Abstract
Flowering time of the short-day plant Chrysanthemum morifolium is largely dependent upon daylength, but it is also distinctly influenced by other environmental factors. Flowering is delayed by summer heat. Here, the underlying basis for this phenomenon was investigated. Heat-induced flowering retardation occurred similarly in C. morifolium and C. seticuspe, a wild-type diploid chrysanthemum. In both plants, this flowering retardation occurred mainly because of inhibition of capitulum development. Concurrently, expression of flowering-related genes in the shoot tip was delayed under high temperature conditions. In chrysanthemums, FLOWERING LOCUS T-like 3 (FTL3) has been identified as a floral inducer produced in the leaves after short-day stimuli and transported to the shoot tip. In C. seticuspe, heat-induced flowering retardation was accompanied by a reduction in FTL3 expression in the leaves. Two C. morifolium cultivars with flowering times that are differently affected by growth temperature were also examined. High temperature-induced FTL3 repression was observed in the leaves of both cultivars, although the degree of repression was greater in the heat-sensitive cultivar than in the heat-tolerant cultivar. When a scion of the heat-sensitive cultivar was grafted onto the stock of the heat-tolerant cultivar, flowering in the shoot tip was less sensitive to heat. Conversely, a scion of the heat-tolerant cultivar grafted onto the heat-sensitive cultivar showed increased heat sensitivity. Thus, several lines of evidence suggest that the reduction of FTL3 signalling from the leaves to the shoot tip at high temperatures is involved in flowering retardation in chrysanthemums.
Key words: Capitulum development, chrysanthemum, floral transition, FT, high temperature, short-day plant.
Introduction
Chrysanthemum morifolium Ramat. is a short-day (SD) herbaceous perennial and a popular ornamental flower worldwide. Its growth habits have been studied in order to establish efficient cropping systems, but some problems remain to be solved. In C. morifolium, flowering time is predominantly determined by daylength. In natural settings, these plants grow vegetatively under the long-day (LD) conditions of spring and summer, and flower under the SD conditions of autumn. For year-round cut-flower production, flowering time in the field is mainly regulated by bringing the photoperiod closer to (e.g. shading) or farther from [e.g. night break (NB)] the appropriate daylength for flowering. However, flowering time also largely depends on the temperature of the planting season, even when daylength is controlled. The optimum flowering temperature for C. morifolium is reported to be ~20 °C, and high temperatures delay flowering (Whealy et al., 1987; Karlsson et al., 1989; Cockshull and Kofranek, 1994; Shibata, 1997; Nozaki and Fukai, 2008). For example, in Japan, where the air temperature is warmer than 20 °C during both day and night in summer, the retardation of flowering during summer and early autumn is a major production problem. Therefore, further study of the linkage between temperature and flowering is important in order to establish a consistent C. morifolium production system.
The development of capitula in chrysanthemums mimics that of a large single flower, in which a shoot apical meristem (SAM) is converted into an inflorescence meristem after floral transition. Bracts are formed around the growing inflorescence meristem. Once the inflorescence meristem has grown, floral meristems appear on the inflorescence meristem and develop into florets. Floral transition in chrysanthemums is reported to occur within 10 SDs, but prolonged SD conditions are required for capitulum development and anthesis (Adams et al., 1998; Oda et al., 2012). High temperature-induced flowering retardation is mainly caused by the inhibition of capitulum development between inflorescence meristem formation and the growth of florets (Cockshull and Kofranek, 1994; Nozaki and Fukai, 2008). Therefore, the SD stimuli required for reproductive growth seem to be counteracted by high temperature.
Studies in molecular biology have shed light on the nature of florigen (Chailakhyan and Krikorian, 1936), a central flowering promoter that transmits floral inductive stimuli perceived in the leaves to the SAM. FLOWERING LOCUS T (FT) in the LD plant Arabidopsis thaliana and Heading date 3a (Hd3a) in the SD plant rice (Oryza sativa) encode a phosphatidylethanolamine binding-like protein that is identified as florigen (Kardailsky et al., 1999; Kobayashi et al., 1999; Kojima et al., 2002). These genes are mainly expressed in the leaves and are up-regulated under floral inductive photoperiods. The corresponding proteins are then translocated to the SAM via the phloem, where they promote flowering (Corbesier et al., 2007; Tamaki et al., 2007). Recently, Oda et al. (2012) characterized FLOWERING LOCUS T-like 3 (FTL3) in C. morifolium and C. seticuspe (Maxim.) Hand.-Mazz. f. boreale (Makino) H. Ohashi & Yoneke, a diploid chrysanthemum species, and demonstrated the florigen-like action of its transcription product. FTL3 induces an early flowering phenotype and acts as a graft-transmissible floral signal when overexpressed in C. morifolium. The expression in the leaves is largely dependent upon daylength and is induced by SDs.
Growth temperature has also been reported to regulate FT. Flowering of Arabidopsis, which is generally cultivated at ~23 °C in many laboratories, is promoted by thermal elevation via FT up-regulation (Blázquez et al., 2003; Balasubramanian et al., 2006; Kumar et al., 2012). Under natural conditions, temperature gradually increases from the cold winter to flowering time in many LD plants. However, the temperature tends to decrease as anthesis approaches in many SD plants that are native to temperate and subpolar zones, including chrysanthemums. The effects of growth temperature on FT and its functional homologues in LD plants may be opposite to those in SD plants, as are the effects of daylength.
In Arabidopsis, FT belongs to a group of so-called ‘floral pathway integrator’ genes, and it controls the genes involved in flower organ morphogenesis (Simpson and Dean, 2002). FT protein translocated to the SAM interacts with FD, a basic leucine zipper protein (Abe et al., 2005; Wigge et al., 2005). In the floral signalling cascade, the FT–FD protein complex is a key element that regulates APETALA1 (AP1) and another floral pathway integrator, SUPPRESSOR OF OVEREXPRESSION OF CONSTANS1 (SOC1; Lee et al., 2000; Samach et al., 2000; Abe et al., 2005; Wigge et al., 2005; Yoo et al., 2005). SOC1 further promotes LEAFY (LFY) expression (Lee et al., 2008). LFY is an Arabidopsis orthologue of the floral homeotic gene in Antirrhinum majus, FLORICAULA (FLO), and is required for floral meristem establishment, along with AP1 (Coen et al., 1990; Irish and Sussex, 1992; Bowman et al., 1993; Weigel and Nilsson, 1995). FRUITFUL (FUL) is closely related to AP1 and acts redundantly with AP1 to regulate floral meristem identity (Ferrándiz et al., 2000). CmSOC1, CmFL, CmAFL1, CDM111, and CDM8/CDM41 of C. morifolium have been identified as the homologues of SOC1, FLO/LFY, AP1/FUL, AP1, and FUL, respectively. The involvement of these genes and C. seticuspe counterparts in flowering has been reported (Shchennikova et al., 2004; Li et al., 2009; Sumitomo et al., 2009; Oda et al., 2012). In accordance with SD requirements during flower development (Adams et al., 1998), SD induction of a highly expressed state of FTL3 seems necessary for the regulation of these genes (Oda et al., 2012). Temperature may affect the activation cascade of flower morphogenesis that begins with FTL3.
This study tested the hypothesis that growth temperature-dependent FTL3 regulation is involved in high temperature-induced flowering retardation in chrysanthemums.
