Abstract
The fruit of melting-flesh peach (Prunus persica L. Batsch) cultivars produce high levels of ethylene caused by high expression of PpACS1 (an isogene of 1-aminocyclopropane-1-carboxylic acid synthase), resulting in rapid fruit softening at the late-ripening stage. In contrast, the fruit of stony hard peach cultivars do not soften and produce little ethylene due to low expression of PpACS1. To elucidate the mechanism for suppressing PpACS1 expression in stony hard peaches, a microarray analysis was performed. Several genes that displayed similar expression patterns as PpACS1 were identified and shown to be indole-3-acetic acid (IAA)-inducible genes (Aux/IAA, SAUR). That is, expression of IAA-inducible genes increased at the late-ripening stage in melting flesh peaches; however, these transcripts were low in mature fruit of stony hard peaches. The IAA concentration increased suddenly just before harvest time in melting flesh peaches exactly coinciding with system 2 ethylene production. In contrast, the IAA concentration did not increase in stony hard peaches. Application of 1-naphthalene acetic acid, a synthetic auxin, to stony hard peaches induced a high level of PpACS1 expression, a large amount of ethylene production and softening. Application of an anti-auxin, α-(phenylethyl-2-one)-IAA, to melting flesh peaches reduced levels of PpACS1 expression and ethylene production. These observations indicate that suppression of PpACS1 expression at the late-ripening stage of stony hard peach may result from a low level of IAA and that a high concentration of IAA is required to generate a large amount of system 2 ethylene in peaches.
Key words: ACC synthase, auxin, ethylene, softening, stony hard peach.
Introduction
Peach (Prunus persica L. Batsch), undergoes textural changes that lead to loss of tissue firmness during ripening accompanied by an increase in ethylene production. Ethylene is biosynthesized by two successive reactions from S-adenosyl-l-methionine (SAM) via 1-aminocyclopropane-1-carboxylic acid (ACC) (Adams and Yang, 1979). The first reaction is catalysed by ACC synthase (ACS), and the second by ACC oxidase (ACO). Both reactions are generally considered as rate limiting in the ethylene biosynthetic pathway. ACS and ACO are encoded by members of multigene families (Kende, 1993).
During peach fruit ripening, the amounts of PpACS1 and PpACO1 transcripts and protein increase significantly, and the enzymes are responsible for the large amount of ethylene production during ripening (Callahan et al., 1992; Lester et al., 1994; Tonutti et al., 1997). Furthermore, these enzymes are also induced in wounded tissues such as leaves and pre-climacteric fruit (Mathooko et al., 2001; Tatsuki et al., 2006). PpACS2 mRNA was also induced rapidly by wounding followed by a decline in its expression, suggesting that this transcript might be negatively regulated by ethylene (Tatsuki et al., 2006).
Peach cultivars are usually classified as melting flesh or non-melting flesh based on their fruit firmness and texture. In melting flesh peaches, rapid softening occurs after harvest, resulting in fruit with a short shelf life. In non-melting flesh peaches, softening is slow and a significant reduction in flesh firmness does not occur. The differences in softening between melting flesh and non-melting flesh cultivars are attributed to the presence of endo-polygalacturonase (PG) activity during ripening (Pressey and Avants, 1978).
Stony hard peaches barely soften on the tree or after harvest, although the fruit change colour normally and contain high amounts of soluble solids (Haji et al., 2001). Genetic analysis indicated that stony hard (hd) is a recessive locus (Yoshida, 1976) and is different from the melting (M)/non-melting (m) locus (Haji et al., 2005). The low level of ethylene production by stony hard peach is responsible for the inhibition of fruit softening because exogenous ethylene induces the expression of some genes encoding cell-wall modification enzymes, e.g. PG (Hayama et al., 2003, 2006; Murayama et al., 2009). Because the fruit softens more rapidly when the applied ethylene concentration is higher, the ethylene concentration is an important factor determining the rate of softening in stony hard peaches (Hayama et al., 2006). Ethylene production occurs and the fruit softens with the application of ACC, a precursor of ethylene. These results indicate that ACC oxidase activity and ethylene sensing are not limiting in stony hard peaches (Haji et al., 2003).
This study group has previously shown that in stony hard peaches, PpACS1 mRNA was not induced during the ripening stage. However, PpACS1 mRNA was induced normally in senescing flowers, wounded leaves, and wounded immature fruit of stony hard peaches (Tatsuki et al., 2006). Furthermore, Begheldo et al. (2008) reported that during low temperature storage, ethylene production in stony hard peaches increased and fruit softened. Because increased levels of PpACS1 were detected in these fruit, low temperature conditions must have induced PpACS1 transcription. These reports indicated that in stony hard peaches, PpACS1 is induced not only at the ripening stage, but also in other tissues, such as those receiving some stress or in some senescing tissues.
This study sought to elucidate the mechanism for suppressing PpACS1 expression in stony hard peaches at the ripening stage. Genes that had similar expression patterns to PpACS1 in the late-ripening stage of melting flesh and mature fruit of stony hard peaches were identified using a microarray approach. Changes in auxin concentration and ethylene production during peach fruit development and ripening were also examined and correlations between auxin levels, ethylene production, and fruit softening at the late-ripening stage of peach is reported.
Materials and methods
Plant materials and treatments
Sampling during fruit development and ripening
Plants of Prunus persica L. Batsch ‘Akatsuki’, ‘Manami’ and ‘Odoroki’ were grown at the National Institute of Fruit Tree Science. ‘Akatsuki’ and ‘Manami’ fruit were sampled during fruit development and ripening. Whole fruit at 14 days after full bloom (DAB) of ‘Akatsuki’ and 11 DAB of ‘Manami’, and pericarp tissues of 21 and 31 DAB of ‘Akatsuki’ and 22 and 32 DAB of ‘Manami’, and mesocarp tissues of the other fruit of both cultivars were sampled. Whole fruit were frozen without cutting, tissue samples were cut into 3-mm cubes and portions were frozen in bulk with liquid nitrogen until use for RNA extraction and measurements for indole-3-acetic acid (IAA) concentrations. The frozen whole young fruit were crushed with a wooden hammer to obtain subsamples for RNA extraction and IAA concentration measurements. Fruit of ‘Odoroki’ was harvested at commercial maturity, and mesocarp tissues were used for measurements of IAA concentrations.
In this study, the same sampling days could not used for ‘Akatsuki’ and ‘Manami’. Therefore, for the statistical analysis of data comparing the two cultivars, the closest time point after full bloom was used between ‘Akatuki’ and ‘Manami’, e.g. ‘Akatsuki’ 14 DAB and ‘Manami’ 11 DAB; ‘Akatsuki’ 21 DAB and ‘Manami’ 22 DAB; ‘Akatsuki’ 31 DAB and ‘Manami’ 32 DAB.
Ethylene, 1-naphthalene acetic acid, and anti-auxin treatments of whole fruit
‘Manami’ and ‘Odoroki’ (stony hard peaches) were harvested from Fukushima and Nagano Prefectures, respectively, and were used at the mature stage and used for ethylene and 1-naphthalene acetic acid NAA treatment. For ethylene treatments, ‘Manami’ and ‘Odoroki’ fruit were placed in 78-l containers and ventilated with continuous flow air or air containing 20 µl l–1 ethylene at 25 °C. Immature ‘Akatsuki’ fruit (88 DAB) were used for NAA treatments. Fruit was sprayed with NAA containing 0.01 % (w/v) Tween 20 every day and stored at 25 °C. Control fruit was sprayed with 0.01 % (w/v) Tween 20 without NAA.
