Abstract
The concentration of CO2 in global surface ocean waters is increasing due to rising atmospheric CO2 emissions, resulting in lower pH and a lower saturation state of carbonate ions. Such changes in seawater chemistry are expected to impact calcification in calcifying marine organisms. However, other physiological processes related to calcification might also be affected, including enzyme activity. In a mesocosm experiment, macroalgal communities were exposed to three CO2 concentrations (380, 665, and 1486 µatm) to determine how the activity of two enzymes related to inorganic carbon uptake and nutrient assimilation in Corallina officinalis, an abundant calcifying rhodophyte, will be affected by elevated CO2 concentrations. The activity of external carbonic anhydrase, an important enzyme functioning in macroalgal carbon-concentrating mechanisms, was inversely related to CO2 concentration after long-term exposure (12 weeks). Nitrate reductase, the enzyme responsible for reduction of nitrate to nitrite, was stimulated by CO2 and was highest in algae grown at 665 µatm CO2. Nitrate and phosphate uptake rates were inversely related to CO2, while ammonium uptake was unaffected, and the percentage of inorganic carbon in the algal skeleton decreased with increasing CO2. The results indicate that the processes of inorganic carbon and nutrient uptake and assimilation are affected by elevated CO2 due to changes in enzyme activity, which change the energy balance and physiological status of C. officinalis, therefore affecting its competitive interactions with other macroalgae. The ecological implications of the physiological changes in C. officinalis in response to elevated CO2 are discussed.
Key words: Calcification, carbon dioxide, carbonic anhydrase, coralline algae, nitrate reductase, ocean acidification.
Introduction
Increasing atmospheric CO2 emissions are changing the chemistry in the surface layer of global oceans. As more CO2 dissolves into the seawater, changes in the speciation of inorganic carbon occur, resulting in more bicarbonate ions (HCO3–), more protons (H+), and fewer carbonate ions (CO32–). The consequences of these changes are a lower pH and CO32– saturation state of the seawater. By the end of this century, the pH of surface oceans is expected to drop by 0.3–0.5 units (Caldeira and Wickett, 2003; Feely et al., 2004; Orr, 2005) due to increasing concentrations of atmospheric CO2 that could reach up to 970 µatm CO2 (Houghton et al., 2001). Such changes in seawater chemistry could have severe impacts on calcifying organisms, which rely on inorganic carbon for producing their shells and skeletons, which consist of calcium carbonate (CaCO3).
Several studies have shown negative responses of corals, macroalgae, and molluscs to elevated seawater CO2 concentrations (Anthony et al., 2008; Jokiel et al., 2008; Martin et al., 2008; Martin and Gattuso, 2009; Albright et al., 2010; Diaz-Pulido et al., 2011; Rodolfo-Metalpa et al., 2011; Hofmann et al., 2012b). However, due to the increase in ocean acidification research in the past few decades, it is now clear that calcifing marine organisms show a variety of responses, due to differences in the substrate (HCO3– or CO32–) used for calcification, their ability to control the pH at the location of calcification, the crystalized form of CaCO3 deposited, and the production of protective organic layers that prevent dissolution (Ries, 2009, 2011; Hurd et al., 2011; Jokiel, 2011a,b; Rodolfo-Metalpa et al., 2011; Roleda et al., 2012). Furthermore, studies have shown that physiological processes other than calcification, such as photosynthesis, nutrient assimilation, and growth, are also affected by elevated CO2 concentrations (Magnusson et al., 1996; Mercado, 1999; Gordillo et al., 2001, 2003; Israel and Hophy, 2002; Zou, 2005; Connell and Russell, 2010; Zou et al., 2011; Hofmann et al., 2012b). The physiological and ecological responses of calcifying organisms to elevated CO2 is therefore species specific, and also depends on local conditions, such as nutrient availability (Ries, 2009; Russell et al., 2009; Fabricius et al., 2011; Price et al., 2011; Hofmann et al., 2012a). It is nevertheless important to understand how all processes, not just calcification, will be affected by elevated CO2, and what implications these changes will have for calcifiying organisms.
In calcifying primary producers, photosynthesis is also affected by increasing CO2 levels. However, the responses of these organisms are again variable, because of different mechanisms and efficiencies of obtaining CO2 for photosynthesis. Because the ambient seawater concentration of HCO3– is much higher than that of CO2, marine algae have mechanisms called carbon-concentrating mechanisms (CCMs) which transport HCO3– across cell membranes using ion transporters, or catalyse the dehydration of HCO3– to CO2 via the membrane-associated external carbonic anhydrase (Johnston, 1991; Badger and Price, 1994; Raven, 1997, 2003; Raven et al., 2002). In non-calcifying algae, CCMs have been shown to be down-regulated under elevated CO2 conditions. This down-regulation relieves algae from the high energy demands of producing ion transporter proteins and enzymes (Raven, 2011; Raven et al., 2012). However, in calcifying algae, this enzyme may play an additional role in calcification, and has been shown to increase under elevated CO2 (Isenberg et al., 1963; Hofmann et al., 2012b).
Nutrient assimilation and uptake are further metabolic processes that may be affected by higher CO2 concentrations. Because the speciation of inorganic nitrogen and phosphate is affected by pH, the preference and uptake of inorganic nutrients may be affected, as well as the enzymatic activity involved in nutrient assimilation. Non-calcifying macroalgae have been shown to decrease nitrate uptake under elevated CO2 (García-Sánchez et al., 1994; Magnusson et al., 1996; Andria et al., 1999). Such changes in metabolism are likely to have significant effects on macroalgal nutritional content, which could have implications for grazers and competitive interactions between species. To date, however, there have not been many studies investigating how inorganic carbon and nutrient-related enzymatic activity in calcifying macroalgae will respond to elevated CO2.
Seasonal changes in temperature, nutrient availability, and light are also likely to interact with the effect of CO2 on metabolic processes in algae (Tyrrell et al., 2008; Martin and Gattuso, 2009; Mercado and Gordillo, 2011). As calcification, photosynthesis, nutrient uptake, growth, and other metabolic processes are affected by temperature, light, and nutrient availability, changes in these factors are likely to have a strong influence on the enzymatic response of macroalgae to increasing CO2. Therefore, mesocosm studies such as this one are useful for monitoring CO2 effects over time during natural temperature, nutrient, and light fluctuations.
