Abstract
Background/Aims
To evaluate the anticancer activity of fenbendazole, a widely used antihelminth with mechanisms of action that overlap with those of the hypoxia-selective nitroheterocyclic cytotoxins/radiosensitizers and the taxanes.
Materials and Methods
We used EMT6 mouse mammary tumor cells in cell culture and as solid tumors in mice to examine the cytotoxic and antitumor effects of fenbendazole as a single agent and in combination regimens.
Results
Intensive treatments with fenbendazole were toxic to EMT6 cells in vitro; toxicity increased with incubation time and under conditions of severe hypoxia. Fenbendazole did not alter the dose-response curves for radiation or docetaxel; instead, the agents produced additive cytotoxicities. Febendazole in maximally-intensive regimens did not alter the growth of EMT6 tumors, or increase the antineoplastic effects of radiation.
Conclusion
These studies provided no evidence that fenbendazole would have value in cancer therapy, but suggested that this general class of compounds merits further investigation.
Keywords: Fenbendazole, benzamidazoles, tumor microenvironments, radiation therapy, hypoxia, EMT6 cells
Fenbendazole, [5-(phenylthio)-1H-benzimidazol-2-yl]carbamic acid methyl ester, is widely used to treat pinworms, other helminthes, and a variety of parasitic infections in laboratory animals, livestock, companion animals, and people (1–3). We became interested in fenbendazole when our university veterinarians recommended that all experimental rodents, including uninfected colonies such as ours, be treated with a fenbendazole-containing diet because of pinworm infections in some colonies. Our research uses tumors in rats and mice to evaluate the effects of new regimens for treating solid tumors with radiation and/or anticancer drugs (4–10). As we researched this proposed prophylactic treatment, we became concerned that the fenbendazole-containing chow might compromise our experiments, but also became intrigued by the possibility that this drug might have antineoplastic effects, by disrupting the tubulin microtubule equilibrium, or by altering the viability or radiosensitivity of cells in the hypoxic environments found in solid tumors.
Fenbendazole acts on helminthes primarily by binding to tubulin and disrupting the tubulin microtubule equilibrium; its utility as an antiparasitic drug results from differences in the structures of tubulin in mammalian cells and in lower organisms, which lead to its greater binding to tubulin, and therefore greater inhibition of polymerization, in the parasites (11–13). In addition, the limited absorption of fenbendazole from the intestine results in low levels of the drug and its active metabolites in tissue relative to the levels within the gut, to which the targeted parasites are exposed (1, 14, 15).
Several widely used anticancer drugs produce their antineoplastic effects by disrupting either microtubule formation (vincristine; vinblastine) or microtubule depolymerization (paclitaxel; docetaxel) (13, 16), suggesting that fenbendazole could have antitumor effects. Some data in the literature support this hypothesis (13). A Fenbendazole-containing diet combined with supplemental high dose of vitamins was reported by Gao et al. to inhibit growth of a human lymphoma xenografted into scid mice (17); it was unclear whether this reflected a direct effect of the drug on tumor cells or stimulation of host immune responses. Bai et al. reported that fenbendazole reduced the engraftment of brain tumors in nude mice (18). Chung et al. reported in a meeting presentation (19) that high doses of fenbendazole, albendazole and mebendazole inhibited the growth of paclitaxel-resistant tumors.
Solid tumors develop regions of severe hypoxia very early in their development, at diameters of less than 1 mm (5, 6). As tumors grow, they concomitantly elicit the development of their vascular beds through angiogenesis, neovascularization, and co-option of vessels from normal tissues. However, tumor vascular beds lack the organization and regulation found in the vasculature supporting healthy normal tissues. The vessels are tortuous and irregular, and lack the musculature that normally regulates blood flow. Blind ends and shunting are common, and vessels frequently have microscopic and macroscopic holes that permit plasma or blood to leak into surrounding tissue. In addition, the growing tumors often invade or compress blood and lymphatic vessels, further compromising perfusion. As a result, solid tumors contain regions, where temporary interruptions in blood flow through individual vessels or persistent regional deficiencies in perfusion produce transient and chronic hypoxia. Because molecular oxygen is a potent chemical radiosensitizer (4–6, 20), hypoxic tumor cells are resistant to radiation, can survive radiation regimens that would eradicate fully aerobic cell populations, and can cause tumors to recur after radiation therapy. Intensive efforts in our laboratory and many others, have therefore been devoted to improving the outcome of radiation therapy by combining radiation with drugs that improve tumor oxygenation, selectively sensitize hypoxic cells to radiation, or preferentially kill hypoxic cells (4–9, 16, 20, 21).
