Abstract
Neurobehavioral deficits have been reported in Egyptian pesticide application teams using organophosphorus (OP) pesticides, but whether these effects are related to OP pesticide exposures has yet to be established. In preparation for a comprehensive study of the relationship between OP pesticide dose and neurobehavioral deficits, we assessed exposure within this population. We conducted occupational surveys and workplace observations, and collected air, dermal patch and biological samples from applicators, technicians and engineers involved in chlorpyrifos applications during cotton production to test the hypotheses that: 1) dermal exposure was an important contributor to internal dose and varied across body regions; and 2) substantial differences would be seen across the three job categories. Applicators were substantially younger and had shorter exposure histories than did technicians and engineers. Applicators and technicians were observed to have relatively high levels of skin or clothing contact with pesticide-treated foliage as they walked through the fields. Both dermal patch loadings of chlorpyrifos and measurements of a chlorpyrifos-specific metabolite (TCPy) in urine confirmed substantial exposure to and skin absorption of chlorpyrifos that varied according to job category; and dermal patch loading was significantly higher on the thighs than on the forearms. These findings support our hypotheses and support the need for research to examine neurobehavioral performance and exposures in this population. More importantly, the exposures reported here are sufficiently high to recommend urgent changes in work practices amongst these workers.
Keywords: Organophosphorus pesticides, chlorpyrifos, pesticide applicators, biomonitoring, urinary metabolites, TCPy, dermal exposure, dermal patches
INTRODUCTION
Potential adverse health impacts of organophosphorus (OP) pesticide exposures are an ongoing public health concern for agricultural workers in low-middle income countries, particularly in regard to acute health effects [e.g., 1–6]. An increasing number of studies have identified neurobehavioral effects associated with occupations involving chronic or subchronic exposures to OP pesticides that did not cause systemic toxicity [7–22]. Most studies inferred their participants’ OP exposure as a result of work group membership, though there is evidence of an association between neurobehavioral deficits and urine metabolites of OP pesticides in one study [11]. Among the studies of occupational OP pesticide exposure, much attention has focused on Egypt [23,24], and two recent studies have reported extensive neurobehavioral deficits in adult workers in Egyptian cotton production [12] and in adolescent Egyptian pesticide applicators [22]. Both studies compared pesticide workers with unexposed workers employed by the Ministry of Agriculture in Menoufia Governorate, Egypt. Whole blood cholinesterase (ChE) levels were significantly lower in samples from pesticide workers relative to samples from the unexposed workers, suggesting higher exposure to OP pesticides in these worker populations than in their ‘control’ comparators.
The objective of the present study was to develop a more detailed understanding of OP pesticide exposure pathways among three categories of Egyptian cotton field workers: pesticide applicators, technicians and engineers. Specifically, we aimed to test the hypotheses that: 1) dermal exposure was an important contributor to internal dose and varied across body regions; and 2) substantial differences would be seen across the three job categories. We considered that evidence of high OP pesticide exposures in this study would be consistent with the hypothesis that OP pesticide exposures were associated with the substantial deficits, relative to controls, seen in these occupations in Egypt [12,22]. The findings from this study are being used to inform the design of a larger epidemiologic study in this worker population.
SETTING AND WORKFORCE
Cotton is treated as a national resource in Egypt, a country in which 32% of the labor force of just over 21 million is employed in agriculture. Although grown and harvested by independent farmers, the national government oversees the country’s entire production of cotton [25]. Once farmers agree to plant cotton in their fields, applications of chemicals on those fields come under control of the national Ministry of Agriculture and that control is delegated to the Ministry’s District Offices in the individual Governorates. Pesticide application equipment and all pesticides are purchased by the national Ministry of Agriculture; the equipment is calibrated at the Governorate District Office and then distributed to the Governorate field stations. Thus, all pesticides, application equipment and application procedures used for cotton production are standardized throughout Egypt.
Menoufia, one of 29 Governorates in Egypt, is situated in the Nile River delta north of Cairo. Menoufia has 270 field stations distributed across 9 agricultural Districts, each of which is responsible for crop management, including sales of chemicals to farmers, worker safety training and pesticide applications [26]. A field station is located close to the cotton fields, and serves as the meeting place for workers and supervisors, as well as the storage area for pesticides and application equipment.
Each field station has a team of employees in three job categories with potential pesticide exposure: (1) Applicators who apply pesticides with backpack sprayers; 2) Technicians who walk each row with the applicator to direct the path of the applicator and point out any heavy insect infestations in the field; and (3) Engineers who periodically walk the fields but more often direct the application process from the edge of the field.
There were 1,068 technicians and engineers assigned to apply pesticides in Menoufia’s 270 field stations in 2007. It is standard procedure to assign four applicators to each field station, so an estimated 1,080 applicators work in the cotton fields during a typical summer in this region. Technicians and engineers are not exposed to pesticides during the rest of the year, as their primary tasks are to review results of the previous season and prepare equipment and plans for pesticide applications in the upcoming season [27]. The applicators are seasonal workers, but their off-season activities are not well documented. There is anecdotal evidence of episodic but not systematic use of pesticides among workers in all three job categories outside their ministry jobs.
METHODS
Occupational surveys, workplace observations, air samples, dermal patch samples, and urine samples were obtained from convenience samples of workers in the Menoufia cotton fields during two growing seasons (July–August 2006 and 2007). Chlorpyrifos was the only OP pesticide used during these application seasons. The procedures followed in this study involving human subjects were approved by the Oregon Health & Science University institutional review board and by the Menoufia University human subjects committee. The study purpose and procedures were explained to participants prior to the beginning of the study, and all participants provided informed consent.
Occupational Surveys
An occupational survey of applicators, technicians and engineers (N=47) was conducted in 2006 to ascertain age, years of education, number of years of work at the specific job, number of years of work in cotton production, and whether the worker had received pesticide safety training. A smaller occupational survey was conducted in 2006 among office managers, applicators and engineers (N=12) to determine the typical frequency of pesticide application and equipment calibration.
