Abstract
Calcium-dependent protein kinases (CDPKs) have been shown to be involved in abscisic acid (ABA)-mediated physiological processes, including seed germination, post-germination growth, stomatal movement, and plant stress tolerance. However, it is not clear whether CDPKs are involved in ABA-induced antioxidant defence. In the present study, the role of the maize CDPK ZmCPK11 in ABA-induced antioxidant defence and the relationship between ZmCPK11 and ZmMPK5, a maize ABA-activated mitogen-activated protein kinase (MAPK), in ABA signalling were investigated. Treatments with ABA and H2O2 induced the expression of ZmCPK11 and increased the activity of ZmCPK11, while H2O2 was required for the ABA-induced increases in the expression and the activity of ZmCPK11. The transient gene expression analysis and the transient RNA interference (RNAi) test in protoplasts showed that ZmCPK11 is involved in ABA-induced up-regulation of the expression and the activities of superoxide dismutase (SOD) and ascorbate peroxidase (APX), and in the production of H2O2. Further, ZmCPK11 was shown to be required for the up-regulation of the expression and the activity of ZmMPK5 in ABA signalling, but ZmMPK5 had very little effect on the ABA-induced up-regulation of the expression and the activity of ZmCPK11. Moreover, the transient gene expression analysis in combination with the transient RNAi test in protoplasts showed that ZmCPK11 acts upstream of ZmMPK5 to regulate the activities of antioxidant enzymes. These results indicate that ZmCPK11 is involved in ABA-induced antioxidant defence and functions upstream of ZmMPK5 in ABA signalling in maize.
Key words: Abscisic acid, antioxidant defence, calcium-dependent protein kinase, maize, mitogen-activated protein kinase, signal transduction.
Introduction
Abscisic acid (ABA) is a plant hormone that plays critical roles in adaptive responses to environmental stresses such as drought and salt stress. ABA accumulates in plant cells under water stress, stimulates stomatal closure, and regulates the expression of many genes, thus increasing the plant’s capacity to cope with stress conditions (Cutler et al., 2010; Hubbard et al., 2010; Umezawa et al., 2010; Joshi-Saha et al., 2011). Accumulating evidence indicates that ABA-enhanced water stress tolerance is associated with the induction of antioxidant defence systems, including reactive oxygen species (ROS)-scavenging enzymes such as superoxide dismutase (SOD), catalase (CAT), ascorbate peroxidase (APX), glutathione peroxidase (GPX), and glutathione reductase (GR), and non-enzymatic antioxidants such as ascorbic acid, glutathione, α-tocopherol, and carotenoids (Jiang and Zhang, 2002a , b ; Hu et al., 2005; Miao et al., 2006; Zhang et al., 2006, 2007; Neill et al., 2008; Xing et al., 2008; Miller et al., 2010; Ye et al., 2011; Ozfidan et al., 2012). Ca2+, calmodulin (CaM), NADPH oxidase, H2O2, nitric oxide (NO), mitogen-activated protein kinase (MAPK), and Ca2+/CaM-dependent protein kinase (CCaMK) are important intermediate components in ABA-induced antioxidant defence (Jiang and Zhang, 2002a , b , 2003; Hu et al., 2005, 2007; Zhang et al., 2006, 2007; Neill et al., 2008; Xing et al., 2008; Ye et al., 2011; Ma et al., 2012; Shi et al., 2012).
Calcium-dependent protein kinases (CDPKs) are serine/threonine protein kinases that include a Ca2+-binding CaM-like domain and are one of the best characterized Ca2+ sensors in plants. CDPKs constitute a large multigene family consisting of 34 genes in Arabidopsis (Cheng et al., 2002; Hrabak et al., 2003) and 29 genes in rice (Asano et al., 2005). A number of studies have shown that CDPKs are involved in the responses of plants to various abiotic stresses, including drought, salt, hormonal stimuli, and oxidative stress (Saijo et al., 2000; Xing et al., 2001; Choi et al., 2005; Ludwig et al., 2005; Mori et al., 2006; Kobayashi et al., 2007, 2012; Ma and Wu, 2007; Zhu et al., 2007; Boudsocq et al., 2010; Mehlmer et al., 2010; Xu et al., 2010; Zou et al., 2010; Asano et al., 2011, 2012; Franz et al., 2011; Zhao et al., 2011; Boudsocq and Sheen, 2012). In Arabidopsis, AtCPK3 and AtCPK6 (Mori et al., 2006), AtCPK4 and AtCPK11 (Zhu et al., 2007), AtCPK10 (Zou et al., 2010), and AtCPK32 (Choi et al., 2005) have been shown to be positive regulators of ABA-mediated physiological processes, including seed germination, post-germination growth, stomatal movement, and plant stress tolerance. In rice, OsCPK12 (Asano et al., 2012) and OsCPK21 (Asano et al., 2011) are involved in positive regulation of the ABA signalling pathway. However, a recent study showed that AtCPK12 is a negative ABA signalling regulator in seed germination and post-germination growth (Zhao et al., 2011). CDPKs have also been shown to be associated with the production of ROS. Ectopic expression of AtCPK1 increases NADPH oxidase activity and ROS production in tomato protoplasts (Xing et al., 2001). AtCPK4, AtCPK5, AtCPK6, and AtCPK11 redundantly regulate the bacterial elicitor flg22-induced ROS burst and pathogen defence (Boudsocq et al., 2010). When potato plants are attacked by pathogens, StCDPK4 and StCDPK5 regulate ROS production by phosphorylating NADPH oxidase (StRbohB) and inducing the oxidative burst (Kobayashi et al., 2007). A further study demonstrated that StCDPK5 can activate StRbohA–D, which mediate the StCDPK5-induced ROS burst (Kobayashi et al., 2012). In rice, a recent study showed that OsCPK12 can induce the expression of the antioxidant genes OsAPX2 and OsAPX8 under salt stress, and reduce the salt-induced accumulation of H2O2 (Asano et al., 2012). These results suggest that OsCPK12 positively regulates ROS detoxification by controlling the expression of antioxidant genes. However, whether CDPKs are involved in ABA-induced antioxidant defence remains to be determined.
The MAPK cascade has been shown to be another major signal transduction pathway that is widely used to adapt cellular metabolism to a changing environment (Colcombet and Hirt, 2008; Pitzschke et al., 2009; Wurzinger et al., 2011). In Arabidopsis, it has been shown that AtMPK3, AtMPK6, AtMPK9, and AtMPK12 are involved in ABA signalling (Colcombet and Hirt, 2008; Xing et al., 2008; Jammes et al., 2009; Liu, 2012). AtMPK6 in Arabidopsis (Xing et al., 2008), ZmMPK5 and ZmMPK3 in maize (Lin et al., 2009; Wang et al., 2010), and OsMPK1 and OsMPK5 in rice (Zhang et al., 2012) are required for ABA-induced antioxidant defence. Although both CDPKs and MAPKs have been shown to be involved in ABA signalling, it is not clear whether there exists a link between the CDPK pathway and the MAPK pathway in ABA signalling.
In this study, the role of the maize CDPK ZmCPK11, which belongs to group I of the CDPK family and is closely related to AtCPK4 and AtCPK11 (Boudsocq and Sheen, 2012), in ABA-induced antioxidant defence was investigated. Previous studies have shown that ZmCPK11 is a component of touch- and wound-induced pathway(s), participating in early stages of local and systemic responses (Szczegielniak et al., 2005, 2012). Moreover, the relationship between ZmCPK11 and ZmMPK5, which is required for the ABA-induced antioxidant defence and for the positive feedback regulation of NADPH oxidase activity (Zhang et al., 2006; Ding et al., 2009; Lin et al., 2009), in ABA signalling was also examined. Here, evidence is provided to show that ZmCPK11 is involved in ABA-induced antioxidant defence and acts upstream of ZmMPK5 in ABA signalling in maize.
Materials and methods
Plant materials and treatments
Seeds of maize (Zea mays L. cv. Nongda 108; from Nanjing Agricultural University, China) were sown in trays of sand in a light chamber at a temperature of 22 °C (night) to 28 °C (day), photosynthetic active radiation of 200 µmol m–2 s–1, and a photoperiod of 14/10h (day/night), and watered daily. For protoplast isolation, maize plants were grown at 26 °C under dark conditions. When the second leaves were fully expanded, they were collected and used for investigations.
The plants were excised at the base of the stem and placed in distilled water for 4h to eliminate wound stress. After treatment, the cut ends of the stems were placed in beakers wrapped with aluminium foil containing 100 µM ABA, 10% (w/v) polyethylene glycol (PEG 6000), 10mM H2O2, or 10mM CaCl2 for the indicated time, with a continuous light intensity of 200 µmol m–2 s–1. To study the effects of inhibitors, the detached plants were pre-treated with 100 µM diphenyleneiodonium chloride (DPI), 10mM dimethylthiourea (DMTU), 200U of CAT, 100 µM trifluoperazine (TFP), 10mM ethylene glycol-bis(2-aminoethyl ether)-N,N,N′,N′- tetraacetic acid (EGTA), 100 µM 2-(2-amino-3-methoxyphenyl)-4H-1-benzopyran- 4-one (PD98059), and 10 µM 1,4-diamino-2,3-dicyano-1,4-bis(o-aminophenyl-mercapto) butadiene (U0126) for 4h, and then subjected to 100 µM ABA treatment. For fluridone treatment, maize seeds were soaked in 100 µM fluridone for 16h, then germinated and grown under the same conditions as described above. Detached plants were treated with distilled water under the same conditions for the whole period and served as controls for the above. After treatments of detached maize plants, the second leaves were sampled and immediately frozen under liquid N2 for further analysis.
Protein extraction and immunocomplex kinase activity assay
Protein was extracted from leaves or protoplasts with an extraction buffer as described previously (Zhang et al., 2006), but without 5mM EGTA in the case of ZmCPK11 assay. After centrifugation at 12 000 g for 30min at 4 °C, the supernatants were transferred into new tubes, immediately frozen with liquid N2, and stored at –80 °C. Protein content was determined according to the method of Bradford (1976) with bovine serum albumin (BSA) as standard.