Several compounds related to fenbendazole have selective effects on hypoxic cells. Some nitroimidazoles and benzimidazoles act as hypoxic cell radiosensitizers, by replacing oxygen in the chemical reactions that lead to the production of DNA damage by ionizing radiation (4, 6, 20, 21). These drugs therefore increase the cytotoxic effects of ionizing radiation in hypoxia, while have no effect on the radiation response of aerobic cells (which are already maximally radiosensitive because of the oxygen levels in their environment). Several modified benzimidazoles synthesized by Gupta et al. were effective hypoxic cell radiosensitizers (21). Moreover, several series of substituted bis-benzimidazoles bind in the minor groove of DNA at specific DNA sequences, thereby inhibiting DNA helicase activity and cell proliferation (22, 23); some bis-benzimidazoles undergo bioreductive activation in hypoxic conditions (24), which could produce selective cytotoxicity toward hypoxic cells (5, 6, 24). Many benzimidazoles alter glucose uptake and carbohydrate metabolism (1, 3, 14), which could produce cytotoxic effects under the conditions of hypoxia, low pH, and nutritional inadequacy occurring in solid tumors.
Because of these considerations, we tested for two possible effects of the fenbendazole-containing therapeutic rodent chow on our primary tumor model system: i) alteration of tumor growth, through a cytotoxic effect of the drug on cells within these solid tumors, and ii) alteration of tumor radiosensitivity (10). We saw no evidence that either effect occurred when our mice were fed the fenbendazole-containing diet, supporting the idea that this prophylactic intervention would not compromise our studies. However, cell culture data from that project also showed that fenbendazole given continuously at doses above those expected in the tissues of rodents fed the therapeutic diet, dramatically inhibited the growth of EMT6 tumor cells in vitro. This prompted us to extend our previously published studies to examine the effects of higher doses of fenbendazole on EMT6 tumor cells in vitro and EMT6 tumors in vivo and to more rigorously define the interactions of this drug with radiation. These studies are reported here.
Materials and Methods
Cells
All experiments were performed using EMT6, a cloned, cell culture-adapted mouse mammary tumor cell line. The origin and characteristics of EMT6 cells in vitro and EMT6 tumors in vivo and the response of the cells and tumors to radiation and many anticancer drugs have been described in our previous publications (4–10).
Cell culture studies
EMT6 cells harvested from exponentially growing monolayers were plated into Petri dishes or glass culture flasks containing Waymouth’s medium supplemented with 15% serum (FetalPlex™) and antibiotics (all from Invitrogen, Carlsbad, CA, USA) (8–10). Cultures were incubated at 37°C in a humid atmosphere of 95% air/5% CO2 for three days and were in the middle of exponential growth at the time of treatment. Cell viability was assayed by trypsinizing and suspending the cells at the end of the treatment, counting the cells with a Coulter counter, and assaying the abilities of the cells to form colonies in cell culture as described in detail previously (4, 7–9). Surviving fractions were calculated by comparing the clonogenicity of treated cells with those of untreated control cells plated on the same day. If the numbers of cells in treated and control cultures differed, cell viability was also assessed using yield-corrected surviving fractions, which consider both the difference in cell number and the difference in clonogenicity (9). Data shown on the graphs are geometric means±SEM from multiple independent experiments. Because some treatment agents used in these experiments are quite effective in killing tumor cells, the surviving fractions in these experiments spanned a wide range (from 1.0 to 0.02); the survival curves are therefore plotted using logarithmic Y axes to allow for rigorous comparison of the data over the full range of the observed survivals.
In experiments examining the effects of hypoxia on cytotoxicity, cultures were grown in glass culture bottles and were made hypoxic by sealing the bottles with rubber gaskets, inserting needles for the influx and efflux of gases, and gassing with a humidified mixture of 95% nitrogen/5% CO2, containing <1 ppm oxygen for 2 h before treatment. Fenbendazole (Sigma, St Louis, MO, USA) was then injected through the septum of the gasket, without breaking the hypoxia, and incubation under hypoxia was continued for 2 h (8, 9). In experiments examining the effects of fenbendazole on the radiation response of hypoxic cells, cultures were grown in permanox Petri dishes (MP Biomedicals, Solon, OH, USA), which allow for rapid outgassing of oxygen from the cultures (4, 8, 9). Immediately after addition of fenbendazole or vehicle, the cultures were placed in stainless steel pressure vessels and gassed with a humidified mixture of 95% nitrogen/5% CO2, containing <1 ppm oxygen for 1 h at 37°C to produce severe hypoxia, then sealed, transported to the irradiator and irradiated. Aerobic cultures were treated analogously, but were incubated under 95% air/5% CO2. Fenbendazole for cell culture studies was dissolved in dimethyl sulfoxide (DMSO; Sigma), diluted in Waymouth’s medium, and added to culture medium. Cell cultures were irradiated with 320 kV X-rays produced by an XRAD irradiator (Precision X-ray, Branford CT, USA) at 12.5 mA, 2 mm Al filtration, and a dose rate of 1.9 Gy/min (hypoxic cells) or 2.4 Gy/min (aerobic cells).
