Abstract
Introduction
Hepatocellular carcinoma (HCC) is a leading cause of cancer-related death worldwide. It is traditionally difficult to cure, especially when discovered at later stages, making early diagnosis and intervention of paramount importance. HCC typically arises in the background of chronic liver disease and can have various morphologic appearances. One of the most difficult of these to recognize on early surveillance imaging is the infiltrative subtype, which can account for up to 13% of all HCC cases, and may be more closely associated with background hepatitis B infection.
Discussion
Certain imaging characteristics can provide vital clues, including differing signal intensity on the T1 and T2 sequences of magnetic resonance imaging (MRI) and the presence/appearance of portal vein thrombus. Owing to the diffuse and infiltrating properties of this tumor, surgical resection and transplantation are rarely if ever viable therapeutic options. Other forms of liver-directed therapy have been attempted with limited success, having minimal efficacy and high morbidity. To date, there is no data available to determine if the various HCC subtypes respond to systemic therapy differently, so this may be the most reasonable approach. Left untreated, observed patients commonly progress to hepatic failure fairly rapidly.
Conclusion
Infiltrative HCC can be extremely subtle, and therefore difficult to detect, especially in the background of cirrhosis. Providers caring for patients with hepatitis, chronic liver disease, and cirrhosis must be extremely vigilant in the evaluation of surveillance imaging in order to potentially discover this HCC subtype as early as possible and initiate a multidisciplinary treatment plan.
Keywords: Hepatocellular, Infiltrating, Imaging, Prognosis, Management
Introduction
Hepatocellular carcinoma (HCC) is the sixth most common cancer worldwide and the third leading cause of cancer-related death.1 HCC most commonly arises in the background of chronic liver disease secondary to viral hepatitis or alcohol use, although other causes such as certain environmental exposures and non-alcoholic fatty liver disease have been implicated.2 Strong geographic variations in the incidence of HCC have been well documented with the highest incidence being in Asia. In the USA, a rising incidence of HCC has been noted, which is believed to be related in part to an increase in the prevalence of hepatitis C. The therapeutic options for HCC depend both on tumor-and liver-specific factors. In patients with advanced tumors and no or minimal evidence of hepatic dysfunction, hepatic resection is the mainstay of surgical therapy. In contrast, among patients with cirrhosis and early-stage HCC, transplantation may be the optimal therapy as it addresses both the neoplasm and the underlying liver disease.3 For small HCC (<3 cm), ablation may also be a reasonable therapeutic option.4 Unfortunately, most patients with HCC present with advanced stage disease in the setting of borderline or decompensated liver function, making them ineligible for potentially curative therapeutic options such as resection, transplantation, or ablation. Surveillance to detect early HCC, therefore, is a key component of the care of high-risk patients.
While the evaluation and diagnosis of HCC has become more standardized among high-risk populations, detection of early-stage disease can still be problematic. HCC can present with different morphological subtypes including “focal/nodular,” “massive,” and “diffuse/infiltrating.”5,6 These subtypes can behave differently with regard to etiology, response to treatment, disease progression, as well as presentation. Focal/nodular HCC most commonly presents as an arterially enhancing mass with well-defined margins and an expansive growth type. In contrast, infiltrating HCC can be difficult to identify as it presents as a spreading ill-defined mass that can blend into the background cirrhotic liver on cross-sectional imaging.5–7 As such, infiltrating HCC is often not diagnosed until it has progressed to an advanced stage. While infiltrating HCC accounts for 7% to 13% of HCC cases,6,7 making it not an uncommon HCC subtype, it remains not well-characterized in the literature. We herein review the current approach to the patient with infiltrating HCC. In particular, we highlight the presentation, natural history, and management options for patients with infiltrating HCC. In addition, we emphasize the potential pitfalls of diagnosing infiltrating HCC on cross-sectional imaging by elucidating and illustrating those radiographic criteria that can potentially aid in the earlier identification of infiltrating HCC.
Risk Factors for Infiltrating HCC
Factors specifically associated with the infiltrating subtype of HCC remain ill defined. There are, however, some emerging data that the etiology of the underlying liver disease and morphologic HCC subtype may be related. The carcinogenic pathway involved with hepatitis B virus (HBV)- and hepatitis C virus (HCV)-derived liver damage and tumorogenesis are distinct. HBV is a deoxyribonucleic acid (DNA) virus that integrates itself into the host cell DNA, allowing it to alter expression levels of various cellular proteins.8,9 The integration of HBV double-stranded DNA into the host genome has been shown to enhance expression of the C-myc and N-myc oncogenes and to inactivate the tumor suppressor gene p53.10 These alterations can adversely affect cell cycle control, signal pathways, and apoptosis, thereby leading to an increased risk of carcinogenesis.11 In contrast to HBV, HCV is a positive-stranded RNA virus that does not integrate into the host DNA but rather most likely leads to carcinogenesis by inducing fibrosis and subsequent cirrhosis.12,13 In turn, HBV-induced HCC may be less a function of a chronic inflammatory process,9 while the chronic inflammatory process appears to be more central to HCV-induced carcinogenesis.14 The varying mechanisms of HBV versus HCV carcinogenesis may in turn influence the development of different HCC subtypes.
