Abstract
The coordination of plant cell division and expansion controls plant morphogenesis, development, and growth. Cyclin-dependent kinases (CDKs) are not only key regulators of cell division but also play an important role in cell differentiation. In plants, CDK activity is modulated by the binding of INHIBITOR OF CDK/KIP-RELATED PROTEIN (ICK/KRP). Previously, ICK2/KRP2 has been shown to mediate auxin responses in lateral root initiation. Here are analysed the roles of all ICK/KRP genes in root growth. Analysis of ick/krp null-mutants revealed that only ick3/krp5 was affected in primary root growth. ICK3/KRP5 is strongly expressed in the root apical meristem (RAM), with lower expression in the expansion zone. ick3/krp5 roots grow more slowly than wildtype controls, and this results not from reduction of division in the proliferative region of the RAM but rather reduced expansion as cells exit the meristem. This leads to shorter final cell lengths in different tissues of the ick3/krp5 mutant root, particularly the epidermal non-hair cells, and this reduction in cell size correlates with reduced endoreduplication. Loss of ICK3/KRP5 also leads to delayed germination and in the mature embryo ICK3/KRP5 is specifically expressed in the transition zone between root and hypocotyl. Cells in the transition zone were smaller in the ick3/krp5 mutant, despite the absence of endoreduplication in the embryo suggesting a direct effect of ICK3/KRP5 on cell growth. It is concluded that ICK3/KRP5 is a positive regulator of both cell growth and endoreduplication.
Key words: Arabidopsis, CDK, cell cycle, cell division, cell expansion, development, root growth
Introduction
Plant growth results from the combined processes of cell division and subsequent cell expansion (Gonzalez et al., 2012). The continuously growing root is a useful system to study the interrelationship of cell division and elongation, as the two processes take place in spatially distinct regions of the root tip (De Smet et al., 2007; Bennett and Scheres, 2010). The root apical meristem (RAM) distal to the quiescent centre (QC) is known as the proximal meristem and contains cells whose size varies by a maximum of approximately 2-fold due to active cell growth and division (De Smet et al., 2007; Perilli et al., 2012). Cells exiting the proximal meristem start to differentiate and elongate in the distal meristem or transition zone, and pass through a zone of rapid elongation and differentiation to end up as fully elongated and differentiated root cells. The balance between cell division and differentiation/elongation is important for the maintenance and size of the primary meristem and controls the overall rate of root growth (Beemster and Baskin, 1998).
The regulation of cell division in plants is achieved through the activity of cyclin-dependent kinase (CDK) complexes. The response of cell division to hormonal, environmental, and development signals is integrated in part through the D-type cyclin (CYCD)- RETINOBLASTOMA-RELATED (RBR) pathway (de Jager et al., 2005; Dewitte et al., 2007; Nieuwland et al., 2009; Sanz et al., 2011; Komaki and Sugimoto, 2012). CYCDs bind and activate the canonical cyclin-dependent kinase CDKA and thereby contribute to phosphorylation of the RBR protein (Dewitte and Murray, 2003; De Veylder et al., 2007). RBR negatively regulates E2F transcription factors, whose activity is required for progress through the cell cycle and the initiation of DNA synthesis in S-phase.
Cellular expansion and differentiation are normally associated in Arabidopsis with progressive endocycles involving the replication of the genome without mitosis, leading to repeated doubling of the nuclear genome content (Galbraith et al., 1991; Ishida et al., 2010). Reduction in the auxin signal, which is highest around the QC, in the more distal region of the RAM is likely to be involved in triggering this switch from mitotic division cycles to endocycles (Ishida et al., 2010).
Ectopic overexpression of CYCD3s promotes cell division and reduces endocycles and associated cell expansion and differentiation (Dewitte et al., 2003, 2007). Overexpression of CYCD2;1 in Arabidopsis roots was similarly shown to cause enhanced cell division and inhibit endoreduplication (Qi and John, 2007) without affecting overall root growth. Together, these results support a role for CYCD activity in promoting mitotic cycles and inhibiting endocycles, a conclusion supported by analysis of a mutant lacking all three Arabidopsis genes encoding CYCD3 (Dewitte et al., 2007).
The INHIBITOR OF CDK/KIP-RELATED PROTEIN (ICK/KRP) family encodes proteins that interact with CYCD and CDKA proteins to modulate their activity. In Arabidopsis, seven ICK/KRP family members have been identified and all have been shown to be able to inhibit CDK activity in vitro (Wang et al., 1997 , 1998; Lui et al., 2000; Verkest et al., 2005; Nakai et al., 2006). The activity of ICK/KRPs has mainly been investigated using ectopic overexpression, and in this case the seven ICK/KRPs show common phenotypes of smaller plant size, reduced cell number, and larger cells (Wang et al., 2000; De Veylder et al., 2001; Zhou et al., 2002; Verkest et al., 2005; Bemis and Torii, 2007; Kang et al., 2007). High levels of overexpression caused a general inhibition of cell cycle progression and reduced ploidy levels (De Veylder et al., 2001; Jasinski et al., 2002; Zhou et al., 2002; Barroco et al., 2006), whereas lower levels of overexpression led to earlier onset of endoreduplication (Coelho et al., 2005; Verkest et al., 2005; Weinl et al., 2005). Therefore, it was proposed that high levels of ICK/KRPs may block both the G1/S and G2/M cell cycle transitions whilst lower levels preferentially block mitotic cycles and promote endoreduplication (Torres Acosta et al., 2011).
The dissection of potential different functions of the genes within the ICK/KRP family has been limited, although the genes show distinct expression patterns (Wang et al., 1998; Lui et al., 2000; De Veylder et al., 2001; Ormenese et al., 2004; Menges et al., 2005; de Almeida Engler et al., 2009; Torres Acosta et al., 2011), intracellular localization (Jakoby et al., 2006; Bird et al., 2007), and phylogenetic relationships (Torres Acosta et al., 2011). The Arabidopsis genes fall into two evolutionary conserved groups, KRP1,2,6,7 and KRP3,4,5. However, of these groupings, only ICK1/KRP1 and ICK2/KRP2 are more closely related to each other than to genes in other species, suggestive of potential conserved distinct functions for the other genes (Torres Acosta et al., 2011).
