Abstract
Rationale
Attention dysfunction is the hallmark of cognitive deficits associated with major psychiatric illnesses including schizophrenia. Cognitive deficits of schizophrenia have been attributed to reduced function of the N-methyl-D-aspartate (NMDA) receptor or reduced expression of the gamma-aminobutyric acid (GABA)-synthesizing enzyme glutamic acid decarboxylase-67, which presumably leads to attenuated neurotransmission at GABAA receptors.
Objective
The present study used a rodent model to compare the inhibition of NMDA and GABAA receptors, and GAD activity on attention. We tested the impact of inhibiting these proteins brain wide or in the anterior cingulate cortex (ACC), a prefrontal cortex region critical for attentional processing.
Methods
Rats were trained on the three choice serial reaction time task (3-CSRT), an attention test. The impact of systemic or intra-ACC injection of drugs on performance was measured in well-trained rats.
Results
Reducing GABAA receptor function within the ACC with the direct antagonist SR95531 (1 or 3 ng/side) or brain wide using systemic injection of the benzodiazepine inverse agonist FG7142 (5 mg/kg) impaired accuracy and increased omissions. Systemic or intra-ACC inhibition of NMDA receptors using MK-801 (at 3 mg/kg or 3 μg, respectively) also impaired performance. Inhibition of GAD with 3-mercaptopropionic acid, even at high doses, had no effect on 3-CSRT accuracy or omissions when administered systemically or within the ACC.
Conclusions
These data demonstrate that, while tonic stimulation of NMDA and GABAA receptors within the ACC are critical for attentional performance, reduction in GAD activity may have little functional significance and is not indicative of reduced GABA neurotransmission.
Keywords: Schizophrenia, Attention, Glutamate, GABA, Glutamic acid decarboxylase
Introduction
Impaired attention is central to the cognitive deficits associated with most psychiatric illnesses, including schizophrenia (Laurent et al. 1999), attention deficit hyperactivity disorder (Huang-Pollock et al. 2012), and mood disorders (Marvel and Paradiso 2004; Porter et al. 2007). In schizophrenia, dysfunction in cognitive domains including attention is considered to be a core feature of this illness (Gold 2004) and is strongly correlated with positive functional outcome (Green et al. 2000). The Cognitive Neuroscience Research to Improve Cognition in Schizophrenia recently selected “control of attention” as the key biomarker for future research focus (Luck et al. 2012).
Current theories on the etiology of the cognitive deficits of schizophrenia emphasize both glutamate and GABA neurotransmission (Lewis and Moghaddam 2006). Antagonists of the N-methyl-D-aspartate (NMDA) receptors cause schizophrenia-like cognitive deficits in healthy controls including impairments in attention (Brier et al. 1992; Krystal et al. 1994). In addition, postmortem studies in schizophrenia have reported abnormalities pointing toward reduced GABAergic tone, such as reduced expression of the GABA transporter (Volk et al. 2001) and reduced expression of the 67 kDa isoform of the GABA synthesizing enzyme glutamic acid decarboxylase (GAD67) (Akbarian 1995; Volk et al. 2000). These postmortem findings have been linked to abnormal cognition and cortical oscillatory activity in schizophrenia (Gonzalez-Burgos et al. 2010; Uhlhaas and Singer 2010). In vivo measures of GABA using magnetic resonance spectroscopy (MRS) in prefrontal cortex (PFC) subregions of individuals with schizophrenia generally indicate an increase in these levels, in particular in medication-free patients (Kegeles et al. 2012; Ongur et al. 2010), inconsistent with the idea that reduced GAD activity reflects reduced GABA neurotransmission in schizophrenia.
There is very little information to date on the consequences of GAD-related metabolic changes in terms of cognitive function; therefore, it is difficult to gauge the relative importance of this system as a target for pharmacotherapeutic remediation for cognitive deficits. Human imaging (Corbetta et al. 1991) and preclinical (Bussey et al. 1997; Chudasama et al. 2003; Muir et al. 1996; Totah et al. 2009) studies suggest that anterior cingulate cortex (ACC) plays an important role in attention. Furthermore, electrophysiological data indicate that ACC neurons respond during attention and serve to detect errors (Totah et al. 2009). Here, we used a rodent model to investigate the effects of manipulating NMDA or GABAA receptors, or GAD activity within the ACC or brain wide on attention-related behaviors. The behavior task used was the three-choice serial reaction time task (3-CSRT; Totah et al. 2009), which was modified from the five-choice serial reaction time task (5-CSRT; Carli et al. 1983). We find impaired performance after systemic or intra-ACC manipulation of GABAA or NMDA receptors, whereas inhibition of GAD did not have an effect on attentional performance.
Materials and methods
Animals
A total of 62 male Sprague–Dawley rats (Harlan Labs, VT) were used in this study. Of those, 26 were used for systemic injection experiments, and 36 were used for microinjection experiments. Rats were pair-housed on a reverse light cycle (i.e., lights on 7 PM–7 AM) in a temperature and humidity controlled environment. Operant task training was conducted in red light, and rats were allowed free access to water in the home cage but were mildly food deprived by limiting food intake to approximately 15 g/day per animal. All experimental procedures were approved by the University of Pittsburgh Institutional Animal Care and Use Committee prior to the start of the study and were in accordance with the NIH’s Guide to the care and use of laboratory animals.