For anti-auxin treatments, ‘Akatsuki’ fruit on the tree was twice sprayed with 1mM α-(phenylethyl-2-one)-IAA (PEO-IAA) (Hayashi et al., 2008, 2012) containing 0.1 % Tween 20 at 91 and 102 DAB, and harvested at 105 DAB. PEO-IAA was dissolved in DMSO as a 200mM stock solution and diluted with water to 1mM; the final concentration of DMSO was 0.5 % (v/v). For the control, fruit was sprayed with 0.5 % (v/v) DMSO and 0.1 % (w/v) Tween 20.
Auxin and anti-auxin treatments of mesocarp discs
For auxin and anti-auxin treatment of mesocarp discs, mature ‘Manami’ fruit and ‘Akatsuki’ mature fruit but slightly firm (firmness about 40 N) that produced ethylene at 0.6–0.9 nl (g freshweight)–1 h–1 were used. Tissue cylinders (9mm in diameter) were excised from ‘Manami’ and ‘Akatsuki’ (using 20 fruits of each cultivar) with a cork borer, 5-mm thick discs were cut with a razor blade from the cylinders, and 42 discs were used for each treatment. The prepared discs were placed on paper filters soaked with auxin (0.5mM IAA, 0.5mM 2,4-dichlorophenoxyacetic acid, or 0.5mM NAA) or anti-auxin (100 µM of PEO-IAA or 100 µM N-propyl-PEO-IAA, a negative control for PEO-IAA), and stored at 28 °C for 18 or 6h, respectively. The control was treated with an aqueous solution of 0.05 % (v/v) DMSO.
Ethylene production and flesh firmness
To measure the ethylene content of whole fruit, each fruit was placed in a 0.49-l or 1.25-l air-tight glass chamber for 1h at 25 °C. To measure the ethylene content in a mesocarp disk, a single disk was placed in 5.52-ml glass vial for 15min at 25 °C. One ml of headspace gas was withdrawn from the chamber for each measurement and injected into a gas chromatograph (model GC-2014, Shimadzu, Kyoto, Japan) equipped with an activated alumina column and flame ionization detectors. The rate of ethylene production was expressed as nl (g freshweight)–1 h–1. Firmness was determined on opposite sides of each fruit using a penetrometer (Italtest, FT011, 8-mm diameter) and expressed in newtons (N).
Measurement of IAA in peach mesocarp tissues
Extraction and purification of IAA were carried out as previously reported (Edlund et al., 1995; Zourelidou et al., 2009) with slight modifications as follows. Frozen samples were ground in liquid nitrogen, 50mg freshweight of powdered sample was mixed with 1ml of 80% methanol containing 1% acetic acid (v/v), and 2ng of [13C6]-IAA was added as an internal standard (Cambridge Isotope Laboratories, Andover, MA, USA). The sample was extracted for 2h at 4 °C under continuous shaking. After centrifugation (10,000 g, 5min), the supernatant was collected and concentrated in vacuo. The sample was resuspended in 1ml 0.01M HCl and slurried for 10 min at 4 °C under continuous shaking with 15mg Amberlite XAD-7HP (Organo, Tokyo, Japan). After removal of the supernatant, the XAD-7HP was washed twice with 1% acetic acid. Samples were then extracted with CH2Cl2 (once with 400 µl and twice with 200 µl), and the combined CH2Cl2 fraction was passed through a 0.2 µm filter. After concentration in vacuo, the sample was analysed by GC-MS or GC-selected ion monitoring. Three independent samples were extracted and analysed.
The GC-MS analyses were carried out under the following conditions. Samples were trimethylsilylated with N-methyl-N-trimethylsilyltrifluoroacetamide (GL Sciences, Tokyo, Japan) at 80 °C for 30min. After conversion to the trimethylsilyl (TMSi) derivatives, the samples were analysed with a mass spectrometer (model JMS-Q1000GCMK II, Jeol) connected to a gas chromatograph (model 7890A, Agilent). The analytical conditions were as follows: GC-column, DB-5 column (0.25mm × 30 m, 0.25 µm film thickness, J&W Scientific, Folsom, CA, USA); injection temperature, 250 °C; carrier gas, helium at flow rate 1ml min–1; ionization, EI (70eV); source temperature, 250 °C; column temperature program, 80 °C for 1min, then raised to 245 °C at a rate of 30 °C min–1 and to 280 °C min–1 at a rate of 5 °C; interface temperature, 250°C; splitless injection. The mass spectrum of IAA after derivatization showed two peaks that contained ions at m/s 202 and m/s 319, corresponding to the base peak and molecular ion of IAA-TMSi (Kobayashi et al., 1989; Wijayanty et al., 1995). The typical retention time was 7.50min. On the other hand, the spectrum of [13C6]IAA after derivatization showed two peaks that contained ions at m/s 208 and m/s 325, corresponding to the base peak and molecular ion of [13C6]IAA-TMSi. The typical retention time was 7.50min, the same retention time as IAA-TMSi. The ions monitored in the GC-selected ion monitoring analysis were follows: m/s 202 and m/s 319 for IAA-TMSi; and m/s 208 and m/s 325 for [13C6]IAA-TMSi. The endogenous levels of IAA were determined as the ratio of the peak areas of the prominent ions, m/s 202 for the endogenous ion, and m/s 208 for the internal standard.
Construction of a peach oligo-DNA microarray
The microarray was constructed using the eArray system (Agilent, https://earray.chem.agilent.com/earray/) according to the system protocol. Probes (60 oligonucleotides per probe) for the peach oligo-DNA microarray were constructed using DNA sequence data from Prunus Unigene v4 (Genome Database for Rosaceae: GDR http://www.rosaceae.org/) (20,654 genes) and this study’s isolated genes (659 genes), including 43,803 probes (21,313 independent genes, CDS and EST). Probes (64.8 %) were functionally annotated with other plants, predominantly Arabidopsis thaliana, and found to be involved putatively in cell-wall metabolism and fatty acid biosynthesis, plant hormone-related processes, transcriptional regulation, and other physiological processes. The remaining probes (35.2 %) were hypothetical or functionally unknown.
RNA extraction, real-time RT-PCR, and microarray analysis
Total RNA was extracted from frozen samples by the hot borate method (Wan and Wilkins, 1994). Preparation of first-strand cDNA and real-time reverse-transcription PCR (real-time RT-PCR) were performed as described before (Tatsuki et al., 2011) using a set of primers designed by Primer Express version 3.0 (Applied Biosystems) for each amplified gene (Supplementary Table S1, available at JXB online). Real-time RT-PCR was conducted with RNA samples isolated independently from two tissue sources, and each tissue source was assayed in triplicate.
For microarray, total RNA (250ng) was labelled with Cy3 according to the instructions for the Quick Amp Labeling Kit (Agilent, Palo Alto, CA, USA). Labelled cRNA was purified using the RNeasy Mini Kit (Qiagen, Hilden, Germany). Hybridization and washing were performed according to Agilent’s instructions. Glass slides were hybridized overnight at 65 °C in a hybridization buffer containing a portion of Cy3-labelled cRNA. After hybridization, slides were washed in Agilent Gene Expression Wash Buffer 1 with 0.005% Triton X-102 for 1min at room temperature and Agilent Gene Expression Wash Buffer 2 with 0.005% Triton X-102 for 1min at 37 °C. After drying the slides, hybridized slides were scanned with a G2565A microarray scanner and the images were extracted using Feature Extraction version 9.5 (Agilent). Data analysis was carried out using GENESPRING GX 10.0 (Agilent). Data normalization was carried out according to the manufacturer’s recommended protocol (Agilent FE for 1 Color).
