Abstract
Background
Volume expansion is a mainstay of therapy in septic shock, although its effect is difficult to predict using conventional measurements. Dynamic parameters, which vary with respiratory changes, appear to predict hemodynamic response to fluid challenge in mechanically ventilated, paralyzed patients. Whether they predict response in patients who are free from mechanical ventilation is unknown. We hypothesized that dynamic parameters would be predictive in patients not receiving mechanical ventilation.
Methods
This is a prospective, observational, pilot study. Patients with early septic shock and who were not receiving mechanical ventilation received 10 ml/kg volume expansion (VE) at their treating physician's discretion after initial resuscitation in the emergency department. We used transthoracic echocardiography to measure vena cava collapsibility index (VCCI) and aortic velocity variation (AoVV) prior to VE. We used a pulse contour analysis device to measure stroke volume variation (SVV). Cardiac index was measured immediately before and after VE using transthoracic echocardiography. Hemodynamic response was defined as an increase in cardiac index ≥ 15%.
Results
14 patients received VE, 5 of which demonstrated a hemodynamic response. VCCI and SVV were predictive (Area under curve = 0.83, 0.92, respectively). Optimal thresholds were calculated: VCCI ≥ 15% (Positive predictive value, PPV 62%, negative predictive value, NPV 100%, p = 0.03); SVV ≥ 17% (PPV 100%, NPV 82%, p = 0.03). AoVV was not predictive.
Conclusions
VCCI and SVV predict hemodynamic response to fluid challenge patients with septic shock who are not mechanically ventilated. Optimal thresholds differ from those described in mechanically ventilated patients.
Keywords: Sepsis, Fluid, Hemodynamics, Echocardiography
Introduction
Volume expansion (VE) with intravenous fluid is a mainstay of therapy for septic shock, and is often one of the first therapies performed. The rationale behind volume expansion is to increase cardiac preload by increasing mean circulatory filling pressure. In early sepsis, aggressive VE appears to be beneficial(1, 2), but evidence suggests that excessive VE is deleterious(3-5). Current consensus statements advocate treatment of septic shock by VE until a goal central venous pressure (CVP) is achieved(6). Despite the widespread clinical use of CVP, multiple studies demonstrate that CVP poorly predicts hemodynamic improvement after VE(7-9). A limitation of CVP is that it is a static measurement, and therefore offers little information about a patient's response to VE. A patient's response to VE is determined by the intersection of his or her Frank-Starling cardiac output curve and venous return curve(10). This curve may vary from patient to patient, as well as within a patient at different points in time.
Measurements that assess the heart-lung interactions that occur with respiratory variations in intrathoracic pressure may offer a better estimate of hemodynamic response to VE. These measures include respiratory variations in pulse pressure(11, 12), left ventricular stroke volume(13-15), aortic blood velocity(16, 17), and vena cava diameter(18-20), and vena cava pressure(21). Many studies of dynamic parameters have largely been confined to intubated patients receiving mechanical ventilation and chemical paralysis(16, 17, 19, 20, 22). These studies caution that dynamic parameters are not useful in spontaneously breathing patients, but make this assessment on patient population largely confined to assisted mode of ventilation(9, 23, 24). Typically, these studies have utilized transesophageal echocardiography(14, 16, 17, 20, 22, 23). In contemporary critical care environments, relatively few patients in septic shock receive mechanical ventilation under chemical paralysis(6). Transthoracic echocardiography is less invasive than transesophageal echocardiography, and is better tolerated by the non-intubated patient. Our goal, therefore, was to use a noninvasive measure that could be obtained easily at bedside, and applied to the non-mechanically ventilated patient in septic shock. We selected three measurements that had been validated as useful predictors of hemodynamic response to VE in the mechanically ventilated, paralyzed patient: vena cava collapsibility index (VCCI), aortic blood velocity variation (AoVV), and stroke volume variation (SVV). We hypothesized that these measurements would be predictive of hemodynamic response to VE in unassisted, spontaneously breathing patients in septic shock.
Materials and Methods
This prospective, observational study was conducted between January 2010 and April 2011 in the 24-bed Shock/Trauma Intensive Care Unit and the 12-bed Respiratory Intensive Care Unit at the Intermountain Medical Center, an academic, tertiary-care 452-bed hospital, with 80 ICU beds, in Murray, Utah. The protocol was approved by the Intermountain Healthcare institutional review board, and all patients or their legally authorized representatives provided written, informed consent.
Patients
We screened patients admitted to the intensive care unit (ICU) with septic shock defined by standard criteria(25). The patients were enrolled within 6 hours from time of admission to the ICU. We included patients who were at least 14 years of age with suspected infection and two or more systemic inflammatory response syndrome (SIRS) criteria (white blood cell count less than 4,000 per mm3 or greater than 12,000 per mm3 or differential with greater than 10% immature forms, heart rate greater than 90 beats per minute, respiratory rate greater than 20 breaths per minute or PaCO2 less than 32 mmHg, temperature less than 36°C or greater than 38°C), evidence of refractory hypotension (a systolic blood pressure less than 90 mmHg despite VE of at least 20ml/kg), and in whom the treating physician intended to perform VE. Inclusion criteria also included presence of a central venous catheter and arterial catheter. When the treating physician decided that VE was clinically indicated, 10 mL/kg crystalloid was infused intravenously over a period of less than 20 minutes. We excluded patients receiving positive pressure ventilation (endotracheal or non-invasive positive pressure ventilation). We excluded patients with known pregnancy, severe aortic stenosis, irregular ventricular rhythm (atrial fibrillation or frequent premature ventricular contractions), or in whom the treating physician deemed aggressive care unsuitable. We excluded anyone with active airways obstruction (concomitant diagnosis of acute exacerbation of asthma or chronic obstructive pulmonary disease). The analysis was restricted to the first VE administered after enrollment. A VE was excluded if there was a change in vasopressor dosage or administration of bolus catecholamine simultaneous with VE. All patients were managed in the emergency department and after admission to the ICU according to Surviving Sepsis Campaign Guidelines 2008 as included in the “sepsis bundle” used at Intermountain Medical Center(6).
