Abstract
A superfamily of integral membrane proteins is characterized by a conserved tryptophan-rich region (called the WWD domain) in an external loop at the inner membrane surface. The three major members of this family (CcmC, CcmF, and CcsBA) are each involved in cytochrome c biosynthesis, yet the function of the WWD domain is unknown. It has been hypothesized that the WWD domain binds heme to present it to an acceptor protein (apoCcmE for CcmC or apocytochrome c for CcmF and CcsBA) such that the heme vinyl group(s) covalently attaches to the acceptors. Alternative proposals suggest that the WWD domain interacts directly with the acceptor protein (e.g., apoCcmE for CcmC). Here, it is shown that CcmC is only trapped with heme when its cognate acceptor protein CcmE is present. It is demonstrated that CcmE only interacts stably with CcmC when heme is present; thus, specific residues in each protein provide sites of interaction with heme to form this very stable complex. For the first time, evidence that the external WWD domain of CcmC interacts directly with heme is presented. Single and multiple substitutions of completely conserved residues in the WWD domain of CcmC alter the spectral properties of heme in the stable CcmC:heme:CcmE complexes. Moreover, some mutations reduce the binding of heme up to 100%. It is likely that endogenously synthesized heme enters the external WWD domain of CcmC either via a channel within this six-transmembrane-spanning protein or from the membrane. The data suggest that a specific heme channel (i.e., heme binding site within membrane spanning helices) is not present in CcmC, in contrast to the CcsBA protein. We discuss the likelihood that it is not important to protect the heme via trafficking in CcmC whereas it is critical in CcsBA.
Keywords: cytochrome, heme, CcmC, CcmE, redox
Introduction
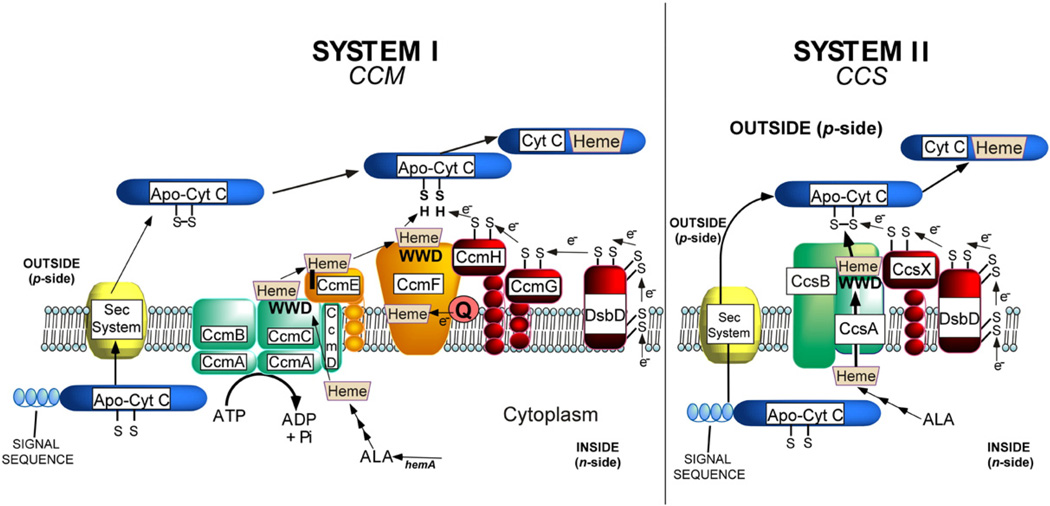
The c-type cytochromes are involved in energy conversion in nearly all organisms. The c-type cytochromes function in electron transport outside the inner membrane of prokaryotes and mitochondria and lumen of chloroplasts. It is thought that their covalently linked heme (to the protein) stabilizes and “hardwires” the cytochromes for their diverse roles in many different respiratory and phototrophic pathways.1–4 The covalent linkages are typically formed between two cysteines of the protein (CXXCH) and the two vinyl groups of reduced (Fe2+) heme.5,6 In all prokaryotes, all plant mitochondria, and all chloroplasts, one of two systems attaches the heme (Fig. 13,7–10). Briefly, in system I, heme is trafficked to a site in CcmC, where the 2-vinyl group of heme is covalently linked to a histidine (His130) in CcmE11–16 (see below). Ultimately, the heme from CcmE is attached to apocytochrome c by the cytochrome c synthetase, the CcmF/H complex.16–18 Other proteins are involved in reducing the cysteines of the CXXCH, which are prone to oxidation outside the inner membrane.19–25 System II is simpler (Fig. 1). Besides the thioredox components,26,27 the CcsBA protein transports heme from inside to an external domain, where it is attached to CXXCH; thus, CcsBA is a heme exporter and a cytochrome c synthetase.28,29
Fig. 1.
Current working models of the system I and II cytochrome c biogenesis pathways. The WWD domain in the three proteins, CcmC, CcmF, and CcsA, is identified with WWD in boldface.
When the system I and II components were first compared by sequence analyses, it was recognized that three of the integral membrane proteins (CcmC, CcmF, and CcsA) were related to each other in a short tryptophan-rich region called the WWD domain.30 It was proposed that the WWD domain may be involved in binding and delivering heme to the apocytochrome c, since the trafficking and presentation of heme to the CXXCH was certainly a common feature of each system.8,31,32 The topology of all transmembrane-spanning segments (TMSs) in all three family members has been experimentally established.16,28,31,32 In all three proteins, the WWD domain is present as an external loop. Two histidines are also conserved, one in each external loop that is adjacent to the WWD domain. The evolution of these three families has been discussed, and the superfamily was termed the “heme-handling proteins”.33 However, the involvement of the diagnostic WWD domain in heme handling is still questioned and there is as yet no evidence that it directly interacts with heme.
Recently, all three members of the WWD superfamily have been purified and trapped with endogenously synthesized heme.16,28,34 For system II, a functional recombinant CcsBA fusion protein possessed heme, and the two conserved external histidines and two other histidines, His77 in TMS-3 and His858 in TMS-8, were each required for function.28 The CcsBA(H858A) mutant contains no heme, and CcsBA(H77A) contains only 25% the level of heme of the wild type. Both mutants were corrected for cytochrome c synthesis function by exogenous imidazole, a result analogous to the proximal histidine substitution of myoglobin.35 A “channel” binding site for heme within the membrane of CcsBA is suggested.28 This initial binding site would protect the heme from oxidation as it presumably moves to the external WWD domain.28,29
For system I, CcmF was also purified with heme.16 The heme b binding site was defined and shown to require a histidine in TMS-5, but not the two histidines adjacent to the external WWD domain.16 In this case, it was shown that the heme is equimolar to the CcmF protein and is reduced by quinols. Thus, CcmF is predicted to be a quinol:heme reductase, where it reduces the heme entering from holoCcmE (Fig. 1). Although apoCcmE has been shown to immunoprecipitate with CcmF,18 it has not been demonstrated that the heme from holoCcmE enters the WWD domain in CcmF for transfer to CXXCH.
CcmC is necessary to attach heme to apoCcmE; thus, it is sometimes referred to as the holoCcmE synthase.12,36 Results from Thony-Meyer et al., where mutations in the CcmC WWD domain were studied, led them to conclude that the function of the WWD domain is to directly interact with the acceptor CcmE37,38 (these data are further reviewed in Discussion). The CcmA ATP-binding cassette (ABC) protein, along with the six TMS CcmB31 and single TMS CcmD,39,40 forms a complex with CcmC and holoCcmE (Fig. 1); the function of this ABC complex is to release the holoCcmE from CcmC.36 This release requires the ATP hydrolysis function of CcmA.36 Recently, it was discovered that in the absence of ccmAB, the CcmC:heme:CcmE complex could be trapped and purified.16 This stable complex exhibited an unusual and diagnostic Nadithionite-reduced visible spectrum at room temperature, with distinct split α absorption maxima at 553 and 559 nm. This absorption was perturbed when either of the two external histidines in CcmC or CcmE His130 was mutated. Therefore, the two histidines of CcmC are the axial ligands to the heme iron in the complex. Here, the trapping of this stable complex and unique spectral characteristics are used to define the involvement of the WWD domain in heme binding. The central role of heme in forming the stable complex is defined, and the mechanisms by which heme may traffic to the WWD domain in CcmC are addressed.
