Abstract
Purpose
Chest wall (CW) pain has recently been recognized as an important adverse effect of stereotactic body radiation therapy (SBRT) for non–small-cell lung cancer (NSCLC). We developed a dose–volume model to predict the development of this toxicity.
Methods and Materials
A total of 126 patients with primary, clinically node-negative NSCLC received three to five fractions of SBRT to doses of 40–60 Gy and were prospectively followed. The dose–absolute volume histograms of two different definitions of the CW as an organ at risk (CW3cm and CW2cm) were examined for all 126 patients.
Results
With a median follow-up of 16 months, the 2-year estimated actuarial incidence of Grade ≥ 2 CW pain was 39%. The median time to onset of Grade ≥ 2 CW pain (National Cancer Institute Common Terminology Criteria for Adverse Events, Version 3.0) was 9 months. There was no predictive advantage for biologically corrected dose over physical dose. Neither fraction number (p = 0.07) nor prescription dose (p = 0.07) were significantly correlated with the development of Grade ≥ 2 CW pain. Cox Proportional Hazards analysis identified significant correlation with a broad range of dose-volume combinations, with the CW volume receiving 30 Gy (V30) as one of the strongest predictors (p < 0.001). CW2cm consistently enabled better prediction of CW toxicity. When a physical dose of 30 Gy was received by more than 70 cm3 of CW2cm, there was a significant correlation with Grade ≥ 2 CW pain (p = 0.004).
Conclusions
CW toxicity after SBRT is common and long-term follow-up is needed to identify affected patients. A volume of CW ≥ 70 cm3 receiving 30 Gy is significantly correlated with Grade ≥ 2 CW pain. We are currently applying this constraint at our institution for patients receiving thoracic SBRT. An actuarial atlas of our data is provided as an electronic supplement to facilitate data-sharing and meta-analysis relating to CW pain.
Keywords: Stereotactic body radiation therapy, Chest wall, Toxicity, Atlas, Lung
INTRODUCTION
Stereotactic body radiation therapy (SBRT) is increasingly used in the treatment of early-stage non–small-cell lung cancer (NSCLC), particularly in patients with medical conditions precluding surgical management. Chest wall (CW) pain has recently been recognized as an important adverse effect of this treatment (1–3). Symptoms can range from mild and transient to severe and chronic. In some cases, pain may be incompletely relieved despite aggressive medical management (1). Therefore, a robust and clinically applicable normal tissue complication probability model is needed to predict for the development of CW toxicity to reduce the incidence of this complication.
Dunlap et al. recently reported that the CW volume receiving 30 Gy predicted for risk of severe CW pain and/or rib fracture in a heterogeneous group of 60 patients treated with SBRT at the University of Colorado and the University of Virginia (4). They observed a threshold of 30 cm3 before CW toxicity was noted.
We have prospectively followed a larger group of early-stage NSCLC patients treated at Memorial Sloan-Kettering Cancer Center with SBRT, assessing for treatment outcomes and toxicities. The aim of this study was to examine the dose–volume histogram (DVH) of the CW for these patients to determine predictors of Grade ≥ 2 CW pain. We also evaluated two separate delineations of the CW contour as an organ at risk to determine which definition best predicted for CW pain. The larger of the two definitions, incorporating more subcutaneous tissue, was a 3 cm two-dimensional expansion of the lung excluding the lung volume, the mediastinal soft tissue, and the anterior vertebral body. This CW definition was used in the analysis by Dunlap et al. and was chosen so that we could compare results.
For the purposes of outcomes comparisons, meta-analysis, and generation of clinical tolerances, QUANTEC (i.e., Quantitative Analysis of Normal Tissue Effects in the Clinic) recently published recommendations for reporting data on the effects of dose–volume parameters on treatment outcome. They called for comprehensive reporting of the dose–volume dependence of complications and the need to adopt a “data-pooling culture” such that better powered and more widely applicable predictive models of normal tissue toxicity could be built (5, 6). Therefore, to facilitate meta-analysis and increase the utility of this article, we provide an actuarial atlas (7) of the dose–volume incidence of Grade ≥ 2 CW pain in an electronic supplement to this article. If dosimetric, CW structure, and endpoint definitions are compatible, the atlas contains sufficient information for others to combine it with their own data in meta-analysis.
