Abstract
Objective
Genetic testing is increasingly part of routine clinical care for women with a family history of breast cancer. Given their substantially elevated risk for breast cancer, BRCA1/BRCA2 mutation carriers must make the difficult decision whether or not to opt for risk reducing mastectomy. To help BRCA1/2 carriers make this decision, we developed a computer-based interactive decision aid which we tested against usual care in a randomized controlled trial.
Design
Following the completion of genetic counseling, 214 female (aged 21–75) BRCA1/BRCA2 mutation carriers were randomized to Usual Care (UC; N=114) or Usual Care plus Decision Aid (DA; N=100) arms. UC participants received no additional intervention. DA participants were sent the CD-ROM decision aid to view at home.
Main Outcome Measures
We measured final management decision, decisional conflict, decisional satisfaction and receipt of risk reducing mastectomy at 1-, 6-, and 12-months post-randomization.
Results
Longitudinal analyses revealed that the DA was effective among carriers who were initially undecided about how to manage their breast cancer risk. Within this group, the DA led to an increased likelihood of reaching a management decision (OR=3.09, 95% CI=1.62, 5.90; p<.001), decreased decisional conflict (B=−.46, z=−3.1, p<.002), and increased satisfaction (B=.27, z=3.1, p=0.002) compared to UC. Among carriers who had already made a management decision by the time of randomization, the DA had no benefit relative to UC.
Conclusion
These results demonstrate that BRCA1/BRCA2 mutation carriers who are having difficulty making a breast cancer risk management decision can benefit from adjunct decision support.
Keywords: BRCA1, BRCA2, decision aid, risk reducing mastectomy
Genetic testing is increasingly part of routine clinical care for women with a strong family history of breast or ovarian cancer. Women who carry a BRCA1 or BRCA2 (BRCA1/2) mutation have a lifetime breast cancer risk of 40–66% and a lifetime ovarian cancer risk of 13–40% (Chen and Parmagiani, 2007). BRCA1/2 carriers who have previously been diagnosed with breast cancer, have a 40–60% lifetime risk of developing a second breast cancer in the contralateral breast (Breast Cancer Linkage Consortium, 1999; Metcalfe et al., 2004). Thus, women who learn that they carry a BRCA1/2 mutation are faced with difficult decisions about how to manage their breast and ovarian cancer risk. Current guidelines for breast cancer risk management recommend enhanced breast cancer surveillance with annual mammography, breast magnetic resonance imaging beginning at age 25–30 and consideration of risk reducing mastectomy (NCCN, 2006; Smith & Robson, 2006). Guidelines for ovarian cancer risk management recommend risk reducing oophorectomy (RRO) at the completion of child-bearing or by age of 35–40 (National Comprehensive Cancer Network, 2006). RRO also reduces breast cancer risk when performed prior to age 50 (Kauff et al., 2002; Rebbeck et al., 2002).
In this study we focused on the decision between surveillance and risk reducing mastectomy (RRM). Although RRM reduces the risk of breast cancer risk by about 90% in both unaffected and previously affected women (Herrinton et al., 2005; McDonnell et al., 2001; Peralta et al., 2000), many women are reluctant to choose RRM due to concerns about body image, sexuality, quality of life, the irreversibility of the procedure, and the aggressive nature of removing healthy breasts. In contrast, breast cancer surveillance is non-invasive and has few adverse effects. However, surveillance does not reduce breast cancer risk and there is limited evidence for the efficacy of enhanced surveillance among BRCA1/2 carriers (Tilanus-Linthorst et al., 2002). Thus, for BRCA1/2 carriers, the decision between surveillance and RRM is based upon individual preferences.