For immunocomplex kinase assay, protein extract (100 µg) was incubated with anti-ZmCPK11 antibody (2 µg) or anti-ZmMPK5 antibody (2 µg) in an immunoprecipitation buffer as described previously (Zhang et al., 2006), but without 2mM EGTA in the case of ZmCPK11 assay, at 4 °C for 12h on a rocker. An ~25 µl volume of protein G–garose was added, and the incubation was continued for another 3h. Agarose bead–protein complexes were pelleted by brief centrifugation. After washing with immunoprecipitation buffer three times, reaction buffer {25mM TRIS, pH 7.5, 100 µM Na3VO4, 1mM dithiothreitol (DTT), 12mM MgCl2, 1mM CaCl2 (not in the ZmMPK5 assay), 200nM ATP plus 50 µCi of [γ-32P]ATP (3000 Ci mM–1), 0.25 µg µl–1 histone S-III for ZmCPK11 or 0.25mg ml–1 MBP for ZmMPK5} was added and reacted for 30min at room temperature. Ten loading samples were then added and boiled for 5min. After centrifugation, the supernatant fraction was electrophoresed on SDS–polyacrylamide gels. The gel was dried onto Whatman 3 MM paper and exposed to Kodak XAR-5 film. Relative activation levels of ZmCPK11 and ZmMPK5 proteins, detected by immunocomplex kinase activity assay and quantificated by Quantity One software (Bio-Rad Laboratories Inc., USA), are presented as values relative to those of the corresponding controls.
RNA preparation and cDNA synthesis
Total RNA was isolated from leaves or protoplasts using RNAiso Plus (TaKaRa Bio Inc., China) according to the manufacturer’s instructions. DNase treatment was included in the isolation step using RNase-free DNase (TaKaRa Bio Inc.). Approximately 2 µg of total RNA was reverse transcribed using oligo d(T)16 primer and M-MLV reverse transcriptase (TaKaRa Bio Inc.) at 42 °C for 75min.
Real-time quantitative RT–PCR expression analysis
Real-time quantitative reverse transcription–PCRs (RT–PCRs) were performed in a DNA Engine Opticon 2 real-time PCR detection system (Bio-Rad Laboratories Inc., USA) using SYBR Premix Ex Taq™ (TaKaRa Bio Inc.) according to the manufacturer’s instructions. cDNA was amplified by PCR using the following primers: ZmCPK11 (GenBank accession no. AAP57564.2), forward CCTCCACGACCCCGACAATG and reverse ACCTCTCCGAG CACCCCAAC; ZmMPK5 (GenBank accession no. BAA74734.1), forward ACTGATGGACCGCAAACC and reverse GGGTGACG AGGAAGTTGG; SOD4 (GenBank accession no. X17565), forward TGGAGCACCAGAAGATGA and reverse CTCGTGTCC ACCCTTTCC; cAPX (GenBank accession no. EU969033), forward TGAGCGACCAGGACATTG and reverse GAGGGCTTTGTCA CTTGGT; and β-actin (GenBank accession no. J01238), forward GTTTCCTGGGATTGCCGAT and reverse TCTGCTGCTGA AAAGTGCTGAG.
Each PCR (20 µl) contained 10 µl of 2× Real-time PCR Mix (containing SYBR Green), 0.2 µM of each primer, and appropriately diluted cDNA. The thermal cycling conditions were 94 °C for 30 s followed by 40 cycles of 94 °C for 10 s, 60 °C for 20 s, and 72 °C for 20 s. To standardize the data, the ratio of the absolute transcript level of the target genes to the absolute transcript level of β-actin was calculated for each sample. The relative expression levels of the target genes were calculated as γ-fold changes relative to the appropriate control experiment for the different chemical treatments.
Plasmids
The full-length cDNA fragment was amplified with the KpnI sites and then cloned between the Cauliflower mosaic virus (CaMV) 35S promoter and yellow fluorescent protein (YFP) of the pXZP008 vector. The primer pairs were: forward GGTA CCCGGGTTTTGCTGGGATTCAAGAGTTCGCCG and reverse GGTACCGAATGCAGCCGGACCCGAGCGGGAA.
In vitro synthesis of dsRNA
DNA templates were produced by PCR using primers containing the T7 promoter sequence (5’-TTAATACGACTCACTATAGGG AGG-3’) on both the 5’ and 3’ ends. The primers used to amplify DNA of ZmCPK11 were: forward TAATACGACTCACTATA GGGAGACCACTGACTTTGGGCTTTCC and reverse TAATA CGACTCACTATA GG GAGACAGCTTCTGGACCATAGCAT. The PCR conditions were as follows: denaturing step at 94 °C for 5min, followed by 34 cycles of 94 °C for 30 s, 58 °C for 30 s, 72 °C for 30 s, and a final extension at 72 °C for 10min. The primers used to amplify DNA of ZmMPK5 were: forward TAATACGACTCACTATAGGGAGAACCTGGTGGAAAAGA TGCT and reverse TAATACGACTCACTATAGGGAGACATG CTGCTCGAAGTCAAA. The PCR conditions were as follows: denaturing step at 94 °C for 5min, followed by 34 cycles of 94 °C for 30 s, 53 °C for 30 s, 72 °C for 30 s, and a final extension at 72 °C for 10min. After PCR product clean up, the DNA templates were used for in vitro synthesis of double-stranded RNAs (dsRNAs) using the Ribomax Express kit (Promega). The dsRNAs were purified by phenol–chloroform–isopropanol extraction, dissolved in RNase-free water, and quantified by UV spectrophotometry.
Protoplast isolation
Maize plants were grown in the dark at 26 °C for ~7 d. When the second leaves were fully expanded, the protoplasts from the second leaf were isolated according to the method described by Ma et al. (2012).
Transfection of protoplasts with plasmid DNA or dsRNAs
The CaMV35S-ZmCPK11-YFP plasmid or dsRNAs were delivered into protoplasts using a PEG–calcium-mediated method described previously (Yoo et al., 2007; Zhai et al., 2009). About 10 µg of plasmid DNA or dsRNAs per 100 µl ×105 protoplasts were used for transient expression analysis. The DNA or dsRNA and protoplast mixtures were added to 40% PEG solution (40% PEG 4000, 0.4mM mannitol, and 100mM CaCl2, adjusted to pH 7.0 with 1M KCl), mixed gently, and incubated for 15min at room temperature in the dark. Protoplasts were washed by 440 µl of W5 solution, and incubated in W5 medium containing 0.1% (w/v) glucose in the dark overnight.
Subcellular localization of ZmCPK11
The protoplasts expressing the ZmCDPK11–YFP fusion protein after 16h incubation were observed using a laser confocal microscope (TCS-SP2, Leica, Bensheim, Germany), with excitation at 530nm and emission at 525nm. For the nuclear staining, 4’,6-diamino-2-phenylindole (DAPI; 1 µg µl–1) was added to the culture medium and incubated for 1h. For the plasma membrane staining, N-[3-triethyl-ammoniumpropyl]-4-[p-diethylaminophenylhexatrienyl] pyridinium dibromide (FM4-64, 5 µg µl–1) was added to the culture medium and incubated for 30min. FM4-64 fluorescence was observed under a microscope using an RFP filter, with excitation at 543nm and emission at 580nm.
H2O2 detection by confocal laser scanning microscopy
H2O2 production in protoplasts was monitored using the H2O2-sensitive fluorescent probe 2′,7′-dichlorofluorescein diacetate (H2DCF-DA; Molecular Probes, Leiden, The Netherlands) using the method described by Bright et al. (2006). Images acquired were analysed using Leica IMAGE software. Data are presented as mean pixel intensities. A total of 120 protoplasts are observed per treatment for three independent replicates.
Enzyme assays
Protoplasts were homogenized in a solution of 50mM potassium phosphate buffer (pH 7.0) containing 1mM EDTA and 1% polyvinylpyrrolidone. The homogenate was centrifuged at 12 000 g for 30min at 4 °C and the supernatant was immediately used for the antioxidant enzyme assays. The total activities of SOD and APX were determined as described previously (Jiang and Zhang, 2001). Protein content was determined according to the method of Bradford (1976) with BSA as standard.
Results
ABA and H2O2 induce the expression of ZmCPK11 and increase the activity of ZmCPK11 in maize leaves
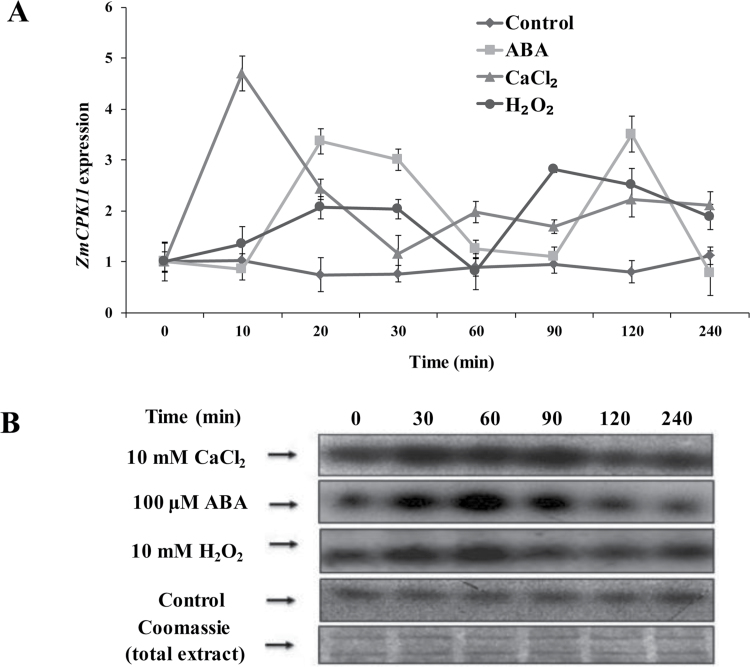
To investigate the effects of ABA and H2O2 on the induction of the expression of ZmCPK11 and the activity of ZmCPK11 in leaves of maize plants, relative quantitative real-time PCR analysis and immunocomplex kinase activity assay were used. Treatments with ABA (100 µM) and H2O2 (10mM) induced a rapid increase in the expression of ZmCPK11 (Fig. 1A). A biphasic response in the expression of ZmCPK11 in maize leaves exposed to ABA and H2O2 treatments was observed, in which the first peak occurred after 20min of treatment, and the second peak appeared after 90min of H2O2 treatment or 120min of ABA treatment (Fig. 1A). Treatments with ABA and H2O2 also caused a rapid increase in the activity of ZmCPK11 in maize leaves (Fig. 1B). Time-course analysis showed that ABA treatment led to a significant increase in the activity of ZmCPK11 within 30min, maximized at 60min, remained high for 90min after ABA treatment, and then decreased to the control level after 120min of ABA treatment. Compared with the ABA treatment, H2O2 treatment also caused a similar change in the activity of ZmCPK11, but the activity of ZmCPK11 returned to the control level after 90min of H2O2 treatment. As a positive control, CaCl2 treatment also induced the increases in the expression of ZmCPK11 (Fig. 1A) and the activity of ZmCPK11 (Fig. 1B).