Experiments examining the interactions of fenbendazole and docetaxel (taxotere) were performed using exponentially-growing cultures in Petri dishes. Docetaxel was dissolved in DMSO (both from Sigma, St Louis, MO, USA), diluted in sterile phosphate buffered saline, and added to the culture medium in volumes giving the desired concentrations. 10 μM fenbendazole was added to the appropriate cultures either a few seconds before or 22 h before docetaxel treatment. To allow ready visual comparison of the survival curves for docetaxel alone and in combination with 10 μM fenbendazole, the data are shown as relative surviving fractions, which were calculated using the clonogenicity of either the untreated control cultures (for docetaxel-alone) or the corresponding fenbendazole-treated cultures from the same experiments (for fenbendazole plus docetaxel).
Tumor studies
Protocols for in vivo experiments were reviewed and approved by the Yale Institutional Animal Care and Use Committee; studies were performed in compliance the policies of Yale University, the National Institutes of Health, and the Association for the Assessment and Accreditation of Laboratory Animal Care, and with the principles outlined in the Guide for the Care and Use of Laboratory Animals (25). Tumors were produced by inoculating 2×105 EMT6 tumor cells, suspended in 0.05 ml of culture medium, intradermally into the skin of the shaved right flank of female BALB/cRw mice, 2.5–3 months of age, which had been bred in our specific-pathogen-free production colony (8–10). The three orthogonal dimensions of the tumors were measured three times a week using vernier calipers and tumor volumes were calculated using the formula for a hemiellipsoid, the geometrical form, best approximating their shape (8–10). When tumors reached an average volume of 100 mm3, approximately two weeks after injection, mice were stratified by tumor volume into treatment and control groups, and treated.
Fenbendazole was dissolved in sterile, pyrogen-free physiologic saline and injected i.p.. Mice to be irradiated were anesthetized using 100 mg/kg of ketamine plus 10 mg/kg xylazine and tumors were irradiated locally with 10 Gy of 250 kV x-rays from a Siemens Stabilipan (Malvern, PA, USA) delivered at 15 mA, 2 mm Al filtration and a dose rate of 6.4 Gy per minute, as described previously (8–10). The bodies and limbs of the mice were shielded so that the dose to critical normal tissues was less than 0.5 Gy and did not cause significant injury.
Each tumor was measured three times per week until it reached a volume of 1000 mm3. Tumor growth was compared by calculating the time needed for each tumor to grow from the volume at randomization to four-times that volume, and comparing these times for groups receiving different treatments (8–10, 26). Because the tumors were measured over a range of volumes from ~1 mm3 to ~1000 mm3, the growth curves on the figure are plotted using a logarithmic Y axis, to allow rigorous comparisons of volumes in the different groups over the full range of tumor growth; points are geometric means±SEM of the volumes of the individual tumors within the group.
At each measurement, mice were also weighed and examined for appearance (e.g. fur condition, appearance of eyes) and behavior (e.g. changes in grooming, spontaneous movement, or response to handling, or breathing rate and pattern) to detect any signs of toxicity from the drug, the radiation treatment or the growing tumors; animals were euthanized if pre-specified toxicity levels (10) occurred. After euthanasia, mice were necropsied to assess local infiltration and metastatic spread by the tumor. The lungs (the most common site of metastases for this tumor line) were removed, fixed in Bouin’s solution, washed in 95% ethanol and stored in ethanol. Tumors on the lung surfaces were counted under a dissecting microscope by a blinded observer.
Results
Effects of fenbendazole on the survival of EMT6 cells in culture
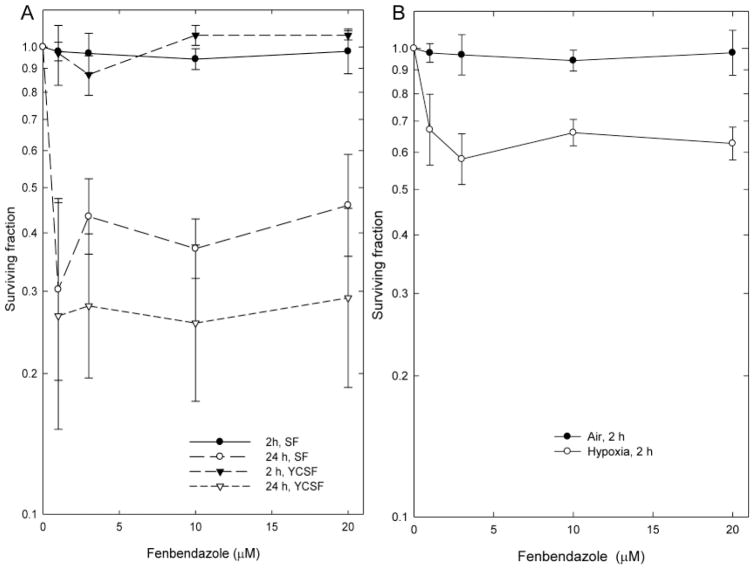
The effect of 2 and 24 h treatments with fenbendazole on the viability of EMT6 cells are shown on Figure 1. The 2-h incubation with fenbendazole was not toxic to aerobic EMT6 cells and produced no changes in the numbers of cells in the monolayer cultures, even at doses approaching the limit of solubility of the drug. The 24-h treatment with fenbendazole resulted in significant decreases in both the numbers of cells in the cultures at the end of treatment and the clonogenicity of those cells. As a result, the yield-corrected surviving fractions were lower than the surviving fractions (Figure 1). Severe hypoxia during treatment increased the toxicity of 2-h treatments with fenbendazole (Figure 1); cell numbers in cultures treated in hypoxia were not significantly different from those in control cultures or cultures treated in air. All survival curves had a similar shape, characterized by a steep decrease in viability at low drug doses, followed by a plateau on which cell viability remained relatively constant as the concentration increased to approach the limit of solubility.
