Abstract
The effects of glucose on aliphatic glucosinolate biosynthesis in Arabidopsis thaliana were investigated in this study by using mutants related to aliphatic glucosinolate biosynthesis and regulation, as well as glucose signalling. The results showed that glucose significantly increased the contents of individual and total aliphatic glucosinolates. Expression of MYB28 and MYB29, two key transcription factors in aliphatic glucosinolate biosynthesis, was also induced by glucose. Consistently, the increased accumulation of aliphatic glucosinolates and the up-regulated expression of CYP79F1 and CYP79F2 induced by glucose disappeared in the double mutant myb28myb29. MYB28 and MYB29 synergistically functioned in the glucose-induced biosynthesis of aliphatic glucosinolates, but MYB28 was predominant over MYB29. Interestingly, the content of total aliphatic glucosinolates and the expression level of MYB28 and MYB29 were substantially reduced in the glucose insensitive mutant gin2-1 and the ABA insensitive 5 (abi5-7) mutant compared with the wild type. In addition, total aliphatic glucosinolates accumulated much less in another sugar-insensitive RGS1 (regulator of G-protein signaling 1) mutant (rgs1-2) than in the wild type. These results suggest that glucose-promoted aliphatic glucosinolate biosynthesis is regulated by HXK1- and/or RGS1-mediated signalling via transcription factors, MYB28, MYB29, and ABI5.
Key words: ABI5, aliphatic glucosinolates, glucose, hexokinase1 (HXK1), regulation, RGS1.
Introduction
Glucosinolates are a group of nitrogen- and sulphur-containing secondary metabolites found mainly in Brassicaceae crops. They can be grouped into aliphatic, aromatic, and indolic glucosinolates based on their different side chain structures (Grubb and Abel, 2006; Agerbirk and Olsen, 2012). It is well known that glucosinolates and their degradation products have diverse biological functions that range from anticarcinogenic activities to plant defence against pathogens and herbivores (Fahey et al., 1997; Grubb and Abel, 2006; Yatusevich, 2008; Kos et al., 2012; Saavedra et al., 2012). Aliphatic glucosinolates have been demonstrated to play an important role in plant–herbivore interactions and non-host resistance in the Arabidopsis–Pseudomonas pathosystem (Beekwilder et al., 2008; Fan et al., 2011).
To date, almost all genes involved in the glucosinolate biosynthetic pathway have been identified (Yan and Chen, 2007; Sønderby et al., 2010). CYP79F1 and CYP79F2 are two important genes whose products catalyse accumulation of long-chain aliphatic glucosinolates, while the product of CYP79F1 also functions in the biosynthesis of short-chain aliphatic glucosinolates (Reintanz et al., 2001; Chen et al., 2003; Tantikanjana et al., 2004). In addition, MYB28, MYB29, and MYB76 have been identified as transcription factors in aliphatic glucosinolate biosynthesis with partial functional redundancy. Among them, MYB28 plays the most important role in aliphatic glucosinolate biosynthesis, followed by MYB29 and MYB76 (Hirai et al., 2007; Gigolashvili et al., 2009). Furthermore, many abiotic factors including nitrogen and sulphur nutrients as well as the plant hormones have been reported to affect the profile and content of glucosinolates (Kliebenstein et al., 2002; Mikkelsen et al., 2003; Mewis et al., 2005; Aires et al., 2006; Chen et al., 2006; Falk et al., 2007; Bano, 2010; Chen et al., 2011; Liu et al., 2011).
Sugars play important roles in plant growth and development as a carbon and energy source. They can also act as effective signalling molecules throughout plant life (Rolland et al., 2006; Ramon et al., 2008; Bolouri-Moghaddam et al., 2010; Smeekens et al., 2010). Hexokinase (HXK), the first enzyme involved in glucose catabolism, can sense glucose and initiate the signalling pathway in Arabidopsis (Moore et al., 2003). The network of HXK1-dependent glucose and ABA signalling has been identified, and the ABA-insensitive abi5 mutant is insensitive to glucose (León and Sheen, 2003; Ramon et al., 2008). Furthermore, it is reported that a regulator of G-protein signalling (AtRGS1) acts as a cell surface receptor and functions in a HXK-independent glucose signalling pathway (Chen and Jones, 2004; Rolland et al., 2006). In previous studies, sugar-regulated plant secondary metabolites were observed in several Brassica vegetables (Guo et al., 2011; Wei et al., 2011). The induction of anthocyanin biosynthesis by sucrose has been clearly illustrated (Teng et al., 2005). Cross-talk between sucrose and hormone signalling pathways in the regulation of the anthocyanin biosynthetic pathway has also been reported (Loreti et al., 2008). Apart from the reports showing that glucose induces the expression of MYB28 in Arabidopsis (Li et al., 2006; Gigolashvili et al., 2007), little information is available on the role of glucose in aliphatic glucosinolate accumulation. In this study, the aim was to investigate the regulatory mechanism of glucose on aliphatic glucosinolate biosynthesis, and to identify the components involved in glucose signalling.
Materials and methods
Plants and growth conditions
Seeds were sterilized for 30 s in 75% ethanol and washed with sterile water twice, and then immersed in 10% sodium hypochlorite for 2min, followed by washing with sterile water four times. Except for the experiments shown in Supplementary Figs S1, S3, and S6, available at JXB online, plant growth conditions in all experiments were as follows. The seeds were stratified for 3 d at 4 °C, and transferred into flasks (~50 seeds per flask) with 40ml of liquid growth medium [full-strength sterilized Murashige–Skoog (MS) salt solution+0.5% glucose]. Plants were grown under a photoperiod of 16h light/8h dark with gentle shaking (135rpm) for 10 d in a plant growth chamber at 21 °C (Loreti et al., 2008). For Supplementary Figs S1 and S3, experiments were carried out with solid culture as follows: surface-sterilized seeds were planted on 0.5% agar plates containing MS salt and 0.5% glucose. In the experiment shown in Supplementary Fig. S6, MS salt solution was replaced with half-strength sterilized MS salt solution. Seeds were grown in a plant growth chamber under the same conditions mentioned above after being incubated at 4 °C for 3 d. Mutant seeds of gin2-1 were generously provided by Dr Sheng Teng (Shanghai Institute of Plant Physiology and Ecology, Chinese Academy of Sciences). The rgs1-2 seeds was obtained from Dr Jirong Huang (Shanghai Institute of Plant Physiology and Ecology, Chinese Academy of Sciences). Mutant seeds of myb28 (SALK_136312) and myb29 (CS121027) were purchased from ABRC (Arabidopsis Biological Resource Center) (Hirai et al., 2007; Beekwilder et al., 2008). The double knock-out mutant myb28myb29 was a gift from Dr Piero Morandini, University of Milan, Italy (Beekwilder et al., 2008). The genetic background of all mutants was Columbia (Col-0), except for gin2-1 which was Landsberg (Ler).
Glucose and sorbitol treatments
Sterilized glucose and sorbitol were added to the flasks after 10 d at final concentrations of 1, 3, and 5% (w/v) with water as a control. For solid culture, 10-day-old seedlings were transferred to MS agar plates with 3% glucose or 3% sorbitol. After treatments, plants were cultured in the same condition as before and were collected at different time points for analysis.