Workplace Observations
Pesticide mixing, loading and application activities were observed by trained staff over the course of one work day in 2007 for six applicators, six technicians, and six engineers. Figure 1 shows a typical pesticide application team consisting of a technician (left) who directs the applicators in the field, an applicator (center), and an engineer (right) who is instructing the applicator on how to hold the tube through which the pesticides are applied.
Figure 1.
Pesticide application team consisting of a technician (left) who directs the applicators in the field, an applicator (center), and an engineer (right) who is instructing the applicator on how to hold the tube through which the pesticides are applied.
The following information was recorded on standardized field data sheets: 1) types and amounts of pesticides mixed and loaded in the sprayer during the day; 2) amount of time spent in the fields; 3) types of work clothing worn; and 4) use of any personal protective equipment (PPE).
Air Samples
Six air samples were collected using XAD-2 OVS tubes and Gilian Hi Flow Samplers (Model HFS 513A) from the breathing zone of six applicators (one sample per applicator). At the end of the work day, the tubes were capped and transported on ice from the field to the laboratory at Menoufia University where they were frozen at −80°C. They were later shipped to the University of Washington (UW) on dry ice for analysis. The samples were extracted with an acetone/toluene solution and analyzed for chlorpyrifos residues using GC/MS by the UW Environmental Health (EH) Laboratory according to the procedures of NIOSH analytical method 5600 for OP pesticides [28]. All samples had measurable amounts of chlorpyrifos in the front section of the OVS tubes. Chlorpyrifos was not detected in the backup section of the OVS tubes, indicating that no breakthrough occurred in these samples. Two field blanks were below the detection limit of 1.4 ng per sample.
Dermal Patch Samples
The traditional dermal patch technique [29] was not used in this preliminary study due to resource constraints. Instead, a limited number of patches were used to determine pesticide loading on two body regions: forearm and upper leg. Patches were attached to the clothing or skin of two applicators, two technicians and two engineers. The patches were constructed of pre-extracted (soxhlet with ethyl acetate) surgical gauze pads (100% cotton) placed in a paper envelope with an aluminum foil lining that revealed a circle cutout (5.6 cm diameter). For each worker, one patch was taped to each forearm (if short-sleeve shirt was worn) or pinned to each sleeve (if long-sleeve shirt was worn), and one patch was pinned to each pant leg at the level of the thigh. The workers wore the patches during the entire workday. At the end of the workday, tweezers that had been triple-rinsed with isopropanol were used to remove the gauze pad from its holder and place it in a separate pre-rinsed glass jar. The jars were transported on ice from the field to the laboratory at Menoufia University where they were frozen at −80°C. The jars with patches from the right side of each participant were then shipped to UW on dry ice for analysis. The remaining set of patches were kept in Egypt as back-up samples due to uncertainties in shipping procedures. Extraction and analysis of the patch samples were conducted by the University of Washington Environmental Health Laboratory (AIHA-certified). The gauze pads were extracted as follows: place in 50 mL tube; add 30 mL ethyl acetate; agitate with a vortex action shaker for 10 minutes; decant solvent into a labeled turbovap flask and evaporate; add additional 30 mL acetate to the tube containing the pads, shake for 5 minutes and transfer to the turbo-vap flask; evaporate extract to <1 mL, then solvent exchange with approximately 5 mL 2,2,4-Trimethylpentane (TMP); evaporate to <1 ml, then add TMP to achieve 1 mL volume. The extracts were analyzed for chlorpyrifos using GC/MS (HP6890 gas chromatograph and HP5973 series mass selective detector). The extraction efficiency of the method was 94.8% +/− 13.1 (n=6). The limit of detection was 10 ng per sample. No field blanks were prepared for this study. Laboratory blanks were below the limit of detection.
Urine Samples
Urine samples were collected from six applicators, six technicians and six engineers. In addition, urine samples were collected from six control participants for each of the three job categories. The control participants worked at the central administrative offices of the Ministry of Agriculture. A standardized protocol was used to collect urine samples at the beginning and end of the work shift on the 16th consecutive day of OP pesticide applications. Samples were collected in pre-washed, wide-mouth plastic bottles and transported in a cooler to the Menoufia University laboratory where they were stored at −80 °C. Due to resource constraints, only half of these samples were analyzed. Samples from three of the six workers in each job category, and samples from three controls for each job category were selected randomly and shipped to the University of Buffalo on dry ice for analysis.
Urine samples were analyzed for the primary metabolite of chlorpyrifos, 3,5,6- trichloro-2-pyridinol (TCPy) using a modification of the method described by Hines and Deddens [30]. A 1 mL aliquot of each urine specimen was thawed and mixed prior to the addition of 100 ng of internal standard (13C – 15N – 3,5,6-TCP, a gift from Steve Hutton at Dow Agrosciences LLC, Indianapolis, IN 46268). Samples were then hydrolyzed at 80°C with 100µL of 12N HCl, and extracted with 1 mL of 20% aqueous NaCl and 1 mL of toluene. The toluene extract was then derivatized with N-(tert-butyldimethylsilyl)-Nmethyltrifluoro- acetamide (MTBSTFA) and analyzed by negative-ion chemical ionization gas chromatography-mass spectrometry (NCI-GC-MSD) on an Agilent Technologies 5973 inert GC-MS (San Jose, CA). Separations were achieved on a DB-5MS capillary column (15m × 0.25mm, 0.1um film thickness, J&W Scientific, Folsom, CA). Helium carrier gas was used at a constant flow of 1.2 mL/min. The initial oven temperature of 100°C was held for 1 minute, followed by a 5°C/min ramp rate to 140°C, followed by a 50°C/min ramp to 300°C and held at 300° C for 2.8 min. The injection port was at 260°C and the transfer line at 250°C. A 2 min splitless injection of 2 uL was made. Methane was used as the reagent gas and ions at m/z 161 for TCPy and 166 for the internal standard were monitored to control for variability in the extraction and assure accurate analysis of TCPy. Each batch of samples was analyzed with a standard curve and spiked samples consistently gave extraction efficiencies of greater that 90%. The limit of detection for the method was 0.5 ng/ml urine. Creatinine concentrations were measured using the Jaffe reaction where creatinine forms a red color on reaction with picric acid under alkaline conditions [31]. TCPy concentrations were adjusted using creatinine concentrations to correct for variable urine dilutions in the spot urine samples.