Results
Screening for genes with similar expression pattern with PpACS1 by microarray analysis
A peach oligo-DNA microarray was constructed using DNA sequence data from Prunus Unigene v4 (Genome Database for Rosaceae: GDR) and this study’s isolated genes. Microarray analysis was carried out using melting peach ‘Akatsuki’ fruit and stony hard peach ‘Manami’ and ‘Odoroki’ fruit. ‘Akatsuki’ fruit were sampled at 92, 98, 104, and 106 DAB; ‘Manami’ and ‘Odoroki’ were harvested at commercial maturity and treated with air or 5000 µl l–1 propylene for 3 days (Tatsuki et al., 2006).
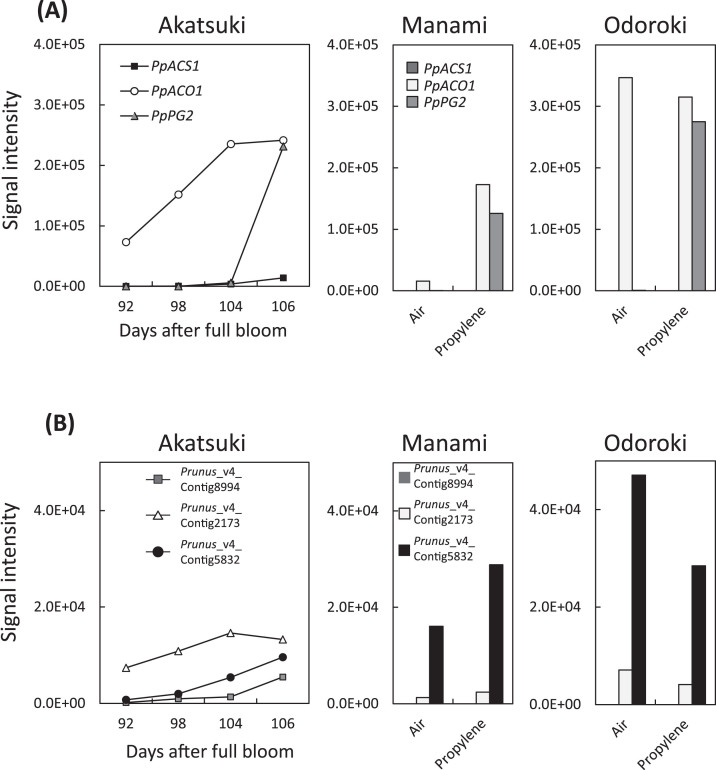
In ‘Akatsuki’, the expression level of PpACS1 increased towards harvest time along with other ripening-related genes (PpACO1, PpPG2) (Fig. 1A). In contrast, the expression level of PpACS1 in stony hard peaches stayed low and did not increase after propylene treatment, although high levels of PpACO1 and PpPG2 expression were detected in propylene-treated stony hard peaches (Fig. 1A).
Fig. 1.
Signal intensity of oligo-DNA microarray analyses of (A) PpACS1, PpACO1, and PpPG2, and (B) IAA-related genes (Prunus_v4_Contig8994, Prunus_v4_Contig2173, Prunus_v4_Contig5832). ‘Akatsuki’ fruit were sampled at 92, 98, 104, and 106 days after full bloom; ‘Manami’ and ‘Odoroki’ were harvested at commercial maturity and treated with air or 5000 µl–1 propylene for 3 days. Signal intensity was normalized by the average of control values calculated by Future Extraction version 10. All genes were passed through a filter based on variances calculated by the cross-gene error model (P < 0.05) in GENESPRING, which performs a variance components analysis for the accurate comparison of mean expression levels between experimental conditions.
In the first step, 652 genes showing a similar expression pattern to PpACS1 in the microarray data for melting flesh peach were selected by the program function of ‘find similar genes’ (minimum Pearson correlation value = 0.85 and raw expression value >1000). Next, the selected 652 genes were filtered to identify genes with less than a 2-fold change between air and propylene treatments of stony hard peaches. Finally, 29 genes were identified to have a similar expression pattern as PpACS1 (Supplementary Table S2). The function of 19 genes of the 29 selected genes was unknown, but the remaining 10 genes have putative gene annotations including IAA-induced protein and gibberellin 2-beta-dioxygenase. Interestingly, IAA-related genes such as the Aux/IAA genes (Chapman and Estelle, 2007) were redundant in this gene list. The patterns of microarray signal intensity for three IAA-related genes (Prunus_v4_Contig8994, Prunus_v4_Contig2173, Prunus_v4_Contig5832) were similar to that of PpACS1 in melting flesh peaches (Fig. 1B). The expression level of Prunus_v4_Contig8994 stayed low throughout fruit development and did not increase after propylene treatment. The expression levels of Prunus_v4_Contig2173 and Prunus_v4_Contig5832 did not increase after propylene treatment, a result similar to that for PpACO1 and PpPG2.
Therefore, this study re-analysed the microarray expression patterns of 13 additional IAA-related genes from the peach oligo-DNA microarray probes (Supplementary Table S3). Among these genes, not only Aux/IAA genes, but also SAUR-like genes (Hagen and Guilfoyle, 2002) were included. The signal intensity of some of these genes increased towards harvest time in ‘Akatsuki’ and remained at a low level in stony hard peaches (Supplementary Table S3). Thus, IAA-related genes seemed to associate with the expression of PpACS1.
Measurements of IAA content during peach fruit development and ripening
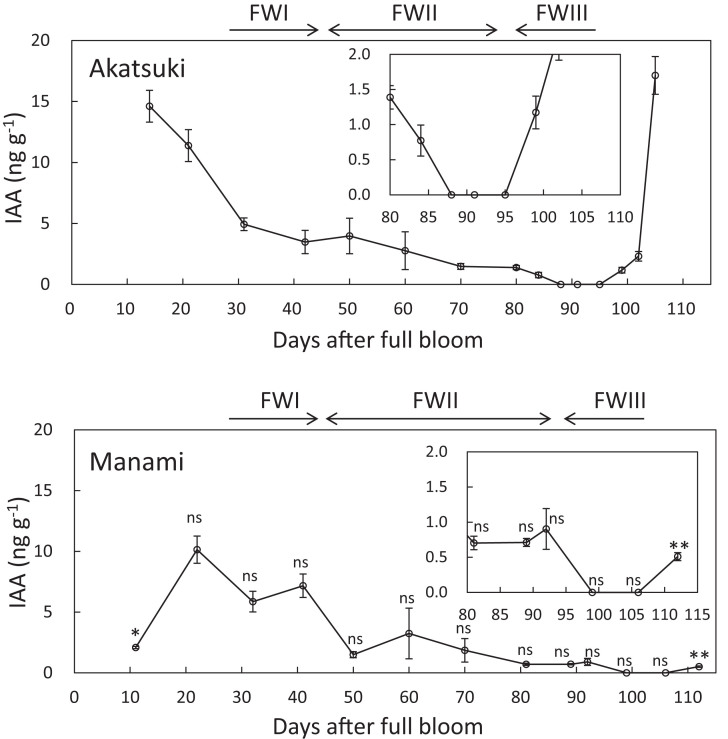
To monitor the changes in IAA concentration during fruit development and ripening in both cultivars, ‘Akatsuki’ and ‘Manami’ fruits were sampled about every 10 days (Fig. 2). The IAA concentration was high in young fruit and then gradually decreased during fruit development, reaching the lowest levels just before the onset of the climacteric rise in both cultivars. After that, in ‘Akatsuki’, IAA levels sharply increased at the late-ripening stage. In contrast, in ‘Manami’ fruit, IAA levels did not increase; the final IAA concentration was 0.5ng (g freshweight)–1. In the fruit of another stony hard cultivar, ‘Odoroki’, the IAA concentration was also low in mature fruit: 0.377±0.131ng (g freshweight)–1.