Figure 1.
Effect of graded doses of fenbendazole on the viability of exponentially growing EMT6 cells in cell culture. A: Survival of cells treated with fenbendazole for 2 or 24 h, then assayed for cell survival using a colony formation assay. Survivals are shown both as surviving fractions and as yield-corrected surviving fractions (YCSF), which are corrected for differences in cell number in treated and control cultures at the end of treatment. B: Effect of severe hypoxia on the survival of cells treated for 2 h with fenbendazole. Points are geometric means±SEM of data from three independent experiments.
Effects of fenbendazole on cellular radiosensitivity
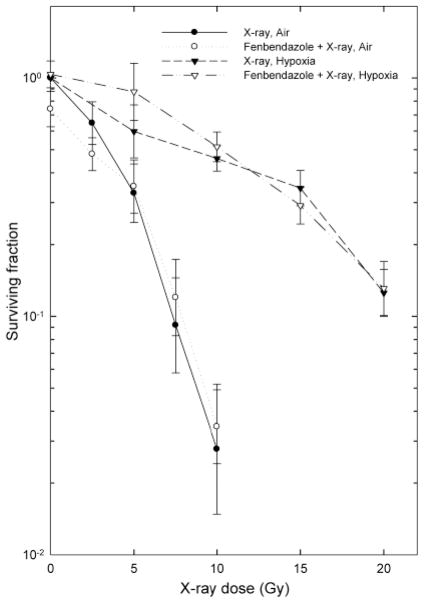
Because the chemical structure of fenbendazole resembles those of compounds known to act as radiosensitizers, we examined the effect of treatment with 10 μM fenbendazole before and during irradiation on the radiation dose-response curves for hypoxic and aerobic cells (Figure 2). Fenbendazole did not alter the radiation response of either aerobic or hypoxic EMT6 cells.
Figure 2.
Effect of treatment with 10 μM fenbendazole on the radiation response of EMT6 cells in vitro. Cultures were treated with graded doses of radiation under aerobic or hypoxic conditions and assayed for cell survival using a colony formation assay. Points are geometric means±SEM of data from three independent experiments.
Experiments with tumors in vivo
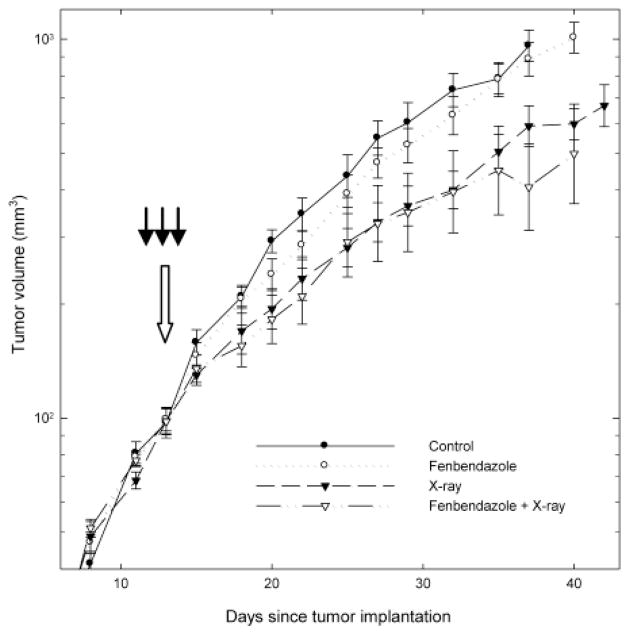
The experiments shown in Figure 3 compared the growth of untreated EMT6 tumors with that of tumors treated with three daily i.p. injections of fenbendazole, 10 Gy of x-rays, or fenbendazole plus x-rays. The growth of the tumors treated with fenbendazole-alone was indistinguishable from that of control tumors (Figure 3). As expected, irradiation of the tumors with 10 Gy produced a significant inhibition of tumor growth. The growth curve for irradiated tumors was not altered by three fenbendazole treatments given one day before, 2 h before, and one day after irradiation (Figure 3).