Data Analysis
Descriptive statistics were calculated for data from the occupational survey. Urinary TCPy concentration differences between groups were analyzed for pre- vs. postapplication concentrations by a paired t-test. Differences between job categories and the controls, and across job categories were compared by an Analysis of Variance (ANOVA).
Urinary TCPy concentrations were used to calculate dose estimates for nine workers, and for each job category. Dose was calculated by the following equation:
Dd = [TCPyc * Cd * IEF * MW ratio]/BW
Where Dd = daily dose (ug/kg); TCPyc = creatinine-adjusted TCPy average concentration (ug/g creatinine) for each worker; Cd = daily creatinine clearance of 1.7 g creatinine/day [32]; IEF = incomplete excretion factor of 1.0/0.7 based on a human oral chlorpyrifos dosing study [33]; MW ratio = molecular weight ratio of chlorpyrifos and TCPy (350.6/198.5 = 1.766; and BW = body weight (kg). Body weights ranged from 65 to 88 kg, with weight for one worker imputed from weights of workers in the same job category.
The mean dose for each job category was calculated as the mean of the three worker mean daily doses in each category. These doses were then used to calculate margins of exposure for each job category. The margin of exposure (MOE) is defined by the U.S. Environmental Protection Agency as the no observed adverse effect level (NOAEL) divided by dose [34]. For occupational exposures the target MOE is >100. In order to calculate a NOAEL for chlorpyrifos suitable for comparison with biologically-based dose estimates, the short-term dermal NOAEL of 5.0 mg/kg-day was adjusted by a 0.03 dermal absorption factor [34], resulting in a NOAEL = 150 ug/kg-day.
RESULTS
Occupational Surveys
Occupational survey results are presented in Table 1. Applicators as a group were much younger and had less education than technicians and engineers. Engineers were about three years older than technicians and had about two more years of education. Applicators had worked at their current job on average two years and in cotton production less than two years, while both technicians and engineers had worked at their current job and in cotton production for about 20 years. None of the applicators reported having had previous pesticide training, whereas 54% of the technicians and 62% of the engineers reported such training.
Table 1.
Occupational survey of the study population: Age, education and work history by job category.
| Job category | Age (yrs) |
Education (yrs) |
Worked at this job (yrs) |
Worked in cotton production (yrs) |
Previous pesticide safety training reported? |
|---|---|---|---|---|---|
| Applicator (N=7) | |||||
| Mean | 17.4 | 11.0 | 2.0 | 1.4 | Yes: none |
| (Std. Dev.) | (0.5) | (1.4) | (0) | (0.5) | No: 100% |
| Range | 17–18 | 9–12 | 2–2 | 1–2 | |
| Technician (N=24) | |||||
| Mean | 47.7 | 12.0 | 20.0 | 19.3 | Yes: 54% |
| (Std. Dev.) | (4.2) | (0) | (7.2) | (7.8) | No: 46% |
| Range | 42–58 | 12–12 | 5–36 | 7–36 | |
| Engineer (N=16) | |||||
| Mean | 50.9 | 13.8 | 20.3 | 22.8 | Yes: 62% |
| (Std. Dev.) | (6.3) | (2.0) | (14.0) | (8.4) | No: 38% |
| Range | 41–59 | 12–16 | 1–37 | 11–35 | |
The smaller survey of workers revealed that teams applied pesticides four to five hours per day, seven days per week during the application season, with the season typically running from mid-late June through mid-August. The backpack sprayers used to apply pesticides were calibrated at the beginning of each season at the central Ministry offices in the Governorate, though the specific steps were not documented. A crude field calibration was performed by the regular practice of sending 3–4 applicators into the field at the same time and observing whether all applicators ran out of pesticide solution at about the same time.
In regard to pesticide applications, biological growth regulators are normally applied from March through May. In June or early July insecticide applications begin based on the inspection of bolls for weevils. Insecticides are usually applied in three waves of approximately 15 days each, according to the following pattern: OP pesticides, pyrethroid pesticides, and OP pesticides. The primary OP pesticide used in Egyptian cotton fields is Pestban™, an emulsifiable concentrate formulation that contains 48% chlorpyrifos as the active ingredient.
According to the Egyptian Ministry of Agriculture, 80 liters of the Pestban™ solution should be applied on each feddan (50 × 40 m2; equivalent to 0.42 hectares or 1.04 acres) of cotton plants by four seasonal applicators, each using a 20-liter backpack sprayer. The applicators are trained to walk 40 steps per minute, and it takes about 20 minutes per feddan. One liter of Pestban with 48% chlorpyrifos active ingredient is mixed with water in a 40-liter barrel, and half that quantity is poured into each backpack sprayer, so the application rate is estimated to be 0.04 liter of diluted Pestban per square meter.
Workplace Observations
Mixing and loading activities were carried out by applicators with supervision by engineers and technicians who sometimes also participated in the mixing/loading (Figure 2). The backpack sprayer was covered with plastic to protect the engine, while the pesticide mixture was transferred from the barrel to the sprayer with a hand-held container. Mixing/loading occurred three times during the observed work periods.
Figure 2.
Pesticide mixing and loading. The pesticide formulation is mixed with water in the 40-liter blue barrel. The mixture is then transferred to the tank of the backpack sprayer. The sprayer is covered with plastic to protect the engine. Note that none of the workers are using gloves or other personal protection.
Applicators formed a staggered line across the field, led or followed by the technicians (Figure 3). Due to the loud noise of the backpack sprayers, the technicians had to remain within ~10 meters of the applicators so they could effectively communicate with them. As is common in this region, farm children congregated around the young applicators and later placed and moved flags at either end of the field to signal the path of each applicator, as directed by the technicians or engineers.