Emerging data suggest that the infiltrating HCC subtype may arise more commonly in the setting of HBV infection. Benvegnu et al. prospectively followed 401 patients over a median duration of 84 months to compare the incidence, risk factors, and morphologic pattern of HCC development in HBV- and HCV-related cirrhosis.15 During follow-up, 77 (19.2%) patients developed HCC, with a 5- and 10-year cumulative incidence of 10% and 27.5%, respectively. Of note, the pattern of HCC was nodular in 63 (81.8%) patients and infiltrating in 14 (18.2%). Interestingly, the authors found several factors that were not only associated with the incidence of HCC but also the specific morphological subtype. Patients with nodular HCC were more likely to be older, had a longer duration and severity of cirrhosis, but did not have a difference in the incidence of HBV relative to HCV. In contrast, development of infiltrating HCC was unrelated to age and duration of cirrhosis, but was more common among patients infected with HBV as well as HBV+HCV co-infection. The authors concluded that although HBV and HCV infection were both associated with the risk of HCC, distinct features in tumor development and in morphogenesis patterns can be identified, with HBV and HBV+HCV patients having a higher incidence of infiltrating HCC.
In a separate study, Myung et al. reported on 35 patients who had been newly diagnosed with infiltrating HCC.16 Patients with infiltrating HCC were compared with patients who had other morphological subtypes of HCC who had been enrolled during the same period of the study. Similar to the study by Benvegnu et al.,15 the authors found that patients with infiltrating HCC were more commonly positive for HBV than those with other subtypes of HCC. Of note, the authors also noted that during regular follow-up, infiltrating HCC tumors were less readily detectable.
Tumor Markers and Infiltrating HCC
Frequently, α-fetoprotein (AFP) is utilized to frame the level of clinical suspicion for an underlying HCC. The clinical usefulness of AFP to detect HCC and therefore assist in the management of high-risk patients is debatable. Farinati et al.17 reported on a large multi-center Italian experience in which a total of 1,158 patients with HCC were analyzed with reference to serum AFP levels at diagnosis. When using the receiver operating characteristic (ROC) curve, the prognostic reliability of AFP was limited with an area under the curve (AUC) of 0.59. As such, while high AFP levels (i.e., >400 ng/mL) tended to be very specific, AFP was not a very sensitive marker of HCC (sensitivity of only 54%). As such, while an elevated AFP level should significantly heighten one’s suspicion of HCC,5,7 a normal AFP level does not exclude an underlying tumor.7,17
An elevated AFP level has been reported to be indicative of a large tumor burden (size and number), as well as more extensive disease. In the study by Farinati et al., statistical correlations with the AFP level were found for, among other things, tumor size and presence of thrombosis.17 Given that infiltrating HCC frequently presents as a large, diffuse process that is often associated with portal vein thrombosis, one might expect that AFP may be a more sensitive indicator of disease in this subset of patients. Indeed, the infiltrating HCC subtype often may be associated with higher AFP levels perhaps owing to its diffuse permeation of the hepatic parenchyma.5 AFP, however, is not a reliable indicator of the presence or absence of disease even among patients with infiltrating HCC. Rather, the range of AFP levels can be quite variable among patients with infiltrating HCC. Kanematsu et al. reported a small series of 15 patients with infiltrating HCC among whom 14 of 18 (78%) patients had an elevated AFP; in fact, 12 patients with infiltrating HCC had an AFP level >1,000 ng/mL.7 However, as the authors noted, a sizeable subset of patients in the study (n=4; 22%) had a normal serum AFP level. In the study by Farinati et al., among the 1,105 included in the study, 97 patients had an HCC classified as an infiltrating morphological subtype.17 The associated AFP levels in this cohort of patients were <20 ng/mL (36%), 21–400 ng/mL (28%), and >400 ng/mL (36%). Taken together, the data suggest that between one fifth and one third of patients with infiltrating HCC will have a completely normal AFP level and perhaps up to a full one half of patients with infiltrating HCC will have an AFP <400 ng/mL. These data have important implications as they strongly suggest that while AFP levels may be helpful in detecting infiltrating HCC, AFP is not a reliable surveillance or diagnostic tool.7