Results
Stable binding of heme to CcmC requires CcmE
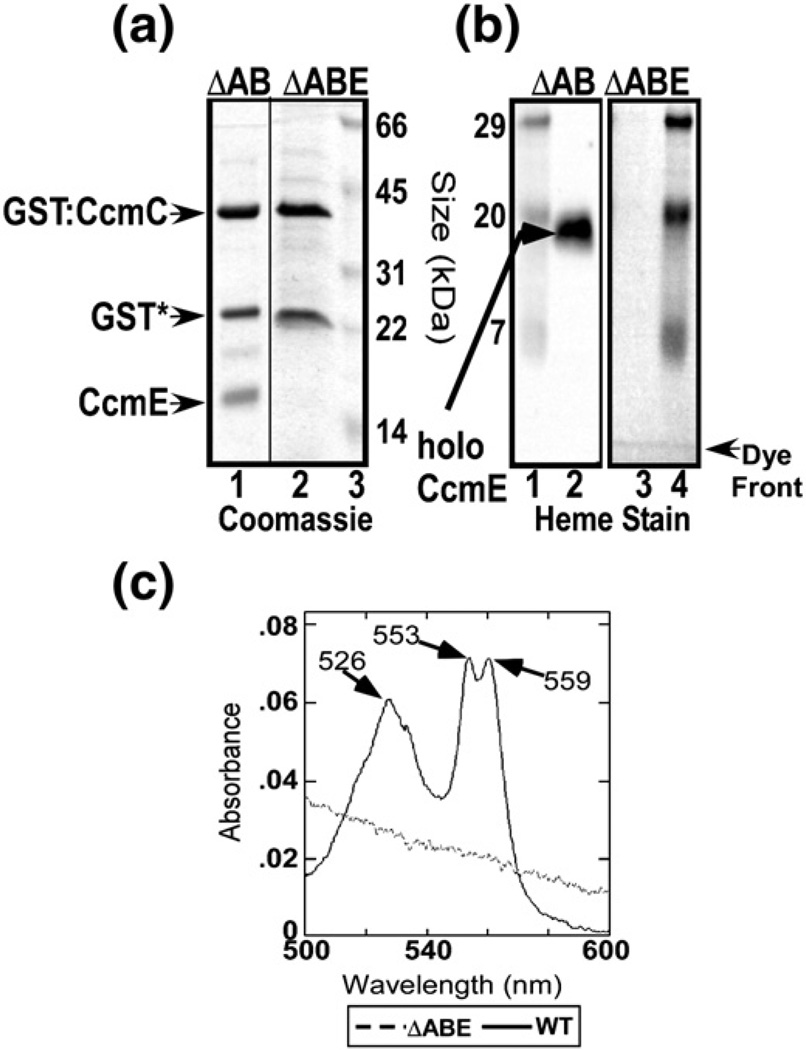
We have recently used a functional glutathione S-transferase (GST) fusion to the N-terminus of CcmC to affinity purify a complex containing CcmC, heme, and CcmE.16 Endogenous heme was trapped in the complex because it was purified from an Escherichia coli strain that was missing the ccmAB genes, shown previously to release the heme as holoCcmE.36 We have also shown previously that the small polypeptide encoded by ccmD, with a single TMS, is not required for complex formation but is required for release.39 Moreover, a tyrosine (Tyr17), the only conserved residue of CcmD, when converted to an alanine, yielded a complex identical with wild type.16 In all studies here, CcmD is included in analyses of complexes. We first examined whether CcmC itself binds (endogenous) heme, as has been proposed37 (see Discussion). All studies use the detergent n-dodecyl β-d-maltoside (DDM) to solubilize the membrane proteins. The CcmC:heme: CcmE complex purifies as three major polypeptides by SDS-PAGE and Coomassie staining (Fig. 2a, lane 1), the GST–CcmC fusion, proteolyzed GST*, and CcmE. (The small 7-kDa polypeptide CcmD is not stainable with Coomassie but has wild type been shown to be part of the complex by silver staining and FLAG tagging.39) CcmE is detected with CcmE antisera (not shown), and it is present as the holoCcmE form, detectable by heme stain (Fig. 2b, lane 2). The holoCcmE is all covalently attached (via the CcmE His130) as established by both the heme stain and pyridine hemochromagen spectra (not shown). The spectra of the wild type complex exhibit a distinct split α at 553 and 559 nm when reduced with Na-dithionite (Fig. 2c, continuous line).16 Surprisingly, no heme is bound to CcmC when CcmE is absent, even though the GST–CcmC fusion protein is as pure and stable (i.e., unproteolyzed), as evidenced by the full-length GST–CcmC polypeptide and GST* (Fig. 2a, lane 2). Heme is not detected by heme stain (Fig. 2b, lane 3) or by spectral analysis (Fig. 2c, broken line). We conclude that stable trapping of heme in CcmC requires the apoCcmE protein.
Fig. 2.
ApoCcmE is necessary for heme to bind to CcmC. (a) Coomassie stain of GST-purified CcmCE complex (from pSysI-ΔccmAB; lane 1) and GST-purified CcmC (from pSysI-ΔccmABE; lane 2). (b) Heme stain of the same purified protein complexes as in (a) with pSysI-ΔccmAB in lane 1 and pSysI-ΔccmABE in lane 2. Standard proteins with indicated molecular masses are shown in lane 3 of (a) and lanes 1 and 4 of (b). (c) Sodium-dithionite-reduced absorption spectra of the purified GST:CcmCE complex (continuous line) and GST:CcmC (broken line). Absorption maxima are indicated with arrows.
The stable binding of CcmE to CcmC requires heme
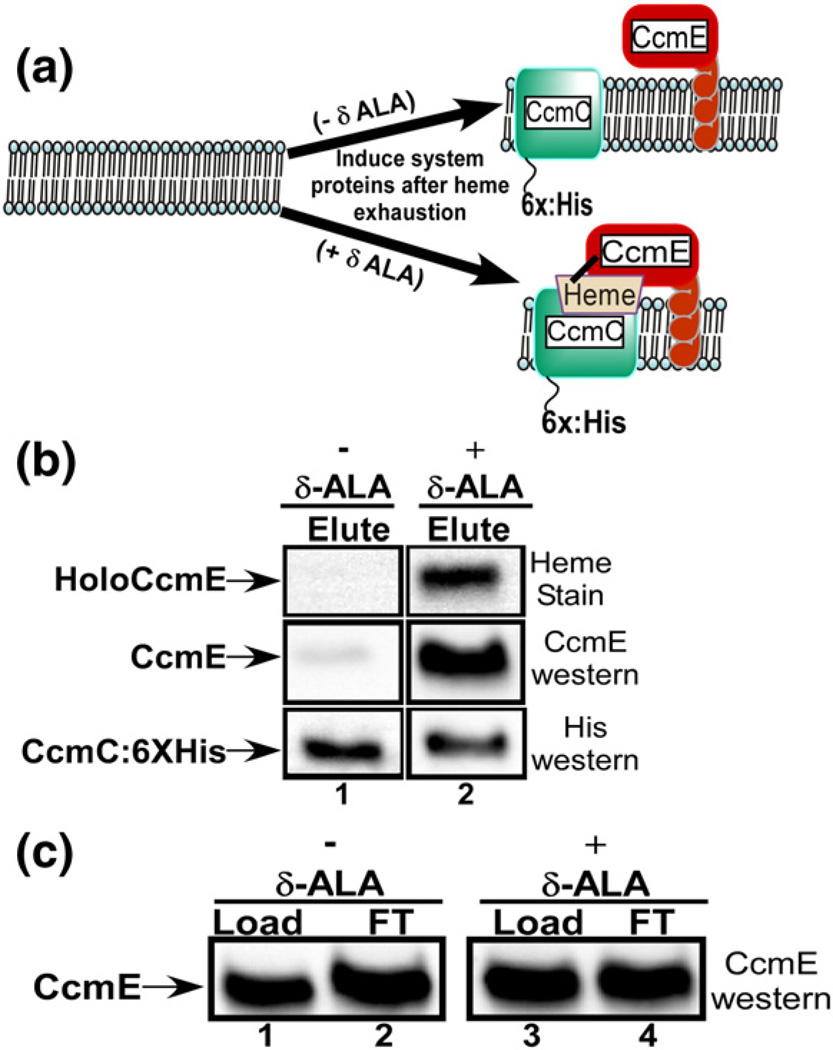
One possibility is that apoCcmE binds to CcmC, resulting in the formation of a heme binding site. An alternative explanation is that heme comprises the central molecule to which both CcmC and apoCcmE bind to form the stable complex. We thus wanted to test whether CcmC and CcmE form a complex without heme. Because we depend on endogenously synthesized heme such that any formation is likely physiological, we needed to deplete heme in vivo and then determine whether a CcmC:CcmE complex forms. For these experiments, we used E. coli (RK105), which is deleted of all ccm genes, and for hemA,41 encoding glutamyl tRNA reductase, the first committed enzyme in heme biosynthesis. E. coli RK105 requires exogenous δ-aminolevulinic acid (δ-ALA) for aerobic growth.41 By using media with either replete or depleted exogenous δ-ALA, then inducing the CcmC and CcmBDEFGH proteins with arabinose and IPTG, respectively, we could determine whether heme is necessary for complex formation (see Fig. 3a). A hexahistidine tag was engineered onto the C-terminus of CcmC,36 allowing affinity purification, and standardization of the amount of CcmC that is purified from each culture (using hexahistidine antisera). The levels of CcmE that are co-purified with CcmC in each condition (i.e., ±δ-ALA, therefore ±heme) were measured (Fig. 3b). By both heme stain and CcmE Western blotting, much less CcmE co-purifies with CcmC-6×: His when heme is depleted (without δ-ALA). We estimate a greater than 90% reduction in CcmE co-purification upon heme depletion compared to replete conditions, even though the same levels of CcmE are produced under each condition (Fig. 3c). We conclude that heme is required for CcmE to stably interact with CcmC (see Fig. 4a diagram).
Fig. 3.
Binding of CcmE to CcmC requires heme. (a) A cartoon of the experimental design for the heme limitation results shown in (b) and (c). (b) Heme stains and anti-CcmE and anti-His Western blots of the elution (Elute) fractions of Ni2+-purified CcmC:6 × His with co-purified CcmE from −-ALA (lane 1) and +δ-ALA (lane 2) cultures. (c) Anti-CcmE Western blots of load and flow-through (FT) fractions from Ni2+-purified CcmC:6 × His experiments with co-purified CcmE from −δ-ALA (lanes 1 and 2) and +δ-ALA (lanes 3 and 4) cultures.
Fig. 4.