Some specificity of biochemical action of KRPs has been found. For example, ICK2/KRP2 has the strongest interaction of KRPs with CYCD2;1, and in vivo, ICK2/KRP2 levels affect both CYCD2;1 activity and cellular localization (Sanz et al., 2011). Indeed, CYCD2;1 was unable to bind to CDKA on its own in yeast two-hybrid assays, but when ICK2/KRP2 was also introduced, CYCD2;1 was able to interact with CDKA suggesting a bridging function for ICK2/KRP2 (Sanz et al., 2011). Several specific ubiquitin-protein ligases (E3) can target different ICK/KRPs for destruction by the ubiquitin/proteasome pathway (Kim et al., 2008; Liu et al., 2008; Ren et al., 2008).
Mutant phenotypes have to date only been identified for ICK2/KRP2, revealing it to be a negative regulator of lateral root formation whose protein levels are reduced by auxin (Sanz et al., 2011). The present study investigates the wider role of ICK/KRP genes in root growth. It demonstrates that ICK3/KRP5 has a specific function in promoting root growth and acts through promoting cell elongation and endoreduplication in the zone of rapid expansion, as well as determining cell size in the embryo transition zone with an impact in the mutant on germination rate.
Materials and methods
Plant material
The wild-type Arabidopsis thaliana ecotypes Col-0 and WS were obtained from the Nottingham Arabidopsis Stock Centre (NASC). The loss-of-function ick1/krp1-1 mutant has been described (Ren et al., 2008; Sanz et al., 2011). The loss-of-function mutant ick6/krp3 was kindly provided by CropDesign (Gent, Belgium) and is in the WS background. Loss-of-function mutants ick7/krp4 (TAIR accession SALK_102417), ick3/krp5-1 (TAIR accession SALK_053533), ick3/krp5-2 (TAIR accession SK27217), ick4/krp6 (TAIR accession SAIL_548_B03), and ick5/krp7 (TAIR accession GK-841D12–025740) were obtained from NASC (Scholl et al., 2000), the SALK (Alonso et al., 2003), SK (Robinson et al., 2009), and GABI-Kat (Kleinboelting et al., 2012) collections.
T-DNA insertion sites were confirmed by PCR on genomic DNA and sequence analysis of the PCR product. Total RNA was extracted to confirm a reduction of the full-length transcript by quantitative real-time (qRT) PCR (Dewitte et al., 2003) spanning the insertion site. qRT-PCR was performed as described (Nieuwland et al., 2009).
Growth conditions
Plants were sterilized in 5% sodium hypochlorite solution and placed on square Petri plates containing Murashige and Skoog (MS) medium with 0.75% sucrose and 0.5g l–1 (N-morpholino)ethanesulfonic acid (MES), pH 5.8, and 1% agar. Seeds were imbibed in the dark at 4 °C for 3 days, and plates were incubated vertically in a Percival CU41-L4D cabinet using a 18/6h light/dark cycle (125 μmol m–2 s–1) at 21 °C.
Primary and lateral root measurements and cell size measurements
Primary root length and lateral root density measurements were performed as described (Nieuwland et al., 2009). Microscopy and sample preparation for cell size measurements were carried out as described (Nieuwland et al., 2009). Cells were measured using ImageJ and using Cell Analyzer on MatLab as described (Quelhas et al., 2011; Schneider et al., 2012).
Plasmid construction
Promoter sequences were amplified from genomic Arabidopsis DNA by high-fidelity PCR using Phusion Taq (New England Biolabs). The start of the KRP5 promotor was chosen from the end of the 3’-untranslated region from the upstream gene (AT3G24800) using primer 5’-CACCGCATATGCTTTCGCTTTGTG-3’. The 3’-end of the genomic fragment was amplified up to the stop codon of KRP5 using primer 5’-CTCCGGGAAGGTGGTTTACTG-3’. Promoter PCR fragments were cloned into pENTR-D-TOPO (Invitrogen) and then subcloned using Gateway technology (Invitrogen) into pKGWFS7 (Karimi et al., 2002). The constructs in binary vectors were introduced into Agrobacterium tumefaciens GV3101 and plants were transformed by floral dipping (Clough and Bent, 1998).
Flow cytometry
Samples of more than 30 roots were harvested after 10 days of vertical growth on plate. Nuclei were released by chopping and analysed as described (Menges and Murray, 2002).
Seed germination assay
Ick3/krp5-1 seeds together with WT controls were sown on a prewetted filter paper which was arranged in square Peri plates and stratified at 4 °C for 3 days in the dark to ensure synchronous germination before moving to a Percival CU41-L4D cabinet and growth under a 18/6h light/dark cycle (125 μmol m–2s–1) at 21 °C. Images were recorded over time and scored for radicle protrusion up to 48h after germination.
Microscopy
Histochemical staining for GUS activity was performed essentially as described (Jefferson et al., 1987). Whole-mount samples were analysed and photographed using a Zeiss Imager M1 microscope. For living imaging of GFP, cell walls in roots were counterstained with 4mg ml–1 propidium iodide in water, and fluorescence was examined with a Zeiss 710 Meta confocal microscope.
Nuclei size measurements
Mature roots were harvested from 10-day-old seedlings and fixed overnight in FAA (3.7% paraformaldehyde, 81% ethanol, 5% glacial acetic acid). After washing with water, samples were mounted in Vectashield with DAPI (Vector Laboratories, Burlingame, CA, USA) on slides. The samples were kept in the dark at 4 °C for at least 2 days before being examined by confocal microscopy. Z-stacks were taken from root cells and the maximum area of the nuclei was selected for measurement.