Figure 3.
Effect of three i.p. injections of Fenbendazole on the growth and radiation response of EMT6 tumors in BALB/cRw mice. Tumor-bearing mice were randomized at a mean tumor volume of 100 mm3 to serve as untreated controls, to receive three daily i.p. injections with 50/mg/kg/day fenbendazole at the times shown by the three dark arrows, to receive 10 Gy of x-rays given at the time indicated by the large open arrow, or to receive Fenbendazole plus x-rays. Points are geometric means±SEM; 7–8 mice/group. Further analyses of these data are shown in Table I.
When each tumor reached a volume of 1000 mm3, the mouse was euthanized and necropsied. Neither local invasion of the body wall nor lymph node metastases were observed in any mice. There were no significant differences in the numbers of lung metastases in Fenbendazole-treated and non-drug-treated mice, either for unirradiated mice (unpaired t-test, p=0.54) or for mice which had received local tumor irradiation (p=0.44).
Tumor growth after different treatments was rigorously compared by calculating the time necessary for each tumor to grow from its volume at the time of randomization for treatment to four times that volume and comparing these times for different groups (Table I). These analyses confirmed that fenbendazole did not alter the growth of unirradiated or irradiated tumors. The Table also includes data from a second experiment examining the effect of the same three-injection fenbendazole regimen on the growth of unirradiated tumors, which confirms the finding from the first experiment (growth curve not shown) and also the time to four-fold volume data from our previous study (10), examining the effect of continuous treatment with a fenbendazole-containing therapeutic diet on the growth of unirradiated and irradiated EMT6 tumors. None of these experiments revealed any significant effect of fenbendazole on the growth or radiation response of EMT6 tumors.
Table I.
Effects of fenbendazole on the growth of EMT6 tumors in BALB/c mice. Tumors in these three experiments were injected i.d.; tumor volume was measured three times per week from the time each tumor became palpable until it reached a volume of 1000 mm3. When tumors reached a mean volume of ~100 mm3, mice were stratified by tumor volume into groups and treated. The time needed for each tumor to grow from its volume at stratification to four-times that volume was calculated. Fenbendazole, whether given in the diet or as three daily i.p. injections, did not alter tumor growth in either unirradiated or irradiated tumors. In addition, fenbendazole did not alter the health of the animals and did not alter the number of spontaneous lung metastases seen on necropsy in any of these experiments.
| Treatment | Days to four-fold volume (mean±SD)
|
|||
|---|---|---|---|---|
| Control | Fenbendazole | 10 Gy | Fenbendazole + 10 Gy | |
| Fenbendazole given as three daily injections, 50 mg/kg/day, i.p. | 10.4±3.0 | 12.4±2.6 | 18.3±5.1 | 17.5±7.5 |
| Growth curves shown in Figure 3 | ||||
| Fenbendazole given as three daily injections 50 mg/kg/day, i.p. | 11.4±3.0 | 11.5±1.0 | ND | ND |
| Growth curves not shown | ||||
| Fenbendazole in diet, 150 ppm, continually during tumor growth | 12.7±0.1 | 12.1±1.2 | 17.5±3.7 | 20.4±2.4 |
| Growth curves published in ref. 10 | ||||
The appearance, behavior, and weights of the mice were monitored at each tumor measurement throughout the course of the experiment shown in Figure 3. There were no differences in the appearance or behavior of the mice in the fenbendazole-treated and non-drug treated groups. ANOVA analyses did not show significant differences between the weights of the mice in the four groups over the course of the experiment. Similar findings were obtained in the other experiments shown on the Table.