Materials and methods
Plant materials
Chrysanthemum seticuspe accession NIFS-3 and C. morifolium cultivar ‘Mona Lisa’ (Japan Agribio Co. Ltd, Tokyo, Japan) and ‘Kurarisu’ (National Federation of Agricultural Cooperative Association, Tokyo, Japan) were used for the experiments. Mother stocks and nursery plants were grown in a glasshouse heated below 18 °C and ventilated above 25 °C. In addition to natural light, 4h of light exposure (2300–0300h) with fluorescent tubes (EFR25ED/22; Toshiba Lighting & Technology Corp., Kanagawa, Japan) was provided as a NB to inhibit flowering. Cuttings (5cm) were rooted for 2 weeks on 1.5cm plastic cell trays that were filled with soil composed mostly of peat moss (Metro-Mix 350; Sun Gro Horticulture Canada Ltd, Canada). Rooted cuttings were cold treated at 5 °C for 4–5 weeks to avoid dormancy. Plants were then transplanted into 7.5cm plastic pots containing gardening soil (Yokabaido, Hokkaido Peatmoss Co. Ltd, Japan) and allowed to become established for 1–2 weeks.
Temperature treatment
Temperature treatment was performed in growth chambers (LPH-0.5P-SH; NK system, Osaka, Japan) with a 10h photoperiod at 20, 25, or 30 °C. Light was supplied via metal halide lamps (MF250EH/BUP, Iwasaki Electric Co. Ltd, Tokyo, Japan) at a photosynthetic photon flux density of 180 µmol m–2 s–1. In the experiments with C. morifolium, shoot tips were pinched off from the potted plants leaving four leaves behind, in order to encourage the growth of two fresh lateral tops. The dates when the bracts became visible and the ligulate flower stood vertically were recorded as the date of visible capitulum and date of anthesis, respectively. The number of leaves and diameter of capitula were measured.
Short-term high temperature treatment
Temperature treatment of C. seticuspe was started at 20 °C with a 10h photoperiod, as described above. After 0, 1, 2, 3, or 4 weeks of cultivation, the plants underwent a short-term high temperature treatment at 30 °C for 1 week and then returned to 20 °C. As a negative control, plants were grown at 30 °C with a 10h photoperiod throughout the experiment.
Microscopic examination
The 3cm shoot tip of each C. seticuspe plant was excised and stored in 100% ethyl alcohol. Leaves and bracts were carefully detached with a surgical knife under a stereoscopic microscope (MZ16; Leica Microsystems K. K., Tokyo, Japan). The developmental stage of each capitulum was classified into one of eight stages (Higuchi et al., 2012): 0, vegetative growth stage; 1, dome-shaped stage; 2, first stage of involucre formation; 3, final stage of involucre formation; 4, first stage of floret formation; 5, final stage of floret formation; 6, first stage of corolla formation; or 7, final stage of corolla formation.
Grafting experiment
Shoot tips (5cm) of ‘Mona Lisa’ and ‘Kurarisu’ were cut from potted plants and used as scions. The remaining parts of the plants, each with 8–10 leaves, were used as stocks. A wedge-shaped/slit grafting technique was used, and the site of union was wrapped with Parafilm. Grafted plants were kept under a mist irrigation system in the glasshouse described above for 1 week to obtain well established plants. Temperature treatment was performed at 20 °C or 27 °C, as described above. Prior to temperature treatment, the shoot tip, leaves, and lateral shoots on the scion were detached, leaving behind one fresh lateral shoot. Leaves and lateral shoots newly formed during temperature treatment were detached continuously.
Total RNA preparation
Expanded leaves of the same node order or shoot tips (~3mm in length) were excised, frozen in liquid nitrogen, and stored at –80 °C until analysis. The samples were ground using a mortar and pestle in the presence of liquid nitrogen. Total RNA was extracted from 15–30mg of the frozen powder by using an RNeasy Plant Mini Kit (Qiagen K. K., Tokyo, Japan) in combination with an RNase-Free DNase Set (Qiagen K. K.), according to the manufacturer’s instructions. The purity and concentration of total RNA were measured using NanoDrop 1000 (Thermo Fisher Scientific K. K., Kanagawa, Japan).
cDNA cloning
A full-length cDNA library was constructed from the leaves and shoot tips of C. seticuspe grown under various conditions by using a SMART cDNA Library Construction Kit (TAKARA BIO INC., Shiga, Japan), according to the manufacturer’s instructions. Over 2 700 000 sequence tags were obtained by the genome sequencer FLX (Roche Diagnostics K. K., Tokyo, Japan; T. Hisamatsu et al., unpublished). Sequences highly homologous to CDM8, CDM19, CDM37, CDM41, CDM44, and CDM86 of C. morifolium (Shchennikova et al., 2003 , 2004) were screened from ~60 000 C. seticuspe contigs by BLAST-N search and are referred to as CsM8 (AB770472), CsM19 (AB770473), CsM37 (AB770474), CsM41 (AB770475), CsM44 (AB770476), and CsM86 (AB770477), respectively.
Quantitative reverse transcription–polymerase chain reaction (RT–PCR)
Reverse transcription was performed using 250ng of total RNA and PrimeScript RT Master Mix Perfect Real Time (TAKARA BIO INC.), according to the manufacturer’s instructions. The resulting cDNA was diluted to 10% with 10mM TRIS-HCl buffer, pH 8.0, containing 1mM EDTA disodium salt. Quantitative PCR of leaf mRNA was performed using cDNA templates and SYBR Premix Ex Taq II Tli RNaseH plus (TAKARA BIO INC.) on a Thermal Cycler Dice Real Time System II (TAKARA BIO INC.). Cycling conditions were as follows: 1min of denaturation at 95 °C, followed by 40 cycles of 95 °C for 5 s and 60 °C for 30 s. Quantitative RT–PCR of shoot tip mRNA was performed with SYBR Premix Ex Taq (TAKARA BIO INC.) on a LightCycler system (Roche Diagnostics K. K.), as described in Oda et al. (2012). Primers newly designed for this study with Primer3 (Rozen and Skaletsky, 2000) are listed in Supplementary Table S1 available at JXB online. ACTIN (AB770470 for CsACTIN, AB770471 for CmACTIN) or EF1α (AB679278) was used as an internal standard to normalize raw data. Specificities and amplification efficiencies of primer pairs were confirmed by preliminary experiments by using target cDNA subcloned in pGEM-T Easy Vector (Promega K. K., Tokyo, Japan). The analysis was performed with three biological replicates through cDNA preparation and two technical replicates in PCR.