Glucosinolate assay
Seedlings were harvested 1, 3, and 5 d after treatment. Glucosinolates were extracted and analysed as previously described, with minor modifications (Sun et al., 2011a, b). Fresh tissues (100mg) were boiled in 1ml of water for 10min. After recovery of the liquid, the residues were washed with water (1ml), and the combined aqueous extract was applied to a DEAE-Sephadex A-25 (30mg) column (pyridine acetate form). The column was washed three times with 20mM pyridine acetate and twice with water. The glucosinolates were converted into their desulpho analogues by overnight treatment with 100 µl of 0.1% (1.4U) aryl sulphatase, and the desulphoglucosinolates were eluted with 2×0.5ml of water. The high-performance liquid chromatography (HPLC) analysis was performed using Shimadzu HPLC (Shimadzu, Kyoto, Japan), consisting of two LC-20AT solvent delivery units, a DGU-20A3 degasser, a CTO-10ASVP column oven, an SIL-20A autosampler, and an SPD-M20A diode array detector. The HPLC system was connected to a computer with LC solution Version 1.25 Software. A Hypersil C18 column (5 µm particle size, 4.6 mm×250mm; Elite Analytical Instruments Co. Ltd, Dalian, China) was used with a mobile phase of acetonitrile and water at a flow rate of 1.0ml min–1. The procedure employed isocratic elution with 1.5% acetonitrile for the first 5min; a linear gradient to 20% acetonitrile over the next 15min; followed by isocratic elution with 20% acetonitrile for the final 13min. A 20 µl sample was injected onto the column by an autosampler. Absorbance was detected at 226nm. Sinigrin (Sigma, St Louis, MO, USA) was used as an internal standard for calculation of molar concentrations of individual glucosinolates, and relative response factors were applied to correct absorbance differences between the standard and other glucosinolates (Brown et al., 2003). Data were given as nmol mg–1 FW (fresh weight).
RNA isolation and expression analysis
Seedlings were collected at different time points (0, 6, 12, 18, 24, and 36h after treatment) and immediately immersed in liquid nitrogen. Total RNA was isolated from ~100mg of Arabidopsis leaves using the Trizol reagent according to the manufacturer’s instruction (Takara, Japan). RNA samples were reversed transcribed into cDNAs using Prime Script RT Master Mix (Takara, Japan).
Real-time quantitative PCR (qPCR) was performed in a total volume of 20 µl, including 2 µl of diluted cDNA, 1 µl of each primer (5 µM), and 10 µl of 2× SYBR Green PCR Master Mix (Takara, Japan) on an Applied Biosystems StepOne Real-Time PCR Systems (Applied Biosystems, USA) according to the kit manual. The qPCR program was conducted at 95 °C for 10 s first, followed by 40 cycles of 95 °C for 5 s, and 60 °C for 30 s. The expression level of Arabidopsis ACTIN2 was used as an internal control and the expression of other genes was computed with the 2–ΔΔCT method (Livak and Schmittgen, 2001). The primers used in this work are listed in Table 1.
Table 1.
Primer sequences used for quantitative real-time polymerase chain reaction (PCR).
| Target gene | Locus code | Primer sequence | Reference |
|---|---|---|---|
| ACTIN2 | AT3G18780 | 5’-TAACTCTCCCGCTATGTATGTCGC-3’ 5’-CCACTGAGCACAATGTTACCGTAC-3’ |
Gigolashvili et al. (2007) |
| CYP79F1 | AT1G16410 | 5’-CCATACCCTTTTCACATCCTACTAGTCT-3’ 5’-GTAGATTGCCGAGGATGGGC-3’ |
Gigolashvili et al. (2007) |
| CYP79F2 | AT1G16400 | 5’- ACTAGGATTTATCGTCTTCATCGCA-3’ 5’-CTAGGACGAGTCATGATTAGTTCGG-3’ |
Gigolashvili et al. (2007) |
| MYB28 | AT5G61420 | 5’-TCCCTGACAAATACTCTTGCTGAAT-3’ 5’-CATTGTGGTTATCTCCTCCGAATT-3’ |
Gigolashvili et al. (2007) |
| MYB29 | AT5G07690 | 5’-CAATACTGGAGGAGGATATAACC-3’ 5’-AGTTCTTGTCGTCATAATCTTGG-3’ |
Beekwilder et al. (2008) |
| MYB76 | AT5G07700 | 5’-ACGTTTAATCGATGATGGCA-3’ 5’-ATGGGCTCAACTGGATTAGG-3’ |
Designed by software www.genscript.com |
Statistical analysis
Statistical analysis was performed using the SPSS package program version 11.5 (SPSS Inc., Chicago, IL, USA). For Figs 1–3, and Supplementary Figs S1, S2, and S4–S6 at JXB online, differences in glucosinolate accumulation among different treatments were tested, while differences in Fig. 5A were tested among different mutants. They all were analysed by one-way analysis of variance (ANOVA), followed by the least significant difference (LSD) test at a 95% confidence level (P < 0.05). For Figs 6A, B, 7A, and Supplementary Fig. S3, differences in glucosinolate accumulation among different mutants were analysed using independent-samples t-test. The values are reported as means with standard error for all results.
Fig. 1.
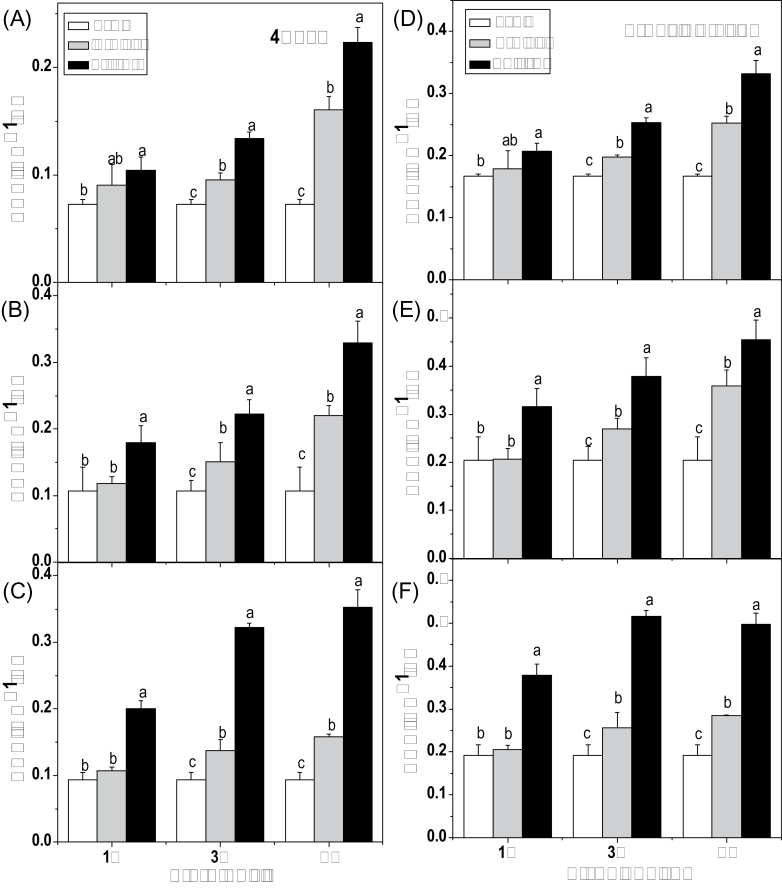
Effect of glucose on accumulation of aliphatic glucosinolates (GS) in a time-course experiment. Total aliphatic glucosinolates and 4MSOB were measured in 10-day-old Arabidopsis seedlings treated with 1, 3, and 5% glucose, and sorbitol. Samples were collected after 1 d (A, D), 3 d (B, E), and 5 d (C, F). Each data point represents the mean of three independent biological replicates per treatment (mean ±SE). Values not sharing a common letter are significantly different at P < 0.05.
Fig. 3.