Fig. 2.
IAA concentrations of melting cultivar ‘Akatsuki’ and stony hard cultivar ‘Manami’. Insets show different scales of graphs. Horizontal arrows above the graphs indicate growth stages: FWI, cell division and enlargement; FWII, period of slow growth (pit hardening stage); FWIII, second period of exponential growth. Data are means ± SE of three individual experiments. Asterisks indicate statistically significant differences compared with ‘Akatsuki’ at a similar stage in development (days after full bloom) using Student’s t-test (*P < 0.05, **P < 0.01). ns indicates that there were no significant differences compared with ‘Akatsuki’.
Ethylene production and softening before harvest
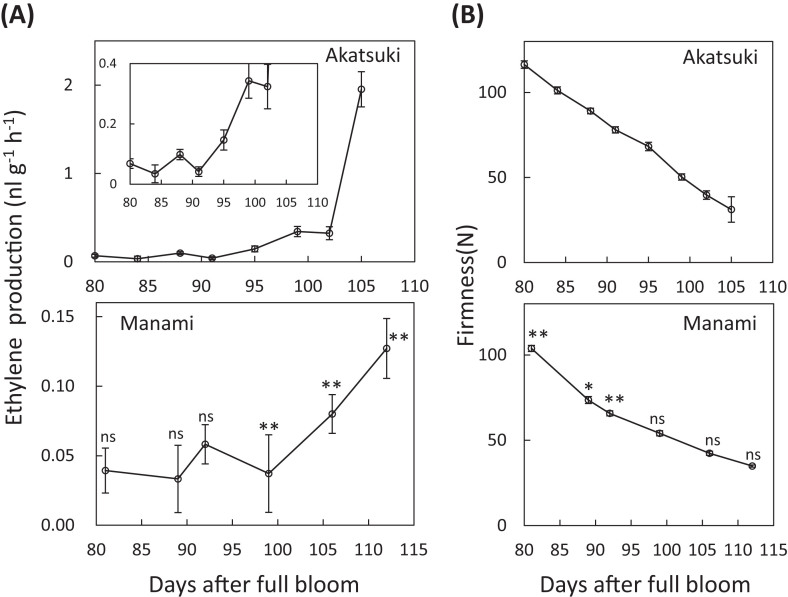
Ethylene production and fruit firmness were measured from the end of pit hardening to harvest (Fig. 3). Ethylene production by ‘Akatsuki’ increased transiently at 88 DAB, decreased at 91 DAB, and sharply increased just before harvest (Fig. 3A). In ‘Manami’, ethylene production increased transiently at 92 DAB. After a decrease at 99 DAB, ethylene production gradually increased; however, the maximum ethylene level attained was much lower than that of ‘Akatsuki’ (Fig. 3A). Flesh firmness decreased linearly in ‘Akatsuki’ (slope = –3.4109, R 2 = 0.9975) between 80 and 105 DAB), but it decreased moderately in ‘Manami’ (slope = –1.6662, R 2 = 0.9916) between 89 and 112 DAB] (Fig. 3B). Although ‘Akatsuki’ fruit is softer on the tree, ‘Manami’ fruit does not continue to soften (data not shown).
Fig. 3.
(A) Ethylene production by ‘Akatsuki’ (insets show different scales of graphs) and ‘Manami’ fruit from completed pit hardening to harvest. (B) Flesh firmness of ‘Akatsuki’ and ‘Manami’. Samples are the same as in Fig. 1. Data are means ± SE of three individual experiments. Asterisks indicate statistically significant differences compared with ‘Akatsuki’ at a similar stage in development (days after full bloom) using Student’s t-test (*P < 0.05, **P < 0.01). ns indicates that there were no significant differences compared with ‘Akatsuki’.
Expression analysis of ethylene biosynthetic enzymes during fruit ripening
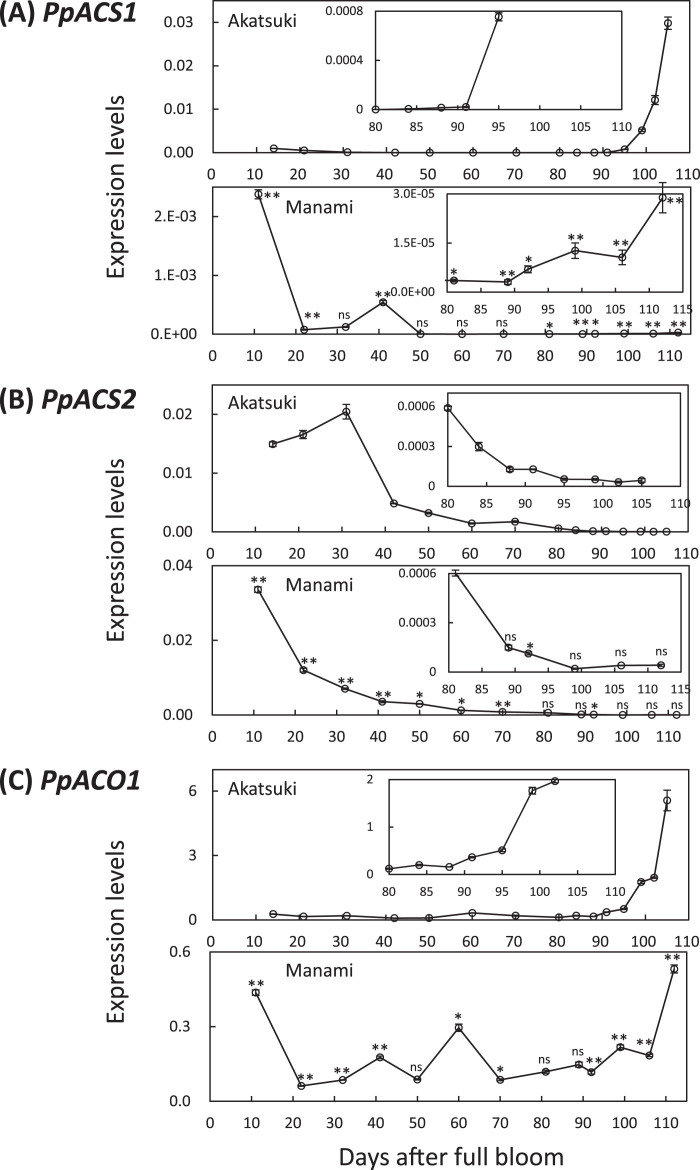
In ‘Akatsuki’, PpACS1 expression was low during fruit development, increased gradually after 80 DAB, and then increased suddenly after 91 DAB to harvest (Fig. 4A). In ‘Manami’, PpACS1 expression levels were high at 11 DAB, declined rapidly by 20 DAB, increased slightly at 41 DAB, declined again, then were maintained at a low level until 90 DAB when it increased a little after that (Fig. 4A). The expression level of PpACS2 was high in young fruit of both cultivars, decreased gradually during fruit development, and reached its lowest level just before optimal maturity (Fig. 4B). Expression levels of PpACO1 were higher 60 DAB in the fruit of both cultivars (Fig. 4C). After pit hardening (80 DAB), PpACO1 expression increased gradually and then increased suddenly after 95 DAB in ‘Akatsuki’ (Fig. 4C). After pit hardening in ‘Manami’, the expression pattern of PpACO1 (Fig. 4C) was similar to that of PpACS1 (Fig. 4A).