Results
Effects of high temperature on flowering in C. seticuspe
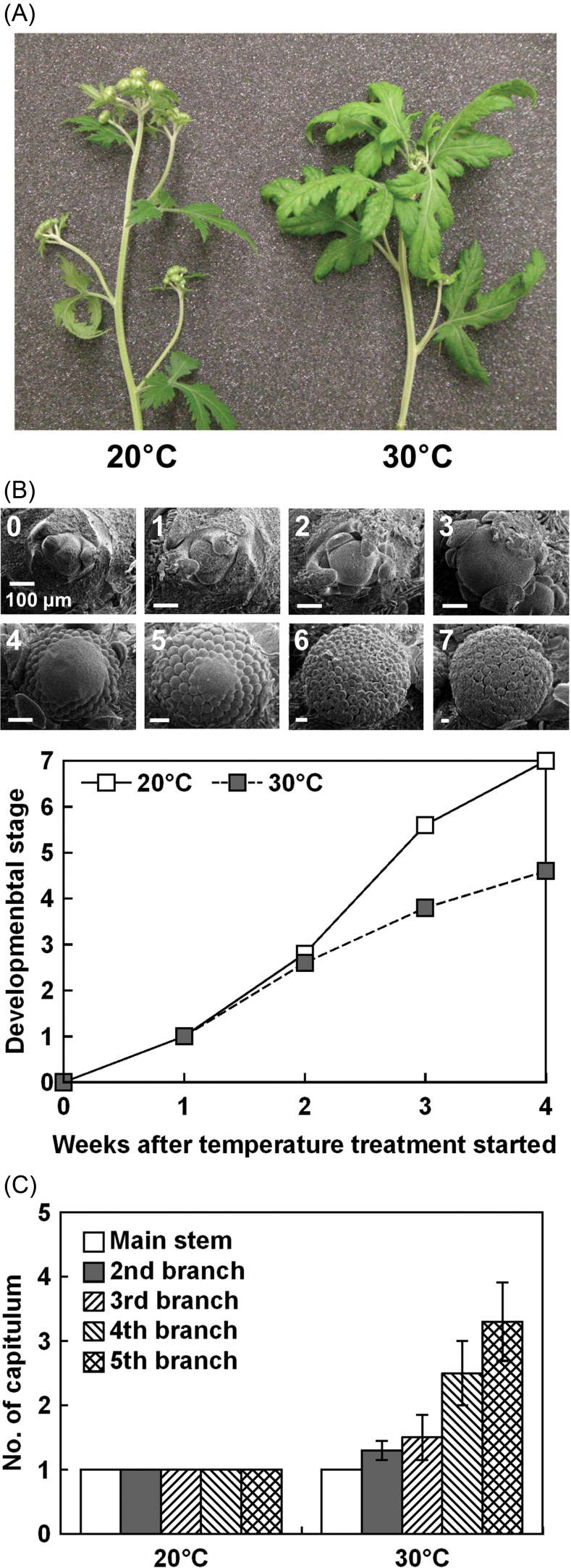
The effect of high temperature on flowering in C. seticuspe was studied at 20 °C, the optimum flowering temperature of these plants (Karlsson, 1989), and 30 °C with a 10h photoperiod (Table 1). There were a slightly greater number of leaves at 30 °C than at 20 °C. There was no difference between temperatures in the number of days to visible capitulum. Plastochron was smaller at 30 °C than at 20 °C. After 35 d of SDs, the capitulum diameter of the plants grown at 30 °C was about half that of the plants grown at 20 °C (Fig. 1A, Table 1). Microscopic examination of the shoot tip showed that inflorescence development was slower at 30 °C than at 20 °C (Fig. 1B). Differentiation of the florets was complete (stage 5) after 3 weeks of SD treatment at 20 °C, while it was delayed at 30 °C. The number of capitula on the uppermost four lateral shoots was larger at 30 °C than at 20 °C (Fig. 1C).
Table 1.
Effects of high temperature on growth and flowering in C. Seticuspe. The plants were cultivated with a 10h photoperiod. Values are means ±SE (n=10).
| Growth temperature | No. of leaves at capitulum initiation | Days to visible capitulum | Capitulum diameter at 35 d (mm) | Plastochrona (d·leaf–1) |
|---|---|---|---|---|
| 20 °C | 18.0±0.4 | 19.3±0.4 | 5.64±0.11 | 2.37±0.11 |
| 30 °C | 19.7±0.3* | 18.9±0.6 | 2.53±0.08** | 1.86±0.05* |
*, ** Significantly different from plants grown at 20 °C (Student’s t-test, P < 0.05, P < 0.01, respectively).
a The plants were cultivated with a 10h photoperiod and 30min of NB for 12 d to measure the number of leaves (n=8).
Fig. 1.
(A) Representative C. seticuspe grown at 20 °C or 30 °C with a 10h photoperiod for 35 d. (B) Progress in the development of terminal inflorescence was observed under a microscope. Development was scored as follows: 0, vegetative growth stage; 1, dome-shaped stage; 2, first stage of involucre formation; 3, final stage of involucre formation; 4, first stage of floret formation; 5, final stage of floret formation; 6, first stage of corolla formation; and 7, final stage of corolla formation. Values are the means of five shoot tips. (C) The number of capitula on each branch surrounding the terminal capitulum. Values are means ±SE (n=9). (This figure is available in colour at JXB online.)
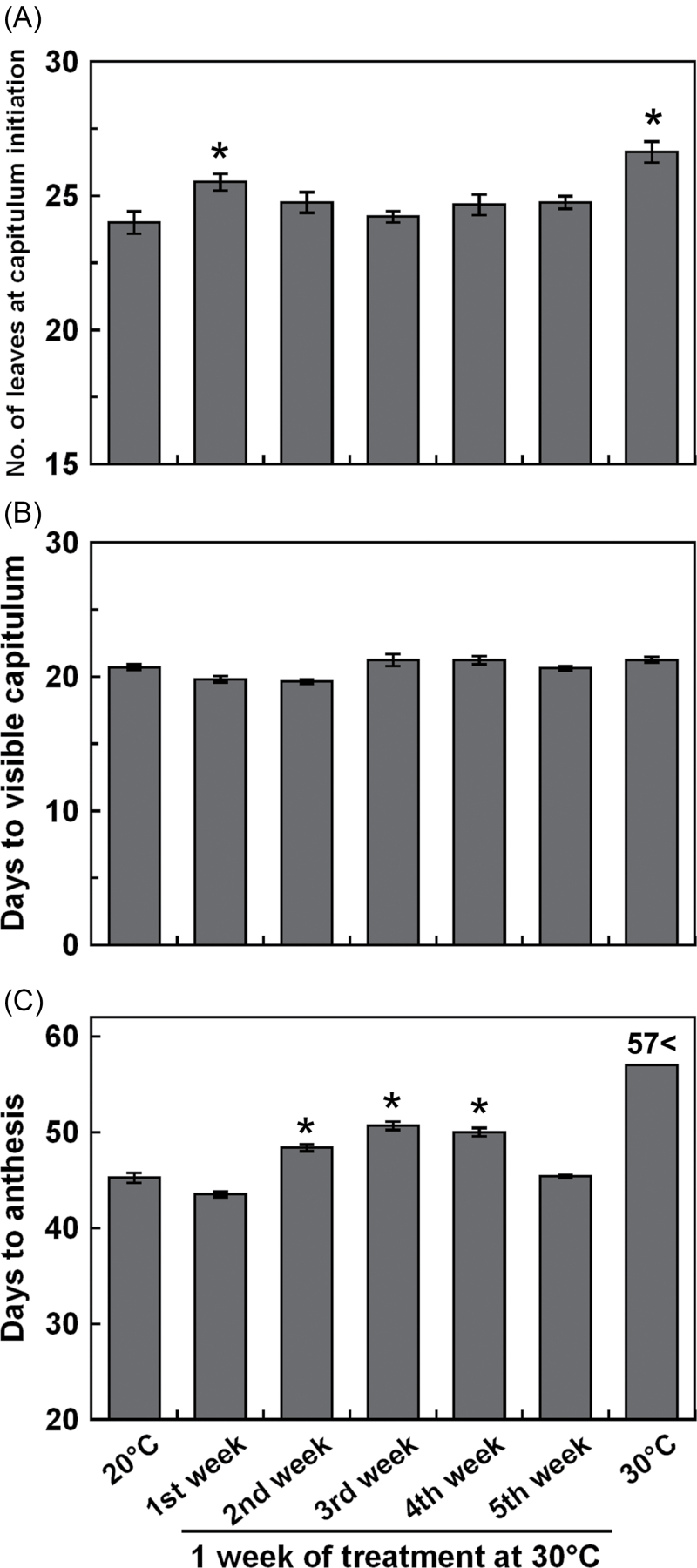
Short-term high temperature treatment at different growth stages was performed using plants cultivated at 20 °C. Exposure of plants to 30 °C during the first week resulted in an increase in the number of leaves (Fig. 2A). The number of days to visible capitulum was not changed by temperature treatment (Fig. 2B). Plants exposed to 30 °C in the second, third, or fourth week flowered late, and plants continually cultivated at 30 °C did not flower within the experimental period (Fig. 2C, Supplementary Fig. S1 at JXB online). High temperature exposure in the fifth week did not affect flowering.