A host of other possible HCC serum biomarkers has been proposed including des-γ-carboxy prothrombin (DCP), glypican-3, transforming growth factor β1 (TGF), Golgi protein-73 (GP73), and Lens culinaris agglutinin reactive AFP (AFP-L3).18 While each of these have varying degrees of sensitivity and specificity for HCC, AFP-L3 may be particularly relevant to infiltrating HCC. AFP-L3 is an alternative glycoform of AFP that differs in its binding affinity to lectins such as Lens culinaris agglutinin. The relative percent increase in AFP-L3 levels has been noted to be related to progression from moderately differentiated to poorly differentiated tumors.18,19 The sensitivity of AFP-L3 changes with HCC tumor size, with a sensitivity of 80–90% when the tumor diameter is >5 cm.18,20 AFP-L3 is also associated with vascular invasion, and therefore, AFL-L3 may be a marker for the aggressiveness of HCC.18 Tada et al. investigated the pathological features of AFP-L3-positive HCC in order to correlate elevations in this serum biomarker with pathological and morphological variants of HCC.21 Of the 111 patients included in the study, the authors identified 33 (29.7%) who were positive for AFP-L3 (i.e., >10%). The prevalence of HCC with an infiltrating growth pattern and portal vein invasion was significantly higher among patients with an elevated AFP-L3. In fact, patients with an infiltrating HCC had a seven-fold increased chance of having an elevated AFP-L3. The authors reported a sensitivity and specificity of AFP-L3 for infiltrating HCC of 75.0% and 73.8%, respectively.21 Okuda et al. have similarly reported that HCC with an infiltrating growth pattern was often found in patients with elevated AFP-L3.22
Imaging of Infiltrating HCC
Although HCC typically has a very characteristic appearance on cross-sectional imaging, detection of the infiltrating HCC subtype can be a challenge. On computed tomography (CT) imaging, HCC generally presents as a mass that is hypervascular in the arterial phase, followed by relative “washout” in the portal venous phase.23 With the advent of high-speed multi-detector CT scanners, even early nodular HCC can now be detected with a relatively high accuracy.24,25 On magnetic resonance imaging (MRI), most HCC lesions have the classic characteristics of hypointensity on T1-weighted images, hyperintensity on the T2-weighted images, and arterial enhancement with washout in the portal venous phase.26 Some studies have suggested that MRI is superior to CT in its sensitivity to detect HCC,27 although the data are conflicting.28
While these “classic” cross-sectional characteristics of HCC apply to nodular HCC, infiltrating HCC is often much more difficult to detect on cross-sectional imaging studies. The imaging characteristics of diffuse HCC are poorly documented in the literature,7,29 and therefore, radiologists may not be familiar with the radiologic findings associated with this variant of HCC. Infiltrating HCC has a diffuse, permeative appearance on cross-sectional imaging and is difficult to detect in the setting of the heterogeneous background of a cirrhotic liver. This is in fact corroborated by histopathologic analysis, which has described tumor nodules having the same gross appearance as regenerative nodules in the cirrhotic liver.5 Unlike nodular HCC, infiltrating HCC often blends into the background of the cirrhotic liver, and there is no discrete well-defined mass (Fig. 1).7 Infiltrating HCC usually presents as a subtle poorly demarcated area within the liver characterized by heterogeneous or homogeneous abnormal signal intensity. Specifically, on MRI, infiltrating HCC often presents as predominantly hypointense on T1-weighted images. On T2-weighted images, infiltrating HCC usually is homogeneous and mild to moderately hyperintense (Fig. 2). Reflecting the histopathologic findings of extensive micronodules, the initial post-contrast images may show “miliary enhancement” of the area involved by the infiltrating HCC.7 This enhancement can be particularly striking in the setting of portal vein thrombosis, which results in greater contribution of overall hepatic blood supply by the hepatic artery. On gadolinium-enhanced dynamic imaging, most infiltrating HCC will show inhomogeneous areas of enhancement on arterial phase images and corresponding washout on more delayed phases of contrast enhancement (Fig. 3).29 HCC, due to the tightly packed cellular arrangement, causes restricted diffusion of water molecules. This manifests as increased signal on diffusion-weighted images (Fig. 2e) and corresponding low signal on the apparent diffusion coefficient (ADC) map.30
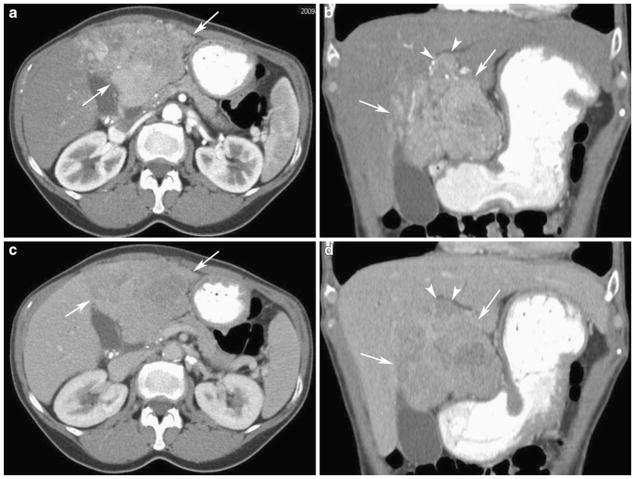
Fig. 1.
Fifty-five-year-old woman with hepatitis C, cirrhosis, and hepatocellular carcinoma. Axial (a) and coronal (b) contrast-enhanced CT in the arterial phase shows a heterogeneously enhancing liver mass in the left hepatic lobe with ill-defined margins (arrows). There is enhancing tumor thrombus in the portal vein (arrowheads). Axial (c) and coronal (d) contrast-enhanced CT in the portal venous phase shows an infiltrative mass in the left hepatic lobe (arrows) with areas of washout consistent with hepatocellular carcinoma
Fig. 2.
