Abstract
The effect of oestrogen replacement therapy (ERT) on stroke incidence and severity has been extensively debated. Clinical trials of ERT have demonstrated an increased risk of stroke in treated women, although the study participants were well past menopause when therapy was initiated. It has been suggested that detrimental effects of ERT may be unmasked after prolonged periods of hypoestrogenicity. To date, very few studies have examined the effect of ERT in aged animals, although the timing of replacement may be critical to the neuroprotective effects of ERT. We hypothesised that chronic ERT initiated in late middle age would decrease infarct size in the brain after an induced stroke, whereas acute ERT would have no beneficial effects in aged females. To test this hypothesis, two paradigms of ERT were administered to aged mice of both sexes aiming to determine the effects on stroke outcome and to explore the possible mechanisms by which ERT interacts with age. Female mice that received chronic ERT from 17–20 months of age showed improved stroke outcomes after experimental stroke, whereas females that had acute ERT initiated at 20 months of age did not. Chronic ERT females exhibited diminished levels of nuclear factor kappa B (NF-κB) translocation compared to acute ERT females after stroke. Acute ERT females demonstrated both an increase in nuclear NF-κB and enhanced expression of pro-inflammatory cytokines. In addition, a sexual dimorphic effect of ERT was seen because males benefited from ERT, regardless of the timing of initiation. Aged males had significantly reduced expression of pro-inflammatory markers after stroke compared to age-matched females, suggesting a pro-inflammatory milieu emerges with age in females. These results are consistent with the emerging clinical literature suggesting that ERT should be initiated at the time of menopause to achieve beneficial effects. The present study demonstrates the importance of using appropriate animal models in preclinical studies.
Keywords: ageing, oestrogen, inflammation, sex differences, NF-κB
Stroke is a disease that mainly affects the elderly. Stroke rates more than double every 10 years after the age of 55 years in both men and women (1). However, functional outcomes after stroke are worse in women than in men, with higher associated disability and mortality (2). Most international databases consistently demonstrate that women enjoy a lower incidence of stroke relative to men until well after menopause, after which stroke incidence in women climbs dramatically (3, 4). Although the age-related risk of stroke is evident, very few studies have examined the neurochemical changes that occur in aged brains after stroke because most of studies have been exclusively performed in young animals.
Oestrogens, primarily 17β-oestradiol (E2), are potent neuroprotective agents in various animal models, including experimental ischaemic stroke (5). Numerous observational and retrospective clinical studies have also suggested that oestrogen replacement therapy (ERT) protects postmenopausal women against age-related diseases, including cardiovascular disease and stroke (6). However, two important clinical ERT trials, the Women Estrogen Stroke Trial (WEST) and the Women Health Initiative (WHI) trial, reported an unexpected increase in stroke incidence in E2-treated women (7, 8). The explanation for these findings has been debated extensively in the literature (9). One major factor is the timing of E2 replacement. Participants recruited in these trials were well beyond menopause when they received ERT; and it has been hypothesised that ERT should be initiated immediately at menopause to achieve its protective effects (10, 11), which is an effect recently modelled in preclinical studies. Prolonged periods of hypo-oestrogenicity led to an enhanced inflammatory response when ERT was administered, which exacerbated stroke damage; however, animals in that study were not aged. Intriguingly, the latest study (12) reporting on post-intervention outcomes in women involved in the WHI trial who were taken off ERT found that, after 10.7 years of follow-up, there was no significant difference in stroke risk between E2 and placebo-treated groups.
Recent studies (13, 14) have revealed that brain ischaemia is a powerful stimulus that triggers a series of events leading to the activation of several transcription factors involved in the inflammatory responses, including nuclear factor kappa B (NF-κB) (15). After ischaemia NF-κB translocates from the cytoplasm to the nucleus and up-regulates the expression of many pro-inflammatory genes, such as tumour necrosis factor (TNF)-α, monocyte chemotactic protein (MCP)-1 and interlukin (IL)-6, etc. (15, 16). A growing body of evidence (17, 18) has shown that E2 interacts with NF-κB through oestrogen receptor (ER)α to down-regulate the expression of inflammatory cytokines. However, whether E2 continues to have these beneficial effects in the aged brain is not yet known.
The present study aimed to evaluate the effects of either chronic or acute ERT on aged mice subjected to ischaemic stroke and to explore the possible mechanism underlying the effects of E2 in the aged brain. The mice utilised in the present study were 17 months old, which is an age equivalent to a 50–55-year-old woman (19) (i.e. the approximate age of menopause). We hypothesised that only chronic ERT initiated early after reproductive senescence would exert neuroprotective effects on mice after stroke, and predicted that the effect is related to the down-regulation of NF-κB activation as a result of continuous exposure to E2 after menopause. We also performed studies in aged males, aiming to determine whether there were sex differences in the response to ERT and to control for other factors associated with ageing.
Materials and methods
Animals
C57BL / 6 mice (17 months old) of both sexes were purchased from the NIA aged mouse colony (Charles River Laboratories, San Diego, CA, USA) All experiments were performed in accordance with NIH guidelines for the care and use of animals in research and under protocols approved by the UCHC Animal Care and Use Committee.
E2 replacement
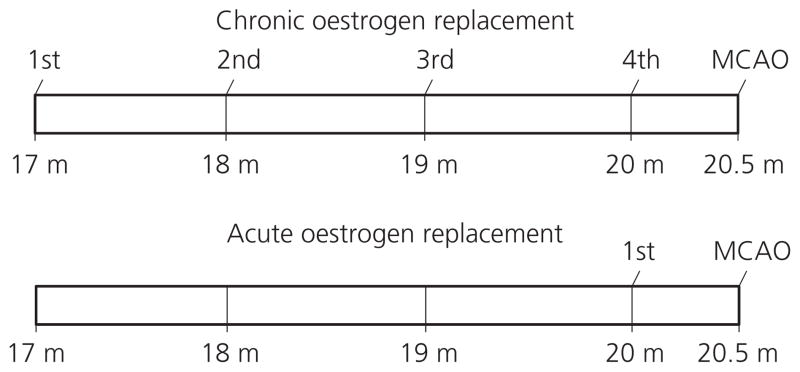
E2 replacement was administered by implanting E2 pellets s.c. as described previously (20). Briefly, E2 was delivered s.c. by a silastic capsule (0.062 inch inner diameter; 0.125 inch outer diameter) filled with 0.035 ml of E2 (180 mg / ml; Sigma, St Louis, MO, USA) in sesame oil or sesame oil alone (vehicle). Two protocols for E2 pellet replacement were developed: for chronic ERT, pellets were implanted in mice at 17 months of age and replaced every month until 20 months of age; for acute ERT, E2 pellets were only implanted once, at 20 months of age. Mice were exposed to anaesthesia at the same frequency as that of the chronic ERT mice to control for repeated anaesthetic effects. Mice from both groups were then subjected to ischaemic stroke at 20.5 months (Fig. 1). Control groups were treated with sesame oil pellets replacement therapy (ORT) correspondingly (chronic or acute ORT). Animals were randomised into treatment groups and stroke was performed by a blinded investigator.
Fig. 1.
Schematic representation of the experimental design of 17β-oestradiol (E2) replacement. In the chronic E2 replacement group, E2 pellets were first implanted to mice at 17 months old and replaced every month until the twentieth month; in the acute E2 replacement group, the pellets were only implanted in the twentieth month. Mice were subjected to MCAO 15 days after the final implant of the pellets. Control groups used pellets that contain oil vehicle and were treated with the same experimental conditions as the E2-treated groups.
Focal cerebral ischaemic model
Focal transient cerebral ischaemia was induced by reversible middle cerebral artery occlusion (MCAO) for 90 min followed by reperfusion as described previously (20). A 6–0 nylon suture, 0.23 mm in dimension, was utilised to occlude the MCA. During surgery and ischaemia, rectal muscle temperature was monitored with a Monotherm system (V WR LabShop, Batavia, IL, USA) and maintained at approximately 37 °C with an automated temperature control feedback system; after surgery, the mice were returned into a cage with a heating pad system that maintained temperature until reperfusion. Cerebral blood flow was measured by Laser Doppler flowmetry (LDF; Moor Instruments Ltd, Axminster, UK) for every single mouse. Occlusion was confirmed by a drop in LDF by 85% of baseline in all mice. Neurological deficit scores were recorded 4 h after stroke and at sacrifice. The scoring system was: 0, no deficit; 1, forelimb weakness and torso turning to the ipsilateral side when held by tail; 2, circling to affected side; 3, unable to bear weight on affected side; 4, no spontaneous locomotor activity or barrel rolling. All mice were examined before MCAO for dermatitis or visible tumours and were subjected to necropsy after sacrifice to exclude any animals with gross tumours including those in the pituitary region or other visible comorbidities.