Results and discussion
Effect of loss-of-function of ICK/KRPs in primary root growth
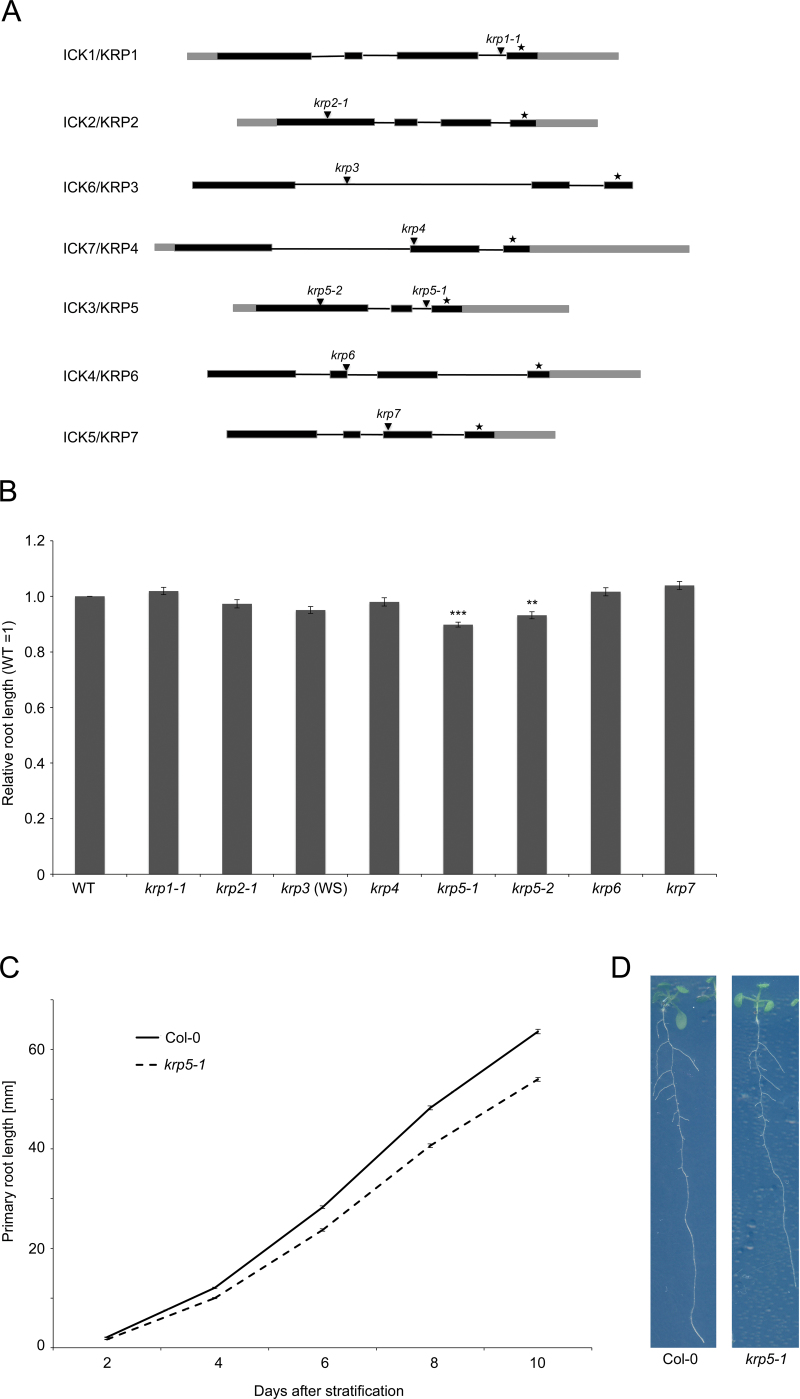
Microarray analyses of sorted cell types and tissues in the Arabidopsis root indicate that ICK/KRPs have distinct expression patterns, suggesting possible specific functions in root growth and development (Brady et al., 2007). To investigate the role of each 7 ICK/KRPs in root growth, the current study collected available null-mutants from several public mutant collections, in addition to the previously published ick1-1/krp1-1 and ick2-1/krp2-1 alleles (Ren et al., 2008; Sanz et al., 2011). The insertion sites and absence of full-length transcripts were confirmed in each mutant line using primers spanning the coding region (Fig. 1A). The ick2/krp2 mutant has been previously described as having increased lateral root initiation and altered response to auxin in lateral root induction (Sanz et al., 2011). The ick/krp mutant lines were grown on vertical plates for 10 days and root growth was measured (Fig. 1B). The ick3/krp5-1 (hereafter krp5-1) loss-of-function mutant showed an approximately 10% reduction (t-test P < 0.001) in primary root growth compared to WT, whereas other mutants were not affected in primary root growth (Fig. 1B, C). Analysis of the rate of growth of krp5-1 mutant roots from days 2–10 demonstrated a reduced growth rate compared to WT (Fig. 1C and Supplementary Table S1, available at JXB online). To ensure that the effect on root growth was not caused by modulation of expression levels of the other six KRP genes in the krp5-1 mutant, all KRP levels were measured and no significant changes in KRP levels except KRP5 were found in krp5-1 (Supplementary Fig. S2). Furthermore, the reduction in root growth was confirmed in the independent allele krp5-2 (7% reduction, t-test P < 0.05) (Fig. 1B). Although ick2/krp2 mutants show an increased lateral root density as reported (Sanz et al., 2011), no difference in lateral root density was observed in other ick/krp mutants (Supplementary Fig. 1).
Fig. 1.
ICK/KRP gene family structure, mutants, and primary root phenotypes. (A) Gene structure of the seven members of the ICK/KRP family. Black boxes and lines indicate predicted exons and introns, respectively, grey boxes indicate untranslated regions, asterisks indicate the most conserved exon, which includes the CDKA and CYCD binding sites, and arrowheads indicate the positions of T-DNA insertions in the loss-of-function mutants used in this study. (B) Primary root length of the ick/krp loss-of-function mutants relative to WT. Seedlings were grown vertically for 10 days and root lengths are relative to Col-0 for all mutants except krp3 which was relative to WS. *** indicates t-test P < 0.001; ** indicates P < 0.05 (n = 40). Error bars represent standard error of the mean. (C) Average primary root lengths of WT and krp5-1 plants at 2, 4, 6, 8, and 10 days after completion of stratification. Error bars represent standard error of the mean (n = 40). (D) Representative examples of 10-day-old Col-0 and krp5-1 seedlings showing a reduction of primary root growth in krp5-1 (this figure is available in colour at JXB online).