Fig. 1.
ABA, H2O2, and CaCl2 induce the expression of ZmCPK11 and the activity of ZmCPK11 in maize leaves. (A) Expression analysis of ZmCPK11 in leaves of maize plants exposed to ABA, H2O2, and CaCl2 treatments. The detached maize plants were treated with ABA (100 µM), H2O2 (10mM), and CaCl2 (10mM) for various times as indicated. The relative expression levels of the ZmCPK11 gene were analysed by real-time quantitative PCR. Values are means ±SE of three independent experiments. Means denoted by the same letter did not differ significantly at P <0.05 according to Duncan’s multiple range test. (B) Induction of the activity of ZmCPK11 by ABA, H2O2, and CaCl2. The detached plants were treated as described in (A). ZmCPK11 was immunoprecipitated from leaves after treatments, and the activity of ZmCPK11 was measured by immunoprecipitation kinase assay using histone S-III as a substrate. Corresponding Coomassie staining was also shown as indicated. Experiments were repeated at least three times with similar results.
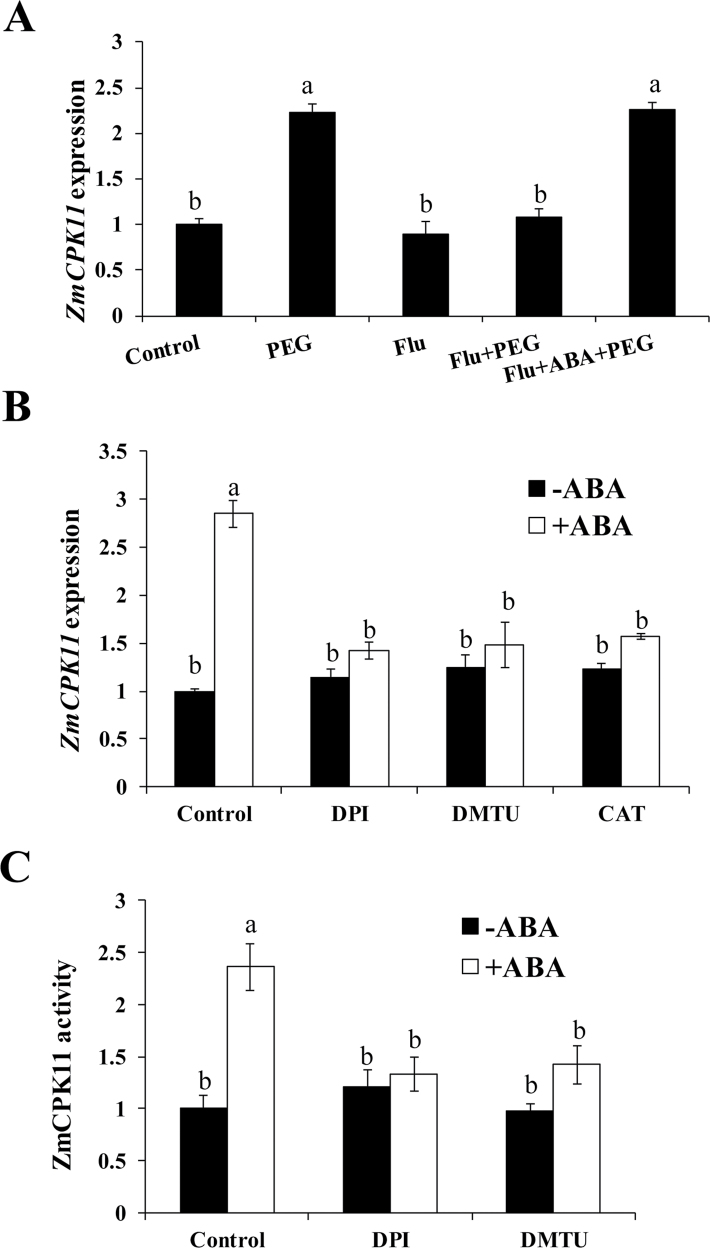
To investigate whether the expression of ZmCPK11 can be regulated by endogenous ABA, maize seeds were pre-treated with an inhibitor of ABA biosynthesis, fluridone, and then the pre-treated plants were exposed to PEG treatment. PEG treatment induced an increase in the expression of ZmCPK11, but the increase was inhibited by the pre-treatment with fluridone (Fig. 2A). The effect of fluridone on the expression of ZmCPK11 was overcome by the application of 100 µM ABA. Pre-treatment with fluridone alone had very little effect on the expression of ZmCPK11 in leaves of maize plants (Fig. 2A). These results suggest that ABA is involved in the up-regulation of ZmCPK11 expression in the leaves of maize plants exposed to water stress.
Fig. 2.
H2O2 is required for ABA-induced activation of ZmCPK11 in maize leaves. (A) Effect of pre-treatment with the ABA biosynthetic inhibitor fluridone (Flu) on the expression of ZmCPK11 in maize leaves exposed to PEG treatment. The fluridone-treated and -untreated seedlings were exposed to 10% PEG treatment for 1h. ABA (100 µM) was added to overcome the effects of fluridone. (B, C) Effects of pre-treatments with the ROS manipulators DMTU, DPI, and CAT on the expression of ZmCPK11 (B) and the activity of ZmCPK11 (C) in maize leaves exposed to ABA treatment. The detached maize plants were pre-treated with 10mM DMTU, 100 µM DPI, and 200U of CAT for 4h, and then exposed to 100 µM ABA for 30min (B) or 60min (C). Values are means ±SE of three independent experiments. Means denoted by the same letter did not significantly differ at P<0.05 according to Duncan’s multiple range test.
To determine whether ABA-induced increases in the expression of ZmCPK11 and the activity of ZmCPK11 are related to the action of endogenous H2O2, several ROS manipulators, such as DPI, an inhibitor of NADPH oxidase, DMTU, a trap for H2O2, and CAT, the enzyme eliminating H2O2, were applied. Pre-treatments with DPI, DMTU, and CAT substantially suppressed the ABA-induced increases in the expression of ZmCPK11 (Fig. 2B) and the activity of ZmCPK11 (Fig. 2C), suggesting that H2O2 is required for the ABA-induced up-regulation of the expression and the activity of ZmCPK11 in maize leaves.
Subcellular localization of ZmCPK11 in maize protoplasts
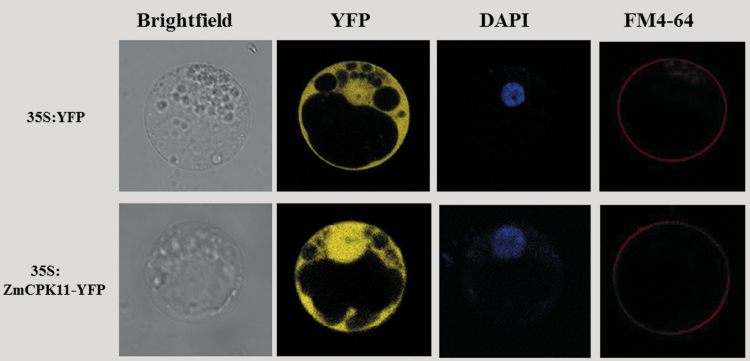
Previous studies showed that different CDPKs have different subcellular localizations, including the plasma membrane, endoplasmic reticulum, actin cytoskeletal system, mitochondria, peroxisomes, cytosol, nucleus, and oil bodies (Zou et al., 2010; Wurzinger et al., 2011; Boudsocq and Sheen, 2012; Kobayashi et al., 2012). To investigate the intracellular localization of ZmCPK11, a reporter gene encoding YFP was fused to ZmCPK11 (ZmCPK11-YFP), which is driven by the 35S:ZmCPK11-YFP promoter, and then transformed into maize protoplasts by PEG–calcium-mediated transformation (Yoo et al., 2007). The nucleus was stained by DAPI, and the plasma membrane was marked by FM4-64. The results showed that ZmCPK11–YFP was localized in the nucleus and the cytoplasm (Fig. 3).
Fig. 3.
Subcellular localization of ZmCPK11 in maize protoplasts. Constructs carrying 35S:ZmCPK11-YFP or 35S:YFP were introduced into protoplasts prepared from the leaves of maize by PEG–calcium-mediated transformation. Transfected protoplasts were observed after 16h incubation by a laser confocal microscope. Nuclei are shown with DAPI staining (blue). The plasma membrane was labelled with FM4-64 (red). Experiments were repeated at least five times with similar results.