Measurements
All measurements were obtained with the patient lying supine, with the head of the bed at 0° elevation for at least 30 seconds prior to recording data. All echocardiographic measurements, including cardiac output, VCCI, and AoVV, were made off-line, with agreement between two of the authors (ML and EH). Both authors are testamurs of the National Board of Echocardiography Examination of Special Competence in Adult Echocardiography (ASCeXAM). Measurements of dynamic parameters were performed blinded to the measurement of hemodynamic response.
Cardiac Index
We used transthoracic echocardiography (TTE) to assess cardiac index, according to standard technique(26-28). Images were obtained using a Philips CX50 ultrasound system (Philips Medical Systems, Bothell, WA). We performed serial limited TTE examinations immediately prior to and immediately after VE. The cardiac index was calculated using velocity-time integration (VTI) and left-ventricular outflow tract (LVOT) diameter, and normalized to body surface area, averaged over 3 consecutive ventricular contractions obtained at end-expiration. The treating physicians were blinded to the results of the limited TTE until after the completion of the study. All patients had an electrical impedence respirometer to assess breathing activity.
Vena cava collapsibility index (VCCI)
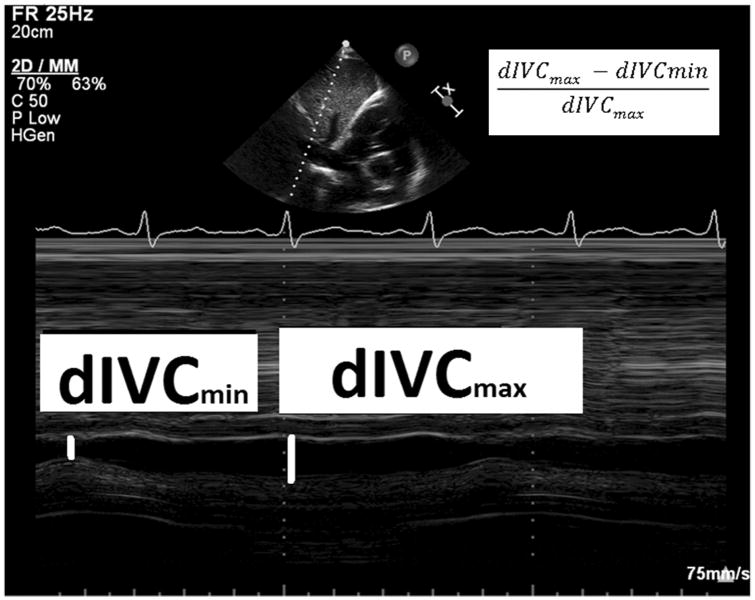
Immediately prior to VE, TTE was used to obtain a two dimensional image, visualizing the inferior vena cava as it entered the right atrium (subcostal window, long axis view)(29). The M-mode cursor was used to create a time-motion image of the inferior vena cava diameter just proximal to the junction of the hepatic veins that lie approximately 0.5 to 3.0 cm proximal to the ostium of the right atrium (Fig.1). Inferior vena cava diameters were collected over a 20 second period of spontaneous respirations, and the VCCI was calculated from the respiratory cycle with the greatest amount of caval collapse. VCCI is calculated as the difference in maximum and minimum diameters divided by the maximum diameter. This index has previously been reported in mechanically ventilated patients, using the superior vena cava(19).
Fig. 1. Vena Cava Collapsibility Index.
Aortic velocity variation (AoVV)
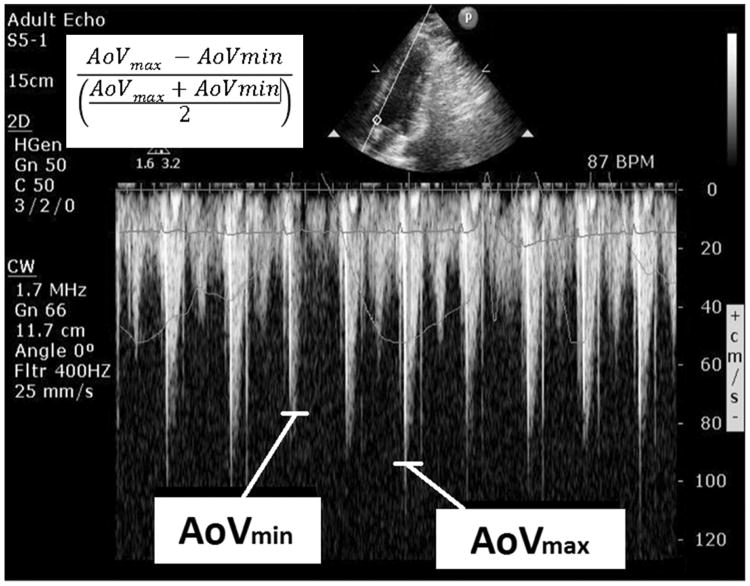
Immediately prior to the fluid challenge, a two dimensional echocardiographic image was obtained, visualizing the left ventricular outflow tract and aortic valve (apical window, 5-chamber view). The continuous wave Doppler cursor was aligned with the direction of blood flow during systole (Fig. 2). Maximum and minimum aortic velocities were collected over a 20 second period of spontaneous respirations, and the AoVV was calculated from the respiratory cycle with the greatest amount of velocity variation. AoVV is calculated as the difference in the maximum and minimum aortic blood velocity divided by the average of the maximum and minimum aortic blood velocity. This index has previously been reported in mechanically ventilated patients and in healthy volunteers(17, 30).
