):349–352. doi: 10.1016/j.biopsych.2007.05.028. [DOI] [PubMed] [Google Scholar]
- Moghaddam B, Adams B, Verma A, Daly D. Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1997;17(8):2921–2927. doi: 10.1523/JNEUROSCI.17-08-02921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohishi H, Ogawa-Meguro R, Shigemoto R, Kaneko T, et al. Immunohistochemical localization of metabotropic glutamate receptors, mGluR2 and mGluR3, in rat cerebellar cortex. Neuron. 1994;13(1):55–66. doi: 10.1016/0896-6273(94)90459-6. [DOI] [PubMed] [Google Scholar]
- Ohishi H, Shigemoto R, Nakanishi S, Mizuno N. Distribution of the messenger RNA for a metabotropic glutamate receptor, mGluR2, in the central nervous system of the rat. Neuroscience. 1993a;53(4):1009–1018. doi: 10.1016/0306-4522(93)90485-x. [DOI] [PubMed] [Google Scholar]
- Ohishi H, Shigemoto R, Nakanishi S, Mizuno N. Distribution of the mRNA for a metabotropic glutamate receptor (mGluR3) in the rat brain: an in situ hybridization study. The Journal of comparative neurology. 1993b;335(2):252–266. doi: 10.1002/cne.903350209. [DOI] [PubMed] [Google Scholar]
- Sengupta S, Peterson TR, Sabatini DM. Regulation of the mTOR complex 1 pathway by nutrients, growth factors, and stress. Molecular cell. 2010;40(2):310–322. doi: 10.1016/j.molcel.2010.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigemoto R, Kinoshita A, Wada E, Nomura S, et al. Differential presynaptic localization of metabotropic glutamate receptor subtypes in the rat hippocampus. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1997;17(19):7503–7522. doi: 10.1523/JNEUROSCI.17-19-07503.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takei N, Kawamura M, Hara K, Yonezawa K, et al. Brain-derived neurotrophic factor enhances neuronal translation by activating multiple initiation processes: comparison with the effects of insulin. The Journal of biological chemistry. 2001;276(46):42818–42825. doi: 10.1074/jbc.M103237200. [DOI] [PubMed] [Google Scholar]
- Tanabe Y, Nomura A, Masu M, Shigemoto R, et al. Signal transduction, pharmacological properties, and expression patterns of two rat metabotropic glutamate receptors, mGluR3 and mGluR4. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1993;13(4):1372–1378. doi: 10.1523/JNEUROSCI.13-04-01372.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi ZX, Baker DA, Shen H, Carson DS, et al. Group II metabotropic glutamate receptors modulate extracellular glutamate in the nucleus accumbens. The Journal of pharmacology and experimental therapeutics. 2002;300(1):162–171. doi: 10.1124/jpet.300.1.162. [DOI] [PubMed] [Google Scholar]
- Zarate CA, Jr., Singh JB, Carlson PJ, Brutsche NE, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Archives of general psychiatry. 2006;63(8):856–864. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]