METHODS AND MATERIALS
Inclusion criteria
The subjects of this study were all the patients with primary, clinically node-negative NSCLC, treated with image-guided SBRT at Memorial Sloan-Kettering Cancer Center (MSKCC) between May 2006 and July 2009. These patients were prospectively followed. Exclusion criteria included recurrent lung cancer or metastatic disease from a distant primary, history of prior thoracic radiation, SBRT to more than one lesion, or treatment with less than three or more than five fractions. A total of 126 consecutive patients met these criteria.
Treatment
Patients underwent immobilization using a patient specific alpha cradle or the MSKCC stereotactic body frame, which has been described in detail elsewhere (8). A computed tomography (CT) simulation was performed, which included a respiratory correlated CT scan (RCCT). Patients were treated using multifield beam arrangements with three to seven coplanar, intensity-modulated 6-MV photon beams (median, 4) concentrated on the ipsilateral side. The intensity-modulated radiation therapy (IMRT) was planned with the MSKCC in-house treatment planning system, which uses a radiological path-length–based pencil beam algorithm for tissue inhomogeneity correction (9, 10), and delivered using the sliding window technique on Varian LINACS. The gross tumor volume (GTV) was modified based on the RCCT to generate an internal target volume (ITV) that accounted for respiratory motion. The clinical target volume (CTV) included the ITV plus 0–2 mm margin for microscopic tumor extension. The planning target volume (PTV) included the CTV plus a 5-mm margin in all directions for setup error. The PTV was treated to doses of 40–60 Gy in three to five fractions. The most common fractionation schedules used were 3 × 20 Gy, 3 × 18 Gy, 4 × 12 Gy, and 5 × 9 Gy. Dose was prescribed to the 100% isodose line. Normal tissue constraints adhered to include a 30 Gy maximal point dose for the esophagus, trachea, and ipsilateral main stem bronchus, and a 24 Gy maximal point dose for the spinal cord. For the brachial plexus, maximal point dose constraints of 27 Gy, 32 Gy, and 35 Gy for three-, four-, and five-fraction treatments, respectively, were observed. Lung constraints were a V20 ≤ 12% for both lungs, and V20 ≤ 25% for the ipsilateral lung. The CW was not a constrained structure during treatment planning. Hot spots were not permitted outside of the PTV.
The LINACS used for treatment were equipped with the Varian-On-Board Imager (OBI, Varian, Palo Alto, CA). After initial setup using skin and cradle marks, a KV cone-beam CT (CBCT) was acquired. The GTV visualized on the CBCT was registered to the planning scan GTV, making sure it was well within the ITV contour. The couch was shifted to correct setup errors ≥2 mm in any direction before treatment. Treatment was given every second day with a median treatment time of 7 days (range, 4–19).
Defining the CW
After CT and treatment-planning data were restored from archive, two definitions of the CW as an organ at risk were contoured on all 126 patients. The first CW definition (CW3cm) was defined as previously described by Dunlap et al. (4). This contour was a 3-cm two-dimensional expansion of the ipsilateral lung excluding the lung volume, the mediastinal soft tissue, and anterior vertebral body. In cases where the 3-cm expansion extended outside the body, the contour was brought in to extend only as far as the external patient surface. The CW contour was extended four slices (1.2 cm) above and below the planning target volume. The second CW definition (CW2cm) was defined as CW3cm, except the lung was expanded only 2 cm. An example of the CW2cm and CW3cm structures is shown in Fig. 1. Dose–absolute volume histograms were saved for both definitions of the CW on all 126 patients.
Fig. 1.

The two definitions of the chest wall (CW) as an organ at risk. CW3cm (green) was a 3-cm two-dimensional expansion of the ipsilateral lung excluding the lung volume, the mediastinal soft tissue, and anterior vertebral body. The CW contour was extended 1.2 cm above and below the planning target volume. CW2cm (red) was defined as CW3cm, except the lung was expanded 2 cm. An example of the CW2cm and CW3cm structures is shown.
Follow-up
Follow-up evaluations after the completion of SBRT occurred 1 month after treatment and every 3 months thereafter. A detailed history and physical exam was performed at each follow-up visit. In addition, beginning at 3 months after treatment, a chest CT scan was performed at every follow-up. Toxicities were scored according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 3.0. For pain, Grade 1 was defined as mild pain not interfering with function. Grade 2 was defined as moderate pain; pain or analgesics interfering with function, but not interfering with activities of daily life. Grade 3 was defined as severe pain; pain or analgesics severely interfering with activities of daily life. Grade 4 was defined as disabling pain. Dates on which the patients experienced CW pain were recorded.