Fig. 2. Aortic blood velocity variation.
SVV
Immediately prior to the fluid challenge, the stroke volume variation was recorded. Stroke volume variation was assessed by pulse contour analysis, using a radial arterial catheter and FloTrac Vigileo system (Edwards LifeSciences, Ivrine, CA). The FloTrac uses a proprietary algorithm to calculate stroke volume variation(31). Criticisms have been raised regarding the accuracy of the FloTrac to measure cardiac output in septic shock(32), but the SVV has demonstrated an ability to predict response to volume expansion in mechanically ventilated, paralyzed patients in septic shock(33).
Statistical analysis
We assessed the diagnostic accuracy of VCCI, AoVV, SVV, and CVP in predicting hemodynamic response to a fluid challenge. Hemodynamic response is defined as an increase in cardiac index ≥ 15% after a fluid challenge. Receiver-operating characteristic curves were calculated for the parameters. Group comparisons used Fisher exact test, Student's t-test or Wilcoxon rank sum, where appropriate. A P value less than 0.05 was considered statistically significant. All analyses were performed using Stata v12.0 (StataCorp, College Station, TX).
Results
Clinical and demographic data
Fourteen patients were studied. Demographic and clinical information regarding patients is displayed in Table 1. Ten patients were initially resuscitated in our emergency department, with a median duration of 1.5 hours prior to ICU admission, and a median amount of 4.6 Liters of crystalloid administered. Two patients were transferred to the ICU from a referring emergency department, and two were transferred from the hospital ward. Over half of the patients (56%) were receiving vasoactive medications at time of enrollment. Median time to acquire all echocardiographic images (including cardiac index) preceding the VE was less than 4 minutes. There were no significant differences in patient demographics, receipt of vasopressors, amount of fluid received prior to study, or in heart rate, blood pressure, or CVP. Patients who were classified as responders to VE had greater values of VCCI (52% vs 11%, p = 0.04) than non-responders, but no difference in SVV (19% vs. 10%, p = 0.08) and AoVV (29% vs 22%, p = 0.41). Neither CVP nor dynamic parameters differed in in patients receiving vasopressor compared to those who did not (Table 2).
Table 1.
Baseline demographics. Continuous data are expressed as median values (interquartile ranges in parentheses), with Mann-Whitney U-test for comparison. Dichotomous data are expressed as proportions with Fisher's exact test for comparison.
| All patients (14) | Nonresponders (9) | Responders (5) | p | |
|---|---|---|---|---|
| Age | 62 (46-81) | 62 (52-81) | 63 (46-74) | 0.61 |
| Percent Female | 64 | 67 | 60 | 1.00 |
| APACHE2 score | 15 (15-19) | 15 (15-17) | 19 (15-19) | 0.44 |
| Percent on vasopressor | 57 | 56 | 60 | 1.00 |
| Fluid administered prior to enrollment (L) | 4.6 (3.0-5.9) | 4.2 (3.0-5.9) | 5.0 (3.1-5.0) | 1.00 |
| Baseline cardiac index (L/min/m2) | 3.32 (2.69-4.15) | 3.13 (2.80-3.64) | 3.51 (2.69-4.15) | 1.00 |
| Heart rate | 102 (80-112) | 97 (77-106) | 109 (107-112) | 0.15 |
| Systolic blood pressure | 91 (86-104) | 93 (87-104) | 90 (81-90) | 0.36 |
| Mean arterial blood pressure | 65 (61-70) | 64 (63-70) | 65 (61-65) | 0.90 |
| Central venous pressure | 9 (7-13) | 9 (8-11) | 7 (6-13) | 0.44 |
| VCCI (percent) | 17 (11-52) | 11 (10-26) | 52 (17-100) | 0.04 |
| AoVV (percent) | 22 (17-33) | 22 (16-33) | 29 (22-49) | 0.41 |
| SVV (percent) | 11 (9-16) | 10 (9-14) | 19 (12-20) | 0.08 |
Table 2.
Differences in dynamic and static parameters, stratified by receipt of vasopressors. Statistical significance calculated using Fisher's exact test.