Fig. 4.
Relative transcript abundance of (A) PpACS1, (B) PpACS2 and (C) PpACO1, in ‘Akatsuki’ (upper) and in ‘Manami’ (lower). Insets show different scales of graphs. The steady-state levels were normalized to actin. Data are means ± SE of two individual experiments, each performed in triplicate. Asterisks indicate statistically significant differences compared with ‘Akatsuki’ at a similar stage in development (days after full bloom) using Student’s t-test (*P < 0.05, **P < 0.01). ns indicates that there were no significant differences compared with ‘Akatsuki’.
The effects of exogenous auxin on peach fruit ripening
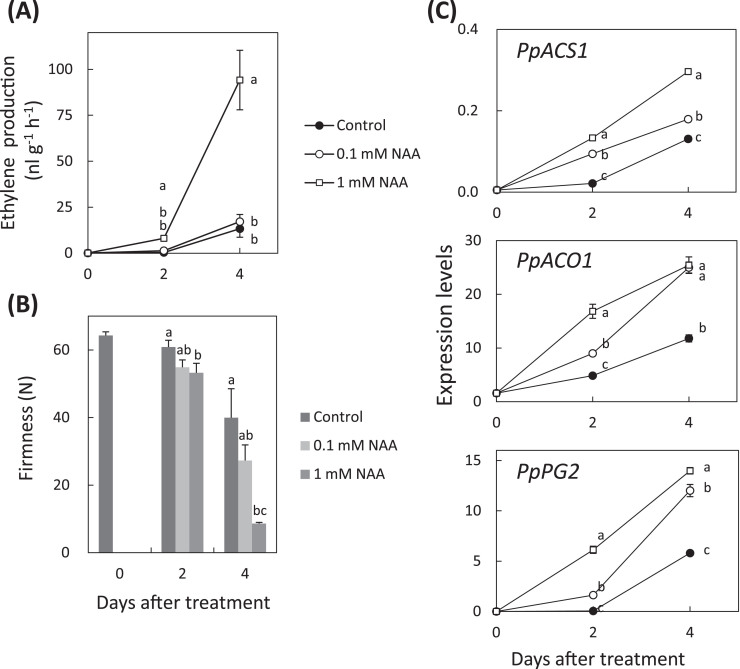
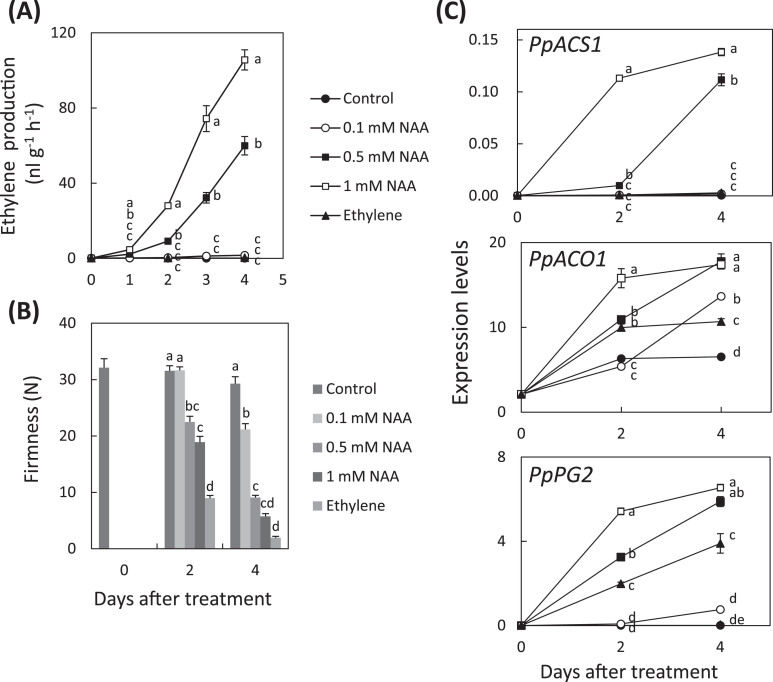
To understand the effect of auxin on ethylene biosynthesis and flesh softening, immature ‘Akatsuki’ fruit that were harvested just before the climacteric rise were treated with a synthetic auxin, NAA. In 1mM NAA-treated fruit, ethylene production was larger and fruit softened faster than in control fruit (Fig. 5A, B). In NAA-treated fruit, expression of PpACS1, PpACO1, and a gene for a cell-wall modification enzyme, PpPG2, were higher than in control fruit (Fig. 5C). Next, ‘Manami’ fruit were treated with NAA and ethylene (Fig. 6). The fruit treated with 0.5mM and 1mM NAA produced abundant ethylene (Fig. 6A). All NAA-treated and ethylene-treated fruit softened (Fig. 6B). The expression level of PpACS1 was higher in both 0.5 and 1mM NAA-treated ‘Manami’ fruit, but expression was not induced in 0.1mM NAA-treated fruit or by ethylene treatment (Fig. 6C). The expression level of PpACO1 increased during storage and was higher in all NAA-treated and ethylene-treated fruit than control fruit at 4 days after treatment (Fig. 6C). The expression level of PpPG2 did not increase in control fruit; however, expression increased in all NAA- and ethylene-treated fruit (Fig. 6C). Similar results for ethylene production, fruit softening, and expression patterns of these genes were obtained in NAA- and ethylene-treated ‘Odoroki’ fruit (Supplementary Fig. S1).
Fig. 5.
Effects of NAA on ‘Akatsuki’ fruit ripening and gene expression. (A) Ethylene production, (B) flesh firmness of NAA-treated ‘Akatsuki’ fruit. Harvested immature fruit of ‘Akatsuki’ were treated with 0.1mM or 1mM NAA each day. Data are means ± SE (n = 10). (C) Relative transcript abundance of PpACS1, PpACO1, and PpPG2. The steady-state levels were normalized to actin. Data are means ± SE of three individual experiments, each performed in triplicate. Different letters indicate significant differences within the same day at P < 0.05 using Tukey’s post hoc test.
Fig. 6.
Effects of NAA on ‘Manami’ fruit ripening and gene expression. (A) Ethylene production. (B) Flesh firmness of NAA-treated ‘Manami’ fruit. Harvested mature fruit of ‘Manami’ were treated with 0.1mM, 0.5mM or 1mM NAA each day or continuous 20 µL–1 ethylene. Data are means ± SE (n = 10). (C) Relative transcript abundance of PpACS1, PpACO1, and PpPG2. The steady-state levels were normalized to actin. Data are means ± SE of three individual experiments, each performed in triplicate. Different letters indicate significant differences within the same day at P < 0.05 using Tukey’s post hoc test.