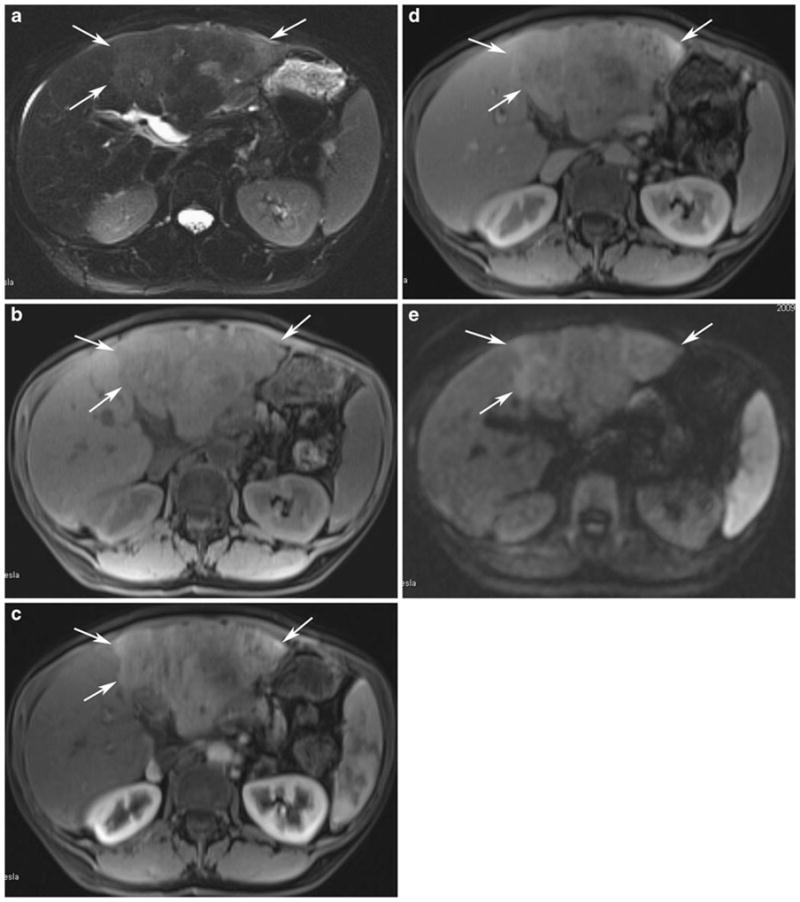
MR images of the same patient as above. a Axial T2-weighted fat saturation image shows subtle T2 hyperintense signal within the infiltrative HCC in the left hepatic lobe (arrows). b Axial precontrast T1-weighted GRE image shows corresponding low T1 signal in the left hepatic lobe tumor (arrows). c Axial postcontrast T1-weighted GRE image in the arterial phase shows heterogeneous enhancement (arrows). d Axial postcontrast T1-weighted GRE image in the portal venous phase demonstrates patchy areas of washout within the tumor (arrows). e Axial diffusion-weighted MRI image shows restricted diffusion within the tumor (arrows)
Fig. 3.
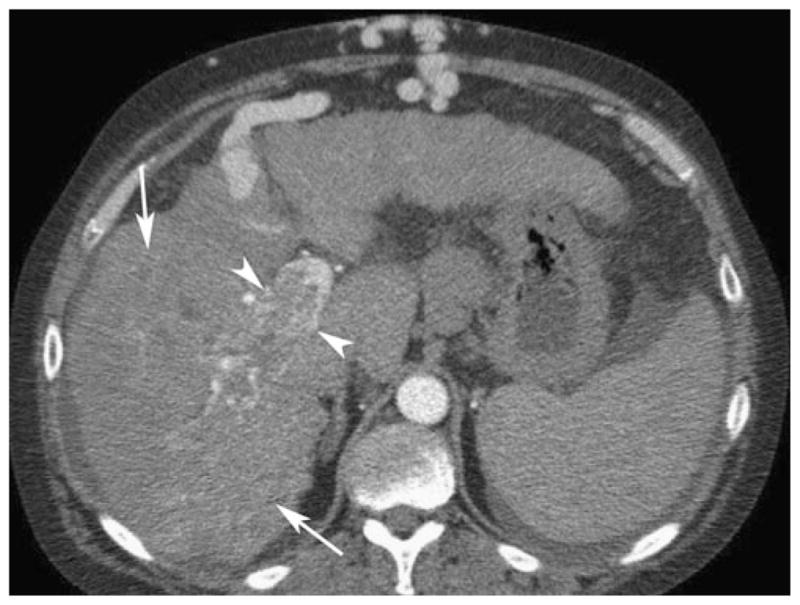
Fifty-three-year-old man with alcoholic cirrhosis and hepatocellular carcinoma. Axial contrast-enhanced CT in the arterial phase shows thrombus in the right portal vein (arrowheads). Small enhancing vessels seen within the thrombus indicate the presence of tumor. The infiltrative HCC (arrows) in the right hepatic lobe is difficult to appreciate. Stigmata of portal hypertension are seen, including ascites and caput medusa
One prominent clue that can be used to identify infiltrating HCC is the presence of portal vein thrombosis, as most patients with infiltrating HCC will have this finding.5,7,26,29 While many patients with cirrhosis and HCC may have portal vein thrombus, the characteristics of the portal vein thrombus in the setting of infiltrating HCC are often unique. The pattern of portal vein invasion with infiltrating HCC is commonly associated with gross distension of the portal vein. When portal vein invasion is extensive, it can fill the peripheral portal vein branches, creating a dilated tumor-filled “cast” of these vessels (Fig. 4). In addition, infiltrating HCC-associated portal vein thrombus commonly displays significant neovascularity or “arterialization” of the tumor thrombus. In fact, it is not uncommon for neovascularity of portal vein thrombus to be the only detectable initial imaging characteristic of an infiltrating HCC. On the corresponding portal venous phase images, tumor thrombus appears as a filling defect in the portal vein, similar to bland thrombus (Fig. 5). Diffusion-weighted images have also emerged as a method of detecting tumor thrombus and differentiating it from bland thrombus. On MRI, portal vein tumor thrombus, similar to the parenchymal mass, has subtle T2 hyperintense signal. The T1 signal is elevated compared to adjacent patent portal veins.
