Abstract
Protein-tyrosine phosphatase 1B (PTP1B), a well-established metabolic regulator, plays an important role in breast cancer. Using whole-body PTP1B knockout mice, recent studies have shown that PTP1B ablation delays HER2/Neu-induced mammary cancer. Whether PTP1B plays a cell-autonomous or a non-cell-autonomous role in HER2/Neu-evoked tumorigenesis and whether it is involved in tumor maintenance was unknown. We generated mice expressing HER2/Neu and lacking PTP1B specifically in the mammary epithelium. We found that mammary-specific deletion of PTP1B delays the onset of HER2/Neu-evoked mammary tumors, establishing a cell autonomous role for PTP1B in such neoplasms. We also deleted PTP1B in established mouse mammary tumors or depleted PTP1B in human breast cancer cell lines grown as xenografts. PTP1B inhibition did not affect tumor growth in either model showing that neither epithelial nor stromal PTP1B is necessary for tumor maintenance. Taken together, our data show that despite the PTP1B contribution to tumor onset, it is not essential for tumor maintenance. This suggests that PTP1B inhibition could be effective in breast tumor prevention.
Keywords: PTPN1, PTP1B, Tyrosine Phosphatases, HER2, Breast Cancer
Introduction
Breast cancer is one of the most common malignancies in women, with ~400,000 deaths annually worldwide (1). The receptor tyrosine kinase c-ErbB2 (HER2/Neu), a member of the epidermal growth factor receptor family, is overexpressed in ~20% of breast cancers (2). Transgenic expression of activated forms of HER2/Neu (NeuNT) in the mammary gland causes mammary adenocarcinoma (3, 4). Furthermore, the success of the anti-HER2 monoclonal antibody trastuzumab (Herceptin) in clinics highlights the importance of HER2/Neu in human breast cancer. In addition to tyrosine kinases such as HER2/Neu, tyrosyl phosphorylation is also regulated by protein-tyrosine phosphatases (PTPs) (5, 6). Because they antagonize the action of tyrosine kinases, PTPs were initially thought to play a negative (signal-attenuating) role in signaling and consequently a tumor-suppressing role in cancer. Recent studies, however, have shown that certain PTPs can also enhance signaling and, thus promote oncogenesis (7).
Protein-tyrosine phosphatase 1B (PTP1B) has signal-attenuating properties downstream of insulin and leptin signaling (8). Knockout mice for PTP1B are insulin- and leptin hypersensitive due to inhibitory effects of PTP1B downstream of the insulin and leptin pathways (9, 10). While PTP1B decreases insulin signaling by dephosphorylating the insulin receptor and IRS proteins, it decreases leptin action by dephosphorylating Jak2. Moreover, PTP1B also attenuates growth hormone-mediated Jak2-Stat signaling, providing another possible mechanism for PTP1B roles in obesity (11). These observations have identified PTP1B as an important target in diabetes and obesity and stimulated the development of PTP1B inhibitors (6).
PTP1B is also involved in oncogenesis, as first suggested by the amplification and overexpression of PTPN1, the gene encoding PTP1B, in breast cancer (12, 13). In contrast to its activity in insulin and leptin receptor signaling, PTP1B was shown to positively regulate IGF-1- and PDGF-induced RAS/ERK signaling in immortalized fibroblasts (14, 15). However, the role of PTP1B downstream of HER2/Neu and other oncogenes was initially controversial, as it was found to enhance or to attenuate the transforming effects depending on the test system (16, 17). Thus, the precise role of PTP1B in breast cancer has remained ill-defined. We and others have shown that global deletion of PTP1B in mice delays or protects against HER2/Neu-induced mammary cancer, depending on the particular HER2/Neu allele and mouse strain studied (18, 19). Therefore, PTP1B is clearly an important positive component of HER2/Neu-evoked transformation, raising the possibility that PTP1B inhibition could be useful for treating breast cancer. However, it is not clear whether this is a direct effect of PTP1B deletion on HER2/Neu signaling in the mammary epithelium or an indirect consequence of the salutary metabolic effects of PTP1B deficiency.
Here, we addressed the site of action of PTP1B (i.e., epithelial vs. non-epithelial) and assessed whether PTP1B is involved in tumor maintenance. We have used a combination of mouse genetics and reverse genetics to address these questions and found that inhibition of PTP1B in the mammary epithelium delays mammary tumor onset, whereas inhibition of PTP1B in established mammary tumors does not affect their growth.
Materials and Methods
Reagents
The pLXSN-NeuNT construct was from L. Petti (Albany Medical College). Rabbit polyclonal anti-mouse PTP1B antibodies were described elsewhere (10). Commercial antibodies included anti-Her2 (Calbiochem), Erk2 (Santa Cruz), phospho-Erk1/2, phospho-Src Y416, Src (Cell Signaling) and Ki-67 (Neomarkers). The dox-inducible lentiviral vector was described elsewhere (20).
Three-Dimensional Cultures
MCF10A cells (from J. Brugge, Harvard Medical School) were infected with pLXSN-NeuNT and pools of cells grown and stained as previously described (21). For experiments with inducible miRs, 500 ng/ml of dox was added to the medium 1 day after seeding the cells and refreshed every 2 days.