Models of the CcmC:heme:CcmE complex and structural information. (a) Cartoon of the four molecules (CcmC:heme:CcmD:CcmE). All conserved residues in bacteria are shown in red, with the exception of CcmE His130 in green (CcmE residue 130 can be a Cys residue in very few bacteria). The ligands to the heme iron and the position of covalent heme attachment in this complex are starred, as well as CcmE Tyr134, which will become the axial ligand upon release. (b) Alignment of the predicted protein sequence of three WWD-domain-containing proteins (CcmC, CcmF, and CcsA). Numbers given above the CcmC sequence represent residues with single or multiple substitutions in the present study. The gray-shaded portion encompasses all invariant residues. (c) Structures of heme in line format or 3D from planar surface (middle) or end-on (right) showing designated atoms. (d) ApoCcmE structures in four different images (i–iv). The residues after Tyr134 are not shown. The His130 and Tyr134 are shown with yellow carbon and blue nitrogen atoms in all images. Image (i) displays all identical residues with atoms in red (spheres). Image (ii) adds all conserved residues as blue (spheres), and image (iii) adds all other atoms as wheat colored (spheres). Image (iv) is rotated approximately 120° to the right and tilted up approximately 30° (from iii), as discussed in the text. (e) Primary sequence of E. coli CcmE shown in (d) with same color representations. (Supplementary Fig. 4 shows clustal W fits.) Organisms used for the determination of amino acid conservation are as follows: Agrobacterium tumefaciens strC58, Caulobacter crescentus CB15, Nitrospira multiformis ATCC 25196, Nitrosomonas europaea ATCC 19718, E. coli strain K12 substrain MG1655, Pseudomonas fluorescens Pf01, Shewanella oneidensis MR-1, Vibrio parahaemolyticus RIMD 2210633, Myxococcus xanthus, Deinococcus geothermalis, and Thermus thermophilus. All 3D structures are presented using PyMOL, HEMsdf for heme in (c) and 1SR3 (Protein Data Bank) for apoCcmE as determined in Enggist et al.42 for (d).
Residues in CcmC required for heme binding and interaction: The WWD domain
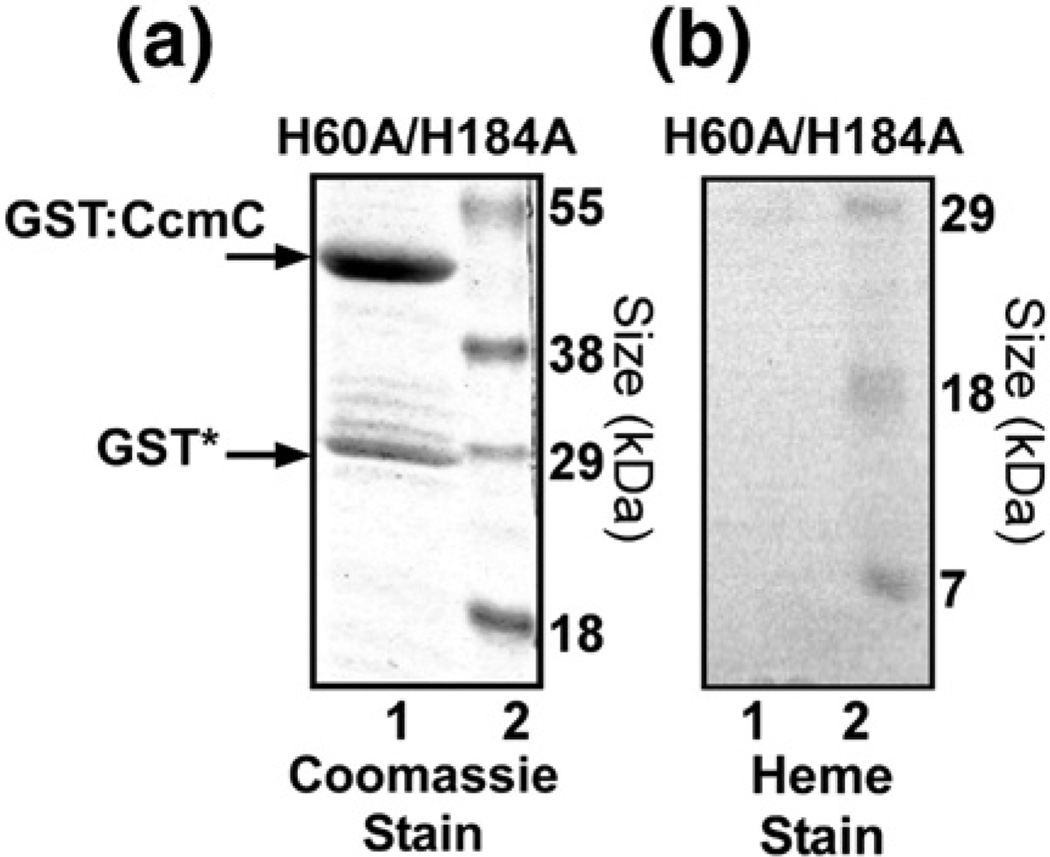
The ability to trap heme in the wild-type complex,36 and its unique split α absorption maxima at room temperature,16 facilitates a comprehensive study on CcmC residues that confer these absorption characteristics. Figure 4a diagrams all residues in CcmC and CcmE that are identically conserved in bacteria (in red; Fig. 4a, d, and e). Heme in the CcmC:heme:CcmE complex is liganded by the CcmC His60 and His184 residues (Fig. 4a, starred). CcmC(H60A) and CcmC(H184A) substitutions significantly perturbed the UV/Vis spectra when compared to the wild-type complex, but each mutant showed significant levels of covalent heme present (on holoCcmE) and they are partially functional16 (Table 1). We extended these results by engineering a CcmC(H60A/H184A) double mutant. The GST-purified CcmC(H60A/H184A) double mutant has stable GST:CcmC with no detectable CcmE (Fig. 5a, lane 1), has no detectable heme associated with it (Fig. 5b, lane 2), and has no function in cytochrome c synthesis (Table 1). These results are consistent with His60 and His184 as axial ligands to the heme iron, at least one of which is required for stable binding of heme. The absence of co-purified CcmE in the double histidine mutant substantiates the requirement of heme for the stable binding of CcmE to form the complex.
Table 1.
Properties of CcmC mutants
| CcmC mutant | Biosynthetic function (cytochrome c4 reporter)a (%) |
% holoCcmE boundb |
% CcmE boundc |
Reduced α absorption peak(s)d |
Pyridine Hemochromee |
|---|---|---|---|---|---|
| Wild type | 100 | 100 | 100 | 553/559 | 552 |
| H60A | 6 (±1) | 50 | 35 | None Detectedf | 553 |
| H184A | 3 (±1) | 50 | 46 | 558 (broad) | 553 |
| H60A/H184A | <1 | <3 | <4 | No hemeg | No heme |
| W119A | 40 (±3) | 100 | 100 | 557 | 552 |
| W125A | 55 (±4) | 100 | 100 | 552/559 | 552 |
| W125A/D126A | 34 (±12) | 59 | 30 | 554/560 | 553 |
| W123A/W125A/D126A | 3 (±1) | 36 | 15 | 558 | 552 |
| W5A/D126Ah | <1 | <3 | <4 | No heme | No heme |
| Y139A | 99 (±12) | 95 | 94 | 552/559 | 552 |
Percentages were determined as described in Materials and Methods from four trials. A representative heme stain of each is shown in Supplementary Fig. 1.
Percentages were determined by comparison of co-purifying holoCcmE (e.g., from heme stain), standardized to the amount of WT co-purifying CcmE (e.g., see Figs. 6 and 7 and Supplementary Fig. 3).
Percentages were determined by comparison of CcmE, standardized to the amount of GST-CcmC, on Coomassie-stained SDS-polyacrylamide gels (e.g., see Fig. 5 and Supplementary Figs. 2 and 3).
Spectroscopically determined as described in Materials and Methods.
Spectroscopically determined as in Berry and Trumpower, 1987.43
No detectable change (from the oxidized or “as purified”) in the α maxima upon Na-dithionite reduction (see Ref. 16, e.g., no detectable α absorption maxima upon reduction).
No detectable heme in the GST-purified protein complex.
W5A corresponds to alanine substitutions at Trp positions 114, 119, 122, 123, and 125 (see Fig. 4a).
Fig. 5.
The double histidine mutant of CcmC(H60A/H184A) does not bind heme. (a) Coomassie stain of the GST-purified GST:CcmC(H60A/H184A) protein and corresponding heme stain shown in (b). All system I proteins except CcmA and CcmB were present in the experiment, including CcmE. Standard proteins with the indicated molecular masses are shown in lane 2 of (a) and lane 1 of (b).