ZmCPK11 is involved in ABA-induced up-regulation of the expression and the activities of antioxidant enzymes and the production of H2O2
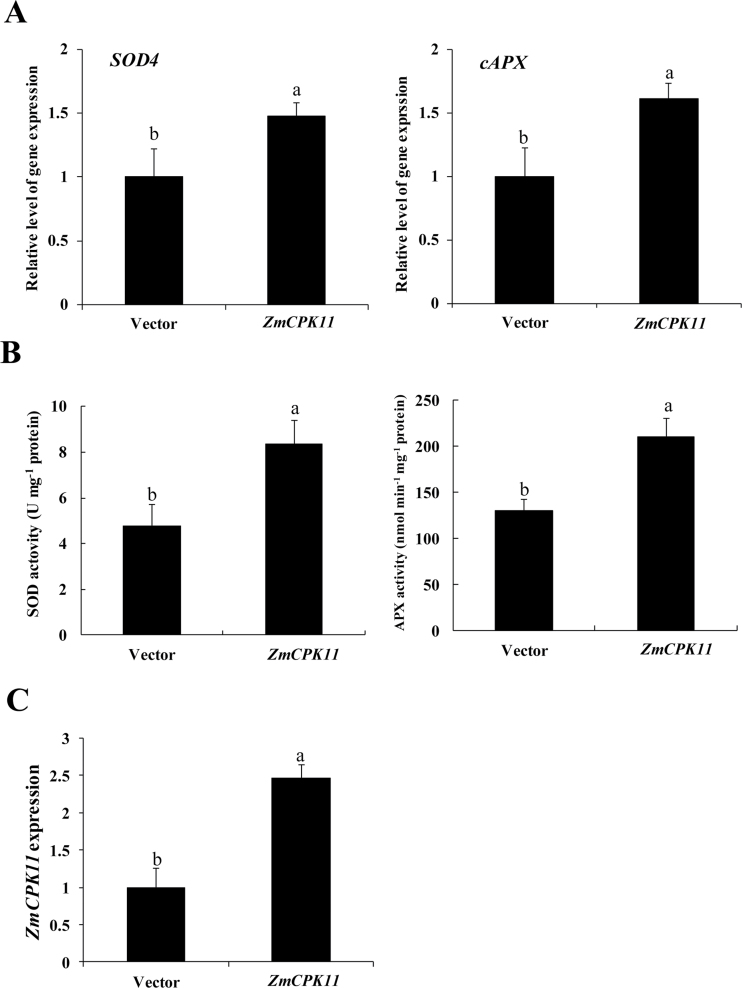
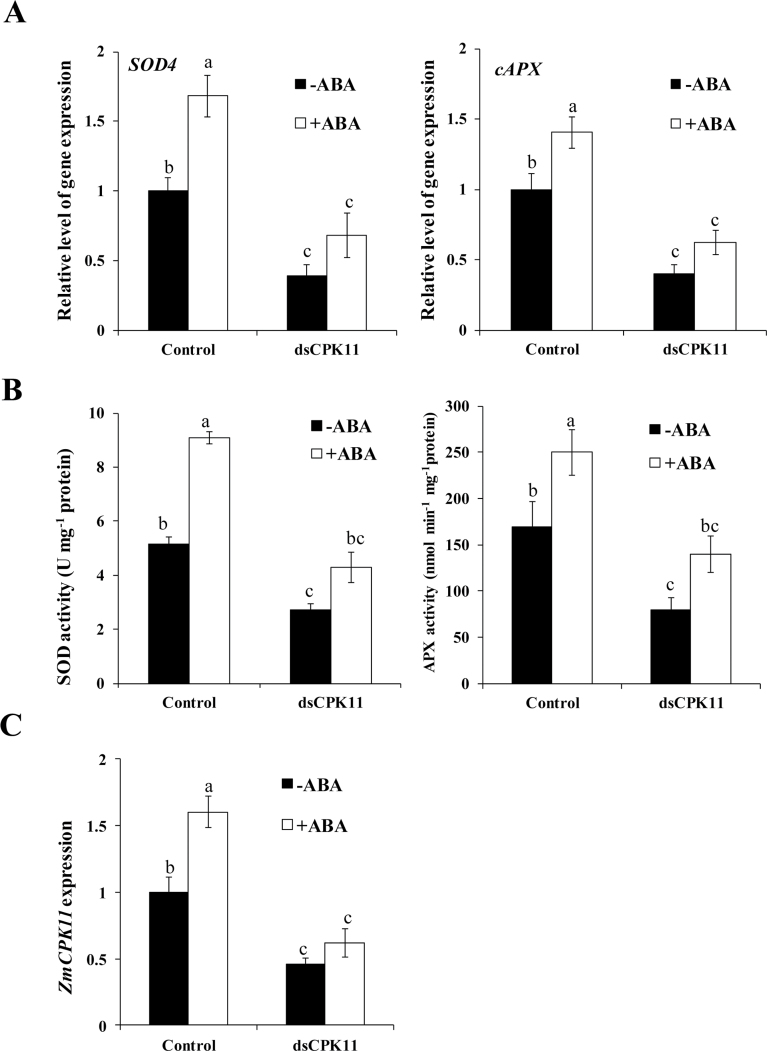
To investigate whether ZmCPK11 is involved in the ABA-induced antioxidant defence response, a transient gene expression analysis (Yoo et al., 2007) and a transient RNA interference (RNAi) test in protoplasts (Zhai et al., 2009), which have been proven to be suitable for functional analysis of plant genes (An et al., 2005; Bart et al., 2006; Chen et al., 2006; Yoo et al., 2007; Zhai et al., 2009; Ma et al., 2012; Shi et al., 2012; Zhang et al., 2012), were used for the functional analysis of ZmCPK11 in ABA signalling. Protoplast transfection with 35S:ZmCPK11-YFP plasmid caused a significant increase in the expression of ZmCPK11 (Fig. 4C), but transfection in protoplasts with an in vitro-synthesized dsRNA against ZmCPK11 (RNAi) resulted in a substantial suppression of the expression of ZmCPK11 (Fig. 5C). Transient expression of ZmCPK11 in protoplasts resulted in significant increases in the expression of the antioxidant genes SOD4, encoding a cytosolic isoform of SOD, and cAPX, encoding a cytosolic isoform of APX, and the activities of the antioxidant enzymes SOD and APX, when compared with those in protoplasts transfected with the empty vector (Fig. 4A, B), but RNAi-mediated silencing of ZmCPK11 decreased the expression of SOD4 and cAPX and the activities of SOD and APX (Fig. 5A, B). Further, treatment with 10 µM ABA induced significant increases in the expression of ZmCPK11 (Fig. 5C), SOD4, and cAPX (Fig. 5A) and the activities of SOD and APX (Fig. 5B) in the control protoplasts, and the increases were blocked by the RNAi silencing of ZmCPK11. These results indicate that ZmCPK11 is required for ABA-induced increases in the expression of SOD4 and cAPX and the activities of SOD and APX.
Fig. 4.
Transient expression of ZmCPK11 up-regulates the expression and the activities of SOD and APX in maize protoplasts. (A) The expression of SOD4 and cAPX in protoplasts with transiently expressed ZmCPK11. (B) The activities of SOD and APX in protoplasts with transiently expressed ZmCPK11. (C) The expression of ZmCPK11 in protoplasts with transiently expressed ZmCPK11. Protoplasts were transfected with ZmCPK11 or empty vector (control) and incubated for 16h. Values are means ±SE of three independent experiments. Means denoted by the same letter did not differ significantly at P<0.05 according to Duncan’s multiple range test.
Fig. 5.
RNAi-mediated silencing of ZmCPK11 inhibits the ABA-induced increases in the expression and activities of SOD and APX in maize protoplasts. (A) The expression of SOD4 and cAPX in protoplasts with transiently silenced ZmCPK11. Protoplasts were treated with 10 µM ABA for 5min, and the relative expression levels of SOD4 and cAPX were analysed by real-time quantitative PCR. (B) The activities of SOD and APX in protoplasts with transiently silenced ZmCPK11. The protoplasts were treated with 10 µM ABA for 5min, and the activities of SOD and APX were measured as described in the Materials and methods. (C) The expression of ZmCPK11 in protoplasts with transiently silenced ZmCPK11. Protoplasts were transfected with dsRNA against ZmCPK11 (dsCPK11) or with water (control) and incubated for 24h. Values are means ±SE of three independent experiments. Means denoted by the same letter did not differ significantly at P<0.05 according to Duncan’s multiple range test.
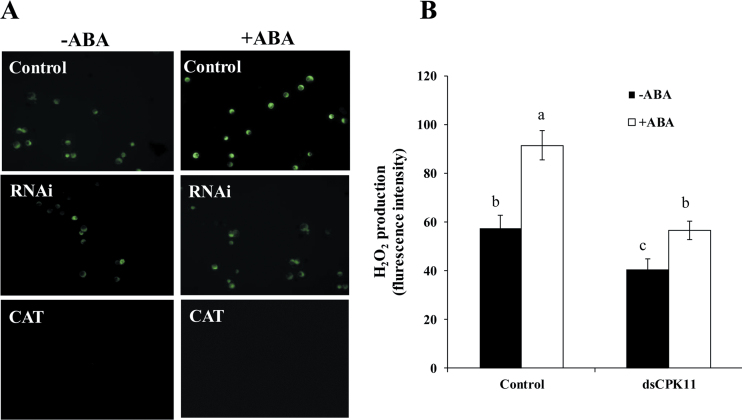
To investigate whether ABA-activated ZmCPK11 also affects ABA-induced H2O2 production, protoplasts transfected with dsRNA against ZmCPK11 were used, and H2O2 production in the protoplasts was monitored using the fluorescent probe H2DCF-DA. The RNAi silencing of ZmCPK11 in the protoplasts not only decreased the H2O2-mediated fluorescence under the control condition, but also blocked the ABA-induced increase in the fluorescence (Fig. 6). The specificity of the H2O2-mediated fluorescence was proven by the application of CAT. These results indicate that ZmCPK11 is involved in ABA-induced H2O2 production.
Fig. 6.
ZmCPK11 mediates ABA-induced production of H2O2 in maize protoplasts. (A) H2O2 fluorescence in protoplasts with transiently silenced ZmCPK11. The protoplasts were treated with 10 µM ABA (+ABA) or the incubation medium (–ABA) for 5min, and then loaded with H2DCF-DA for 10min. CAT (20U) was also added to the control protoplasts in the presence or absence of ABA. The protoplasts transfected with water were used as controls. H2O2 was visualized by confocal microscopy. Experiments were repeated at least three times with similar results. (B) Changes in the fluorescence intensity in (A). Values are means ±SE of three independent experiments. Means denoted by the same letter did not significantly differ at P<0.05 according to Duncan’s multiple range test.