Both calcifying and non-calcifying algae provide important habitat and shelter for many marine organisms, and erect calcifying algae such as Corallina officinalis contribute to the strength of the intertidal community structure and provide refugia for organisms in environments with high wave action (Dommasnes, 1968; Stewart, 1982; Coull and Wells, 1983; Kelaher, 2002, 2003). Corallina officinalis is an upright calcifying alga found in the inter- and subtidal zones on rocky coastlines, often at exposed locations and in tidal drainage channels. It is a late successional species with a complex morphological structure (Littler and Littler, 1980). Corallina spp. often form extensive macroalgal beds that cover large areas of the intertidal zone and provide substratum, habitat, and refugia for a number of important marine organisms (Coull and Wells, 1983; Hicks, 1977; Akioka et al., 1999; Kelaher, 2002, 2003). The important ecological roles served by this alga could be interrupted under high CO2 conditions, as its skeleton contains high-Mg calcite, the most soluble form of CaCO3 found in calcifying marine macroalgae (Andersson et al., 2008). It is therefore important to understand how its metabolism may be affected in the future. Therefore, a mesocosm study was conducted with macroalgal communities containing the calcifying rhodophyte C. officinalis grown under three different CO2 concentrations. The competitive interactions between C. officinalis and non-calcifying macroalgae as well as the overall macroalgal community response are discussed in a separate paper (Hofmann et al., 2012a). Here the focus is on inorganic nutrient uptake rates and the ezymatic activity of carbonic anydrase and nitrate reductase in C. officinalis grown under elevated CO2 conditions.
Materials and methods
Experimental design and seawater chemistry
The experiment was conducted in 75 litre mesocosms on the German island of Sylt in the North Sea. Experimental conditions including mesocosm set-up, duration, and the inorganic carbon chemistry of the seawater are outlined in Hofmann et al. (2012a). Temperature, salinity, and pH were monitored daily. Seawater samples for inorganic nutrient analysis were taken weekly from the seawater source flowing into all tanks. Nutrient uptake rates were calculated based on a 3h incubation of C. officinalis in 5 litre plexiglass chambers continuously bubbled with mixed gas (386, 665, or 1486 µatm CO2). Rates were calculated after doubling the projected surface area of the algal thalli, which was measured using the imaging analysis software ImageJ (National Institute of Mental Health, Bethesda, MD, USA).
Tissue sampling and analysis
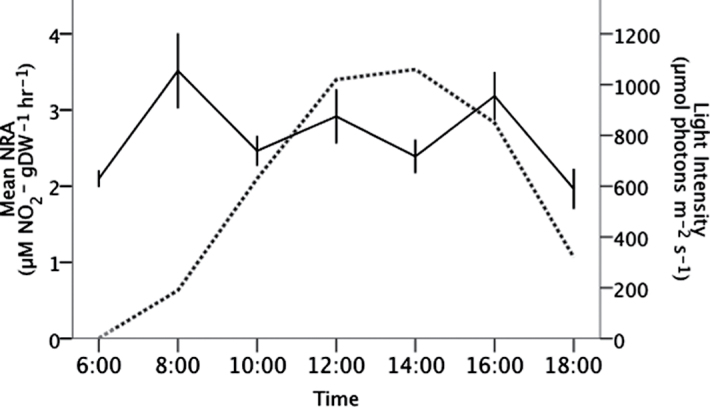
Corallina officinalis tissue samples were taken weekly for analysis of nitrate reductase and carbonic anhydrase activity, as well as total inorganic carbon content of the skeleton. Nitrate reductase activity of C. officinalis was determined based on the in situ method of Corzo and Niell (1991). Fresh algal tissue (200–400mg) was placed into 5ml amber vials containing 3ml of anoxic assay buffer (0.1M phosphate buffer, pH 8.0, 0.5mM EDTA, 0.1% 1-propanol, 30mM KNO3, 10 µM glucose) that had been previously bubbled with N2 gas for at least 5min. Each vial was individually bubbled with N2 gas for an additional 1min before being placed into a 30 °C water bath in the dark for 30min. After the incubation, 1ml of the assay buffer was removed and the nitrite concentrations were determined colorimetrically (Snell and Snell, 1949) after the addition of 200 µl of 4% sulphanilimide and 300 µl of 0.1% n-(1-naphthyl) ethylenediamine dihydrochloride. Following the assay, the algal tissue was dried at 60 °C for 48h to determine the dry weight (DW), and nitrate reductase activity was calculated as µmol NO2– g DW–1 h–1. Prior to the experiment, nitrate reductase activity was measured hourly under ambient CO2 conditions in the light (Fig. 1). Following this analysis, tissue was sampled for enzyme activity each week between 10:00h and 14:00h, when the activity was most stable, to ensure that the nitrate reductase activity measurements in C. officinalis were not confounded by daily fluctuations.
Fig. 1.
Daily cycle of mean (±SE, n=3) nitrate reductase activity in C. officinalis in the light in April 2011. The hourly light intensity is shown by the dotted lines.
Total carbonic anhydrase activity of C. officinalis was measured according to Haglund et al. (1992). Algal tissue [50–100mg fresh weight (FW)] was ground with liquid nitrogen using a chilled mortar and pestle and immersed in 15ml of chilled assay buffer (50mM TRIS, pH 8.5, 25mM dithiothreitol, 25mM isoascorbic acid, 5mM EDTA). Aliquots of 3ml of the extract were added to clean tubes followed by 2ml of ice-cold CO2-saturated water. The time it took for the pH to drop 0.4 units during continuous mixing was recorded. Three aliquots from each extract were measured and the mean of these measurements was considered as one replicate. External carbonic anhydrase activity was measured using the same method, but with intact algal thalli (200–400mg FW) immersed in assay buffer rather than algal extract. Total and external carbonic anhydrase activity were calculated as (Tb/Ts–1)/FW, where Tb=the time it took for a blank sample with just assay buffer to drop 0.4 pH units, Ts=the time it took for the algal extract (total) or buffer with an intact thallus (external) to drop 0.4 pH units, and FW=fresh weight of the algae in grams. External carbonic anhydrase activity was normalized to the dry weight of the thalli. The internal carbonic anhydrase activity was calculated by subtracting the external from the total carbonic anhydrase activity.
The percentage of C. officinalis tissue made of CaCO3 was determined in fragments that were used in the enzyme activity analysis and was measured by determining the ash free DW of the dried tissue after removing the organic material by burning at 400 °C for 12h. No effect of the enzyme assay buffers was apparent on the skeletal material, as the amount of CaCO3 in algae grown under 385 µatm CO2 did not differ from previous measurements on algae that had not been exposed to any enzyme buffer. Furthermore, the CaCO3 content of algae exposed to the nitrate reductase assay buffer did not differ from that of algae exposed to the carbonic anhydrase assay buffer, and algae exposed to the assay buffers showed the same trend with respect to CO2 concentration.