Fig. 2.
The effects of short-term high temperature treatment at different growth stages on flowering in C. seticuspe. (A) The number of leaves at capitulum initiation. (B) Days to visible capitulum. (C) Days to anthesis. Plants were grown at 20 °C with a 10h photoperiod and transferred to 30 °C for 1 week. Plants continuously grown at 20 °C or 30 °C were also prepared. Values are means ±SE (n=8–9). *Significantly larger than the plants grown at 20 °C (Dunnett’s test, P < 0.05).
Expression analysis of flowering-related genes in the shoot tip and of CsFTL3 in the leaves of C. seticuspe
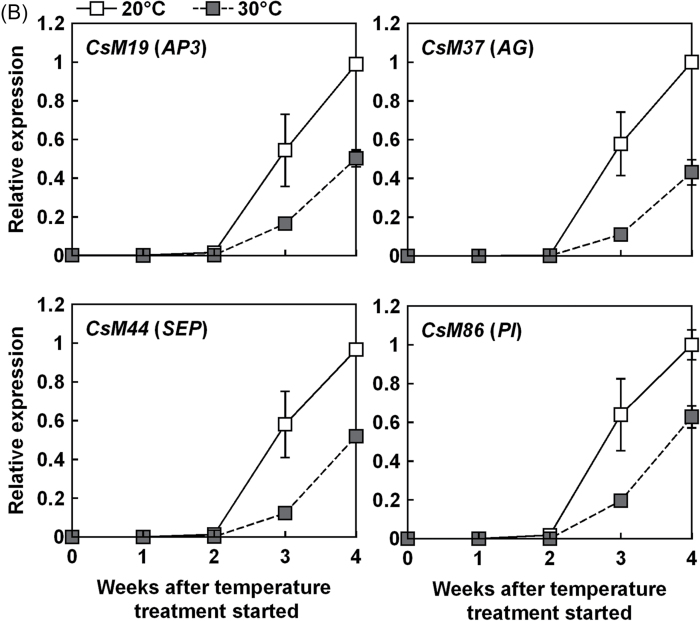
The amount of flowering-related mRNA in plants grown at 20 °C and 30 °C was compared. Expression levels of CsSOC1 (AB679276), CsFL (AB679274), CsAFL1 (AB679273), and CsM8 in the shoot tip increased to their maximum after 2 weeks at 20 °C (Fig. 3A). Thereafter, these genes were down-regulated at 20 °C. In plants grown at 30 °C, expression of these genes remained at high levels until the end of the experiment. Expression levels of CsM111 (AB679275) and CsM41 increased after 2 weeks at 20 °C, while they started to increase after 3 weeks at 30 °C (Fig. 3A). CsM37, CsM44, CsM19, and CsM86 were identified in C. seticuspe as the homologues of the Arabidopsis floral homeotic genes AGAMOUS (AG), SEPALLATA (SEP), APETALA3 (AP3), and PISTILLATA (PI), respectively. Transcripts of CsM86, CsM19, CsM37, and CsM44 increased after 3 weeks at both 20 °C and 30 °C (Fig. 3B). The transcript level of each gene was lower at 30 °C than at 20 °C.
Fig. 3.
Expression analysis of several floral pathway integrator and floral meristem identity genes (A) and floral homeotic genes (B) in the shoot tip of C. seticuspe by using quantitatitve real-time PCR. Corresponding Arabidopsis genes are shown in parentheses. Shoot tips (3mm) were harvested every week from plants grown at 20 °C or 30 °C with a 10h photoperiod. CsEF1α was used as an internal standard. Values are means ±SE (n=3).
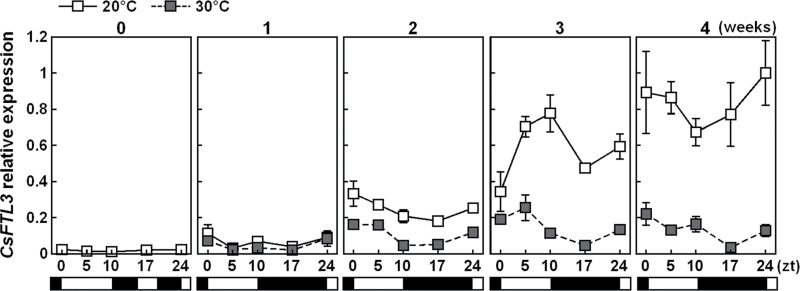
Expression of CsFTL3 (AB679272) in the leaves of plants grown at 20 °C and 30 °C was studied (Fig. 4). Compared with NB conditions, transcription of CsFTL3 increased after 1 week of SDs at both temperatures. After that, the expression level became higher at 20 °C than at 30 °C.
Fig. 4.
Expression analysis of CsFTL3 in the leaves of C. seticuspe by using quantitatitve real-time PCR. Plants were grown at 20 °C or 30 °C with a 10h photoperiod. The leaves were harvested every week. Open and filled horizontal bars represent light and dark periods, respectively. CsACT was used as an internal standard. Values are means ±SE (n=3).
Differences in high temperature effect on flowering and CmFTL3 expression in two C. morifolium cultivars
Chrysanthemum morifolium ‘Mona Lisa’ and ‘Kurarisu’ were cultivated with a 10h photoperiod at 20, 25, or 30 °C. The number of leaves at capitulum initiation increased with temperature in ‘Mona Lisa’ (Fig. 5A). The number of days to visible capitulum was slightly greater at 25 °C, and considerably greater at 30 °C, than at 20 °C (Fig. 5B). In ‘Mona Lisa’, capitulum diameter after 35 d of SDs decreased, and flowering was increasingly delayed as the temperature increased (Fig. 5C, D). In ‘Kurarisu’, flowering time did not differ between 20 °C and 25 °C (Fig. 5C, D). However, at 30 °C, there were a significantly greater number of leaves and days to visible capitulum and a smaller capitulum size than at 20 °C.
Fig. 5.