ZmCPK11 regulates the expression of ZmMPK5 and the activity of ZmMPK5 in ABA signalling
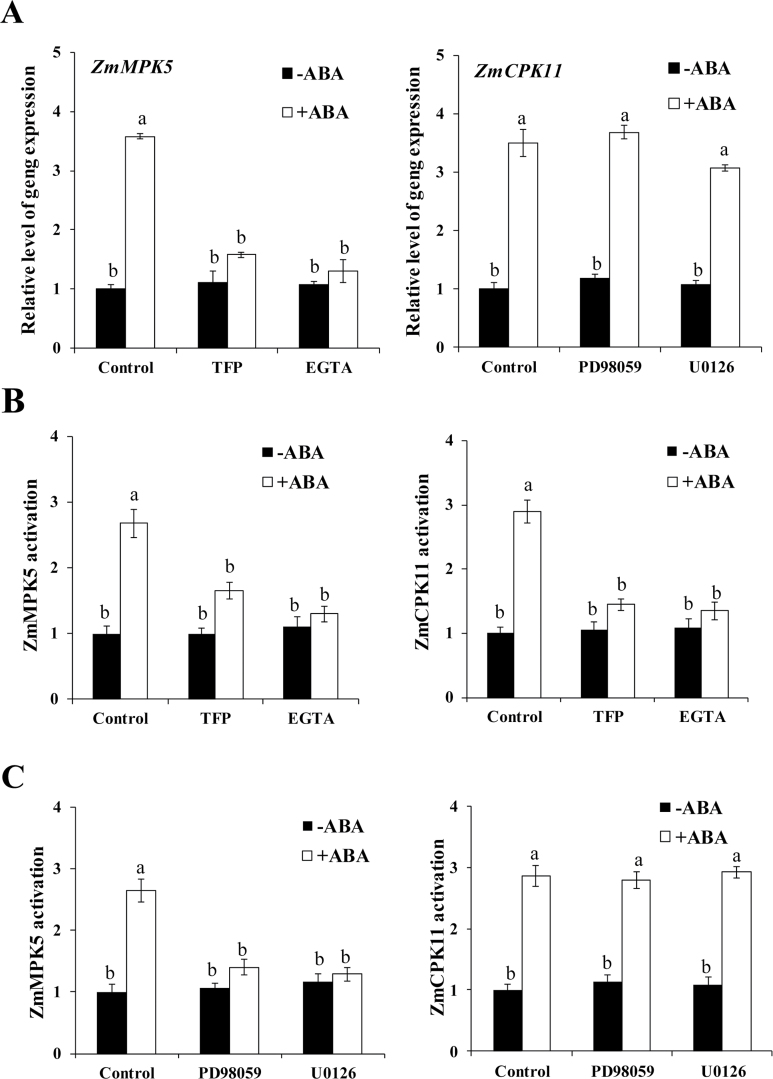
Previous studies showed that ABA and H2O2 induced the expression of ZmMPK5 and the activity of ZmMPK5 in leaves of maize plants, and ZmMPK5 is required for ABA-induced antioxidant defence (Zhang et al., 2006; Ding et al., 2009; Lin et al., 2009). To establish a possible link between ZmCPK11 and ZmMPK5 in ABA signalling, the detached maize plants were pre-treated with the Ca2+ chelator EGTA and the CDPK inhibitor TFP, and the MAPK kinase (MAPKK) inhibitors PD98059 and U0126, respectively, and then exposed to ABA treatment. Experimental results showed that pre-treatments with EGTA and TFP substantially suppressed the ABA-induced increase in the activity of ZmCPK11 in maize leaves (Fig. 7B, right), and also blocked the ABA-induced increases in the expression of ZmMPK5 (Fig. 7A, left) and the activity of ZmMPK5 (Fig. 7B, left). However, pre-treatments with PD98059 and U0126 almost completely blocked the ABA-induced increase in the activity of ZmMPK5 (Fig. 7C, left), but had very little effect on the ABA-induced up-regulation of the expression of ZmCPK11 (Fig. 7A, right) and the activity of ZmCPK11 (Fig. 7C, right).
Fig. 7.
Effects of pre-treatments with EGTA and TFP on the ABA-induced activation of ZmMPK5 and the effects of pre-treatments with PD98059 and U0126 on the ABA-induced activation of ZmCPK11 in maize leaves. The detached maize plants were pre-treated with 10mM EGTA, 100 µM TFP, 100 µM PD98059, and 10 µM U0126 for 4h, and then exposed to 100 µM ABA treatment. Values are means ±SE of three independent experiments. Means denoted by the same letter did not differ significantly at P<0.05 according to Duncan’s multiple range test.
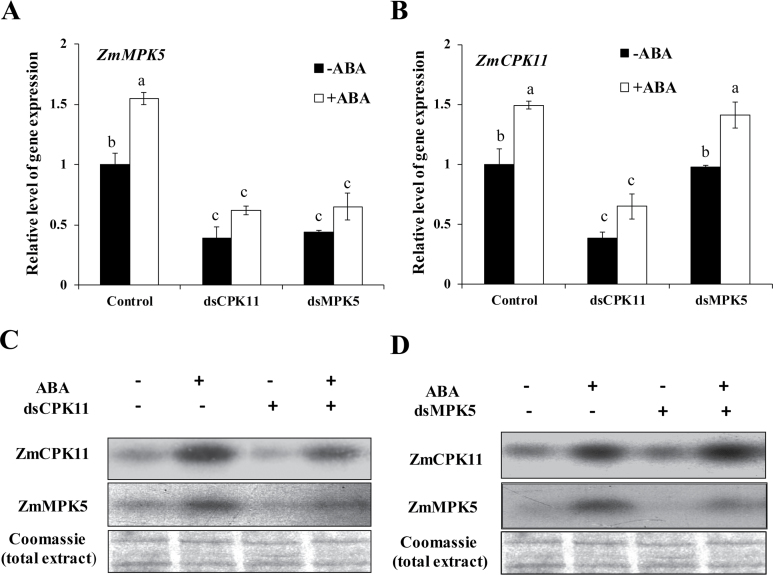
To determine the relationship between ZmCPK11 and ZmMPK5 in ABA signalling, transient RNAi analysis in maize protoplasts was used. RNAi-mediated silencing of ZmCPK11 in protoplasts decreased the expression of ZmCPK11 (Fig. 8B) and the activity of ZmCPK11 (Fig. 8C) under control conditions, and also decreased the expression of ZmMPK5 (Fig. 8A) and the activity of ZmMPK5 (Fig. 8C). In contrast, RNAi silencing of ZmMPK5 inhibited the expression of ZmMPK5 (Fig. 8A) and the activity of ZmMPK5 (Fig. 8D), but had very little effect on the expression of ZmCPK11 (Fig. 8B) and the activity of ZmCPK11 (Fig. 8D). Further, ABA-induced increases in the expression of ZmMPK5 (Fig. 8A) and the activity of ZmMPK5 (Fig. 8C) were substantially suppressed by RNAi silencing of ZmCPK11, but RNAi silencing of ZmMPK5 had very little effect on the ABA-induced increases in the expression of ZmCPK11 (Fig. 8B) and the activity of ZmCPK11 (Fig. 8D). These results suggest that ZmCPK11 regulates the expression of ZmMPK5 and the activity of ZmMPK5 in ABA signalling, and ZmMPK5 does not mediate the ABA-induced up-regulation of the expression and activity of ZmCPK11.
Fig. 8.
ZmCPK11 regulates the expression of ZmMPK5 and the activity of ZmMPK5 in ABA signalling. (A) The expression of ZmMPK5 in protoplasts with transiently silenced ZmCPK11 (dsCPK11) and ZmMPK5 (dsMPK5). The protoplasts were treated with 10 µM ABA for 5min, and the relative expression level of ZmMPK5 was analysed by real-time quantitative PCR. (B) The expression of ZmCPK11 in protoplasts with transiently silenced ZmCPK11 (dsCPK11) and ZmMPK5 (dsMPK5). The protoplasts were treated with 10 µM ABA for 5min, and the relative expression level of ZmCPK11 was analysed by real-time quantitative PCR. (C) The activity of ZmCPK11 and ZmMPK5 in maize protoplasts with transiently silenced ZmCPK11 (dsCPK11). (D) The activity of ZmCPK11 and ZmMPK5 in maize protoplasts with transiently silenced ZmMPK5 (dsMPK5). In A and B, values are means ±SE of three independent experiments. Means denoted by the same letter did not significantly differ at P<0.05 according to Duncan’s multiple range test. In C and D, experiments were repeated at least three times with similar results.