Figure 3.
Applicators walk through the fields in a staggered pattern to avoid spraying each other. A technician can be seen leading the applicators, while another technician is just in front of the lead applicator on the left. Other technicians who are not in the picture are following the applicators. Note that the applicators are spraying in front of their bodies and walking into the spray.
Engineers spent an average of 37 min actually in the cotton fields, while the technicians and applicators spent an average of 52 min in the fields. All six of the applicators were observed emerging from the field after application with pants drenched in pesticide solution as a result of walking through the plants in front of them that they had just sprayed. In contrast, only two of the six technicians and none of the engineers were observed emerging from the fields with wet pants after pesticides had been applied.
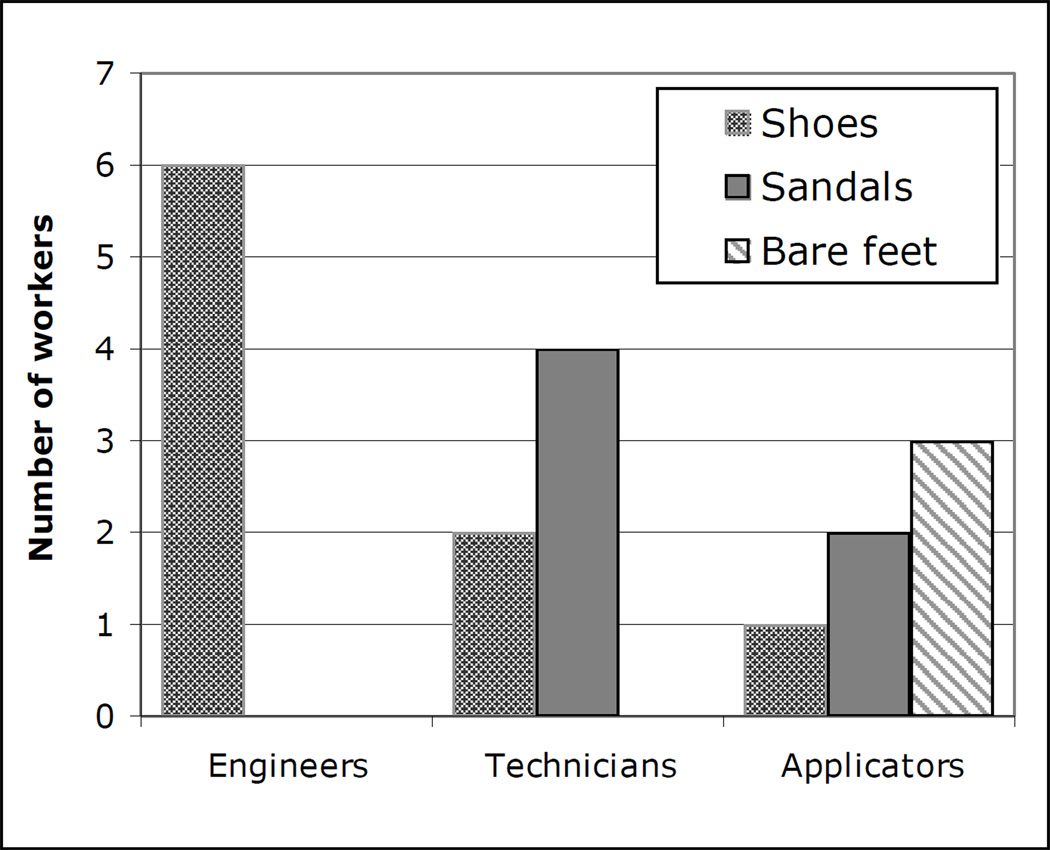
All 18 workers wore long pants, half wore long-sleeve shirts and half wore short-sleeve shirts. The most striking difference across job category was the use of footwear (Figure 4). It was observed that all of the engineers wore shoes, whereas three of the six applicators were barefoot and two others wore sandals. The technicians were intermediate, with two wearing shoes and four wearing sandals. None of the workers wore gloves or any other protective clothing, and none used respirators. Some participants wore dust masks, but often not over the nose, and some wore glasses or goggles but not always appropriately.
Figure 4.
Footwear worn by engineers, technicians, and applicators during workplace observations of chlorpyrifos applications in cotton.
Air Samples
Chlorpyrifos air concentrations in the six applicators’ breathing zones were generally quite low. Concentrations averaged 23 ug/m3, with a range of 13–54 ug/m3. The total air sampling time was approximately 15 minutes, with spraying occurring during about half of that period.
Dermal Patch Samples
Dermal patch loadings and loading rates are listed in Table 2. Chlorpyrifos patch loadings for the two engineers were a small fraction of the loadings for most applicators and technicians. Loading on the thighs was higher than loading on the forearms in five of six cases, consistent with observations of contact with sprayed foliage, and with our hypothesis that differences would be seen between lower body and upper body loadings. Variability within worker category was high for applicators and technicians. The highest arm loading (197 ug/cm2) was measured on a technician, whereas the highest thigh loading (422 ug/cm2) was measured on an applicator.
Table 2.
Chlorpyrifos loading and loading rates on dermal patches1 for three job categories during pesticide applications in cotton production
| Applicator | Technician | Engineer | ||||
|---|---|---|---|---|---|---|
| Subject | 1 | 2 | 3 | 4 | 5 | 6 |
| Time2 (min) | 55 | 60 | 55 | 60 | 40 | 40 |
| Loading (ug/cm2) | ||||||
| Arm | 12.6 | 49.6 | 0.8 | 197.0 | 3.1 | 1.0 |
| Thigh | 49.0 | 422.0 | 24.5 | 70.8 | 4.2 | 5.6 |
| Loading rate (ug/cm2/hr) | ||||||
| Arm | 13.8 | 49.6 | 0.9 | 197.0 | 4.6 | 1.4 |
| Thigh | 53.5 | 422.0 | 26.7 | 70.8 | 6.2 | 8.3 |
Dermal patches were 5.2 cm2 exposed gauze patches attached to subjects’ pant leg and shirt sleeve or taped to the arm. All patches analyzed were located on the right side.