Fig. 4.
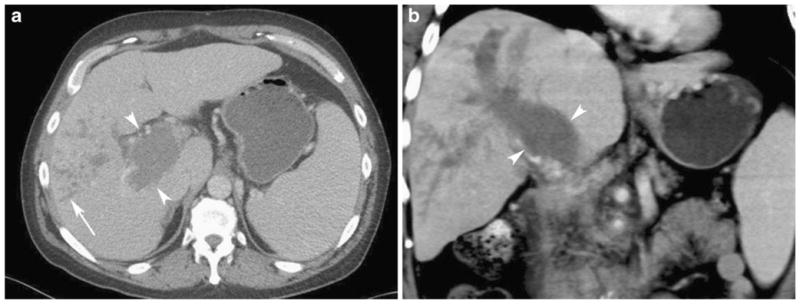
Fifty-three-year-old man with hepatitis C, cirrhosis, and hepatocellular carcinoma. Axial (a) and coronal (b) contrast-enhanced CT in the portal venous phase shows expansion of the portal vein with tumor thrombus (arrowheads). The branching low attenuation structures in the right hepatic lobe represent dilated tumor-filled peripheral portal vein branches (arrow in a)
Fig. 5.
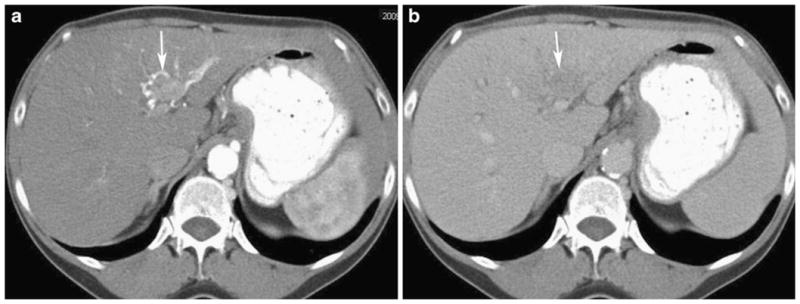
Sixty-six-year-old man with infiltrative hepatocellular carcinoma. a Axial contrast-enhanced CT in the arterial phase shows early enhancement or “arterializations” of the left portal vein (arrow) indicating tumor involvement. b Axial contrast-enhanced CT in the portal venous phase demonstrates tumor thrombus in the left portal vein as a low attenuation filling defect (arrow)
Treatment and Outcomes of Patients with Infiltrating HCC
The prognosis of patients with infiltrating HCC is considerably worse compared with patients who have a focal/nodular subtype. Benvegnu et al. reported that the cumulative probabilities of survival at 1 and 3 years after tumor development were 75.4% and 46.0% in patients with focal/nodular HCC versus 33.3% and 13.6% in patients with infiltrating HCC, independent of treatment received.15 Given that most patients present at a late stage of disease with diffuse disease and associated major vascular invasion, the therapeutic options for infiltrating HCC are limited. Patients with infiltrating HCC are almost always outside the Milan criteria and are not candidates for transplantation. While surgical resection is feasible in patients with large HCC,31 patients with major vascular invasion have a prohibitively poor prognosis with surgical resection.32 In addition, most patients with infiltrating HCC also have advanced cirrhosis, making resection not a tenable option. Several studies have reported on patients who underwent resection or transplant in situations where the infiltrating subtype was underestimated (i.e., a seemingly small tumor nodule is seen, but the remainder of the disease blends into the cirrhotic background) or altogether unrecognized.33,34 Han et al. reported a case involving a 41-year-old patient with HBV who was transplanted for advanced cirrhosis.33 On pathological assessment of the explant, a previously unrecognized infiltrating HCC was detected. While the patient had yet to recur at 18 months of follow-up, the authors noted that pathology revealed that virtually the entire liver parenchyma was replaced with malignant nodules. In a separate study, Ochiai et al. reported that the infiltrating HCC subtype, especially among those patients with an AFP >400 ng/mL, was associated with a very poor prognosis following surgical resection.34 Specifically, patients with an infiltrating HCC subtype had over a three-fold increased risk of disease-specific death following resection with an associated 5-year survival of 16%. Patients with infiltrating HCC almost invariably do very poorly following transplantation or resection, and therefore, an infiltrating morphological subtype should be considered a strong or absolute contraindication to surgical therapy.
Given that surgical options are not applicable to patients with infiltrating HCC, there has been interest in intra-arterial therapy (IAT). IAT, largely in the form of transarterial chemoembolization (TACE), has been advocated as a potentially efficacious modality of liver-directed therapy for infiltrating HCC. IAT involves the delivery of cytotoxic agents via specific arterial tumor-feeding blood vessels, followed by induced ischemia secondary to embolization of those vessels.35 Two randomized controlled trials have demonstrated a statistically significant improvement in survival using TACE as compared to supportive/symptomatic care for HCC—most of which was of the focal/nodular subtype.36,37 The role and efficacy of IAT for diffuse, infiltrating HCC are less defined.