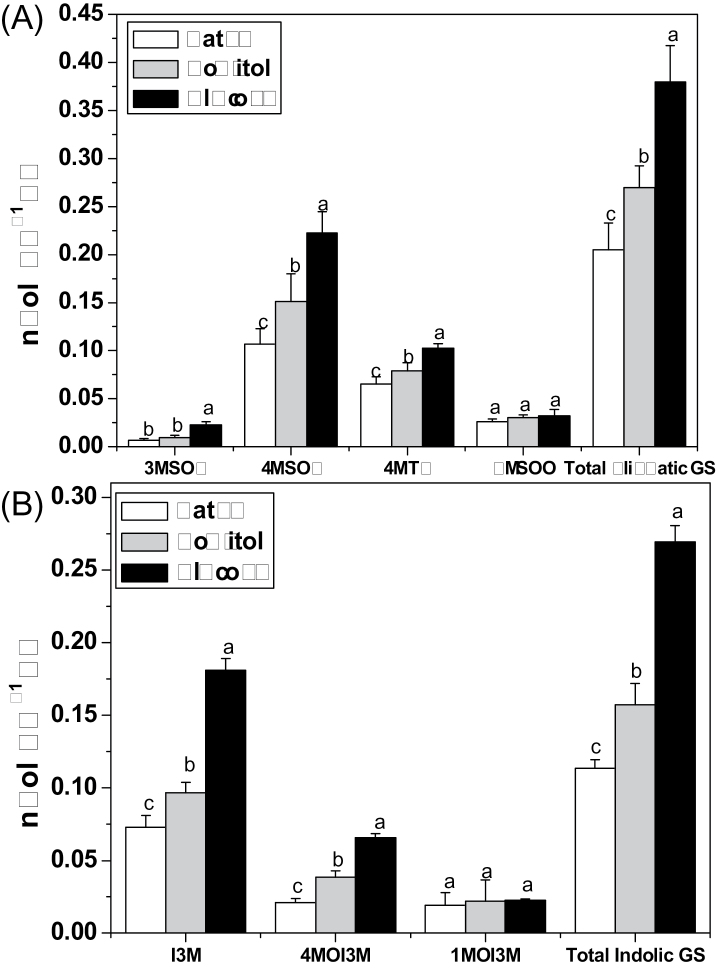
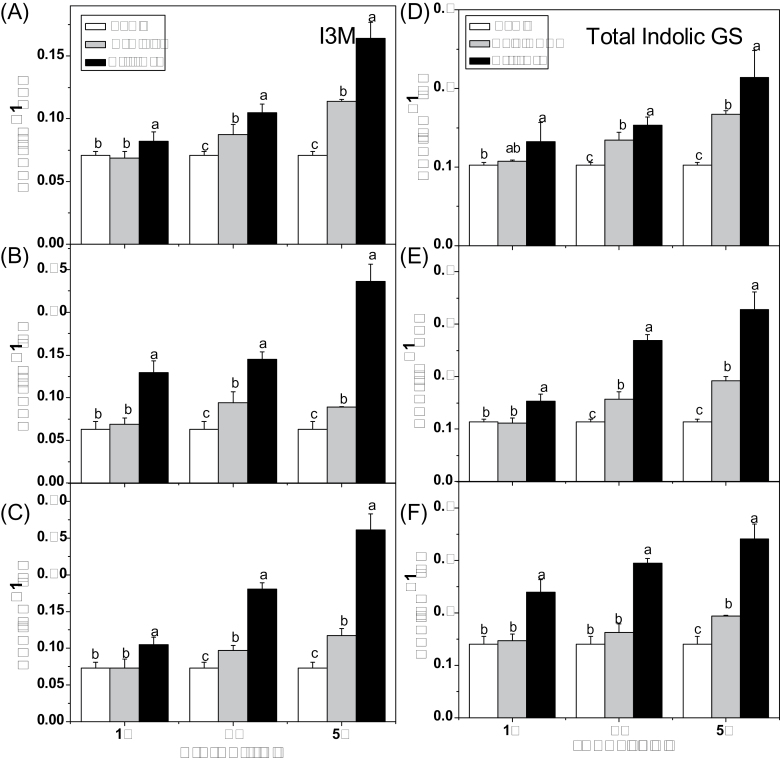
Effect of glucose on accumulation of individual and total aliphatic and indolic glucosinolates. (A) Total aliphatic glucosinolates and four aliphatic glucosinolates 3MSOP, 4MSOB, 4MTB, and 8MSOO (8-methylsulphinyloctyl glucosinolate). (B) Total indolic glucosinolates and three indolic glucosinolates I3M, 4MOI3M, and 1MOI3M (1-methoxyindol-3-ylmethyl) were measured in 10-day-old Arabidopsis seedlings treated with 3% glucose and sorbitol. Samples were collected 3 d after treatment. Each data point represents the mean of three independent biological replicates per treatment (mean ±SE). Values not sharing a common letter are significantly different at P < 0.05.
Fig. 5.
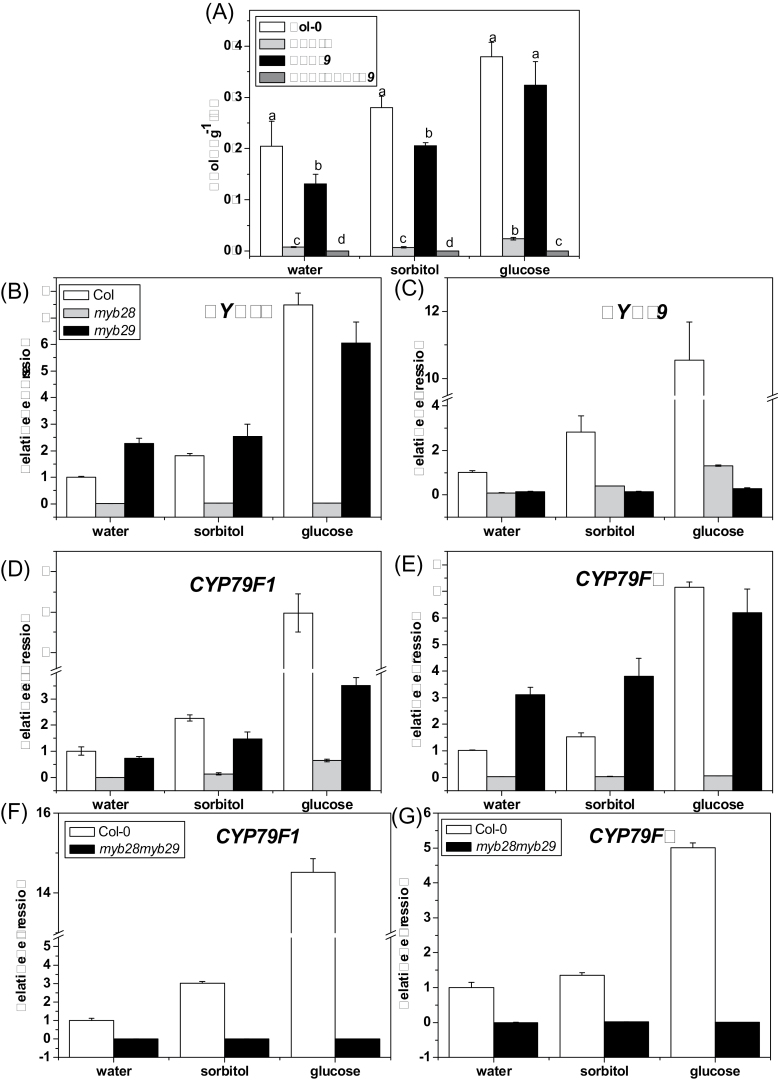
Total aliphatic glucosinolates content of myb28 and myb29 (A). Effect of glucose on MYB28 (B), MYB29 (C), CYP79F1 (D), and CYP79F2 (E) expression level in myb28 and myb29, and of CYP79F1 (F), CYP79F2 (G) in the myb28myb29 double mutant. Samples used for glucosinolate assay were collected 3 d after treatment. Samples used for qPCR experiment were collected 18h after treatment. Each data point represents the mean of three independent biological replicates per treatment (mean ±SE). Values not sharing a common letter are significantly different at P < 0.05. Relative expression values are given compared with seedlings treated by water.
Fig. 6.