Analysis
The time between last treatment and the first onset of Grade ≥ 2 CW pain was chosen for the analysis. The Kaplan-Meier (KM) method was used to estimate the overall incidence of Grade ≥ 2 CW pain, local control, and survival. We also used the log–rank test to assess the significance of patient gender, age, and the number of fractions; and we used the Cox proportional hazards (CPH) method to assess the univariate influence of prescription dose on CW pain. A multivariate CPH model based on prescription dose and number of fractions was also assessed.
To test the impact of the various dose–fractionation schedules used in the study, we analyzed the data in terms of both physical dose and biologically effective dose (BED). The basic dosimetric data came from the absolute dose–absolute volume DVHs for the two CW definitions for each patient. The BED was calculated using the linear quadratic model such that BED = D (1+ (β/ α) D/N), where D is the total dose and N is the number of fractions, for α/β ratios ranging from 2 to 10 Gy, including an α/β ratio of 3 Gy, which is often used for late effects (11, 12). To test the significance of different dose (or BED)/volume combinations in relation to CW pain, statistical analysis using the CPH model and the log–rank test was performed. For the Cox models, those based on the volume exposed to a given dose D (VD) were constructed for values of D from zero to the maximum dose in the total population at intervals of 1 Gy; those based on the dose received by a given volume (DV) were constructed for values of V from zero to the maximum volume in the total population at intervals of 1 cm3. For the log–rank test, at each dose D, patients with volumes VD ≥ Vmed(D) were compared with those with VD < Vmed(D), where VD is the volume exposed to at least D, and Vmed(D) is the median volume exposed to at least D in the patient population. Doses D were chosen at 1 Gy intervals.
Provision of data for meta-analysis
To facilitate use of this data set in meta-analysis, the dose–volume, complication, and follow-up information are provided in an electronic supplement in the form of actuarial dose-volume atlases (7). If dosimetric, CW structure, and endpoint definitions are compatible, the atlases contain sufficient information for others to combine them with their own data in meta-analysis. A dose–volume atlas is a summary of all the patient DVHs and complication information for the endpoint in question. In its most basic form, an atlas is a two-dimensional plot with the same axes as conventional DVHs. At each point on a grid of locations (Di,Vj), we record two numbers: np(Di,Vj) and nc(Di,Vj). np(Di,Vj) is the number of patients with at least volume Vj of their organ exposed to at least dose Di (i.e., the number of patients whose DVHs pass through or above the point (Di,Vj)), and nc(Di,Vj) is the number of those patients with complications. Note that along the line Vj = 0, we count patients whose maximum dose, Dmax, is at least Di. The raw incidence of the complication for patients exposed to at least this dose and volume is nc(Di,Vj)/np(Di,Vj).
In our study, the patient follow-up time was variable and comparable with the time to onset of CW pain. Therefore, the data we provide must enable others to account for the temporal pattern of censoring and its influence on the probability of observing complications. To adapt the atlas to this circumstance requires the addition of time as another dimension, either follow-up time if the patient does not develop the complication, or time to onset of the complication. Specifically, np(Di,Vj,tk) is the number of patients whose DVHs pass through or above (Di,Vj) who either developed the complication or were censored at or before the time tk, whereas nc(Di,Vj,tk) is the number of those patients who developed the complication.
Further details of the format of the atlases, together with instructions for the use of this material in actuarial analysis are presented in Appendix e1. The atlases are included in a zipped file Appendix e2. The atlas consists of Microsoft Excel files, which are compressed by WinRAR, software that can be downloaded from the website http://www.win-rar.com/download.html. Links to this supplementary material can be found at the red journal web site (www.redjournal.org), alongside the links to the electronic version of this article.
RESULTS
Patients
Patient and tumor characteristics are described in Table 1. The median patient age was 77 years and the median tumor diameter on pretreatment imaging was 2.1 cm. Seventy-five percent of patients had T1, 23% had T2, and 2% had T3 tumors. With a median follow-up time of 16 months, the 2-year KM estimated local control and overall survival rates were 93% and 61%, respectively (Fig. 2).
Table 1.