Three-choice serial reaction time task
This task is based on the 5-CRST (Carli et al. 1983) and has been described in detail elsewhere (Totah et al. 2009, 2012). Briefly, rats were required to attend to one wall of an operant chamber, which displayed three equally spaced cue holes with internal light-emitting diodes, until a brief stimulus light was presented randomly in one of the cue holes. A correct response was counted if the rat responded with a nose poke in the same cue hole within 5 s of the cue being extinguished and resulted in delivery of a 45 mg food pellet in a food trough located on the opposing wall. An incorrect response was registered if the rat responded within 5 s with a nose poke into either of the other two cue holes. Failure to respond within 5 s of the cue being extinguished resulted in an omission, while a nose-poke response before the cue was lit resulted in a premature response. Premature responses led to a “time out” of 5 s in duration where the house light was turned off and nose pokes into the food trough or cue holes had no programmed consequences. Similarly, incorrect responses and omissions also led to a time out; however, this time out differed in that rats could start the next trial by poking into the food trough. Food pellets were not delivered after omissions, incorrect responses, or premature responses. After a trial was completed, an intertrial interval (ITI) of 5 s passed before the start of the next trial. Training occurred in six “levels” of difficulty where the duration of the cue was reduced gradually from 15 s to 300 ms as rats met training criteria at each level. In addition, a criterion of minimal performance was set a priori consisting of completed choice trials (correct and incorrect) of at least 10 % of the total trials completed by the vehicle group in order to obtain meaningful accuracy and omissions measures in the task. Any animals not passing the criterion were eliminated and not included in the final data sets. Rats were trained in 47±2 sessions. This value is similar to that reported in previous studies (Totah et al. 2009).
Surgical procedure
After training, rats used in the microinjection experiment were anesthetized with isoflurane and placed on an electrical heating pad within a stereotaxic frame. A minimal incision was made in the skin over the skull, into which a 2 % lidocaine solution was perfused. Holes were drilled into the skull bilaterally at a stereotaxically determined location, and the dura was removed prior to lowering a bilateral guide cannula, 2 mm above the ACC (anteroposterior, +1.9 mm; mediolateral, +0.7 mm; dorsoventral, −1.4 mm; all measurements relative to bregma), based on the atlas of Paxinos and Watson (1998). The guide cannula was secured in a head cap consisting of dental acrylic and was fastened to the skull using skull screws. Rats were housed individually and were given a minimum of 1 week to recover after surgery.
Drugs
MK801, 3-mercaptopropionic acid (3-MPA), FG7142 (β-carboline-3-carboxylic acid N-methylamide) and SR95531 were obtained from Sigma-Aldrich (St. Louis, MO, USA). For microinjection experiments, all drugs were dissolved in a ringer’s solution containing 145 mM NaCl, 2.7 mM KCl, 1.0 mM MgCl2, and 1.2 mM CaCl2. For experiments using systemic injections, MK801 and 3-MPA were dissolved in a 0.9 % saline solution, while FG7142 was dissolved in deionized water with 2 % Tween 80 (Sigma-Aldrich, St. Louis, MO, USA). For MK801 and SR95531, aliquots were frozen for a maximum of 1 month prior to use, while 3-MPA and FG7142 were mixed freshly before use on each test day.
Drug administration
For local microinjections, drugs were delivered bilaterally through an injection cannula that protruded 2 mm past the end of the guide cannulas. A total volume of 0.5 μL per side at a rate of 0.5 μL/min was injected 15 min before testing. All systemic injections were administered intraperitoneally (i.p.) at a volume of 1 mL/kg of body weight 30 min before testing. The order of all doses, applied systemically or locally, was determined using a randomized Latin square design. In some cases, a rat was used in more than one dose–effect curve. Animals were given a minimum of a 1-week washout period after drug administration and were required to restore baseline performance levels before they could be tested again.
Histology
Rats from microinjection experiments were anesthetized with chloral hydrate (400 mg/kg) and transcardially perfused with saline followed by a 10 % neutral buffered formaldehyde solution. Brains were removed and stored in formaldehyde for several days prior to being cut in serial sections at 250 μm intervals. In order to verify cannula placements, slices were mounted on slides and stained using cresyl violet. Only data from placements within the brain region of interest were used for further analysis (Fig. 1).
Fig. 1.
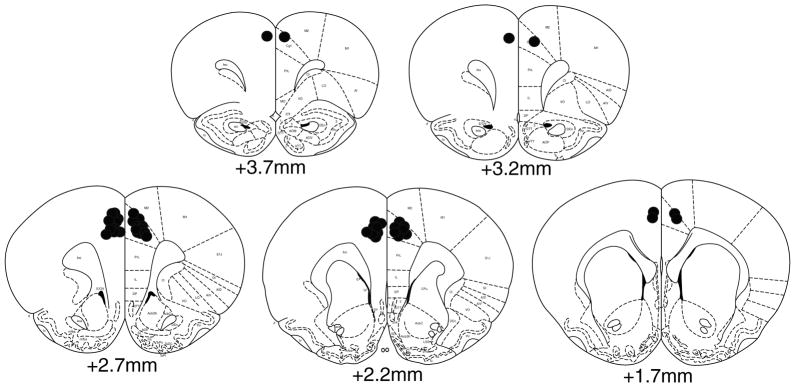
Histology. A schematic representation of cannula placements in the anterior cingulate cortex
Data analysis
The following behavioral measures were analyzed: percent accuracy [100×(number of correct responses/number of correct and incorrect responses)]; percent omissions [100×(number of omissions/number of total trials)]; and total trials and premature (ITI) responses (number per session). Data for each dependent measure (i.e., percent accuracy, percent omissions, premature responses, and total responses) on the test day are expressed as a group mean±SEM. Data from individual test sessions meeting Pierce’s criteria for outliers (Ross 2003) were omitted from the statistical analysis of that dependent measure. Statistical analyses were conducted using one-way between subjects ANOVAs or Student’s t tests where appropriate. Where significant main effects or interactions were indicated, post hoc comparisons were made using the Newman–Keuls test. Statistical significance was set at p<0.05 for all analyses. Data sets were excluded from analysis for the following reasons: incomplete fluid delivery during microinjection, animals’ failure to complete a criterion of minimal performance in a session defined a priori as at least 10 % of total trials completed by vehicle rats, or satisfying Pierce’s criterion for outliers.