The current data suggest that, among the ICK/KRP genes, only KRP5 has a rate-limiting role in primary root growth. However, since transcripts of other ICK/KRPs are present in the root (Brady et al., 2007; Winter et al., 2007), it is possible that they have overlapping and redundant functions in root growth and development.
Loss of KRP5 function leads not to an increase in root growth as might be expected from its supposed function as a CDK inhibitor, but rather to decreased growth. Previous analysis shows that ICK2/KRP2 promotes the assembly of CYCD2;1–CDKA complexes (Sanz et al., 2011), indicating that ICK/KRP proteins could have both positive and negative effects on overall growth rate.
Expression analysis of KRP5 in the root
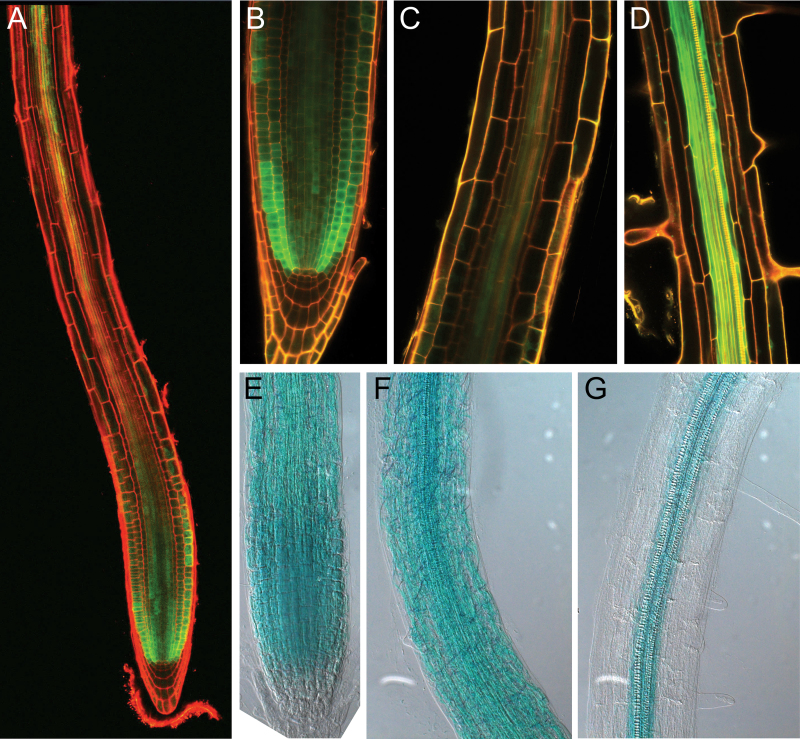
To examine the expression pattern of KRP5 in the root, a GUS-GFP fusion protein was placed under control of the KRP5 promoter. In the root apical meristem, GFP signal controlled by the KRP5 promoter was highest in the epidermis and cortex, although lower signal could be detected in all other tissues except lateral root cap and columella (Fig. 2A, B). Overall GUS activity revealed that strongest expression was found in the proximal meristem region (Fig. 2E). In the transition zone, the GFP signal was found to become weaker distally in the epidermis, cortex, and endodermis whereas GFP or GUS remained stronger in the stele (Figure 2C, F). In the mature root, GFP signal and corresponding GUS activity was found only in the stele (Figure 2D, G). Since root growth is dependent on both cell division in the RAM and cell elongation in the transition/elongation zones, the expression pattern of KRP5 is compatible with a function in root growth acting in either region.
Fig. 2.
Expression analysis of KRP5 in the root. GUS-GFP was driven by the promoter of KRP5. (A) In general, KRP5 is expressed in the RAM and in the stele of the mature root. (B, E) Accumulation of GFP and GUS activity is highest in epidermal and cortical cells in the apex of the RAM. (C, F) Expression is maintained at a lower level in the elongation zone. (D, G) KRP5 expression is restricted to the stele outside the elongation zone.
KRP5 is a positive regulator of cell elongation and endoreduplication during root growth
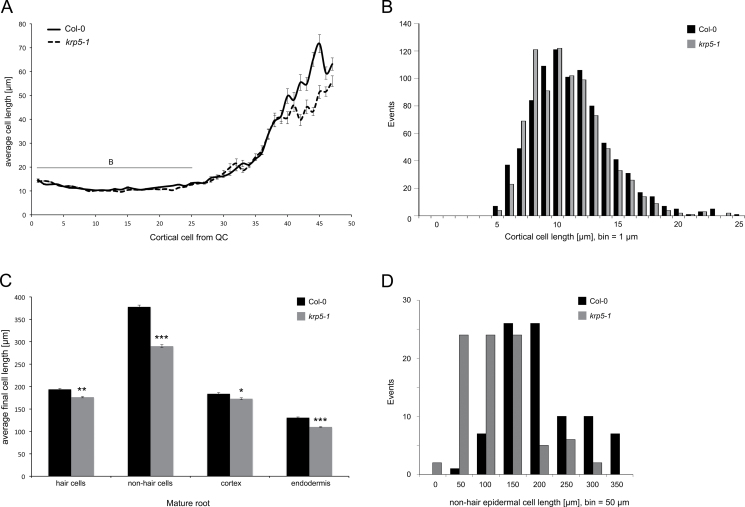
To establish the site of action of KRP5, cells in these parts of the root were analysed in detail. Cortical cell lengths were measured as cell sizes in the RAM and transition zone, starting from the QC in both WT and krp5-1 roots (n = 50; Fig. 3A), and the average cortical cell lengths in both the proximal and distal regions of the RAM were unaffected in krp5-1 mutants. The distribution of cortical cell lengths was also unaffected (Fig. 3B), suggesting no difference in either growth rate or cell size at division for krp5-1 RAM cells. The overall meristem size of the root can be estimated by measuring the distance between the QC and where cortical cells double in length. Using this estimation, the results show that meristem sizes in krp5-1 roots are equivalent to WT.
Fig. 3.