Combination of fenbendazole with docetaxel
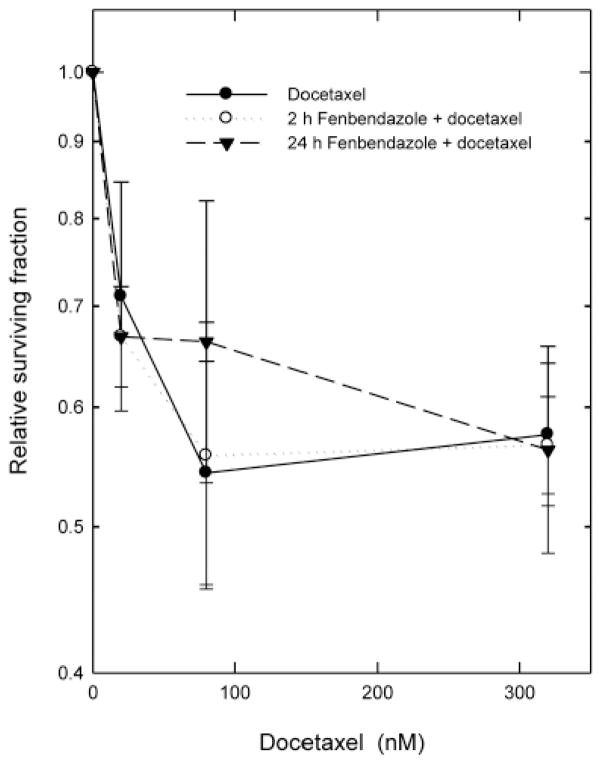
Because of the overlap in the mechanisms of action of fenbendazole and the taxanes, we examined the effect of 10 μM fenbendazole, added to the cultures either a few seconds before or 22 h before docetaxel, on the survival curves for cells treated with graded doses of docetaxel for 2 h in vitro (Figure 4). To allow for ready visual comparison of the survival curves for cells treated with docetaxel-alone and docetaxel in combination with 10 μM fenbendazole, the relative surviving fractions shown on Figure 3 were calculated using the clonogenicity of either the untreated control cultures (for docetaxel-alone) or the corresponding fenbendazole-treated cultures from the same experiments (for fenbendazole plus docetaxel). In agreement with the data shown in Figure 1, the 2-h treatment with fenbendazole had no significant cytotoxicity in these experiments, while the 24-h treatment with fenbendazole reduced the surviving fraction to 0.18±0.02 relative to the untreated control. Fenbendazole did not alter the shape of the dose-response curve for docetaxel. Instead, the survival curves with and without fenbendazole were superimposed when the survivals were normalized to account for the toxicity of fenbendazole-alone. These findings show that the 2 drugs produced additive toxicities, with no evidence for significant interactions between the two agents. Additivity was confirmed by isobologram analyses (27).
Figure 4.
Effect of 10 μM fenbendazole on the response of EMT6 cells to graded doses of docetaxel. Fenbendazole was added to the cultures either a few seconds or 22 h before the start of a 2-h treatment with graded doses of docetaxel. Relative surviving fractions were calculated using either the untreated control cultures (for docetaxel alone) or the corresponding Fenbendazole-treated cultures (for fenbendazole + docetaxel) from the same experiments. Points are geometric means±SEM from three independent experiments.
Discussion
Studies by others (17–19) have shown that fenbendazole can alter the growth of some tumors in mice. Because of this, previous studies in our laboratory examined the effects of a standard commercial therapeutic diet, commonly used to prevent or treat pinworms in experimental rodent colonies on the growth and radiation response of EMT6 tumors, to ascertain whether use of the diet would compromise our experimental cancer therapy studies (10). We saw no evidence that the therapeutic diet altered the growth or radiation response of EMT6 tumors. During that project, limited cell growth studies were performed to ascertain whether EMT6 cultures were sensitive to this drug when given in continuous incubations. Fenbendazole did not produce significant changes in the growth of EMT6 cells in vitro at concentrations of 0.11 and 0.33 μM, but produced striking growth inhibition at doses of 1 and 3 μM. At these higher doses, we also noted changes in the appearance of the cultures: cells in treated cultures were rounder than the control cells and less firmly attached to growth surface, in keeping with effects expected from disruption of the tubulin microtubule equilibrium. The limited data available on the pharmacokinetics of fenbendazole in rodents (1, 14, 15) suggested that the experimental diet used in our previous studies should produce maximal tissue levels of ~0.1 μM or lower. Thus, our cell culture data were in agreement with tumor data for mice fed the fenbendazole-containing antihelminthic diet. However, the in vitro data also showed that higher drug concentrations did have very significant effects on the growth of these tumor cells in culture.
The studies presented here extended this previous work to examine the effects of higher doses of fenbendazole on the viability of EMT6 cells in vitro, using a rigorous colony formation assay to measure for cell survival. These data showed that 24-h incubation with high doses of fenbendazole reduced the clonogenicity of EMT6 cells, as well as reducing the number of cells in the cultures. Fenbendazole, therefore had cytotoxic, as well as cytostatic, effects on these tumor cells when used at high concentrations and with long incubations.
The cytotoxicity of 2-h treatments with fenbendazole increased when tumor cells were treated under severe hypoxia. However, the preferential toxicity of fenbendazole towards hypoxic cells was relatively modest, and the small difference suggests that fenbendazole is unlikely to be of therapeutic utility as a hypoxia-selective anticancer drug.
We also examined the effect of fenbendazole on the radiation response of aerobic and hypoxic EMT6 cells in vitro, using a protocol that we have extensively used in our laboratory to identify and characterize radiosensitizing compounds (5, 6, 8, 9). Fenbendazole did not alter the radiation response of EMT6 cells under either aerobic or hypoxic conditions.