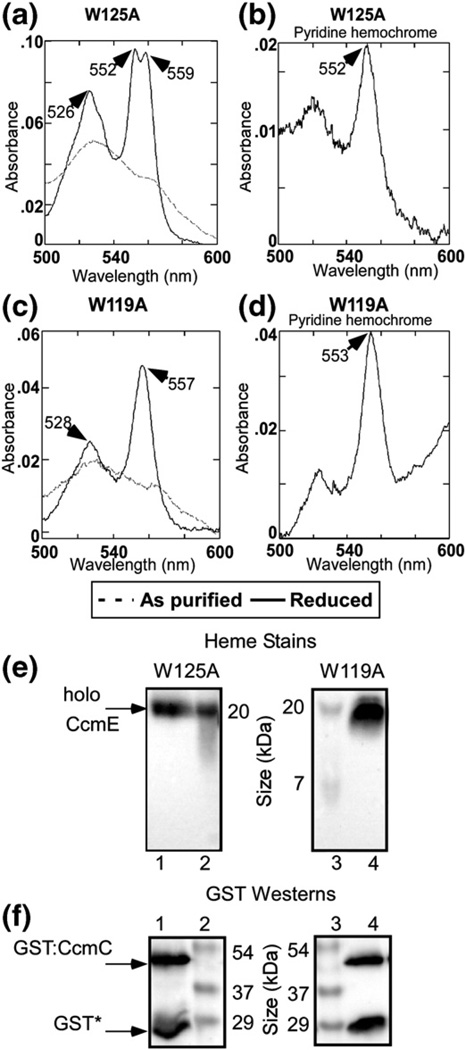
We next studied the residues in the WWD domain of CcmC (Fig. 4b), focusing on the residues that are invariable in all three family members (CcmC, CcmF, and CcsA). Because a variety of single mutations have been shown to have partial function,31,32,44 we decided to analyze single, double, and triple mutants for function (e.g., Supplementary Fig. 1); levels of (holo)CcmE bound; levels of CcmE bound (Supplementary Fig. 2); and spectral properties (Figs. 6 and 7, quantified and summarized in Table 1). A single CcmC(W125A) mutant shows wild-type levels of heme and (holo)CcmE that co-purifies (Fig. 6a and e and Table 1). The spectra of the purified, Nadithionite-reduced, and pyridine hemochromagen look identical with that of wild type (Fig. 6a and b), and function is 55% of that of wild type. Note that treatment with pyridine and reduction typically yields an absorption maximum of 552 nm (±1 nm) when one covalent linkage to a heme vinyl is present. The CcmC(W119A) complex shows 40%function and wild-type levels of holoCcmE (Fig. 6c and e) and pyridine hemochromagen spectra (Fig. 6d). However, a notable difference is observed in the α absorption maxima upon Na-dithionite reduction of the CcmC (W119A) complex (Fig. 6c). The split α maxima are no longer present, and it yields a single absorption maximum at 557 nm, intermediate to the unique split peaks observed in the wild type. The simplest interpretation is that Trp119 of CcmC interacts directly with the heme in the complex, and the indole side chain of Trp119 is important to the heme environment, which yields the split α absorption.
Fig. 6.
Purification and spectral analysis of GST-purified CcmC single point mutant complexes. Absorption spectra and pyridine hemochromes of purified GST: CcmC(W125A)E [(a) and (b), respectively] and purified GST: CcmC(W119A)E [(c) and (d), respectively]. As-purified (broken line) and Na-dithionite-reduced (continuous dark line) spectra are shown. The absorption maxima are denoted with arrows. (e) Heme stains and (f) anti-GST Western blots of GST-purified CcmC(W125A)E (lane 1) and CcmC(W119A)E (lane 4). The positions of holoCcmE, GST: CcmC, and GST* are shown with arrows to the left of lane 1. Standard proteins with indicated molecular masses are shown in lanes 2 and 3.
Fig. 7.
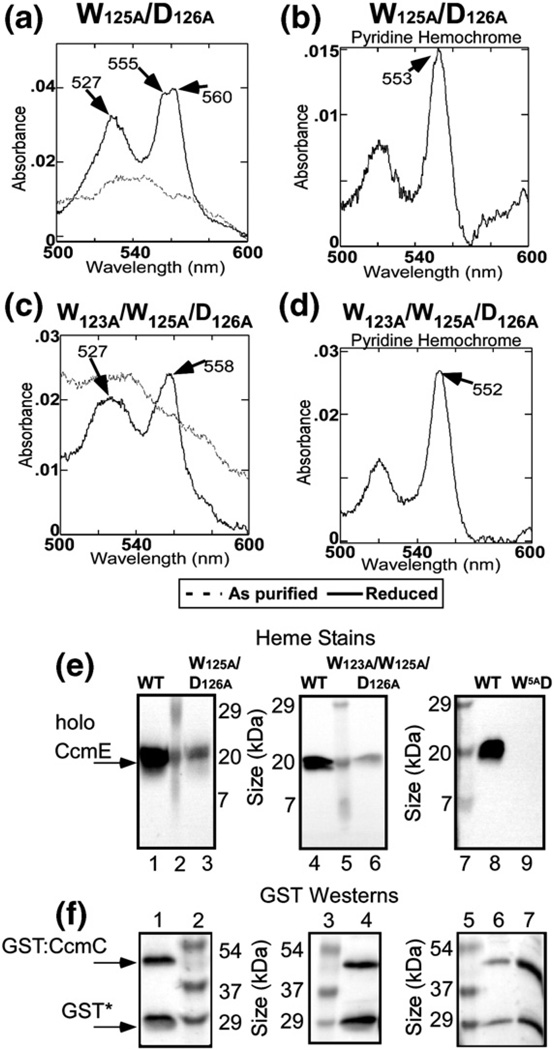
Purification and spectral analysis of GST-purified CcmC multiple point mutant complexes. Absorption spectra and pyridine hemochromes of purified GST: CcmC(W125A/D126A)E [(a) and (b), respectively] and purified GST:CcmC(W123A/W125A/D126A)E [(c) and (d), respectively]. As-purified (broken line) and Na-dithionite-reduced (continuous dark line) spectra are shown. The absorption maxima are denoted with arrows. (e) Heme stains and (f) anti-GST Western blots of GST-purified wild-type CcmCE (e: lanes 1, 4, and 8; f: lane 6), CcmC(W125A/D126A) E (e: lane 3; f: lane 1), CcmC(W123A/W125A/D126A)E (e: lane 6; f: lane 4), and CcmC(W 5A /D126A)E (e: lane 9; f: lane 7). The designation “W5AD” represents GST-purified CcmC(W114A/W119A/W121A/W123A/W125A/D126A)E. The positions of holoCcmE, GST:CcmC, and GST* are shown with arrows to the left of lane 1. Standard proteins with indicated molecular masses are shown in lanes 2, 5, and 7 of (e) and lanes 2, 3, and 5 of (f).
A double mutant, CcmC(W125A/D126A), exhibits 34% function and 59% of trapped heme as wild type, all of which by heme stain, and pyridine hemochrome spectra are covalently linked to CcmE (Fig. 7a, b, and e and Table 1). The spectra of this Nadithionite-reduced complex show a broadening and slight red shift of the split α but the absorption maxima are close to that of wild type (Fig. 7a). A triple mutant, CcmC(W123A/W125A/D126A), shows 3% function and has approximately one-third the levels of heme in the complex as wild type (Fig. 7c–e and Table 1). In this case, the spectra of the heme were again significantly perturbed in the Nadithionite-reduced state, with no split α absorption maxima and a single α maximum at 558 nm (Fig. 7c). The pyridine hemochrome spectrum (Fig. 7d) is identical with that of the wild type, again suggesting that of the heme that is present, all is covalently attached to the CcmE His130 residue. A CcmC mutant with all five tryptophans substituted for alanines (marked with numbers in Fig. 4b) and a D126A is completely nonfunctional and exhibits no bound heme, and no CcmE co-purifies (Table 1).We conclude that the WWD domain and the conserved tryptophans that define this superfamily directly interact with heme, forming the specific heme binding site. In Discussion, we present a unified model that explains prior results and further reflects on the heme binding site.
Heme entry into the WWD domain (external heme binding site)
Note that our results define CcmC residues important for interaction with heme and that residues in CcmE also play a major role in forming the stable heme complex diagrammed in Fig. 4a. It is still unclear how heme travels to the CcmC WWD domain to interact with CcmE. The only conserved residue in TMS regions in CcmC, other than glycine/alanine/leucine, is Tyr139. Tyr139 in TMS-4 is spatially deeper in the bilayer below the periplasmic WWD domain (Fig. 4a). This position is topologically equivalent to His858 in the CcsBA protein, shown to be necessary for heme binding in a “channel” site in CcsBA and proposed to protect the heme from oxidation as it moves to the WWD domain.28 CcmC Tyr139 has previously been mutated and shown to be functional,44 but we wanted to test the possibility that it may affect levels of heme that move to the WWD domain. Both histidines and tyrosines can serve as axial ligands to heme iron. The CcmC(Y139A) mutant shows wild type levels of heme and identical spectral properties as the wild-type CcmC:heme:CcmE complex (Supplementary Fig. 3). We discuss below how heme may move to the WWD domain of CcmC and implications with respect to the formation of the CcmE covalent adduct.
Discussion
Requirements for formation of a stable CcmC:heme:CcmE complex
The purified CcmC:heme:CcmE complex yielding the unusual split α maxima (553 and 559 nm) is stable for months on ice with no additional changes in the UV/Vis spectrum upon Na-dithionite reduction (Ref. 16, this study). These distinct UV/Vis spectrum is a ramification of the heme’s unique environment, including a covalent linkage to the CcmE His130 residue and axial ligands to CcmC His60 and His18416 (see below). Surprisingly, only when apoCcmE is present is the stable complex formed. [Recall that CcmE(H130A) still forms a stable holoCcmCDE(H130A) complex with non-covalent heme16 (see below).] GST–CcmC, without CcmE, does not purify with endogenous heme even where levels 100 times lower could be measured in the complex. All experiments included the small polypeptide CcmD, thus indicating that CcmE is essential for trapping heme. Our data and, consequently, conclusion are contrary to the data and conclusion of Ren and Thony-Meyer, who showed that CcmC alone binds to a heme agarose column.37 The heme is attached to this agarose matrix via the heme propionate group(s) such that the vinyl side chains are presumably accessible, yet, in theory, the heme naturally bound in CcmC “presents” the vinyl groups for interaction with CcmE (see Fig. 4a and c). Accordingly, it is hard to envision how the matrix: propionate-attached heme would specifically bind its site in CcmC without major steric hindrances. It is well known that heme columns are hydrophobic matrices, and in our hands, many membrane proteins nonspecifically bind to heme columns. Experiments presented here have all been performed with endogenously synthesized heme such that any heme trapped is likely physiological.