Histological assessment
For evaluation of stroke outcomes, the mice were allowed to survive for 24 h and then were sacrificed with pentobarbital overdose (i.p.). Animals were perfused transcardially with cold phosphate-buffered saline followed by 4% paraformaldehyde; the brain was removed from the skull and post-fixed for 18 h and subsequently placed in cyroprotectant (30% sucrose). The brain tissue was cut into 30-μm free-floating coronal sections on a freezing microtome and every eighth slice was stained by cresyl violet (CV) staining for evaluation of ischaemic cell damage (21). Then images were digitalised by a charge-coupled device camera (Qiamging, Surrey, BC, Canada), and the infarct volumes [expressed as a percentage of (%) the contralateral hemispheric structure to correct for oedema] (Swanson’s method) (22) and analysed using SIGMASCAN PRO5 (Systat Software Inc., Chicago, IL, USA) as described previously (20).
Subcellular fractionation
For analysis of protein expression, subcellular fractionation was performed as described previously (23). Briefly, brain samples were obtained by rapid removal of the brain, with the subsequent removal of the cerebellum / occipital pole and olfactory / frontal pole to enrich for tissue supplied by the MCA. The ipsilateral hemispheres were homogenised in Dounce homogenisers with cold lysis solution [50 mM HEPES, pH 7.5, 1 mM ethylenediamine tetraacetic acid (EDTA), 1 mM ethylene glycol tetraacetic acid (EGTA), 50 mM NaF, 0.25 mM sucrose, 1 mM dithiothreitol, Protease cocktail (dilution 1 : 50; Roche Diagnostics Corp., Basel, Switzerland)]. Homogenates were filtered and centrifuged at 800 g for 10 min at 4 °C. The pellet (P1) contained the nuclear fraction; the supernatant (S1) was further centrifuged at 15 000 g for 10 min to obtain the cytosolic (supernatant, S2) and the mitochondrial fraction (pellet, P2). The P2 pellet was washed in SEE buffer [10 mM HEPES, pH 7.4, 1 mg / ml bovine serum albumin (BSA), 0.5 mM EDTA, pH 8.0, 0.5 mM EGTA, pH 8.0], then centrifuged at 15 000 g for 10 min. The S2 supernatant (cytosolic fraction) was centrifuged at 20 200 g for 30 min. The resulting supernatant is the cytosolic fraction. The P1 pellet was resuspended in a Tris buffer and run through a sucrose gradient composed of 1.8 and 2.3 M sucrose and centrifuged in a SW41 rotor at 30 000 g for 45 min. The pellet (P3) was washed with a nuclei pure storage buffer (Sigma-Aldrich, St Louis, MO, USA), and centrifuged at 960 g for 10 min. The pellet (P4) was resolved with nuclear extraction buffer (20 mM HEPES, pH 7.9, with 1.5 mM MgCl2, 0.42 M NaCl, 0.2 mM EDTA and 25% glycerol) (Sigma-Aldrich), sonicated, and stored at −80 °C as the nuclear fraction.
Western blots
The fractionated protein concentration was determined by BCA Protein Assay Kit (Thermo Fisher Scientific Inc., Rockford, IL, USA) and subjected to western blotting as described previously (23). Sample proteins were resolved on 4–15% sodium dodecylsulphate electrophoresis gels and transferred to a polyvinylidene fluoride membrane and then probed with NF-κB (p65; dilution 1 : 1000; Abcam, Cambridge, MA, USA), ERα (dilution 1 : 500; Abcam), insulin-like growth factor (IGF)-1 receptor β (IGF-1Rβ) (dilution 1 : 1000; Santa Cruz Biotechnology, Santa Cruz, CA, USA) and Bcl-2 (dilution 1 : 500; Cell Signaling Technology, Beverly, MA, USA). Macrophage migration inhibitory factor (MIF; dilution 1 : 1000; Cell Sciences, Canton, MA, USA) and Histone H3 (dilution 1 : 4000; Abcam) were used as loading controls for cytosolic and nuclear fractions, respectively. All blots were incubated overnight in primary antibodies at 4 °C in Tris-buffered saline containing 4% BSA and 0.1% Tween 20. Secondary antibodies (goat anti-rabbit IgG, dilution 1 : 5000; goat anti-mouse IgG, dilution 1 : 5000; donkey anti-goat IgG, dilution 1 : 1000; Santa Cruz Biotechnology) were diluted and ECL detection kit (Amersham Pharmacia, Piscataway, NJ, USA) was used for signal detection. Densitometry of western blots was analysed with SCION IMAGE (Scion Corp., Frederick, MD, USA) as described previously (20).
Immunhistochemistry (IHC)
Floating brain sections were prepared as described previously (24). Briefly, free-floating sections were blocked in normal goat serum block solution (PBTGS buffer: phosphate buffer + 0.3% Triton X-100 + 10% goat serum) for 1 h at room temperature followed by incubation overnight at room temperature with primary antibodies diluted with PBTGS buffer: anti-NF-κB p65 (dilution 1 : 200; Abcam); anti-neuronal nucleus (NeuN) (dilution 1 : 200; Chemicon International Inc., Temecula, CA, USA). The secondary antibodies (dilution 1 : 1000; Invitrogen, Carlsbad, CA, USA) were tagged with red or green fluorescent Fluor dye and diluted in PBTGS buffer. They were either goat anti-mouse or goat anti-rabbit depending on the primary antibody and were incubated for 2 h at room temperature. 4′,6-diamidino-2-phenylindole (DAPI) (dilution 1 : 1000; Invitrogen) was added to the sample together with the secondary antibodies. The signal was visualised with a Zeiss Axiovert 200M microscope (Carl Zeiss, Oberkochen, Germany) using a X-Cite 120Q fluorescence illumination system (Lumen Dynamics Group Inc., Mississauga, ON, Canada) using Zeiss image acquisition software (Zeiss LSM 510). All slices (from the same brain as for CV staining) were approximately 0.5 mm anterior to bregma and three × 20 fields / animal (n = 6 animals per group) were analysed in the penumbral area of the infarct. Cells with NF-κB and NeuN co-localised in the nuclei were counted using MACBIOPHOTONICS IMAGEJ software (http://www.macbiophotonics.ca) after a DAPI counterstain to confirm nuclear localisation. For each animal, the total numbers of cells in three × 20 fields were used for the statistical analysis.
E2, testosterone and cytokine analysis
Serum levels of E2, testosterone, MCP-1, IL-6 and TNF-α were measured in each group by enzyme-linked immunosorbent assay (ELISA) (E2; IBL Hamburg, Hamburg, Germany; testosterone; Calbiotech, Spring Valley, CA, USA; MCP-1, IL-6, TNF-α; eBioscience, San Diego, CA, USA). The uterus was removed at sacrifice in all female mice and weighed at sacrifice to confirm the loss of end-organ oestrogen effects.
Statistical analysis
Data from individual experiments are presented as the mean ± SEM and analysed with a t-test for two groups, and two-way ANOVA (with Tukey’s post-hoc correction for multiple comparisons where appropriate) for the comparison of the means between the experimental groups. Neurological deficit scores were analysed with a Mann–Whitney U-test. P < 0.05 was considered statistically significant. Induction of ischaemia and all behavioural and histological assessments were performed by an investigator who was blinded to vehicle or E2 replacement.