Time = total time worker was involved in spraying activities on that day.
Urine Samples
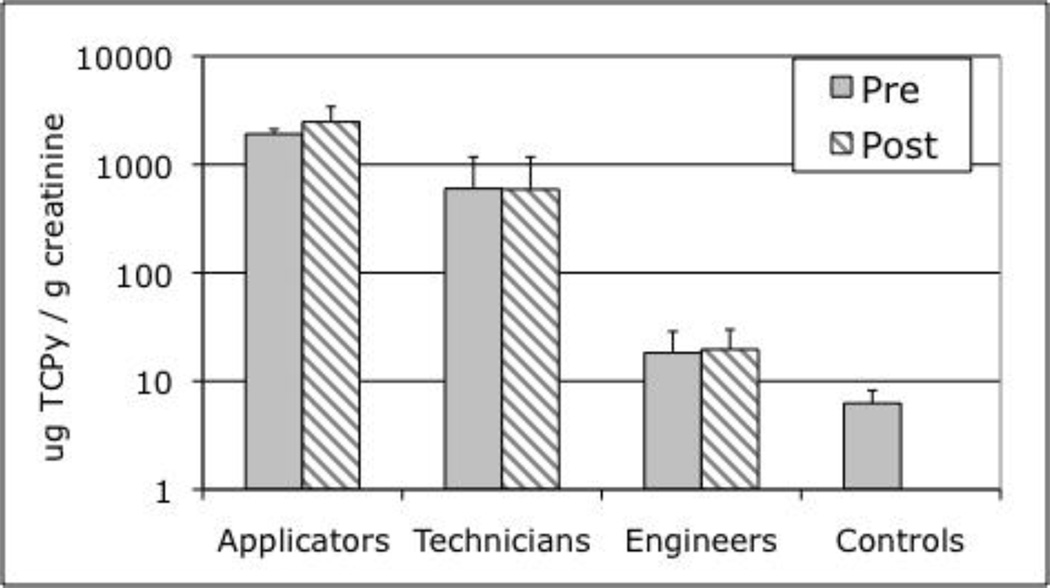
Urinary concentrations of TCPy from three applicators, three technicians, and three engineers, before (pre) and after (post) chlorpyrifos application, together with controls are shown in Figure 5. There were no significant differences between pre- and post-application concentrations within job categories (p=0.40). This finding was expected, since these workers had been applying pesticides for about 15 consecutive days prior to the study day. Significant differences were found, however, between applicators, technicians, engineers, and controls; these groups had mean TCPy levels of 2,471, 590, 19.6, and 6.25 µg/g creatinine, respectively (ANOVA; p<0.001), with all four groups being distinct (p<0.01 for each pair-wise comparison).
Figure 5.
Estimates of exposure / internal dose were made by analysis of urinary TCPy (µg/g creatinine), a chlorpyrifos-specific metabolite, in applicators, technicians, engineers and controls before and after the chlorpyrifos application (data presented as the mean ± SD, n=9 for controls and n=3 for occupational groups, note log scale). While there were no significant differences in pre- and post-application levels within a job category, there were significant differences between applicators, technicians, engineers, and controls.
Dose Estimates
Mean daily dose estimates were highest in applicators, followed by technicians, and then engineers (Table 3). The mean daily dose estimates by job category were 141 ug/kg for applicators, 30 ug/kg for technicians, and 1 ug/kg for engineers, translating to margins of exposure (MOE) of 1, 5 and 150, respectively.
Table 3.
Chlorpyrifos dose estimates for Egyptian cotton production workers during 2007 observational study.
| Job category |
Worker ID |
Worker mean daily dose (ug/kg)1 |
Category mean daily dose (ug/kg)2 |
Margin of exposure (target MOE >100)3 |
|---|---|---|---|---|
| Engineer | 1 | 1.69 | 1.0 | 150 |
| 3 | 0.74 | |||
| 4 | 0.59 | |||
| Technician | 1 | 61.3 | 29.7 | 5 |
| 2 | 14.3 | |||
| 3 | 13.6 | |||
| Applicator | 3 | 118.3 | 141.4 | 1 |
| 4 | 134.6 | |||
| 5 | 171.4 |
Worker mean daily dose based on two measurements for each worker; dose was calculated by the following equation: dose (ug/kg) = TCPy (ug/g creatinine) * 1.7 g creatinine/day * 1/0.7 * 1.766)/body weight (kg); creatinine clearance = 1.7 gram/day (ICRP Report of the Task Group on Reference Man, 1994); incomplete excretion factor = 0.7 (human oral dosing study by Nolan et al. 1984); chlorpyrifos molecular weight = 350.6, TCPy molecular weight = 198.5, chlorpyrifos equivalent factor = 1.766; body weights ranged from 65 to 88 kg, with weight for one worker imputed from weights of workers in the same job category.
Category mean dose is the mean of the three worker mean daily doses in each worker category
For comparison with biologically-based dose estimates, a dermal NOAEL of 5.0 mg/kgday * 0.03 dermal absorption factor was used (32, Table 8), resulting in a NOAEL = 150 ug/kg-day; Margin of Exposure (MOE) = NOAEL/dose; Target MOE > 100.
DISCUSSION
The information gathered in these field investigations provides convincing evidence that applicators, technicians, and engineers working in Egyptian cotton production had chlorpyrifos exposures substantially greater than unexposed workers during pesticide applications to cotton plants. Both dermal patch loadings of chlorpyrifos and measurements of a chlorpyrifos-specific metabolite (TCPy) in urine indicated that applicators were the most highly exposed, followed by technicians and then engineers, supporting our hypothesis that exposure levels vary significantly between job categories.
The occupational survey indicated that applicators were much younger, much less experienced, and without adequate safety training, based on self report. These characteristics likely put them at risk for greater exposure relative to the technicians and engineers, independent of the particular tasks performed.