| On pressor (8) | Not on pressor (6) | P value | |
|---|---|---|---|
| Fluid administered prior to enrollment | 5.0 (2.5 - 6.0) | 5.0 (4.2 - 5.5) | 0.77 |
| CVP | 11 (7.5-15.5) | 8 (6-11) | 0.17 |
| VCCI (percent) | 17 (14-49) | 20 (11-100) | 1.00 |
| AoVV (percent) | 34 (23-61) | 22 (16-37) | 0.20 |
| SVV (percent) | 9.5 (9-11) | 16 (14-19) | 0.12 |
Hemodynamic Response
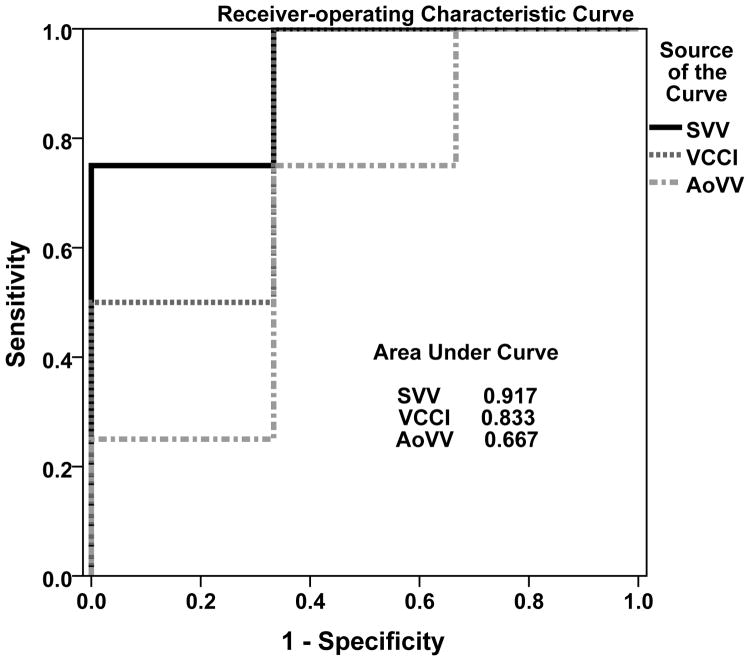
Five of 14 VEs resulted in an increase in cardiac index ≥ 15%. The median increase in cardiac index for all VEs was 11.6%. There were statistically significant differences between responders and non-responders in VCCI. Receiver operating-characteristic curves predicting hemodynamic response to VE demonstrated good diagnostic accuracy for VCCI (AUC = 0.83, 95% confidence interval = 0.58-1.00, Fig. 3), excellent accuracy for SVV (AUC = 0.92, 95% confidence interval= 0.73-1.0), and poor accuracy for AoVV (AUC = 0.67, 95% confidence interval 0.32-1.00). We performed sensitivity analyses to determine optimal thresholds for each parameter. A threshold for VCCI ≥ 15% had poor positive predictive value (PPV, 62%), but excellent negative predictive value (NPV, 100%, p = 0.03, Table 3). A threshold for VCCI ≥ 50% had fair positive (75%) and good negative (80%) predictive value, although did not achieve statistical significance (p = 0.09). A threshold for AoVV ≥ 25% was not predictive (PPV = 50%, NPV = 85%, p = 0.27). A threshold for SVV ≥ 17% had excellent negative predictive value and good positive predictive value (100% and 82%, respectively, p = 0.03).
Fig. 3.
Receiver-operating characteristic curve of dynamic parameters predicting hemodynamic response to volume expansion. AoVV: Aortic Velocity Variation. VCCI: Vena Cava Collapsibility. SVV: Stroke Volume Variation.
Table 3.
Response to volume expansion (n= 14). Statistical significance calculated using Fisher's exact test. Only 13 patients are included for AoVV analysis, as one patient did not have a recorded image.
| N = 14 | VCCI ≥ 15% | VCCI < 15% | VCCI ≥ 50% | VCCI < 50 % | AoVV ≥ 25% | AoVV < 25 % | SVV ≥ 17% | SVV < 17% |
|---|---|---|---|---|---|---|---|---|
| Non-responders | 3 | 6 | 1 | 8 | 3 | 6 | 0 | 9 |
| Responders | 5 | 0 | 3 | 2 | 3 | 1 | 3 | 2 |
| Positive predictive value | 62% | 75% | 50% | 100% | ||||
| Negative predictive value | 100% | 80% | 85% | 82% | ||||
| p | 0.03 | 0.09 | 0.27 | 0.03 | ||||
In a sensitivity analysis, we also examined the value of VCCI and AoVV in predicting hemodynamic response to volume expansion if variation were calculated from the minimum and maximum values over a 20 second period. In this case, AoVV had an AUC of 0.86 (0.65-1.00) and VCCI had an AUC of 0.84 (0.63-1.0).
Discussion
This is the first study to demonstrate that SVV can predict hemodynamic response to volume expansion in patients with septic shock who are not receiving mechanical ventilation, and the second study to demonstrate VCCI can predict volume expansion in the same population(34). Prior studies in this area have largely been confined to the mechanically ventilated patient receiving fully controlled ventilation(14, 16, 17, 20, 22, 23). Prior to this study and the study by Muller and colleagues, the only dynamic parameter that reliably predicted hemodynamic response to volume expansion in the non-intubated septic patient is the passive leg raise maneuver(35). However, the passive leg raise maneuver requires manipulation of patient position, as well as a device to measure changes in cardiac output or stroke volume. Furthermore, the passive leg raise may be inaccurate in the setting of abdominal compartment syndrome(36). The parameters in this study require no cooperation from or movement of the patient, and can be easily obtained in less than 1 minute by a trained clinician, and they do not require measurement of cardiac output after the volume expansion.
Dynamic fluctuations in intrathoracic pressure will occur not only in the passive mechanical breath, but also in the spontaneously breathing patient(37). A measurable delta in intrathoracic pressure, whether positive or negative, may allow the clinician to infer the patient's position on the Frank-Starling curve. In the patient receiving passive mechanical ventilation, the change in intrathoracic pressure is positive deflection, while in the patient free from mechanical ventilation, the change is a negative deflection. In a patient receiving a mechanically assisted breath, the net effect of intrathoracic pressure change may be difficult to assess(38).