Results
Chronic and acute ERT exerted different effects on stroke outcomes in males and females
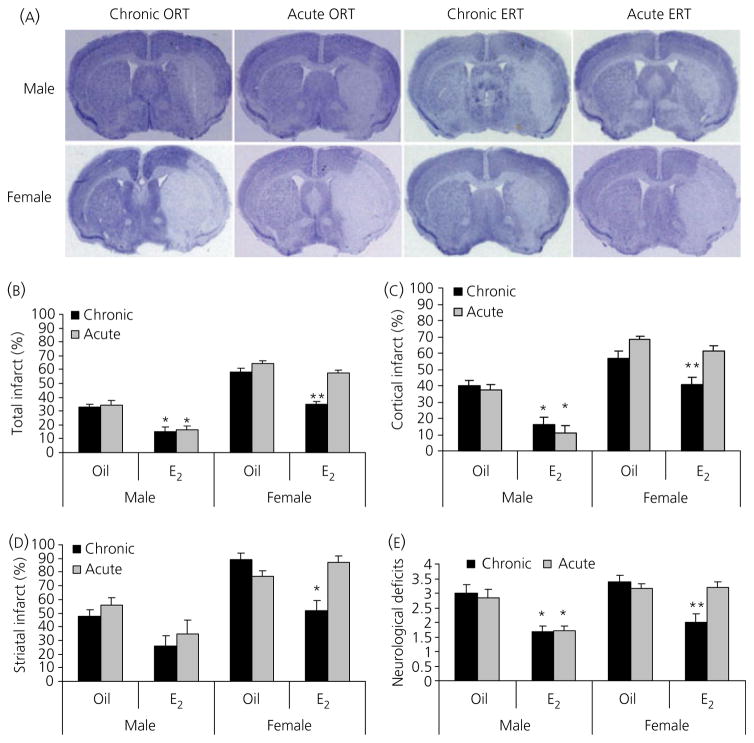
To evaluate the effects of E2 replacement initiated at different times during the lifespan on ischaemic stroke, we designed two experimental paradigms: chronic and acute ERT (Fig. 1). We first measured stroke outcome 24 h after the induction of a 90-min MCAO. Brain slices were stained with CV and infarct volumes were digitally calculated (Fig. 2A–D). Consistent with our previous study performed in middle-aged mice (20), both chronic or acute ORT males had significantly smaller infarct volumes than their counterpart females. Both chronic and acute ERT decreased infarct volumes significantly in males compared to vehicle-treated groups (total infarct: chronic ERT versus chronic ORT 15.1 ± 3.1% versus 33.2 ± 2.0%, acute ERT versus acute ORT 16.4 ± 2.9% versus 34.4 ± 3.1%; n = 12 per group, P < 0.05). A different pattern was seen in females. Only chronic ERT reduced infarct volume compared to oil-treated mice (total infarct: chronic ERT versus chronic ORT 34.7 ± 2.4% versus 58.2 ± 2.6%; n = 9 per group, P < 0.05) and there was no difference in infarct size between acute ERT and ORT groups (total infarct: acute ERT versus acute ORT 57.8 ± 1.8% versus 64.1 ± 2.4%; n = 10 per group, P > 0.05). Neurological deficit scores reflected these changes in infarct size (Fig. 2E). There were no differences in preischaemic or intraischaemic arterial blood pressure, and blood gas measurements between any two groups; however, female mice in both the ORT and chronic ERT groups were significantly lighter than their male counterparts (n=6 per group, P < 0.05) (See Table 1).
Fig. 2.
Effect of 17β-oestradiol (E2) replacement on infarct size in mice. (A) Representative image of a cresyl violet (CV)-stained brain for each group. The area lacking staining (white) reflects the infarct. (B–D) Quantification of infarct volumes based on CV staining in total hemisphere (B), cortex (C) and striatum (D). (E) Neurological deficit scores at 4 h of stroke. In all figures: *P < 0.05 versus oil-treated counterparts in males; **P < 0.05 versus chronic vehicle or acute E2-treated female mice; n = 9–12 animals per group. ORT, oil pellets replacement therapy; ERT, oestrogen replacement therapy.
Table 1.
Physiological Measurements in Each Group After Middle Cerebral Artery Occlusion MCAO).
| Group | pH | PaCO2 (mm Hg) | PaO2 (mm Hg) | Glucose (mg / dl) | Mean arterial blood pressure (mmHg) | HCO3 (mM) | Body weight (g) |
|---|---|---|---|---|---|---|---|
| C ORT – M | 7.38 ± 0.02 | 38.1 ± 2.9 | 105.3 ± 7.8 | 128 ± 12 | 67 ± 4 | 26.6 ± 2.3 | 41.8 ± 5.0 |
| A ORT – M | 7.35 ± 0.05 | 38.3 ± 4.3 | 89.3 ± 7.8 | 130 ± 21 | 76 ± 8 | 25.2 ± 1.4 | 45.7 ± 6.0 |
| C ERT – M | 7.41 ± 0.03 | 39.9 ± 4.4 | 90.7 ± 14.2 | 131 ± 14 | 67 ± 7 | 25.2 ± 2.8 | 42.6 ± 5.2 |
| A ERT – M | 7.38 ± 0.03 | 41.0 ± 4.6 | 97.3 ± 5.8 | 130 ± 5 | 65 ± 3 | 26.1 ± 1.5 | 37.3 ± 3.5 |
| C ORT – F | 7.40 ± 0.04 | 39.1 ± 4.3 | 91.3 ± 19.8 | 126 ± 8 | 79 ± 3 | 24.5 ± 1.1 | 31.8 ± 4.1 |
| A ORT – F | 7.38 ± 0.01 | 39.8 ± 3.8 | 81.7 ± 7.6 | 110 ± 20 | 69 ± 5 | 26.1 ± 3.2 | 32.7 ± 1.5 |
| C ERT – F | 7.38 ± 0.03 | 40.0 ± 3.7 | 92.7 ± 6.9 | 105 ± 16 | 71 ± 6 | 26.8 ± 2.5 | 33.6 ± 4.7 |
| A ERT – F | 7.38 ± 0.06 | 37.4 ± 2.8 | 92.7 ± 4.2 | 115 ± 17 | 65 ± 6 | 25.0 ± 2.3 | 33.3 ± 2.7 |
No differences were seen in physiological variables between oil- and E2-treated mice of either sex prior to (not shown) and 60 min after MCAO, except body weight (*P < 0.05 versus male counterpart) (n= 4 per group, except body weight group, where n= 6 per group). C, chronic; A, acute; M, male; F, female; ORT, oil pellets replacement therapy; ERT, oestrogen replacement therapy.
NF-κB translocation differed between the sexes after MCAO
With ischaemic injury, NF-κB is activated and translocates from the cytosol to the nucleus to regulate gene expression of inflammatory cytokines (15). To examine the translocation of NF-κB, we performed immunofluorescent staining to co-label brain slices with NF-κB and NeuN. Because NeuN is also expressed in cytoplasm (25, 26), we further exposed the slices with the nuclear stain, DAPI, to confirm the nuclear localisation of NF-κB. As shown in Fig. 3(A), NF-κB co-labelled with NeuN in the nucleus in stroke brains (NF-κB translocation positive cells; indicated by arrows in the upper panel of Fig. 3A); however, in sham brains, NF-κB was localised only in the cytoplasm (NF-κB translocation negative cells; indicated by arrow heads in the lower panel of Fig. 3A). Quantification was performed in all the stroke groups (Fig. 3B–D). There were significantly less NF-κB translocation positive cells in chronic ORT or acute ORT males compared to their counterpart females, and ERT did not decrease NF-κB translocation significantly in males. Interestingly, chronic ERT females had significantly fewer positive cells than chronic ORT females; however, no difference in NF-κB positive cell numbers was seen between acute ERT and ORT females.
Fig. 3.
Immunofluorescence of nuclear factor kappa B (NF-κB), neuronal nucleus (NeuN) and 4′,6-diamidino-2-phenylindole (DAPI). (A) Co-localisation of NF-κB and NeuN (NF-κB translocation positive cells indicated as arrows; negative cells as arrow heads) in the nucleus (upper panel; stroke groups) and the cytosol (lower panel; sham groups) (× 100 magnification; scale bar = 20 μm). (B,C) Co-labelling of NF-κB and NeuN in aged male (B) and female (C) mice brain after stroke. Boxed areas in a cresyl violet stained slice illustrate the three examined regions in each slice (× 20 magnification; scale bar = 50 μm). (D) Semi-quantification of co-localisation of NF-κB and NeuN in the nucleus. Each column stands for nine fields from six animals. *P < 0.05 versus oil or E2 counterparts in males. **P < 0.05 versus chronic oil or acute E2-treated group. ORT, oil pellets replacement therapy; ERT, oestrogen replacement therapy.