Transgenic mice
MMTV-NeuNT (strain TG.NK) and SCID-Beige mice were purchased from Jackson Labs. PTP1Bfl/fl mice (22) were backcrossed with FVB/J (Harlan) mice for six generations. MMTV-Cre and Actin-CreERT mice were described previously (23, 24). All mice were kept as virgins throughout the entire study.
Animal Experiments
PTP1Bfl/fl or PTP1Bwt/wt mice containing one copy of MMTV-NeuNT and MMTV-Cre were monitored twice weekly for tumor onset.
PTP1Bfl/fl - MMTV-NeuNT - Actin CreERT mice and wild-type littermates PTP1Bwt/wt - MMTV-NeuNT - Actin CreERT were monitored twice weekly for tumor onset. Once palpable tumors were formed, the mice were injected every day intraperitoneally with 0.5 mg of Tamoxifen (Sigma) for a total of 10 days.
For xenograft studies, 106 MCF10A-NeuNT cells or 500,000 MDA-MB-231 cells (from ATCC) were suspended in a 100-μl mixture of Basement Membrane Matrix Phenol Red-free (BD Biosciences) and PBS 1:1 and injected orthotopically into SCID-Beige mice. Expression of CTRL or PTP1B miR was induced by doxycycline (Sigma) in the drinking water (2 g/l in a 5% sucrose solution, refreshed every 2 days). Tumor volume was measured every 5 days using calipers.
Protein Analysis
Snap-frozen mammary glands or mammary tumors were lysed in a tissue homogeniser with RIPA buffer containing 50 mM Tris-HCl pH 7.5, 1% Triton X-100, 150 mM NaCl, 0.5% Na-deoxycholate, 0.1% SDS. The following inhibitors were added to the buffer just before lysis: 10 mM sodium pyrophosphate, 5 mM EGTA, 2 μg/ml each of aprotinin, leupeptin, pepstatin and antipain, 2mM sodium orthovanadate, 10 mM sodium fluoride, 10 mM β-glycero-phosphate and 2 mM PMSF. Tumor lysates were resolved by SDS-PAGE, transferred to Immobilon-FL membranes (Millipore) and immunoblotted with the indicated antibodies.
Immunohistochemistry
Excised mammary glands or tumors were fixed with Formal-Fix (Thermoscientific) for 24 h at 4°C. Fixed samples were processed and embedded in paraffin. Sections of 4 μm were cut and dried overnight at 37°C. Staining was performed automatically using a Discovery XT automated stainer (Ventana Medical Systems (vms). In brief, for Ki-67 (1:100) and PTP1B (1:100) antibodies, the Research IHC DABMAP XT procedure was used with mild CC1 and Protease 1 (vms) pretreatment (for 4 min), respectively. Primary antibodies were incubated for 1 h at 37°C. Biotinylated secondary donkey anti-rabbit antibodies (Jackson Labs, 1:100) were then added for 32 min at 37°C. All sections were counterstained with Hematoxylin II (vms) and bluing reagent (vms) for 4 min before washing, dehydrating and mounting. We quantified the percentage of Ki67-positive cells using the ImagePro Software. We analyzed 6 tumors/group and ~10,000 cells/tumor.
Statistical Analysis
Survival curves were generated using the Kaplan-Meier method and significance evaluated with the log-rank test. Paired data were evaluated by Student’s t-test and tumor growth was analyzed by Wilcoxon rank sum test using JMP software.
Results
Epithelial Specific Deletion of PTP1B Delays Mammary Tumor Onset
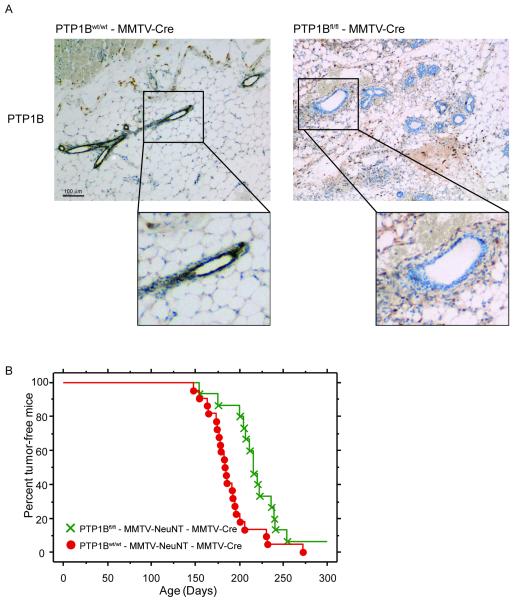
To determine whether epithelial expression of PTP1B is important for mammary tumor onset, we crossed PTP1Bfl/fl mice to MMTV-Cre mice, in which expression of the Cre recombinase is under the control of the Mouse Mammary Tumor Virus Promoter (MMTV), and to MMTV-NeuNT mice, which express the activated HER2/Neu oncogene NeuNT. We generated PTP1Bfl/fl - MMTV-Cre - MMTV-NeuNT mice and PTP1Bwt/wt - MMTV-Cre - MMTV-NeuNT mice. As expected, immunohistochemistry analysis showed specific deletion of PTP1B in the mammary glands of PTP1Bfl/fl - MMTV-Cre, but not PTP1Bwt/wt - MMTV-Cre, mice (Fig. 1A). Tumor latency in PTP1Bwt/wt - MMTV-Cre- MMTV-NeuNT mice was ~189 days. Interestingly, tumor onset in PTP1Bfl/fl - MMTV-Cre - MMTV-NeuNT mice was significantly delayed to ~217 days (Fig. 1B). We found that 4 out of 19 tumors from PTP1Bfl/fl - MMTV-Cre - MMTV-NeuNT mice retained significant levels of PTP1B protein as detected by immunohistochemistry (Supplementary Fig. 1). This could reflect the known mosaic expression of Cre in this line (25) and/or a selective growth advantage of tumor cells that retain PTP1B. In any event, these mice were excluded from the analysis. Our data show that the absence of PTP1B in mammary epithelium delays tumor onset, arguing for a cell-autonomous role of PTP1B in the initiation of mammary tumors of this subtype.