Decision aids have been used for a variety of preference-based medical decisions such as decisions about hormone replacement therapy (O’Connor et al., 1998), treatment for breast and prostate cancers (Goel, Sawka, Thiel, Gort, & O’Connor, 2001; Molenaar et al., 2001; Whelan et al., 2004), and prostate cancer screening (Volk, Spann, Cass, & Hawley, 2003). Unlike traditional patient education, decision aids are designed to improve the quality of medical decision making by providing patients with balanced information about the potential benefits, risks, and likely outcomes associated with each decision, and by helping patients consider the personal importance they place on each option (O’Connor et al., 1999; O’Connor et al., 2003). Decision aids have been shown to increase knowledge and reduce decisional conflict (O’Connor et al., 2003). Although the impact of decision aids on actual decision making is less clear (O’Connor et al., 2003), a recent meta analysis suggested that breast cancer patients who used decision aids were 25% more likely to choose breast conserving surgery compared to patients who did not use a decision aid (Waljee, Rogers, & Alderman, 2007).
Several decision aids have been developed to help individuals make decisions about whether or not to pursue BRCA1/2 testing. Green and colleagues (2004) found that a CD-based interactive decision aid led to increased knowledge and decreased intentions for genetic testing among low-risk participants. These results are consistent with an earlier study among women at low-risk for a BRCA1/2 mutation (Schwartz et al., 2001). Wakefield and colleagues (2007) found that a print decision aid led to increased knowledge about genetic testing but had no impact on decision regret or genetic testing decision. There have been fewer studies focused on management decision making following BRCA1/2 testing. A randomized trial that compared individual, multi-session decision counseling to usual care among BRCA1/2 carriers found that decision counseling led to decreased distress, but did not impact management decisions, decision uncertainty or satisfaction (van Roosmalen et al., 2004a). A recent study using a pre-post design found reduced decisional conflict and increased knowledge among BRCA1/2 carriers after using a decision aid (Metcalfe et al., 2007).
We developed and tested a CD-based, interactive decision aid designed for home use by women who had recently learned that they carried a BRCA1/2 mutation. Development of the decision aid (DA) was guided by the Ottawa Framework for Informed Decision Making (O’Connor et al., 1998; O’Connor et al., 1999). The Ottawa Framework highlights impediments to quality decision making such as poor knowledge, unrealistic outcome expectations, unclear values, high uncertainty, and decisional conflict (O’Connor et al., 1999). We targeted these impediments through tailored information about the risks, benefits and likely outcomes of each option (O’Connor et al., 1998; O’Connor, Jacobsen, & Stacey, 2002). To help participants clarify their preferences, the DA included an interactive, individually tailored value clarification exercise (Kaufman et al., 2003) that was based on multiattribute value theory (Edwards & Newman, 1982; Keeney & Raiffa, 1993).
We evaluated the impact of our DA as an adjunct to usual care against usual care (UC) alone in a randomized trial of women who had recently learned that they carry a BRCA1/2 mutation. In this report we focus on the impact of the DA on breast cancer risk management decision making outcomes. We predicted that women who were randomized to the DA would be more likely to reach a definitive breast cancer risk management decision, report lower decisional conflict and increased decision satisfaction relative to participants who were randomized to UC. We further predicted that the DA would be most beneficial to women who were undecided about their risk management strategy at the time of randomization.
METHOD
Participants
Between the years of 2001–2005, we recruited participants from the genetic testing research programs at the Lombardi Comprehensive Cancer Center (Washington, DC), Mount Sinai School of Medicine (New York, NY), and Englewood Hospital and Medical Center (Englewood, NJ). Eligible participants were women between the ages of 25 to 75 who had received a positive BRCA1/2 gene test result through the clinical research program at one of the participating sites, had not had prior bilateral mastectomy, and did not have metastatic breast or ovarian cancer. Genetic counseling was provided free of charge.