Effects of auxin on ‘Manami’ fruit disks
The effect of some auxins on ethylene production and expression patterns of ripening-related genes of stony hard peach was examined. Disks were treated with IAA, 2,4-dichlorophenoxyacetic acid, or NAA. Ethylene production increased in all auxin-treated disks and was most abundant in NAA-treated disks (Supplementary Fig. S2A). Expression levels of PpACS1 and PpPG2 were higher in all auxin-treated disks and were highest in the NAA-treated disks. PpACO1 was only slightly induced by these auxin treatments (Supplementary Fig. S2B).
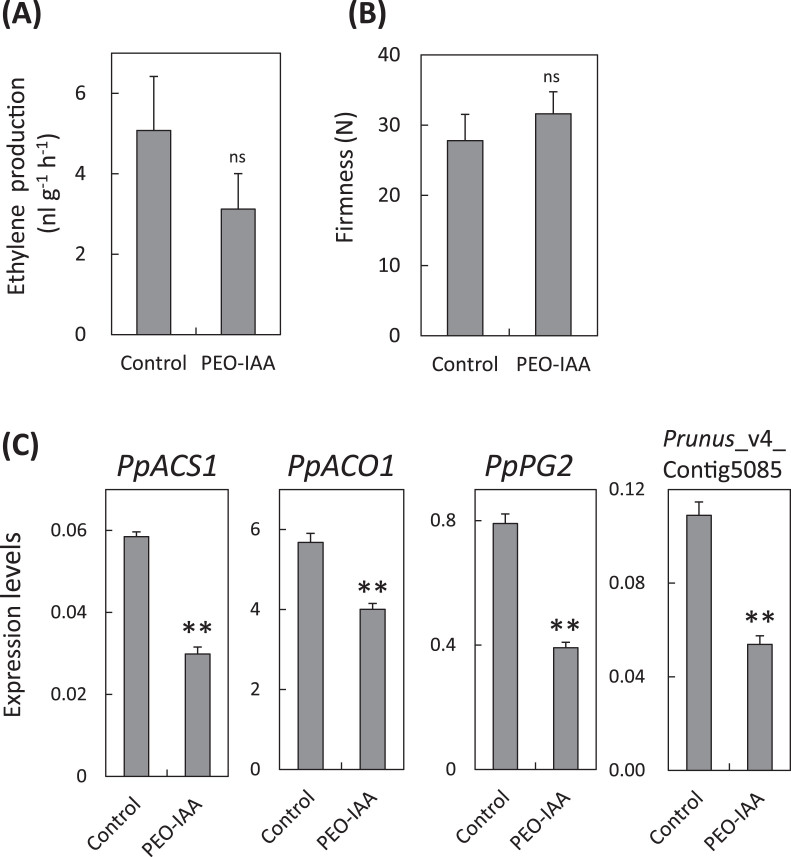
Effects of anti-auxin on ‘Akatsuki’ fruit
PEO-IAA is an effective auxin blocker that specifically binds to the auxin-binding site of TIR/AFB receptors and influences plant growth and developmental processes that are regulated by auxin (Hayashi et al., 2008, 2012). PEO-IAA treatments were performed twice, at 14 and 3 days before harvest (91 and 102 DAB). Although ethylene production was lower in PEO-IAA-treated fruit than control fruit, and flesh firmness was higher in PEO-IAA-treated fruit than control fruit, the data are not significantly different (Fig. 7A, B). Expression of PpACS1, PpACO1, and PpPG2 were lower in PEO-treated fruit than in control fruit (Fig. 7C). Prunus_v4 Contig 5085 is the peach homologue of one of the indole-3-acetic acid inducible genes and was used as an indicator of endogenous auxin responsive genes. Expression of Prunus_v4 Contig 5085 was also lower in PEO-IAA-treated fruit than in control fruit (Fig. 7C).
Fig. 7.
Effect of PEO-IAA on fruit ripening and expression patterns of ripening-related genes of ‘Akatsuki’ at harvest. (A, B) Ethylene production (A) and flesh firmness (B) of ‘Akatsuki’ fruit treated with PEO-IAA. Fruit on the tree was twice sprayed with 1mM α-(phenylethyl-2-one)-IAA (PEO-IAA) at 91 and 99 DAB, and harvested at 105 DAB. Data are means ± SE (n = 34). (C) Relative transcript abundance of PpACS1, PpACO1, PpPG2, and peach IAA-related gene (Prunus_v4_Contig5085), in ‘Akatsuki’ fruit that were the same samples used for measurements in (A). Data are means ± SE of three individual experiments, each performed in triplicate. Asterisks indicate statically significant differences between the control and PEO-IAA using Student’s t-test (P < 0.01). ns indicates that there were no significant differences compared with the control.
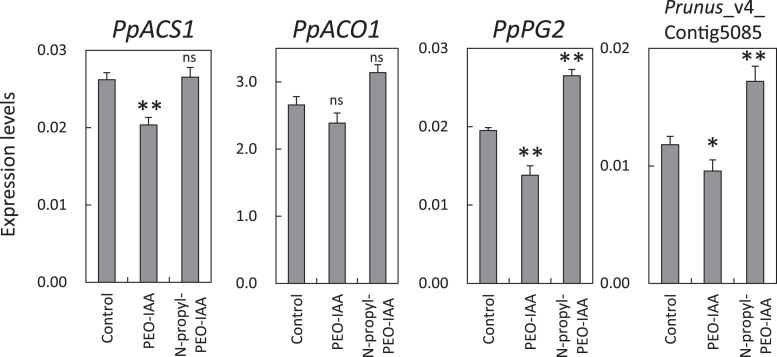
Mesocarp disks prepared from ‘Akatuski’ fruit were slightly firm (firmness about 40 N) and produced ethylene at 0.6–0.9 nl (g freshweight)–1 h–1. Disks were treated with 100 µM PEO-IAA or 100 µM N-propyl-PEO-IAA, a negative control for PEO-IAA (Hayashi et al., 2012). Expression of PpACS1 and PpPG2 were lower in PEO-IAA-treated disks than in the control and N-propyl-PEO-IAA treated discs (Fig. 8). Expression of Prunus_v4 Contig 5085 was slightly lower in PEO-IAA-treated disks than in control disks (Fig. 8). There was little difference in the expression of PpACO1 between the control and PEO-IAA-treated disks (Fig. 8).
Fig. 8.
Relative transcript abundance of PpACS1, PpACO1, PpPG2, and a peach IAA-related gene (Prunus_v4_Contig5085) in mesocarp disks of ‘Akatsuki’ treated with 100 µM of PEO-IAA or 100 µM N-propyl-PEO-IAA. Data are means ± SE of three individual experiments, each performed in triplicate. Asterisks indicate statically significant differences compared with the control using Student’s t-test (*P < 0.05, **P < 0.01). ns indicates that there were no significant differences compared with the control.
Discussion
In several plant organs, several ACS isogenes have been identified that are induced by exogenous auxin treatment (Kende, 1993; Zarembinski and Theologis, 1994). Whether these ACS isogenes are really induced by auxin in vivo or not has remained unknown.