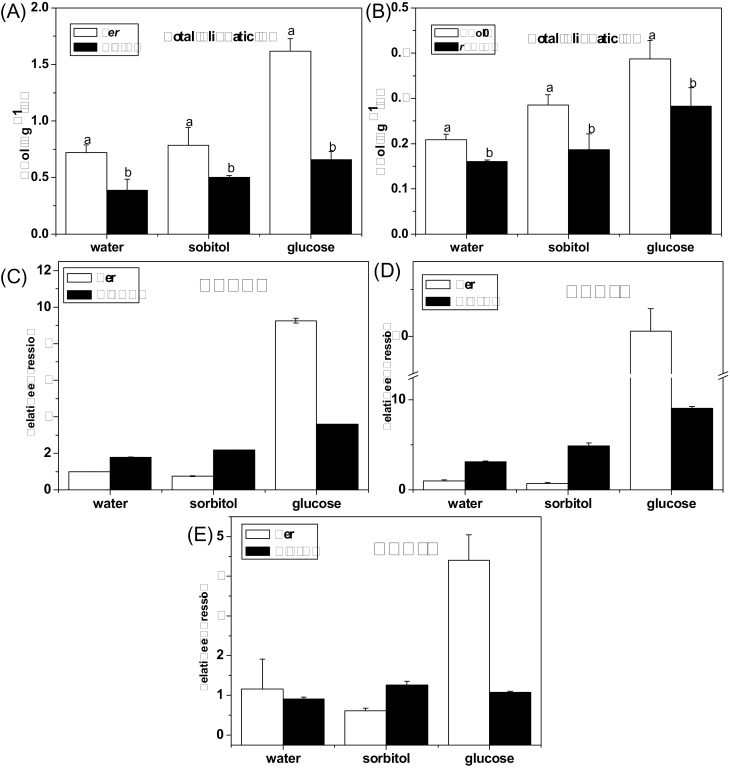
Total aliphatic glucosinolate content of gin2-1 and rgs1-2 (A, B). Effect of glucose on the MYB28, MYB29, and MYB76 expression level in the gin2-1 mutant (C, D, E). Arabidopsis seedlings (10-day-old) were treated with 3% glucose or sorbitol. Samples used for glucosinolate assay were collected 3 d after treatment. Samples used for qPCR experiment were collected 18h after treatment. Each data point represents the mean of three independent biological replicates per treatment (mean ±SE). Values not sharing a common letter are significantly different at P < 0.05. Relative expression values are given compared with seedlings treated by water.
Fig. 7.
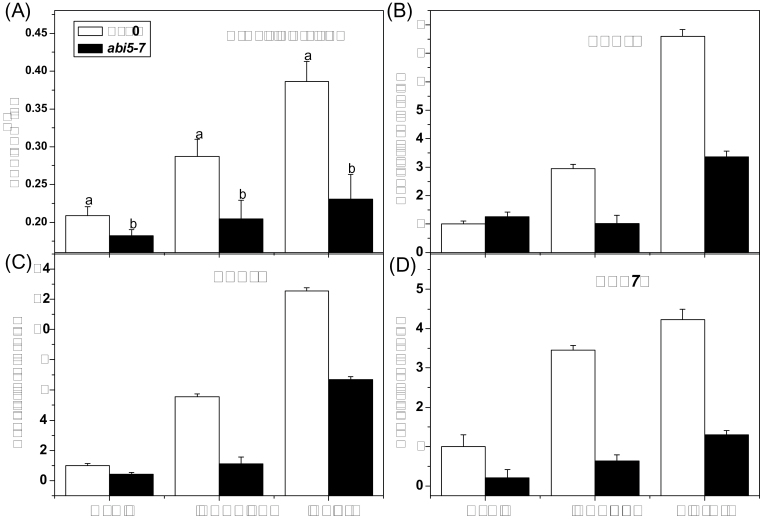
Total aliphatic glucosinolate content and effect of glucose on the MYB28, MYB29, and MYB76 expression level in abi5-7. Arabidopsis seedlings (10-day-old) were treated with 3% glucose or sorbitol. (A) Samples were collected 3 d after treatment. (B–D) Samples were collected 18h after treatment. Each data point represents the mean of three independent biological replicates per treatment (mean ±SE). Values not sharing a common letter are significantly different at P < 0.05. Relative expression values are given compared with seedlings treated by water.
Results
Effect of glucose on glucosinolate contents in Arabidopsis
To study the role of glucose in glucosinolate biosynthesis, 10-day-old seedlings of Arabidopsis wild-type (Col-0) were treated with different concentrations (1, 3, and 5%) of glucose or sorbitol (as an osmotic control) and sampled 1, 3, and 5 d after each treatment. As shown in Fig. 1, the contents of 4MSOB (4-methylsulphinylbutyl glucosinolate, the predominant aliphatic glucosinolate) and the total aliphatic glucosinolates increased significantly at almost all time points under all the tested concentrations except for 1 d after 1% glucose or sorbitol treatment. Similarly, the contents of I3M (indol-3-ylmethyl glucosinolate, the predominant indolic glucosinolate) and the total indolic glucosinolates were also enhanced significantly by glucose (Fig. 2). Furthermore, glucose promoted glucosinolate accumulation in a time-dependent manner (Figs 1, 2). It should be noted that glucose stimulated accumulation of glucosinolates much more than sorbitol, indicating that the regulatory mechanism of glucose and sorbitol on glucosinolate biosynthesis is different.
Fig. 2.
Effect of glucose on accumulation of indolic glucosinolates in a time-course experiment. Total indolic glucosinolates and I3M were measured in 10-day-old Arabidopsis seedlings treated with 1, 3, and 5% glucose, and sorbitol. Samples were collected after 1 d (A, D), 3 d (B, E), and 5 d (C, F). Each data point represents the mean of three independent biological replicates (mean ±SE). Values not sharing a common letter are significantly different at P < 0.05.
Since vitrification occurred in some seedlings 5 d after treatment, the effect of glucose on glucosinolate biosynthesis was studied using plants treated with a moderate concentration (3%) for 3 d. Under this condition, the contents of major aliphatic glucosinolates, 3MSOP (3-methylsulphinylpropyl glucosinolate), 4MTB (4-methylthiobutyl glucosinolate), and 4MSOB, and total aliphatic glucosinolates notably increased by 146, 30, 38, and 31%, respectively, compared with sorbitol treatment (Fig. 3A). In addition, significant increases in 4MOI3M (4-methoxyindol-3-ylmethyl glucosinolate), I3M, and total indolic glucosinolates were also observed 3 d after 3% glucose treatment compared with sorbitol treatment (Fig. 3B).
To verify the effect of glucose on aliphatic glucosinolate biosynthesis, solid media were used to grow seedlings (for details see the Materials and methods). The results showed the same trend as the liquid culture (Supplementary Fig. S1 at JXB online). Because there exists an interaction between glucose and nitrogen (Price et al., 2004), liquid growth medium including half-strength MS salt solution with 0.5% glucose, which contains a lower nitrogen concentration, was used to confirm the induction of glucose, and strong induction of glucose still can be observed (Supplementary Fig. S6). In addition, the content of total aliphatic glucosinolates was also measured at 0, 6, 12, 24, and 36h after glucose treatment to investigate the time-course accumulation of aliphatic glucosinolates (Supplementary Fig. S2). A significant difference in total aliphatic glucosinolates between glucose and sorbitol treatment occurred 24h after treatment.