The effects of high temperature on flowering in C. morifolium ‘Mona Lisa’ and ‘Kurarisu’. (A) The number of leaves at capitulum initiation. (B) Days to visible capitulum. (C) Capitulum diameter at 35 d. Plants were grown at 20, 25, or 30 °C with a 10h photoperiod. Values are means ±SE (n=13–15). Different letters within a cultivar indicate significant differences (Tukey–Kramer HSD test, P < 0.05). (D) Representative ‘Mona Lisa’ and ‘Kurarisu’ at 50 SDs. (This figure is available in colour at JXB online.)
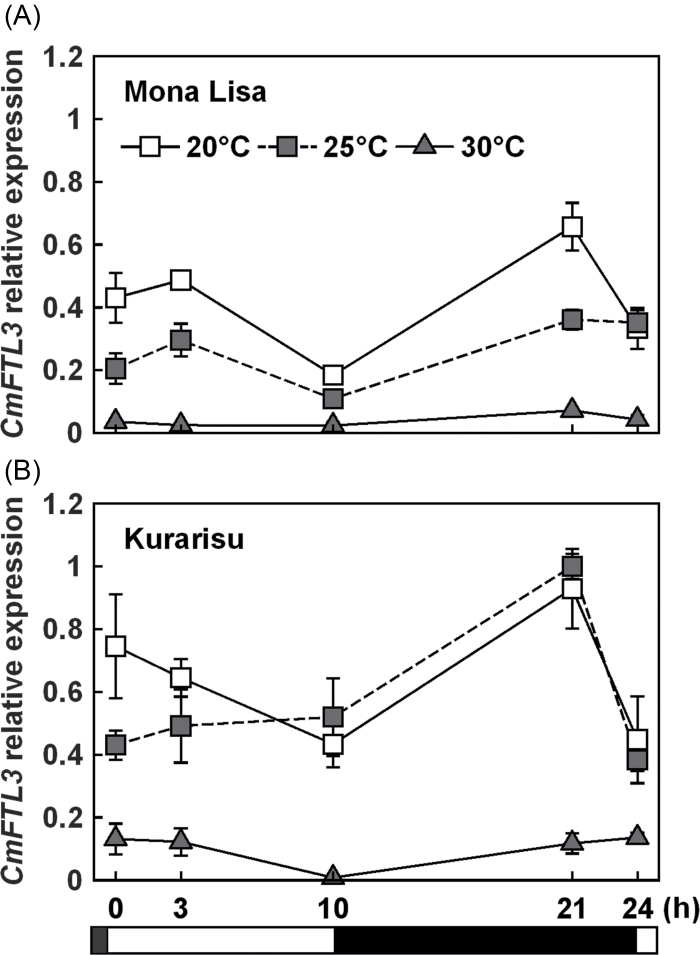
CmFTL3 (AB770479) transcript levels in these cultivars under different temperature conditions were compared after 3 weeks of SD. Expression of CmFTL3 in ‘Mona Lisa’ was reduced at 25 °C, while that in ‘Kurarisu’ was not affected (Fig. 6). CmFTL3 expression was lower at 30 °C than at 20 °C and 25 °C in both cultivars.
Fig. 6.
Expression analysis of CmFTL3 in the leaves of C. morifolium ‘Mona Lisa’ (A) and ‘Kurarisu’ (B) by using quantitatitve real-time PCR. Plants were grown at 20, 25, or 30 °C with a 10h photoperiod. The leaves were harvested after 3 weeks. Open and filled horizontal bars represent light and dark periods, respectively. CmACT was used as an internal standard. Values are means ±SE (n=3).
Grafting experiment: effects of different stock cultivars on high temperature-induced flowering retardation in C. morifolium
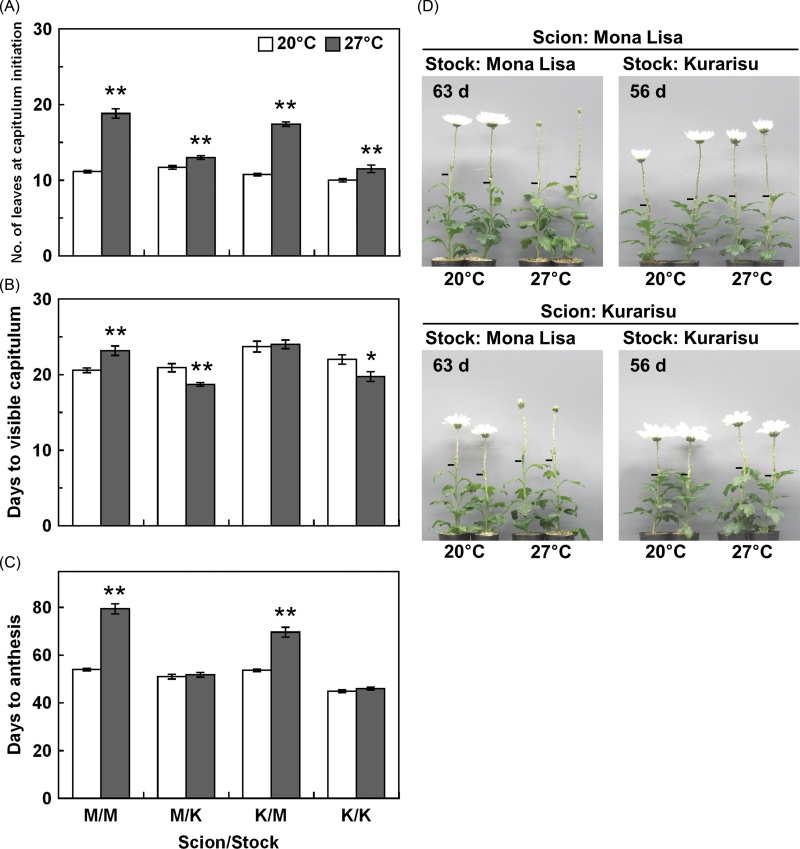
To determine whether the sensitivity of CmFTL3 expression to high temperatures in the leaves reflects flowering time, the shoot tip and stock of ‘Mona Lisa’ and ‘Kurarisu’ were recombined by grafting. Since 30 °C strongly inhibited flowering and CmFTL3 expression in both cultivars, 27 °C was used for the high temperature treatment in this experiment. There were more leaves at 27 °C than at 20 °C in all combinations of scions and stocks tested (Fig. 7A). However, the impact of high temperature was greater for scions grafted onto ‘Mona Lisa’ stock than for those grafted onto ‘Kurarisu’. The number of days to visible capitulum at 27 °C was slightly greater than that at 20 °C for the ‘Mona Lisa’ scion combined with the ‘Mona Lisa’ stock, while there were fewer days to visible capitulum at 27 °C than at 20 °C when ‘Kurarisu’ was used as stock (Fig. 7B). Both scions took longer to reach anthesis at 27 °C than at 20 °C when paired with ‘Mona Lisa’ stock (Fig. 7C, D). In contrast, high temperature did not affect the flowering time of the scions grafted onto ‘Kurarisu’ stock.
Fig. 7.