ZmCPK11 functions upstream of ZmMPK5 to regulate the activities of antioxidant enzymes
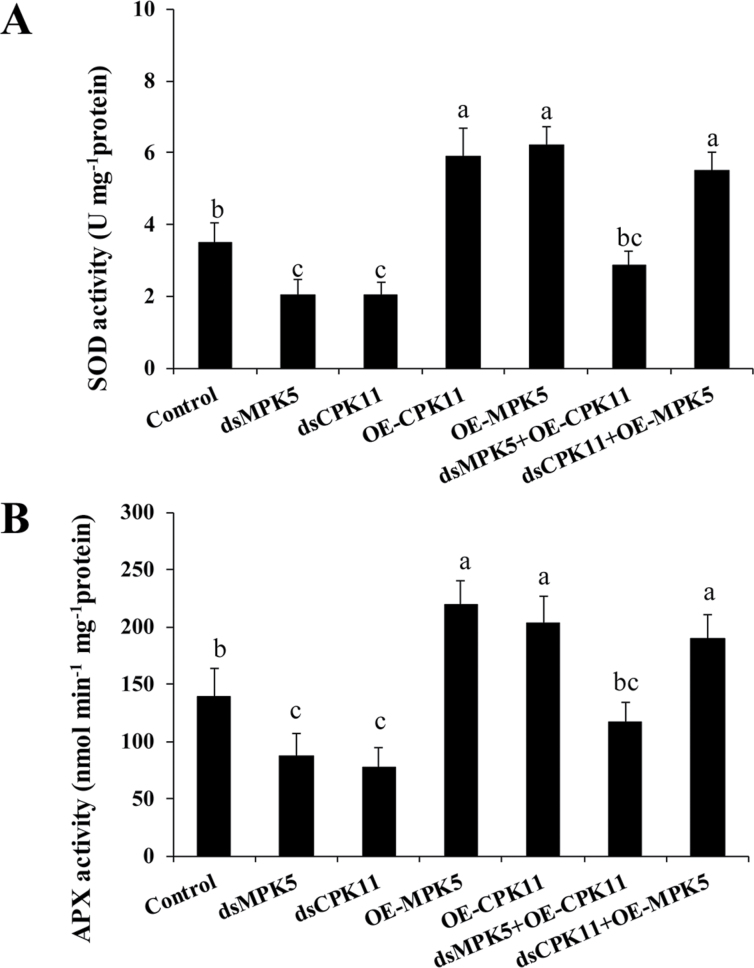
To determine the relationship between ZmCPK11 and ZmMPK5 in the regulation of antioxidant enzyme activity, transient RNAi analysis in combination with the transient expression test in maize protoplasts was conducted. Transient expression of ZmCPK11 or ZmMPK5 in protoplasts induced the increases in the activities of SOD and APX, but RNAi-mediated silencing of ZmCPK11 or ZmMPK5 decreased the activities of SOD and APX (Fig. 9A, B). However, in the protoplasts with transiently silenced ZmMPK5, the transient expression of ZmCPK11 could hardly induce the increases in the activities of SOD and APX, but in the protoplasts with transiently silenced ZmCPK11, the transient expression of ZmMPK5 induced a similar increase in the activities of SOD and APX, compared with those in the protoplasts with transiently expressed ZmMPK5 alone (Fig. 9A, B). These results suggest that ZmCPK11 acts upstream of ZmMPK5 to regulate the activities of antioxidant enzymes.
Fig. 9.
ZmCPK11 functions upstream of ZmMPK5 to regulate the activities of antioxidant enzymes. (A) The activity of SOD in protoplasts with transiently expressed ZmCPK11 (OE-CPK11) and ZmMPK5 (OE-MPK5), transiently silenced ZmCPK11 (dsCPK11) and ZmMPK5 (dsMPK5), transiently expressed ZmCPK11 in combination with transiently silenced ZmMPK5 (dsMPK5+OE-CPK11), or transiently expressed ZmMPK5 in combination with transiently silenced ZmCPK11 (dsCPK11+OE-MPK5). (B) The activity of APX in protoplasts with transiently expressed ZmCPK11 (OE-CPK11) and ZmMPK5 (OE-MPK5), transiently silenced ZmCPK11 (dsCPK11) and ZmMPK5 (dsMPK5), transiently expressed ZmCPK11 in combination with transiently silenced ZmMPK5 (dsMPK5+ OE-CPK11), or transiently expressed ZmMPK5 in combination with transiently silenced ZmCPK11 (dsCPK11+OE-MPK5). Values are means ±SE of three independent experiments. Means denoted by the same letter did not differ significantly at P<0.05 according to Duncan’s multiple range test.
Discussion
With 34 members in Arabidopsis (Cheng et al., 2002; Hrabak et al., 2003) and 29 members in rice (Asano et al., 2005), CDPKs constitute a large multigene family. In Arabidopsis and rice, several CDPKs, such as AtCPK3 and AtCPK6 (Mori et al., 2006), AtCPK4 and AtCPK11 (Zhu et al., 2007), AtCPK10 (Zou et al., 2010), AtCPK32 (Choi et al., 2005), and OsCPK12 (Asano et al., 2012) and OsCPK21 (Asano et al., 2011) have been reported to be positive regulators of ABA-mediated physiological processes, including seed germination, post-germination growth, stomatal movement, and plant stress tolerance. Under conditions of high salinity, the accumulation of H2O2 in OsCPK12-overexpressing plants was less than that in wild-type plants, whereas the accumulation was more in oscpk12 mutant and OsCPK12 RNAi plants (Asano et al., 2012). The levels of ROS accumulation were correlated with altered expression levels of the antioxidant genes OsAPX2 and OsAPX8 in the OsCPK12-overexpressing and loss-of-function plants under the conditions of high salinity. These results suggest that OsCPK12 positively regulates ROS detoxification by controlling the expression of OsAPX2 and OsAPX8. However, the detoxification of ROS regulated by OsCPK12 under salt stress seems to be ABA independent (Asano et al., 2012). Therefore, whether CDPKs are involved in ABA-induced antioxidant defence is not yet clear. In this study, a functional analysis was performed of ZmCPK11, which belongs to group I of the CDPK family and is closely related to AtCPK4 and AtCPK11 (Boudsocq and Sheen, 2012), in ABA-induced antioxidant defence in maize. The results showed that ABA treatment induced increases in the expression of ZmCPK11 and the activity of ZmCPK11 in leaves of maize (Fig. 1), and ABA was required for the PEG-induced increase in the expression of ZmCPK11 (Fig. 2A). The transient expression of ZmCPK11 in maize protoplasts enhanced the expression of the antioxidant genes SOD4 and cAPX and the activities of SOD and APX (Fig. 4). In contrast, the RNAi silencing of ZmCPK11 in protoplasts decreased the expression and the activities of SOD and APX (Fig. 5). Further, ABA treatment induced increases in the expression and activities of these antioxidant enzymes in control protoplasts, and the increases were inhibited in protoplasts transfected with dsRNA against ZmCPK11 (Fig. 5). These results clearly indicate that ZmCPK11 is involved in ABA-induced up-regulation of the expression and activities of antioxidant enzymes in maize.
The MAPK cascade has been demonstrated to play a crucial role in plant responses to environmental stresses (Colcombet and Hirt, 2008; Pitzschke et al., 2009). AtMPK6 in Arabidopsis and its homologues in maize and rice, ZmMPK5 and OsMPK1, have been shown to be involved in ABA-induced antioxidant defence (Xing et al., 2008; Lin et al., 2009; Zhang et al., 2012). However, it is not clear whether there exists a cross-talk between the CCaMK pathway and the MAPK pathway in ABA signalling. Previous studies showed that the application of the CDPK inhibitor N-(6-aminohexyl)-5-chloro-1-naphthalene sulphonamide hydrochloride (W7) inhibited the activation of MAPKs induced by cold and heat (Sangwan et al., 2002) or by heavy metals (Yeh et al., 2007), suggesting that the stress-induced activation of MAPKs may occur through the action of CDPKs. However, W7 is also a well-known CaM antagonist. This implies that the inhibition of the activities of MAPKs by W7 under environmental stresses is not certain from the action of CDPKs. Genetic evidence shows that there exists a complex relationship between CDPKs and MAPKs in plant responses to environmental stresses. In an early study addressing cross-talk between CDPK and MAPK signalling in response to biotic stress, it was found that elevated CDPK signalling inhibited stress-induced MAPK activation (Ludwig et al., 2005). However, several recent studies demonstrated that, in response to pathogen- or microbe-associated molecular patterns (Boudsocq et al., 2010; Kobayashi et al., 2012) and salt stress (Mehlmer et al., 2010), CDPKs and MAPKs act in parallel and no direct cross-talk exists between them. Therefore, to elucidate the relationship between CDPKs and MAPKs in ABA signalling appears to be particularly interesting. In the present study, three lines of evidence indicate that there exists a link between ZmCPK11 and ZmMPK5 in ABA signalling in maize. First, pre-treatments with EGTA and TFP suppressed the ABA-induced increase in the activity of ZmCPK11 in maize leaves, and also inhibited the ABA-induced increases in the expression of ZmMPK5 and the activity of ZmMPK5 (Fig. 7). Secondly, RNAi-mediated silencing of ZmCPK11 in maize protoplasts not only decreased the expression of ZmMPK5 and the activity of ZmMPK5 under control conditions, but also blocked the ABA-induced increases in the expression and the activity of ZmMPK5 (Fig. 8). In contrast, RNAi-mediated silencing of ZmMPK5 in protoplasts affected neither the expression and activity of ZmCPK11 under control conditions, nor the ABA-induced increases in the expression and activity of ZmCPK11. Finally, in the protoplasts with transiently silenced ZmMPK5, the transient expression of ZmCPK11 could hardly induce the increases in the activities of SOD and APX, but the RNAi silencing of ZmCPK11 had very little effect on the ZmCPK5-induced increases in the activities of these antioxidant enzymes (Fig. 9). Taken together, these results clearly indicate that ZmCPK11 functions upstream of ZmMPK5 in ABA-induced antioxidant defence in maize. Moreover, recent studies showed that treatments with ABA, H2O2, and PEG induced the expression of the rice CCaMK gene OsDMI3 and the maize CCaMK gene ZmCCaMK in the leaves of these plants, and these CCaMKs were required for ABA-induced antioxidant defence and oxidative stress tolerance under water stress (Ma et al., 2012; Shi et al., 2012). A further study revealed that in rice leaves, OsDMI3 is required for the up-regulation of the expression and activity of the MAPK OsMPK1 in ABA signalling (unpublished data), indicating that there also exists a cross-talk between the CCaMK pathway and the MAPK pathway in ABA signalling. The present results suggest that MAPK is a convergence point of the CDPK pathway and the CCaMK pathway in ABA signalling.