Loss of KRP5 leads to a reduction in cell size in the root. (A) Cortical cell lengths measured from the QC in 10-day-old roots. No difference in cell lengths within the meristem could be detected between krp5-1 and WT, resulting a similar RAM size. Krp5-1 cortical cells in the elongation zone are impaired in growth (n = 50). (B) Histogram of cortical cell lengths from cells in the meristem (cells 1–25 in part A) showing the distribution of cell lengths in WT and krp5-1. (C) The final cell lengths of mature epidermal hair and non-hair cells, cortical cells and endodermal cells were all found to be smaller in krp5-1 compared to WT (n > 100). *** indicates t-test P < 0.001; ** indicates P < 0.01; * indicates P < 0.05. Error bars represent standard error of the mean. (D) Histogram showing the distribution of mature cell lengths of non-hair epidermal cells in WT and krp5-1.
However, in the elongation zone, cells in krp5-1 mutants slowed their rate of expansion earlier than in WT roots (Fig. 3A). To establish whether elongated cells eventually reach a WT size in the krp5-1 mutant or remain smaller in the mature root, cell lengths were measured in the mature root where cells have reached their final size (Fig. 3C). Epidermal, cortical, and endodermal cells remained smaller in krp5-1 mutant plants, the largest reduction of cell length being in non-hair epidermal cells. The distribution of lengths of non-hair epidermal cells was shifted towards smaller cells for krp5-1 but the overall shape of the distribution curve was maintained (Fig. 3D).
Taken together, these results show that KRP5 promotes cell elongation in the root distally from the RAM, and the reduction in cell elongation rate corresponds to a smaller mature cell size sufficient to explain the reduced growth rate.
ICK3/KRP5 promotes progression of endoreduplication in roots
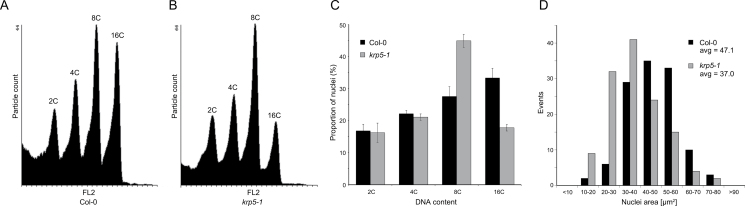
There are several examples of correlation between cell elongation and endoreduplication (Melaragno et al., 1993; Gendreau et al., 1998; Kato and Lam, 2003; Barow, 2006). Furthermore, KRPs have generally been implicated as positive regulators of endoreduplication (Coelho et al., 2005; Verkest et al., 2005; Weinl et al., 2005), although examples also exist where they are inhibitory, probably due to a highly elevated expression level (Schnittger et al., 2003; Verkest et al., 2005). To test whether the krp5-1 mutant has a different DNA ploidy profile, mutant roots were analysed in comparison with WT using flow cytometry (Fig. 4A–C). The total amount of nuclei with higher DNA content than 4C was not changed in the mutant, but the proportion of 16C nuclei was drastically reduced and that of 8C nuclei increased (Fig. 4C). This suggests that KRP5 promotes higher endoreduplication rounds in roots or that the reduced cell growth in krp5-1 leads to an earlier cessation of endocycles.
Fig. 4.
Loss of KRP5 leads to a reduced progression through endoreduplication in the root and is correlated with smaller nuclei. (A, B) Representative ploidy histograms for nuclei (n > 5000) isolated from roots of WT and krp5-1. 2C peak, G1 DNA content; 4C peak, G2 DNA content; 8C and 16C peaks, endoreduplicating cells. (C) Flow cytometry analysis of ploidy levels in roots of WT and krp5-1 (n = 4). The proportion of nuclei with 16C DNA is decreased and of 8C DNA is increased (t-test P < 0.01) in the krp5-1 root without changing the proportion of 2C and 4C. (D) Nuclear size (area) of mature root epidermal cells from WT and krp5-1 plants (n > 120). Mean sizes of WT and krp5-1 nuclei are 47.1 μm2 (SEM 1.18) and 37.0 μm2 (SEM 1.13), respectively (t-test P << 0.001).
Since flow cytometry analysis is based on nuclei released from all root cell types, this study sought to establish whether the reduced epidermal cell size of the krp5-1 mutant was specifically correlated with reduced ploidy. Nuclear size correlates with DNA content (Galbraith et al., 1991; Friedman, 1999; Beeckman et al., 2001; Tian et al., 2005), so the maximal cross-sectional nuclear area of epidermal cells was quantified using confocal stacks. Krp5-1 mutants showed a 10% reduction in average nuclear size in epidermal cells compared to WT, corresponding to a higher proportion of smaller nuclei (Fig. 4D). In conclusion, KRP5 promotes subsequent rounds of endoreduplication in roots, which correlates with larger cells and nuclei.
KRP5 enhances germination and cell size in the embryonic root
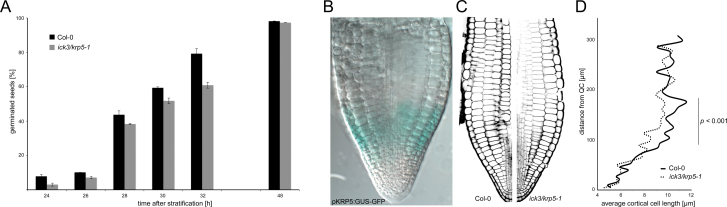
Germination is a process driven largely by cell expansion (Bewley, 1997; Koornneef et al., 2002), and therefore the rate of germination in krp5-1 and krp5-2 mutants was investigated (Fig. 5A and Supplementary Fig. S3). To reduce possible effects of parental growth conditions, krp5 mutant plants were grown together with their respective Col-0 control plants, and seeds were harvested at the same time. Loss of function of KRP5 led to a delay in germination in both independent alleles, corresponding to an average delay of approximately 2h in reaching 50% germination. The expression pattern of KRP5 during germination was investigated and, in seeds imbibed at 4 °C for 3 days, the KRP5 promoter is active in the region known as the transition zone at the hypocotyl–radicle junction (Fig. 5B) (Esau, 1965; Lin and Schiefelbein, 2001). Therefore, this study determined the sizes of cortical cells from the radicle tip into the transition zone in krp5-1 and WT embryos. In krp5-1, the average cortical cell length was significantly reduced in the transition zone where KRP5 is expressed (Figure 5C, D).
Fig. 5.