The poor demarcation and difficulty of defining the extent of infiltrating HCC on cross-sectional imaging may impact patient selection, the ability to adequately target the lesion as well as determine subsequent treatment response. Lopez et al. compared patients with unresectable infiltrating versus focal HCC who were treated with TACE.38 In this study, patients in both groups underwent TACE using a drug combination of doxorubicin, cisplatin, and mitomycin. A total of 157 TACE treatments were performed in 88 patients with unresectable HCC: 132 treatments in 69 patients with focal HCC and 25 treatments in 19 patients with infiltrating HCC. Patients with infiltrating HCC did significantly worse following TACE. Specifically, after TACE, patients with infiltrating HCC had a longer hospital stay, more procedure-related mortalities, and a higher readmission rate. In fact, three out of the 19 (16%) patients with infiltrating HCC treated with TACE suffered from a procedure-related death (n=2, liver failure; n=1, tumor rupture). The long-term outcome following TACE for infiltrating HCC was similarly disappointing. While patients with focal HCC had a mean survival of 42.5 months and a 1-year survival of 63%, mean survival following TACE for infiltrating HCC patients was only 10.3 months, and no patient was alive at 1 year. Lopez et al. concluded that their data demonstrated that TACE for infiltrating HCC can be associated with significant morbidity, mortality, and yielded poor long-term outcomes. The authors cautioned that the poor outcomes were related to underestimation of the tumor burden and hepatic functional reserve among patients with infiltrating HCC.38 As such, while IAT should be considered in situations where surgical intervention is not possible, the clinician needs to be cognizant of the possible increased risk of complications among patients with infiltrating HCC. Future studies will need to better define the role, safety, and efficacy of IAT therapy for infiltrating HCC.
While systemic chemotherapy has traditionally been largely ineffectual for HCC, data from the Sorafenib Hepatocellular Carcinoma Assessment Randomized Protocol (SHARP) trial showed an improvement in progression-free survival for HCC patients treated with sorafenib. The median time to radiographic progression was 5.5 months in the sorafenib group and 2.8 months in the placebo group. Overall median survival was also significantly longer in the sorafenib 0group (10.7 months) than in the placebo group (7.9 months; hazard ratio, 0.69).39 Based on these findings, sorafenib is approved for the treatment of advanced HCC. Whether sorafenib is safe and efficacious in the treatment of patients with infiltrating HCC remains, however, to be determined. It is important to note that only a minority of patients in the SHARP trial had HBV infection (19%) or macroscopic vascular invasion (36%), and most patients were Child-Pugh A. Given this, the role of sorafenib for patients with infiltrating HCC—most of whom have HBV, portal vein thrombosis, and more advanced underlying liver disease—is uncertain. While sorafenib and other emerging targeted agents hold some therapeutic promise, these agents will need to be better studied in patients with infiltrating HCC before the relative risks versus benefits can be determined.
Conclusion
Infiltrating HCC is relatively uncommon, representing 7% to 13% of HCC lesions. Infiltrating HCC appears to have a stronger correlation with HBV infection. Timely identification of infiltrating HCC can be challenging as AFP levels are unreliable and the tumor lacks a well-demarcated boundary on cross-sectional imaging. Infiltrating HCC often “blends” into the background of the cirrhotic liver, making it difficult to “see the tree through the forest”. We have here highlighted the important radiologic features of infiltrating HCC. Specifically, infiltrating HCC most commonly is characterized by hypointensity on T1-weighted images and homogeneous, mild to moderately hyperintensity on T2-weighted images. Gadolinium-enhanced MR images usually show patchy contrast enhancement. Most patients with infiltrating HCC will also have portal vein thrombosis with characteristic distention of the vessel and “arterialization” of the thrombus. Due in part to the higher risk of being missed on cross-sectional imaging leading to a late diagnosis, treatment options for infiltrating HCC are limited. Surgery is almost always not a consideration for patients with infiltrating HCC. Unfortunately, liver-directed therapy with IAT is also difficult to apply given the difficulty assessing tumor extent and the reported increased rates of complications. As such, the prognosis of patients with infiltrating HCC is poor with most patients succumbing to disease progression and worsening of underlying liver function. Infiltrating HCC remains a challenging variant of primary liver cancer that demands more attention if we hope to improve the outcome of patients afflicted with this disease.
Contributor Information
Aram Demirjian, Department of Surgery, The Johns Hopkins University School of Medicine, 600 N Wolfe St., Harvey 611, Baltimore, MD 21287, USA.
Peter Peng, Department of Surgery, The Johns Hopkins University School of Medicine, 600 N Wolfe St., Harvey 611, Baltimore, MD 21287, USA.
Jean-Francois H. Geschwind, Department of Interventional Radiology, The Johns Hopkins University School of Medicine, Baltimore, MD, USA
David Cosgrove, Department of Medical Oncology, The Johns Hopkins University School of Medicine, Baltimore, MD, USA.
Jacob Schutz, Department of Radiology, The Johns Hopkins University School of Medicine, Baltimore, MD, USA.