Figure 1. MMTV-Cre-mediated deletion of PTP1B delays NeuNT-induced mammary tumor onset.
A. PTP1B is deleted specifically in the mammary epithelial cells of PTP1Bfl/fl - MMTV-Cre mice. Immunohistochemical staining of PTP1B in the mammary glands of PTP1Bfl/fl - MMTV-Cre and PTP1Bwt/wt - MMTV-Cre mice. PTP1B staining is shown in brown. Inserts show a higher magnification of the boxed areas.
B. Epithelial-specific deletion of PTP1B delays NeuNT-induced mammary tumor onset. Kaplan-Meier curves showing mammary tumor onset in PTP1Bwt/wt - MMTV-Cre - MMTV-NeuNT mice (n=22) and PTP1Bfl/fl - MMTV-Cre - MMTV-NeuNT mice (n=15). PTP1Bwt/wt - MMTV-Cre - MMTV-NeuNT mice developed palpable tumors within a mean probability of 189 days, whereas PTP1Bfl/fl - MMTV-Cre - MMTV-NeuNT mice developed tumors by 217 days. P=0.006, log-rank test.
Epithelial Specific PTP1B Deletion Does not Affect the Growth of Mammary Tumors
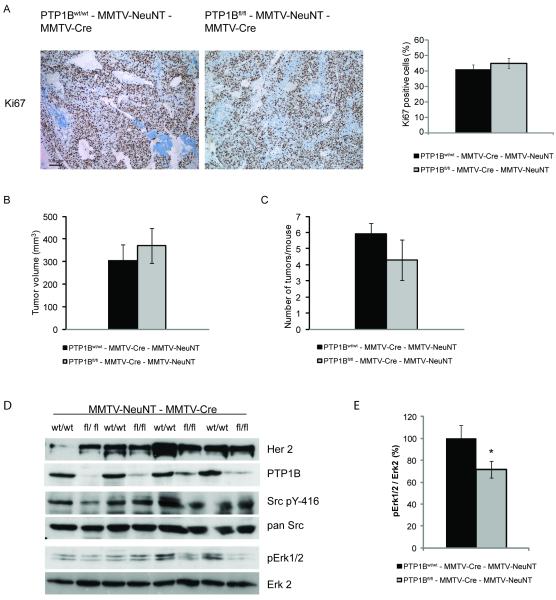
To gain insight into the involvement of PTP1B in tumor growth, we stained mammary tumors from PTP1Bfl/fl - MMTV-Cre - MMTV-NeuNT mice and PTP1Bwt/wt - MMTV-Cre - MMTV-NeuNT mice for the proliferation marker Ki67. No differences between tumors with and without PTP1B were found (Fig. 2A). Consistently, the absence of PTP1B did not affect tumor volume or the number of tumors per mouse (Fig. 2B, C).
Figure 2. Epithelial deletion of PTP1B does not affect proliferation and signaling of NeuNT-induced mammary tumors.
A. Immunohistochemical analysis of mammary tumors from PTP1Bwt/wt - MMTV-Cre - MMTV-NeuNT and PTP1Bfl/fl - MMTV-Cre - MMTV-NeuNT mice 5 weeks after their onset. Representative images of Ki67-stained sections of mammary tumors as indicated. Bar graph showing the quantification of Ki67 staining in mammary tumors (n=6). P=0.41 student’s t-test.
B, C. Tumor volume and the number of tumors per mouse of PTP1Bwt/wt - MMTV-Cre - MMTV-NeuNT (n=12) and PTP1Bfl/fl - MMTV-Cre - MMTV-NeuNT mice (n=6) 30-35 days after tumor onset. P=0.56 for tumor volume and P=0.24 for the number of tumors per mouse, student’s t-test.
D. Immunoblots of mammary tumor lysates from PTP1Bwt/wt - MMTV-Cre - MMTV-NeuNT and PTP1Bfl/fl - MMTV-Cre - MMTV-NeuNT mice 5 weeks after tumor onset.
E. Densitometric quantification of pErk1/2 normalized to Erk2 levels (n=4). P=0.03, student’s t-test.
We then assessed changes in the phosphorylation of key signaling molecules downstream of HER2/Neu. Immunoblotting of lysates from mammary tumors showed differences in the phosphorylation status of Erk between some PTP1Bfl/fl - MMTV-Cre - MMTV-NeuNT mice and PTP1Bwt/wt - MMTV-Cre - MMTV-NeuNT littermates, but these changes were not consistent across all animals. Furthermore, there were no consistent differences in the phosphorylation of Akt, pY-416Src or p70S6k (Fig. 2D, E and data not shown). These data indicate that tumors developing in the absence of PTP1B activated other pathways circumventing the effect of PTP1B on tumor growth.