The effects of high temperature on flowering in grafted C. morifolium plants. (A) The number of leaves at capitulum initiation. (B) Days to visible capitulum. (C) Days to anthesis. Plants were grown at 20 °C or 27 °C with a 10h photoperiod. ‘Mona Lisa’ and ‘Kurarisu’ are abbreviated to M and K, respectively, in the graph label. Values are means ±SE (n=5–12). *, **Significantly different from plants grown at 20 °C (Student’s t-test, P < 0.05, P < 0.01, respectively). (D) Representatives of each plant were photographed when plants grown at 20 °C reached the flowering stage. Bars indicate graft junctions. (This figure is available in colour at JXB online.)
CmFTL3 transcript levels after 20 d of SDs were significantly repressed in ‘Mona Lisa’ and slightly repressed in ‘Kurarisu’ at 27 °C (Supplementary Fig. S2 at JXB online).
Discussion
The results of these experiments suggest that impaired FTL3 signalling from leaves to the SAM at high temperatures is involved in flowering retardation of chrysanthemums. This is different from de-vernalization of mother stock, a memory of summer heat that inhibits growth and flowering of suckers in the following seasons (Schwabe, 1955). This is the first report that describes the negative effect of growth temperature elevation on FTL gene-mediated floral promotion.
Flowering retardation by high temperature in chrysanthemums
In order to study the molecular mechanisms of temperature response in chrysanthemums, a diploid chrysanthemum, C. seticuspe, was used because C. morifolium are complex hybrids derived from several species. In this study, it was confirmed that the flowering of C. seticuspe shows a high temperature response similar to C. morifolium (Whealy et al., 1987; Cockshull and Kofranek, 1994; Shibata, 1997).
In C. seticuspe, the terminal capitulum is determined within 4 d and morphological changes are observed at around 8 d when daylength and temperature are appropriate for flowering (Oda et al., 2012). In the experiments described here, plants differentiated a larger number of leaves before floral transition at 30 °C than at 20 °C (Table 1), as has been previously reported for C. morifolium (Cockshull and Kofranek, 1994). Nevertheless, the number of days to visible capitulum (Table 1) and inflorescence meristem initiation (Fig. 1B) were not different between the two tested temperatures. These results indicate that although high temperature increases the flowering node, it does not affect the timing of floral transition so much. Faster initiation of leaves at 30 °C, as indicated by the small plastochron (Table 1), supports this idea. However, subsequent development of the capitulum was retarded by high temperature (Fig. 1A, Table 1). After the morphological change of the SAM to a dome shape under floral inductive conditions, it is reported to shift to inflorescence meristem development, floral meristem differentiation, and floret development within 10, 15, and 20 d, respectively (Zhang et al., 1998). In this study, the capitula developed in a similar manner at 20 °C (Fig. 1B). At 30 °C, the capitula grew to visible size but were not followed by floral meristem initiation and development. Additionally, plants exposed to 30 °C for 1 week, at times corresponding to the stages between floral meristem differentiation and floret development (second, third, or fourth week of SDs), flowered later than plants kept at 20 °C (Fig. 2C). On the other hand, high temperature exposure at the fifth week of temperature treatment did not affect flowering time, probably because capitulum development had passed the heat-sensitive stages by that time.
Once established above the terminal leaf, the terminal inflorescence meristem is surrounded by axillary bud primordia that develop into lateral inflorescence meristems or vegetative shoots, depending on growth conditions. There were more capitula on these side branches at 30 °C than at 20 °C (Fig. 1C), indicating that determinate growth was delayed by high temperature. Previous studies indicate that, after induction of terminal capitulum differentiation on the main stem by 8 SDs, LDs increase the number of capitula on lateral shoots (Post, 1950). It has been suggested that in plants that form a cyme, regulation of determinate growth in the axillary meristems depends on competition between FT protein, a central flowering promoter signal from leaves, and TERMINAL FLOWER 1 (TFL1) protein, an FT antagonistic floral inhibitor (reviewed by McGarry and Ayre, 2012). High temperature seems to shift the balance between vegetative and reproductive growth of the SAM to vegetative in C. seticuspe. However, expression of CsTFL1, a TFL1 homologue of C. seticuspe, in the shoot tip was not so different between the two temperatures (Supplementary Fig. S3 at JXB online), suggesting that inhibition of reproductive growth by CsTFL1 protein was not involved in high temperature-induced flowering retardation.
Effect of high temperature on the expression of flowering-related genes in the shoot tip
The analysis of the effects of high temperature on the expression of flowering-related gene counterparts of C. seticuspe suggests that high temperature causes flowering retardation by inhibiting the activation cascade of floral homeotic genes. Under SD conditions, expression of CsSOC1, CsFL, CsM8, and CsAFL1 increased for 2 weeks at both 20 °C and 30 °C. Expression of these genes decreased after this time at 20 °C, but down-regulation of these genes was inhibited at 30 °C (Fig. 3A). Up-regulation of CsM111 and CsM41 after 2 weeks and that of floral homeotic gene homologues (CsM19, CsM37, CsM44, and CsM86) that occurred after 3 weeks at 20 °C were delayed at 30 °C (Fig. 3). These results might explain why high temperature had little effect on floral transition and significantly inhibited capitulum development (Fig. 1A, B, Table 1). In Arabidopsis, down-regulation of SOC1 by the action of AP1 protein is required for normal expression of floral homeotic genes and establishment of floral meristem identity (Liu et al., 2007). It is possible that such a system is conserved in C. seticuspe. Delayed induction of CsM111/CsM41 might have inhibited the down-regulation of CsSOC1 and up-regulation of floral homeotic genes (Fig. 3).
Although the expression profiles of the floral integrator and floral meristem identity genes were delayed under high temperature conditions, the data did not indicate that there was a heat-sensitive key step in the gene activation cascades involved in the capitulum development.
Effect of high temperature on expression of CsFTL3 in leaves
Flowering retardation under high temperature conditions is accompanied by a reduction in the expression of CsFTL3, an SD-induced FT/Hd3a homologue, in the leaves of C. seticuspe (Fig. 4; Oda et al., 2012). The inhibition of CsFTL3 expression by high temperature is small in comparison with the inhibition of CsFTL3 expression by NB (Fig. 4). Therefore, the amount of CsFTL3 mRNA amplified in the leaves by 1 week of SD seems to be enough to induce floral transition in C. seticuspe (Fig. 1B, Table 1), even under high temperature conditions. This is supported by the failure of high temperature to affect expression of floral pathway integrator genes significantly in the shoot tip at 1 week of SDs (Fig. 3A). Oda et al. (2012) have suggested that although floral transition occurs within a few SDs, continuous SD and CsFTL3 stimuli are necessary for floral gene regulation and inflorescence development. CsFTL3 expression continued to increase at 20 °C by repeated SD stimuli, while the amplitude of induction was small at 30 °C (Fig. 4). CsFTL3 signal translocated from the leaves to the shoot tip may not reach the threshold for smooth inflorescence development at 30 °C, as indicated by the phenotype (Fig. 1B, Table 1) and the delayed expression patterns of flowering-related gene mRNA in the shoot tip (Fig. 3). These data suggest that inhibition of CsFTL3 amplification by high temperature causes a delay in the flowering programme at the SAM.
There are at least two other FT/Hd3a homologues, CsFTL1 (AB679270) and CsFTL2 (AB679271), in C. seticuspe. Although the role of CsFTL1 has not been well studied, it is repressed by SD and is insensitive to temperature (Oda et al., 2012; Supplementary Fig. S4 at JXB online). Moreover, the absolute expression level of CsFTL2 seems substantially lower than the expression of the other two homologues (Oda et al., 2012). Therefore, CsFTL1 and CsFTL2 are not likely to be involved in the temperature response studied here.