Patient and tumor characteristics
| Characteristic | Median (Range) |
|---|---|
| Age (y) | 77 (55–95) |
| Follow-up (months) | 16 (3–43) |
| Dose (Gy) | 54 (40–60) |
| Treatment time (days) | 7 (4–19) |
| Total fractions (n) | 3 (3–5) |
| 3 fractions | 73 |
| 4 fractions | 38 |
| 5 fractions | 15 |
| Tumor diameter (cm) | 2.1 (0.6–8.0) |
| T stage | |
| T1a | 63 |
| T1b | 32 |
| T2a | 27 |
| T2b | 2 |
| T3 | 2 |
| Tumor location | |
| Right superior | 41 |
| Right inferior | 26 |
| Right middle | 11 |
| Left superior | 26 |
| Left inferior | 22 |
| Histology | |
| Adenocarcinoma | 93 (74%) |
| Unspecified non–small-cell lung cancer | 2 (2%) |
| Not determined | 1 (1%) |
| Squamous | 30 (24%) |
Fig. 2.
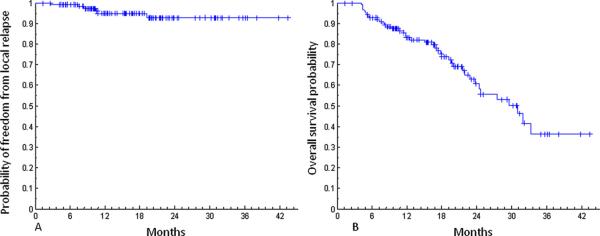
Freedom from local relapse (A) and overall survival (B) probability in 126 patients.
Chest wall toxicity
Of the 126 patients treated with SBRT, 19 (15%) developed Grade 1 CW pain, 16 (13%) developed Grade 2 CW pain, and 19 (15%) developed Grade 3 CW pain (Table 2). No patients to date have developed Grade 4 CW pain. Figure 3 demonstrates the KM curve for incidence of Grade ≥ 2 CW pain for the entire group. The 2-year estimated actuarial incidence of Grade ≥ 2 CW pain was 39%. The median time to onset of Grade ≥ 2 CW pain was 9 months (range, 1–36). The symptoms of 18 of the 35 patients in this study who developed Grade ≥ 2 CW pain have resolved. In patients with a history of Grade ≥ 2 CW pain, the median time to resolution of CW pain was 6 months (range, 1–27). Patients with CW pain were generally managed with one or a combination of nonsteroidal anti-inflammatory drugs, opioids, topical anesthetics, or the anticonvulsant gabapentin.
Table 2.
Chest wall toxicity information (n = 126)
| Variable | Value |
|---|---|
| Pain | |
| Grade 1 | 19 (15%) |
| Grade 2 | 16 (13%) |
| Grade 3 | 19 (15%) |
| Grade 4 | 0 |
| Rib fracture | 5 (4%) |
Fig. 3.

Kaplan-Meier curve describing cumulative incidence of developing Grade ≥ 2 chest wall pain.
Five (4%) patients developed a total of eight rib fractures believed to be related to radiation treatment. The median time to rib fracture diagnosis was 27 months (range, 21–32). Three patients with rib fractures were asymptomatic at the time of diagnosis and two patients had Grade 3 CW pain. Of the three patients who were asymptomatic at the time of rib fracture diagnosis, one had developed transient Grade 1 CW pain 17 months prior, whereas the other 2 patients had no history of CW pain.
Correlation of CW pain with dose–volume parameters
Median values of absolute dose–absolute volume histograms together with envelopes containing the central 68% and 95% of the histograms for patients with and without Grade ≥ 2 CW pain for the CW2cm and the CW3cm definitions of the CW are shown in Fig. 4. CPH analysis of the physical dose–volume effects found broad regions of dose and volume that significantly predicted the development of Grade ≥ 2 CW pain for both definitions of the CW (Fig. 5, A–D). For CW2cm, the CPH analysis indicated significant correlations of CW pain with VD for D between 9 and 47 Gy, and with DV for V up to 506 cm3. For CW3cm, VD and DV were significantly correlated with CW pain when D was between 9 and 46 Gy, and when V was less than 782 cm3, respectively.
Fig. 4.