The function of KRP5 in germination. (A) Germination of krp5-1 seeds compared to Col-0 control seeds harvested at the same time (n > 70). (B) The KRP5 promoter is specifically expressed in the transition zone of the embryo. Embryo was isolated in mid-imbibition and GUS-stained. (C,D) Average cortical cell lengths in WT and krp5-1 embryos, measured from the QC (n = 14). Cortical cell lengths remain significantly smaller in the transition zone of krp5-1 embryos (P < 0.001).
Within the mature Arabidopsis seed, all cells are present at a ploidy of 2C (Masubelele et al., 2005), and the smaller size of these cells in the krp5-1 mutant is therefore consistent with a reduced cell growth independent of endoreduplication in this tissue.
Concluding remarks
This paper shows that KRP5 has a specific function in root growth in promoting both cell growth in the expansion zone and endoreduplication, and in determining cell size in the transition zone of the mature embryo. Loss-of-function of KRP5 leads to smaller roots and reduced final cell sizes in the root, suggesting that KRP5 acts as a promoter of cell elongation. Furthermore, KRP5 promotes progression of endoreduplication in roots and that KRP5 is rate limiting for nuclear size in mature root epidermal cells since loss of KRP5 leads to a reduction of nuclear size. The action of KRP5 in promoting higher/later endocycles is distinct from the previously reported effects of ICK2/KRP2 and ICK1/KRP1 on promoting endocycles when ectopically expressed, since in these examples the effect occurs as a consequence of promoting an earlier exit from the mitotic cell cycle (De Veylder et al., 2001; Verkest et al., 2005; Weinl et al., 2005). Indeed high-level expression of ICK/KRP has been found to inhibit both mitotic cycles and endocycles (Schnittger et al., 2003; Verkest et al., 2005).
In embryos, KRP5 is specifically expressed in the transition zone and loss of function causes a delay in germination correlated with a reduction of cell length in cortical transition zone cells. Since endoreduplication initiates only after radicle protrusion (Barroco et al., 2005; Masubelele et al., 2005), the promoting effect of KRP5 on cell size during germination does not seem to correlate with a stimulation of endoreduplication, as shown here in roots. This raises the possibility that KRP5 controls cell expansion independently of the cell cycle. Taken together, the data suggest that ICK3/KRP5 has promotive effect on growth by stimulating endoreduplication and promoting cell elongation.
Supplementary material
Supplementary data are available at JXB online.
Supplementary Fig. S1. Lateral root density in 10-day-old seedlings for WT and ick/krp mutants.
Supplementary Fig. S2. Expression of KRP genes in 10-day-old seedlings in krp5-1 relative to Col-0 by quantitative real-time PCR.
Supplementary Fig. S3. Germination of krp5-2 seeds compared to Col-0 control seeds harvested at the same time.
Supplementary Table S1. Raw data for Fig. 1C.
Acknowledgements
The authors thank Angela Marchbank and Jo Kilby for excellent technical support and Hong Wang for help on the ick5/krp7 mutant. BW and JN were supported by BBSRC grant BB/G00482X.
References
- Alonso JM, Stepanova AN, Leisse TJ, et al. 2003. Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301, 653–657 [DOI] [PubMed] [Google Scholar]
- Barow M. 2006. Endopolyploidy in seed plants. Bioessays 28, 271–281 [DOI] [PubMed] [Google Scholar]
- Barroco RM, Peres A, Droual AM, et al. 2006. The cyclin-dependent kinase inhibitor Orysa;KRP1 plays an important role in seed development of rice. Plant Physiology 142, 1053–1064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barroco RM, Van Poucke K, Bergervoet JH, De Veylder L, Groot SP, Inze D, Engler G. 2005. The role of the cell cycle machinery in resumption of postembryonic development. Plant Physiology 137, 127–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeckman T, Burssens S, Inze D. 2001. The peri-cell-cycle in Arabidopsis. Journal of Experimental Botany 52, 403–411 [DOI] [PubMed] [Google Scholar]
- Beemster GT, Baskin TI. 1998. Analysis of cell division and elongation underlying the developmental acceleration of root growth in Arabidopsis thaliana . Plant Physiology 116, 1515–1526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bemis SM, Torii KU. 2007. Autonomy of cell proliferation and developmental programs during Arabidopsis aboveground organ morphogenesis. Developmental Biology 304, 367–381 [DOI] [PubMed] [Google Scholar]
- Bennett T, Scheres B. 2010. Root development-two meristems for the price of one?. Current topics in developmental biology 91, 67–102 [DOI] [PubMed] [Google Scholar]
- Bewley JD. 1997. Seed Germination and Dormancy. The Plant Cell 9, 1055–1066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird DA, Buruiana MM, Zhou Y, Fowke LC, Wang H. 2007. Arabidopsis cyclin-dependent kinase inhibitors are nuclear-localized and show different localization patterns within the nucleoplasm. Plant Cell Reports 26, 861–872 [DOI] [PubMed] [Google Scholar]
- Brady SM, Orlando DA, Lee JY, Wang JY, Koch J, Dinneny JR, Mace D, Ohler U, Benfey PN. 2007. A high-resolution root spatiotemporal map reveals dominant expression patterns. Science 318, 801–806 [DOI] [PubMed] [Google Scholar]
- Clough SJ, Bent AF. 1998. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana . The Plant Journal 16, 735–743 [DOI] [PubMed] [Google Scholar]
- Coelho CM, Dante RA, Sabelli PA, Sun Y, Dilkes BP, Gordon-Kamm WJ, Larkins BA. 2005. Cyclin-dependent kinase inhibitors in maize endosperm and their potential role in endoreduplication. Plant Physiology 138, 2323–2336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Almeida Engler J, De Veylder L, De Groodt R, et al. 2009. Systematic analysis of cell-cycle gene expression during Arabidopsis development. The Plant Journal 59, 645–660 [DOI] [PubMed] [Google Scholar]