PTP1B is not Essential for Tumor Maintenance
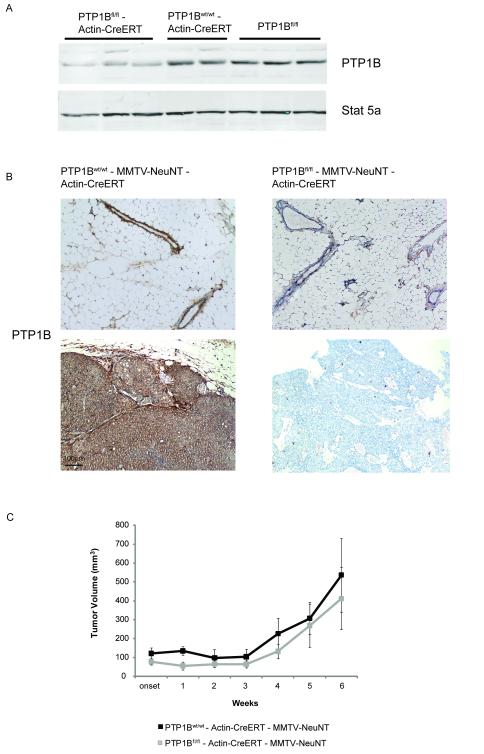
Previous studies and our new data show that PTP1B is important for tumor onset but do not reveal whether PTP1B is involved in tumor maintenance. To address this question, we crossed PTP1Bfl/fl mice to MMTV-NeuNT mice and to mice expressing a tamoxifen-regulated Cre recombinase (Actin-CreERT) (23). We generated two cohorts of mice: PTP1Bfl/fl - Actin-CreERT - MMTV-NeuNT and PTP1Bwt/wt - Actin-CreERT - MMTV-NeuNT mice. Upon tamoxifen injection, PTP1B was deleted in glands from PTP1Bfl/fl - Actin-CreERT mice but not from PTP1Bwt/wt - Actin-CreERT or PTP1Bfl/fl mice (Fig. 3A). To determine whether PTP1B affects tumor maintenance, we administered tamoxifen to mice once tumors became palpable and found that tamoxifen treatment led to PTP1B deletion (Fig. 3B). Surprisingly, deletion of PTP1B had no effect on tumor growth, proliferation or apoptosis (Fig. 3C and Supplementary Fig. 2A, B) and is, thus, not essential for the maintenance of HER2/Neu-induced mammary tumors.
Figure 3. Deletion of PTP1B after overt tumor development does not affect tumor growth.
A. Deletion of PTP1B in the mammary gland following tamoxifen injection into PTP1Bfl/fl - Actin-CreERT mice. Immunoblots of lysates of mammary glands from PTP1Bwt/wt - Actin-CreERT mice and PTP1Bfl/fl mice are also shown.
B. Representative images of PTP1B-stained sections of mammary tumors (upper panel) and adjacent non-tumor (lower panel) glands from PTP1Bfl/fl - Actin-CreERT - MMTV-NeuNT and PTP1Bwt/wt - Actin-CreERT - MMTV-NeuNT mice 4 weeks after tamoxifen injection.
C. Volume of mammary tumors from PTP1Bwt/wt - Actin-CreERT - MMTV-NeuNT mice (n=7 for weeks 1-4 and n=4 for weeks 5 and 6) and PTP1Bfl/fl - Actin-CreERT - MMTV-NeuNT mice (n=8 for weeks 1-4 and n=3 for weeks 5 and 6) after tamoxifen injection (P=0.91 at week 4, P=0.85 at week 6, Wilcoxon rank sum test).