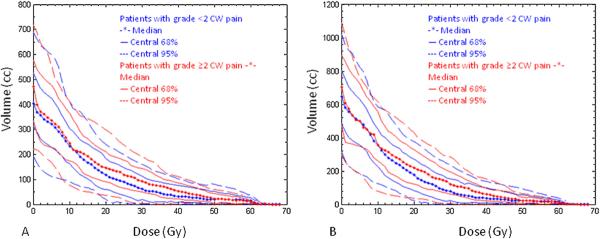
Median values of absolute dose-absolute volume histograms (stars), together with envelopes containing the central 68% (solid lines) and 95% (dashed lines) of the histograms for patients with (red) and without (blue) Grade ≥ 2 chest wall pain, for the CW2cm (A) and the CW3cm (B) definitions of the chest wall.
Fig. 5.
Cox proportional hazards analysis evaluating the significance of dose-volume variables of Grade $ 2 chest wall pain incidence. For CW2cm, the volume exposed to a given dose D (VD) (A) was a better model than the dose received by a given volume (DV) (B), and the 68% and 95% confidence intervals of the best fit were (19.8 Gy, 31.8 Gy) and (16.2 Gy, 38.2 Gy) respectively (C). For CW3cm also, the VD (D) was a better model than the DV (E), and the 68% and 95% confidence intervals of the best fit were (18.9 Gy, 24.2 Gy) and (15.8 Gy, 34.7 Gy), respectively (F). The dose variable in these plots is physical dose (β/α = 0).
Best CPH models were those based on VD. Likelihood values for the CPH models show that to 68% confidence, the best CW2cm VD models had D between 19.8 and 31.8 Gy (for 95% confidence, D was between 16.2 and 38.2 Gy). For the CW3cm VD models, the 68% confidence interval for D was 18.9–24.2 Gy (the 95% confidence interval for D was 15.8–34.7 Gy). These ranges are consistent with the regions of separation of the DVH distributions of patients with and without complications seen in Figs. 4A and B. For the CW2cm, V30 was one of the strongest predictors of CW pain (p < 0.001) and for CW3cm, V30 was within the 95% confidence interval of the strongest predictor. The fitted parameters and their uncertainties for CPH models based on V30 and V20 for both of the CW definitions are given in Table 3. The CW volume receiving 30 Gy was previously reported by others to best predict for severe CW pain and/or rib fracture and these authors recommended that the V30 be limited if possible to <30 cm3 (4). We therefore further analyzed the 30 Gy dose level in our group of patients, using the log–rank test to evaluate the significance of KM curves of complication incidence for volumes above and below 30 cm3 and other prospectively selected cut points.
Table 3.
Parameter B of Cox proportional hazards models for V20 and V30 for the two chest wall definitions
| B(CW2cm, VD) (cm−3) | B(CW3cm, VD) (cm−3) | |
|---|---|---|
| V20 | 0.0086 ± 0.0025 | 0.0058 ± 0.0017 |
| V30 | 0.011 ± 0.0032 | 0.007 ± 0.0022 |
The hazard function in the Cox proportional hazards model is given by: h(t) = h0(t) * exp(B*VD). Here exp(B) is the relative risk for patients with volumes increased by 1 cm3.
In our study, just 19 of 126 patients met the cut point of V30 <30cm3 and the cut point of 30 cm3 did not significantly predict for Grade ≥ 2 CW pain. We next prospectively selected a median split to group patients. The median values of V30 for the CW2cm and CW3cm definitions were 70 cm3 and 88 cm3, respectively. For both CW definitions, receiving physical dose of 30 Gy to more than the median V30 was significantly correlated with Grade ≥ 2 CW pain, with a stronger significance for CW2cm (p = 0.004 Fig. 6) than CW3cm (p = 0.02). We consistently found for a range of dose–volume parameters that CW2cm enabled more robust prediction of clinically significant CW pain than the previously reported CW3cm definition (data not shown).
Fig. 6.
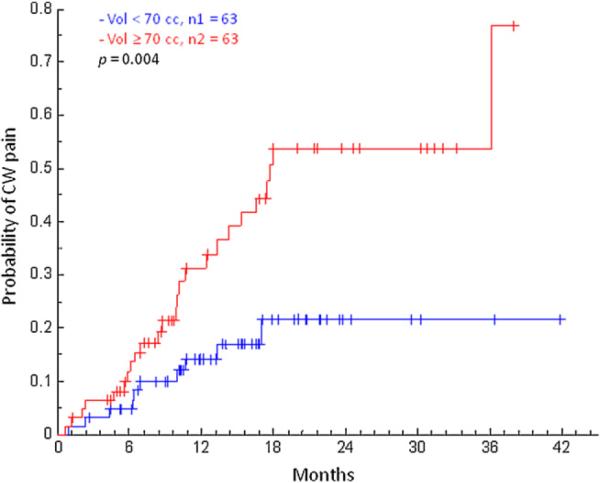
Kaplan-Meier curves describing cumulative incidence of developing Grade ≥ 2 chest wall pain. Patients were categorized using CW2cm V30 ≥ or <70 cm3, the median value. The log-rank test was used to compare the complication incidence in the two groups.