We suspect that reported poor prediction of dynamic parameters in the spontaneously breathing, mechanically ventilated patient may be due to two factors. First, spontaneous breathing in assisted mechanical ventilation may have more complex hemodynamic effects than either unassisted breathing or controlled ventilation(38-40). Kimura and colleagues demonstrated that the IVC collapsibility may be affected by the amount of diaphragmatic excursion vs. the amount of chest excursion(41). Second, the tidal volumes and intrathoracic pressures are inconsistent in the spontaneously breathing patient. One of the few positive studies in which dynamic parameters predicted hemodynamic response to VE in non-mechanically breathing subjects did so in healthy volunteers(30). That study required the subjects to synchronize their breathing to a metronome set at 6 breaths/min in order to decrease breath-to-breath variability. Our study made no attempts to pace the breathing of the patient.
This study is the second study to demonstrate VCCI is predictive in spontaneously breathing patient. Muller and colleagues examined VCCI in a group of spontaneously breathing patients with acute circulatory failure, 24 of which were in septic shock. In their non-blinded study, they demonstrated that a VCCI of 40% or greater was likely to predict hemodynamic response to volume expansion, while a VCCI of < 40% was not very useful(34). Taken together with our study, it appears that either very large or very small values of VCCI may have some utility in predicting response to volume expansion, although VCCI may have a wide range where it is clinically indeterminate in the spontaneously breathing patient.
In our study, the echocardiographically-derived AoVV was poorly predictive, while the SVV, derived from arterial pulse contour, had excellent predictive value. In most cases, the peak aortic blood velocity should be proportional with the stroke volume, although it does not correlate with stroke volume as well as the velocity-time integration (VTI). One possible explanation for the discrepancy between AoVV and SVV is that the FloTrac uses an algorithm that calculates SVV from all data points, continuously averaged over a period of time (20s), while our AoVV was calculated from the largest single variation over a 20 second period. Calculation of the AoVV from the largest and smallest values over a 20 second period yielded a greater AUC (0.86, 0.64 - 1.0). Another possible explanation for the difference between AoVV and SVV is that the AoVV in dependent on the ultrasound probe angle, and therefore may be prone to artifact from cardiac movement or from operator technique. If either the heart or the ultrasound probe moved during recording, it would alter the measurement of aortic blood velocity. We attempted to mitigate the possibility of cardiac translation during respiration by always measuring cardiac output at end-expiration. However, respiration-induced cardiac translation cannot be eliminated from the AoVV, as it is measured throughout respiration.
We chose the largest single respiratory variation during a 20 second observation (to capture the largest signal) because it may difficult for a clinician at beside to average several consecutive measurements in real-time. This methodology is similar to previous studies in mechanically ventilated patients, which measured changes over the course of a single breath, or an average over several consecutive respiratory cycles(17, 19, 20, 22). However, in these previous studies, there was minimal breath-to-breath variation, and thus no preference was given to one respiratory cycle over another.
The thresholds of the studied parameters are somewhat larger than similar values reported in mechanically ventilated patients receiving chemical paralysis. Vieillard-Baron and colleagues assessed superior vena cava collapsibility using transesophageal echocardiography in septic shock, finding a threshold of 36% had good sensitivity and specificity(19). Barbier and colleagues assessed inferior vena cava distensibility in mechanically ventilated patient in septic shock, with a threshold of 18%(22). Feissel and colleagues calculated an AoVV of 12% in mechanically ventilated septic patients(17), while Skulec and colleagues calculated a threshold value for AoVV of 14% in spontaneously breathing, healthy volunteers(30). SVV obtained from FloTrac device has been validated in mechanically ventilated patients recovering from cardiac surgery(13, 15), but at the time of this writing, has not yet been validated in patients with septic shock(31). The useful threshold for SVV in mechanically ventilated post-operative patients is 10%(15). We posit two possible explanations for why the optimal thresholds for VCCI and AoVV in this study were larger than those calculated in previous studies: First, we used the largest single respiratory variation observed over a 20 second period rather than an average breath-to-breath calculation. Second, there may be greater efficiency in the work of breathing in patients free from mechanical ventilation compared to patients receiving passive mechanical ventilation. This increased efficiency may result in decreased delta to the intrathoracic pressure for a comparable tidal volume, and therefore may require a larger signal to achieve comparable effect.
This study enrolled patients after their initial emergency department resuscitation. Every enrolled patient had received 2-5 Liters of intravenous fluid prior to enrollment. Therefore, this study is relevant to the intensivist managing early septic shock after the initial emergency department resuscitation. In our study, 64% (8 of 14) of volume expansions, all of which were administered for clinical indications, failed to improve hemodynamics. This number is within range of other similar studies (28-70%)(10, 22, 42). These studies, combined with the known harms of excess VE, serve as a reminder to the intensivist that continued administration of fluid may not be indicated in the early ICU resuscitation of septic patients(3-5).
This is a pilot study, and therefore is limited by its small size. However, the sample size is not much smaller than similar studies in the field. Feissel and colleagues demonstrated AoVV to be predictive in 19 mechanically ventilated patients(17), and Barbier and colleagues demonstrated IVC distention was predictive in 23 mechanically ventilated patients(22). All echocardiographic measurements were obtained by a single physician, which eliminates interobserver variability, but does not allow for assessment of reproducibility. The study only assessed a single 10mL/kg volume expansion, and cannot inform on the value of larger or smaller volume expansions. Clinical parameters, such as increase in urine output or improved survival, were not studied. Strengths of this study include its prospective nature and its measurement of cardiac index by echocardiography, which is more relevant to current clinical practice, and avoids the risks of assessing cardiac index with a pulmonary artery catheter(26). This study also more closely replicates the clinical setting, as the intensivist typically treats patients in septic shock who have already received initial emergency department resuscitation.