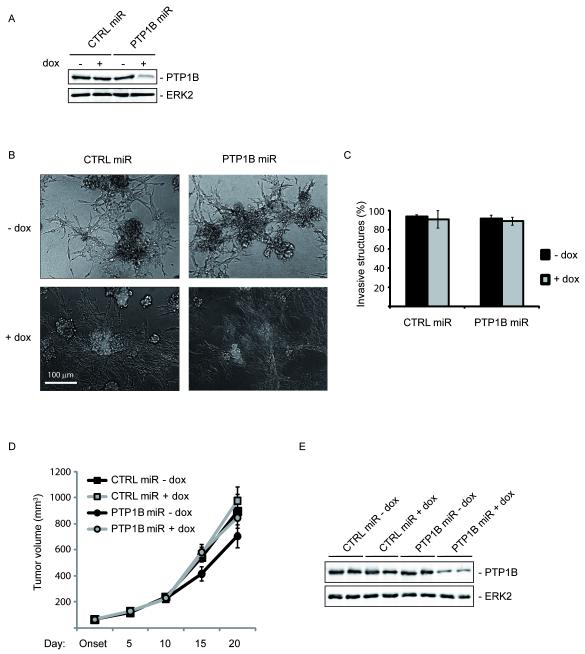
To further assess a potential role of PTP1B in tumor maintenance, we generated a doxycycline (dox)-inducible lentiviral vector (20) expressing a PTP1B shRNAmiR (PTP1B miR) to knockdown PTP1B in the transformed breast epithelial cell line MCF10A-NeuNT. As control, we used cells expressing a lentiviral vector targeting firefly luciferase (CTRL miR). Dox treatment suppressed PTP1B expression (79.8%) in cells infected with PTP1B miR but not in cells infected with CTRL miR (Fig. 4A). In the absence of dox, MCF10A-NeuNT cells expressing CTRL miR or PTP1B miR formed invasive structures when grown in 3D culture (Fig. 4B). Knockdown of PTP1B by dox treatment 1 day after seeding cells in 3D cultures did not affect the invasiveness of MCF10A-NeuNT cells expressing PTP1B miR (Fig. 4B, C). We then injected MCF10A-NeuNT CTRL miR or PTP1B miR cells into the fat pad of immunodeficient mice and, once tumors became palpable, administered dox to achieve PTP1B knockdown. Consistent with our results using the inducible Cre in PTP1Bfl/fl - Actin-CreERT - MMTV-NeuNT mice (Fig. 3C), we found no difference in the maintenance of MCF10A-NeuNT tumors at this level of PTP1B knockdown (Fig. 4D). Immunoblotting of protein lysates obtained from these tumors at the end of the experiment confirmed knockdown of PTP1B upon dox administration (Fig. 4D). Similar results were obtained upon dox-inducible knockdown of PTP1B in xenografts of the MDA-MB-231 breast cancer cell line and in shRNA-mediated constitutive knockdown of PTP1B in MCF10A-NeuNT cells (Supplementary Fig. 3A, B and data not shown). Taken together, these data show that, once tumors are formed, knockdown of PTP1B does not affect tumor growth, which suggests that PTP1B is not essential for breast tumor maintenance.
Figure 4. PTP1B is not essential for tumor maintenance.
A. Immunoblotting of lysates from pools of MCF10A-NeuNT cells expressing a firefly control (CTRL) or PTP1B miR after 5 days of culture in the presence or absence of dox. Dox treatment of MCF10A-NeuNT cells deleted PTP1B (79.8%) in cells expressing PTP1B miR, but not in control cells.
B. PTP1B knockdown does not affect invasiveness of MCF10A-NeuNT cells. MCF10A-NeuNT cells expressing control or PTP1B miR were grown in 3D cultures in the presence or absence of dox. Phase contrast images showing the invasive structures.
C. Bar graph showing the mean percentage of MCF10A-NeuNT invasive structures ±SEM (n=3; P=0.85 student’s t-test).
D. Growth curves of MCF10A-NeuNT tumors in the presence or absence of PTP1B, showing the mean tumor volume (mm3) ±SEM (n=8; P=0.42 student’s t-test).
E. Immunoblot of lysates from MCF10A-NeuNT tumors in the presence or absence of PTP1B.
Discussion
Previous in vivo studies showing that inhibition of PTP1B delays or prevents NeuNT-induced mammary tumorigenesis were performed in PTP1B whole-body knockout mice. Thus, the site of action of PTP1B (epithelial vs. non-epithelial) remained unclear. We have now deleted PTP1B specifically in the mammary epithelium and discovered that epithelial PTP1B is important for NeuNT-evoked mammary cancer.
There was a delay of ~28 days in the onset of mammary tumors when PTP1B was deleted in the mammary epithelium of nulliparous MMTV-NeuNT mice in the FVB/J background. The magnitude of this delay differs from earlier studies. Using nulliparous mice in a mixed genetic background (FVB/J, 129Sv, C57B6/J), we showed previously that PTP1B deletion delayed tumor onset by ~86 days in about one-third of cases and completely protected the remaining mice against NeuNT-evoked mammary tumors (18). This difference in tumor onset may be attributable to the different genetic backgrounds of the mice, but a further, more interesting possibility is that PTP1B also plays a non-cell autonomous role in mammary tumorigenesis. This possibility warrants further studies because a) PTP1B is involved in immune cell signaling (26), b) the level of circulating insulin is lower in PTP1B knockout mice than in wild-type littermates and increased insulin levels have been associated with a high risk of developing breast cancer (27, 28), and c) PTP1B regulates leptin and growth hormone signaling both of which were linked to breast cancer (29, 30). It has been reported that whole-body knockout of PTP1B delayed HER2/Neu-induced mammary tumor onset by ~57 days in multiparous mice in an FVB/J background. In this case, the mice expressed an in-frame deletion in the extracellular domain of HER2/Neu (NDL2, Neu deletion in extracellular domain 2 mice) and tumor onset was assessed in multiparous mice. These factors may explain the observed difference in tumor onset (19).
In the present study, we found no differences in the growth rate of tumors, or in the number of tumors per animal expressing or lacking PTP1B. There also were no consistent changes in the phosphorylation status of Erk, Akt, c-Src or p70S6k. These data suggest that PTP1B may not be relevant for the progression of the disease once tumors are formed, most likely because the tumors activate other oncogenic pathways not requiring PTP1B. Studies in cancer cell lines grown as monolayers or 3D cultures have suggested that PTP1B knockdown suppresses activation of c-SRC (31, 32). In the present study, epithelial deletion of PTP1B did not affect c-Src activation downstream of HER2/Neu, which is consistent with previous in vivo studies using whole-body knockouts of PTP1B (18, 19).