Results
Effects of GABA synthesis inhibiton
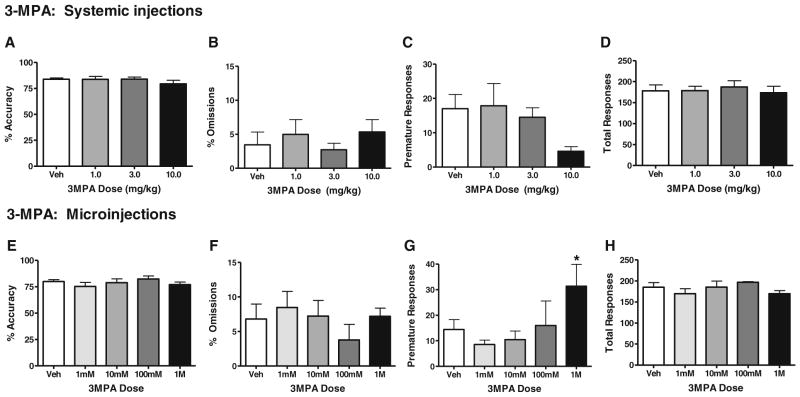
The effects of systemic administration and local microinjections in ACC of the GABA synthesis inhibitor 3-MPA (Lamar 1970) are shown in Fig. 2a–h. Administration (i.p.) of 3-MPA had no effects on accuracy in the 3-CSRT [F(3, 23)=0.74, p=n.s.], omissions [F(3, 23)=0.50, p=n.s.], premature responses [F(3,19)=1.8, p=n.s.], or total responses [F(3, 23)=0.17, p=n.s., n= 6–7/group] (Fig. 2a–d). Examination of 3-MPA’s systemic effects on 3CSRT performance was limited to doses of 10.0 mg/kg or below. Higher doses were found to produce severe seizures, and it was felt that an examination of attention-related behavior after such an event would be neither scientifically useful nor ethical. ACC microinjections were used to examine the effects of higher 3-MPA concentrations on 3CSRT performance.
Fig. 2.
Intra-ACC infusion or systemic administration of 3-MPA has no significant effects on 3-CSRT performance. 3-MPAwas administered i.p. at doses of0, 1, 3, or 10mg/kg (a–d; n=7, 7, 7, and 6, respectively), or locally within the ACC at 0, 1, 10, 100, or 1 M concentrations (e–h; n=9, 7, 6, 3, and 5, respectively). Performance was measured in terms of percent accuracy (a, e), percent omissions (b, f), premature responses (c, g), and total responses (d, h). Data are presented as mean±SEM. Asterisks indicate significant differences from vehicle (*p<0.05; **p<0.01)
ACC microinfusions of the GAD inhibitor 3-MPA at concentrations of 1 mM, 10 mM, 100 mM, or 1M/side had no significant effects on accuracy [F(4, 25)=0.65, p=n.s.], omissions [F(4, 25)=0.38, p=n.s.] or total trials [F(4, 25)=0.72, p=n.s.] (Fig. 2e–h, respectively, n=3–9/group), but a significant overall effect was obtained in premature responding [F(4, 25)=3.12, p<0.05], with post hoc analyses determining a significant increase in premature pokes at the highest microinjection dose of 1 M/side (Fig. 2g). ACC microinfusions of 3-MPA did not visibly produce seizures at any dose tested.
Effects of GABAA receptor inhibition
Given that direct GABAA antagonists produce seizures when injected systemically, we used an inverse agonist at the benzodiazepine receptor (FG7142) to reduce the function of GABAA channels for the systemic injection studies in place of a selective GABAA antagonist (SR95531) that was used in the ACC microinjection studies. FG7142 was administered i.p. to rats prior to performance on the attention task (Fig. 3a–d, n=14–15/group). At the dose of 5 mg/kg, FG7142 significantly impaired accuracy (t27=3.29, p<0.01) and increased omissions (t27=2.45, p<0.05). There was a trend toward reduced premature responses (t27=1.97, p=0.06) but no significant changes in total trials (t27=0.4, p=n.s.). A higher dose of 10 mg/kg also was administered but produced >95% omissions in 6 out of 12 animals, and thus, the impact on accuracy could not be assessed reliably (data not shown).
Fig. 3.