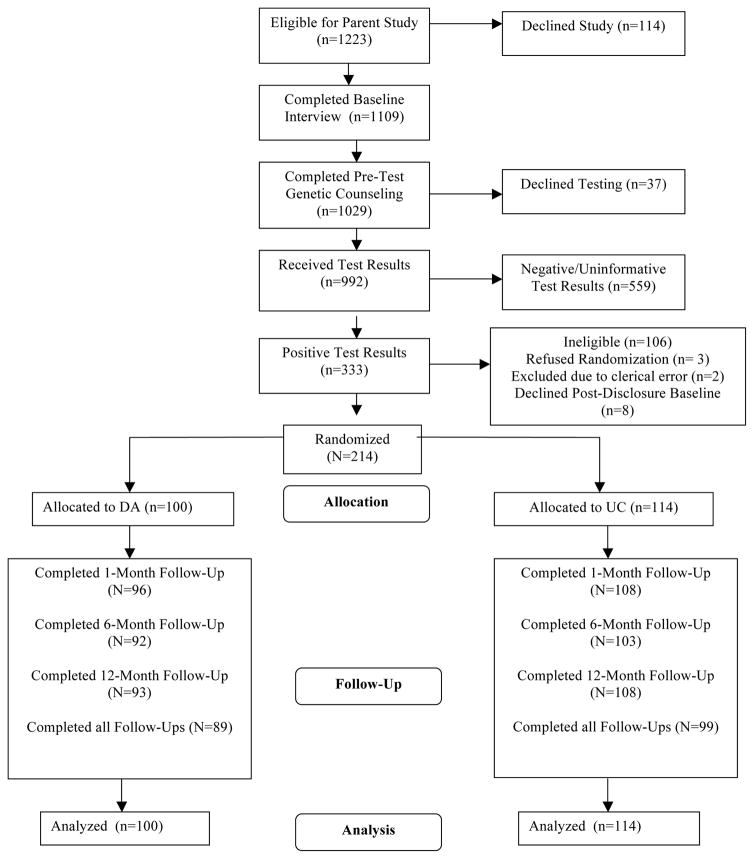
As seen in Figure 1, of 1223 individuals who contacted the clinical research programs across the sites, 1109 (91%) completed a baseline telephone interview. Of the 1109 who completed a baseline interview, 1029 (93%) completed pre-test genetic counseling, 992 (89%) chose to receive BRCA1/2 test results, and 333 received positive BRCA1/2 test results making them potentially eligble for this trial. Of the 333 BRCA1/2 carriers, 106 were ineligible for randomization for the following reasons: male (N=46); prior bilateral mastectomy (N=44); under age 25 (N=2); age 75 or older (N=6); metastatic disease (N=7); non-English speaker (N=1). Thus, 227 women were eligible for randomization. Of these, 3 (1%) declined randomization, 8 (3%) did not complete the post-disclosure baseline interview, and 2 (.9%) were excluded from the study due to clerical errors. Thus, 214 (94%) women were randomized to either UC (N=114) or DA (N=100). Of these, 188 (88%) completed all follow-up assessments. There was no difference in the drop-out rate in the DA versus the UC groups (χ2 (df=1, N=214)=.08, p=.78), nor were there any baseline demographic, medical, or family history differences between those who completed all follow-ups and those who did not.
FIGURE 1.
Study Flow Chart.
Of the final sample, 93% were Caucasian, 76% were college educated, 67% were married, 48% were employed full-time and 49% were Jewish. The mean age of participants was 44 years (Range: 21–74 years). Thirty-seven percent were affected with breast cancer and 10% with ovarian cancer (mean time since diagnosis = 7.7 years).