- de Jager SM, Maughan S, Dewitte W, Scofield S, Murray JA. 2005. The developmental context of cell-cycle control in plants. Seminars in Cell and Developmental Biology 16, 385–396 [DOI] [PubMed] [Google Scholar]
- De Smet I, Tetsumura T, De Rybel B, et al. 2007. Auxin-dependent regulation of lateral root positioning in the basal meristem of Arabidopsis . Development 134, 681–690 [DOI] [PubMed] [Google Scholar]
- De Veylder L, Beeckman T, Beemster GT, Krols L, Terras F, Landrieu I, van der Schueren E, Maes S, Naudts M, Inze D. 2001. Functional analysis of cyclin-dependent kinase inhibitors of Arabidopsis . The Plant Cell 13, 1653–1668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Veylder L, Beeckman T, Inze D. 2007. The ins and outs of the plant cell cycle. Nature Reviews Molecular Cell Biology 8, 655–665 [DOI] [PubMed] [Google Scholar]
- Dewitte W, Murray JA. 2003. The plant cell cycle. Annual Review of Plant Biology 54, 235–264 [DOI] [PubMed] [Google Scholar]
- Dewitte W, Riou-Khamlichi C, Scofield S, Healy JM, Jacqmard A, Kilby NJ, Murray JA. 2003. Altered cell cycle distribution, hyperplasia, and inhibited differentiation in Arabidopsis caused by the D-type cyclin CYCD3. The Plant Cell 15, 79–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewitte W, Scofield S, Alcasabas AA, et al. 2007. Arabidopsis CYCD3 D-type cyclins link cell proliferation and endocycles and are rate-limiting for cytokinin responses. Proceedings of the National Academy of Sciences, USA 104, 14537–14542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esau K. 1965. Plant anatomy. New York: John Wiley; [Google Scholar]
- Friedman WE. 1999. Expression of the cell cycle in sperm of Arabidopsis: implications for understanding patterns of gametogenesis and fertilization in plants and other eukaryotes. Development 126, 1065–1075 [DOI] [PubMed] [Google Scholar]
- Galbraith DW, Harkins KR, Knapp S. 1991. Systemic endopolyploidy in Arabidopsis thaliana . Plant Physiology 96, 985–989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendreau E, Hofte H, Grandjean O, Brown S, Traas J. 1998. Phytochrome controls the number of endoreduplication cycles in the Arabidopsis thaliana hypocotyl. The Plant Journal 13, 221–230 [DOI] [PubMed] [Google Scholar]
- Gonzalez N, Vanhaeren H, Inze D. 2012. Leaf size control: complex coordination of cell division and expansion. Trends in Plant Science 17, 332–340 [DOI] [PubMed] [Google Scholar]
- Ishida T, Adachi S, Yoshimura M, Shimizu K, Umeda M, Sugimoto K. 2010. Auxin modulates the transition from the mitotic cycle to the endocycle in Arabidopsis . Development 137, 63–71 [DOI] [PubMed] [Google Scholar]
- Jakoby MJ, Weinl C, Pusch S, Kuijt SJH, Merkle T, Dissmeyer N, Schnittger A. 2006. Analysis of the subcellular localization, function, and proteolytic control of the Arabidopsis cyclin-dependent kinase inhibitor ICK1/KRP1. Plant Physiology 141, 1293–1305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasinski S, Riou-Khamlichi C, Roche O, Perennes C, Bergounioux C, Glab N. 2002. The CDK inhibitor NtKIS1a is involved in plant development, endoreduplication and restores normal development of cyclin D3;1-overexpressing plants. Journal of Cell Science 115, 973–982 [DOI] [PubMed] [Google Scholar]
- Jefferson RA, Kavanagh TA, Bevan MW. 1987. Gus fusions: beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO Journal 6, 3901–3907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang J, Mizukami Y, Wang H, Fowke L, Dengler NG. 2007. Modification of cell proliferation patterns alters leaf vein architecture in Arabidopsis thaliana . Planta 226, 1207–1218 [DOI] [PubMed] [Google Scholar]
- Karimi M, Inze D, Depicker A. 2002. GATEWAY((TM)) vectors for Agrobacterium-mediated plant transformation. Trends in Plant Science 7, 193–195 [DOI] [PubMed] [Google Scholar]
- Kato N, Lam E. 2003. Chromatin of endoreduplicated pavement cells has greater range of movement than that of diploid guard cells in Arabidopsis thaliana . Journal of Cell Science 116, 2195–2201 [DOI] [PubMed] [Google Scholar]
- Kim HJ, Oh SA, Brownfield L, Hong SH, Ryu H, Hwang I, Twell D, Nam HG. 2008. Control of plant germline proliferation by SCF(FBL17) degradation of cell cycle inhibitors. Nature 455, 1134–1137 [DOI] [PubMed] [Google Scholar]
- Kleinboelting N, Huep G, Kloetgen A, Viehoever P, Weisshaar B. 2012. GABI-Kat SimpleSearch: new features of the Arabidopsis thaliana T-DNA mutant database. Nucleic Acids Research 40, D1211–D1215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komaki S, Sugimoto K. 2012. Control of the plant cell cycle by developmental and environmental cues. Plant and Cell Physiology 53, 953–64 [DOI] [PubMed] [Google Scholar]
- Koornneef M, Bentsink L, Hilhorst H. 2002. Seed dormancy and germination. Current Opinion in Plant Biology 5, 33–36 [DOI] [PubMed] [Google Scholar]
- Lin Y, Schiefelbein J. 2001. Embryonic control of epidermal cell patterning in the root and hypocotyl of Arabidopsis . Development 128, 3697–3705 [DOI] [PubMed] [Google Scholar]
- Liu JJ, Zhang YY, Qin GJ, et al. 2008. Targeted degradation of the cyclin-dependent kinase inhibitor ICK4/KRP6 by RING-type E3 ligases is essential for mitotic cell cycle progression during Arabidopsis gametogenesis. The Plant Cell 20, 1538–1554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lui H, Wang H, DeLong C, Fowke LC, Crosby WL, Fobert PR. 2000. The Arabidopsis Cdc2a-interacting protein ICK2 is structurally related to ICK1 and is a potent inhibitor of cyclin-dependent kinase activity in vitro. The Plant Journal 21, 379–385 [DOI] [PubMed] [Google Scholar]