Exposures by inhalation were low, as would be expected in an outdoor workplace using equipment such as backpack sprayers that produce relatively large aerosol particles. There is a long-standing consensus among exposure scientists that dermal contact is the primary route of exposure and contributes most of the internal dose for pesticide handlers [35–37]. This hypothesis was recently confirmed for chlorpyrifos exposures among pest control applicators in the United States [30]. In Egyptian cotton production it seems clear from both the patch and urinary metabolite data that substantial exposure occurs through work pants that become saturated with pesticides during application due to contact with wet, treated foliage. These findings are very consistent with a study of greenhouse applicators demonstrating that wet foliage contact can result in very high dermal exposures [38]. These observations also suggest the potential for dermal exposure to continue after work in the fields until the pants are removed.
The dermal patch data can be compared directly with a study of chlorpyrifos exposure among handgunners in Florida greenhouses [39]. Handgunning is quite similar to the type of spraying conducted in Egyptian cotton fields; that is, applicators walk through overhanging foliage while treating plants with a pressurized spray wand. Stamper et al. [39] placed forearm and thigh patches outside the protective clothing of three applicators, as was the case in the present study. They collected data over 24 separate work days. The average forearm and thigh patch values were 0.28 and 1.8 ug/cm2/hr, respectively; maximum values were 2.3 and 8.8 µg/cm2/hr, respectively. As was the case with the workers in our study, thigh patch values were substantially higher than forearm patch values, presumably due to direct contact with wet foliage. However, the magnitude of exposure was quite different: forearm patch values for Egyptian applicators were six times greater than Florida values in one case and 22 times greater in the other; thigh patch values were six times greater in one case and 48 times greater in the other. The exposures of the Egyptian applicators thus appear to be unprecedented in the published pesticide exposure literature. Since the Florida applicators wore chemical protective coveralls, their actual chlorpyrifos loadings on skin were substantially lower than the outside patch loading values. The lack of use of chemical protective clothing by the Egyptian workers, therefore, greatly exacerbates the differences in actual dermal exposures.
Urinary TCPy
The NHANES 2001–2002 Third National Report of Human Exposure to Environmental Chemicals found that measurable concentrations of the chlorpyrifos (and chlorpyrifos-methyl) specific metabolite, 3, 5, 6- trichloro-2-pyridinol (TCPy), were present in the urine of 91% of the general population in the United States [40]. The median and 95th percentile levels of TCPy in urine of adult males were 1.87 and 10.3 ug/g creatinine, respectively. The mean level in Egyptian controls (unexposed male workers) was 6.25 µg/g creatinine, which is about three times greater than background levels in adult males in the United States.
Alexander et al. [41] assessed urinary TCPy levels in 34 families of licensed pesticide applicators in the United States. Applicators’ peak TCPy levels were observed the day after application (geometric mean, 10.5 µg/g creatinine). A previous study of male termite control applicators reported geometric mean levels of TCPy in urine samples ranging from 169–262 µg/g creatinine [30]. A recent study of airblast orchard applicators in Washington State collected 572 morning void urine samples over three weeks of spraying, and reported median and 95th percentile TCPy levels of 12 and 49 µg/g creatinine, respectively [42].
The present study of Egyptian cotton field workers reports a wide range of exposures to chlorpyrifos, based on urine TCPy levels (Figure 6). The mean level in applicators of 2,471 µg/g creatinine is 10-fold higher than termite control applicators [30], 50-fold higher than the 95th percentile of orchard airblast applicators [42], more the 200- fold higher than farm applicators [41] and about 1300-fold higher than background levels reported in the U.S. [40].
The target margin of exposure (MOE, NOAEL/dose) of >100, based on the chlorpyrifos NOAEL established by the U.S. Environmental Protection Agency [34], was greatly exceeded by the applicators (MOE of 1) and the technicians (MOE of 5), indicating the potential for health risks of concern. It also consistent with the hypothesis that the extensive neurobehavioral deficits of workers seen recently in this same Egyptian agricultural workforce [12,22], compared to controls, was due in part to extensive pesticide exposures reported here. Finally, while Egypt has some exposure maxima comparable to Maximum Allowable Concentrations (MACs), Threshold Limit Values (TLVs) or Permissible Exposure Limits (PELs), it does not have an exposure maximum for chlorpyrifos.
This study had a number of limitations. First, the sample size was small; however, the differences in exposure measurements across the job categories were substantial. Second, there are a number of uncertainties in the dose estimates. Some nonoccupational exposure to chlorpyrifos or TCPy may be occurring. There may be interindividual differences in uptake and elimination of chlorpyrifos and its metabolites. There are likely inter-individual differences in creatinine excretion. The dose estimation is based on an assumed 1.7 g/day creatinine excretion value; however, it is not clear whether this value is applicable to Egyptian males and whether there may be differences between 17 year-old males (applicators) vs. 50 year-old males (technicians and engineers). Overall though, it is unlikely that the substantial differences between estimated doses observed in the three work groups could be explained by these dose estimation uncertainties. Third, measures that would have more completely characterized this population were not included in the present study; however, the data described in this article have been used to design a followon epidemiologic study that includes neurobehavioral performance, cholinesterase activity and other measures to provide a more complete analysis of the exposures reported here.
Conclusion
The information gathered in these field investigations provides convincing evidence that Egyptian engineers, technicians and applicators have substantial exposures during OP pesticide applications to cotton, and strongly suggests that their work practices (e.g. contacting treated foliage) and the lack of PPE exacerbate these exposures, resulting in elevated internal doses. The National Ministry of Agriculture carefully controls pesticide application equipment, calibration, and pesticide product use, suggesting that the exposures observed in this study are representative of exposures throughout Egypt’s cotton fields. Together, these observations support a recommendation for changes in work practices and PPE in these workers, and suggest that future research in this population should examine the relationships between neurobehavioral effects and exposures.