Procedure
Participants were self- or physician-referred to the genetic counseling program at one of the participating sites. The randomized trial was nested within an observational study evaluating the outcomes of BRCA1/2 testing. Prior to genetic counseling, participants consented to participation in the observational study and to participation in the decision aid trial if they were to be eligible. After completing a standard pre-counseling telephone interview to collect information on demographics, personal/family cancer history, and psychosocial variables, participants attended a genetic counseling session and provided a blood sample for BRCA1/2 mutation testing. When test results were available, participants completed a genetic counseling disclosure session and a routine 2-week follow-up telephone contact. At 1-month post-disclosure, we contacted participants for the post-disclosure baseline interview to collect information on management intentions, behaviors, decisional conflict, decision satisfaction, and psychosocial outcomes. At the conclusion of this interview, eligible participants provided verbal consent and were randomized via computer-generated random number in a 1:1 ratio to either the Usual Care (UC; N=114) or Usual Care plus Decision Aid (DA; N=100) arm. Participants in the UC arm received no further intervention. Participants in the DA arm were mailed the CD-ROM via priority mail. We recontacted participants at 1-, 6-, and 12-months post-randomization for follow-up telephone interviews.
Usual Care
All participants received standard genetic counseling. Details of standard genetic counseling for BRCA1/2 have been described in detail in previous reports (Schwartz et al., 2002). Briefly, the pre-test genetic counseling session included the following topics: risk assessment, cancer risks associated with mutations in BRCA1/2, the process of BRCA1/2 testing and interpretation of results, options for cancer prevention and surveillance, and potential benefits and risks of testing. Participants received their results at a genetic counseling disclosure session during which the implications of their test result and management options were discussed. All carriers were provided with explicit recommendations for breast cancer surveillance, information about other management options, physician referrals, and a summary letter outlining all guidelines and recommendations. Approximately 2–4 weeks following the disclosure session participants were contacted for a brief telephone follow-up in which the genetic counselor answered any new patient questions, discussed ongoing concerns, and made additional referrals if indicated.
Decision Aid Intervention
After completing usual care, DA participants were sent the DA via express mail. We have previously described the content and development of the DA (Kaufman et al., 2003). Briefly, the DA was delivered via interactive CD-ROM. After a brief introduction and tutorial, the user answered several questions that were used for individually tailoring the DA content. The DA was tailored to the participant’s age, menopausal status, breast cancer history, prior tamoxifen use, oophorectomy history, and current breast cancer risk management intention. The informational content of the DA was divided into four sections: 1) The Breast Cancer Information section contained general information on breast cancer including a definition, a pictorial description, epidemiology and stage information, and a general overview of treatment; 2) The Risk Communication section provided individually tailored breast and ovarian cancer risk graphs along with interpretive information; 3) The Risk Management Options section comprised the bulk of the DA. In this section the pros and cons of each management option were reviewed in detail with a focus on the tradeoffs made in choosing one option over another; 4) The Interactive Decision Task guided participants through a multi-attribute value model (Keeney & Raiffa, 1993) and based on the results of this task, patients were provided with feedback on the management option that they appeared to favor.
Measures
Control Variables
Sociodemographics
Participants provided the following demographic information: age, race, education, marital status, employment status, health insurance status, and religion. These variables were dichotomized as follows: age (≤ 50 vs. > 50), race (Caucasian vs. other), marital status (married/partnered vs. other), education (college graduate vs. < college graduate), employment (full time vs. other), insurance status (yes vs. no), and religion (Jewish vs. other).
Medical/Family History
Upon entry into the genetic counseling program, we assessed family and personal cancer history, screening behavior, and surgical history.
Outcome Variables
Decisional Conflict
We administered the Decisional-Conflict Scale (DCS) (O’Connor, 1995) at post-disclosure and all follow-ups. The DCS consists of 16 items measured on a 5-point Likert scale. The DCS measures uncertainty about the decision (3 items), feeling uninformed about the decision (3 items), feeling unsupported in decision making (3 items), feeling unclear about values (3 items), and the perceived quality of the decision (4 items). Because the perceived decision quality items ask about decisions that have already been made, women who had not reached a final decision at a particular assessment were not asked these questions. In order to create comparable scales for women who had made a final decision and those who had not, only the 12 items from the first four subscales were used. The total score was calculated by averaging the individual item scores so that higher scores indicated higher decisional conflict. Cronbach’s alphas in this study ranged from 0.87 to 0.94.