Further work in this area, in addition to external validation, should explore composite measurements of these parameters and others to create a robust model to predict a patient's likelihood to respond to fluid administration.
e-Table 1. List of patient's ventricular function, lactate, ScVO2, and dynamic parameters.
| Patient | RV size | RVSF | PASP | LVSF | Lactate | ScVO2 | VCCI | AoVV | SVV | CVP | Responsive |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | normal | normal | normal | normal | 1.9 | 50.6 | 16.7 | 66.2 | 20 | 7 | Yes |
| 2 | normal | normal | normal | hyperdynamic | 3.4 | 56.6 | 8.7 | 16.8 | 10 | 17 | No |
| 3 | normal | normal | normal | hyperdynamic | 3.9 | 70.0 | 52.1 | NA | 9 | 13 | Yes |
| 4 | Mildly enlarged | normal | Moderately elevated | normal | 0.5 | 70.0 | 11.1 | 36.7 | 9 | 6 | No |
| 5 | normal | normal | normal | hyperdynamic | 2.9 | N/A | 100 | 22.0 | 42 | 5 | Yes |
| 6 | normal | normal | normal | hyperdynamic | 3.7 | 76.4 | 10.5 | 36.9 | 4 | 11 | No |
| 7 | Mildly enlarged | normal | normal | normal | 0.8 | 50.0 | 9.5 | 16.0 | 9 | 9 | No |
| 8 | normal | Mildly reduced | normal | normal | 1.9 | 50.6 | 16.7 | 34.3 | 12 | 14 | Yes |
| 9 | normal | normal | Mildly elevated | normal | 5.0 | N/A | 60.0 | 30.5 | 16 | 7 | No |
| 10 | Mildly enlarged | normal | normal | normal | 1.5 | 65.7 | 5.6 | 22.2 | 14 | 9 | No |
| 11 | normal | normal | normal | hyperdynamic | 7.4 | 35.6 | 25.9 | 10.9 | 19 | 6 | Yes |
| 12 | normal | normal | Mildly elevated | hyperdynamic | 1.9 | 65.1 | 100 | 42.4 | 6 | 8 | No |
| 13 | Mildly enlarged | Mildly reduced | normal | normal | 3.6 | 68.2 | 10.5 | 36.7 | 9 | 11 | No |
| 14 | Mildly enlarged | normal | Mildly elevated | normal | 1.2 | 71.7 | 60.0 | 61.0 | 10 | 19 | No |
Acknowledgments
This study was supported by grants from the Easton Family Fund and the Deseret Foundation Dr. Brown is supported by a career development award from National Institute of General Medical Sciences (K23GM094465)
We acknowledge Ben Briggs for screening patients, Naresh Kumar for consenting patients, and Amanda Borba for administrative support
Sources of Support: This study was supported by grants from the Easton Family Fund and the Intermountain Medical Research Foundation. Dr. Brown is supported by a career development award from National Institute of General Medical Sciences (K23GM094465).
Footnotes
All authors report no relevant conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Durairaj L, Schmidt GA. Fluid therapy in resuscitated sepsis: Less is more. Chest. 2008;133:252–263. doi: 10.1378/chest.07-1496. [DOI] [PubMed] [Google Scholar]
- 2.Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, Peterson E, Tomlanovich M. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368–1377. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- 3.Maitland K, Kiguli S, Opoka RO, Engoru C, Olupot-Olupot P, Akech SO, Nyeko R, Mtove G, Reyburn H, Lang T, Brent B, Evans JA, Tibenderana JK, Crawley J, Russell EC, Levin M, Babiker AG, Gibb DM. Mortality after fluid bolus in african children with severe infection. N Engl J Med. 2011;364:2483–2495. doi: 10.1056/NEJMoa1101549. [DOI] [PubMed] [Google Scholar]
- 4.Humphrey H, Hall J, Sznajder I, Silverstein M, Wood L. Improved survival in ards patients associated with a reduction in pulmonary capillary wedge pressure. Chest. 1990;97:1176–1180. doi: 10.1378/chest.97.5.1176. [DOI] [PubMed] [Google Scholar]
- 5.Wiedemann HP, Wheeler AP, Bernard GR, Thompson BT, Hayden D, deBoisblanc B, Connors AF, Jr, Hite RD, Harabin AL. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med. 2006;354:2564–2575. doi: 10.1056/NEJMoa062200. [DOI] [PubMed] [Google Scholar]
- 6.Dellinger RP, Levy MM, Carlet JM, Bion J, Parker MM, Jaeschke R, Reinhart K, Angus DC, Brun-Buisson C, Beale R, Calandra T, Dhainaut JF, Gerlach H, Harvey M, Marini JJ, Marshall J, Ranieri M, Ramsay G, Sevransky J, Thompson BT, Townsend S, Vender JS, Zimmerman JL, Vincent JL. Surviving sepsis campaign: International guidelines for management of severe sepsis and septic shock: 2008. Intensive Care Med. 2008;34:17–60. doi: 10.1007/s00134-007-0934-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marik PE, Baram M, Vahid B. Does central venous pressure predict fluid responsiveness? A systematic review of the literature and the tale of seven mares. Chest. 2008;134:172–178. doi: 10.1378/chest.07-2331. [DOI] [PubMed] [Google Scholar]
- 8.Shippy CR, Appel PL, Shoemaker WC. Reliability of clinical monitoring to assess blood volume in critically ill patients. Crit Care Med. 1984;12:107–112. doi: 10.1097/00003246-198402000-00005. [DOI] [PubMed] [Google Scholar]