Acknowledgements
This project could not have been carried out without the support of Ahmed Ahmed Al-Jaodi, Menoufia’s former Deputy Agricultural Minister, and Hamdi Farahat, General Director for Supervision, who arranged access to the field stations. Dr. Taghreed M. Farahat of Menoufia University arranged support from the Ministries of Agriculture (for access to the workforce) and Health (for shipping samples). Ms. Barb McGarrigle (University of Buffalo) analyzed the urine samples. Dr. Jianbo Yu (University of Washington) analyzed the air and dermal patch samples. Mike Lasarev (Oregon Health & Science University) provided the statistical analyses. The work was supported by the Center for Research on Occupational and Environmental Toxicology (CROET) at Oregon Health & Science University and by grant number R01 ES016308 (Anger and Lein, MPI) from the National Institute of Environmental Health Sciences (NIEHS). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIEHS or CROET. The project, consent forms and data forms were approved by Institutional Review Boards at the Oregon Health & Science University and Menoufia University. We appreciate the careful and thorough reading by the journal reviewers and editor that have led to substantial improvements in this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest/Disclosures
All authors have submitted conflict of interest statements to OHSU or their own University to indicate that they do not have a conflict of interest with respect to this submission.
References
- 1.Keifer M, McConnell R, Pacheco AF, Daniel W, Rosenstock L. Estimating underreported pesticide poisonings in Nicaragua. Am. J. Ind. Med. 1996;30:195–201. doi: 10.1002/(SICI)1097-0274(199608)30:2<195::AID-AJIM10>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 2.Vergara AE, Fuortes L. Surveillance and epidemiology of occupational pesticide poisonings on banana plantations in Costa Rica. Int. J. Occup. Environ. Health. 1998;4:199–201. doi: 10.1179/oeh.1998.4.3.199. [DOI] [PubMed] [Google Scholar]
- 3.Litchfield MH. Estimates of acute pesticide poisoning in agricultural workers in less developed countries. Toxicol. Rev. 2005;24:271–278. doi: 10.2165/00139709-200524040-00006. [DOI] [PubMed] [Google Scholar]
- 4.Recena M, Pires D, Caldas E. Acute poisoning with pesticides in the state of Mato Grosso do Sul, Brazil. Sci. Total Environ. 2006;357:88–95. doi: 10.1016/j.scitotenv.2005.04.029. [DOI] [PubMed] [Google Scholar]
- 5.Rodriguez T, Younglove L, Lu C, Funez A, Weppner S, Barr DB, Fenske RA. Biological monitoring of pesticide exposures among applicators and their children in Nicaragua. Int. J. Occup. Environ. Health. 2006;12:312–320. doi: 10.1179/oeh.2006.12.4.312. [DOI] [PubMed] [Google Scholar]
- 6.Dharmani C, Jaga K. Epidemiology of acute organophosphate poisoning in hospital emergency room patients. Rev. Environ. Health. 2005;20:215–232. doi: 10.1515/reveh.2005.20.3.215. [DOI] [PubMed] [Google Scholar]
- 7.Stephens R, Spurgeon A, Calvert IA, Beach J, Levy LS, Berry H, Harrington JM. Neuropsychological effects of long-term exposure to organophosphates in sheep dip. Lancet. 1995;345:1135–1139. doi: 10.1016/s0140-6736(95)90976-1. [DOI] [PubMed] [Google Scholar]
- 8.De Silva HJ, Samarawickrema NA, Wickremasinghe AR. Toxicity due to organophosphorus compounds: what about chronic exposure? Trans. R. Soc. Trop. Med. Hyg. 2006;100:803–806. doi: 10.1016/j.trstmh.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 9.Cole DC, Carpio F, Julian J, Leon N, Carbotte R, De Almeida H. Neurobehavioral Outcomes Among Farm and Nonfarm Rural Ecuadorians. Neurotoxicol. Teratol. 1997;19:277–286. doi: 10.1016/s0892-0362(97)00019-6. [DOI] [PubMed] [Google Scholar]
- 10.Rohlman D, Lasarev M, Anger W, Scherer J, Stupfel J, McCauley L. Neurobehavioral Performance of Adult and Adolescent Agricultural Workers. Neurotoxicology. 2007;28:374–380. doi: 10.1016/j.neuro.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 11.Rothlein J, Rohlman D, Lasarev M, Phillips J, Muniz J, McCauley L. Organophosphate pesticide exposure and neurobehavioral performance in agricultural and non-agricultural Hispanic workers. Environ. Health Perspect. 2006;114:691–696. doi: 10.1289/ehp.8182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Farahat TM, Abdelrasoul GM, Amr MM, Shebl MM, Farahat FM, Anger WK. Neurobehavioural effects among workers occupationally exposed to organophosphorous pesticides. Occup. Environ. Med. 2003;60:279–286. doi: 10.1136/oem.60.4.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kamel F, Rowland AS, Park LP, Anger WK, Baird DD, Gladen BC, Moreno T, Stallone L, Sandler DP. Neurobehavioral performance and work experience in Florida farmworkers. Environ. Health Perspect. 2003;111:1765–1772. doi: 10.1289/ehp.6341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reidy TJ, Bowler RM, Rauch SS, Pedroza GI. Pesticide exposure and neuropsychological impairment in migrant farm workers. Arch. Clin. Neuropsychol. 1992;7:85–95. [PubMed] [Google Scholar]
- 15.Bazylewicz-Walczak B, Majczakowa W, Szymczak M. Behavioral effects of occupational exposure to organophosphorous pesticides in female greenhouse planting workers. Neurotoxicology. 1999;20:819–826. [PubMed] [Google Scholar]
- 16.Gomes J, Lloyd O, Revitt M, Basha M. Morbidity among farm workers in a desert country in relation to long-term exposure to pesticides. Scand. J. Work Environ. and Health. 1998;24:213–219. doi: 10.5271/sjweh.301. [DOI] [PubMed] [Google Scholar]