Clearly, PTP1B plays an important role in tumor onset downstream of HER2/Neu but the question of whether PTP1B is involved in the maintenance of established mammary tumors had until now not been answered. Studies using an inhibitor targeting PTP1B did not report its effect on established mammary tumors (19). Here we report studies using mouse genetics and xenograft models which have shown that neither epithelial nor stromal PTP1B is required for tumor maintenance. A similar discrepancy between the effects on tumor onset and maintenance was observed upon deletion of Jak2 in mammary tumors (33).
Earlier studies showing that PTP1B is required for tumor onset raised the exciting possibility that PTP1B inhibitors, as currently developed for the treatment of diabetes and obesity, might also be useful for breast cancer therapy. We now show that PTP1B is not essential for breast tumor maintenance in HER2/Neu-evoked mammary tumors but that inhibitors of PTP1B may be relevant as chemopreventive agents in breast cancer.
Supplementary Material
Suppl. Figure 1. Immunohistochemical staining of PTP1B in tumors from PTP1Bfl/fl – MMTV-Cre – MMTV-NeuNT mice.
Immunohistochemical analysis of mammary tumors from PTP1Bfl/fl – MMTV-Cre – MMTV-NeuNT mice revealed either the absence (middle) or presence (right) of PTP1B. PTP1B-positive tumors within the PTP1Bfl/fl group were excluded from the Kaplan-Meier analysis (see Fig. 1B). A representative picture of a tumor from PTP1Bwt/wt – MMTV-Cre – MMTV-NeuNT mice is shown on the left as a positive control (left). PTP1B staining is shown in brown.
Suppl. Figure 2. Deletion of PTP1B after NeuNT-induced mammary tumor formation does not affect proliferation and apoptosis.
A. Immunohistochemical staining of mammary tumors from PTP1Bwt/wt - Actin-CreERT - MMTV-NeuNT and PTP1Bfl/fl - Actin-CreERT - MMTV-NeuNT mice 4 weeks after tamoxifen injection. Representative images of Ki67 and cleaved caspase 3 stainings are shown.
B. Bar graph showing the percentage of Ki67- or cleaved caspase 3-positive cells in mammary tumors (n=3). P=0.53 for Ki67 and P=0.8 for cleaved caspase 3, student’s t-test.
Suppl. Figure 3. Knockdown of PTP1B using shPTP1BmiR does not affect the growth of MDAMB 231 xenografts.
A. Growth curves of MDA-MB-231 tumors in the presence or absence of PTP1B, showing the mean tumor volume (mm3) ±SEM (n=10; P=0.97 student’s t-test).
B. Immunoblot of lysates from MDA-MB-231 tumors in the presence or absence of PTP1B.
ACKNOWLEDGEMENTS
We thank A. Dolemeyer (Novartis Institute of Biomedical Research) for quantification of Ki67 staining, T. Westbrook (Baylor College of Medicine) and S. Elledge (Harvard Medical School) for the dox-inducible shRNAmiR lentiviral vector, J. Brugge (Harvard Medical School) for the MCF10A cells, L. Petti (Albany Medical College) for the PLXSN-NeuNT vector, C. Lobe (Sunnybrook and Women’s College Health Science Centre, Toronto) for the Actin CreERT mice, W. Muller (Mc Gill University) for the MMTV-Cre mice, members of the Bentires-Alj lab for advice and discussions, and various colleagues for reagents. Research in the lab of M.B-A. is supported by the Novartis Research Foundation, a Marie-Curie re-integration grant, the European Research Council (ERC starting grant 243211-PTPsBDC), the Association of International Cancer Research (AICR), and the Krebsliga Beider Basel. K.K. Balavenkatraman was supported by a grant from the AICR. BGN is supported by NIH R37CA49132 and funds from the Ontario Ministry of Long Term Care and the Princess Margaret Hospital Foundation.
Abbreviations
- PTP
protein-tyrosine phosphatase
- MMTV
mouse mammary tumor virus
References
- 1.http://www.who.int.