To ensure that the predictions of our cell culture studies translated to tumors in vivo, we tested the effect of fenbendazole on the growth and radiation response of EMT6 tumors, using three daily i.p. injections of fenbendazole at the maximal concentration allowed by the solubility of the drug. This regimen was designed to produce maximal levels of fenbendazole in tumors at the time of irradiation, therefore maximizing the effects of any preferential cytotoxicity to hypoxic tumor cells or any radiosensitization. Because the uptake of oral fenbendazole is very limited (1, 14, 15), we were concerned that the lack of effect seen in our previous studies could have been a false-negative, resulting from poor absorption of the ingested drug rather than from a true lack of antitumor activity. However, the studies reported here showed that i.p. administration of fenbendazole in a maximally intensive three-day regimen also did not alter the growth or radiation response of EMT6 tumors. Overall, our data provide no evidence to support further examination of fenbendazole as a potential agent for the treatment of solid tumors, either alone or in combination with radiation.
The effects of Fenbendazole on the tubulin microtubule equilibrium could synergize with, or antagonize the effects of anticancer drugs having a mechanism of action involving stabilization or disruption of microtubules, such as paclitaxel, docetaxel, vincristine, vinblastine, colchicine or podo-phyllotoxin. Such synergism has been reported for the related benzimidazole, flubendazole (13). To examine this possibility, we examined the effect of treatments with fenbendazole for 2 h (which did not produce significant cytotoxicity) and 24 h (which produced pronounced cytotoxicity), on the survival curves for EMT6 cells treated with graded doses of docetaxel for 2 h. Our data provided no evidence for interactions between these two agents; the toxicities of docetaxel and fenbendazole were strictly additive.
In conclusion, despite the overlap of the mechanisms of action of fenbendazole with those of the hypoxia-selective nitroheterocyclic cytotoxins and radiosensitizers, the taxanes, and the vinca alkaloids, our studies provided no evidence that fenbendazole warrants further testing as a potential agent for use in cancer therapy. However, it is very possible that related compounds could be valuable anticancer drugs. Given the current interest by the US Food and Drug Administration in examining the possibility of ‘repurposing’ previously approved drugs with well-defined characteristics and good toxicology data for new uses, it could be worth exploring other antihelminths developed in the past to ascertain whether this class of agents includes compounds that might be valuable in cancer therapy, either because of significant differential effects on hypoxic cells or through their effects on the tubulin microtubule equilibrium. It is very possible that agents that were not pursued for use as antiparasitic, agents because of greater absorption from the intestines (and therefore greater host toxicity), might provide new anticancer agents or new lead compounds of value in developing novel anticancer drugs.
Acknowledgments
We thank Jacqueline Mendes for her assistance with the experiments. This research was supported by grants P01 CA129186 and CA129186-03S2 from the National Cancer Institute (NCI). Core facilities supported by the Yale Cancer Center and NCI center grant 16359 were used in the performance of the studies.
References
- 1.Keller WC. Fenbendazole. [Last accessed January 2, 2013];WHO Food Additive Series 29. available at http://www.inchem.org/documents/jecfa/jecmono/v29je04.htm.
- 2.Pritchett KR, Johnson NA. A review of treatments for the eradication of pinworm infections from laboratory rodent colonies. Contemp Top Lab Anim Sci. 2002;41:36–46. [PubMed] [Google Scholar]
- 3.Villar D, Cray C, Zaias J, Altman NH. Biological effects of fenbendazole in rats and mice: A review. J Am Assoc Lab Anim Sci. 2007;46:8–15. [PubMed] [Google Scholar]
- 4.Rockwell S. In vivo-in vitro tumor systems: New models for studying the response of tumors to therapy. Lab Anim Sci. 1972;27:831–851. [PubMed] [Google Scholar]
- 5.Rockwell S. Use of hypoxia-directed drugs in the therapy of solid tumors. Semin Oncol. 1992;19:29–40. [PubMed] [Google Scholar]
- 6.Rockwell S, Dobrucki IT, Kim EY, Marrison ST, Vu VT. Hypoxia and radiation therapy: Past history, ongoing research, and future promise. Cur Mol Med. 2009;9:441–459. doi: 10.2174/156652409788167087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rockwell S, Liu Y, Higgins SA. Alteration of the effects of cancer therapy agents on breast cancer cells by the herbal medicine black cohosh. Breast Cancer Res Treat. 2005;90:233–239. doi: 10.1007/s10549-004-4260-x. [DOI] [PubMed] [Google Scholar]