In peach, several reports have indicated a possible relationship of auxin with fruit development and ripening (Miller et al., 1987; Agusti et al., 1999; Ohmiya, 2000). Peach fruit displays a double sigmoidal growth curve and this pattern of growth is customarily divided into three stages: FWI, cell division and enlargement; FWII, period of slow growth (pit hardening stage); FWIII, second period of exponential growth. IAA concentrations in peach are relatively high during FWI and FWIII and their lowest levels are during FWII (Miller et al., 1987; Ohmiya, 2000). The IAA concentration during FWI and FWII were assumed to effect the enlargement of fruit mesocarp (Ohmiya, 2000). Tonutti et al. (1991) demonstrated that the peak of ethylene production occurred at the late stage of peach fruit development, concurrently with an increase in mesocarp IAA concentration, suggesting that modulation of ethylene biosynthesis may be regulated by auxin.
Previous studies have shown that application of synthetic auxin enhances peach fruit development, ripening, and ethylene production (Agusti et al., 1999; Ohmiya, 2000; Ohmiya and Haji, 2002). Furthermore, Trainotti et al. (2007) demonstrated that the expression of auxin-related genes increased during ripening and the existence of important cross-talk between auxin and ethylene using a genomic approach. In this study, the IAA concentration in mesocarp tissues of stony hard peach cultivars of ‘Manami’ and ‘Odoroki’ were low and did not increase at the climacteric stage, although IAA levels significantly increased in the melting flesh cultivar, ‘Akatsuki’ (Fig. 2). Based on previous work and this study group’s own reports, the suppressed expression of PpACS1 at the late-ripening stage of stony hard peach could result from low levels of IAA. Similar results were reported by El-Sharkawy et al. (2010); treatment of fruit from the late ripening Japanese plum cultivar ‘Shiro’ with auxin significantly accelerated auxin-induced ethylene production, suggesting that a scarcity of auxin might affect the levels of autocatalytic ethylene production in ‘Shiro’. The endogenous IAA concentration was, however, not measured in the El-Sharkawy et al. (2010) study.
In the experiments of the anti-auxin treatment of fruit on the tree, although the expression levels of genes were suppressed by anti-auxin, there were no significant differences in ethylene or firmness at harvest. One possible reason for these results is that treatments were given at the immature stage when there were some differences in the ripening stage among the fruit and some differences among the individual fruit. In this study, anti-auxin treatments were given at 91 DAB (low level of IAA) and 102 DAB (start of increase of IAA). To date, the stage that is most effective for applying an inhibitor to inhibit auxin and ethylene production remains unknown.
Because there were few differences in the IAA concentration at the FWI and FWII stages in ‘Manami’ and ‘Akatsuki’ (Fig. 2), and also few differences in tree growth and development in stony hard peaches, melting flesh, and non-melting flesh peaches (data not shown), this study assumed that differences in IAA concentration occurred only at the fruit climacteric stage. However, the mechanism causing the low IAA concentration in stony hard peaches could only be inferred. Endogenous IAA is made either by de novo synthesis or by release from conjugates (Bartle, 1997). Low IAA concentrations result from the following: (1) the suppression of IAA biosynthesis; (2) the acceleration of IAA inactivation and degradation; (3) an imbalance in the transition from free IAA to IAA storage forms (IAA conjugates and indole-3-butyric acid); and (4) the inhibition of IAA transport from biosynthetic tissues. Further study will be required to demonstrate which possible mechanism functions.
Two systems of ethylene regulation have been proposed to operate during fruit development (Lelièvre et al., 1997). In this study, at the pre-climacteric stage of both cultivars, expression of PpACS2 decreased (Fig. 4B), whereas PpACS1 and PpACO1 expression started to slightly increase (Fig. 4A, C). These two genes were responsible for a small increase in ethylene production, assumed to be from system 1. In pre-climacteric fruit, the IAA concentration was at the lowest level, thus IAA concentration may not affect system 1 ethylene production. After system 1 ethylene production, the expression of PpACS1 and PpACO1 strongly increased concomitant with a significant increase in IAA concentration. These results indicated that a high concentration of IAA may be required to induce high levels of expression of these genes to result in large amounts of system 2 ethylene production.
Because stony hard peaches change colour normally and contain high levels of soluble solids without a large amount of ethylene production, a small amount of ethylene is sufficient to cause these ripening phenomena. Johnston et al. (2009) reported in apple that early ripening events showed a low dependency for ethylene; in contrast, later ripening events such as softening of the flesh showed a high dependency for ethylene but were less sensitive to low concentrations. In peach, flesh softening may be less sensitive and requires a high concentration of ethylene.
In conclusion, the IAA level in stony hard peaches is not high during the late-ripening stage, resulting in low ethylene production and inhibition of fruit softening. These findings show that stony hard peaches will be a good model for investigating the effect of auxin on fruit ripening and softening.
Supplementary material
Supplementary data are available at JXB online.
Supplementary Table S1. Sequences of the oligonucleotide primers used for real-time RT-PCR.
Supplementary Table S2. Pearson’s correlation coefficient (PCC) and signal intensity of 29 filtered genes with similar expression patterns for PpACS1.
Supplementary Table S3. Pearson’s correlation coefficient (PCC) and signal intensity of IAA related genes with similar expression patterns for PpACS1.
Supplementary Fig. S1. Ethylene production and flesh firmness of NAA- or ethylene-treated ‘Odoroki’ fruit.
Supplementary Fig. S2. Effect of some auxins on ethylene production and expression patterns of ripening-related genes of ‘Manami’.
Acknowledgements
The authors thank Dr H Yaegaki (National Institute of Fruit Tree Science) for his gift of ‘Odoroki’ fruit, and K. Amano (National Institute of Fruit Tree Science) for technical support. This work was supported by the Japan Society for the Promotion of Science [Grant-in-Aid for Young Scientists (B) no. 19780031 to MT, and Grant-in-Aid for Scientific Research (C) no. 22580044 to MT].