We carried out experiments whereby the endogenous heme synthesis was depleted, demonstrating that apoCcmE only binds to hexahistidine-tagged CcmC when heme is present. We conclude that distinct atoms in heme interact with CcmC and CcmE to form a stable complex. We previously demonstrated that formation of the CcmC:heme: CcmE complex does not require the CcmE His130 covalent adduct, although the split α absorption is perturbed in the CcmE(H130A) mutant and the heme is not covalently attached to CcmE.16 Some residues in CcmE have been studied for the in vitro binding of free heme to mutated soluble CcmE*,13,15,45 but the relevance to the interactions in formation of the complex described here is unknown. An NMR-derived structure for apoCcmE* has been determined,42,45 and prior models have speculated on where heme might reside in the released holoCcmE.42,46,47 We focus and speculate here on where heme presented by CcmC may interact with CcmE to form the complex. It can be surmised that the features of heme that interact with apoCcmE are near the vinyl groups (Fig. 4c) and this is the side of heme presented by CcmC. It is likely that surface residues in CcmE near the His130 imidazole play a role in this interaction46 (see Fig. 4d for possible residues). Figure 4d presents two possible surfaces of CcmE that may interact with heme in the complex, represented by images i–iii and image iv. From the CcmE orientation in images i–iii, it is clear that nearly all completely conserved (identical) residues other than the His130 and Tyr134 are buried in the protein (see residues colored red). However, some semi-conserved residues (blue in Fig. 4d and e) are accessible and near His130, as designated in Fig. 4d, images ii and iii. Clearly, the CcmE surface presented in Fig. 4d, image iii, could specifically accommodate the heme atoms labeled in Fig. 4c. However, if one rotates apoCcmE as shown in Fig. 4d, image iv, a new surface shows that the His130 imidazole nitrogens are exposed in a pocket (labeled “N of H130”). This surface has many more completely and partially conserved residues (red and blue, with some labeled), which could also accommodate the vinyl-group heme elements and position the β-carbon of 2-vinyl nearby the Nπ atom of His130 imidazole (see below). We favor this orientation because of more conserved residues and because an H130A mutant would have no impact on formation of the complex (i.e., in images i–iii, the His130 side chain is more prominent in a potential interaction with heme). After release from the CcmC:heme:CcmE complex, CcmE Tyr134 and other adjoining residues in CcmE might show significant movements to further bury the heme, as has been previously discussed.46 It is also likely that residues in CcmC and CcmE specifically interact with each other transiently or that heme transiently interacts with CcmC or CcmE, but these interactions were not predominant enough to uncover in our studies. Clearly, heme is the central molecule in formation of the CcmC:heme: CcmE complex.
Function of residues in the CcmC WWD domain and flanking histidines in binding heme
The His60 and His184 of CcmC are likely the axial ligands to the heme in the complex, yet all heme in the CcmC(H60A) and CcmC(H184A) mutants was still covalently linked to acceptor CcmE.16 Although only approximately 50%of the heme is present in these His mutants compared to wild type, they are still partially functional16,37 (Table 1). In the present study, a double CcmC(H60A/H184A) mutant has no heme bound, shows no function, and has no bound CcmE. Data shown in Table 1 with all CcmC mutants, including WWD mutants described below, lead us to the following conclusion: the levels of CcmE bound to CcmC are always directly proportional to the levels of stably bound heme. Indeed, levels of the CcmE:heme adduct formed determine the levels of holocytochrome c produced; thus, any holoCcmE (released by CcmAB via formation of the CcmABCDE complex) is probably used by the putative CcmF/H synthetase for ligation to apocytochrome c.
Results of site-directed mutational analysis of conserved residues in the WWD domain demonstrate that, indeed, this domain directly interacts with heme. A single mutation, CcmC(W119A), resulted in a complex where the distinct split α absorption is changed to a single α absorption maxima at 557 nm. This mutant complex still has covalently bound holoCcmE and wild type levels of heme and it is functional for cytochrome c biosynthesis (Table 1). Other combinations of mutations [e.g., CcmC(W123A/W125A/D126A)] also lead to an altered α absorption maximum, while some mutations have no effect on UV/Vis spectra. A mutant with six alterations in the WWD domain does not have heme or CcmE bound. The simplest explanation for the spectral perturbations that result when tryptophans are removed is that these indole side chains interact directly with heme; possibly, this interaction is with the heme pyrroles where the α absorption emanates in part (via iron liganding). Interestingly, none of the CcmC mutations in the WWD domain or histidines prevent covalent ligation to the CcmE His130, suggesting that as long as the heme 2-vinyl is positioned correctly with respect to the His130, the covalent adduct will form. Of course, the interaction of specific WWD domain residues with heme may be obligatory to the proper positioning of heme to form the covalent adduct.
Ren and Thony-Meyer have proposed that the function of the WWD domain (and His60 and His184) is to bind CcmE because CcmC WWD mutants (and a H60A, H184A double mutant) still bound to the heme agarose columns discussed earlier, yet CcmE did not co-immunoprecipitate with these CcmC mutant proteins.37 Having addressed the heme agarose experiments above, our data explain the second observation. That is, heme is absolutely necessary for apoCcmE to bind to CcmC and because WWD mutants bind less heme, they will bind less CcmE. The same is true for the double H60A, H184A mutant.
Heme trafficking to the CcmC WWD domain and formation of the CcmE:heme adduct
A question of considerable importance in the heme-handling protein family is how heme traffics to the WWD domain. For CcmF, heme in principle enters directly from released holoCcmE, thus via the periplasm, but this is poorly understood. For CcsBA, the data indicate that heme enters from the cytoplasm or as it is released by ferrochelatase to a specific heme binding site in the CcsBA channel (Fig. 1). This channel binding site is defined by two completely conserved histidines within TMS-3 (His77) and TMS-8 (His858).28 TMS-8 is immediately after and thus topologically below the WWD domain of CcsBA. Both TMS histidines of CcsBA are required for cytochrome c biosynthesis, and CcsBA (His858A) no longer binds heme. Other evidence supports the idea that CcsBA His77 and His858 form a heme binding site and protect the heme before it moves into the external heme binding domain (theoretically the WWD domain). CcmC does not have a conserved histidine in TMSs, yet it does have a conserved tyrosine (Tyr139) that is topologically located equivalent to CcsBA His858 (Fig. 4a). However, CcmC(Y139A) is completely functional and the complex with heme and CcmE is identical with wild type (Supplementary Fig. 3). Although we do not know the function of Tyr139, we conclude that it is unlikely that a specific heme binding site within a channel is present in CcmC as it is in CcsBA. We suggest that heme moves to the WWD domain either through a nonspecific “channel” or “vestibule” in CcmC or from the lipid bilayer. As we have noted for CcsBA,28 in the case of CcmC, we cannot rule out whether an energy source is necessary for movement of heme to the WWD domain, and hence, the proper use of the terms “channel” or “transporter” awaits further study.
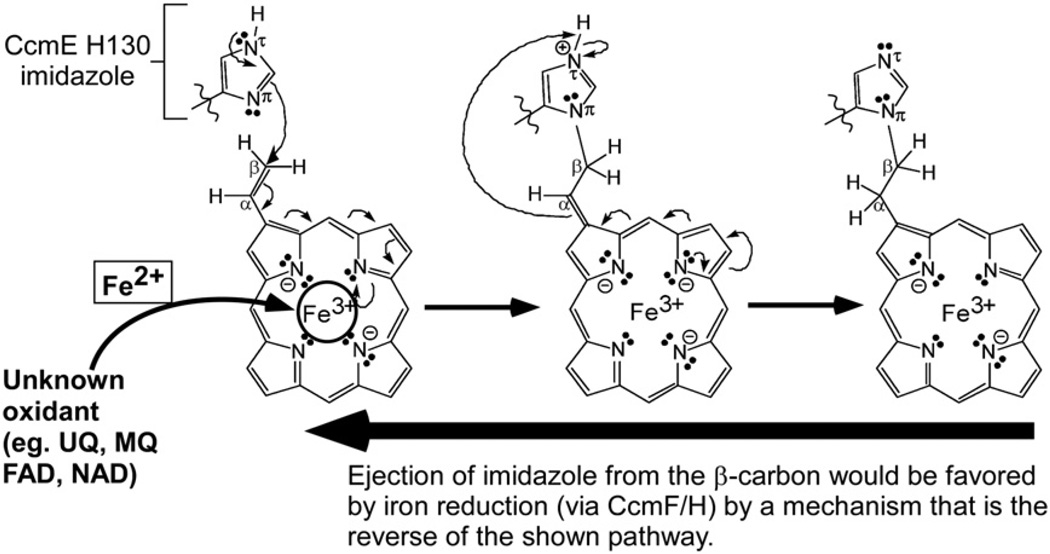
Why would heme moving to the WWD domain in CcmC, whether through the channel or bilayer, not require protection from oxidation, as suggested for CcsBA heme trafficking? CcsBA is the cytochrome c synthetase, and it attaches the trafficked heme directly to the CXXCH apocytochrome c acceptor. Recall that for attachment to the α-carbon of 2- and 4-vinyl groups, the reduced cysteines (of CXXCH) require the heme in the +2 reduced state.3,5,6,10,48 Lee et al. have shown that the CcmE His130 adduct is at the β-carbon of the 2-vinyl group of heme.49 As we have discussed recently, we propose that the adduct to CcmE His130 is formed using heme with iron in the oxidized (+3) state.10,16 According to a Michael reaction mechanism, the CcmE His130 imidazole would preferentially attach to the β-carbon of the 2-vinyl of heme when heme is in the iron +3 state (Fig. 8). At the CcmF/H cytochrome c synthetase, ejection of that imidazole (of CcmE His130) would be favored by iron reduction, which would also leave the vinyl groups prepared for attachment to CXXCH. In this scheme, not only is heme iron protection from oxidation by CcmC unnecessary, but a mechanism for oxidation would be essential as well. We suggest that any oxidant such as ubiquinone or redox-active molecule that has access to the heme in the WWD domain or before entering it could suffice. Heme moieties that are exposed to aqueous environments often have very low redox potentials in general.46,50,51 We also predict that the redox potential of the heme in this site will be very negative and thus easily oxidizable. Future work will be dedicated to purifying enough complexes to determine the redox potential, find candidate oxidants, and reconstitute in vitro the adduct formation using CcmC, CcmE, and heme.