In ABA signalling, ROS are important signal molecules (Neill et al., 2008; Wang and Song, 2008; Mittler et al., 2011) and NADPH oxidase is a major source of ROS (Kwak et al., 2003). In this study, H2O2 treatment induced the expression of ZmCPK11 and the activity of ZmCPK11 in maize leaves (Fig. 1), and H2O2 is required for ABA-induced increases in the expression and the activity of ZmCPK11 (Fig. 2), suggesting that H2O2 might function upstream of ZmCPK11 in ABA signalling. In addition, another important signal molecule, NO, has also been shown to be involved in the regulation of CDPK and CCaMK. The NO donor sodium nitroprusside (SNP) induced the activation of a 50kDa CDPK in cucumber (Lanteri et al., 2006) and ZmCCaMK in maize (Ma et al., 2012). H2O2-dependent NO production plays an important role in ABA-induced activation of ZmCCaMK (Ma et al., 2012). These results suggest that NO might also be involved in the regulation of ZmCPK11 in ABA signalling. On the other hand, CDPKs have also been shown to be associated with the production of ROS. In Arabidopsis, several CDPKs such as AtCPK4, AtCPK5, AtCPK6, and AtCPK11 from group I have been demonstrated to play a key role in defence-induced ROS production (Boudsocq et al., 2010). StCDPK4 and StCDPK5, close homologues of AtCPK5/AtCPK6, can directly phosphorylate the NADPH oxidases StRbohB and StRbohC to induce ROS production (Kobayashi et al., 2007, 2012). Interestingly, ROS and NO production induced by ABA was not impaired in the cpk6 guard cells of Arabidopsis (Munemasa et al., 2011). In this study, RNAi-mediated silencing of ZmCPK11 in maize protoplasts blocked the ABA-induced increase in the production of H2O2 (Fig. 6), suggesting that ZmCPK11 mediates the ABA-induced up-regulation of the production of H2O2. Previous studies showed that H2O2 is required for the activation of ZmMPK5 in maize leaves (Ding et al., 2009; Lin et al., 2009). This might be a reason why ZmCPK11 induced the activation of ZmMPK5 in ABA signalling. However, in this study, the expression of ZmMPK5 was also up-regulated by ZmCPK11 in ABA signalling, suggesting that the CDPK can activate the transcription of the MAPK. The mechanism whereby ZmCPK11 regulates the expression of ZmMPK5 and the activity of ZmMPK5 remains to be elucidated.
In conclusion, the present data indicate that ZmCPK11 is required for ABA-induced antioxidant defence in maize leaves. ABA-induced H2O2 production activates ZmCPK11, which induces the activation of ZmMPK5, thus resulting in the up-regulation of the expression and the activities of antioxidant enzymes in ABA signalling.
Acknowledgements
This work was supported by the National Basic Research Program of China (grant no. 2012CB114300), the National Natural Science Foundation of China (grant nos. 30970238, 31070254, 31071344, and 31271631), the Fundamental Research Funds for the Central Universities (grant nos. KYZ200905 and KYZ201157), the Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions, the Natural Science Foundation of Jiangsu Province (grant no. BK2010455), the Research Fund for the Doctoral Program of Higher Education of China (grant no. 20090097110017), and the Program for New Century Excellent Talents in University (grant no. NCET-10–0498).
References
- An CI, Sawada A, Kawaguchi Y, Fukusaki E, Kobayashi A. 2005. Transient RNAi induction against endogenous genes in Arabidopsis protoplasts using in vitro-prepared double-stranded RNA. Bioscience, Biotechnology, and Biochemistry 69, 415–418 [DOI] [PubMed] [Google Scholar]
- Asano T, Hakata M, Nakamura H, Aoki N, Komatsu S, Ichikawa H, Hirochika H, Ohsugi R. 2011. Functional characterisation of OsCPK21, a calcium-dependent protein kinase that confers salt tolerance in rice. Plant Molecular Biology 75, 179–191 [DOI] [PubMed] [Google Scholar]
- Asano T, Hayashi N, Kobayashi M, et al. 2012. A rice calcium-dependent protein kinase OsCPK12 oppositely modulates salt-stress tolerance and blast disease resistance. The Plant Journal 69, 26–36 [DOI] [PubMed] [Google Scholar]
- Asano T, Tanaka N, Yang G, Hayashi N, Komatsu S. 2005. Genome-wide identification of the rice calcium-dependent protein kinase and its closely related kinase gene families: comprehensive analysis of the CDPKs gene family in rice. Plant and Cell Physiology 46, 356–366 [DOI] [PubMed] [Google Scholar]
- Bart R, Chern M, Park CJ, Bartley L, Ronald PC. 2006. A novel system for gene silencing using siRNAs in rice leaf and stem-derived protoplasts. Plant Methods 2, 13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudsocq M, Willmann MR, McCormack M, Lee H, Shan L, He P, Bush J, Cheng SH, Sheen J. 2010. Differential innate immune signalling via Ca2+ sensor protein kinases. Nature 464, 418–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudsocq M, Sheen J. 2012. CDPKs in immune and stress signaling. Trends in Plant Science (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Analytical Biochemistry 72, 248–254 [DOI] [PubMed] [Google Scholar]
- Bright J, Desikan R, Hancock JT, Weir IS, Neill SJ. 2006. ABA-induced NO generation and stomatal closure in Arabidopsis are dependent on H2O2 synthesis. The Plant Journal 45, 113–122 [DOI] [PubMed] [Google Scholar]
- Chen S, Tao L, Zeng L, Vega-Sanchez ME, Umemura K, Wang GL. 2006. A highly efficient transient protoplast system for analyzing defence gene expression and protein–protein interactions in rice. Molecular Plant Pathology 7, 417–427 [DOI] [PubMed] [Google Scholar]
- Cheng SH, Willmann MR, Chen HC, Sheen J. 2002. Calcium signaling through protein kinases. The Arabidopsis calcium-dependent protein kinase gene family. Plant Physiology 129, 469–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi H, Park HJ, Park JH, Kim S, Im MY, Seo HH, Kim YW, Hwang I, Kim SY. 2005. Arabidopsis calcium-dependent protein kinase CPK32 interacts with ABF4, a transcriptional regulator of abscisic acid-responsive gene expression, and modulates its activity. Plant Physiology 139, 1750–1761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colcombet J, Hirt H. 2008. Arabidopsis MAPKs: a complex signaling network involved in multiple biological processes. Biochemical Journal 413, 217–226 [DOI] [PubMed] [Google Scholar]
- Cutler SR, Rodriguez PL, Finkelstein RR, Abrams SR. 2010. Abscisic acid: emergence of a core signaling network. Annual Review of Plant Biology 61, 651–679 [DOI] [PubMed] [Google Scholar]
- Ding H, Zhang A, Wang J, Lu R, Zhang H, Zhang J, Jiang M. 2009. Identity of an ABA-activated 46kDa mitogen-activated protein kinase from Zea mays leaves: partial purification, identification and characterization. Planta 230, 239–251 [DOI] [PubMed] [Google Scholar]
- Franz S, Ehlert B, Liese A, Kurth J, Cazale A-C, Romeis T. 2011. Calcium-dependent protein kinase CPK21 functions in abiotic stress response in Arabidopsis thaliana . Molecular Plant 4, 83–96 [DOI] [PubMed] [Google Scholar]
- Hrabak EM, Chan CWM, Gribskov M, et al. 2003. The Arabidopsis CDPK–SnRK superfamily of protein kinases. Plant Physiology 132, 666–680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Jiang M, Zhang A, Lu J. 2005. Abscisic acid-induced apoplastic H2O2 accumulation up-regulates the activities of chloroplastic and cytosolic antioxidant enzymes in maize leaves. Planta 223, 57–68 [DOI] [PubMed] [Google Scholar]
- Hu X, Jiang M, Zhang J, Zhang A, Lin F, Tan M. 2007. Calcium–calmodulin is required for abscisic acid-induced antioxidant defense and functions both upstream and downstream of H2O2 production in leaves of maize (Zea mays) plants. New Phytologist 173, 27–38 [DOI] [PubMed] [Google Scholar]
- Hubbard KE, Nishimura N, Hitomi K, Getzoff ED, Schroeder JI. 2010. Early abscisic acid signal transduction mechanisms: newly discovered components and newly emerging questions. Genes and Development 24, 1695–1708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jammes F, Song C, Shin D, et al. 2009. MAP kinases MPK9 and MPK12 are preferentially expressed in guard cells and positively regulate ROS-mediated ABA signaling. Proceedings of the National Academy of Sciences, USA 106, 20520–20525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang M, Zhang J. 2001. Effect of abscisic acid on active oxygen species, antioxidative defence system and oxidative damage in leaves of maize seedlings. Plant and Cell Physiology 42, 1265–1273 [DOI] [PubMed] [Google Scholar]
- Jiang M, Zhang J. 2002a. Involvement of plasma membrane NADPH oxidase in abscisic acid- and water stress-induced antioxidant defense in leaves of maize seedlings. Planta 215, 1022–1030 [DOI] [PubMed] [Google Scholar]
- Jiang M, Zhang J. 2002b. Water stress-induced abscisic acid accumulation triggers the increased generation of reactive oxygen species and up-regulates the activities of antioxidant enzymes in maize leaves. Journal of Experimental Botany 53, 2401–2410 [DOI] [PubMed] [Google Scholar]
- Jiang M, Zhang J. 2003. Cross-talk between calcium and reactive oxygen species originated from NADPH oxidase in abscisic acid-induced antioxidant defense in leaves of maize seedlings. Plant, Cell and Environment 26, 929–939 [DOI] [PubMed] [Google Scholar]
- Joshi-Saha A, Valon C, Leung J. 2011. Abscisic acid signal off the starting block. Molecular Plant 4, 562–580 [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Ohura I, Kawakita K, Yokota N, Fujiwara M, Shimamoto K, Doke N, Yoshioka H. 2007. Calcium-dependent protein kinases regulate the production of reactive oxygen species by potato NADPH oxidase. The Plant Cell 19, 1065–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi M, Yoshioka M, Asai S, Nomura H, Kuchimura K, Mori H, Doke H, Yoshioka H. 2012. StCDPK5 confers resistance to late blight pathogen but increases susceptibility to early blight pathogen in potato via reactive oxygen species burst. New Phytologist 196, 223–237 [DOI] [PubMed] [Google Scholar]