To account for the effects of the different dose–fractionation schemes used in the study, we examined whether using BED, as defined according to the linear quadratic model, improved the correlation of dosimetric parameters with Grade ≥ 2 CW pain. We analyzed α/β ratios ranging from 2 to 10 Gy and found no advantage of biologically effective dose over physical dose in predicting pain.
To confirm that we were observing an underlying dose–volume effect, we evaluated the patient parameters of age and gender, and neither was associated with the development of CW pain. In addition, fraction number (p = 0.07) and prescription dose (p = 0.07) did not significantly predict for the development of Grade ≥ 2 CW pain, nor did the multivariate combination of fraction number and prescription dose.
DISCUSSION
Chest wall pain and rib fracture were common adverse effects of treatment in this prospective analysis of 126 patients with NSCLC treated with SBRT. The 2-year estimated actuarial incidence of Grade ≥ 2 CW pain in our study was 39%. The rate of clinically significant CW pain reported here was similar to that of a previous study of SBRT for thoracic malignancies (4). The median time to onset of Grade ≥ 2 CW pain was 9 months after the completion of therapy. The late presentation of CW toxicity underlines the importance of long-term follow-up by radiation oncologists in this patient population and education of other health care providers. Indeed, SBRT-associated CW pain can be easily confused with other serious medical conditions, leading to unnecessary, costly, and potentially harmful tests and interventions.
Dunlap et al. previously reported, with a median follow-up of just over 11 months, that a dose of 30 Gy to the CW3cm showed a threshold of 30 cm3 before severe CW toxicity was observed. They recommended, if possible, that the V30 be limited to less than 30 cm3 (4). In our experience, a CW3cm volume >30 cm3 receiving 30 Gy was not a significant predictor of Grade ≥ 2 CW pain. Just 15% of our patients and 10% of patients in a series from the M.D. Anderson Cancer Center had a chest wall V30 ≤ 30 cm3 (13). These findings raise concerns that a cut point at that location would result in a prohibitively high false-positive rate and that achieving such a stringent dose–volume parameter may not be clinically feasible in peripherally located tumors.
Our analysis revealed that a relatively broad range of CW physical doses, centering around 16–38 Gy, were significantly associated with CW pain. Confirming that the CW2cm V30 was a significant predictor of CW pain, we prospectively determined that a V30 >70cm3 (the median value) was significantly correlated with Grade ≥ 2 CW pain. Initial treatment planning studies at our institution confirm the feasibility of applying this normal tissue constraint in many cases without compromising target coverage. In the future, the volume of CW irradiated may be reduced further by the implementation of respiratory management techniques such as respiratory gating or real-time tumor tracking to reduce the ITV expansion.
Differences in patient population, treatment technique, the longer follow-up time in our study, or other yet to be determined factors could account for some differences between our results and data reported by other groups. For example, at MSKCC, patients receiving SBRT for NSCLC are treated using IMRT and three to six coplanar beams with rather homogeneous target coverage. More commonly at other institutions, however, a stereotactic radiosurgery technique with 10 or more beam directions (often noncoplanar, sometimes IMRT) and 20% or more dosimetric inhomogeneity in the target is used for early-stage NSCLC. Like us, others have reported favorable control rates and toxicity profiles using IMRT (14), and the local control rates reported here are comparable to previously reported large experiences of SBRT for NSCLC (15–17). It is also noteworthy that we are the first group to use actuarial methods (the log–rank test and CPH models) to determine dose–volume parameters that best predict for the development of CW pain. Given the late onset of CW pain in many patients, these methods appropriately take into account the varying length of follow-up and the temporal pattern of censoring, which influences the probability of observing CW pain (7).