Results
Seasonal variability of temperature and inorganic nutrients
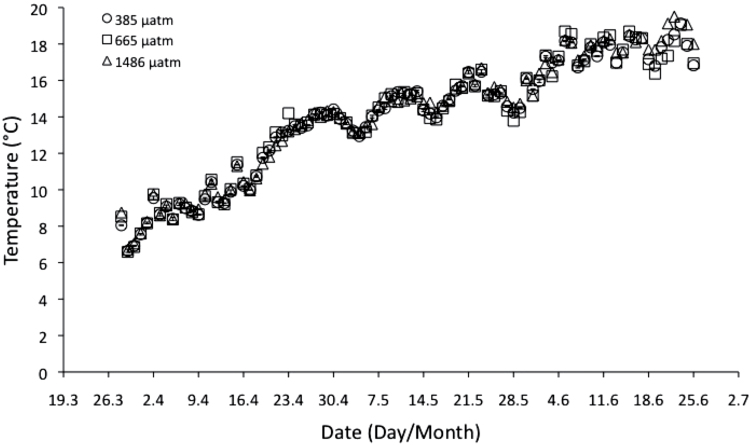
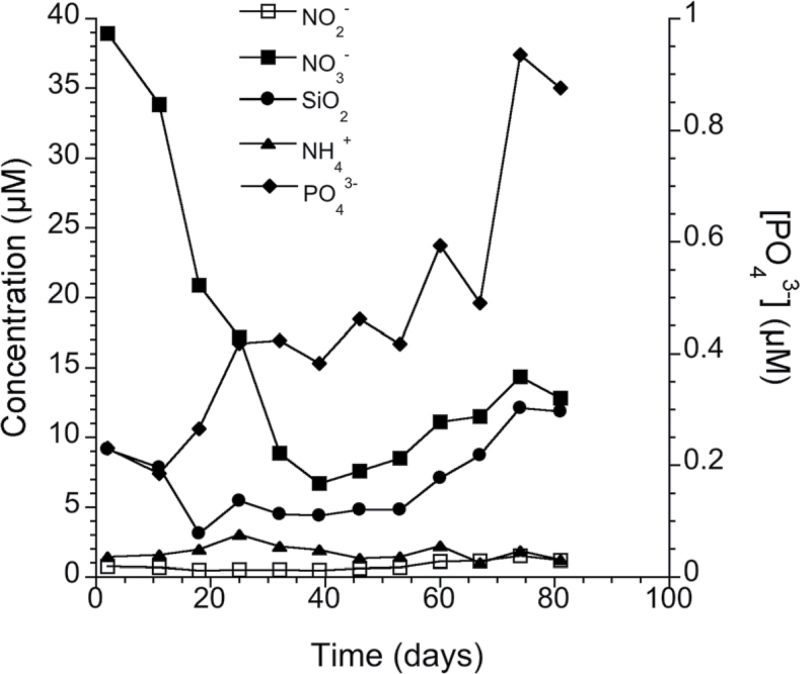
The mean seawater temperature in the mesocosm tanks during the experimental period is shown in Fig. 2. Temperature increased linearly with time from the end of March to the beginning of July 2011, and ranged from 6 °C to 19 °C. The seawater concentrations of nitrate, nitrite, ammonium, phosphate, and silicate are shown in Fig. 3. Nitrate concentrations in the seawater ranged from 6.7 µM to 38.9 µM and were highest in March, at the beginning of the experiment, and declined rapidly to a minumum 40 d after the experiment began. Nitrate levels then began to increase again, but only reached 37% of the initial concentration by the end of the experiment. Silicate concentrations followed a similar pattern, but reached higher than initial levels at the end of the experiment. On the other hand, phosphate concentrations increased during the experiment, and ammonium concentrations ranged from 1.03 µM to 3.06 µM.
Fig. 2.
Mean (±SE, n=4) seawater temperature in the mesocosm tanks during the experimental period. Circles, 385; squares, 665; and triangles, 1486 µatm CO2.
Fig. 3.
Inorganic nutrient concentrations of the ambient seawater throughout the duration of the experiment, from 30 March to 17 June 2011.
Nutrient uptake rates and nitrate reductase activity
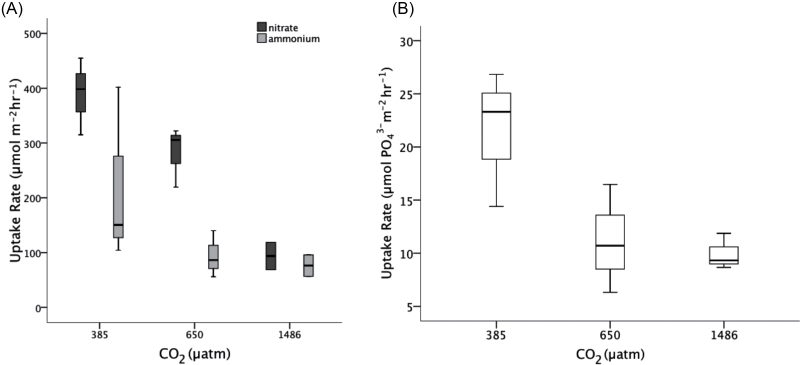
Nutrient uptake rates of nitrate, ammonium, and phosphate by C. officinalis were measured after 35 d of exposure to the CO2 treatments and are shown in Fig. 4. There was a negative correlation between nitrate uptake and CO2 concentration (Pearson’s correlation coefficient= –0.81, P=0.002). There was no significant treatment effect of CO2 on ammonium or phosphate uptake rates.
Fig. 4.
Boxplots showing the median, minimum, maximum, and first and third quartiles of C. officinalis uptake rates for (A) nitrate and ammonium and (B) phosphate as a function of CO2 concentration.