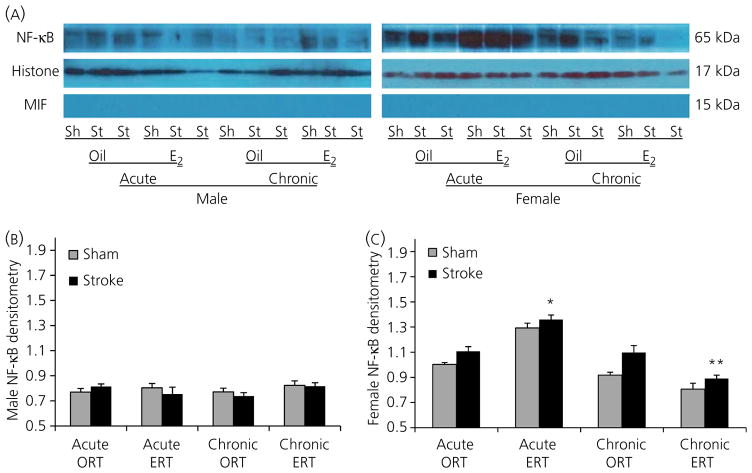
Western blotting showed a reduction in NF-κB expression in chronic ERT females
To confirm the IHC findings, and quantify nuclear NF-κB expression levels, western blotting was performed to examine nuclear NF-κB expression. Consistent with the IHC data, chronic ERT females had significantly less nuclear NF-κB compared to either chronic ORT or acute ERT females (Fig. 4A–C). Acute ERT females exhibited higher NF-κB expression in the nuclear fragment compared to acute ORT females. No significant difference in NF-κB expression levels was seen between male cohorts.
Fig. 4.
Nuclear factor kappa B (NF-κB) expression in the nucleus after stroke. (A) Representative western blots of NF-κB protein levels in the nuclear fraction of brain homogenates from male (right) and female (right) mice 24 h after stroke onset. Histone H3 was used as a loading control and migration inhibitory factor (MIF) confirmed the lack of cytosolic contamination. (B,C) The optical density of samples in males (B) and females (C) was expressed as the ratio of NF-κB bands to control bands (Histone). Six stroke and six sham brains were randomly aliquoted into three separate experiments for statistical analysis. *P < 0.05 versus stroke mice in acute oil pellets replacement therapy (ORT) group; **P < 0.05 versus stroke mice in acute oestrogen replacement therapy (ERT) or chronic ORT group. Sh, sham; St, stroke.
Expression of pro-inflammatory cytokines differed after stroke in males and females
Because NF-κB can regulate the expression of IL-6, MCP-1 and TNF-α, etc., we next examined the serum level of these cytokines by ELISA. We selected to assess cytokines 24 h after stroke. Proinflammatory signalling occurs in two peaks after stroke: the first peak is usually within 6 h and the second peak occurs 24–36 h after stroke (27). Stroke induced a significant increase in levels of these cytokines in every group compared to sham (see Supporting information, Tables S1–S3). Both MCP-1 and IL-6 levels in chronic ERT females were significantly lower than those in chronic ORT or acute ERT females; chronic ERT also reduced TNF-α levels compared to chronic ORT females (Fig. 5A–C; n = 6 per group, P < 0.05). In males, E2 treatment did not decrease these cytokines compared to oil vehicle groups after stroke; however, chronic ORT or acute ORT males had significantly lower MCP-1 compared to their counterpart females. IL-6 and TNF-α levels were significantly lower in chronic ORT males compared to chronic ORT females.
Fig. 5.
Serum levels of monocyte chemotactic protein (MCP)-1 (A), interleukin (IL)-6 (B) and tumour necrosis factor (TNF)-α (C) after stroke. *P < 0.05 versus female counterparts; **P < 0.05 versus chronic oil or acute 17β-oestradiol (E2)-treated group in females; ΔP < 0.05 versus chronic oil group in females (n = 6 per group).
Serum E2, testosterone levels and uterine weights
Serum E2 and testosterone levels were assessed in each group by ELISA. As expected, both chronic ERT or acute ERT paradigms led to significantly higher serum E2 levels compared to those seen in the chronic ORT or acute ORT mice of both sexes (Fig. 6A), and were equivalent to basal circulating oestrous levels of cycling female mice (28–30). No difference in E2 levels were seen between chronic and acute ERT mice. Uterine weights in all female mice showed the same pattern as serum E2 levels (Fig. 6B). The mean testosterone level was below 1 ng / ml in all groups regardless of sexes, which is lower than the previously reported levels in young C57BL6 male mouse (> 10 ng / ml) (31, 32), and showed no significant differences between groups (see Supporting information, Fig. S1).
Fig. 6.
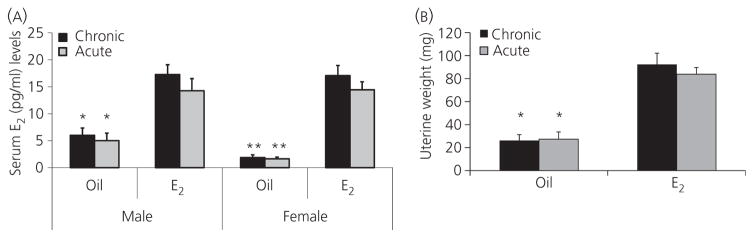
Serum levels of 17β-oestradiol (E2) and uterine weights after middle cerebral artery occlusion. (A) Serum E2 levels in stroke mice. *P < 0.05 versus E2-treated counterparts in males. **P < 0.01 versus E2-treated counterparts in females (n = 6 per group). (B) Uteruses of both oil and E2-treated female mice were separated, cut, and weighed after brains were removed. All the uteruses were handled and measured by the same investigator (n = 6 per group). *P < 0.05 versus E2-treated counterparts.
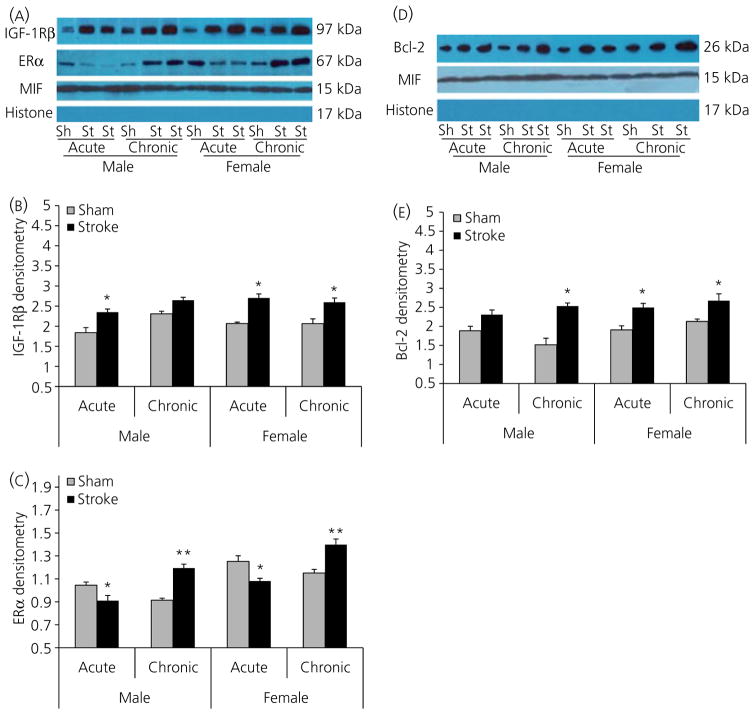
Effects of ERT on ERα, IGF-1Rβ, Bcl-2 expression
E2 replacement led to a higher expression of cytosolic ERα in both chronic ERT males and females compared to their acute ERT counterparts after stroke. Intriguingly, acute ERT decreased ERα levels after stroke compared to sham groups in both males and females (Fig. 7A,C). ERα levels in both male groups were significantly lower than their female counterparts. E2 can also regulate the expression of the anti-apoptotic protein Bcl-2, by interacting with IGF-1 (33). Therefore, we also examined IGF-1Rβ and Bcl-2 expression by western blotting. We found that these two protein levels significantly increased after stroke in each group compared to sham mice, except for IGF-1Rβ in chronic ERT males (Fig. 7A,B) and Bcl-2 in acute ERT males (Fig. 7D,E); however, no significant differences were seen between sexes and treatments.
Fig. 7.