Fig. 8.
Proposed Michael addition (nucleophilic) reaction mechanism for covalent adduct formation at CcmE His130. The imidazole group of the CcmE His130 is indicated and is attached to the β-carbon of the heme 2-vinyl. Full arrows represent two electron transfers. The oxidation state of the heme is shown for each step. Mechanism of initial oxidation of heme (Fe2+ to Fe3+) is unknown. UQ, ubiquinone; MQ, menaquinone; FAD, flavin adenine dinucleotide; NAD, nicotinamide dinucleotide (figure is modified from Kranz et al.10).
Materials and Methods
Bacterial strains and growth conditions
The E. coli strains (Table 2) for protein over-expression and purification were grown at 37 °C, shaking at 240 rpm, unless otherwise noted, in Luria–Bertani broth (LB; Difco). Concentrations of antibiotics (Sigma-Aldrich) and other media additives were as follows: carbenicillin, 50 µg ml−1; chloramphenicol, 20 µg ml−1; tetracycline, 15 µg ml−1; IPTG (Gold Biotechnology), 1 mM; arabinose (Gold Biotechnology), 0.2% (wt/vol); δ-ALA (Sigma-Aldrich), 50 µg ml−1; and 4-(2-aminoethyl)benzenesulfonyl fluoride hydrochloride (Gold Biotechnology), 1 mM.
Table 2.
Oligonucleotides, strains, and plasmids used in this study
| Name | Sequence 5′–3′ | Purpose |
|---|---|---|
| ccmC-F.BglII | GCGAGATCTATGTGGAAAACACTGCATC | pSysI-ccmC derivatives |
| ccmE-R. NdeI/EcoRI | GCGAATTCGGCATCATATGGCTGGGTCCTTATAAAC | pSysI-ccmC derivatives |
| ccmC H60A.R | GCCGCAGGCACAGCCAGGTAG | pRGK392 [pGEXccmC (H60A/H184A)DE] |
| ccmC W119A-R | CACCAGGTGCCCGCCATCGGTTTTC | pRGK393 [pGEXccmC (W119A)DE] |
| ccmC W125A-R | GACGTGCATCCGCTACCCACCAGGTGCC | pRGK394 [pGEXccmC (W125A)DE] |
| ccmC W125A/D126A-R | CAGACGTGCAGCCGCTACCCACCAGGTGCC | pRGK395 [pGEXccmC (W125A/D126A)DE] |
| ccmC W123A/W125A/D126A-R | CAGACGTGCAGCCGCTACCGCCCAGGTGCCCCAC | pRGK396 [pGEXccmC (W123A/W125A/D126A)DE] |
| ccmC W114A/W119A/W122A-R | CGCTACCGCCGCGGTGCCCGCCATCGGTTTTCCCGCTGCAGAGCC | pRGK397 [pGEXccmC (W5A/D126A)DE] |
| ccmC Y139A-R | ACCCACAGCCAAAAACAGCAG | pRGK398 [pGEXccmC (Y139A)DE] |
| Description | References | |
| Strains | ||
| RK103 | Δccm | 17 |
| RK105 | Δccm ΔhemA | 41 |
| RK111 | Δccm-c4:6×His integrate | Unpublished |
| Plasmids | ||
| pRGK333 | pSysI | 17 |
| pRGK354 | pSysI-ΔccmAB | 36 |
| pRGK363 | pBADccmC:6×His | 36 |
| pRGK365 | pUCA10-compccmAB | 36 |
| pRGK375 | pGEXccmCDE | 16 |
| pRGK376 | pGEXccmC (H60A)DE | 16 |
| pRGK377 | pGEXccmC (H184A)DE | 16 |
| pRGK382 | pSysI-ΔccmAC | 16 |
| pRGK383 | pSysI-ΔccmABE | 16 |
| pRGK388 | pBAD-ccmF:6×HisGH | 16 |
| pRGK391 | pGEXccmC (H60A/H184A)DE | This work |
| pRGK392 | pGEXccmC (W119A)DE | This work |
| pRGK393 | pGEXccmC (W125A)DE | This work |
| pRGK394 | pGEXccmC (W125A/D126A)DE | This work |
| pRGK395 | pGEXccmC (W123A/W125A/D126A)DE | This work |
| pRGK396 | pGEXccmC (W5A/D126A)DE | This work |
| pRGK397 | pGEXccmC (Y139A)DE | This work |
Construction of strains and plasmids
All oligonucleotide primer sequences, plasmids, and strains are given in Table 2. The cytochrome c4:6 × His chromosomal integrate strain (RK111) will be described elsewhere. E. coli strain TB1 was the initial host for all plasmids used in generating ccmC mutations. System I ccmC-derived plasmids that are pGEX 4T-1 (GE Healthcare) based have an IPTG-inducible GST fusion to the N-terminus of CcmC for over-expression and purification. The final construct for each system-I-derived plasmid was verified by DNA sequencing. Unless noted, pRGK33317 (pSysI) was used as the template in all PCR reactions used to generate the system I ccmC-derived plasmids. A plasmid containing ccmC(H60A/H184A)DE was constructed by using oligonucleotides ccmC-F.BglII and ccmCH60A-R to generate a short PCR product. This short PCR product was then used as a forward oligonucleotide primer with ccmE-R.NdeI/EcoRI to amplify ccmC(H60A/H184A)DE from pRGK37716 [pGEXccmC(H184A) DE], which was digested with BglII/EcoRI and ligated to BamHI/EcoRI-digested pGEX4T-1 to generate pRGK391. A similar two-step process was used to construct pRGK392 [pGEXccmC(W119A)DE], pRGK393 [pGEXccmC(W125A)DE], pRGK394 [pGEXccmC(W125A/D126A)DE], pRGK395 [pGEXccmC(WWD-A)DE], and pRGK397 [pGEXccmC(Y139A) DE]. First, the corresponding point mutant primer and ccmC-F.BglII were used to generate a short PCR product that contained the desired point mutation. Second, the short PCR product was used as an oligonucleotide primer, along with ccmE-R.NdeI/EcoRI, to amplify ccmCXXDE, digested with BglII/EcoRI, and ligated to BamHI/EcoRI-digested pGEX 4T-1. To engineer a ccmC-derived plasmid that has all five Trp residues and the Asp residue in the WWD domain mutated to alanines, we first amplified a short PCR product with ccmC-F.BglII and ccmCW114A/W119A/W122A-R. This short PCR product was then used with ccmE-R.NdeI/EcoRI and pRGK396 [pGEXccmC(WWD-A)DE] as a template to generate a ccmCXXDE PCR product with all six ccmC Ala substitutions, digested with BglII/EcoRI, and ligated to BamHI/EcoRI-digested pGEX 4T-1 to generate pRGK396 .
Holocytochrome c4 activity by ccmC point mutants
Cytochrome c4:6 × His production was assayed using E. coli RK111 (Δccm-c4:6 × His integrate) harboring pRGK36536 (pUCA10-compccmAB), pRGK38816 (pBAD-ccmF: 6 × HisGH), and one of the following pGEXccmCXX DE-based ccmC point mutant plasmids: pRGK376, pRGK377, and pRGK392-397. Five-milliliter cultures in LB media were started with a 1% (vol/vol) inoculum from a fresh overnight culture and grown to an OD600 (optical density at 600 nm) of approximately 0.5, and the system I genes and c4:6 × His reporter were induced with 1 mM IPTG and 0.2% arabinose for 3 h with shaking at 300 rpm at 37 °C. B-Per (Pierce ThermoScientific) fractionations and mini His Bind affinity columns were performed as previously described.41
Heme limitation
The δ-ALA (heme) limitation experiments were performed as previously described.41 Briefly, cultures of E. coli RK10541 (ΔccmΔhemA) harboring pRGK38216 (pGEX-ΔccmAC), pRGK36336 (pBADccmC:6 × His), and pHPEX252 were grown overnight in 100 ml of LB media containing carbenicillin, chloramphenicol, and δ-ALA. For each experimental condition, 1 L of fresh LB media, containing δ-ALA, was inoculated to 1% (vol/vol) with fresh overnight culture and incubated at 37°°C with shaking at 300 rpm for 2.5 h (OD600 ~ 1.0). Following the initial growth step, the cells were collected by centrifugation at 4000g for 10 min, washed once with 250 ml of LB media devoid of δ-ALA, and resuspended in 1 L of LB media with antibiotics but devoid of δ-ALA. To exhaust the cellular supply of heme (δ-ALA), we incubated the washed cultures at 37 °C with shaking at 300 rpm for 2.5 h. A second 1-L culture that contained δ-ALA was grown and treated identically to the culture devoid of δ-ALA. The pGEX-ΔccmAC and pBADccmC:His were induced with 1 mM IPTG and 0.2% arabinose, respectively, for 14–16 h. The cells were harvested at 6000g for 10 min, and the cell pellets were stored at −80 °C for at least 1 h.