Glucose induces expression of transcription factors and biosynthetic genes in the aliphatic glucosinolate pathway
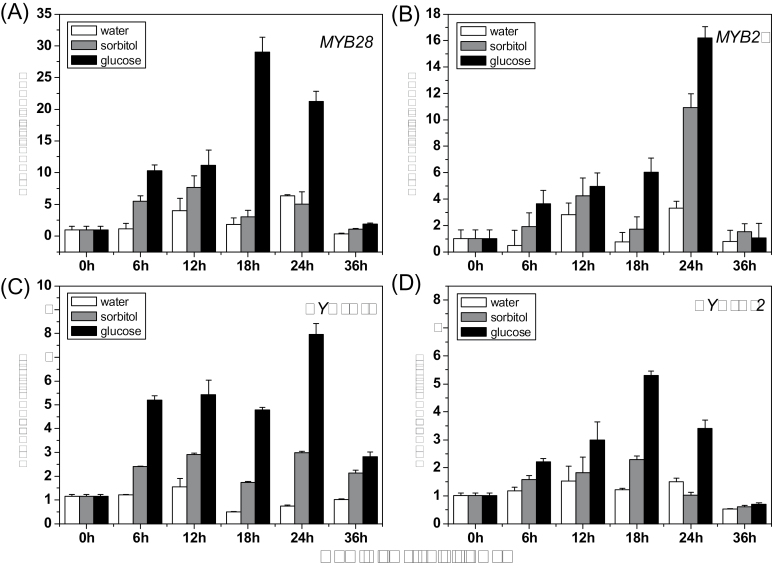
MYB28 and MYB29 are two major regulators for aliphatic glucosinolate biosynthesis, and the double mutant myb28myb29 does not accumulate any aliphatic glucosinolates (Gigolashvili et al., 2007, 2008; Hirai et al., 2007; Sønderby et al., 2007; Beekwilder et al., 2008). CYP79F1 and CYP79F2 encode two core biosynthetic enzymes catalysing the production of aliphatic glucosinolates (Tantikanjana et al., 2004; Sønderby et al., 2010). Here the data showed that the expression of all the four genes responded positively to exogenous glucose treatment at a time point as early as 6h (Fig. 4). The expression of the genes was induced with a steady rise after 6h, peaked at 18h (MYB28 and CYP79F2) or 24h (MYB29 and CYP79F1), and then decreased sharply at 36h. The expression levels of MYB28, MYB29, CYP79F1, and CYP79F2 increased by ~8.4-, 2.5-, 1.8-, and 1.3-fold, respectively, at 18h after the 3% glucose treatment when compared with sorbitol treatment. So, 18h after treatment was set as the harvest point of Arabidopsis plants for further analysis of gene expression.
Fig. 4.
Effect of glucose on the expression level of MYB28 (A), MYB29 (B), CYP79F1 (C), and CYP79F2 (D). The expression level was measured in 10-day-old Arabidopsis seedlings treated with 3% glucose or sorbitol. Samples used for qPCR experiment were collected 0, 6, 12, 18, 24, and 36h after treatment, respectively. Each data point represents the mean of three independent biological replicates per treatment (mean ±SE). Relative expression values are given compared with 0h non-treated seedlings (0 h=1).
Effect of glucose on aliphatic glucosinolate biosynthesis in myb28, myb29 and myb28myb29 mutants
The level of total aliphatic glucosinolates was examined in myb28 and myb29 under glucose treatment. As shown in Fig. 5A, the level of total aliphatic glucosinolates decreased significantly in both mutants, especially in myb28. In myb28, although the contents of 4MTB and 4MSOB increased with glucose treatment (data not shown), the content of total aliphatic glucosinolates was much lower than that in the wild type. Interestingly, glucose treatment rescued the chemotype of low contents of individual and total aliphatic glucosinolates in the myb29 mutant.
The transcriptional level of MYB28, MYB29, CYP79F1, and CYP79F2 was determined in myb28 and myb29 mutants. As shown in Fig. 5B, glucose treatment dramatically increased the expression level of MYB28 in both wild-type and myb29 plants compared with sorbitol treatment, which produced a slightly increased MYB28 expression level. MYB29 expression was also substantially induced by glucose in the wild type, but glucose-induced expression of MYB29 was very weak in myb28 (Fig. 5C). The expression pattern of CYP79F1 and CYP79F2 was similar to that of MYB28 (Fig. 5D, E). These results indicate that MYB28 is a key transcription factor for expression of genes involved in aliphatic glucosinolate biosynthesis. No detectable aliphatic glucosinolates were found in the myb28myb29 double mutant with or without glucose treatment (Fig. 5A). Consistently, the expression levels of CYP79F1 and CYP79F2 were almost undetectable in the double mutant (Fig. 5F, G).
Effect of glucose on aliphatic glucosinolate biosynthesis in gin2-1 and rgs1-2 mutants
The HXK1 null mutant gin2-1 is glucose insensitive (Moore et al., 2003), while rgs1-2, a null mutant of the gene encoding RGS protein (AtRGS1), is insensitive to glucose and sucrose (Chen and Jones, 2004). Accumulation of total aliphatic glucosinolates was analysed in these two mutants along with their wild types under liquid culture. As shown in Fig. 6A and B, the level of total aliphatic glucosinolates in gin2-1 and rgs1-2 decreased by 46% and 23%, respectively, when compared with the corresponding wild types. Similar results were also observed when the mutants were grown on the solid culture medium (Supplementary Fig. S3 at JXB online). Furthermore, glucose treatment did not rescue the chemotype of low glucosinolates in the mutant plants. The total aliphatic glucosinolate content was significantly lower in gin2-1 and rgs1-2 plants than that in the control under glucose treatment. These results indicate that HXK1 and RGS1 are involved in the glucose-regulated aliphatic glucosinolate biosynthesis.
Considering that HXK1 controls gene transcription via interaction with several protein families including MYB in the nucleus (Rolland et al., 2006), the expression level of three transcription factors involved in aliphatic glucosinolate biosynthesis (MYB28, MYB29, and MYB76) was investigated in gin2-1 mutant plants. Although glucose treatment increased the expression of MYB28 and MYB29 in the gin2-1 mutant when compared with sorbitol treatment (Fig. 6C, D), severely lower expression levels of MYB28, MYB29, and MYB76 (Fig. 6C, D, E) were found in gin2-1 as compared with Ler after glucose treatment.
Effect of glucose on aliphatic glucosinolate biosynthesis in the abi5 mutant
A significant decrease in total aliphatic glucosinolates was observed in the abi5-7 mutant in comparison with the wild type with or without glucose treatment (Fig. 7A). Furthermore, expression levels of MYB28, MYB29, and MYB76 in abi5-7 were substantially lower than that in the wild type after glucose treatment (Fig. 7B–D).
Discussion
Glucose-induced aliphatic glucosinolate accumulation
Sugars were first recognized to be the prime carbon supply and energy source in plants. However, reports on their regulatory functions have been increasing in recent years (Smeekens et al., 2010). The regulatory mechanism of sucrose in anthocyanin biosynthesis has been well elucidated (Teng et al., 2005). In addition, previous studies indicated that sugars could boost the accumulation of health-promoting compounds in Brassica vegetables (Guo et al., 2011; Wei et al., 2011). As a signalling molecule, glucose controls plant growth, development, metabolism, and stress resistance (Ramon et al., 2008). In the current study, the results indicated that glucose positively regulated aliphatic glucosinolate biosynthesis. Aliphatic glucosinolates can be greatly induced through MYB transcription factors by glucose in a time- and dose-dependent manner (Figs 1, 4), while aliphatic glucosinolate accumulation was severely disrupted in the glucose signalling mutants gin2-1 and rgs1-2 (Fig. 6A, B). In addition, because glucosinolate contents could be affected by several abiotic stress factors, such as salt, drought, and water (López-Berenguer et al., 2008; Khan et al., 2010; Yuan et al., 2010), sorbitol was taken as an osmotic stress control and its effect on glucosinolates was not as strong as that of glucose. Moreover, fructose, the isomer of glucose, which can interconvert with glucose and enter the metabolic pathway of glucosinolates with the form of glucose-6-phosphate as intermediate (Henry et al., 1991; Bhagavan and Ha, 2011), could not mimic the induction by glucose of glucosinolate accumulation (Supplementary Fig. S4 at JXB online). In addition, the glucose analogue mannose, the substrate of HXK1 phosphorylation, which can also trigger the signalling function of HXK1 (Ramon et al., 2008), did not have a similar induction to glucose (Supplementary Fig. S5). To sum up, glucose induces aliphatic glucosinolate accumulation as a signalling molecule in Arabidopsis thaliana through a mechanism different from mannose.