Ihab R. Kamel, Department of Radiology, The Johns Hopkins University School of Medicine, Baltimore, MD, USA
Timothy M. Pawlik, Email: tpawlik1@jhmi.edu, Department of Surgery, The Johns Hopkins University School of Medicine, 600 N Wolfe St., Harvey 611, Baltimore, MD 21287, USA
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55(2):74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362(9399):1907–17. doi: 10.1016/S0140-6736(03)14964-1. [DOI] [PubMed] [Google Scholar]
- 3.Cunningham SC, Tsai S, Marques HP, et al. Management of early hepatocellular carcinoma in patients with well-compensated cirrhosis. Ann Surg Oncol. 2009;16(7):1820–31. doi: 10.1245/s10434-009-0364-1. [DOI] [PubMed] [Google Scholar]
- 4.Huang J, Yan L, Cheng Z, et al. A randomized trial comparing radiofrequency ablation and surgical resection for HCC conforming to the Milan criteria. Ann Surg. 252(6):903–12. doi: 10.1097/SLA.0b013e3181efc656. [DOI] [PubMed] [Google Scholar]
- 5.Okuda K, Noguchi T, Kubo Y, et al. A clinical and pathological study of diffuse type hepatocellular carcinoma. Liver. 1981;1 (4):280–9. doi: 10.1111/j.1600-0676.1981.tb00044.x. [DOI] [PubMed] [Google Scholar]
- 6.Trevisani F, Caraceni P, Bernardi M, et al. Gross pathologic types of hepatocellular carcinoma in Italian patients. Relationship with demographic, environmental, and clinical factors. Cancer. 1993;72(5):1557–63. doi: 10.1002/1097-0142(19930901)72:5<1557::aid-cncr2820720512>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 7.Kanematsu M, Semelka RC, Leonardou P, et al. Hepatocellular carcinoma of diffuse type: MR imaging findings and clinical manifestations. J Magn Reson Imaging. 2003;18(2):189–95. doi: 10.1002/jmri.10336. [DOI] [PubMed] [Google Scholar]
- 8.Chemin I, Zoulim F. Hepatitis B virus induced hepatocellular carcinoma. Cancer Lett. 2009;286(1):52–9. doi: 10.1016/j.canlet.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 9.Brechot C. Pathogenesis of hepatitis B virus-related hepatocellular carcinoma: old and new paradigms. Gastroenterology. 2004;127(5 Suppl 1):S56–61. doi: 10.1053/j.gastro.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 10.Wang XW, Forrester K, Yeh H, et al. Hepatitis B virus X protein inhibits p53 sequence-specific DNA binding, transcriptional activity, and association with transcription factor ERCC3. Proc Natl Acad Sci U S A. 1994;91(6):2230–4. doi: 10.1073/pnas.91.6.2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shiratori Y, Shiina S, Imamura M, et al. Characteristic difference of hepatocellular carcinoma between hepatitis B- and C-viral infection in Japan. Hepatology. 1995;22(4 Pt 1):1027–33. doi: 10.1016/0270-9139(95)90605-3. [DOI] [PubMed] [Google Scholar]
- 12.Castells L, Vargas V, Gonzalez A, et al. Long interval between HCV infection and development of hepatocellular carcinoma. Liver. 1995;15(3):159–63. doi: 10.1111/j.1600-0676.1995.tb00664.x. [DOI] [PubMed] [Google Scholar]
- 13.Vauthey JN, Walsh GL, Vlastos G, Lauwers GY. Importance of field cancerisation in clinical oncology. Lancet Oncol. 2000;1(1):15–6. doi: 10.1016/S1470-2045(00)00105-4. [DOI] [PubMed] [Google Scholar]
- 14.Levrero M. Viral hepatitis and liver cancer: the case of hepatitis C. Oncogene. 2006;25(27):3834–47. doi: 10.1038/sj.onc.1209562. [DOI] [PubMed] [Google Scholar]
- 15.Benvegnu L, Noventa F, Bernardinello E, et al. Evidence for an association between the aetiology of cirrhosis and pattern of hepatocellular carcinoma development. Gut. 2001;48(1):110–5. doi: 10.1136/gut.48.1.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Myung SJ, Yoon JH, Kim KM, et al. Diffuse infiltrative hepatocellular carcinomas in a hepatitis B-endemic area: diagnostic and therapeutic impediments. Hepatogastroenterology. 2006;53(68):266–70. [PubMed] [Google Scholar]
- 17.Farinati F, Marino D, De Giorgio M, et al. Diagnostic and prognostic role of alpha-fetoprotein in hepatocellular carcinoma: both or neither? Am J Gastroenterol. 2006;101(3):524–32. doi: 10.1111/j.1572-0241.2006.00443.x. [DOI] [PubMed] [Google Scholar]
- 18.Malaguarnera G, Giordano M, Paladina I, et al. Serum markers of hepatocellular carcinoma. Dig Dis Sci. 55(10):2744–55. doi: 10.1007/s10620-010-1184-7. [DOI] [PubMed] [Google Scholar]
- 19.Miyaaki H, Nakashima O, Kurogi M, et al. Lens culinaris agglutinin-reactive alpha-fetoprotein and protein induced by vitamin K absence II are potential indicators of a poor prognosis: a histopathological study of surgically resected hepatocellular carcinoma. J Gastroenterol. 2007;42(12):962–8. doi: 10.1007/s00535-007-2117-x. [DOI] [PubMed] [Google Scholar]
- 20.Taketa K. Alpha-fetoprotein: reevaluation in hepatology. Hepatology. 1990;12(6):1420–32. doi: 10.1002/hep.1840120625. [DOI] [PubMed] [Google Scholar]