Western blots of inslulin-like growth factor (IGF)-1Rβ, oestrogen receptpr (ER)α and Bcl-2 in each group. (A) Expression of IGF-1Rβ and ERα in the cytosolic fraction of brain homogenates from E2-treated male and female mice 24 h after stroke onset. Migration inhibitory factor (MIF) was used as a loading control and Histone H3 analysis confirmed the lack of nuclear contamination. (B,C) The optical density of samples in (A) was expressed as the ratio of IGF-1Rβ (B) and ERα (C) bands to control bands (MIF). (B) *P < 0.05 versus sham groups. (C) *P < 0.05 versus acute sham groups; **P < 0.05 versus chronic sham groups. (D) Expression of Bcl-2 in the cytosolic fraction of brain homogenates from E2-treated male and female mice 24 h after stroke onset. MIF was used as a loading control and Histone H3 confirmed the lack of nuclear contamination. (E) The optical density of samples in (D) was expressed as the ratio of Bcl-2 bands to control bands (MIF). *P < 0.05 versus sham groups. Six stroke and sham brains were randomly aliquoted into three separate experiments for statistical analysis. Sh, sham; St, stroke.
Discussion
The present study demonstrates several important new findings. First, the timing of administration of ERT is important, although only in the female brain. Both chronic ERT and acute ERT led to neuroprotection after induced stroke in males; however, only chronic ERT improved infarct outcome in females, whereas acute ERT did not. Oil-treated males had significantly smaller infarcts than oil-treated females, which is consistent with previous findings in middle-aged animals (20). Second, the expression of nuclear NF-κB was different in males versus females after stroke. Male mice had less NF-κB translocation after stroke compared to their counterpart females, except in the chronic ERT group. Chronic ERT robustly inhibited NF-κB translocation in females after stroke compared to chronic ORT females, which reduced the level to that of chronic ERT males; however, acute ERT increased NF-κB translocation beyond that seen with acute ORT, consistent with the proinflammatory effects of acute ERT. Third, the pro-inflammatory cytokines MCP-1, IL-6, and TNF-α induced by NF-κB were inhibited by chronic ERT but not acute ERT in females. Fourth, the timing of E2 therapy had an effect on expression of ERα after stroke (i.e. acute ERT decreased, whereas chronic ERT increased, cytosolic ERα levels in both males and females after stroke compared to shams). Two-way ANOVA also showed a sex effect in ERα expression (i.e. females had significantly higher level of ERα compared to males in each respective treatment group). Finally, although IGF-1 and Bcl-2 levels increased after stroke in both male and female mice, neither sex, nor the timing of E2 therapy had an effect on levels of either protein.
Extensive debate continues regarding the potential neuroprotective effects of E2 in both pre-clinical and clinical populations (9); the importance of the timing therapy of ERT has been increasingly recognised. The interval between menopause and the start of ERT plays a crucial role in the effectiveness of ERT in the vascular system (34). The present study found that only chronic ERT reduced infarct in aged females. We designed the chronic ERT treatment to initiate ERT in mice beginning at an age equivalent to a 50–55-year-old woman (19), the approximate age of menopause. Interestingly in both the WEST and WHI trial, both of which showed an increase in stroke incidence in treated women, the mean age of the participants at entry was 63 years (WHI) or 71 years (WEST), representing an age well past menopause. In a previous study (12) where young female mice were ovariectomised (OVX), it was found that E2 treatment was neuroprotective only if treatment was initiated immediately after OVX, which is consistent with our results demonstrating that chronic ERT was neuroprotective. If E2 treatment was delayed for 10 weeks after OVX, the infarct size was significantly larger (12); however, in the present study, acute ERT had no effect in aged females. The subtle difference between the previous and the present study may be a result of the different menopausal models used. In the present study, we used intact aged mice (17 months old), which have low circulating E2, testosterone, and low uterine weights to model natural menopause and to determine the effects of ERT in the ageing brain. By contrast, the previous study was performed in young females after surgical menopause (11). Additionally, we utilised a reperfusion injury, where the suture was removed after 90 min, allowing for reperfusion, whereas a permanent occlusion model was used in the previous study. This model leads to larger infarcts (35, 36) that may be less responsive to neuroprotectants (37) compared to that of the reperfusion model. Previous studies of ERT in middle-aged rats subjected to MCAO demonstrated similar results to those reported in the present study. Immediate ERT after OVX decreased infarct volume in 9–12-month-old female rats (38), whereas ERT initiated after an additional 9 weeks of E2-deficiency significantly increased infarct size (39). Intriguingly, long-term ovarian hormone deprivation not only blocks the beneficial effects of ERT in ischaemic injury, but also attenuates the ability of E2 to improve hippocampus-dependent memory in both young adult and middle-aged rats (40).
The mechanism by which E2 loses its protective effects after a prolonged period of hypoestrogenicity is not yet known. E2 is known to have profound effects on inflammation, which, in turn, can influence stroke outcome (41). In most cases, at least in models that have examined young animals, E2 suppresses the inflammatory response. This occurs in central nervous system disorders (42), as well as in models of arthritis (43), wound repair (44) and trauma (45). However, the role E2 plays in inflammation is complex. It is becoming increasingly clear that ERT administered at different time points during the lifespan can elicit very different effects (46). This is evident in many species, including primates. In surgically ovariectomised primates, a 70% reduction of atherosclerosis was seen when ERT was initiated simultaneously with an atherosclerotic diet but, when ERT was delayed 2 years after diet initiation, no protection against atherosclerosis was observed (47). The inflammatory responses induced by NF-κB activation play crucial roles in the development of post-stroke injury (48). E2 has long been known to inhibit NF-κB activity in various diseases, including stroke (18, 49); the loss of E2 with menopause promotes a pro-inflammatory state that is unmasked with acute injury. The present study revealed that chronic, but not acute, E2 replacement suppresses this NF-κB proinflammatory response, which likely contributes to the reduction of infarct volume after MCAO.
The timing effect of E2 on ischaemic stroke in aged mice also showed a subtle sexual dimorphic effect in the present study. To date, no studies have examined the effects of ERT after MCAO in aged mice. Young mice have been increasingly used in focal ischaemia models as a result of the availability of transgenic and knockout strains (21). Only a few studies on ischaemic stroke have been performed in aged rats (50–52), and the results from these studies were quite controversial. This may be because of the variability of the sex of the animals examined. Sex differences have been well documented in ischaemic stroke studies using young animals (5, 23). The neuroprotective effects of E2 are a major contributor to the smaller infarcts seen in young gonadally intact females. Numerous neurochemical and physiological changes occur with ageing (53), which likely lead to differences in ischaemic sexual dimorphism between young and aged animals. We have previously found that middle-aged male mice (15 months) had significantly smaller infarct volumes than females after MCAO (20), and the present study now extends these findings into the aged brain. These findings are consistent with clinical data demonstrating that aged women have higher disability and mortality than men after stroke (2). Despite the significant difference in infarct in oil-treated groups, no difference in neurological deficits were seen between males and females, suggesting that infarct size does not necessarily correlate with behavioural deficits, especially in aged animals.
Inflammatory responses have long been known to contribute to ischaemic injury (14), although how the inflammatory response changes with age and sex is not yet known. Serum levels of IL-6 differed in middle-aged male and female mice after stroke (20), which we investigated further in the present study. The expression of several pro-inflammatory markers, NF-κB, MCP-1 and IL-6, were significantly up-regulated after stroke in females compared to males. The higher level of pro-inflammatory markers is consistent with clinical data (54) showing that women enjoy immunological and metabolic advantages until menopause, which then decrease as women age. The results of the present study suggest that differential inflammatory responses after stroke contribute, at least in part, to differences in stroke outcomes in aged male and female mice.
Chronic and acute ERT not only led to different stroke outcomes and inflammatory responses, but also induced age-related differences in ERα expression. E2 receptor α (and not β) is a critical to E2-mediated protection against brain injury (55) and was therefore the focus of these investigations. Oestrogens are known to autoregulate ER (56), and the expression of ERα increases greatky in the ischaemic cortex after stroke injury (41); therefore, it is not unexpected that chronic ERT induced high levels of ERα in both male and female mice after stroke. However, there was a different response of ERα to ERT in the acute ERT groups, with decreased ERα expression in both sexes after stroke. It has been reported that the expression of both ERα and ERβ decreases with age in the cortex and hippocampus (57, 58), which could contribute to a decreased responsiveness to E2 in the acute ERT groups. The results obtained in the present study suggest that the decreased expression of ERα in aged animals cannot be reversed by acute ERT, in contrast to chronic ERT. To counteract the decreased responsiveness caused by ageing, it may be necessary to start E2 replacement early after menopause so that ER levels can be maintained. This is suggested by our results demonstrating that chronic ERT groups of both sexes had increased ERα expression compared to the corresponding acute ERT groups. The mechanism by which ERα expression decreases with age has not been elucidated; however, a recent study reported that methylation of 5′ untranslated exons in DNA encoding ERα increases with age, leading to silencing of the ERα gene (59). Methylation of DNA is an epigenetic mechanism underlying gene silencing (31). Interestingly, increased methylation of DNA with age also exists in X-chromosome inactivation (XCI), a major mechanism for dosage compensation of X-linked genes in females. XCI becomes unstable with ageing, and XCI skewing may occur in ageing populations, as demonstrated by increasing levels of methylation of target genes (60). Gene silencing by methylation of DNA in ageing subjects is being increasingly recognised and requires further study.