- Kwak JM, Mori IC, Pei Z-M, Leonhardt N, Torres MA, Dangl JL, Bloom RE, Bodde S, Jones JDG, Schroeder JI. 2003. NADPH oxidase AtrbohD and AtrbohF genes function in ROS-dependent ABA signaling in Arabidopsis . EMBO Journal 22, 2623–2633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanteri ML, Pagnussat GC, Lamattina L. 2006. Calcium and calcium-dependent protein kinases are involved in nitric oxide- and auxin-induced adventitious root formation in cucumber. Journal of Experimental Botany 57, 1341–1351 [DOI] [PubMed] [Google Scholar]
- Lin F, Ding H, Wang J, Zhang H, Zhang A, Zhang Y, Tan M, Dong W, Jiang M. 2009. Positive feedback regulation of maize NADPH oxidase by mitogen-activated protein kinase cascade in abscisic acid signaling. Journal of Experimental Botany 60, 3221–3238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y. 2012. Roles of mitogen-activated protein kinase cascades in ABA signaling. Plant Cell Reports 31, 1–12 [DOI] [PubMed] [Google Scholar]
- Ludwig AA, Saitoh H, Felix G, Freymark G, Miersch O, Wasternack C, Boller T, Jones JDG, Romeis T. 2005. Ethylene-mediated cross-talk between calcium-dependent protein kinase and MAPK signaling controls stress responses in plants. Proceedings of the National Academy of Sciences, USA 102, 10736–10741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma F, Lu R, Liu H, Shi B, Zhang J, Tan M, Zhang A, Jiang M. 2012. Nitric oxide-activated calcium/calmodulin-dependent protein kinase regulates the abscisic acid-induced antioxidant defence in maize. Journal of Experimental Botany 63, 4835–4847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma SY, Wu WH. 2007. AtCPK23 functions in Arabidopsis responses to drought and salt stresses. Plant Molecular Biology 65, 511–518 [DOI] [PubMed] [Google Scholar]
- Mehlmer N, Wurzinger B, Stael S, Hofmann-Rodrigues D, Csaszar E, Pfister B, Bayer R, Teige M. 2010. The Ca2+-dependent protein kinase CPK3 is required for MAPK-independent salt-stress acclimation in Arabidopsis. The Plant Journal 63, 484–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao Y, Lu D, Wang P, Wang X, Chen J, Miao C, Song CP. 2006. An Arabidopsis glutathione peroxidase functions as both a redox transducer and a scavenger in abscisic acid and drought stress responses. The Plant Cell 18, 2749–2766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G, Suzuki N, Ciftci-Yilmaz S, Mittler R. 2010. Reactive oxygen species homeostasis and signaling during drought and salinity stresses. Plant, Cell and Environment 33, 453–467 [DOI] [PubMed] [Google Scholar]
- Mittler R, Vanderauwera S, Suzuki N, Miller G, Tognetti VB, Vandepoele K, Gollery M, Shulaev V, Van Breusegem F. 2011. ROS signaling: the new wave? Trends in Plant Science 16, 300–309 [DOI] [PubMed] [Google Scholar]
- Mori IC, Murata Y, Yang Y, et al. 2006. CDPKs CPK6 and CPK3 function in ABA regulation of guard cell S-type anion- and Ca2+-permeable channels and stomatal closure. PLoS Biology 4, 1749–1761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munemasa S, Hossain MA, Nakamura Y, Mori IC, Murata Y. 2011. The Arabidopsis calcium-dependent protein kinase, CPK6, functions as a positive regulator of methyl jasmonate signaling in guard cells. Plant Physiology 155, 553–561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neill S, Barros R, Bright J, Desikan R, Hancock J, Harrison J, Morris P, Ribeiro D, Wilson I. 2008. Nitric oxide, stomatal closure, and abiotic stress. Journal of Experimental Botany 59, 165–176 [DOI] [PubMed] [Google Scholar]
- Ozfidan C, Turkan I, Sekmen AH, Seckin B. 2012. Abscisic acid-regulated responses of aba2-1 under osmotic stress: the abscisic acid-inducible antioxidant defence system and reactive oxygen species production. Plant Biology 14, 337–346 [DOI] [PubMed] [Google Scholar]
- Pitzschke A, Schikora A, Hirt H. 2009. MAPK cascade signaling networks in plant defence. Current Opinion in Plant Biology 12, 421–426 [DOI] [PubMed] [Google Scholar]
- Saijo Y, Hata S, Kyozuka J, Shimamoto K, Izui K. 2000. Over-expression of a single Ca2+-dependent protein kinase confers both cold and salt/drought tolerance on rice plants. The Plant Journal 23, 319–327 [DOI] [PubMed] [Google Scholar]
- Sangwan V, Orvar BL, Beyerly J, Hirt H, Dhindsa RS. 2002. Opposite changes in membrane fluidity mimic cold and heat stress activation of distinct plant MAP kinase pathways. The Plant Journal 31, 629–638 [DOI] [PubMed] [Google Scholar]
- Shi B, Ni L, Zhang A, Cao J, Zhang H, Qin T, Tan M, Zhang J, Jiang M. 2012. OsDMI3 is a novel component of abscisic acid signaling in the induction of antioxidant defense in leaves of rice. Molecular Plant 5, 1359–1374 [DOI] [PubMed] [Google Scholar]
- Szczegielniak J, Borkiewicz L, Szurmak B, Lewandowska-Gnatowska E, Statkiewicz M, Klimecka M, Ciesla J, Muszynska G. 2012. Maize calcium-dependent protein kinase (ZmCPK11): local and systemic response to wounding, regulation by touch and components of jasmonate signaling. Physiologia Plantarum 146, 1–14 [DOI] [PubMed] [Google Scholar]
- Szczegielniak J, Klimecka M, Liwosz A, Ciesielski A, Kaczanowski S, Dobrowolska G, Harmon AC, Muszynska G. 2005. A wound-responsive and phospholipid-regulated maize calcium-dependent protein kinase. Plant Physiology 139, 1970–1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umezawa T, Nakashima K, Miyakawa T, Kuromori T, Tanokura M, Shinozaki K, Yamaguchi-Shinozaki K. 2010. Molecular basis of the core regulatory network in ABA responses: sensing, signaling and transport. Plant and Cell Physiology 51, 1821–1839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Ding H, Zhang A, Ma F, Cao J, Jiang M. 2010. A novel mitogen-activated protein kinase gene in maize (Zea mays), ZmMPK3, is involved in response to diverse environmental cues. Journal of Integrative Plant Biology 52, 442–452 [DOI] [PubMed] [Google Scholar]
- Wang P, Song CP. 2008. Guard-cell signalling for hydrogen peroxide and abscisic acid. New Phytologist 178, 703–718 [DOI] [PubMed] [Google Scholar]
- Wurzinger B, Mair A, Pfister B, Teige M. 2011. Cross-talk of calcium-dependent protein kinase and MAP kinase signaling. Plant Signaling and Behavior 6, 8–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing T, Wang XJ, Malik K, Miki BL. 2001. Ectopic expression of an Arabidopsis calmodulin-like domain protein kinase-enhanced NADPH oxidase activity and oxidative burst in tomato protoplasts. Molecular Plant-Microbe Interactions 14, 1261–1264 [DOI] [PubMed] [Google Scholar]
- Xing Y, Jia W, Zhang J. 2008. AtMKK1 mediates ABA-induced CAT1 expression and H2O2 production via AtMPK6-coupled signaling in Arabidopsis . The Plant Journal 54, 440–451 [DOI] [PubMed] [Google Scholar]
- Xu J, Tian YS, Peng RI, Xiong AS, Zhu B, Jin XF, Gao F, Fu XY, Hou XL, Yao QH. 2010. AtCPK6, a functionally redundant and positive regulator involved in salt/drought stress tolerance in Arabidopsis. Planta 231, 1251–1260 [DOI] [PubMed] [Google Scholar]
- Ye N, Zhu G, Liu Y, Li Y, Zhang J. 2011. ABA controls H2O2 accumulation through the induction of OsCATB in rice leaves under water stress. Plant and Cell Physiology 52, 689–698 [DOI] [PubMed] [Google Scholar]
- Yeh CM, Chien PS, Huang HJ. 2007. Distinct signalling pathways for induction of MAP kinase activities by cadmium and copper in rice roots. Journal of Experimental Botany 58, 659–671 [DOI] [PubMed] [Google Scholar]
- Yoo SD, Cho YH, Sheen J. 2007. Arabidopsis mesophyll protoplasts: a versatile cell system for transient gene expression analysis. Nature Protocols 2, 1565–1572 [DOI] [PubMed] [Google Scholar]
- Zhai Z, Sooksa-nguan T, Vatamaniuk OK. 2009. Establishing RNA interference as a reverse- genetic approach for functional analysis in protoplasts. Plant Physiology 149, 642–652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang A, Jiang M, Zhang J, Ding H, Xu S, Hu X, Tan M. 2007. Nitric oxide induced by hydrogen peroxide mediates abscisic acid-induced activation of the mitogen-activated protein kinase cascade involved in antioxidant defense in maize leaves. New Phytologist 175, 36–50 [DOI] [PubMed] [Google Scholar]
- Zhang A, Jiang M, Zhang J, Tan M, Hu X. 2006. Mitogen-activated protein kinase is involved in abscisic acid-induced antioxidant defense and acts downstream of reactive oxygen species production in leaves of maize plants. Plant Physiology 141, 475–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Ni L, Liu Y, Wang Y, Zhang A, Tan M, Jiang M. 2012. The C2H2-type zinc finger protein ZFP182 is involved in abscisic acid-induced antioxidant defense in rice. Journal of Integrative Plant Biology 54, 500–510 [DOI] [PubMed] [Google Scholar]
- Zhao R, Sun HL, Mei C, Wang XJ, Yan L, Liu R, Zhang XF, Wang XF, Zhang DP. 2011. The Arabidopsis Ca2+-dependent protein kinase CPK12 negatively regulates abscisic acid signaling in seed germination and post-germination growth. New Phytologist 192, 61–73 [DOI] [PubMed] [Google Scholar]
- Zhu SY, Yu XC, Wang XJ, et al. 2007. Two calcium-dependent protein kinases, CPK4 and CPK11, regulate abscisic acid signal transduction in Arabidopsis. The Plant Cell 19, 3019–3036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou JJ, Wei FJ, Wang C, Wu JJ, Ratnasekera D, Liu WX, Wu WH. 2010. Arabidopsis calcium-dependent protein kinase CPK10 functions in abscisic acid- and Ca2+-mediated stomatal regulation in response to drought stress. Plant Physiology 154, 1232–1243 [DOI] [PMC free article] [PubMed] [Google Scholar]