- 21.Tada T, Kumada T, Toyoda H, et al. Relationship between Lens culinaris agglutinin-reactive alpha-fetoprotein and pathologic features of hepatocellular carcinoma. Liver Int. 2005;25(4):848–53. doi: 10.1111/j.1478-3231.2005.01111.x. [DOI] [PubMed] [Google Scholar]
- 22.Okuda H, Nakanishi T, Takatsu K, et al. Clinicopathologic features of patients with hepatocellular carcinoma seropositive for alpha-fetoprotein-L3 and seronegative for des-gamma-carboxy prothrombin in comparison with those seropositive for des-gamma-carboxy prothrombin alone. J Gastroenterol Hepatol. 2002;17(7):772–8. doi: 10.1046/j.1440-1746.2002.02806.x. [DOI] [PubMed] [Google Scholar]
- 23.Sherman M. The radiological diagnosis of hepatocellular carcinoma. Am J Gastroenterol. 105(3):610–2. doi: 10.1038/ajg.2009.663. [DOI] [PubMed] [Google Scholar]
- 24.Baron RL, Brancatelli G. Computed tomographic imaging of hepatocellular carcinoma. Gastroenterology. 2004;127(5 Suppl 1):S133–43. doi: 10.1053/j.gastro.2004.09.027. [DOI] [PubMed] [Google Scholar]
- 25.de Ledinghen V, Laharie D, Lecesne R, et al. Detection of nodules in liver cirrhosis: spiral computed tomography or magnetic resonance imaging? A prospective study of 88 nodules in 34 patients. Eur J Gastroenterol Hepatol. 2002;14(2):159–65. doi: 10.1097/00042737-200202000-00010. [DOI] [PubMed] [Google Scholar]
- 26.Willatt JM, Hussain HK, Adusumilli S, Marrero JA. MR Imaging of hepatocellular carcinoma in the cirrhotic liver: challenges and controversies. Radiology. 2008;247(2):311–30. doi: 10.1148/radiol.2472061331. [DOI] [PubMed] [Google Scholar]
- 27.Burrel M, Llovet JM, Ayuso C, et al. MRI angiography is superior to helical CT for detection of HCC prior to liver transplantation: an explant correlation. Hepatology. 2003;38(4):1034–42. doi: 10.1053/jhep.2003.50409. [DOI] [PubMed] [Google Scholar]
- 28.Noguchi Y, Murakami T, Kim T, et al. Detection of hepatocellular carcinoma: comparison of dynamic MR imaging with dynamic double arterial phase helical CT. AJR Am J Roentgenol. 2003;180(2):455–60. doi: 10.2214/ajr.180.2.1800455. [DOI] [PubMed] [Google Scholar]
- 29.Kim YK, Han YM, Kim CS. Comparison of diffuse hepatocellular carcinoma and intrahepatic cholangiocarcinoma using sequentially acquired gadolinium-enhanced and Resovist-enhanced MRI. Eur J Radiol. 2009;70(1):94–100. doi: 10.1016/j.ejrad.2008.01.015. [DOI] [PubMed] [Google Scholar]
- 30.Catalano OA, Choy G, Zhu A, et al. Differentiation of malignant thrombus from bland thrombus of the portal vein in patients with hepatocellular carcinoma: application of diffusion-weighted MR imaging. Radiology. 254(1):154–62. doi: 10.1148/radiol.09090304. [DOI] [PubMed] [Google Scholar]
- 31.Pawlik TM, Poon RT, Abdalla EK, et al. Critical appraisal of the clinical and pathologic predictors of survival after resection of large hepatocellular carcinoma. Arch Surg. 2005;140(5):450–7. doi: 10.1001/archsurg.140.5.450. discussion 57–8. [DOI] [PubMed] [Google Scholar]
- 32.Pawlik TM, Poon RT, Abdalla EK, et al. Hepatectomy for hepatocellular carcinoma with major portal or hepatic vein invasion: results of a multicenter study. Surgery. 2005;137(4):403–10. doi: 10.1016/j.surg.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 33.Han YS, Choi DL, Park JB. Cirrhotomimetic type hepatocellular carcinoma diagnosed after liver transplantation—eighteen months of follow-up: a case report. Transplant Proc. 2008;40(8):2835–6. doi: 10.1016/j.transproceed.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 34.Ochiai T, Sonoyama T, Ichikawa D, et al. Poor prognostic factors of hepatectomy in patients with resectable small hepatocellular carcinoma and cirrhosis. J Cancer Res Clin Oncol. 2004;130(4):197–202. doi: 10.1007/s00432-003-0533-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lencioni R. Loco-regional treatment of hepatocellular carcinoma. Hepatology. 52(2):762–73. doi: 10.1002/hep.23725. [DOI] [PubMed] [Google Scholar]
- 36.Lo CM, Ngan H, Tso WK, et al. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology. 2002;35(5):1164–71. doi: 10.1053/jhep.2002.33156. [DOI] [PubMed] [Google Scholar]
- 37.Llovet JM, Real MI, Montana X, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359(9319):1734–9. doi: 10.1016/S0140-6736(02)08649-X. [DOI] [PubMed] [Google Scholar]
- 38.Lopez RR, Jr, Pan SH, Hoffman AL, et al. Comparison of transarterial chemoembolization in patients with unresectable, diffuse vs focal hepatocellular carcinoma. Arch Surg. 2002;137(6):653–7. doi: 10.1001/archsurg.137.6.653. discussion 57–8. [DOI] [PubMed] [Google Scholar]
- 39.Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378–90. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]