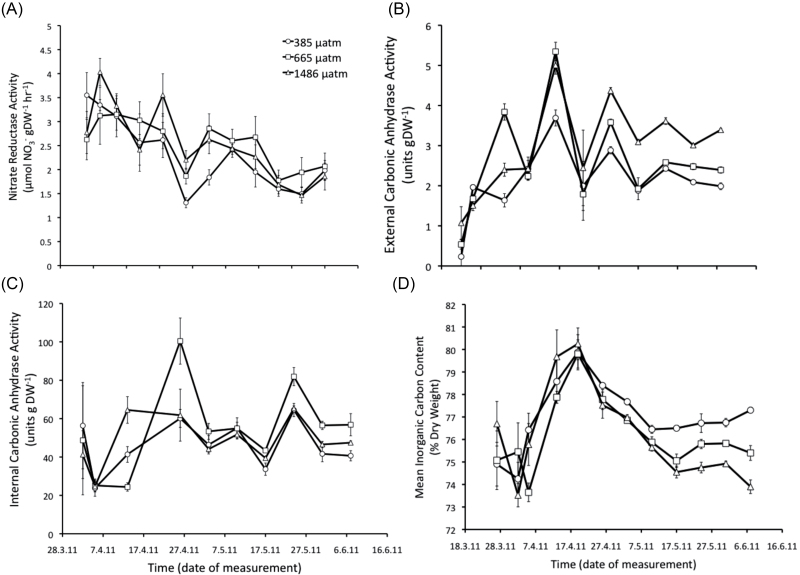
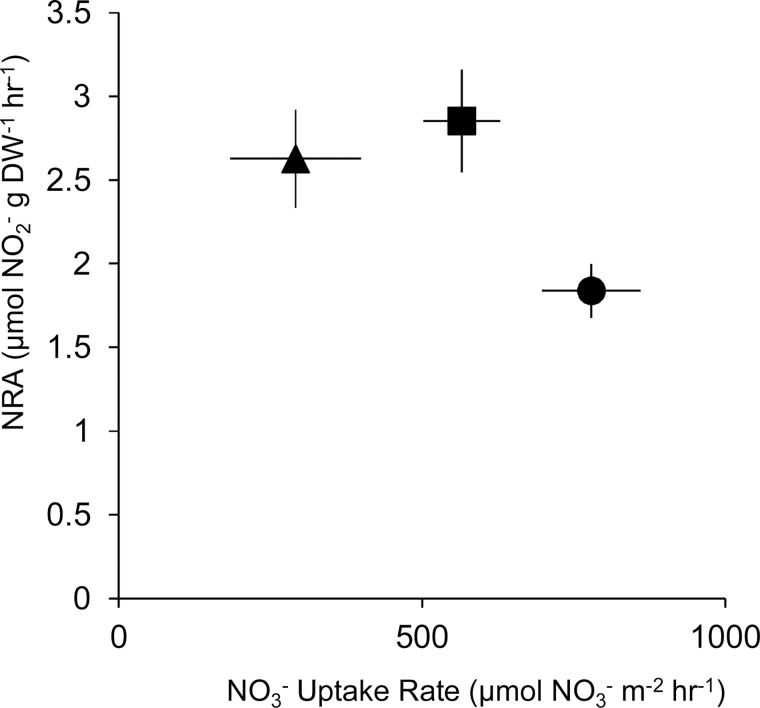
Throughout the experimental period, there was a significant effect of time on nitrate reductase activity, as it decreased in all CO2 treatments after 12 weeks. There was also a significant effect of CO2 on nitrate reductase activity (Table 1). Algae grown under ambient CO2 levels had the lowest enzyme activity, while algae grown under 665 µatm CO2 had the highest (Fig. 5A). The relationship between nitrate reductase activity and nitrate uptake rate differed between the CO2 treatments (Fig. 6). The algae grown under elevated CO2 had higher nitrate reductase activity, but lower nitrate uptake rates compared with algae grown in the ambient CO2 treatment.
Table 1.
Results from a MANOVA test on enzyme activity and CaCO3 content (Cinorg) of C. officinalis with time as a within-subject factor and CO2 as a between-subject factor. F-ratios are given with degrees of freedom in parentheses, followed by the P-values significant at the 95% confidence level.
| Response variable | Time (within-subject factor) | CO2 (between-subject factor) | Time×CO2 (interaction) |
|---|---|---|---|
| tCAA | F(9, 81)=15.5, P=2.1E-14 | – | – |
| eCAA | F(11, 99)=45.6, P=7.8E-34 | F(2, 9)=36.2, P=5.0E-5 | F(22, 99)=3.4, P=1.6E-5 |
| iCAA | F(10, 90)=15.5, P=1.0E-15 | – | F(20, 90)=2.4, P=0.003 |
| NRA | F(11, 99)=9.4, P=2.5E-11 | F(2, 9)=5.0, P=0.034 | – |
| Cinorg | F(11, 99)=21.4, P=1.5E-21 | F(22, 99)=2.1, P=0.006 | F(2, 9)=12.1, P=0.003 |
tCAA, total carbonic anhydrase activity; eCAA, external carbonic anhydrase activity; iCAA, internal carbonic anhydrase activity; NRA, nitrate reductase activity.
Fig. 5.
Time series of mean (±SE, n=4) (A) nitrate reductase activity, (B) external carbonic anhydrase activity, (C) internal carbonic anhydrase activity, and (D) percentage inorganic carbon of C. officinalis exposed to three carbon dioxide concentrations. Circles, 385; squares, 665; and triangles, 1486 µatm CO2.
Fig. 6.
Mean nitrate reductase activity (±SE, n=4) as a function of mean nitrate uptake rates in C. officinalis exposed to the three CO2 levels. Circles, 385; squares, 665; and triangles, 1486 µatm CO2.
Carbonic anhydrase activity
All carbonic anhydrase activity (total, internal, and external) was significantly affected by time. External carbonic anhydrase activity was affected by CO2, and internal and external carbonic anhydrase activity was affected by an interaction between time and CO2 (Table 1). Internal carbonic anhydrase showed no observable pattern over time, except for two peaks in the 1486 µatm CO2 treatment after 4 and 8 weeks, and a large drop after 12 weeks. On the other hand, external carbonic anhydrase increased equally in all treatments during the first half of the experiment until week 7 when all treatments levelled off, but the enzyme activity was highest in the 1486 µatm CO2 treatment and subsequently decreased with decreasing CO2 level (Fig. 5B, C).
CaCO3 content
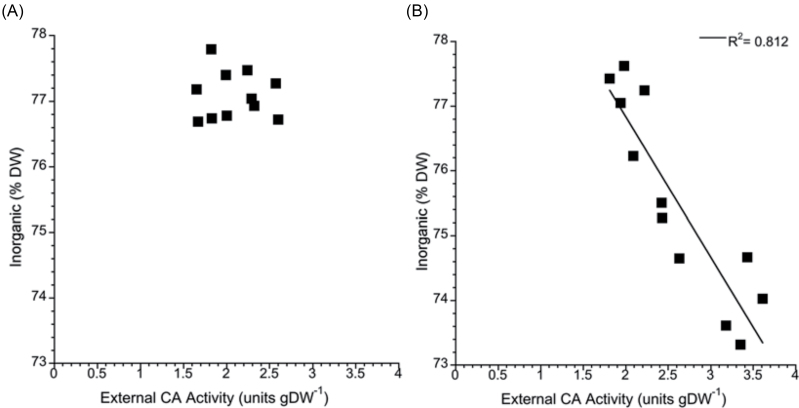
Inorganic carbon content of the C. officinalis skeleton peaked in all treatments after 3 weeks, and afterwards the CO2 treatment effect became apparent, as the skeletal inorganic carbon content decreased with increasing CO2 concentration (Fig. 5D). By the end of the experiment, there was a negative linear relationship between skeletal inorganic content (% DW of CaCO3) and external carbonic anhydrase activity which was not apparent after shot-term exposure (36 d) to elevated CO2 (Fig. 7A, B).