Decision Satisfaction
We measured satisfaction with breast cancer management decision at each follow-up assessment using the 6-item Satisfaction With Decision Scale (SWD) (Holmes-Rovner et al., 1996). The six items are rated on a 5-point Likert-style scale and the mean score across all items is used as the total score (higher scores indicate greater satisfaction). Cronbach’s alpha in the present study ranged from 0.91 to 0.92.
Management Decision
At post-disclosure and each follow-up assessment, we asked participants ‘Have you made a final decision about how to manage your risk for breast cancer?’ Participants responded either yes or no at each timepoint. At each assessment, we also asked participants whether they had obtained an RRM since the previous assessment.
Statistical Analyses
We conducted preliminary analyses to identify baseline group differences to control in multivariate analyses. Next, we evaluated the impact of recruitment site on each of our study outcomes. We also evaluated the impact of recruiting multiple members of the same family. However, the impact of family clustering was negligible so we did not consider family clustering in subsequent analyses. To evaluate the longitudinal impact of the intervention across our follow-up timepoints, we used generalized estimating equations (GEE) in both linear and logistic regression models. These analyses employed an intention-to-treat approach in which all participants were included in the analyses regardless of whether they reported viewing the DA or not. Thus within the DA group participants who reported that they did not view the DA prior to their 1-month follow-up assessment were included in all analyses. This approach provides the most conservative estimate of the impact of the intervention. In all analyses, we controlled for baseline scores on the outcome of interest and for potential confounders that differed between groups at baseline. We tested our hypothesis that the DA intervention would lead to improved decision making outcomes by examining the main effect of group. In each model, we included a time factor and examined the group by time interaction effect to determine whether the group effect varied across the follow-up timepoints. To test our hypothesis that the DA group would be most effective among women who were undecided about management at the time of randomization, we evaluated the interaction between baseline management decision status and group. Finally, we conducted post-hoc analyses to determine whether having a personal history of breast and/or ovarian cancer moderated the impact of the DA by entering the cancer history main effect and cancer history by group interaction term in our multivariate models.
RESULTS
Baseline Group Comparisons
At baseline, DA participants were more likely to have been diagnosed with ovarian cancer (15% vs. 6%; χ2 (df=1, N=214)= 4.5, p=.03) and were less likely to have intact ovaries (73% vs. 87%; χ2 (df=1, N=214)=8.0, p=.005). Because of the overlap between these variables, we could not control for both in multivariate analyses. We decided to adjust for baseline ovary status because the association between ovary status and group assignment was stronger than the association between ovarian cancer history and group assignment. Further, since ovary status impacts on breast cancer risk, it is important to account for ovary status even among unaffected participants. Controlling for ovarian cancer history rather than ovary status does not alter the study results (data not shown).
Study Site
Compared to New York/New Jersey participants (N=99) those recruited at GU (N=115). reported lower decisional conflict at 1-month (t(196)=2.0, p=.04) and higher decisional satisfaction at 1- (t(187)=2.2, p=.03) and 12-months (t(193)=2.6, p=.01). Thus, we controlled for site in multivariate analyses of decisional conflict and decision satisfaction.
Use of the Decision Aid
Within the DA arm, 64 (63%) of participants reported that they used the DA at least once, 36 (35%) reported that they did not use the DA and 2 (2%) were missing this item. Since we employed a conservative intention-to-treat approach, all DA participants are included in subsequent analyses regardless of whether they report having used the DA or not.