The mechanism of CW pain following SBRT is uncertain. One possibility may be injury of the large peripheral intercostal nerves. Forquer et al. recently reported on the frequency of brachial plexopathy in early-stage apical NSCLC treated with SBRT (11). They noted a 2-year risk of brachial plexopathy of 46% with a maximum brachial plexus dose of >26 Gy. Bone injury may also be a cause of CW pain. Interestingly in our study, however, just 2 of the 5 patients with treatment-related rib fractures had histories of clinically significant CW pain. Rib fracture after SBRT has previously been reported to be associated with small-volume/high-dose regions, specifically the dose to 2 cm3 of the rib (18). We found that a definition of CW as an organ at risk consisting of a 2-cm expansion of the lungs minus the lung volume was a better predictor of Grade ≥ 2 CW pain than the previously reported 3 cm definition. This finding suggests that the neurovascular structures, muscle, and ribs may be more relevant to the pathogenesis of radiation-induced CW pain than the soft tissues encompassed more peripherally by the larger CW definition. We recommend, therefore, that the 2-cm definition described herein be contoured to constrain the CW during treatment planning for thoracic SBRT.
Our report is limited in that this was a single-institution study. Although the patients were monitored prospectively for toxicity, the CW contouring and DVH analyses were performed retrospectively. For some patients, there may be significant intra- and interfractional uncertainty in delivered dose to the CW resulting from respiratory motion (19). In our study, respiratory motion of the target was accounted for by using the simulation RCCT to define an ITV and positioning the patient at treatment to place the target visualized with CBCT within the contours of the ITV. We did not, however, use either the RCCT or the CBCTs in our analyses to take into account CW respiratory motion. Further study will be needed to confirm that CW pain will be reduced by applying our dosimetric model and to assess its applicability outside of our institution.
The use of SBRT is growing rapidly, and the dose–volume dependence of its toxicity is yet to be fully established. To ensure the best possible understanding of the limits of safety of SBRT as clinical practice evolves, it is vital that the toxicities of individual clinical series be adequately reported. Without uniform and comprehensive reporting, thorough meta-analysis will not be possible, and the literature on toxicity will remain fragmented into individual studies suggesting different dose–volume limits that are difficult to relate to each other (20). With these points in mind, we have included an actuarial atlas of the dose–volume incidence of CW pain in an electronic supplement, as described in the Appendix. This format will allow others to combine their own dosimetric data and outcomes with those reported in this article and test existing and new hypotheses.
In conclusion, CW toxicity after SBRT is common, and long-term follow-up is needed to identify affected patients. CW dosimetry predicts for Grade ≥ 2 CW pain; therefore, the CW dose and volume should be constrained during treatment planning and in future clinical trial design. A 2 cm definition of the CW (CW2cm) as an organ at risk appears to be more robust than a 3 cm definition at predicting CW pain and has become our standard method of contouring the CW at MSKCC. A volume of CW2cm >70 cm3 receiving 30 Gy is significantly correlated with Grade ≥ 2 CW pain. We are currently applying this constraint to patients receiving thoracic SBRT in our clinic.
Supplementary Material
Acknowledgments
Supported in part by Grant Number 1RO1CA129182-01A2 from the National Cancer Institute.
Footnotes
Conflict of interest: none.
REFERENCES
- 1.Voroney JP, Hope A, Dahele MR, et al. Chest wall pain and rib fracture after stereotactic radiotherapy for peripheral non-small cell lung cancer. J Thorac Oncol. 2009;4:1035–1037. doi: 10.1097/JTO.0b013e3181ae2962. [DOI] [PubMed] [Google Scholar]
- 2.Collins BT, Erickson K, Reichner CA, et al. Radical stereotactic radiosurgery with real-time tumor motion tracking in the treatment of small peripheral lung tumors. Radiat Oncol. 2007;2:39. doi: 10.1186/1748-717X-2-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baumann P, Nyman J, Hoyer M, et al. Outcome in a prospective phase II trial of medically inoperable stage I non-small-cell lung cancer patients treated with stereotactic body radiotherapy. J Clin Oncol. 2009;27:3290–3296. doi: 10.1200/JCO.2008.21.5681. [DOI] [PubMed] [Google Scholar]