Sex differences in ERα expression were also seen in the present study (i.e. males had significantly lower cytosolic ERα level than females in each group), consistent with previous studies (61, 62). Activation of ERα can have inhibitory effects on NF-κB activity via enhancement of endogenous inhibitors of NF-κB, or by direct inhibition of NF-κB DNA binding activity (17). This is consistent with our finding that chronic ERT female mice had less nuclear NF-κB than that of acute ERT females, which also had lower levels of ERα expression. However, acute ERT females, despite their higher ERα levels, had larger infarcts than acute ERT males. This suggests that acute ERT is unable to inhibit NF-κB in females despite the higher level of ERα expression, and demonstrates that ERα levels do not correlate with infarct size. Both chronic and acute ERT males had low level of NF-κB, although chronic ERT males had higher expression levels of ERα compared to acute ERT males. However, both acute and chronic ERT were neuroprotective in males. The complicated nature of the relationship between E2 signalling and NF-κB seen in aged male animals suggested that E2 signalling may not be a major contributor to NF-κB activity in males.
One major limitation of these results is that ERα levels were only measured in the cytosolic fractions. Therefore, the results must be interpreted with caution. It is possible that acute E2 treatment led to receptor dimerisation with subsequent DNA binding and nuclear translocation. Indeed, decreased levels of cytosolic ER could be secondary to nuclear translocation and subsequent enhancement of ER signalling. However, it is clear that differences exist in the response of the ER to both E2 treatment regimen and by sex. Further studies that directly evaluate nuclear ER levels or, even more importantly, directly assess the downstream consequences of ER activation in the aged stroke brain are needed. This will require the use of ER knockout mice.
Another important pathway through which E2 may exert its neuroprotective effects is via interaction with IGF-1, leading to activation of the extracellular regulated kinase / mitogen-activated protein kinase signalling cascade and activation of transcription factor such as cAMP-response element binding protein, whose target genes include Bcl-2 (63, 64). In the present study, both IGF-1Rβ and Bcl-2 were increased after stroke compared to sham in each group, reflecting the activation of neuroprotective E2–IGF-1 axis. However, no differences in the expression of these two proteins were seen between the acute and chronic ERT groups of both sexes, indicating that sex differences and timing effects of E2 in ischaemic stroke in aged mice are not caused by activity of E2–IGF-1 axis.
The present study has several limitations that must be kept in mind when interpreting the results. MCAO in mice is not a perfect model to compare to the WHI trial because we induced a stroke and infarct size was examined subsequently. This is very different from the WHI trial in which the outcome assessed was stroke incidence. In the present study, we administered ERT to mice before MCAO; however, in most clinical neuroprotection trials, agents are usually applied a few hours after stroke onset. It is possible that E2 may be equally effective in both sexes if administered as an acute neuroprotectant. We also only evaluated acute stroke outcomes after 24 h of stroke; therefore, the long-term effects of ERT in aged mice of both sexes remain unknown. However, previous work in this model has shown that infarct is complete by 24 h (65) and infarct size at 24 h correlates well with that seen at 30 days, even in aged mice (66).
In conclusion, the effect of ERT on stroke in aged mice is modulated by several factors. Overall, sex differences are evident in that ERT led to neuroprotection in aged male mice regardless of the timing of ERT; whereas the effect is modulated in aged females based on the timing of initiation of therapy. Chronic ERT treated females showed improved stroke outcomes, whereas acute ERT had no beneficial effect on stroke induced brain injury. Inflammatory responses may mediate the differential effect of ERT in aged mice because NF-κB translocation and serum levels of MCP-1, IL-6 and TNF-α paralleled the changes in infarct size. ERα expression exhibited sex differences in aged mice after stroke, and may contribute to the inflammatory response to ischaemic stroke in aged females.
Supplementary Material
Acknowledgments
This work was supported by the NINDS (NS076293, NS066406 and NS055215 to L.D.M.).
Footnotes
The authors declare that there are no conflicts of interest.
Fig. S1. Serum testosterone levels in each group. The mean level of testosterone in each group was below 1 ng / ml. There was no significant difference in testosterone level between any two groups.
Table S1. Serum monocyte chemotactic protein (MCP)-1 levels (pg / ml) in stroke and sham mice.
Table S2. Serum interleukin (IL)-6 levels (pg / ml) in stroke and sham mice.
Table S3. Serum tumour necrosis factor (TNF)-α levels (pg / ml) in stroke and sham mice.
Please note: Wiley-Blackwell is not responsible for the content or functionality of any supplementary material supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- 1.Rojas JI, Zurru MC, Romano M, Patrucco L, Cristiano E. Acute ischemic stroke and transient ischemic attack in the very old – risk factor profile and stroke subtype between patients older than 80 years and patients aged less than 80 years. Eur J Neurol. 2007;14:895–899. doi: 10.1111/j.1468-1331.2007.01841.x. [DOI] [PubMed] [Google Scholar]
- 2.Glader EL, Stegmayr B, Norrving B, Terent A, Hulter-Asberg K, Wester PO, Asplund K. Sex differences in management and outcome after stroke: a Swedish national perspective. Stroke. 2003;34:1970–1975. doi: 10.1161/01.STR.0000083534.81284.C5. [DOI] [PubMed] [Google Scholar]
- 3.Rosamond W, Flegal K, Furie K, Go A, Greenlund K, Haase N, Hailpern SM, Ho M, Howard V, Kissela B, Kittner S, Lloyd-Jones D, McDermott M, Meigs J, Moy C, Nichol G, O’Donnell C, Roger V, Sorlie P, Steinberger J, Thom T, Wilson M, Hong Y. Heart disease and stroke statistics – 2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008;117:e25–e146. doi: 10.1161/CIRCULATIONAHA.107.187998. [DOI] [PubMed] [Google Scholar]
- 4.Bushnell CD, Hurn P, Colton C, Miller VM, del Zoppo G, Elkind MS, Stern B, Herrington D, Ford-Lynch G, Gorelick P, James A, Brown CM, Choi E, Bray P, Newby LK, Goldstein LB, Simpkins J. Advancing the study of stroke in women: summary and recommendations for future research from an NINDS-Sponsored Multidisciplinary Working Group. Stroke. 2006;37:2387–2399. doi: 10.1161/01.STR.0000236053.37695.15. [DOI] [PubMed] [Google Scholar]
- 5.McCullough LD, Hurn PD. Estrogen and ischemic neuroprotection: an integrated view. Trends Endocrinol Metab. 2003;14:228–235. doi: 10.1016/s1043-2760(03)00076-6. [DOI] [PubMed] [Google Scholar]
- 6.Wise PM, Dubal DB, Rau SW, Brown CM, Suzuki S. Are estrogens protective or risk factors in brain injury and neurodegeneration? Reevaluation after the Women’s health initiative. Endocr Rev. 2005;26:308–312. doi: 10.1210/er.2004-0014. [DOI] [PubMed] [Google Scholar]
- 7.Wassertheil-Smoller S, Hendrix SL, Limacher M, Heiss G, Kooperberg C, Baird A, Kotchen T, Curb JD, Black H, Rossouw JE, Aragaki A, Safford M, Stein E, Laowattana S, Mysiw WJ. Effect of estrogen plus progestin on stroke in postmenopausal women: the Women’s Health Initiative: a randomized trial. JAMA. 2003;289:2673–2684. doi: 10.1001/jama.289.20.2673. [DOI] [PubMed] [Google Scholar]