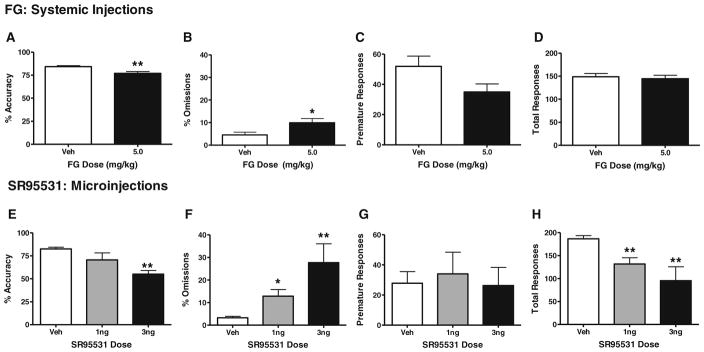
Systemic FG-7142 administration and intra-ACC infusion of SR95531 impair 3-CSRT performance. Rats were administered vehicle or 5 mg/kg of i.p. FG-7142 (a–d; n=15 and 14, respectively) or SR95531 at doses of 0, 1, or 3 ng per side (ACC microinjection e–h; n=8, 5, and 3, respectively). Performance was measured in terms of percent accuracy (a, e), percent omissions (b, f), premature responses (c, g), and total responses (d, h). Data are presented as mean±SEM. Asterisks indicate significant differences from vehicle (*p<0.05; **p<0.01)
The effects of intra-ACC microinjections of the GABAA antagonist SR95531 are depicted in Fig. 3e–h (n=3–8/group). Specifically, ACC microinjections of SR95531 caused dramatic dose-dependent deficits in accuracy [F(2, 13)=7.56, p<0.01] at the 3.0 ng/side dose, as well as omissions [F(2, 13)=14.17, p<0.005] and total trials completed [F(2, 13)=11.71, p<0.005] at the 3.0 and 10.0 ng/side doses (Fig. 3e–h). Analyses of premature responding data indicated no overall ANOVA effects in the attention task [F(2, 13)=0.12, p=n.s.] (Fig. 3g).
Effects of systemic and ACC microinjection administration of the noncompetitive NMDA antagonist MK-801 on attentional performance
The effects of systemic administration and local microinfusions in ACC of the noncompetitive NMDA antagonist MK-801 are shown in Fig. 4a–h. Intraperitoneal injections of MK801 caused significant deficits in accuracy [F(3, 23)= 14.44, p<0.001], omissions [F(3, 23)=19.94, p<0.001], and total trials [F(3, 23)=9.87, p<0.001] (Fig. 4, panels a, b and d, respectively, n=3–8/group). Post hoc analyses comparing individual drug dose groups found significant performance impairments in each of these measures at the 0.3 mg/kg dose. Moreover, MK801 had no significant effects on premature responding in the operant attention task [F(3, 23)=2.00, p=n.s.] at any of the doses tested (Fig. 4c).
Fig. 4.
Systemic or intra-ACC MK-801 administration impairs 3-CSRT performance. MK-801 was administered either i.p. at doses of 0, 0.03, 0.1, or 0.3 mg/kg (a–d; n=8, 8, 8, and 3, respectively) or locally within the ACC at doses of 0, 1, or 3 μg/side (e–h n=15, 11, and 12, respectively). Performance was measured in terms of percent accuracy (a, e), percent omissions (b, f), premature responses (c, g), and total responses (d, h). Data are presented as mean±SEM. Asterisks indicate significant differences from vehicle (*p<0.05; **p<0.01)
Furthermore, the effect of local application of NMDA antagonist MK-801 in the ACC was investigated in the context of 3-CSRT performance (Fig. 4e–h). Microinjections of MK-801 induced a significant overall ANOVA effect in accuracy [F(2,35)=3.4, p<0.05] although individual groups were not significantly different than vehicle-treated animals (Fig. 4e). MK-801 also induced an increase in omissions [F(2, 35)=4.88, p<0.05] on the 3-CSRT (Fig. 4f), with post hoc analyses between individual groups revealing a significant increase at the highest dose of 3.0 μg/side compared to the vehicle condition. Conversely, no significant effects were noted in premature responses [F(2, 33)=1.99, p=n.s.] or total trials completed [F(2, 35)=1.55, p=n.s.] (Fig. 4g and h, respectively, n=11–15/group).
Discussion
Cognitive deficiencies and disturbances in attention assumed a prominent role in the original descriptions of schizophrenia, and extensive psychological research has since characterized the nature of these cognitive deficits (Andreasen 1999; Gold et al. 1999; Goldberg and Weinberger 1987; Payne 1961) and suggests that their frequency and severity are the major contributing factors to impaired social and vocational functioning and poor treatment outcome (Green 1996). In fact, “control of attention” has been selected recently as the key biomarker for future research focus in schizophrenia (Luck et al. 2012). Reduced NMDA receptor function, as well as reduced GABA synthesis and neurotransmission, has been implicated in the cognitive deficits associated with schizophrenia (Adler et al. 1999; Gonzalez-Burgos et al. 2010; Krystal et al. 1994; Lewis et al. 2005). Here, we examined the effects of pharmacological inhibition of the GABA synthesizing enzyme GAD, NMDA, or GABAA receptors on attentional performance in rats. We find that intra-ACC application, as well as systemic administration of GABAA or NMDA receptor inhibitors, dose dependently impaired performance, suggesting that tonic activation of these receptors in the ACC is critical for attention. In contrast, neither systemic nor intra-ACC inhibition of a GAD inhibitor had significant effects on attention-related behavior, suggesting that transient reduction in GAD function does not affect attention.
Local application of the GABAA antagonist SR95531 produced dose-dependent deficits in 3-CSRT performance, characterized by profound impairments in accuracy and omissions. These data are consistent with reports that intra-PFC GABAA receptor activation (Murphy et al. 2012; Paine et al. 2011) or inhibition (Paine et al. 2011) impairs accuracy and increases omissions in the 5-CSRT, as well as other PFC-dependent cognitive tasks (Ragozzino and Rozman 2007), suggesting that GABAA receptor-mediated neurotransmission may have an “inverted U” relationship with attention. Systemic administration of FG7142 similarly impaired accuracy and omissions, but did so in a qualitatively modest fashion. FG7142 is an inverse agonist at benzodiazepine binding site of GABAA receptors and decreases the function of active GABAA receptors by decreasing chloride conductance (Rudolph and Knoflach 2011). These data demonstrate that GABA neurotransmission, systemically and particularly within the ACC, is critical for normal performance in attention tests. Furthermore, the adverse effects of FG7142 are in line with a recent clinical study reporting that another bezodiazepine inverse agonist, iomazenil, worsened symptoms and perceptual alterations in individuals with schizophrenia (Ahn et al. 2011).