Fig. 7.
Inorganic content of C. officinalis as a function of external carbonic anhydrase activity after (A) 36 d and (B) 71 d of exposure to three CO2 concentrations
Discussion
The present results suggest that elevated CO2 will significantly affect enzyme activity and subsequently many metabolic processes in C. officinalis, including photosynthesis, calcification, and inorganic nutrient uptake and assimilation. The enzyme external carbonic anhydrase is important in the CCM of many macroalgae. Although its activity has been shown to decrease in non-calcifying macroalgae in response to elevated CO2 (Björk et al., 1993; García-Sánchez et al., 1994; Haglund and Pedersén, 2009), an increase in external carbonic anhydrase activity with increasing CO2 concentration was observed. In non-calcifying macroalgae, there is evidence that less enzyme is produced because more CO2 is available for photosynthesis, so less HCO3– must be converted to CO2 (Giordano et al., 2005a, and references therein; Matsuda et al., 2011). However, in the case of calcifying macroalgae, it is likely that external carbonic anhydrase plays a role in metabolic processes other than photosynthesis, particularly calcification. In corals, carbonic anhydrase has been reported to be an important enzyme in the calcification process (Kingsley and Watabe, 1987; Nimer et al., 1994; Al-Horani et al., 2003; Rahman et al., 2008; Tambutté, 2007). Hofmann et al. (2012a) showed that calcification rates in C. officinalis had a parabolic relationship to CO2 concentration, and Hofmann et al. (2012b) showed that external carbonic anhydrase showed an increasing trend with elevated CO2 in the same species. As photosynthesis was not stimulated by CO2 in this species, despite an increase in external carbonic anhydrase activity, it is hypothesized that external carbonic anhydrase activity is related to calcification, and that its activity is up-regulated under elevated CO2. In this way, the algae may regulate the calcification mechanism despite changes in seawater inorganic carbon chemistry that are unfavourable for CaCO3 deposition. This hypothesis is supported by the relationship observed between external carbonic anhydrase activity and the skeletal inorganic carbon content of C. officinalis, which was only revealed after long-term exposure to elevated CO2. Such a response of the algae could be to compensate for higher dissolution rates under elevated CO2 conditions (Ries, 2011; Rodolfo-Metalpa et al., 2011). If external carbonic anhydrase does indeed play a role in calcification, higher dissolution rates would explain why an increase in skeletal inorganic carbon was not seen despite an increase in external carbonic anhydrase activity under elevated CO2.
The overall decrease in nitrate reductase activity in C. officinalis grown at all CO2 concentrations during the first 6 weeks was most probably due to a decline in the seawater nitrate concentration, as the enzyme has been shown to be dependent on external nitrate availability (Solomonson and Barber, 1990; Gordillo et al., 2006). Algae generally prefer ammonium over nitrate as their nitrogen source, as it is less energy costly to assimilate (Losada and Guerro, 1979; Syrett, 1981). The ammonium concentrations were sufficient to supply the algae with an alternative source of nitrogen when the seawater nitrate concentrations, and subsequently nitrate reductase activity, decreased.
The decrease in nitrate uptake rates by C. officinalis under elevated CO2 conditions is consistent with other results found for non-calcifying macroalgae and seagrass (García-Sánchez et al., 1994; Magnusson et al., 1996; Andria et al., 1999; Alexandre et al., 2012) as well as the observed increase in nitrate reductase activity (Mercado et al., 1999; Gordillo et al., 2001; Alexandre et al., 2012). Mercado et al. (1999) reported that the reduction and assimilation of nitrate in Porphyra leucosticta grown under elevated CO2 was uncoupled, which also seems to be the case in C. officinalis. Changes in the intracellular ATP:NADP+/NADPH ratio could affect nitrate reductase activity, due to the requirement for NADPH as a reducing agent to convert nitrate to nitrite (Corzo and Niell, 1991). Chlamydomonas sp. cells grown under normal CO2 conditions require higher ATP:NADPH ratios for CO2 assimilation than high CO2-grown cells (Spalding et al., 1984). Therefore, if algae grown under elevated CO2 have a lower ATP:NADPH requirement, the excess NADPH could stimulate nitrate reductase activity. However, this may only be the case when CCMs are down-regulated. In high CO2-grown C. officinalis, the protein content decreases, indicating a likely decrease in Rubisco concentration. This reduction in Rubisco content could be interpreted as a partial down-regulation of the CCM in C. officinalis, despite the increase in external carbonic anhydrase activity. However, another possible reason for stimulation of nitrate reductase under elevated CO2 is a change in the plastoquinone pool. Giordano et al. (2005b) reported that nitrate reductase activity is controlled by the redox state of the plastoquinone pool in Chlamydomonas reinhardtii, in that nitrate reductase activity is stimulated by a reduced plastoquinone pool. A reduced plastoquinone pool generally occurs under high light conditions, when the electron transport chain is saturated (Behrenfeld et al., 1998). The lower protein content in C. officinalis grown under elevated CO2 (Hofmann et al., 2012a) suggests that Rubisco content may be lower in algae grown under high CO2 conditions. When CO2 concentrations are saturating for photosynthesis, activation of Rubisco can be rate limiting to photosynthesis rather than electron flow (Dietz and Herber, 1984). The combination of high light conditions during summer and lower Rubisco content under elevated CO2 could have resulted in a more reduced plastoquinone pool in C. officinalis grown under elevated CO2, causing the stimulation of nitrate reductase activity.
The absolute values and seasonal pattern of seawater temperature in the mesocosm tanks and ambient seawater nutrient concentrations were consistent with previously recorded seasonal trends in the Wadden Sea (van Beusekom et al., 2001, 2010). Therefore, the changes in both enzyme activities during the experimental period indicate that there was an effect of seasonally changing temperature and nutrient conditions on C. officinalis metabolism. However, the enzymes responded differently to seasonal fluctuations, as nitrate reductase increased and external carbonic anhydrase decreased during the first 6 weeks of the experiment. The increase in external carbonic anhydrase activity in all treatments during the first 6 weeks was most probably a response to increasing seawater temperature, as enzymes have optimum temperatures for maximum activity, and C. officinalis growth is optimal at temperatures between 12 °C and 18 °C (Colthart and Johansen, 1973), which were reached after the first half of the experiment. The stimulation of carbonic anhydrase by elevated temperature has been previously reported for Chlorella vulgaris (Shiraiwa and Miyachi, 1985). This temperature effect could have masked the CO2 effect during the first 6 weeks of the experiment, as there was no difference in external carbonic anhydrase activity between the CO2 treatments until after 6 weeks. Therefore, the enzymatic activity, reliant metabolic mechanisms, and cellular products of the calcifying red alga C. officinalis will be affected by CO2, but will also depend on seasonal effects such as nutrient availability and temperature.