- 8.Viscoli CM, Brass LM, Kernan WN, Sarrel PM, Suissa S, Horwitz RI. A clinical trial of estrogen-replacement therapy after ischemic stroke. N Engl J Med. 2001;345:1243–1249. doi: 10.1056/NEJMoa010534. [DOI] [PubMed] [Google Scholar]
- 9.Lobo RA. Menopause and stroke and the effects of hormonal therapy. Climacteric. 2007;10(Suppl 2):27–31. doi: 10.1080/13697130701550903. [DOI] [PubMed] [Google Scholar]
- 10.Sherwin BB. The critical period hypothesis: can it explain discrepancies in the oestrogen-cognition literature? J Neuroendocrinol. 2007;19:77–81. doi: 10.1111/j.1365-2826.2006.01508.x. [DOI] [PubMed] [Google Scholar]
- 11.Suzuki S, Brown CM, Dela Cruz CD, Yang E, Bridwell DA, Wise PM. Timing of estrogen therapy after ovariectomy dictates the efficacy of its neuroprotective and antiinflammatory actions. Proc Natl Acad Sci USA. 2007;104:6013–6018. doi: 10.1073/pnas.0610394104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.LaCroix AZ, Chlebowski RT, Manson JE, Aragaki AK, Johnson KC, Martin L, Margolis KL, Stefanick ML, Brzyski R, Curb JD, Howard BV, Lewis CE, Wactawski-Wende J. Health outcomes after stopping conjugated equine estrogens among postmenopausal women with prior hysterectomy: a randomized controlled trial. JAMA. 2011;305:1305–1314. doi: 10.1001/jama.2011.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dirnagl U, Simon RP, Hallenbeck JM. Ischemic tolerance and endogenous neuroprotection. Trends Neurosci. 2003;26:248–254. doi: 10.1016/S0166-2236(03)00071-7. [DOI] [PubMed] [Google Scholar]
- 14.Han HS, Yenari MA. Cellular targets of brain inflammation in stroke. Curr Opin Investig Drugs. 2003;4:522–529. [PubMed] [Google Scholar]
- 15.Wang Q, Tang XN, Yenari MA. The inflammatory response in stroke. J Neuroimmunol. 2007;184:53–68. doi: 10.1016/j.jneuroim.2006.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Altinoz MA, Korkmaz R. NF-kappaB, macrophage migration inhibitory factor and cyclooxygenase-inhibitions as likely mechanisms behind the acetaminophen- and NSAID-prevention of the ovarian cancer. Neoplasma. 2004;51:239–247. [PubMed] [Google Scholar]
- 17.Kalaitzidis D, Gilmore TD. Transcription factor cross-talk: the estrogen receptor and NF-kappaB. Trends Endocrinol Metab. 2005;16:46–52. doi: 10.1016/j.tem.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 18.Stein B, Yang MX. Repression of the interleukin-6 promoter by estrogen receptor is mediated by NF-kappa B and C / EBP beta. Mol Cell Biol. 1995;15:4971–4979. doi: 10.1128/mcb.15.9.4971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harrison DE. Life span as a biomarker. The Jackson Laboratory website; http://researchjaxorg/faculty/harrison/ger1vLifespan1html. [Google Scholar]
- 20.Liu F, Yuan R, Benashski SE, McCullough LD. Changes in experimental stroke outcome across the life span. J Cereb Blood Flow Metab. 2009;29:792–802. doi: 10.1038/jcbfm.2009.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li X, Blizzard KK, Zeng Z, DeVries AC, Hurn PD, McCullough LD. Chronic behavioral testing after focal ischemia in the mouse: functional recovery and the effects of gender. Exp Neurol. 2004;187:94–104. doi: 10.1016/j.expneurol.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 22.Swanson RA, Morton MT, Tsao-Wu G, Savalos RA, Davidson C, Sharp FR. A semiautomated method for measuring brain infarct volume. J Cereb Blood Flow Metab. 1990;10:290–293. doi: 10.1038/jcbfm.1990.47. [DOI] [PubMed] [Google Scholar]
- 23.Liu F, Li Z, Li J, Siegel C, Yuan R, McCullough LD. Sex differences in caspase activation after stroke. Stroke. 2009;40:1842–1848. doi: 10.1161/STROKEAHA.108.538686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McCullough LD, Zeng Z, Blizzard KK, Debchoudhury I, Hurn PD. Ischemic nitric oxide and poly (ADP-ribose) polymerase-1 in cerebral ischemia: male toxicity, female protection. J Cereb Blood Flow Metab. 2005;25:502–512. doi: 10.1038/sj.jcbfm.9600059. [DOI] [PubMed] [Google Scholar]
- 25.Mullen RJ, Buck CR, Smith AM. NeuN, a neuronal specific nuclear protein in vertebrates. Development. 1992;116:201–211. doi: 10.1242/dev.116.1.201. [DOI] [PubMed] [Google Scholar]
- 26.Lind D, Franken S, Kappler J, Jankowski J, Schilling K. Characterization of the neuronal marker NeuN as a multiply phosphorylated antigen with discrete subcellular localization. J Neurosci Res. 2005;79:295–302. doi: 10.1002/jnr.20354. [DOI] [PubMed] [Google Scholar]
- 27.Ceulemans AG, Zgavc T, Kooijman R, Hachimi-Idrissi S, Sarre S, Michotte Y. The dual role of the neuroinflammatory response after ischemic stroke: modulatory effects of hypothermia. J Neuroinflammation. 2010;7:74. doi: 10.1186/1742-2094-7-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nelson JF, Felicio LS, Osterburg HH, Finch CE. Differential contributions of ovarian and extraovarian factors to age-related reductions in plasma estradiol and progesterone during the estrous cycle of C57BL / 6J mice. Endocrinology. 1992;130:805–810. doi: 10.1210/endo.130.2.1733727. [DOI] [PubMed] [Google Scholar]
- 29.Wise PM, Camp-Grossman P, Barraclough CA. Effects of estradiol and progesterone on plasma gonadotropins, prolactin, and LHRH in specific brain areas of ovariectomized rats. Biol Reprod. 1981;24:820–830. doi: 10.1095/biolreprod24.4.820. [DOI] [PubMed] [Google Scholar]
- 30.Porter KL, Chanda S, Wang HQ, Gaido KW, Smart RC, Robinette CL. 17beta-estradiol is a hormonal regulator of mirex tumor promotion sensitivity in mice. Toxicol Sci. 2002;69:42–48. doi: 10.1093/toxsci/69.1.42. [DOI] [PubMed] [Google Scholar]
- 31.Feng J, Fan G. The role of DNA methylation in the central nervous system and neuropsychiatric disorders. Int Rev Neurobiol. 2009;89:67–84. doi: 10.1016/S0074-7742(09)89004-1. [DOI] [PubMed] [Google Scholar]
- 32.Lacombe A, Lelievre V, Roselli CE, Muller JM, Waschek JA, Vilain E. Lack of vasoactive intestinal peptide reduces testosterone levels and reproductive aging in mouse testis. J Endocrinol. 2007;194:153–160. doi: 10.1677/JOE-07-0102. [DOI] [PubMed] [Google Scholar]
- 33.Selvamani A, Sohrabji F. The neurotoxic effects of estrogen on ischemic stroke in older female rats is associated with age-dependent loss of insulin-like growth factor-1. J Neurosci. 2010;30:6852–6861. doi: 10.1523/JNEUROSCI.0761-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maas AH, van der Schouw YT, Grobbee DE, van der Graaf Y. Rise and fall of hormone therapy in postmenopausal women with cardiovascular disease. Menopause. 2004;11:228–235. doi: 10.1097/01.gme.0000087980.28957.86. [DOI] [PubMed] [Google Scholar]
- 35.Stumm RK, Zhou C, Schulz S, Endres M, Kronenberg G, Allen JP, Tulipano G, Hollt V. Somatostatin receptor 2 is activated in cortical neurons and contributes to neurodegeneration after focal ischemia. J Neurosci. 2004;24:11404–11415. doi: 10.1523/JNEUROSCI.3834-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kuhmonen J, Haapalinna A, Sivenius J. Effects of dexmedetomidine after transient and permanent occlusion of the middle cerebral artery in the rat. J Neural Transm. 2001;108:261–271. doi: 10.1007/s007020170071. [DOI] [PubMed] [Google Scholar]
- 37.Greco R, Amantea D, Blandini F, Nappi G, Bagetta G, Corasaniti MT, Tassorelli C. Neuroprotective effect of nitroglycerin in a rodent model of ischemic stroke: evaluation of Bcl-2 expression. Int Rev Neurobiol. 2007;82:423–435. doi: 10.1016/S0074-7742(07)82024-1. [DOI] [PubMed] [Google Scholar]