References
- Adams DO, Yang SF. 1979. Ethylene biosynthesis: identification of ACC as an intermediate in the conversion of methionine to ethylene. Proceedings of the National Academy of Sciences, USA 76, 170–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agusti M, Almela V, Andreu I, Juan M, Zacarias L. 1999. Synthetic auxin 3,5,6-TPA promotes fruit development and climacteric in Prunus persica L. Batsch. Journal of Horticultural Science and Biotechnology 74, 556–560 [Google Scholar]
- Bartle B. 1997. Auxin biosynthesis. Annual Review of Plant Physiology and Plant Molecular Biology 48, 51–66 [DOI] [PubMed] [Google Scholar]
- Begheldo M, Manganaris GA, Bonghi C, Tonutti P. 2008. Different postharvest conditions modulate ripening and ethylene biosynthetic and signal transduction pathways in stony hard peaches. Postharvest Biology and Technology 48, 84–91 [Google Scholar]
- Callahan AM, Morgens PH, Wright P, Nichols KE., Jr 1992. Comparison of pch313 (pTOM13 homolog) RNA accumulation during fruit softening and wounding of two phenotypically different peach cultivars. Plant Physiology 100, 482–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman EJ, Estelle M. 2007. Mechanism of auxin-regulated gene expression in plants. Annual Review of Genetics 43, 265–285 [DOI] [PubMed] [Google Scholar]
- Edlund A, Eklof S, Sundberg B, Moritz T, Sandberg G. 1995. A microscale technique for gas chromatography-mass spectrometry measurements of picogram amounts of indole-3-acetic acid in plant tissues. Plant Physiology 108, 1043–1047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Sharkawy I, Mila I, Bouzayen M, Jayasankar S. 2010. Regulation of two germin-like protein genes during plum fruit development. Journal of Experimental Botany 61, 1761–1770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagen G, Guilfoyle T. 2002. Auxin-responsive gene expression: genes, promoters, and regulatory factors. Plant Molecular Biology 49, 373–385 [PubMed] [Google Scholar]
- Haji T, Yaegaki H, Yamaguchi M. 2001. Changes in ethylene production and flesh firmness of melting, nonmelting and stony hard peaches after harvest. Journal of the Japanese Society for Horticultural Science 70, 458–459 [Google Scholar]
- Haji T, Yaegaki H, Yamaguchi M. 2003. Softening of stony hard peach by ethylene and the induction of endogenous ethylene by 1-aminocyclopropane-1-carboxylic acid (ACC). Journal of the Japanese Society for Horticultural Science 72, 212–217 [Google Scholar]
- Haji T, Yaegaki H, Yamaguchi M. 2005. Inheritance and expression of fruit texture melting, non-melting and stony hard in peach. Scientia Horticulturae 105, 241–248 [Google Scholar]
- Hayama H, Ito A, Moriguchi T, Kashimura Y. 2003. Identification of a new expansin gene closely associated with peach fruit softening. Postharvest Biology and Technology 29, 1–10 [Google Scholar]
- Hayama H, Tatsuki M, Ito A, Kashimura Y. 2006. Ethylene and fruit softening in the stony hard mutation in peach. Postharvest Biology and Technology 41, 16–21 [Google Scholar]
- Hayashi K, Neve J, Hirose M, Kuboki A, Shimada Y, Kepinski S, Nozaki H. 2012. Rational design of an auxin antagonist of the SCF (TIR1) auxin receptor complex. ACS Chemical Biology 7, 590–598 [DOI] [PubMed] [Google Scholar]
- Hayashi K, Tan X, Zheng N, Hatate T, Kimura Y, Kepinski S, Nozaki H. 2008. Small-molecule agonists and antagonists of F-box protein-substrate interactions in auxin perception and signaling. Proceedings of the National Academy of Sciences, USA 105, 5632–5637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston JW, Gunaseelan K, Pidakala P, Wang M, Schaffer RJ. 2009. Co-ordination of early and late ripening events in apples is regulated through differential sensitivities to ethylene. Journal of Experimental Botany 60, 2689–2699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kende H. 1993. Ethylene biosynthesis. Annual Review of Plant Physiology and Plant Molecular Biology 44, 283–307 [Google Scholar]
- Kobayashi M, Sakurai A, Saka H, Takahashi N. 1989. Fluctuation of the endogenous IAA level in rice during its life cycle. Agricultural and Biological Chemistry 53, 1089–1094 [Google Scholar]
- Lelièvre JM, Latche A, Jones B, Bouzayen M, Pech JC. 1997. Ethylene and fruit ripening. Physiologia plantarum 101, 727–739 [Google Scholar]
- Lester DR, Speirs J, Orr G, Brady CJ. 1994. Peach (Prunus persica) endopolygalacturonase cDNA isolation and mRNA analysis in melting and nonmelting peach cultivars. Plant Physiology 105, 225–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathooko FM, Tsunashima Y, Owino WZO, Kubo Y, Inaba A. 2001. Regulation of genes encoding ethylene biosynthetic enzymes in peach (Prunus persica L.) fruit by carbon dioxide and 1-methylcyclopropene. Postharvest Biology and Technology 21, 265–281 [Google Scholar]
- Miller AN, Walsh CS, Cohen JD. 1987. Measurement of indole-3-acetic acid in peach fruits (Prunus persica L. Batsch cv. Redhaven) during development. Plant Physiology 84, 491–494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murayama H, Arikawa M, Sasaki Y, Dal, Cin V, Mitsuhashi W, Toyomasu T. 2009. Effect of ethylene treatment on expression of polyuronide-modifying genes and solubilization of polyuronides during ripening in two peach cultivars having different softening characteristics. Postharvest Biology and Technology 52, 196–201 [Google Scholar]
- Ohmiya A. 2000. Effects of auxin on growth and ripening of mesocarp discs of peach fruit. Scientia Horticulturae 84, 309–319 [Google Scholar]
- Ohmiya A, Haji T. 2002. Promotion of ethylene biosynthesis in peach mesocarp discs by auxin. Plant Growth Regulation 36, 209–214 [Google Scholar]
- Pressey R, Avants JK. 1978. Difference in polygalacturonase composition of clingstone and freestone peaches. Journal of Food Science 43, 1415–1423 [Google Scholar]
- Tatsuki M, Haji T, Yamaguchi M. 2006. The involvement of 1-aminocyclopropane-1-carboxylic acid synthase isogene, Pp-ACS1, in peach fruit softening. Journal of Experimental Botany 57, 1281–1289 [DOI] [PubMed] [Google Scholar]
- Tatsuki M, Hayama H, Yoshioka H, Nakamura Y. 2011. Cold pre-treatment is effective for 1-MCP efficacy in ‘Tsugaru’ apple fruit. Postharvest Biology and Technology 62, 282–287 [Google Scholar]
- Tonutti P, Bonghi C, Ruperti B, Tornielli GB, Ramina A. 1997. Ethylene evolution and 1-aminocyclopropane-1-carboxylate oxidase gene expression during early development and ripening of peach fruit. Journal of the American Society of Horticultural Science 122, 642–647 [Google Scholar]
- Tonutti P, Casson P, Ramina A. 1991. Ethylene biosynthesis during peach fruit development. Journal of the American Society for Horticultural Science 116, 274–279 [Google Scholar]
- Trainotti L, Tadiello A, Casadoro G. 2007. The involvement of auxin in the ripening of climacteric fruits comes of age: the hormone plays a role of its own and has an intense interplay with ethylene in ripening peaches. Journal of Experimental Botany 58, 3299–3308 [DOI] [PubMed] [Google Scholar]
- Wan CY, Wilkins TA. 1994. A modified hot borate method significantly enhances the yield of high-quality RNA from cotton (Gossypium hirsutum L.). Analytical Biochemistry 223, 7–12 [DOI] [PubMed] [Google Scholar]
- Wijayanty L, Kobayashi M, Fujioka S, Yoshizawa K, Sakurai A. 1995. Identification and quantification of abscisic acid, indole-3-acetic acid and gibberellins in phloem exudates of Pharbitis nil . Bioscience, Biotechnology and Biochemistry 59, 1533–1535 [Google Scholar]
- Yoshida M. 1976. Genetical studies on the fruit quality of peach varieties. III. Texture and keeping quality. Bulletin of the Fruit Tree Research Station 3, 1–16 [Google Scholar]
- Zarembinski TI, Theologis A. 1994. Ethylene biosynthesis and action: a case of conservation. Plant Molecular Biology 26, 1579–1597 [DOI] [PubMed] [Google Scholar]
- Zourelidou M, Müller I, Willige BC, Nill C, Jikumaru Y, Li H, Schwechheimer C. 2009. The polarly localized D6 PROTEIN KINASE is required for efficient auxin transport in Arabidopsis thaliana . Development 136, 627–636 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.