- Masubelele NH, Dewitte W, Menges M, Maughan S, Collins C, Huntley R, Nieuwland J, Scofield S, Murray JA. 2005. D-type cyclins activate division in the root apex to promote seed germination in Arabidopsis. Proceedings of the National Academy of Sciences, USA 102, 15694–15699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melaragno JE, Mehrotra B, Coleman AW. 1993. Relationship between endopolyploidy and cell size in epidermal tissue of Arabidopsis . The Plant Cell 5, 1661–1668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menges M, de Jager SM, Gruissem W, Murray JA. 2005. Global analysis of the core cell cycle regulators of Arabidopsis identifies novel genes, reveals multiple and highly specific profiles of expression and provides a coherent model for plant cell cycle control. The Plant Journal 41, 546–566 [DOI] [PubMed] [Google Scholar]
- Menges M, Murray JA. 2002. Synchronous Arabidopsis suspension cultures for analysis of cell-cycle gene activity. The Plant Journal 30, 203–212 [DOI] [PubMed] [Google Scholar]
- Nakai T, Kato K, Shinmyo A, Sekine M. 2006. Arabidopsis KRPs have distinct inhibitory activity toward cyclin D2-associated kinases, including plant-specific B-type cyclin-dependent kinase. FEBS Letters 580, 336–340 [DOI] [PubMed] [Google Scholar]
- Nieuwland J, Maughan S, Dewitte W, Scofield S, Sanz L, Murray JA. 2009. The D-type cyclin CYCD4;1 modulates lateral root density in Arabidopsis by affecting the basal meristem region. Proceedings of the National Academy of Sciences, USA 106, 22528–22533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ormenese S, de Almeida Engler J, De Groodt R, De Veylder L, Inze D, Jacqmard A. 2004. Analysis of the spatial expression pattern of seven Kip related proteins (KRPs) in the shoot apex of Arabidopsis thaliana . Annals of Botany 93, 575–580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perilli S, Di Mambro R, Sabatini S. 2012. Growth and development of the root apical meristem. Current Opinion in Plant Biology 15, 17–23 [DOI] [PubMed] [Google Scholar]
- Qi R, John PCL. 2007. Expression of genomic AtCYCD2;1 in Arabidopsis induces cell division at smaller cell sizes: implications for the control of plant growth. Plant Physiology 144, 1587–1597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quelhas P, Nieuwland J, Dewitte W, Mendonca AM, Murray J, Campilho A. 2011. Arabidopsis thaliana automatic cell file detection and cell length estimation. Springer Lecture Notes in Computer Science 6754, 1–11 [Google Scholar]
- Ren H, Santner A, del Pozo JC, Murray JA, Estelle M. 2008. Degradation of the cyclin-dependent kinase inhibitor KRP1 is regulated by two different ubiquitin E3 ligases. The Plant Journal 53, 705–716 [DOI] [PubMed] [Google Scholar]
- Robinson SJ, Tang LH, Mooney BAG, et al. 2009. An archived activation tagged population of Arabidopsis thaliana to facilitate forward genetics approaches. BMC Plant Biology 9, 101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz L, Dewitte W, Forzani C, et al. 2011. The Arabidopsis D-type cyclin CYCD2;1 and the inhibitor ICK2/KRP2 modulate auxin-induced lateral root formation. The Plant Cell 23, 641–660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider CA, Rasband WS, Eliceiri KW. 2012. NIH Image to ImageJ: 25 years of image analysis. Nature methods 9, 671–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnittger A, Weinl C, Bouyer D, Schobinger U, Hulskamp M. 2003. Misexpression of the cyclin-dependent kinase inhibitor ICK1/KRP1 in single-celled Arabidopsis trichomes reduces endoreduplication and cell size and induces cell death. The Plant Cell 15, 303–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholl RL, May ST, Ware DH. 2000. Seed and molecular resources for Arabidopsis . Plant Physiology 124, 1477–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian H, Yuan T, Russell S. 2005. Relationship between double fertilization and the cell cycle in male and female gametes of tobacco. Sexual Plant Reproduction 17, 243–252 [Google Scholar]
- Torres Acosta JA, Fowke LC, Wang H. 2011. Analyses of phylogeny, evolution, conserved sequences and genome-wide expression of the ICK/KRP family of plant CDK inhibitors. Annals of Botany 107, 1141–1157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkest A, Manes CLD, Vercruysse S, Maes S, Van der Schueren E, Beeckman T, Genschik P, Kuiper M, Inze D, De Veylder L. 2005. The cyclin-dependent kinase inhibitor KRP2 controls the onset of the endoreduplication cycle during Arabidopsis leaf development through inhibition of mitotic CDKA;1 kinase complexes. The Plant Cell 17, 1723–1736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Fowke LC, Crosby WL. 1997. A plant cyclin-dependent kinase inhibitor gene. Nature 386, 451–452 [DOI] [PubMed] [Google Scholar]
- Wang H, Qi QG, Schorr P, Cutler AJ, Crosby WL, Fowke LC. 1998. ICK1, a cyclin-dependent protein kinase inhibitor from Arabidopsis thaliana interacts with both Cdc2a and CycD3, and its expression is induced by abscisic acid. The Plant Journal 15, 501–510 [DOI] [PubMed] [Google Scholar]
- Wang H, Zhou YM, Gilmer S, Whitwill S, Fowke LC. 2000. Expression of the plant cyclin-dependent kinase inhibitor ICK1 affects cell division, plant growth and morphology. The Plant Journal 24, 613–623 [DOI] [PubMed] [Google Scholar]
- Weinl C, Marquardt S, Kuijt SJ, Nowack MK, Jakoby MJ, Hulskamp M, Schnittger A. 2005. Novel functions of plant cyclin-dependent kinase inhibitors, ICK1/KRP1, can act non-cell-autonomously and inhibit entry into mitosis. The Plant Cell 17, 1704–1722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter D, Vinegar B, Nahal H, Ammar R, Wilson GV, Provart NJ. 2007. An ‘Electronic Fluorescent Pictograph’ browser for exploring and analyzing large-scale biological data sets. PloS ONE 2, e718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Fowke LC, Wang H. 2002. Plant CDK inhibitors: studies of interactions with cell cycle regulators in the yeast two-hybrid system and functional comparisons in transgenic Arabidopsis plants. Plant Cell Reports 20, 967–975 [Google Scholar]