Systemic or local inhibition of the GABA synthesizing enzyme GAD, however, did not affect accuracy and omissions at the doses tested. It is possible that our maximum systemic dose of 3-MPA, which was chosen in order to avoid 3-MPA’s known seizure-inducing properties, was insufficient to inhibit GAD to a behaviorally relevant degree. Previous data has shown, however, that 3-MPA causes approximately 90 % GAD inhibition at concentrations of only 10 mM (Lamar 1970). Therefore, the high concentrations of 100 mM and 1 M used in this study are likely sufficient to reduce GAD activity in our preparation beyond the modest reductions reported in postmortem studies (Curley and Lewis 2012). The idea that the concentrations used in this study are physiologically relevant is supported by the significant increases in premature responding observed at the 1 M concentration, as well as the fact that other research groups have found significant behavioral (Paxinos and Watson 1998; Vanini et al. 2008) or neurochemical effects (Herbison et al. 1990) after 3-MPA microinjections at 10–100 mM concentrations. These data underscore the idea that, while GABA neurotransmission within the ACC is critical for attentional performance, reduction in GAD activity may have little functional relevance for this behavior.
There is a relative paucity of data with which to compare our results with the GAD inhibitor, as very few studies have looked at the cognitive effects of manipulating GABA metabolism. Miner and Sarter (1999) found that local administration of antisense oligodeoxynucleotides (ODNs) for the 65 kDa isoform of GAD (GAD65) impaired sustained attention performance. This effect did not extend to local administration of ODNs for GAD67. Moreover, these data are in line with the interpretation that GAD65 is involved in synthesizing GABA for neural transmission, while GAD67 is involved in synthesizing GABA for more general metabolic purposes (Martin and Rimvall 1993). Another study found that a single administration of the GABA transaminase inhibitor vigabatrin significantly decreased accuracy and the number of trials completed (Mazurkiewicz et al. 1992). Thus, in contrast to pharmacological inhibition of GAD, it appears that increasing GABA availability through GABA transaminase inhibition significantly impairs attention. The apparent lack of behavioral effects brought about by transient GAD inhibition may be explained by the fact that GABA synthesis and catabolism are interconnected and tightly regulated by the GABA shunt. This metabolic system serves both to produce and conserve the available supply of GABA and makes it possible to have reduced GABA metabolism without reducing GABA availability.
Consistent with both preclinical (Grottick and Higgins 2000; Paine and Carlezon 2009; Paine et al. 2007) and clinical data (Adler et al. 1999; Krystal et al. 1994) using analogous tasks, our results demonstrate that NMDA receptors are critical for attention. SystemicMK-801 administration significantly reduced accuracy and profoundly increased the number of omitted trials, while also reducing the total number of trials completed. Local MK-801 application in the ACC also caused significant increases in omitted trials, suggesting that MK-801-induced increases in omissions are mediated at least in part by glutamate neurotransmission in the ACC. Moreover, the observation that competitive NMDA receptor inhibition in the medial prefrontal cortex is able to impair performance in the 5-CSRT task both in terms of accuracy and omissions (Carli et al. 2006; Murphy et al. 2012) may point to separable roles for glutamate neurotransmission in these two cortical regions within the context of regulating attention.
GABA neurotransmission has been strongly implicated in the etiology and pathophysiology of psychiatric illnesses, particularly schizophrenia and bipolar depression. Postmortem evidence in schizophrenia points to several GABA abnormalities including increased expression of the GABAA receptors and reductions in GAD67 expression (Akbarian 1995; Thompson et al. 2009; Volk et al. 2000). These postmortem findings have been interpreted as reduced GABA neurotransmission in schizophrenia (Volk and Lewis 2002). However, MRS studies measuring in vivo tissue levels of GABA in individuals with schizophrenia have been equivocal. These studies have reported reductions (Yoon et al. 2010), elevations (Kegeles et al. 2012), or no change (Goto et al. 2009; Tayoshi et al. 2010) in cortical GABA levels. The discrepancies may be attributed to antipsychotic medication in patients. A notable recent report in unmedicated patients with schizophrenia showed a 30 % elevation in GABA levels in medial prefrontal cortex compared with controls (Kegeles et al. 2012). Taken together, these results do not support the hypothesis that cortical GAD67 reductions found by postmortem schizophrenia studies reflect reduced GABA neurotransmission. It may be the case that reduced GAD67 expression in the schizophrenia brain is not a primary pathology, but instead reflects a compensatory response to other changes. A number of preclinical experiments have now demonstrated that reduced GAD67 expression can be caused by altering glutamate neurotransmission during development (Belforte et al. 2010; Turner et al. 2010) or by neonatal ventral hippocampal lesions (Francois et al. 2009; Lipska et al. 2003).
Conclusion
These results suggest that activation of NMDA and GABAA receptors in the ACC plays a critical role in attention as measured by 3-CSRT. In contrast, a transient pharmacological inhibition of GAD activity in the ACC has little functional significance within the context of this task. While it is unknown whether sustained GAD inhibition will have similar effects, the broader implication of these results is that reduced GAD expression in postmortem tissue may not reflect GABA-mediated neurotransmission deficits or be a cause of the attention deficits observed in schizophrenia.
Acknowledgments
This study was supported by National Institute of Mental Health Grant MH84906, Andrew Mellon Foundation Pre-doctoral Fellowship Grant (to N. K. B. T.), and NRSA Training Grant T32 MH018273 (to C. O. B.)