- 4.Dunlap NE, Cai J, Biedermann GB, et al. Chest wall volume receiving >30 Gy predicts risk of severe pain and/or rib fracture after lung stereotactic body radiotherapy. Int J Radiat Oncol Biol Phys. 2010;76:796–801. doi: 10.1016/j.ijrobp.2009.02.027. [DOI] [PubMed] [Google Scholar]
- 5.Deasy JO, Bentzen SM, Jackson A, et al. Improving normal tissue complication probability models: The need to adopt a “data-pooling” culture. Int J Radiat Oncol Biol Phys. 2010;76:S151–S154. doi: 10.1016/j.ijrobp.2009.06.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jackson A, Marks LB, Bentzen SM, et al. The lessons of QUANTEC: Recommendations for reporting and gathering data on dose-volume dependencies of treatment outcome. Int J Radiat Oncol Biol Phys. 2010;76:S155–S160. doi: 10.1016/j.ijrobp.2009.08.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jackson A, Yorke ED, Rosenzweig KE. The atlas of complication incidence: A proposal for a new standard for reporting the results of radiotherapy protocols. Semin Radiat Oncol. 2006;16:260–268. doi: 10.1016/j.semradonc.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 8.Lovelock DM, Hua C, Wang P, et al. Accurate setup of paraspinal patients using a noninvasive patient immobilization cradle and portal imaging. Med Phys. 2005;32:2606–2614. doi: 10.1118/1.1951042. [DOI] [PubMed] [Google Scholar]
- 9.Mohan R, Barest G, Brewster LJ, et al. A comprehensive three-dimensional radiation treatment planning system. Int J Radiat Oncol Biol Phys. 1988;15:481–495. doi: 10.1016/s0360-3016(98)90033-5. [DOI] [PubMed] [Google Scholar]
- 10.Spirou SV, Chui CS. A gradient inverse planning algorithm with dose-volume constraints. Med Phys. 1998;25:321–333. doi: 10.1118/1.598202. [DOI] [PubMed] [Google Scholar]
- 11.Forquer JA, Fakiris AJ, Timmerman RD, et al. Brachial plexopathy from stereotactic body radiotherapy in early-stage NSCLC: Dose-limiting toxicity in apical tumor sites. Radiother Oncol. 2009;93:408–413. doi: 10.1016/j.radonc.2009.04.018. [DOI] [PubMed] [Google Scholar]
- 12.Hall EJ. Radiobiology for the radiologist. 5th ed. Lippincott Williams & Wilkins; Philadelphia: 2000. [Google Scholar]
- 13.Welsh J, Thomas J, Shah D, et al. Obesity increases the risk of chest wall pain from thoracic stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys. 2010;81:91–96. doi: 10.1016/j.ijrobp.2010.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Videtic GM, Stephans K, Reddy C, et al. Intensity-modulated radiotherapy-based stereotactic body radiotherapy for medically inoperable early-stage lung cancer: Excellent local control. Int J Radiat Oncol Biol Phys. 2010;77:344–349. doi: 10.1016/j.ijrobp.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 15.Fritz P, Kraus HJ, Blaschke T, et al. Stereotactic, high single-dose irradiation of stage I non-small cell lung cancer (NSCLC) using four-dimensional CT scans for treatment planning. Lung Cancer. 2008;60:193–199. doi: 10.1016/j.lungcan.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 16.Timmerman R, Paulus R, Galvin J, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA. 2010;303:1070–1076. doi: 10.1001/jama.2010.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Onishi H, Shirato H, Nagata Y, et al. Hypofractionated stereotactic radiotherapy (HypoFXSRT) for stage I non-small cell lung cancer: Updated results of 257 patients in a Japanese multi-institutional study. J Thorac Oncol. 2007;2:S94–S100. doi: 10.1097/JTO.0b013e318074de34. [DOI] [PubMed] [Google Scholar]
- 18.Pettersson N, Nyman J, Johansson KA. Radiation-induced rib fractures after hypofractionated stereotactic body radiation therapy of non-small cell lung cancer: A dose- and volume-response analysis. Radiother Oncol. 2009;91:360–368. doi: 10.1016/j.radonc.2009.03.022. [DOI] [PubMed] [Google Scholar]
- 19.Morrow NV, Stepaniak C, White J, et al. Intra- and interfractional variations for prone breast irradiation: An indication for image-guided radiotherapy. Int J Radiat Oncol Biol Phys. 2007;69:910–917. doi: 10.1016/j.ijrobp.2007.06.056. [DOI] [PubMed] [Google Scholar]
- 20.Bentzen SM, Constine LS, Deasy JO, et al. Quantitative Analyses of Normal Tissue Effects in the Clinic (QUANTEC): An introduction to the scientific issues. Int J Radiat Oncol Biol Phys. 2010;76:S3–S9. doi: 10.1016/j.ijrobp.2009.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