Management Decision Status
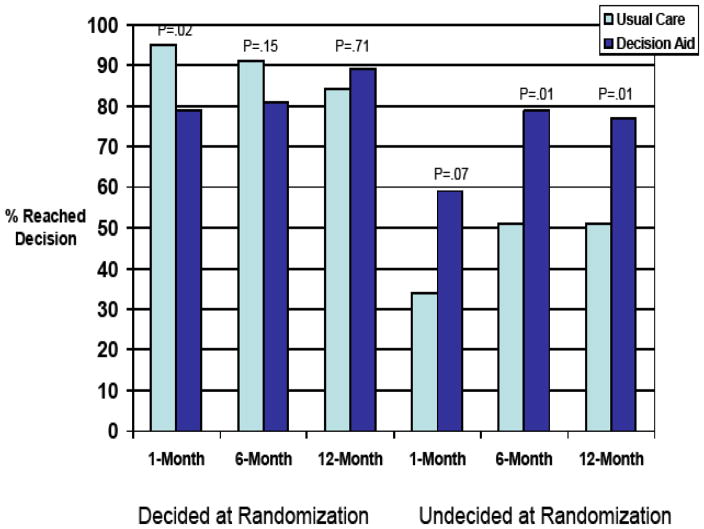
Logistic regression with GEE (controlling for baseline ovary status, and baseline management decision status) revealed a significant longitudinal impact of the DA (z=−3.0, p=.003) which was consistent across the follow-up timepoints (group by time; z=1.7, p = .09) but was moderated by baseline decision status (group by baseline decision status; z=3.00, p<.003). As reflected in Figure 2, relative to UC, the DA led to a three-fold increase in the odds of reaching a final management decision among those who were undecided at the time of randomization (OR=3.09, 95% CI=1.62, 5.90; p<.001). Among those who had already reached a management decision prior to randomization, the DA did not significantly impact the likelihood of remaining decided over the course of the study (OR=0.56 95% CI=0.24, 1.29; p=.17).
FIGURE 2.
Final Management Decision by Group and Baseline Management Decision
In post-hoc analyses we tested the possibility that the DA might differentially impact participants with and without a personal history of breast and/or ovarian cancer by entering the cancer history main effect and the cancer history by group interaction term in our final multivariate models. Cancer history did not moderate the effect of the DA among those who had reached a management decision prior to randomization (z=−0.87; p=.39) or among those who had not (z=−0.70, p=0.49).
Decisional Conflict
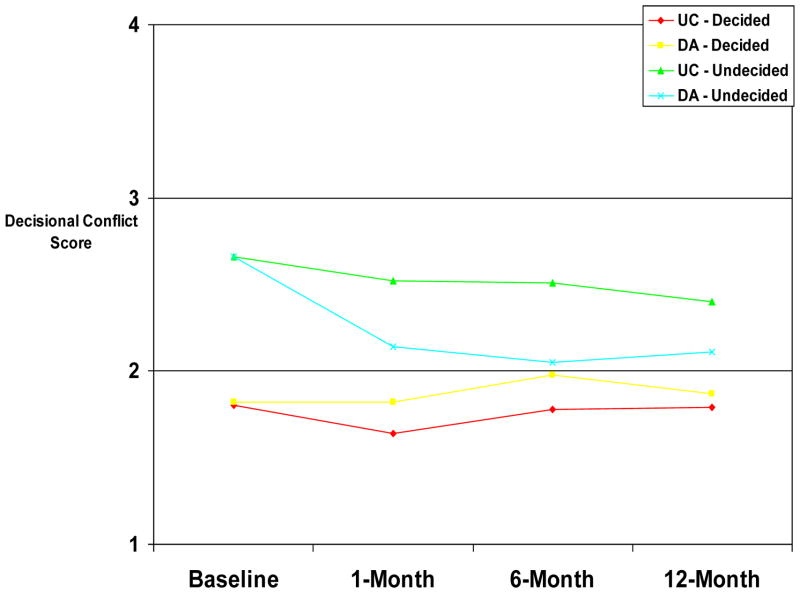
Multiple linear regression with GEE (controlling for baseline decisional conflict, study site, and baseline ovary status) revealed a significant longitudinal impact of the DA (B=.57, z=2.2, p=.03) which was consistent across the follow-up timepoints (group by time; B=−.001, z=−.03, p=.98) but was moderated by baseline decision status (group by baseline management decision status; B=−.46, z=−3.1, p=.002). As shown in Figure 3, relative to UC, the DA led to significant decreases in decisional conflict for those who were undecided at the time of randomization (B=−.35, z=−3.6, p<.001) but not for those who were decided at randomization (B=.10, z=.98, p=0.33).