Induction of aliphatic glucosinolates by glucose through MYB transcription factors
MYB28 and MYB29 are the two vital transcription factors in aliphatic glucosinolate biosynthesis in A. thaliana (Gigolashvili et al., 2009). Almost all the biosynthetic genes in the aliphatic glucosinolate pathway can be positively regulated by these two transcription factors (Yan and Chen, 2007). To test whether aliphatic glucosinolate biosynthesis induced by glucose was due to the up-regulation of MYB transcription factors, qPCR was applied to determine the expression pattern of MYB28 and MYB29 after glucose treatment. As expected, induction of these two genes was similar at different time points except that the expression of MYB28 had an earlier peak than that of MYB29. This exactly reflected that MYB28 could regulate the expression of MYB29 (Yatusevich, 2008) (Fig. 4A, B). In addition, the expression levels of CYP79F1 and CYP79F2, the key structural genes in aliphatic glucosinolate biosynthesis, were also enhanced after glucose treatment (Fig. 4C, D). The induction occurred within the first 6h, which was a little earlier than glucosinolate enhancement. This indicates that the expression of transcription factors can sense changes in the environment in a shorter time than the production of secondary metabolites. The results are consistent with previous reports on Brassica crops with sucrose treatment (Guo et al., 2011; Wei et al., 2011).
To obtain a deeper understanding of the role that the main transcriptional regulators and structural genes played in the regulation of aliphatic glucosinolate biosynthesis in response to glucose, three mutants, myb28, myb29, and myb28myb29, were used for further analysis. MYB28 is the predominant transcription factor belonging to the MYB family controlling the profiles of aliphatic glucosinolates, and it is mainly responsible for long-chain and short-chain aliphatic glucosinolates, while MYB29 participates more in short-chain aliphatic glucosinolates. In the present study, myb28 showed a very severe lack of aliphatic glucosinolates compared with myb29 (Hirai et al., 2007; Gigolashvili et al., 2008; Yatusevich, 2008) (Fig. 5A). Surprisingly, the reduction could be recovered by glucose supplementation in myb29 but not in the case of myb28 (Fig. 5A). This is due to the fact that these two transcription factors have functional redundancy in regulation of aliphatic glucosinolate, in which MYB28 takes a master role while MYB29 has an accessory role (Hirai et al., 2007). According to previous reports (Yatusevich, 2008), MYB29 can be induced by MYB28. Thus, when myb29 was treated with glucose, the strong expression of MYB28, induced by glucose, could compensate the decreased level of MYB29 expression (Fig. 5B, C). When MYB28 is knocked out, the expression of MYB29 is still lower than that in Col-0 even under glucose treatment (Fig. 5C). Interestingly, the induction by glucose of total aliphatic glucosinolates and the expression of MYB29 in mutant myb28 were much weaker than that in Col-0. This observation indicated that the strong induction by glucose of the expression of MYB29 in Col-0 is partly due to the regulation of MYB28 (Fig. 4). These results may explain why myb28 contained a significantly reduced level of aliphatic glucosinolates with or without glucose treatment.
Aliphatic glucosinolates and the expression of CYP79F1 and CYP79F2 could not be detected in the loss-of-function mutant myb28myb29 (Fig. 5F, G), which is consistent with previous surveys (Sønderby et al., 2007). Interestingly, it contained no detectable aliphatic glucosinolates even when treated with glucose. Previous research has illustrated that CYP79F1 and CYP79F2 could be regulated by MYB factors (MYB28, MYB29, and MYB76) (Gigolashvili et al., 2008). Here the up-regulated expression of CYP79F1 and CYP79F2 in Col-0 was not observed in myb28myb29 before and after glucose treatment. These findings suggest that the MYB transcript factors are induced by glucose, and then regulate the expression of structural genes which finally lead to the accumulation of aliphatic glucosinolate.
HXK1- and RGS1-dependent regulation of aliphatic glucosinolate biosynthesis by glucose
HXKs are one of the most conserved sugar sensors together with other sugar kinases in the plant kingdom. In A. thaliana, six HXK and HXK-like genes have been identified so far (Ramon et al., 2008) and they carry out diverse and distinct functions in glucose metabolism and signalling. AtHXK1 has been characterized as an intracellular glucose sensor (Bolouri-Moghaddam et al., 2010). HXK1-dependent glucose signalling can affect plant growth, which relies on the endogenous glucose level and the sensitivity to glucose in plants (Ramon et al., 2008). In addition, there are other glucose-sensing and signalling pathways independent of HXK1. It has been reported that the signalling pathway of sugar-induced anthocyanin accumulation is independent of HXK1 (Xiao et al., 2000; Teng et al., 2005). Furthermore, the fructose signalling can function independently of the HXK1 glucose sensor (Li et al., 2011). In A. thaliana, RGS1 is a regulator of G-protein signalling protein, which modulates plant cell proliferation and may serve as a glucose sensor located on the cell surface, independent of HXK1 (Chen and Jones, 2004; Ramon et al., 2008).
In the present study, the relationship between HXK1/RGS1 and aliphatic glucosinolates was analysed using their corresponding mutants, gin2-1 and rgs1-2. Both mutants are glucose insensitive. According to the present results, gin2-1 and rgs1-2 mutants showed a notable decrease in the level of total aliphatic glucosinolates whether treated with glucose or not, when compared with their corresponding controls (Fig. 6A, B). This suggested that HXK1 and RGS1 might both participate in the regulation of aliphatic glucosinolate biosynthesis. It has been reported that HXK1 regulates transcription and proteasome-mediated degradation of the EIN3 (ETHYLENE INSENSITIVE3) transcription factor in the nucleus, and several types of transcription factors are involved in sugar-regulated transcription (Rolland et al., 2006; Ramon et al., 2008). Therefore, the gene expression pattern of three MYB transcription factors involved in aliphatic glucosinolate biosynthesis (MYB28, MYB29, and MYB76) was analysed in gin2-1 mutants, and a substantial decrease in the expression level in response to glucose was observed when compared with the wild type (Fig. 6C–E). This is undoubtedly another piece of evidence for HXK1-dependent induction of aliphatic glucosinolate biosynthesis by glucose signalling through MYB factors.
ABI5 is involved in the regulation of aliphatic glucosinolate biosynthesis by glucose
Previous studies have indicated that glucose signalling is a complex network, and cross-talk between glucose signalling and phytohormone signalling was elucidated (Gazzarrini and McCourt, 2001; Dekkers et al., 2008). ABA signalling and sugar sensing share several common components, and many glucose-insensitive mutants are also allelic to ABA biosynthetic or signalling mutants (Finkelstein and Gibson, 2002; Rook et al., 2006). ABI5 (ABA-insensitive 5) encodes a transcription factor belonging to a large basic leucine zipper (bZIP) gene family, conferring on abi5-7 a glucose-insensitive phenotype. ABI5 is therefore a putative glucose signalling component downstream of HXK1 (Ramon et al., 2008; Reeves et al., 2011). In this study, abi5-7 was deficient in total aliphatic glucosinolate content before and after glucose treatment when compared with the wild type, as well as the expression level of MYB transcription factors after glucose treatment (Fig. 7). These results suggested that ABI5 might be involved in the regulation of aliphatic glucosinolate biosynthesis as a glucose signalling component downstream of HXK1.
In conclusion, glucose induces the accumulation of aliphatic glucosinolate as a signalling molecule by modulating MYB transcription factors (MYB28 and MYB29), which participated in the regulation of aliphatic glucosinolate biosynthesis, with MYB28 as a master component. Furthermore, two distinct glucose sensors, HXK1 and RGS1, as well as ABI5, a putative glucose signalling component located downstream of HXK1, are proved to be involved in the network of glucose signalling to regulate aliphatic glucosinolate biosynthesis. The abolishment of increased expression of MYB28, MYB29, and MYB76 induced by glucose in gin2-1 and abi5-7 mutants in the present study indicates an interaction between HXK1 or ABI5 and the MYB factors involved in regulation of aliphatic glucosinolate biosynthesis.