CmFTL3 expression and flowering in high temperature-sensitive and less sensitive C. morifolium cultivars
There is a varietal difference in C. morifolium cultivars with respect to flowering ability under high temperature, depending on breeding background (Whealy et al., 1987; Shibata, 1997; Nozaki and Fukai, 2008). ‘Kurarisu’ is a cultivar bred for summer production in Japan, where the average temperature is >25 °C in summer. In contrast, ‘Mona Lisa’ was bred in The Netherlands, where the average temperature is <20 °C in summer. Although 30 °C severely inhibited flowering in both cultivars, flowering of ‘Kurarisu’ was not affected at 25 °C, while ‘Mona Lisa’ was prevented from smooth flowering at that temperature (Fig. 5). Therefore, the flowering of ‘Mona Lisa’ is more sensitive to high temperature than that of ‘Kurarisu’. In accordance with flowering rate, high temperature caused a striking repression of CmFTL3 expression in ‘Mona Lisa’ (Fig. 6A). The expression was similarly repressed in ‘Kurarisu’ at 30 °C, and not at 25 °C (Fig. 6B). Therefore, high temperature-induced flowering retardation is associated with CmFTL3 repression in C. morifolium, as it is in C. seticuspe, and this flowering retardation seems quantitatively alleviated by the CmFTL3 expression level.
In chrysanthemums, FTL3 acts as a graft-transmissible floral inducer (Oda et al., 2012). A grafting experiment was performed in order to investigate whether levels of CmFTL3 expression in the leaves affect the magnitude of high temperature-induced flowering retardation in the SAM. Each scion that was combined with ‘Mona Lisa’ stock flowered late at 27 °C (Fig. 7). In contrast, there was no flowering retardation at 27 °C when ‘Kurarisu’ was used as stock (Fig. 7). The expression of CmFTL3 was more strongly reduced by high temperature in ‘Mona Lisa’ leaves than in ‘Kurarisu’ leaves (Fig. 6; Supplementary Fig. S2 at JXB online). Although CmFTL3 expression in ‘Kurarisu’ was slightly reduced at 27 °C, it was comparable with that in ‘Mona Lisa’ at 20 °C (Supplementary Fig. S2). Thus, the signal might have reached the threshold for prosperous flowering. These data suggest that the degree of flowering retardation that results from high temperature in chrysanthemums largely depends on the amount of transmissible FTL3 signal from the leaves to the SAM.
Conclusion
Several lines of evidence described herein support the hypothesis that the regulation of FTL3 signalling by temperature is responsible for high temperature-induced flowering retardation in chrysanthemums. Interestingly, this is in contrast to the spring bloomer Arabidopsis, in which LDs and high temperature promote flowering via FT up-regulation (Blázquez et al., 2003; Balasubramanian et al., 2006; Kumar et al., 2012). In chrysanthemums, FTL3 seems to be one of the common outputs from daylength- (Oda et al., 2012) and temperature-sensing pathways, but it causes chrysanthemums to flower in autumn. The inhibitory effect of high temperature on floral transition is weaker than the powerful inhibition caused by LDs or NB. Meanwhile, high temperature inhibits further FTL3 signalling amplified by repeated SD stimuli, which seems to have a large effect on the rate of inflorescence development. FTL3 regulation by a temperature-sensing mechanism is a steady determinant of flowering time in chrysanthemums. The mechanism by which environments affect FTL3 expression and the reason why expression in C. morifolium cultivars is differently influenced by temperature remain to be clarified. Further studies will contribute to a better understanding of the flowering mechanism in chrysanthemums.
Supplementary data
Supplementary data are available at JXB online.
Figure S1. Representative C. seticuspe exposed to 30 °C for 1 week at different growth stages.
Figure S2. Expression of CmFTL3 in the leaves of C. morifolium ‘Mona Lisa’ and ‘Kurarisu’ stocks at 20 °C and 27 °C.
Figure S3. Expression of CsTFL1 in the shoot tip of C. seticuspe at 20 °C and 30 °C.
Figure S4. Expression of CsFTL1 in the leaves of C. seticuspe at 20 °C and 30 °C.
Table S1. List of PCR primers used in this study.
Acknowledgements
This work was supported by a grant-in-aid from the Ministry of Agriculture, Forestry and Fisheries of Japan (Development of mitigation and adaptation techniques to global warming in the sectors of agriculture, forestry, and fisheries). We thank A. Oda for sequence analysis. We are very grateful to S. Kamei and T. Hashimoto for sample preparation.
Glossary
Abbreviations:
- LD
long day
- NB
night break
- RT–PCR
reverse transcription–polymerase chain reaction
- SAM
shoot apical meristem
- SD
short day.
References
- Abe M, Kobayashi Y, Yamamoto S, Daimon Y, Yamaguchi A, Ikeda Y, Ichinoki H, Notaguchi M, Goto K, Araki T. 2005. FD, a bZIP protein mediating signals from the floral pathway integrator FT at the shoot apex. Science 309, 1052–1056 [DOI] [PubMed] [Google Scholar]
- Adams SR, Pearson S, Hadley P. 1998. An appraisal of the use of reciprocal transfer experiments: assessing the stages of photoperiod sensitivity in chrysanthemum cv. Snowdon (Chrysanthemum morifolium Ramat.). Journal of Experimental Botany 49, 1405–1411 [Google Scholar]
- Balasubramanian S, Sureshkumar S, Lempe J, Weigel D. 2006. Potent induction of Arabidopsis thaliana flowering by elevated growth temperature. PLoS Genetics 2, e106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blázquez MA, Ahn JH, Weigel D. 2003. A thermosensory pathway controlling flowering time in Arabidopsis thaliana. Nature Genetics 33, 168–171 [DOI] [PubMed] [Google Scholar]
- Bowman JL, Alvarez J, Weigel D, Meyerowitz EM, Smyth DR. 1993. Control of flower development in Arabidopsis thaliana by APETALA1 and interacting genes. Development 119, 721–743 [Google Scholar]
- Chailakhyan MK, Krikorian AD. 1975. Forty years of research on the hormonal basis of plant development—some personal reflections. Botanical Review 41, 1–29 [Google Scholar]
- Cockshull KE, Kofranek AM. 1994. High night temperatures delay flowering, produce abnormal flowers and retard stem growth of cut-flower chrysanthemums. Scientia Horticulturae 56, 217–234 [Google Scholar]
- Coen ES, Romero JM, Doyle S, Elliott R, Murphy G, Carpenter R. 1990. floricaula: a homeotic gene required for flower development in antirrhinum majus. Cell 63, 1311–1322 [DOI] [PubMed] [Google Scholar]
- Corbesier L, Vincent C, Jang S, et al. 2007. FT protein movement contributes to long-distance signaling in floral induction of Arabidopsis. Science 316, 1030–1033 [DOI] [PubMed] [Google Scholar]