The present results indicate that the response of C. officinalis to elevated CO2 is complex, and involves many metabolic processes other than just calcification and photosynthesis. The observed changes in enzyme activity, combined with changes in photosynthesis, calcification, and cell nutritional content reported by Hofmann et al. (2012a), will alter the competitive status of C. officinalis under future oceanic CO2 conditions, which could have implications for macroalgal communities and their grazers. However, it is still unclear if calcifying coralline algae, such as C. officinalis, will be able to adapt to increasing CO2 concentrations that will allow them to maintain their current competitive status within macroalgal communities. Their ability to adapt will most probably depend on other abiotic factors and seasonal patterns. Therefore, it will be important to conduct future experiments on different life history stages of this alga, as well as to follow the responses of multiple generations to elevated CO2 under conditions which simulate seasonal changes.
Supplementary Material
Acknowledgements
The authors would like to thank the scientists and staff at the Wadden Sea Station of the Alfred-Wegener Institute for Polar and Marine Research for their support and contributions to this project, in particular Dr Ragnhild Asmus, Petra Kadel, Reimer Magens, Birgit Hussel, Margit Ludwig-Schweikert, Tatyana Romanova, Michael Klett, and Rene Gerrits. Finally, we thank the reviewers of this manuscript for their thorough examination and thoughtful comments that greatly improved its scientific quality. Funding for this project was provided by the German Federal Ministry of Education and Research (BMBF) through the cooperative research project Biological Impacts of Ocean Acidification (BIOACID).
References
- Akioka H, Baba M, Masaki T, William Johansen H. 1999. Rocky shore turfs dominated by Corallina (Corallinales, Rhodophyta) in northern Japan. Phycological Research 47, 199–206 [Google Scholar]
- Albright R, Mason B, Miller M, Langdon C. 2010. Ocean acidification compromises recruitment success of the threatened Caribbean coral Acropora palmata . Proceedings of the National Academy of Sciences, USA 107, 20400–20404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexandre A, Silva J, Buapet P, Björk M, Santos R. 2012. Effects of CO2 enrichment on photosynthesis, growth, and nitrogen metabolism of the seagrass Zostera noltii. Ecology and Evolution 2, 2625–2635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Horani FA, Al-Moghrabi SM, De Beer D. 2003. The mechanism of calcification and its relation to photosynthesis and respiration in the scleractinian coral Galaxea fascicularis . Marine Biology 142, 419–426 [Google Scholar]
- Andria J, Vergara J, Perez-Llorens JL. 1999. Biochemical responses and photosynthetic performance of Gracilaria sp. (Rhodophyta) from Cádiz, Spain, cultured under different inorganic carbon and nitrogen levels. European Journal of Phycology 34, 497–504 [Google Scholar]
- Andersson AJ, Mackenzie FT, Bates NR. 2008. Life on the margin: implications of ocean acidification on Mg-calcite, high latitude and cold-water marine calcifiers. Marine Ecology Progress Series 373, 265–273 [Google Scholar]
- Anthony KRN, Kline DI, Diaz-Pulido G, Dove S, Hoegh-Guldberg O. 2008. Ocean acidification causes bleaching and productivity loss in coral reef builders. Proceedings of the National Academy of Sciences, USA 105, 17442–17446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badger MR, Price GD. 1994. The role of carbonic anhydrase in photosynthesis. Annual Review of Plant Biology 45, 369–392 [Google Scholar]
- Behrenfeld MJ, Prasil O, Kolber ZS, Babin M, Falkowski PG. 1998. Compensatory changes in photosystem II electron turnover rates protect photosynthesis from photoinhibition. Photosynthesis Research 58, 259–268 [Google Scholar]
- Björk M, Haglund K, Ramazanov Z, Garcia-Reina G, Pedersén M. 1992. Inorganic-carbon assimilation in the green seaweed Ulva rigida C. Ag. (Chlorophyta). Planta 187, 152–156 [DOI] [PubMed] [Google Scholar]
- Caldeira K, Wickett ME. 2003. Anthropogenic carbon and ocean pH. Nature 425, 365–365 [DOI] [PubMed] [Google Scholar]
- Colthart BJ, Johansen HW. 1973. Growth rates of Corallina officinalis (Rhodophyta) at different temperatures. Marine Biology 18, 46–49 [Google Scholar]
- Connell SD, Russell BD. 2010. The direct effects of increasing CO2 and temperature on non-calcifying organisms: increasing the potential for phase shifts in kelp forests. Proceedings of the Royal Society B: Biological Sciences 277, 1409–1415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corzo A, Niell FX. 1991. Determination of nitrate reductase activity in Ulva rigida C. Agardh by the in situ method. Journal of Experimental Marine Biology and Ecology 146, 181–191 [Google Scholar]
- Coull BC, Wells JBJ. 1983. Refuges from fish predation: experiments with phytal meiofauna from the New Zealand rocky intertidal. Ecology 64, 1599–1609 [Google Scholar]
- Diaz-Pulido G, Gouezo M, Tilbrook B, Dove S, Anthony KR. 2011. High CO2 enhances the competitive strength of seaweeds over corals. Ecological Letters 14, 156–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz K-J, Heber U. 1984. Rate-limiting factors in leaf photosynthesis. I. Carbon fluxes in the Calvin cycle. Biochimica et Biophysica Acta 767, 432–443 [Google Scholar]
- Dommasnes A. 1968. Variations in the meiofauna of Corallina officinalis L. with wave exposure. Sarsia 34, 117–124 [Google Scholar]
- Fabricius KE, Langdon C, Uthicke S, Humphrey C, Noonan S, De’ath G, Okazaki R, Muehllehner N, Glas MS, Lough JM. 2011. Losers and winners in coral reefs acclimatized to elevated carbon dioxide concentrations. Nature Climate Change 1, 165–169 [Google Scholar]
- Feely RA, Sabine CL, Lee K, Berelson W, Kleypas J, Fabry VJ, Millero FJ. 2004. Impact of anthropogenic CO2 on the CaCO3 system in the oceans. Science 305, 362–366 [DOI] [PubMed] [Google Scholar]