References
- Adler CM, Malhotra AK, Elman I, Goldberg T, Egan M, Pickar D, Breier A. Comparison of ketamine-induced thought disorder in healthy volunteers and thought disorder in schizophrenia. American Journal of Psychiatry. 1999;156:1646–1649. doi: 10.1176/ajp.156.10.1646. [DOI] [PubMed] [Google Scholar]
- Ahn K, Gil R, Seibyl J, Sewell RA, D’Souza DC. Probing GABA receptor function in schizophrenia with iomazenil. Neuropsychopharmacology. 2011;36:677–683. doi: 10.1038/npp.2010.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akbarian Rt. Reduced inhibitory capacity in prefrontal cortex of schizophrenics. Archives of General Psychiatry. 1995;52:267–278. doi: 10.1001/archpsyc.1995.03950160017003. [DOI] [PubMed] [Google Scholar]
- Andreasen NC. A unitary model of schizophrenia—Bleuler’s “fragmented phrene” as schizencephaly. Archives of General Psychiatry. 1999;56:781–787. doi: 10.1001/archpsyc.56.9.781. [DOI] [PubMed] [Google Scholar]
- Belforte JE, Zsiros V, Sklar ER, Jiang Z, Yu G, Li Y, Quinlan EM, Nakazawa K. Postnatal NMDA receptor ablation in corticolimbic interneurons confers schizophrenia-like phenotypes. Nature neuroscience. 2010;13:76–83. doi: 10.1038/nn.2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brier A, Davis O, Buchanan R, Listwak SJ, Holmes C, Pickar D, Goldstein DS. Effects of alprazolam on pituitary-adrenal and catecholaminergic response to metabolic stress in humans. Biological Psychiatry. 1992;32:880–890. doi: 10.1016/0006-3223(92)90177-2. [DOI] [PubMed] [Google Scholar]
- Bussey T, Muir J, Everitt B, Robbins T. Triple dissociation of anterior cingulate, posterior cingulate, and medial frontal cortices on visual discrimination tasks using a touchscreen testing procedure for the rat. Behavioral Neuroscience. 1997;11:920–936. doi: 10.1037//0735-7044.111.5.920. [DOI] [PubMed] [Google Scholar]
- Carli M, Robbins TW, Evenden JL, Everitt BJ. Effects of lesions to ascending noradrenergic neurones on performance of a 5-choice serial reaction task in rats; implications for theories of dorsal noradrenergic bundle function based on selective attention and arousal. Behav Brain Res. 1983;9:361–380. doi: 10.1016/0166-4328(83)90138-9. [DOI] [PubMed] [Google Scholar]
- Carli M, Baviera M, Invernizzi RW, Balducci C. Dissociable contribution of 5-HT1A and 5-HT2A receptors in the medial prefrontal cortex to different aspects of executive control such as impulsivity and compulsive perseveration in rats. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2006;31:757–767. doi: 10.1038/sj.npp.1300893. [DOI] [PubMed] [Google Scholar]
- Chudasama Y, Passetti F, Rhodes SE, Lopian D, Desai A, Robbins TW. Dissociable aspects of performance on the 5-choice serial reaction time task following lesions of the dorsal anterior cingulate, infralimbic and orbitofrontal cortex in the rat: differential effects on selectivity, impulsivity and compulsivity. Behav Brain Res. 2003;146:105–119. doi: 10.1016/j.bbr.2003.09.020. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Miezin FM, Dobmeyer S, Shulman GL, Petersen SE. Selective and divided attention during visual discriminations of shape, color, and speed: functional anatomy by positron emission tomography. J Neurosci. 1991;11:2383–2402. doi: 10.1523/JNEUROSCI.11-08-02383.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curley AA, Lewis DA. Cortical basket cell dysfunction in schizophrenia. J Physiol. 2012;590:715–724. doi: 10.1113/jphysiol.2011.224659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francois J, Ferrandon A, Koning E, Angst MJ, Sandner G, Nehlig A. Selective reorganization of GABAergic transmission in neonatal ventral hippocampal-lesioned rats. Int J Neuropsychopharmacol. 2009;12:1097–1110. doi: 10.1017/S1461145709009985. [DOI] [PubMed] [Google Scholar]
- Gold JM. Cognitive deficits as treatment targets in schizophrenia. Schizophr Res. 2004;72:21–28. doi: 10.1016/j.schres.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Gold S, Arndt S, Nopoulos P, O’Leary DS, Andreasen NC. Longitudinal study of cognitive function in first-episode and recent-onset schizophrenia. American Journal of Psychiatry. 1999;156:1342–1348. doi: 10.1176/ajp.156.9.1342. [DOI] [PubMed] [Google Scholar]
- Goldberg TE, Weinberger DR. Methodological issues in the neuropsychological approach to schizophrenia. Elsevier Science; New York: 1987. [Google Scholar]
- Gonzalez-Burgos G, Hashimoto T, Lewis DA. Alterations of cortical GABA neurons and network oscillations in schizophrenia. Curr Psychiatry Rep. 2010;12:335–344. doi: 10.1007/s11920-010-0124-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto N, Yoshimura R, Moriya J, Kakeda S, Ueda N, Ikenouchi-Sugita A, Umene-Nakano W, Hayashi K, Oonari N, Korogi Y, Nakamura J. Reduction of brain gamma-aminobutyric acid (GABA) concentrations in early-stage schizophrenia patients: 3 T Proton MRS study. Schizophr Res. 2009;112:192–193. doi: 10.1016/j.schres.2009.04.026. [DOI] [PubMed] [Google Scholar]