- 17.London L, Myers J, Nell V, Taylor T, Thompson M. An Investigation into Neurologic and Neurobehavioral Effects of Long-Term Agrichemical Use among Deciduous Fruit Farm Workers in the Western Cape, South Africa. Environ. Res. 1997;73:132–145. doi: 10.1006/enrs.1997.3715. [DOI] [PubMed] [Google Scholar]
- 18.Richter ED, Chuwers P, Levy Y, Gordon M, Grauer F, Marzouk J, Levy S, Barron S, Gruener N. Health effects from exposure to organophosphate pesticides in workers and residents in Israel. Isr. J. Med. Sci. 1992;28:584–598. [PubMed] [Google Scholar]
- 19.Fiedler N, Kipen H, McNeil K, Fenske R. Long-term use of organophosphates and neuropsychological performance. Am. J. Ind. Med. 1997;5:487–496. doi: 10.1002/(sici)1097-0274(199711)32:5<487::aid-ajim8>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 20.Korsak RJ, Sato MM. Effects of chronic organophosphate pesticide exposure on the Central Nervous System. Clin. Toxicol. 1977;11:83–95. doi: 10.3109/15563657708989822. [DOI] [PubMed] [Google Scholar]
- 21.Steenland K, Dick RB, Howell RJ, Chrislip DW, Jines CJ, Reid TM, Lehman E, Laber P, Krieg EF, Knott C. Neurologic functioning among termiticide applicators exposed to chlorpyifos. Environ. Health Perspect. 2000;108:293–300. doi: 10.1289/ehp.00108293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abdel Rasoul GM, Abou Salem ME, Mechael AA, Hendy OM, Rohlman DS, Ismail AA. Effects of occupational pesticide exposure on children applying pesticides. Neurotoxicology. 2008;29:833–838. doi: 10.1016/j.neuro.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 23.Amr M, Halim Z, Moussa S. Psychiatric disorders among Egyptian pesticide aplicators and formulators. Environ. Toxicol. 1997;73:193–199. doi: 10.1006/enrs.1997.3744. [DOI] [PubMed] [Google Scholar]
- 24.Amr M. Pesticide monitoring and its health problems in Egypt, a Third World country. Toxicol. Lett. 1999;107:1–13. doi: 10.1016/s0378-4274(99)00026-0. [DOI] [PubMed] [Google Scholar]
- 25.Mostafa S. Head, Menoufia Pest Control administration; Menoufia Governorate. Shebin El-Kom, Egypt. 2008 [Google Scholar]
- 26.Egyptian Ministry of Agriculture. Website of the Egyptian Ministry of Agriculture. 2008 < http://www.agri.gov.eg>. [Google Scholar]
- 27.Hesham S, Mazen M, El-Damaty M. Quality of training service for agricultural workers in some Egyptian governorates. Egyptian J. Agric. Res. 2006;84:1646–1650. [Google Scholar]
- 28.NIOSH. NIOSH Manual of Analytical Methods (NMAM) Fourth ed 1994. Organophosphorus Pesticides. Method 5600. [Google Scholar]
- 29.Durham WF, Wolfe HR. Measurement of the exposure of workers to pesticides. Bull. World Health Organ. 1962;26:75–91. [PMC free article] [PubMed] [Google Scholar]
- 30.Hines CJ, Deddens JA. Determinants of chlorpyrifos exposures and urinary 3,5,6-trichloro-2-pyridinol levels among termiticide applicators. Ann. Occup. Hyg. 2001;45:309–321. [PubMed] [Google Scholar]
- 31.Fabiny D, Ertingshausen G. Automated reaction-rate method for determination of serum creatinine with the CentrifiChem. Chem. Clin. Chem. 1971;17:696–700. [PubMed] [Google Scholar]
- 32.ICRP. International Commission on Radiological Protection. 1994. ICRP Publication 23, (ed.), Reference Manual: anatomical, physiological and metabolic characteristics. [Google Scholar]
- 33.Nolan RJ, Rick DL, Freshour NL, Saunders JH. Chlorpyrifos: pharmacokinetics in human volunteers. Toxicol. Appl. Pharmacol. 1984;73:8–15. doi: 10.1016/0041-008x(84)90046-2. [DOI] [PubMed] [Google Scholar]
- 34.Office of Pesticide Programs, Registration Eligibility Document (RED) for Chlorpyrifos, Table 8, U.S. Environmental Protection Agency. 2006 < http://www.epa.gov/opp00001/reregistration/status_page_c.htm>.
- 35.Durham WF, Wolfe HR, Elliott JW. Absorption and excretion of parathion by spraymen. Arch. Environ. Health. 1972;24:381–387. doi: 10.1080/00039896.1972.10666113. [DOI] [PubMed] [Google Scholar]
- 36.Wester R, Maiback H. Ch. 43. In: Krieger RI, editor. Handbook of Pesticide Toxicology. London: Academic Press; 2001. pp. 905–912. [Google Scholar]
- 37.Riegart R, Roberts J, editors. USEPA. Recognition and management of pesticide poisoning. Washington DC: US Environmental Protection Agency; 1999. [Google Scholar]
- 38.Methner M, Fenske R. Pesticide exposure during greenhouse applications, Part II. Chemical permeation through protective clothing in contact with treated foliage. Appl. Occup. Environ. Hyg. 1994;9:567–574. [Google Scholar]
- 39.Stamper JH, Nigg HN, Mahon WD, Nielsen AP, Royer MD. Pesticide exposure to greenhouse handgunners. Arch. Environ. Contam. Toxicol. 1989;18:515–529. doi: 10.1007/BF01055018. [DOI] [PubMed] [Google Scholar]
- 40.CDC. Third National Report of Human Exposure to Environmental Chemicals. Centers for Disease Control; 2005. [Google Scholar]
- 41.Alexander BH, Burns CJ, Bartels MJ, Acquavella JF, Mandel JS, Gustin C, Baker BA. Chlorpyrifos exposure in farm families: results from the farm family exposure study. J. Expo. Sci. Environ. Epidemiol. 2006;16:447–456. doi: 10.1038/sj.jes.7500475. [DOI] [PubMed] [Google Scholar]
- 42.Kibogy J. Master of Science Thesis. Department of Environmental and Occupational Health Sciences, University of Washington; 2008. Assessment of chlorpyrifos exposure in agricultural workers during airblast applications. [Google Scholar]