Membrane preparation and protein purification
E. coli cultures containing the pGEX4T-1-based, system-I-derived (ccmCXXDE) plasmids were grown as given above in LB media supplemented with the appropriate antibiotics to an OD600 of approximately 1.8 and induced with 1 mM IPTG for 14–16 h. The cells were harvested at 6000g for 10 min and the cell pellets were stored at −80 °C for at least 1 h. For each gram of thawed cell pellet, 3.5 ml of either 1 × GST buffer (4.3 mM Na3HPO4, 1.47 mM KH2PO4, 137 mM NaCl, and 2.7 mM KCl, pH 7.3) or, for CcmC:6 × His purification, 1 × His bind buffer (300 mM NaCl, 50 mM sodium phosphate buffer, and 10 mM imidazole, pH 8.0) was used to resuspend each cell pellet. The cell suspension was treated with 4-(2-aminoethyl) benzenesulfonyl fluoride hydrochloride (1 mM) and egg white lysozyme (100 µg ml−1 ; Sigma-Aldrich) on ice with shaking for 20 min and sonicated using a Branson 250 sonicator (50% duty, 80% output) fitted with a medium (flat tip) twice for 5 min each. Membrane proteins were isolated at 207,000g for 1 h and 15 min, washed three times with either 1 × GST buffer or 1 × His bind buffer, resuspended in the respective buffers, and solubilized with 1% DDM (Anatrace). Prior to column chromatography, the solubilized membranes were spun at 5000g for 10 min. GST:CcmC or CcmC:6 × His was purified over either glutathione agarose resin (Pierce ThermoScientific) or Ni-NTA His Bind resin (EMD Biosciences) as per manufacturers’ recommendations except that the wash buffers were supplemented with 0.02% DDM.
Other methods
Heme stains and Western blots were performed as previously described17,53 on proteins that were first separated by 12.5% SDS-PAGE and then transferred to Hybond C nitrocellulose membranes (GE Healthcare). Antibodies were used at the following dilutions: anti-CcmE,17 1:5000; anti-GST (Sigma Aldrich), 1:5000; and anti-His (Santa Cruz Biotechnology), 1:5000. Protein A peroxidase (Sigma Aldrich) was used as the secondary label. The chemiluminescent signal for heme stains and anti-His Westerns was developed using the SuperSignal Femto kit (Pierce ThermoScientific) and the signal from anti-CcmE and anti-GST Westerns was developed with the Immobilon Western Chemiluminescent horseradish peroxidase substrate (Millipore). Chemiluminescent signals were detected with an LAS-1000 plus detection system (Fujifilm-GE Healthcare). The purity of purified protein complexes was determined by digitizing Coomassie-stained SDS-polyacrylamide gels with the LAS-1000 plus. Quantification of holoCcmE, CcmE, GST: CcmC, and CcmC:His was by comparison of either chemiluminescent signals using Science Lab Image Gauge quantification software (Fujifilm-GE Healthcare) or digitized Coomassie stains (Image J54). Protein concentrations were determined using the BCA assay (Pierce ThermoScientific) with bovine serum albumin as a standard. Pyridine hemochrome analysis was performed by the method of Berry and Trumpower with analytical-grade pyridine (Sigma Aldrich).43 Reduced (Na-dithionite; Sigma Aldrich) and oxidized absorption spectra were obtained as previously described16 on concentrated (Amicon Ultra C-10; Millipore) protein samples with a UV2101 scanning spectrophotometer (Shimadzu). The spectral resolution is ±1 nm.
Supplementary Material
Acknowledgements
We thank Dr. John Taylor and Dr. Elaine Frawley for helpful discussions. We also thank Brian San Francisco for generation of the c4:6 × His chromosomal integrate and manuscript comments. This study was funded by National Institutes of Health grant GM47909 to R.G.K.
Abbreviations used
- TMS
transmembrane-spanning segment
- ABC
ATP-binding cassette
- GST
glutathione S-transferase
- DDM
n-dodecyl β-d-maltoside
- δ-ALA
δ-aminolevulinic acid.
Footnotes
Supplementary Data
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.jmb.2010.06.041
References
- 1.Moore GR, Pettigrew GW. Springer Series in Molecular Biology. Berlin, Germany: Springer-Verlag; 1990. Cytochromes c: Evolutionary, Structural, and Physicochemical Aspects. [Google Scholar]
- 2.Ow YP, Green DR, Hao Z, Mak TW. Cytochrome c: functions beyond respiration. Nat. Rev., Mol. Cell Biol. 2008;9:532–542. doi: 10.1038/nrm2434. [DOI] [PubMed] [Google Scholar]
- 3.Ferguson SJ, Stevens JM, Allen JW, Robertson IB. Cytochrome c assembly: a tale of ever increasing variation and mystery? Biochim. Biophys. Acta. 2008;1777:980–984. doi: 10.1016/j.bbabio.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 4.Richardson DJ. Bacterial respiration: a flexible process for a changing environment. Microbiology. 2000;146:551–571. doi: 10.1099/00221287-146-3-551. [DOI] [PubMed] [Google Scholar]
- 5.Barker PD, Ferrer JC, Mylrajan M, Loehr TM, Feng R, Konishi Y, et al. Transmutation of a heme protein. Proc. Natl Acad. Sci. USA. 1993;90:6542–6546. doi: 10.1073/pnas.90.14.6542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nicholson DW, Neupert W. Import of cytochrome c into mitochondria: reduction of heme, mediated by NADH and flavin nucleotides, is obligatory for its covalent linkage to apocytochrome c. Proc. Natl Acad. Sci. USA. 1989;86:4340–4344. doi: 10.1073/pnas.86.12.4340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hamel P, Corvest V, Giege P, Bonnard G. Biochemical requirements for the maturation of mitochondrial c-type cytochromes. Biochim. Biophys. Acta. 2009;1793:125–138. doi: 10.1016/j.bbamcr.2008.06.017. [DOI] [PubMed] [Google Scholar]
- 8.Kranz R, Lill R, Goldman B, Bonnard G, Merchant S. Molecular mechanisms of cytochrome c biogenesis: three distinct systems. Mol. Microbiol. 1998;29:383–396. doi: 10.1046/j.1365-2958.1998.00869.x. [DOI] [PubMed] [Google Scholar]
- 9.Thony-Meyer L. Cytochrome c maturation: a complex pathway for a simple task? Biochem. Soc. Trans. 2002;30:633–638. doi: 10.1042/bst0300633. [DOI] [PubMed] [Google Scholar]
- 10.Kranz RG, Richard-Fogal C, Taylor JS, Frawley ER. Cytochrome c biogenesis: mechanisms for covalent modifications and trafficking of heme and for heme-iron redox control. Microbiol. Mol. Biol. Rev. 2009;73:510–528. doi: 10.1128/MMBR.00001-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schulz H, Hennecke H, Thony-Meyer L. Prototype of a heme chaperone essential for cytochrome c maturation. Science. 1998;281:1197–1200. doi: 10.1126/science.281.5380.1197. [DOI] [PubMed] [Google Scholar]
- 12.Schulz H, Fabianek RA, Pellicioli EC, Hennecke H, Thony-Meyer L. Heme transfer to the heme chaperone CcmE during cytochrome c maturation requires the CcmC protein, which may function independently of the ABC-transporter CcmAB. Proc. Natl Acad. Sci. USA. 1999;96:6462–6467. doi: 10.1073/pnas.96.11.6462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stevens JM, Daltrop O, Higham CW, Ferguson SJ. Interaction of heme with variants of the heme chaperone CcmE carrying active site mutations and a cleavable N-terminal His tag. J. Biol. Chem. 2003;278:20500–20506. doi: 10.1074/jbc.M212925200. [DOI] [PubMed] [Google Scholar]
- 14.Uchida T, Stevens JM, Daltrop O, Harvat EM, Hong L, Ferguson SJ, Kitagawa T. The interaction of covalently bound heme with the cytochrome c maturation protein CcmE. J. Biol. Chem. 2004;279:51981–51988. doi: 10.1074/jbc.M408963200. [DOI] [PubMed] [Google Scholar]
- 15.Stevens JM, Uchida T, Daltrop O, Kitagawa T, Ferguson SJ. Dynamic ligation properties of the Escherichia coli heme chaperone CcmE to non-covalently bound heme. J. Biol. Chem. 2006;281:6144–6151. doi: 10.1074/jbc.M508765200. [DOI] [PubMed] [Google Scholar]
- 16.Richard-Fogal CL, Frawley ER, Bonner ER, Zhu H, San Francisco B, Kranz RG. A conserved haem redox and trafficking pathway for cofactor attachment. EMBO J. 2009;28:2349–2359. doi: 10.1038/emboj.2009.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feissner RE, Richard-Fogal CL, Frawley ER, Loughman JA, Earley KW, Kranz RG. Recombinant cytochromes c biogenesis systems I and II and analysis of haem delivery pathways in Escherichia coli. Mol. Microbiol. 2006;60:563–577. doi: 10.1111/j.1365-2958.2006.05132.x. [DOI] [PubMed] [Google Scholar]
- 18.Ren Q, Ahuja U, Thony-Meyer L. A bacterial cytochrome c heme lyase. CcmF forms a complex with the heme chaperone CcmE and CcmH but not with apocytochrome c. J. Biol. Chem. 2002;277:7657–7663. doi: 10.1074/jbc.M110979200. [DOI] [PubMed] [Google Scholar]
- 19.Allen JW, Barker PD, Ferguson SJ. A cytochrome b562 variant with a c-type cytochrome CXXCH heme-binding motif as a probe of the Escherichia coli cytochrome c maturation system. J. Biol. Chem. 2003;278:52075–52083. doi: 10.1074/jbc.M307196200. [DOI] [PubMed] [Google Scholar]