- 38.Dubal DB, Wise PM. Neuroprotective effects of estradiol in middle-aged female rats. Endocrinology. 2001;142:43–48. doi: 10.1210/endo.142.1.7911. [DOI] [PubMed] [Google Scholar]
- 39.Selvamani A, Sohrabji F. The neurotoxic effects of estrogen on ischemic stroke in older female rats is associated with age-dependent loss of insulin-like growth factor-1. J Neurosci. 2010;30:6852–6861. doi: 10.1523/JNEUROSCI.0761-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bohacek J, Bearl AM, Daniel JM. Long-term ovarian hormone deprivation alters the ability of subsequent oestradiol replacement to regulate choline acetyltransferase protein levels in the hippocampus and prefrontal cortex of middle-aged rats. J Neuroendocrinol. 2008;20:1023–1027. doi: 10.1111/j.1365-2826.2008.01752.x. [DOI] [PubMed] [Google Scholar]
- 41.Suzuki S, Brown CM, Wise PM. Neuroprotective effects of estrogens following ischemic stroke. Front Neuroendocrinol. 2009;30:201–211. doi: 10.1016/j.yfrne.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lelu K, Delpy L, Robert V, Foulon E, Laffont S, Pelletier L, Engelhardt B, Guery JC. Endogenous estrogens, through estrogen receptor alpha, constrain autoimmune inflammation in female mice by limiting CD4+ T-cell homing into the CNS. Eur J Immunol. 2010;40:3489–3498. doi: 10.1002/eji.201040678. [DOI] [PubMed] [Google Scholar]
- 43.Subramanian S, Tovey M, Afentoulis M, Krogstad A, Vandenbark AA, Offner H. Ethinyl estradiol treats collagen-induced arthritis in DBA / 1LacJ mice by inhibiting the production of TNF-alpha and IL-1beta. Clin Immunol. 2005;115:162–172. doi: 10.1016/j.clim.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 44.Ashcroft GS, Mills SJ, Lei K, Gibbons L, Jeong MJ, Taniguchi M, Burow M, Horan MA, Wahl SM, Nakayama T. Estrogen modulates cutaneous wound healing by downregulating macrophage migration inhibitory factor. J Clin Invest. 2003;111:1309–1318. doi: 10.1172/JCI16288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yokoyama Y, Schwacha MG, Samy TS, Bland KI, Chaudry IH. Gender dimorphism in immune responses following trauma and hemorrhage. Immunol Res. 2002;26:63–76. doi: 10.1385/ir:26:1-3:063. [DOI] [PubMed] [Google Scholar]
- 46.Straub RH. The complex role of estrogens in inflammation. Endocr Rev. 2007;28:521–574. doi: 10.1210/er.2007-0001. [DOI] [PubMed] [Google Scholar]
- 47.Mikkola TS, Clarkson TB. Estrogen replacement therapy, atherosclerosis, and vascular function. Cardiovasc Res. 2002;53:605–619. doi: 10.1016/s0008-6363(01)00466-7. [DOI] [PubMed] [Google Scholar]
- 48.Harari OA, Liao JK. NF-kappaB and innate immunity in ischemic stroke. Ann N Y Acad Sci. 2010;1207:32–40. doi: 10.1111/j.1749-6632.2010.05735.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Paimela T, Ryhanen T, Mannermaa E, Ojala J, Kalesnykas G, Salminen A, Kaarniranta K. The effect of 17beta-estradiol on IL-6 secretion and NF-kappaB DNA-binding activity in human retinal pigment epithelial cells. Immunol Lett. 2007;110:139–144. doi: 10.1016/j.imlet.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 50.Kharlamov A, Kharlamov E, Armstrong DM. Age-dependent increase in infarct volume following photochemically induced cerebral infarction: putative role of astroglia. J Gerontol A Biol Sci Med Sci. 2000;55:B135–B141. doi: 10.1093/gerona/55.3.b135. [DOI] [PubMed] [Google Scholar]
- 51.Sutherland GR, Dix GA, Auer RN. Effect of age in rodent models of focal and forebrain ischemia. Stroke. 1996;27:1663–1667. doi: 10.1161/01.str.27.9.1663. [DOI] [PubMed] [Google Scholar]
- 52.Shapira S, Sapir M, Wengier A, Grauer E, Kadar T. Aging has a complex effect on a rat model of ischemic stroke. Brain Res. 2002;925:148–158. doi: 10.1016/s0006-8993(01)03270-x. [DOI] [PubMed] [Google Scholar]
- 53.Anyanwu EC. Neurochemical changes in the aging process: implications in medication in the elderly. Scientific World Journal. 2007;7:1603–1610. doi: 10.1100/tsw.2007.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yang Y, Kozloski M. Sex differences in age trajectories of physiological dysregulation: inflammation, metabolic syndrome, and allostatic load. J Gerontol A Biol Sci Med Sci. 2011;66:493–500. doi: 10.1093/gerona/glr003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dubal DB, Zhu H, Yu J, Rau SW, Shughrue PJ, Merchenthaler I, Kindy MS, Wise PM. Estrogen receptor alpha, not beta, is a critical link in estradiol-mediated protection against brain injury. Proc Natl Acad Sci USA. 2001;98:1952–1957. doi: 10.1073/pnas.041483198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Marin-Castano ME, Elliot SJ, Potier M, Karl M, Striker LJ, Striker GE, Csaky KG, Cousins SW. Regulation of estrogen receptors and MMP-2 expression by estrogens in human retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2003;44:50–59. doi: 10.1167/iovs.01-1276. [DOI] [PubMed] [Google Scholar]
- 57.Adams MM, Fink SE, Shah RA, Janssen WG, Hayashi S, Milner TA, McEwen BS, Morrison JH. Estrogen and aging affect the subcellular distribution of estrogen receptor-alpha in the hippocampus of female rats. J Neurosci. 2002;22:3608–3614. doi: 10.1523/JNEUROSCI.22-09-03608.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mehra RD, Sharma K, Nyakas C, Vij U. Estrogen receptor alpha and beta immunoreactive neurons in normal adult and aged female rat hippocampus: a qualitative and quantitative study. Brain Res. 2005;1056:22–35. doi: 10.1016/j.brainres.2005.06.073. [DOI] [PubMed] [Google Scholar]
- 59.Westberry JM, Trout AL, Wilson ME. Epigenetic regulation of estrogen receptor alpha gene expression in the mouse cortex during early postnatal development. Endocrinology. 2010;151:731–740. doi: 10.1210/en.2009-0955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hatakeyama C, Anderson CL, Beever CL, Penaherrera MS, Brown CJ, Robinson WP. The dynamics of X-inactivation skewing as women age. Clin Genet. 2004;66:327–332. doi: 10.1111/j.1399-0004.2004.00310.x. [DOI] [PubMed] [Google Scholar]
- 61.Cao J, Patisaul HB. Sexually dimorphic expression of hypothalamic estrogen receptors alpha and beta and kiss1 in neonatal male and female rats. J Comp Neurol. 2011;519:2954–2977. doi: 10.1002/cne.22648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Orikasa C, Kondo Y, Hayashi S, McEwen BS, Sakuma Y. Sexually dimorphic expression of estrogen receptor beta in the anteroventral periventricular nucleus of the rat preoptic area: implication in luteinizing hormone surge. Proc Natl Acad Sci USA. 2002;99:3306–3311. doi: 10.1073/pnas.052707299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jover-Mengual T, Zukin RS, Etgen AM. MAPK signaling is critical to estradiol protection of CA1 neurons in global ischemia. Endocrinology. 2007;148:1131–1143. doi: 10.1210/en.2006-1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cardona-Gomez GP, Mendez P, DonCarlos LL, Azcoitia I, Garcia-Segura LM. Interactions of estrogens and insulin-like growth factor-I in the brain: implications for neuroprotection. Brain Res Brain Res Rev. 2001;37:320–334. doi: 10.1016/s0165-0173(01)00137-0. [DOI] [PubMed] [Google Scholar]
- 65.Liu F, Schafer DP, McCullough LD. TTC, fluoro-Jade B and NeuN staining confirm evolving phases of infarction induced by middle cerebral artery occlusion. J Neurosci Methods. 2009;179:1–8. doi: 10.1016/j.jneumeth.2008.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu F, Benashski SE, Persky R, Xu Y, Li J, McCullough LD. Age-related changes in AMP-activated protein kinase after stroke. Age (Dordr) 2011 Mar 1; doi: 10.1007/s11357-011-9214-8. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.