- Green M. What are the functional consequences of neurocognitive deficits in schizophrenia. American Journal of Psychiatry. 1996;153:321–330. doi: 10.1176/ajp.153.3.321. [DOI] [PubMed] [Google Scholar]
- Green MF, Kern RS, Braff DL, Mintz J. Neurocognitive deficits and functional outcome in schizophrenia: are we measuring the “right stuff”? Schizophr Bull. 2000;26:119–136. doi: 10.1093/oxfordjournals.schbul.a033430. [DOI] [PubMed] [Google Scholar]
- Grottick AJ, Higgins GA. Effect of subtype selective nicotinic compounds on attention as assessed by the five-choice serial reaction time task. Behav Brain Res. 2000;117:197–208. doi: 10.1016/s0166-4328(00)00305-3. [DOI] [PubMed] [Google Scholar]
- Herbison AE, Heavens RP, Dyer RG. Endogenous release of gamma-aminobutyric acid from the medial preoptic area measured by microdialysis in the anaesthetised rat. J Neurochem. 1990;55(5):1617–1623. doi: 10.1111/j.1471-4159.1990.tb04947.x. [DOI] [PubMed] [Google Scholar]
- Huang-Pollock CL, Karalunas SL, Tam H, Moore AN. Evaluating vigilance deficits in ADHD: a meta-analysis of CPT performance. J Abnormal Psychol. 2012;121:360–371. doi: 10.1037/a0027205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kegeles LS, Mao X, Stanford AD, Girgis R, Ojeil N, Xu X, Gil R, Slifstein M, Abi-Dargham A, Lisanby SH, Shungu DC. Elevated prefrontal cortex gamma-aminobutyric acid and glutamate-glutamine levels in schizophrenia measured in vivo with proton magnetic resonance spectroscopy. Arch Gen Psychiatry. 2012;69:449–459. doi: 10.1001/archgenpsychiatry.2011.1519. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD, Heninger GR, Bowers MB, Jr, Charney DS. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry. 1994;51:199–214. doi: 10.1001/archpsyc.1994.03950030035004. [DOI] [PubMed] [Google Scholar]
- Lamar C., Jr Mercaptopropionic acid: a convulsant that inhibits glutamate decarboxylase. J Neurochem. 1970;17:165–170. doi: 10.1111/j.1471-4159.1970.tb02197.x. [DOI] [PubMed] [Google Scholar]
- Laurent A, Saoud M, Bougerol T, d’Amato T, Anchisi AM, Biloa-Tang M, Dalery J, Rochet T. Attentional deficits in patients with schizophrenia and in their non-psychotic first-degree relatives. Psychiatry Res. 1999;89:147–159. doi: 10.1016/s0165-1781(99)00109-2. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Moghaddam B. Cognitive dysfunction in schizophrenia: convergence of gamma-aminobutyric acid and glutamate alterations. Arch Neurol. 2006;63:1372–1376. doi: 10.1001/archneur.63.10.1372. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci. 2005;6:312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- Lipska BK, Lerman DN, Khaing ZZ, Weickert CS, Weinberger DR. Gene expression in dopamine and GABA systems in an animal model of schizophrenia: effects of antipsychotic drugs. Eur J Neurosci. 2003;18:391–402. doi: 10.1046/j.1460-9568.2003.02738.x. [DOI] [PubMed] [Google Scholar]
- Luck SJ, Ford JM, Sarter M, Lustig C. CNTRICS final biomarker selection: control of attention. Schizophrenia bulletin. 2012;38:53–61. doi: 10.1093/schbul/sbr065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin DL, Rimvall K. Regulation of gamma-aminobutyric acid synthesis in the brain. Journal of Neurochemistry. 1993;60:395–407. doi: 10.1111/j.1471-4159.1993.tb03165.x. [DOI] [PubMed] [Google Scholar]
- Marvel CL, Paradiso S. Cognitive and neurological impairment in mood disorders. Psychiatr Clin North Am. 2004;27:19–36. vii–viii. doi: 10.1016/S0193-953X(03)00106-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazurkiewicz M, Sirvio J, Riekkinen P., Sr Effects of single and repeated administration of vigabatrin on the performance of rats in a 5-choice serial reaction time task. Epilepsy Res. 1992;13:231–237. doi: 10.1016/0920-1211(92)90057-z. [DOI] [PubMed] [Google Scholar]
- Miner LA, Sarter M. Intra-accumbens infusions of antisense oligodeoxynucleotides to one isoform of glutamic acid decarboxylase mRNA, GAD65, but not to GAD67 mRNA, impairs sustained attention performance in the rat. Brain Res Cogn Brain Res. 1999;7:269–283. doi: 10.1016/s0926-6410(98)00030-5. [DOI] [PubMed] [Google Scholar]
- Muir JL, Everitt BJ, Robbins TW. The cerebral cortex of the rat and visual attentional function: dissociable effects of mediofrontal, cingulate, anterior dorsolateral, and parietal cortex lesions on a five-choice serial reaction time task. Cerebral Cortex. 1996;6:470– 481. doi: 10.1093/cercor/6.3.470. [DOI] [PubMed] [Google Scholar]