- Ferrándiz C, Gu Q, Martienssen R, Yanofsky MF. 2000. Redundant regulation of meristem identity and plant architecture by FRUITFULL. APETALA1 and CAULIFLOWER. Development 127, 725–734 [DOI] [PubMed] [Google Scholar]
- Higuchi Y, Sumitomo K, Oda A, Shimizu H, Hisamatsu T. 2012. Day light quality affects the night-break response in the short-day plant chrysanthemum, suggesting differential phytochrome-mediated regulation of flowering. Journal of Plant Physiology 169, 1789–1796 [DOI] [PubMed] [Google Scholar]
- Irish VF, Sussex IM. 1990. Function of the apetala-1 gene during Arabidopsis floral development. The Plant Cell 2, 741–753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kardailsky I, Shukla VK, Ahn JH, Dagenais N, Christensen SK, Nguyen JT, Chory J, Harrison MJ, Weigel D. 1999. Activation tagging of the floral inducer FT. Science 286, 1962–1965 [DOI] [PubMed] [Google Scholar]
- Karlsson MG, Heins RD, Erwin JE, Berghage RD, Carlson WH, Biernbaum JA. 1989. Irradiance and temperature effects on time of development and flower size in chrysanthemum. Scientia Horticulturae 39, 257–267 [Google Scholar]
- Kobayashi Y, Kaya H, Goto K, Iwabuchi M, Araki T. 1999. A pair of related genes with antagonistic roles in mediating flowering signals. Science 286, 1960–1962 [DOI] [PubMed] [Google Scholar]
- Kojima S, Takahashi Y, Kobayashi Y, Monna L, Sasaki T, Araki T, Yano M. 2002. Hd3a, a rice ortholog of the Arabidopsis FT gene, promotes transition to flowering downstream of Hd1 under short-day conditions. Plant and Cell Physiology 43, 1096–1105 [DOI] [PubMed] [Google Scholar]
- Kumar SV, Lucyshyn D, Jaeger KE, Alós E, Alvey E, Harberd NP, Wigge PA. 2012. Transcription factor PIF4 controls the thermosensory activation of flowering. Nature 484, 242–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Suh SS, Park E, Cho E, Ahn JH, Kim SG, Lee JS, Kwon YM, Lee I. 2000. The AGAMOUS-LIKE 20 MADS domain protein integrates floral inductive pathways in Arabidopsis. Genes and Development 14, 2366–2376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Oh M, Park H, Lee I. 2008. SOC1 translocated to the nucleus by interaction with AGL24 directly regulates LEAFY. The Plant Journal 55, 832–843 [DOI] [PubMed] [Google Scholar]
- Li T, Niki T, Nishijima T, Douzono M, Koshioka M, Hisamatsu T. 2009. Roles of CmFL. CmAFL1, and CmSOC1 in the transition from vegetative to reproductive growth in Chrysanthemum morifolium Ramat. Journal of Horticultural Science and Biotechnology 84, 447–453 [Google Scholar]
- Liu C, Zhou J, Bracha-Drori K, Yalovsky S, Ito T, Yu H. 2007. Specification of Arabidopsis floral meristem identity by repression of flowering time genes. Development 134, 1901–1910 [DOI] [PubMed] [Google Scholar]
- McGarry RC, Ayre BG. 2012. Manipulating plant architecture with members of the CETS gene family. Plant Science 188–189 71–81 [DOI] [PubMed] [Google Scholar]
- Nozaki K, Fukai S. 2008. Effects of high temperature on floral development and flowering in spray chrysanthemum. Journal of Applied Horticulture 10, 8–14 [Google Scholar]
- Oda A, Narumi T, Li T, Kando T, Higuchi Y, Sumitomo K, Fukai S, Hisamatsu T. 2012. CsFTL3, a chrysanthemum FLOWERING LOCUS T-like gene, is a key regulator of photoperiodic flowering in chrysanthemums. Journal of Experimental Botany 63, 1461–1477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post K. 1950. Controlled photoperiod and spray formation of chrysanthemums. Proceedings of the American Society for Horticultural Science 55, 467–472 [Google Scholar]
- Rozen S, Skaletsky HJ. 2000. Primer3 on the WWW for general users and for biologist programmers. Methods in Molecular Bbiology 132, 365–386 [DOI] [PubMed] [Google Scholar]
- Samach A, Onouchi H, Gold SE, Ditta GS, Schwarz-Sommer Z, Yanofsky MF, Coupland G. 2000. Distinct roles of CONSTANS target genes in reproductive development of Arabidopsis. Science 288, 1613–1616 [DOI] [PubMed] [Google Scholar]
- Shchennikova AV, Shulga OA, Angenent GC, Skryabin KG. 2003. Regulatory network of chrysanthemum inflorescence development. Doklady Akademii Nauk 391, 1–3 [DOI] [PubMed] [Google Scholar]
- Shchennikova AV, Shulga OA, Richard Immink R, Skryabin KG, Angenent GC. 2004. Identification and characterization of four chrysanthemum MADS-box genes, belonging to the APETALA1/FRUITFULL and SEPALLATA3 subfamilies. Plant Physiology 134, 1632–1641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwabe WW. 1955. Factors controlling flowering in the chrysanthemum V. De-vernalization in relation to high temperature and low light intensity treatments. Journal of Experimental Botany 6, 435–450 [Google Scholar]
- Shibata M. 1997. Studies on responses to temperature and photoperiod and breeding of spray type chrysanthemums with summer-to-autumn flowering. Bulletin of the National Research Institute of Vegetables, Ornamental Plants and Tea 12, 1–71 (In Japanese with English abstract) [Google Scholar]
- Simpson GG, Dean C. 2002. Arabidopsis, the rosetta stone of flowering time?. Science 296, 285–289 [DOI] [PubMed] [Google Scholar]
- Sumitomo K, Li T, Hisamatsu T. 2009. Gibberellin promotes flowering of chrysanthemum by upregulating CmFL, a chrysanthemum FLORICAULA/LEAFY homologous gene. Plant Science 176, 643–649 [Google Scholar]
- Tamaki S, Matsuo S, Wong HL, Yokoi S, Shimamoto K. 2007. Hd3a protein is a mobile flowering signal in rice. Science 316, 1033–1036 [DOI] [PubMed] [Google Scholar]
- Weigel D, Nilsson O. 2002. A developmental switch sufficient for flower initiation in diverse plants. Nature 377, 495–500 [DOI] [PubMed] [Google Scholar]
- Whealy CA, Nell TA, Barrett JE, Larson RA. 1987. . High temperature effects on growth and floral development of chrysanthemum. Journal of the American Society for Horticultural Science 112, 464–468 [Google Scholar]
- Wigge PA, Kim MC, Jaeger KE, Busch W, Schmid M, Lohmann JU, Weigel D. 2005. Integration of spatial and temporal information during floral induction in Arabidopsis. Science 309, 1056–1059 [DOI] [PubMed] [Google Scholar]
- Yoo SK, Chung KS, Kim J, Lee JH, Hong SM, Yoo SJ, Yoo SY, Lee JS, Ahn JH. 2005. CONSTANS activates SUPPRESSOR OF OVEREXPRESSION OF CONSTANS 1 through FLOWERING LOCUS T to promote flowering in Arabidopsis. Plant Physiology 139, 770–778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Fukai S, Goi M. 1998. Morphology of capitulum initiation and floret development of Dendrathema species native to Japan. Journal of the Japanese Society for Horticultural Science 67, 347–351 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.