- 9.Bendjelid K, Romand JA. Fluid responsiveness in mechanically ventilated patients: A review of indices used in intensive care. Intensive Care Med. 2003;29:352–360. doi: 10.1007/s00134-002-1615-9. [DOI] [PubMed] [Google Scholar]
- 10.Osman D, Ridel C, Ray P, Monnet X, Anguel N, Richard C, Teboul JL. Cardiac filling pressures are not appropriate to predict hemodynamic response to volume challenge. Crit Care Med. 2007;35:64–68. doi: 10.1097/01.CCM.0000249851.94101.4F. [DOI] [PubMed] [Google Scholar]
- 11.De Backer D, Heenen S, Piagnerelli M, Koch M, Vincent JL. Pulse pressure variations to predict fluid responsiveness: Influence of tidal volume. Intensive Care Med. 2005;31:517–523. doi: 10.1007/s00134-005-2586-4. [DOI] [PubMed] [Google Scholar]
- 12.Michard F, Boussat S, Chemla D, Anguel N, Mercat A, Lecarpentier Y, Richard C, Pinsky MR, Teboul JL. Relation between respiratory changes in arterial pulse pressure and fluid responsiveness in septic patients with acute circulatory failure. American journal of respiratory and critical care medicine. 2000;162:134–138. doi: 10.1164/ajrccm.162.1.9903035. [DOI] [PubMed] [Google Scholar]
- 13.Cannesson M, Musard H, Desebbe O, Boucau C, Simon R, Henaine R, Lehot JJ. The ability of stroke volume variations obtained with vigileo/flotrac system to monitor fluid responsiveness in mechanically ventilated patients. Anesth Analg. 2009;108:513–517. doi: 10.1213/ane.0b013e318192a36b. [DOI] [PubMed] [Google Scholar]
- 14.Marx G, Cope T, McCrossan L, Swaraj S, Cowan C, Mostafa SM, Wenstone R, Leuwer M. Assessing fluid responsiveness by stroke volume variation in mechanically ventilated patients with severe sepsis. Eur J Anaesthesiol. 2004;21:132–138. doi: 10.1017/s0265021504002091. [DOI] [PubMed] [Google Scholar]
- 15.Hofer CK, Senn A, Weibel L, Zollinger A. Assessment of stroke volume variation for prediction of fluid responsiveness using the modified flotrac and piccoplus system. Crit Care. 2008;12:R82. doi: 10.1186/cc6933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Monnet X, Rienzo M, Osman D, Anguel N, Richard C, Pinsky MR, Teboul JL. Esophageal doppler monitoring predicts fluid responsiveness in critically ill ventilated patients. Intensive care medicine. 2005;31:1195–1201. doi: 10.1007/s00134-005-2731-0. [DOI] [PubMed] [Google Scholar]
- 17.Feissel M, Michard F, Mangin I, Ruyer O, Faller JP, Teboul JL. Respiratory changes in aortic blood velocity as an indicator of fluid responsiveness in ventilated patients with septic shock. Chest. 2001;119:867–873. doi: 10.1378/chest.119.3.867. [DOI] [PubMed] [Google Scholar]
- 18.Jue J, Chung W, Schiller NB. Does inferior vena cava size predict right atrial pressures in patients receiving mechanical ventilation? J Am Soc Echocardiogr. 1992;5:613–619. doi: 10.1016/s0894-7317(14)80327-1. [DOI] [PubMed] [Google Scholar]
- 19.Vieillard-Baron A, Chergui K, Rabiller A, Peyrouset O, Page B, Beauchet A, Jardin F. Superior vena caval collapsibility as a gauge of volume status in ventilated septic patients. Intensive Care Med. 2004;30:1734–1739. doi: 10.1007/s00134-004-2361-y. [DOI] [PubMed] [Google Scholar]
- 20.Feissel M, Michard F, Faller JP, Teboul JL. The respiratory variation in inferior vena cava diameter as a guide to fluid therapy. Intensive Care Med. 2004;30:1834–1837. doi: 10.1007/s00134-004-2233-5. [DOI] [PubMed] [Google Scholar]
- 21.Westphal GA, Silva E, Caldeira Filho M, Roman Goncalves AR, Poli-de-Figueiredo LF. Variation in amplitude of central venous pressure curve induced by respiration is a useful tool to reveal fluid responsiveness in postcardiac surgery patients. Shock. 2006;26:140–145. doi: 10.1097/01.shk.0000227439.76418.7d. [DOI] [PubMed] [Google Scholar]
- 22.Barbier C, Loubieres Y, Schmit C, Hayon J, Ricome JL, Jardin F, Vieillard-Baron A. Respiratory changes in inferior vena cava diameter are helpful in predicting fluid responsiveness in ventilated septic patients. Intensive care medicine. 2004;30:1740–1746. doi: 10.1007/s00134-004-2259-8. [DOI] [PubMed] [Google Scholar]
- 23.Pinsky MR. Hemodynamic evaluation and monitoring in the icu. Chest. 2007;132:2020–2029. doi: 10.1378/chest.07-0073. [DOI] [PubMed] [Google Scholar]
- 24.Coudray A, Romand JA, Treggiari M, Bendjelid K. Fluid responsiveness in spontaneously breathing patients: A review of indexes used in intensive care. Crit Care Med. 2005;33:2757–2762. doi: 10.1097/01.ccm.0000189942.24113.65. [DOI] [PubMed] [Google Scholar]
- 25.American college of chest physicians/society of critical care medicine consensus conference: Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med. 1992;20:864–874. [PubMed] [Google Scholar]