- 8.Rockwell S, Liu Y, Seow HA, Ishiguro K, Baumann RP, Penketh PG, Shyam K, Glazer PM, Sartorelli AC. Preclinical evaluation of laromustine for use in combination with radiation therapy in the treatment of solid tumors. Int J Radiat Biol. 2012;88:277–285. doi: 10.3109/09553002.2012.638359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim EY, Liu Y, Akintujoye OM, Shyam K, Grove TA, Sartorelli AC, Rockwell S. Preliminary studies with a new hypoxia-selective cytotoxin, KS119W, in vitro and in vivo. Radiat Res. 2012;178:126–137. doi: 10.1667/rr2934.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duan Q, Liu Y, Booth C, Rockwell S. Use of fenbendazole-containing therapeutic diets for mice in experimental cancer therapy studies. J Am Assoc Lab Anim Sci. 2012;51:224–230. [PMC free article] [PubMed] [Google Scholar]
- 11.Dawson PJ, Gutteridge WE, Gull K. A comparison of the interaction of antihelminth benzimidazoles with tubulin isolated from mammalian tissue and the parasitic nematode Ascaridia galli. Biochem Pharm. 1984;33:1069–1074. doi: 10.1016/0006-2952(84)90515-x. [DOI] [PubMed] [Google Scholar]
- 12.Lacey E. The role of the cytoskeletal protein, tubulin, in the mode of action and mechanism of drug resistance to benzimidazoles. Int J Paracytol. 1988;7:885–936. doi: 10.1016/0020-7519(88)90175-0. [DOI] [PubMed] [Google Scholar]
- 13.Spagnuolo PA, Hu J, Hurren R, Wang X, Gronda M, Sukhai MA, Di Meo A, Boss J, Ashall I, Zavareh RB, Fine N, Simpson CD, Sharmeen S, Rottapel R, Schimmer AD. The antihelmintic flubendazole inhibits microtubule function through a mechanism distinct from Vinca alkaloids and displays preclinical activity in leukemia and myeloma. Blood. 2009;115:4824–4833. doi: 10.1182/blood-2009-09-243055. [DOI] [PubMed] [Google Scholar]
- 14.Düwel D. Fenbendazole II. Biological properties and activity. Pestic Sci. 1977;8:550–555. [Google Scholar]
- 15.Pritchard RK, Kelly JD, Bolin TD, Duncombe VM, Fagan MR. The effect of iron and protein deficiency on plasma levels and parasite uptake of [14C]fenbendazole in rats infected with Nippostrongylus brasiliensis. Aust J Exper Biol Med Sci. 1981;59:567–573. doi: 10.1038/icb.1981.49. [DOI] [PubMed] [Google Scholar]
- 16.Tannock I, Hill R, Bristow R, Harrington L. The Basic Science of Oncology. 4. McGraw-Hill; New York, NY: 2004. [Google Scholar]
- 17.Bai R-Y, Staedtke V, Aprhys CM, Gallia GL, Riggins GJ. Antiparasitic mebendazole shows survival benefit in two preclinical models of glioblastoma multiforme. Neuro Oncol. 2011;13:974–892. doi: 10.1093/neuonc/nor077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gao P, Dang CV, Watson J. Unexpected antitumorigenic effect of fenbendazole when combined with supplementary vitamins. J Am Assoc Lab Anim Sci. 2008;47:37–40. [PMC free article] [PubMed] [Google Scholar]
- 19.Chung I, Barrows C, Wilson A, Rummel N, Badaruddin S, Mizokami A, Banyard J, Zetter B. Benzimidazole as novel therapeutic agent for metastatic prostate cancer. Poster session presented at the Annual Meeting of the American Association for Cancer Research; Washington DC. April 17–21, 2010. [Google Scholar]
- 20.Overgaard J. Hypoxic radiosensitization: Adored and ignored. J Clin Oncol. 2007;25:4066–4074. doi: 10.1200/JCO.2007.12.7878. [DOI] [PubMed] [Google Scholar]
- 21.Gupta RP, Larroquette CA, Agrawal KC. Potential radiosensitizing agents. 5. 2-Substituted benzimidazole derivatives. J Med Chem. 1982;25:1342–1346. doi: 10.1021/jm00353a014. [DOI] [PubMed] [Google Scholar]
- 22.Seaton A, Higgins C, Mann J, Baron A, Bailly C, Neidle S, van den Berg H. Mechanistic and antiproliferative studies of two novel, biologically-active bis-benzimidazoles. Eur J Cancer. 2003;39:2548–2555. doi: 10.1016/s0959-8049(03)00621-x. [DOI] [PubMed] [Google Scholar]
- 23.Soderlind K-J, Gorodetsky B, Singh AK, Bacher NR, Miller GG, Lown JW. Bis-benzimidazole anticancer agents: targeting human tumor helicases. Anticancer Drug Des. 1999;14:19–36. [PubMed] [Google Scholar]
- 24.Singh AK, Lown JW. Design, synthesis and antitumor cytotoxicity of novel bis-benzimidazoles. Anticancer Drug Des. 2000;15:265–275. [PubMed] [Google Scholar]
- 25.Institute of Laboratory Animal Sciences, National Research Council. Guide for the care and use of laboratory animals. National Academy Press; Washington DC: 1996. [Google Scholar]
- 26.Kallman RF. Rodent Tumors Models in Experimental Cancer Therapy. Plenum Press; New York, NY: 1987. [Google Scholar]
- 27.Steel GG. Terminology in the description of drug radiation interactions. Int J Radiat Oncol Biol Phys. 1979;5:1145–1150. doi: 10.1016/0360-3016(79)90634-5. [DOI] [PubMed] [Google Scholar]