Further analysis of the interplay between HXK1/ABI5 and MYB28/MYB29/MYB76 will help to elucidate the regulatory mechanism of aliphatic glucosinolate biosynthesis induced by glucose signalling. Another possibility of glucose enhancing sulphate assimilation should also be considered. Glucosinolates are sulphur-rich plant metabolites, and sulphur fertilization usually causes an increase in glucosinolate content (Falk et al., 2007). Previous reports showed that sugars (sucrose and glucose) at low concentration induced APR (adenosine 5-phosphosulphate reductase) activity and enhanced sulphate uptake (Kopriva et al., 2002; Hesse et al., 2003; Kopriva, 2006). Therefore, further investigation is needed to elucidate whether glucose induces the accumulation of glucosinolates by regulating sulphate assimilation and uptake.
Supplementary data
Supplementary data are available at JXB online.
Figure S1. Effect of glucose on accumulation of total aliphatic glucosinolates with the solid culture method.
Figure S2. Effect of glucose on accumulation of total aliphatic glucosinolates during 36h after treatment.
Figure S3. Total content of aliphatic glucosinolates of mutants gin2-1 and rgs1-2 grown on solid culture medium.
Figure S4. Effect of fructose on the accumulation of total aliphatic glucosinolates.
Figure S5. Effect of mannose on the accumulation of total aliphatic glucosinolates.
Figure S6. Effect of glucose on the accumulation of total aliphatic glucosinolates in Arabidopsis cultured in half-strength MS salt solution.
Acknowledgements
This work was supported by grants from National Science Foundation of China (NO. 31270343), National Key Laboratory of Plant Molecular Genetics (2010–2011), National High-tech R&D Program of China (863 program 2008AA10Z111), Fok Ying Tong Education Foundation (104034), and NCET-05-0516.
Glossary
Abbreviations:
- ABI5
ABA insensitive5
- gin2
glucose insensitive2
- HXK1
hexokinase1
- RGS
regulator of G-protein signalling.
References
- Agerbirk N, Olsen CE. 2012. Glucosinolate structures in evolution. Phytochemistry 77, 16–45 [DOI] [PubMed] [Google Scholar]
- Aires A, Rosa E, Carvalho R. 2006. Effect of nitrogen and sulfur fertilization on glucosinolates in the leaves and roots of broccoli sprouts (Brassica oleracea var. italica). Journal of the Science of Food and Agriculture 86, 1512–1516 [Google Scholar]
- Bano A. 2010. Nutritive values of Brassica campestris L. oil as affected by growth regulator treatments. Journal of the Chemical Society of Pakistan 31, 819–822 [Google Scholar]
- Beekwilder J, Van Leeuwen W, Van Dam NM, Bertossi M, Grandi V, Mizzi L, Soloviev M, Szabados L, Molthoff JW, Schipper B. 2008. The impact of the absence of aliphatic glucosinolates on insect herbivory in Arabidopsis. PLoS One 3, e2068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolouri-Moghaddam MR, Le Roy K, Xiang L, Rolland F, Van den Ende W. 2010. Sugar signalling and antioxidant network connections in plant cells. FEBS Journal 277, 2022–2037 [DOI] [PubMed] [Google Scholar]
- Brown PD, Tokuhisa JG, Reichelt M, Gershenzon J. 2003. Variation of glucosinolate accumulation among different organs and developmental stages of Arabidopsis thaliana. Phytochemistry 62, 471–481 [DOI] [PubMed] [Google Scholar]
- Chen JG, Jones AM. 2004. AtRGS1 function in Arabidopsis thaliana. Methods in Enzymology 389, 338–350 [DOI] [PubMed] [Google Scholar]
- Chen SX, Glawischnig E, Jørgensen K, Naur P, Jørgensen B, Olsen CE, Hansen CH, Rasmussen H, Pickett JA, Halkier BA. 2003. CYP79F1 and CYP79F2 have distinct functions in the biosynthesis of aliphatic glucosinolates in Arabidopsis. The Plant Journal 33, 923–937 [DOI] [PubMed] [Google Scholar]
- Chen XJ, Zhu ZJ, Ni XL, Qian QQ. 2006. Effect of nitrogen and sulfur supply on glucosinolates in Brassica campestris ssp. chinensis. Agricultural Sciences in China 5, 603–608 [Google Scholar]
- Chen YZ, Yan XF, Chen SX. 2011. Bioinformatic analysis of molecular network of glucosinolate biosynthesis. Computational Biology and Chemistry 35, 10–18 [DOI] [PubMed] [Google Scholar]
- Dekkers BJW, Schuurmans JAMJ, Smeekens SCM. 2008. Interaction between sugar and abscisic acid signalling during early seedling development in Arabidopsis. Plant Molecular Biology 67, 151–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahey JW, Zhang Y, Talalay P. 1997. Broccoli sprouts: an exceptionally rich source of inducers of enzymes that protect against chemical carcinogens. Proceedings of the National Academy of Sciences, USA 94, 10367–10372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk KL, Tokuhisa JG, Gershenzon J. 2007. The effect of sulfur nutrition on plant glucosinolate content: physiology and molecular mechanisms. Plant Biology 9, 573–581 [DOI] [PubMed] [Google Scholar]
- Fan J, Crooks C, Creissen G, Hill L, Fairhurst S, Doerner P, Lamb C. 2011. Pseudomonas sax genes overcome aliphatic isothiocyanate-mediated non-host resistance in Arabidopsis. Science’s STKE 331, 1185–1188 [DOI] [PubMed] [Google Scholar]
- Finkelstein RR, Gibson SI. 2002. ABA and sugar interactions regulating development: cross-talk or voices in a crowd?. Current Opinion in Plant Biology 5, 26–32 [DOI] [PubMed] [Google Scholar]
- Gazzarrini S, McCourt P. 2001. Genetic interactions between ABA, ethylene and sugar signaling pathways. Current Opinion in Plant Biology 4, 387–391 [DOI] [PubMed] [Google Scholar]
- Gigolashvili T, Berger B, Flügge UI. 2009. Specific and coordinated control of indolic and aliphatic glucosinolate biosynthesis by R2R3-MYB transcription factors in Arabidopsis thaliana. Phytochemistry Reviews 8, 3–13 [Google Scholar]
- Gigolashvili T, Engqvist M, Yatusevich R, Müller C, Flügge UI. 2008. HAG2/MYB76 and HAG3/MYB29 exert a specific and coordinated control on the regulation of aliphatic glucosinolate biosynthesis in Arabidopsis thaliana. New Phytologist 177, 627–642 [DOI] [PubMed] [Google Scholar]
- Gigolashvili T, Yatusevich R, Berger B, Müller C, Flügge UI. 2007. The R2R3-MYB transcription factor HAG1/MYB28 is a regulator of methionine-derived glucosinolate biosynthesis in Arabidopsis thaliana. The Plant Journal 51, 247–261 [DOI] [PubMed] [Google Scholar]
- Grubb CD, Abel S. 2006. Glucosinolate metabolism and its control. Trends in Plant Science 11, 89–100 [DOI] [PubMed] [Google Scholar]
- Guo RF, Yuan GF, Wang QM. 2011. Sucrose enhances the accumulation of anthocyanins and glucosinolates in broccoli sprouts. Food Chemistry 129, 1080–1087 [DOI] [PubMed] [Google Scholar]
- Henry RR, Crapo PA, Thorburn AW. 1991. Current issues in fructose metabolism. Annual Review of Nutrition 11, 21–39 [DOI] [PubMed] [Google Scholar]
- Hesse H, Trachsel N, Suter M, Kopriva S, Von Ballmoos P, Rennenberg H, Brunold C. 2003. Effect of glucose on assimilatory sulphate reduction in Arabidopsis thaliana roots. Journal of Experimental Botany 54, 1701–1709 [DOI] [PubMed] [Google Scholar]
- Hirai MY, Sugiyama K, Sawada Y, Tohge T, Obayashi T, Suzuki A, Araki R, Sakurai N, Suzuki H, Aoki K. 2007. Omics-based identification of Arabidopsis Myb transcription factors regulating aliphatic glucosinolate biosynthesis. Proceedings of the National Academy of Sciences, USA 104, 6478–6483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan M, Ulrichs C, Mewis I. 2010. Influence of water stress on the glucosinolate profile of Brassica oleracea var. italica and the performance of Brevicoryne brassicae and Myzus persicae. Entomologia Experimentalis et Applicata 137, 229–236 [Google Scholar]