- 26.Colebourn CL, Barber V, Salmon JB, Young JD. The accuracy of diagnostic and haemodynamic data obtained by transthoracic echocardiography in critically ill adults: A systematic review. Journal of the Intensive Care Society. 2008;9:7. [Google Scholar]
- 27.McLean AS, Needham A, Stewart D, Parkin R. Estimation of cardiac output by noninvasive echocardiographic techniques in the critically ill subject. Anaesth Intensive Care. 1997;25:250–254. doi: 10.1177/0310057X9702500307. [DOI] [PubMed] [Google Scholar]
- 28.Noritomi DT, Vieira ML, Mohovic T, Bastos JF, Cordioli RL, Akamine N, Fischer CH. Echocardiography for hemodynamic evaluation in the intensive care unit. Shock. 2010;34(1):59–62. doi: 10.1097/SHK.0b013e3181e7e8ed. [DOI] [PubMed] [Google Scholar]
- 29.Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, Solomon SD, Louie EK, Schiller NB. Guidelines for the echocardiographic assessment of the right heart in adults: A report from the american society of echocardiography endorsed by the european association of echocardiography, a registered branch of the european society of cardiology, and the canadian society of echocardiography. J Am Soc Echocardiogr. 2010;23:685–713. doi: 10.1016/j.echo.2010.05.010. quiz 786-688. [DOI] [PubMed] [Google Scholar]
- 30.Skulec R, Cermak O, Skalicka H, Kolar J. Variability of aortic blood flow predicts fluid responsiveness in spontaneously breathing healthy volunteers. Kardiol Pol. 2009;67:265–271. [PubMed] [Google Scholar]
- 31.Compton FD, Zukunft B, Hoffmann C, Zidek W, Schaefer JH. Performance of a minimally invasive uncalibrated cardiac output monitoring system (flotrac/vigileo) in haemodynamically unstable patients. Br J Anaesth. 2008;100:451–456. doi: 10.1093/bja/aem409. [DOI] [PubMed] [Google Scholar]
- 32.Sakka SG, Bredle DL, Reinhart K, Meier-Hellmann A. Comparison between intrathoracic blood volume and cardiac filling pressures in the early phase of hemodynamic instability of patients with sepsis or septic shock. J Crit Care. 1999;14:78–83. doi: 10.1016/s0883-9441(99)90018-7. [DOI] [PubMed] [Google Scholar]
- 33.Khwannimit B, Bhurayanontachai R. Prediction of fluid responsiveness in septic shock patients: Comparing stroke volume variation by flotrac/vigileo and automated pulse pressure variation. Eur J Anaesthesiol. 2012;29:64–69. doi: 10.1097/EJA.0b013e32834b7d82. [DOI] [PubMed] [Google Scholar]
- 34.Muller L, Bobbia X, Toumi M, Louart G, Molinari N, Ragonnet B, Quintard H, Leone M, Zoric L, Lefrant JY. Respiratory variations of inferior vena cava diameter to predict fluid responsiveness in spontaneously breathing patients with acute circulatory failure: Need for a cautious use. Crit Care. 2012;16:R188. doi: 10.1186/cc11672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Preau S, Saulnier F, Dewavrin F, Durocher A, Chagnon JL. Passive leg raising is predictive of fluid responsiveness in spontaneously breathing patients with severe sepsis or acute pancreatitis. Crit Care Med. 2010;38:819–825. doi: 10.1097/CCM.0b013e3181c8fe7a. [DOI] [PubMed] [Google Scholar]
- 36.Mahjoub Y, Touzeau J, Airapetian N, Lorne E, Hijazi M, Zogheib E, Tinturier F, Slama M, Dupont H. The passive leg-raising maneuver cannot accurately predict fluid responsiveness in patients with intra-abdominal hypertension. Crit Care Med. 2010;38:1824–1829. doi: 10.1097/CCM.0b013e3181eb3c21. [DOI] [PubMed] [Google Scholar]
- 37.Mead J, Gaensler EA. Esophageal and pleural pressures in man, upright and supine. J Appl Physiol. 1959;14:81–83. doi: 10.1152/jappl.1959.14.1.81. [DOI] [PubMed] [Google Scholar]
- 38.MacIntyre NR. Respiratory function during pressure support ventilation. Chest. 1986;89:677–683. doi: 10.1378/chest.89.5.677. [DOI] [PubMed] [Google Scholar]
- 39.Grinnan DC, Truwit JD. Clinical review: Respiratory mechanics in spontaneous and assisted ventilation. Crit Care. 2005;9:472–484. doi: 10.1186/cc3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Putensen C, Muders T, Varelmann D, Wrigge H. The impact of spontaneous breathing during mechanical ventilation. Curr Opin Crit Care. 2006;12:13–18. doi: 10.1097/01.ccx.0000198994.37319.60. [DOI] [PubMed] [Google Scholar]
- 41.Kimura BJ, Dalugdugan R, Gilcrease GW, 3rd, Phan JN, Showalter BK, Wolfson T. The effect of breathing manner on inferior vena caval diameter. European journal of echocardiography: the journal of the Working Group on Echocardiography of the European Society of Cardiology. 2011;12:120–123. doi: 10.1093/ejechocard/jeq157. [DOI] [PubMed] [Google Scholar]
- 42.Michard F, Ruscio L, Teboul JL. Clinical prediction of fluid responsiveness in acute circulatory failure related to sepsis. Intensive Care Med. 2001;27:1238. doi: 10.1007/s001340100974. [DOI] [PubMed] [Google Scholar]