- Murphy ER, Fernando AB, Urcelay GP, Robinson ES, Mar AC, Theobald DE, Dalley JW, Robbins TW. Impulsive behaviour induced by both NMDA receptor antagonism and GABA(A) receptor activation in rat ventromedial prefrontal cortex. Psychopharmacology. 2012;219:401–410. doi: 10.1007/s00213-011-2572-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ongur D, Prescot AP, McCarthy J, Cohen BM, Renshaw PF. Elevated gamma-aminobutyric acid levels in chronic schizophrenia. Biological psychiatry. 2010;68:667–670. doi: 10.1016/j.biopsych.2010.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paine TA, Carlezon WA., Jr Effects of antipsychotic drugs on MK-801-induced attentional and motivational deficits in rats. Neuropharmacology. 2009;56:788–797. doi: 10.1016/j.neuropharm.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paine TA, Tomasiewicz HC, Zhang K, Carlezon WA., Jr Sensitivity of the five-choice serial reaction time task to the effects of various psychotropic drugs in Sprague–Dawley rats. Biol Psychiatry. 2007;62:687–693. doi: 10.1016/j.biopsych.2006.11.017. [DOI] [PubMed] [Google Scholar]
- Paine TA, Slipp LE, Carlezon WA., Jr Schizophrenia-like attentional deficits following blockade of prefrontal cortex GABAA receptors. Neuropsychopharmacology. 2011;36:1703–1713. doi: 10.1038/npp.2011.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic; San Diego: 1998. [DOI] [PubMed] [Google Scholar]
- Payne R. Cognitive abnormalities. Basic Books; New York: 1961. [Google Scholar]
- Porter RJ, Bourke C, Gallagher P. Neuropsychological impairment in major depression: its nature, origin and clinical significance. Aust N Z J Psychiatry. 2007;41:115–128. doi: 10.1080/00048670601109881. [DOI] [PubMed] [Google Scholar]
- Ragozzino ME, Rozman S. The effect of rat anterior cingulate inactivation on cognitive flexibility. Behav Neurosci. 2007;121:698–706. doi: 10.1037/0735-7044.121.4.698. [DOI] [PubMed] [Google Scholar]
- Ross SM. Peirce’s criterion for the elimination of suspect experimental data. J Eng Technol. 2003;20:38–41. [Google Scholar]
- Rudolph U, Knoflach F. Beyond classical benzodiazepines: novel therapeutic potential of GABAA receptor subtypes. Nat Rev Drug Discov. 2011;10:685–697. doi: 10.1038/nrd3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tayoshi S, Nakataki M, Sumitani S, Taniguchi K, Shibuya-Tayoshi S, Numata S, Iga J, Ueno S, Harada M, Ohmori T. GABA concentration in schizophrenia patients and the effects of antipsychotic medication: a proton magnetic resonance spectroscopy study. Schiz research. 2010;117:83–91. doi: 10.1016/j.schres.2009.11.011. [DOI] [PubMed] [Google Scholar]
- Thompson M, Weickert CS, Wyatt E, Webster MJ. Decreased glutamic acid decarboxylase(67) mRNA expression in multiple brain areas of patients with schizophrenia and mood disorders. J Psychiatr Res. 2009;43:970–977. doi: 10.1016/j.jpsychires.2009.02.005. [DOI] [PubMed] [Google Scholar]
- Totah NK, Kim YB, Homayoun H, Moghaddam B. Anterior cingulate neurons represent errors and preparatory attention within the same behavioral sequence. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2009;29:6418–6426. doi: 10.1523/JNEUROSCI.1142-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Totah NK, Jackson ME, Moghaddam B. Preparatory attention relies on dynamic interactions between prelimbic cortex and anterior cingulate cortex. Cerebral Cortex. 2012 doi: 10.1093/cercor/bhs057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner CP, DeBenedetto D, Ware E, Stowe R, Lee A, Swanson J, Walburg C, Lambert A, Lyle M, Desai P, Liu C. Postnatal exposure to MK801 induces selective changes in GAD67 or parvalbumin. Exp Brain Res. 2010;201:479–488. doi: 10.1007/s00221-009-2059-z. [DOI] [PubMed] [Google Scholar]
- Uhlhaas PJ, Singer W. Abnormal neural oscillations and synchrony in schizophrenia. Nat Rev Neurosci. 2010;11:100–113. doi: 10.1038/nrn2774. [DOI] [PubMed] [Google Scholar]
- Vanini G, Watson CJ, Lydic R, Baghdoyan HA. Gamma-aminobutyric acid-mediated neurotransmission in the pontine reticular formation modulates hypnosis, immobility, and breathing during isoflurane anesthesia. Anesthesiology. 2008;109(6):978–988. doi: 10.1097/ALN.0b013e31818e3b1b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk DW, Lewis DA. Impaired prefrontal inhibition in schizophrenia: relevance for cognitive dysfunction. Physiol Behav. 2002;77:501–505. doi: 10.1016/s0031-9384(02)00936-8. [DOI] [PubMed] [Google Scholar]
- Volk DW, Austin MC, Pierri JN, Sampson AR, Lewis DA. Decreased glutamic acid decarboxylase67 messenger RNA expression in a subset of prefrontal cortical gamma-aminobutyric acid neurons in subjects with schizophrenia. Arch Gen Psychiatry. 2000;57:237–245. doi: 10.1001/archpsyc.57.3.237. [DOI] [PubMed] [Google Scholar]
- Volk D, Austin M, Pierri J, Sampson A, Lewis D. GABA transporter-1 mRNA in the prefrontal cortex in schizophrenia: decreased expression in a subset of neurons. Am J Psychiatry. 2001;158:256–265. doi: 10.1176/appi.ajp.158.2.256. [DOI] [PubMed] [Google Scholar]
- Yoon JH, Maddock RJ, Rokem A, Silver MA, Minzenberg MJ, Ragland JD, Carter CS. GABA concentration is reduced in visual cortex in schizophrenia and correlates with orientation-specific surround suppression. J Neurosci. 2010;30:3777–3781. doi: 10.1523/JNEUROSCI.6158-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]