- Kliebenstein DJ, Figuth A, Mitchell-Olds T. 2002. Genetic architecture of plastic methyl jasmonate responses in Arabidopsis thaliana. Genetics 161, 1685–1696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopriva S. 2006. Regulation of sulfate assimilation in Arabidopsis and beyond. Annals of Botany 97, 479–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopriva S, Suter M, Von Ballmoos P, Hesse H, Krähenbühl U, Rennenberg H, Brunold C. 2002. Interaction of sulfate assimilation with carbon and nitrogen metabolism in Lemna minor. Plant Physiology 130, 1406–1413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kos M, Houshyani B, Wietsma R, Kabouw P, Vet LEM, van Loon JJA, Dicke M. 2012. Effects of glucosinolates on a generalist and specialist leaf-chewing herbivore and an associated parasitoid. Phytochemistry 77, 162–170 [DOI] [PubMed] [Google Scholar]
- León P, Sheen J. 2003. Sugar and hormone connections. Trends in Plant Science 8, 110–116 [DOI] [PubMed] [Google Scholar]
- Li P, Wind JJ, Shi X, Zhang H, Hanson J, Smeekens SC, Teng S. 2011. Fructose sensitivity is suppressed in Arabidopsis by the transcription factor ANAC089 lacking the membrane-bound domain. Proceedings of the National Academy of Sciences, USA 108, 3436–3441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Lee KK, Walsh S, Smith C, Hadingham S, Sorefan K, Cawley G, Bevan MW. 2006. Establishing glucose- and ABA-regulated transcription networks in Arabidopsis by microarray analysis and promoter classification using a Relevance Vector Machine. Genome Research 16, 414–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu QX, Guo J, Yan XF. 2011. Effect of exogenous jasmonic acid on glucosinolate content in Arabidopsis thaliana rosette leaves. Journal of Northeast Agricultural University 42, 133–137 [Google Scholar]
- Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2-[Delta][Delta] CT method. Methods 25, 402–408 [DOI] [PubMed] [Google Scholar]
- López-Berenguer C, Martínez-Ballesta MC, García-Viguera C, Carvajal M. 2008. Leaf water balance mediated by aquaporins under salt stress and associated glucosinolate synthesis in broccoli. Plant Science 174, 321–328 [Google Scholar]
- Loreti E, Povero G, Novi G, Solfanelli C, Alpi A, Perata P. 2008. Gibberellins, jasmonate and abscisic acid modulate the sucrose-induced expression of anthocyanin biosynthetic genes in Arabidopsis. New Phytologist 179, 1004–1016 [DOI] [PubMed] [Google Scholar]
- Mewis I, Appel HM, Hom A, Raina R, Schultz JC. 2005. Major signaling pathways modulate Arabidopsis glucosinolate accumulation and response to both phloem-feeding and chewing insects. Plant Physiology 138, 1149–1162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikkelsen MD, Petersen BL, Glawischnig E, Jensen AB, Andreasson E, Halkier BA. 2003. Modulation of CYP79 genes and glucosinolate profiles in Arabidopsis by defense signaling pathways. Plant Physiology 131, 298–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore B, Zhou L, Rolland F, Hall Q, Cheng WH, Liu YX, Hwang I, Jones T, Sheen J. 2003. Role of the Arabidopsis glucose sensor HXK1 in nutrient, light, and hormonal signaling. Science’s STKE 300, 332–336 [DOI] [PubMed] [Google Scholar]
- Price J, Laxmi A, Martin SKS, Jang JC. 2004. Global transcription profiling reveals multiple sugar signal transduction mechanisms in Arabidopsis. The Plant Cell 16, 2128–2150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramon M, Rolland F, Sheen J. 2008. Sugar sensing and signaling. The Arabidopsis Book 6, e0117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves WM, Lynch TJ, Mobin R, Finkelstein RR. 2011. Direct targets of the transcription factors ABA-Insensitive (ABI) 4 and ABI5 reveal synergistic action by ABI4 and several bZIP ABA response factors. Plant Molecular Biology 75, 347–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reintanz B, Lehnen M, Reichelt M, Gershenzon J, Kowalczyk M, Sandberg G, Godde M, Uhl R, Palme K. 2001. Bus, a bushy Arabidopsis CYP79F1 knockout mutant with abolished synthesis of short-chain aliphatic glucosinolates. The Plant Cell 13, 351–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolland F, Baena-Gonzalez E, Sheen J. 2006. Sugar sensing and signaling in plants: conserved and novel mechanisms. Annual Review of Plant Biology 57, 675–709 [DOI] [PubMed] [Google Scholar]
- Rook F, Hadingham SA, Li Y, Bevan MW. 2006. Sugar and ABA response pathways and the control of gene expression. Plant, Cell and Environment 29, 426–434 [DOI] [PubMed] [Google Scholar]
- Sønderby IE, Geu-Flores F, Halkier BA. 2010. Biosynthesis of glucosinolates—gene discovery and beyond. Trends in Plant Science 15, 283–290 [DOI] [PubMed] [Google Scholar]
- Sønderby IE, Hansen BG, Bjarnholt N, Ticconi C, Halkier BA, Kliebenstein DJ. 2007. A systems biology approach identifies a R2R3 MYB gene subfamily with distinct and overlapping functions in regulation of aliphatic glucosinolates. PLoS One 2, e1322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saavedra MJ, Dias C, Martinez-Murcia A, Bennett RN, Aires A, Rosa E. 2012. Antibacterial effects of glucosinolate-derived hydrolysis products against enterobacteriaceae and enterococci isolated from pig ileum segments. Foodborne Pathogens and Disease 9, 338–345 [DOI] [PubMed] [Google Scholar]
- Smeekens S, Ma J, Hanson J, Rolland F. 2010. Sugar signals and molecular networks controlling plant growth. Current Opinion in Plant Biology 13, 274–279 [DOI] [PubMed] [Google Scholar]
- Sun B, Liu N, Zhao YT, Yan HZ, Wang QM. 2011. a .Variation of glucosinolates in three edible parts of Chinese kale (Brassica alboglabra Bailey) varieties. Food Chemistry 124, 941–947 [Google Scholar]
- Sun B, Yan HZ, Liu N, Wei J, Wang QM. 2011. b Effect of 1-MCP treatment on postharvest quality characters, antioxidants and glucosinolates of Chinese kale. Food Chemistry 131, 519–526 [Google Scholar]
- Tantikanjana T, Mikkelsen MD, Hussain M, Halkier BA, Sundaresan V. 2004. Functional analysis of the tandem-duplicated P450 genes SPS/BUS/CYP79F1 and CYP79F2 in glucosinolate biosynthesis and plant development by Ds transposition-generated double mutants. Plant Physiology 135, 840–848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng S, Keurentjes J, Bentsink L, Koornneef M, Smeekens S. 2005. Sucrose-specific induction of anthocyanin biosynthesis in Arabidopsis requires the MYB75/PAP1 gene. Plant Physiology 139, 1840–1852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei J, Miao HY, Wang QM. 2011. Effect of glucose on glucosinolates, antioxidants and metabolic enzymes in Brassica sprouts. Scientia Horticulturae 129, 535–540 [Google Scholar]
- Xiao W, Sheen J, Jang JC. 2000. The role of hexokinase in plant sugar signal transduction and growth and development. Plant Molecular Biology 44, 451–461 [DOI] [PubMed] [Google Scholar]
- Yan XF, Chen SX. 2007. Regulation of plant glucosinolate metabolism. Planta 226, 1343–1352 [DOI] [PubMed] [Google Scholar]
- Yatusevich R. 2008. Analysis of the MYB28, MYB29 and MYB76 transcription factors involved in the biosynthesis of aliphatic glucosinolates in Arabidopsis thaliana. PhD thesis, Universität zu Köln: [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.