- 2.Hynes NE, Lane HA. ERBB receptors and cancer: the complexity of targeted inhibitors. Nat Rev Cancer. 2005;5:341–54. doi: 10.1038/nrc1609. [DOI] [PubMed] [Google Scholar]
- 3.Muller WJ, Sinn E, Pattengale PK, Wallace R, Leder P. Single-step induction of mammary adenocarcinoma in transgenic mice bearing the activated c-neu oncogene. Cell. 1988;54:105–15. doi: 10.1016/0092-8674(88)90184-5. [DOI] [PubMed] [Google Scholar]
- 4.Ursini-Siegel J, Schade B, Cardiff RD, Muller WJ. Insights from transgenic mouse models of ERBB2-induced breast cancer. Nat Rev Cancer. 2007;7:389–97. doi: 10.1038/nrc2127. [DOI] [PubMed] [Google Scholar]
- 5.Tonks NK. Protein tyrosine phosphatases: from genes, to function, to disease. Nat Rev Mol Cell Biol. 2006;7:833–46. doi: 10.1038/nrm2039. [DOI] [PubMed] [Google Scholar]
- 6.Julien SG, Dube N, Hardy S, Tremblay ML. Inside the human cancer tyrosine phosphatome. Nat Rev Cancer. 2011;11:35–49. doi: 10.1038/nrc2980. [DOI] [PubMed] [Google Scholar]
- 7.Ostman A, Hellberg C, Bohmer FD. Protein-tyrosine phosphatases and cancer. Nat Rev Cancer. 2006;6:307–20. doi: 10.1038/nrc1837. [DOI] [PubMed] [Google Scholar]
- 8.Yip SC, Saha S, Chernoff J. PTP1B: a double agent in metabolism and oncogenesis. Trends Biochem Sci. 2010;35:442–9. doi: 10.1016/j.tibs.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elchebly M, Payette P, Michaliszyn E, Cromlish W, Collins S, Loy AL, et al. Increased insulin sensitivity and obesity resistance in mice lacking the protein tyrosine phosphatase-1B gene. Science. 1999;283:1544–8. doi: 10.1126/science.283.5407.1544. [DOI] [PubMed] [Google Scholar]
- 10.Klaman LD, Boss O, Peroni OD, Kim JK, Martino JL, Zabolotny JM, et al. Increased energy expenditure, decreased adiposity, and tissue-specific insulin sensitivity in protein-tyrosine phosphatase 1B-deficient mice. Mol Cell Biol. 2000;20:5479–89. doi: 10.1128/mcb.20.15.5479-5489.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gu F, Dube N, Kim JW, Cheng A, Ibarra-Sanchez Mde J, Tremblay ML, et al. Protein tyrosine phosphatase 1B attenuates growth hormone-mediated JAK2-STAT signaling. Mol Cell Biol. 2003;23:3753–62. doi: 10.1128/MCB.23.11.3753-3762.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tanner MM, Tirkkonen M, Kallioniemi A, Isola J, Kuukasjarvi T, Collins C, et al. Independent amplification and frequent co-amplification of three nonsyntenic regions on the long arm of chromosome 20 in human breast cancer. Cancer Res. 1996;56:3441–5. [PubMed] [Google Scholar]
- 13.Wiener JR, Kerns BJ, Harvey EL, Conaway MR, Iglehart JD, Berchuck A, et al. Overexpression of the protein tyrosine phosphatase PTP1B in human breast cancer: association with p185c-erbB-2 protein expression. J Natl Cancer Inst. 1994;86:372–8. doi: 10.1093/jnci/86.5.372. [DOI] [PubMed] [Google Scholar]
- 14.Dube N, Cheng A, Tremblay ML. The role of protein tyrosine phosphatase 1B in Ras signaling. Proc Natl Acad Sci U S A. 2004;101:1834–9. doi: 10.1073/pnas.0304242101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buckley DA, Cheng A, Kiely PA, Tremblay ML, O’Connor R. Regulation of insulin-like growth factor type I (IGF-I) receptor kinase activity by protein tyrosine phosphatase 1B (PTP-1B) and enhanced IGF-I-mediated suppression of apoptosis and motility in PTP-1B-deficient fibroblasts. Mol Cell Biol. 2002;22:1998–2010. doi: 10.1128/MCB.22.7.1998-2010.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brown-Shimer S, Johnson KA, Hill DE, Bruskin AM. Effect of protein tyrosine phosphatase 1B expression on transformation by the human neu oncogene. Cancer Res. 1992;52:478–82. [PubMed] [Google Scholar]
- 17.Liu F, Sells MA, Chernoff J. Transformation suppression by protein tyrosine phosphatase 1B requires a functional SH3 ligand. Mol Cell Biol. 1998;18:250–9. doi: 10.1128/mcb.18.1.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bentires-Alj M, Neel BG. Protein-tyrosine phosphatase 1B is required for HER2/Neu-induced breast cancer. Cancer Res. 2007;67:2420–4. doi: 10.1158/0008-5472.CAN-06-4610. [DOI] [PubMed] [Google Scholar]
- 19.Julien SG, Dube N, Read M, Penney J, Paquet M, Han Y, et al. Protein tyrosine phosphatase 1B deficiency or inhibition delays ErbB2-induced mammary tumorigenesis and protects from lung metastasis. Nat Genet. 2007;39:338–46. doi: 10.1038/ng1963. [DOI] [PubMed] [Google Scholar]
- 20.Meerbrey KL, Hu G, Kessler JD, Roarty K, Li MZ, Fang JE, et al. The pINDUCER lentiviral toolkit for inducible RNA interference in vitro and in vivo. Proc Natl Acad Sci U S A. 2011;108:3665–70. doi: 10.1073/pnas.1019736108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bentires-Alj M, Gil SG, Chan R, Wang ZC, Wang Y, Imanaka N, et al. A role for the scaffolding adapter GAB2 in breast cancer. Nat Med. 2006;12:114–21. doi: 10.1038/nm1341. [DOI] [PubMed] [Google Scholar]