- Frost-Christensen H, Sand-Jensen K. 1992. The quantum efficiency of photosynthesis in macroalgae and submerged angiosperms. Oecologia 91, 377–384 [DOI] [PubMed] [Google Scholar]
- García-Sánchez MJ, Fernández JA, Niell X. 1994. Effect of inorganic carbon supply on the photosynthetic physiology of Gracilaria tenuistipitata . Planta 194, 55–61 [Google Scholar]
- Giordano M, Beardall J, Raven JA. 2005a. CO2 concentration mechanisms in algae: mechanisms, environmental modulation, and evolution. Annual Review of Plant Biology 56, 99–131 [DOI] [PubMed] [Google Scholar]
- Giordano M, Chen Y-B, Koblizek M, Falkowski PG. 2005. b Regulation of nitrate reductase in Chlamydomonas reinhardtii by the redox state of the plastoquinone pool. European Journal of Phycology 40, 345–352 [Google Scholar]
- Gordillo FJ, Aguilera J, Jiménez C. 2006. The response of nutrient assimilation and biochemical composition of Arctic seaweeds to a nutrient input in summer. Journal of Experimental Botany 57, 2661–2671 [DOI] [PubMed] [Google Scholar]
- Gordillo FJ, Figueroa FL, Niell FX. 2003. Photon- and carbon-use efficiency in Ulva rigida at different CO2 and N levels. Planta 218, 315–322 [DOI] [PubMed] [Google Scholar]
- Gordillo FJ, Niell FX, Figueroa FL. 2001. Non-photosynthetic enhancement of growth by high CO2 level in the nitrophilic seaweed Ulva rigida C. Agardh (Chlorophyta). Planta 213, 64–70 [DOI] [PubMed] [Google Scholar]
- Haglund K, Björk M, Ramazanov Z, García-Reina G, Pedersén M. 1992. Role of carbonic anhydrase in photosynthesis and inorganic-carbon assimilation in the red alga Gracilaria tenuistipitata . Planta 187, 275–28. [DOI] [PubMed] [Google Scholar]
- Haglund K, Pedersen M. 2009. Growth of the red alga Gracilaria tenuistipitata at high pH. Influence of some environmental factors and correlation to an increased carbonic-anhydrase activity. Botanica Marina 35, 579–588 [Google Scholar]
- Hicks GRF. 1977. Species associations and seasonal population densities of marine phytal harpacticoid copepods from Cook Strait. New Zealand Journal of Marine and Freshwater Research 11, 621–643 [Google Scholar]
- Hofmann LC, Straub S, Bischof K. 2012. a .Competition between calcifying and noncalcifying temperate marine macroalgae under elevated CO2 levels. Marine Ecology Progress Series 464, 89–105 [Google Scholar]
- Hofmann LC, Yildiz G, Hanelt D, Bischof K. 2012. b Physiological responses of the calcifying rhodophyte Corallina officinalis (L.) to future CO2 levels. Marine Biology 159, 783–792 [Google Scholar]
- Houghton JT, Ding Y, Griggs DJ, Noguer M, van der Linden PJ, Dai X, Maskell K, Johnson CA. 2001. Climate change 2001: the scientific basis. Contribution of Working Group I to the Third Assessment Report of the Intergovernmental Panel on Climate Change Cambridge: Cambridge University Press; [Google Scholar]
- Hurd CL, Cornwall CE, Currie K, Hepburn CD, McGraw CM, Hunter KA, Boyd PW. 2011. Metabolically-induced pH fluctuations by some coastal calcifiers exceed projected 22nd century ocean acidification: a mechanism for differential susceptibility?. Global Change Biology 17, 3254–3262 [Google Scholar]
- Isenberg HD, Lavine LS, Weissfellner H. 1963. The suppression of mineralization in a coccolithophorid by an inhibitor of carbonic anhydrase. Journal of Eukaryotic Microbiology 10, 477–479 [DOI] [PubMed] [Google Scholar]
- Israel A, Hophy M. 2002. Growth, photosynthetic properties and Rubisco activities and amounts of marine macroalgae grown under current and elevated seawater CO2 concentrations. Global Change Biology 8, 831–840 [Google Scholar]
- Johnston AM. 1991. The acquisition of inorganic carbon by marine macroalgae. Canadian Journal of Botany 69, 1123–1132 [Google Scholar]
- Jokiel PL. 2011. a .Ocean acidification and control of reef coral calcification by boundary layer limitation of proton flux. Bulletin of Marine Science 87, 639–657 [Google Scholar]
- Jokiel PL. 2011. b The reef coral two compartment proton flux model: a new approach relating tissue-level physiological processes to gross corallum morphology. Journal of Experimental Marine Biology and Ecology 409, 1–12 [Google Scholar]
- Jokiel PL, Rodgers KS, Kuffner IB, Andersson AJ, Cox EF, Mackenzie FT. 2008. Ocean acidification and calcifying reef organisms: a mesocosm investigation. Coral Reefs 27, 473–483 [Google Scholar]
- Kelaher BP. 2002. Influence of physical characteristics of coralline turf on associated macrofaunal assemblages. Marine Ecology Progress Series 232, 141–148 [Google Scholar]
- Kelaher BP. 2003. Changes in habitat complexity negatively affect diverse gastropod assemblages in coralline algal turf. Oecologia 135, 431–441 [DOI] [PubMed] [Google Scholar]
- Kingsley RJK, Watabe NW. 1987. Role of carbonic anhydrase in calcification in the Gorgonian Leptogorgia virgulata . Journal of Experimental Zoology 241, 171–180 [Google Scholar]
- Littler MM, Littler DS. 1980. The evolution of thallus form and survival strategies in benthic marine macroalgae: field and laboratory tests of a functional form model. American Naturalist 116, 25–44 [Google Scholar]
- Losada M, Guerrero MG. 1979. The photosynthetic reduction of nitrate and its regulation. In: Barber J, ed. Photosynthesis in relation to model systems Amsterdam: Elsevier; 365–408 [Google Scholar]
- Magnusson G, Larsson C, Lennart A. 1996. Effects of high CO2 treatment on nitrate and ammonium uptake by Ulva lactuca grown in different nutrient regimes. Scientia Marina 60, (Suppl. 1)179–189 [Google Scholar]
- Martin S, Gattuso JP. 2009. Response of Mediterranean coralline algae to ocean acidification and elevated temperature. Global Change Biology 15, 2089–2100 [Google Scholar]
- Martin S, Rodolfo-Metalpa R, Ransome E, Rowley S, Buia MC, Gattuso JP, Hall-Spencer J. 2008. Effects of naturally acidified seawater on seagrass calcareous epibionts. Biological Letters 4, 689–692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda Y, Nakajima K, Tachibana M. 2011. . Recent progresses on the genetic basis of the regulation of CO2 acquisition systems in response to CO2 concentration. Photosynthesis Research 109, 191–203 [DOI] [PubMed] [Google Scholar]