- 20.Fabianek RA, Hennecke H, Thony-Meyer L. The active-site cysteines of the periplasmic thioredoxin-like protein CcmG of Escherichia coli are important but not essential for cytochrome c maturation in vivo. J. Bacteriol. 1998;180:1947–1950. doi: 10.1128/jb.180.7.1947-1950.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Metheringham R, Tyson KL, Crooke H, Missiakas D, Raina S, Cole JA. Effects of mutations in genes for proteins involved in disulphide bond formation in the periplasm on the activities of anaerobically induced electron transfer chains in Escherichia coli K12. Mol. Gen. Genet. 1996;253:95–102. doi: 10.1007/pl00013815. [DOI] [PubMed] [Google Scholar]
- 22.Beckman DL, Kranz RG. Cytochromes c biogenesis in a photosynthetic bacterium requires a periplasmic thioredoxin-like protein. Proc. Natl Acad. Sci. USA. 1993;90:2179–2183. doi: 10.1073/pnas.90.6.2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Monika EM, Goldman BS, Beckman DL, Kranz RG. A thioreduction pathway tethered to the membrane for periplasmic cytochromes c biogenesis; in vitro and in vivo studies. J. Mol. Biol. 1997;271:679–692. doi: 10.1006/jmbi.1997.1227. [DOI] [PubMed] [Google Scholar]
- 24.Deshmukh M, Turkarslan S, Astor D, Valkova-Valchanova M, Daldal F. The dithiol: disulfide oxidoreductases DsbA and DsbB of Rhodobacter capsulatus are not directly involved in cytochrome c biogenesis, but their inactivation restores the cytochrome c biogenesis defect of CcdA-null mutants. J. Bacteriol. 2003;185:3361–3372. doi: 10.1128/JB.185.11.3361-3372.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sanders C, Deshmukh M, Astor D, Kranz RG, Daldal F. Overproduction of CcmG and CcmFH(Rc) fully suppresses the c-type cytochrome biogenesis defect of Rhodobacter capsulatus CcmI-null mutants. J. Bacteriol. 2005;187:4245–4256. doi: 10.1128/JB.187.12.4245-4256.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beckett CS, Loughman JA, Karberg KA, Donato GM, Goldman WE, Kranz RG. Four genes are required for the system II cytochrome c biogenesis pathway in Bordetella pertussis a unique bacterial model. Mol. Microbiol. 2000;38:465–481. doi: 10.1046/j.1365-2958.2000.02174.x. [DOI] [PubMed] [Google Scholar]
- 27.Schiott T, von Wachenfeldt C, Hederstedt L. Identification and characterization of the ccdA gene, required for cytochrome c synthesis in Bacillus subtilis. J. Bacteriol. 1997;179:1962–1973. doi: 10.1128/jb.179.6.1962-1973.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frawley ER, Kranz RG. CcsBA is a cytochrome c synthetase that also functions in heme transport. Proc. Natl Acad. Sci. USA. 2009;106:10201–10206. doi: 10.1073/pnas.0903132106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Merchant SS. His protects heme as it crosses the membrane. Proc. Natl Acad. Sci. USA. 2009;106:10069–10070. doi: 10.1073/pnas.0905189106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beckman DL, Trawick DR, Kranz RG. Bacterial cytochromes c biogenesis. Genes Dev. 1992;6:268–283. doi: 10.1101/gad.6.2.268. [DOI] [PubMed] [Google Scholar]
- 31.Goldman BS, Beck DL, Monika EM, Kranz RG. Transmembrane heme delivery systems. Proc. Natl Acad. Sci. USA. 1998;95:5003–5008. doi: 10.1073/pnas.95.9.5003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hamel PP, Dreyfuss BW, Xie Z, Gabilly ST, Merchant S. Essential histidine and tryptophan residues in CcsA, a system II polytopic cytochrome c biogenesis protein. J. Biol. Chem. 2003;278:2593–2603. doi: 10.1074/jbc.M208651200. [DOI] [PubMed] [Google Scholar]
- 33.Lee JH, Harvat EM, Stevens JM, Ferguson SJ, Saier MH., Jr Evolutionary origins of members of a superfamily of integral membrane cytochrome c biogenesis proteins. Biochim. Biophys. Acta. 2007;1768:2164–2181. doi: 10.1016/j.bbamem.2007.04.022. [DOI] [PubMed] [Google Scholar]
- 34.Ahuja U, Kjelgaard P, Schulz BL, Thony-Meyer L, Hederstedt L. Haem-delivery proteins in cytochrome c maturation System II. Mol. Microbiol. 2009;73:1058–1071. doi: 10.1111/j.1365-2958.2009.06833.x. [DOI] [PubMed] [Google Scholar]
- 35.Barrick D. Replacement of the proximal ligand of sperm whale myoglobin with free imidazole in the mutant His-93→Gly. Biochemistry. 1994;33:6546–6554. doi: 10.1021/bi00187a023. [DOI] [PubMed] [Google Scholar]
- 36.Feissner RE, Richard-Fogal CL, Frawley ER, Kranz RG. ABC transporter-mediated release of a haem chaperone allows cytochrome c biogenesis. Mol. Microbiol. 2006;61:219–231. doi: 10.1111/j.1365-2958.2006.05221.x. [DOI] [PubMed] [Google Scholar]
- 37.Ren Q, Thony-Meyer L. Physical interaction of CcmC with heme and the heme chaperone CcmE during cytochrome c maturation. J. Biol. Chem. 2001;276:32591–32596. doi: 10.1074/jbc.M103058200. [DOI] [PubMed] [Google Scholar]
- 38.Schulz H, Pellicioli EC, Thony-Meyer L. New insights into the role of CcmC, CcmD and CcmE in the haem delivery pathway during cytochrome c maturation by a complete mutational analysis of the conserved tryptophan-rich motif of CcmC. Mol. Microbiol. 2000;37:1379–1388. doi: 10.1046/j.1365-2958.2000.02083.x. [DOI] [PubMed] [Google Scholar]
- 39.Richard-Fogal CL, Frawley ER, Kranz RG. Topology and function of CcmD in cytochrome c maturation. J. Bacteriol. 2008;190:3489–3493. doi: 10.1128/JB.00146-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goldman BS, Beckman DL, Bali A, Monika EM, Gabbert KK, Kranz RG. Molecular and immunological analysis of an ABC transporter complex required for cytochrome c biogenesis. J. Mol. Biol. 1997;268:724–738. doi: 10.1006/jmbi.1997.0992. [DOI] [PubMed] [Google Scholar]
- 41.Richard-Fogal CL, Frawley ER, Feissner RE, Kranz RG. Heme concentration dependence and metalloporphyrin inhibition of the system I and II cytochrome c assembly pathways. J. Bacteriol. 2007;189:455–463. doi: 10.1128/JB.01388-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Enggist E, Thony-Meyer L, Guntert P, Pervushin K. NMR structure of the heme chaperone CcmE reveals a novel functional motif. Structure (London) 2002;10:1551–1557. doi: 10.1016/s0969-2126(02)00885-7. [DOI] [PubMed] [Google Scholar]
- 43.Berry EA, Trumpower BL. Simultaneous determination of hemes a, b, and c from pyridine hemochrome spectra. Anal. Biochem. 1987;161:1–15. doi: 10.1016/0003-2697(87)90643-9. [DOI] [PubMed] [Google Scholar]
- 44.Ahuja U, Thony-Meyer L. Dynamic features of a heme delivery system for cytochrome C maturation. J. Biol. Chem. 2003;278:52061–52070. doi: 10.1074/jbc.M310077200. [DOI] [PubMed] [Google Scholar]
- 45.Arnesano F, Banci L, Barker PD, Bertini I, Rosato A, Su XC, Viezzoli MS. Solution structure and characterization of the heme chaperone CcmE. Biochemistry. 2002;41:13587–13594. doi: 10.1021/bi026362w. [DOI] [PubMed] [Google Scholar]
- 46.Harvat EM, Redfield C, Stevens JM, Ferguson SJ. Probing the heme-binding site of the cytochrome c maturation protein CcmE. Biochemistry. 2009;48:1820–1828. doi: 10.1021/bi801609a. [DOI] [PubMed] [Google Scholar]
- 47.Sanders C, Turkarslan S, Lee DW, Daldal F. Cytochrome c biogenesis: the Ccm system. Trends Microbiol. 2010;18:266–274. doi: 10.1016/j.tim.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Turkarslan S, Sanders C, Daldal F. Extracytoplasmic prosthetic group ligation to apoproteins: maturation of c-type cytochromes. Mol. Microbiol. 2006;60:537–541. doi: 10.1111/j.1365-2958.2006.05148.x. [DOI] [PubMed] [Google Scholar]
- 49.Lee D, Pervushin K, Bischof D, Braun M, Thony-Meyer L. Unusual heme-histidine bond in the active site of a chaperone. J. Am. Chem. Soc. 2005;127:3716–3717. doi: 10.1021/ja044658e. [DOI] [PubMed] [Google Scholar]
- 50.Stellwagen E. Haem exposure as the determinate of oxidation-reduction potential of haem proteins. Nature. 1978;275:73–74. doi: 10.1038/275073a0. [DOI] [PubMed] [Google Scholar]
- 51.Tezcan F, Winkler J, Gray H. Effects of ligation and folding on reduction potentials of heme proteins. J. Am. Chem. Soc. 1998;120:13383–13388. [Google Scholar]
- 52.Varnado CL, Goodwin DC. System for the expression of recombinant hemoproteins in Escherichia coli. Protein Expr. Purif. 2004;35:76–83. doi: 10.1016/j.pep.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 53.Feissner R, Xiang Y, Kranz RG. Chemiluminescent-based methods to detect subpicomole levels of c-type cytochromes. Anal. Biochem. 2003;315:90–94. doi: 10.1016/s0003-2697(02)00658-9. [DOI] [PubMed] [Google Scholar]
- 54.Rasband W. Image J. Bethesda, MD: U.S. National Institutes of Health; 1997–2008. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.