- 22.Bence KK, Delibegovic M, Xue B, Gorgun CZ, Hotamisligil GS, Neel BG, et al. Neuronal PTP1B regulates body weight, adiposity and leptin action. Nat Med. 2006;12:917–24. doi: 10.1038/nm1435. [DOI] [PubMed] [Google Scholar]
- 23.Guo C, Yang W, Lobe CG. A Cre recombinase transgene with mosaic, widespread tamoxifen-inducible action. Genesis. 2002;32:8–18. doi: 10.1002/gene.10021. [DOI] [PubMed] [Google Scholar]
- 24.Andrechek ER, Hardy WR, Siegel PM, Rudnicki MA, Cardiff RD, Muller WJ. Amplification of the neu/erbB-2 oncogene in a mouse model of mammary tumorigenesis. Proc Natl Acad Sci U S A. 2000;97:3444–9. doi: 10.1073/pnas.050408497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.White DE, Kurpios NA, Zuo D, Hassell JA, Blaess S, Mueller U, et al. Targeted disruption of beta1-integrin in a transgenic mouse model of human breast cancer reveals an essential role in mammary tumor induction. Cancer Cell. 2004;6:159–70. doi: 10.1016/j.ccr.2004.06.025. [DOI] [PubMed] [Google Scholar]
- 26.Simoncic PD, McGlade CJ, Tremblay ML. PTP1B and TC-PTP: novel roles in immune-cell signaling. Can J Physiol Pharmacol. 2006;84:667–75. doi: 10.1139/y06-012. [DOI] [PubMed] [Google Scholar]
- 27.Del Giudice ME, Fantus IG, Ezzat S, McKeown-Eyssen G, Page D, Goodwin PJ. Insulin and related factors in premenopausal breast cancer risk. Breast Cancer Res Treat. 1998;47:111–20. doi: 10.1023/a:1005831013718. [DOI] [PubMed] [Google Scholar]
- 28.Gunter MJ, Hoover DR, Yu H, Wassertheil-Smoller S, Rohan TE, Manson JE, et al. Insulin, insulin-like growth factor-I, and risk of breast cancer in postmenopausal women. J Natl Cancer Inst. 2009;101:48–60. doi: 10.1093/jnci/djn415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cirillo D, Rachiglio AM, la Montagna R, Giordano A, Normanno N. Leptin signaling in breast cancer: an overview. J Cell Biochem. 2008;105:956–64. doi: 10.1002/jcb.21911. [DOI] [PubMed] [Google Scholar]
- 30.Kleinberg DL, Wood TL, Furth PA, Lee AV. Growth hormone and insulin-like growth factor-I in the transition from normal mammary development to preneoplastic mammary lesions. Endocr Rev. 2009;30:51–74. doi: 10.1210/er.2008-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arias-Romero LE, Saha S, Villamar-Cruz O, Yip SC, Ethier SP, Zhang ZY, et al. Activation of Src by protein tyrosine phosphatase 1B Is required for ErbB2 transformation of human breast epithelial cells. Cancer Res. 2009;69:4582–8. doi: 10.1158/0008-5472.CAN-08-4001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bjorge JD, Pang A, Fujita DJ. Identification of protein-tyrosine phosphatase 1B as the major tyrosine phosphatase activity capable of dephosphorylating and activating c-Src in several human breast cancer cell lines. J Biol Chem. 2000;275:41439–46. doi: 10.1074/jbc.M004852200. [DOI] [PubMed] [Google Scholar]
- 33.Sakamoto K, Lin WC, Triplett AA, Wagner KU. Targeting janus kinase 2 in Her2/neu-expressing mammary cancer: Implications for cancer prevention and therapy. Cancer Res. 2009;69:6642–50. doi: 10.1158/0008-5472.CAN-09-0746. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Suppl. Figure 1. Immunohistochemical staining of PTP1B in tumors from PTP1Bfl/fl – MMTV-Cre – MMTV-NeuNT mice.
Immunohistochemical analysis of mammary tumors from PTP1Bfl/fl – MMTV-Cre – MMTV-NeuNT mice revealed either the absence (middle) or presence (right) of PTP1B. PTP1B-positive tumors within the PTP1Bfl/fl group were excluded from the Kaplan-Meier analysis (see Fig. 1B). A representative picture of a tumor from PTP1Bwt/wt – MMTV-Cre – MMTV-NeuNT mice is shown on the left as a positive control (left). PTP1B staining is shown in brown.
Suppl. Figure 2. Deletion of PTP1B after NeuNT-induced mammary tumor formation does not affect proliferation and apoptosis.
A. Immunohistochemical staining of mammary tumors from PTP1Bwt/wt - Actin-CreERT - MMTV-NeuNT and PTP1Bfl/fl - Actin-CreERT - MMTV-NeuNT mice 4 weeks after tamoxifen injection. Representative images of Ki67 and cleaved caspase 3 stainings are shown.
B. Bar graph showing the percentage of Ki67- or cleaved caspase 3-positive cells in mammary tumors (n=3). P=0.53 for Ki67 and P=0.8 for cleaved caspase 3, student’s t-test.
Suppl. Figure 3. Knockdown of PTP1B using shPTP1BmiR does not affect the growth of MDAMB 231 xenografts.
A. Growth curves of MDA-MB-231 tumors in the presence or absence of PTP1B, showing the mean tumor volume (mm3) ±SEM (n=10; P=0.97 student’s t-test).
B. Immunoblot of lysates from MDA-MB-231 tumors in the presence or absence of PTP1B.