- Mercado JM, Javier F, Gordillo L, Xavier Niell F, Figueroa FL. 1999. Effects of different levels of CO2 on photosynthesis and cell components of the red alga Porphyra leucosticta . Journal of Applied Phycology 11, 455–461 [Google Scholar]
- Mercado JM, Gordillo FJ. 2011. Inorganic carbon acquisition in algal communities: are the laboratory data relevant to the natural ecosystems?. Photosynthesis Research 109, 257–267 [DOI] [PubMed] [Google Scholar]
- Nimer NA, Guan Q, Merrett MJ. 1994. Extra- and intra-cellular carbonic anhydrase in relation to culture age in a high-calcifying strain of Emiliania huxleyi Lohmann. New Phytologist 126, 601–607 [Google Scholar]
- Orr JC, Fabry VJ, Aumont O, et al. 2005. Anthropogenic ocean acidification over the twenty-first century and its impact on calcifying organisms. Nature 437, 681–686 [DOI] [PubMed] [Google Scholar]
- Price NN, Hamilton SL, Smith JE. 2011. Species-specific consequences of ocean acidification for the calcareous tropical green algae Halimeda . Marine Ecology Progress Series 440, 67–78 [Google Scholar]
- Rahman MA, Oomori T, Uehara T. 2008. Carbonic anhydrase in calcified endoskeleton: novel activity in biocalcification in alcyonarian. Marine Biotechnology 10, 31–38 [DOI] [PubMed] [Google Scholar]
- Raven JA. 1997. Inorganic carbon acquisition by marine autotrophs. Advances in Botanical Research 27, 85–209 [Google Scholar]
- Raven JA. 2003. Inorganic carbon concentrating mechanisms in relation to the biology of algae. Photosynthesis Research 77, 155–171 [DOI] [PubMed] [Google Scholar]
- Raven JA. 2011. The cost of photoinhibition. Physiologia Plantarum 142, 87–104 [DOI] [PubMed] [Google Scholar]
- Raven JA, Giordano M, Beardall J, Maberly SC. 2012. Algal evolution in relation to atmospheric CO2: carboxylases, carbon-concentrating mechanisms and carbon oxidation cycles. Philosophical Transactions of the Royal Society B: Biological Sciences 367, 493–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raven JA, Johnston AM, Kübler JE, Korb R, Mcinroy SG, Handley LL, Scrimgeour CM, Walker DI, Beardall J, Clayton MN. 2002. Seaweeds in cold seas: evolution and carbon acquisition. Annals of Botany 90, 525–536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ries JB. 2009. Effects of secular variation in seawater Mg/Ca ratio (calcite-aragonite seas) on CaCO3 sediment production by the calcareous algae Halimeda . Penicillus and Udotea—evidence from recent experiments and the geological record. Terra Nova 21, 323–339 [Google Scholar]
- Ries J. 2011. Biodiversity and ecosystems: acid ocean cover up. Nature Climate Change 1, 294–295 [Google Scholar]
- Rodolfo-Metalpa R, Houlbèque F, Tambutté É, et al. 2011. Coral and mollusc resistance to ocean acidification adversely affected by warming. Nature Climate Change 1, 308–312 [Google Scholar]
- Roleda MY, Boyd PW, Hurd CL. 2012. Before ocean acidification: calcifier chemistry lessons. Journal of Phycology 48, 840–843 [DOI] [PubMed] [Google Scholar]
- Russell BD, Thompson J-A, Falkenberg LJ, Connell SD. 2009. Synergistic effects of climate change and local stressors: CO2 and nutrient-driven change in subtidal rocky habitats. Global Change Biology 15, 2153–2162 [Google Scholar]
- Shiraiwa Y, Miyachi S. 1985. Effects of temperature and CO2 concentration on induction of carbonic anhydrase and changes in efficiency of photosynthesis in Chlorella vulgaris 11h. Plant and Cell Physiology 26, 543–549 [Google Scholar]
- Snell FD, Snell CT. 1949. Colorimetric methods of analysis , Vol. 2, 3rdedn. Princeton, NJ: Van Nostrand; [Google Scholar]
- Solomonson LP, Barber MJ. 1990. Assimilatory nitrate reductase: functional properties and regulation. Annual Review of Plant Biology 41, 225–253 [Google Scholar]
- Spalding MH, Critchley C, Orgren WL. 1984. Influence of carbon dioxide concentration during growth on fluorescence induction characteristics of the green alga Chlamydomonas reinhardii . Photosynthesis Research 5, 169–176 [DOI] [PubMed] [Google Scholar]
- Stewart JG. 1982. Anchor species and epiphytes in intertidal algal turf. Pacific Science 36, 45–60 [Google Scholar]
- Syrett PJ. 1981. Nitrogen metabolism of microalgae. Canadian Bulletin of Fisheries and Aquatic Sciences 210, 182–210 [Google Scholar]
- Tambutté S, Tambutté E, Zoccola D, Caminiti N, Lotto S, Moya A, Allemand D, Adkins J. 2007. Characterization and role of carbonic anhydrase in the calcification process of the azooxanthellate coral Tubastrea aurea . Marine Biology 151, 71–83 [Google Scholar]
- Tyrrell T, Schneider B, Charalampopoulou A, Riebesell U. 2008. Coccolithophores and calcite saturation state in the Baltic and Black Seas. Biogeosciences 5, 485–494 [Google Scholar]
- van Beusekom JEE, Fock H, de Jong F, Diehl-Christiansen S, Christiansen B. 2001. Wadden Sea specific eutrophication criteria. Wadden Sea Ecosystem No. 14 Wilhelmshaven, Germany: Common Wadden Sea Secretariat; [Google Scholar]
- van Beusekom JEE, Buschbaum C, Loebl M, Martens P, Reise K. 2010. Long-term ecological change in the northern Wadden Sea. In: Müller F, Baessler C, Schubert H, Klotz S, eds. Long-term ecological research: between theory and application Dordrech, The Netherlands: Springer, 145–153 [Google Scholar]
- Zou D. 2005. Effects of elevated atmospheric CO2 on growth, photosynthesis and nitrogen metabolism in the economic brown seaweed, Hizikia fusiforme (Sargassaceae, Phaeophyta). Aquaculture 250, 726–735 [Google Scholar]
- Zou D, Gao K, Luo H. 2011. Short- and long-term effects of elevated CO2 on photosynthesis and respiration in the marine macroalga Hizikia fusiformis (Sargassaceae, Phaeophyta) grown at low and high N supplies. Journal of Phycology 47, 87–97 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.