FIGURE 3.
Decisional Conflict by Group and Baseline Management Decision.
Post-hoc analyses in which we entered the cancer history main effect and the cancer history by group interaction term in our final multivariate models, revealed that personal cancer history did not moderate the impact of the DA on decisional conflict among participants who had reached a decision at the time of randomization (z =0.18, p=.85) or those who were undecided (z =1.8, p = .07).
Satisfaction With Decision Making
Since satisfaction with decision making was not measured at baseline (i.e., prior to a decision), the multiple regression with GEE controlled for baseline ovary status and study site only. There was neither a main effect of group assignment (B=−.39, z=−1.7, p=0.09) nor an interaction between group and time (B=−.003, z=−0.1, p=0.95). However, a significant group by baseline decision status interaction (B=.33, z=2.51, p=.01) revealed that the DA led to significantly increased satisfaction compared to UC (B=.27, z=3.1, p=0.002) among those who were undecided at randomization, but not among those who had made a management decision prior to randomization (B=−.07, z=−0.7, p=0.48).
Post-hoc analyses in which we entered the cancer history main effect and the cancer history by group interaction term in our final multivariate models, revealed that personal history of breast and/or ovarian cancer did not moderate the impact of the DA on decision satisfaction among participants who had reached a decision at randomization (z=−0.93, p=.35) or those who remained undecided at randomization (z=−1.42, p=.16).
Risk Reducing Mastectomy
Overall, 18 (18%) DA participants and 15 (13%) UC participants obtained RRM by the 12-month follow-up (χ2 (df=1, N=214)=0.96, p=.33). Bivariate analyses of RRM by timepoint revealed a difference in the timing of RRM across the two groups. At the 1-month follow-up, none (0%) of the DA participants and 5 (5.1%) of the UC participants had obtained a RRM (2-tailed Fisher Exact Test: p=.06). Of those who had not previously obtained RRM (N=209), 8 (8%) DA participants and 7 (6.4%) UC participants obtained RRM between the 1- and 6-month follow-ups (χ2 (df=1, N=209) = 0.44, p=.51). Of those who had not obtained a RRM by six months (N=194), 10 (10.9%) DA participants and 3 (2.9%) UC participants obtained a RRM between 6- and 12-months (χ2 (df=1, N=194)=3.80, p=.05).
Logistic regression controlling for baseline ovary status and site, indicated that neither group assignment (OR=1.27, 95% CI=0.37 to 1.7) nor the group by baseline decision status interaction (χ2 (df=1) = 0.56, p=.45) were significantly associated with obtaining a RRM by the 12-month follow-up assessment. Post-hoc analyses to evaluate the potential role of personal cancer history revealed that neither personal history of breast and/or ovarian cancer nor the group by cancer history interaction approached statistical significance (p’s > .20).
Discussion
Women who learn that they carry a BRCA1/2 mutation are faced with a complex and emotion-laden decision about how to manage their risk for breast cancer (Schwartz, Peshkin, Tercyak, Taylor, & Valdimarsdottir, 2005). Given the lack of definitive guidance, the decision about whether or not to obtain RRM must be made based on individual values. In this study, we tested a computer-based interactive DA designed to help BRCA1/2 mutation carriers make this decision. We found that the DA did not benefit the 48% of mutation carriers who had reached a management decision prior to randomization (i.e., within one month of receiving their test result). In contrast, the DA was highly effective among women who were undecided about how to manage their risk. For these women, the DA led to significant increases in the odds of reaching a management decision, increased satisfaction and decreased decisional conflict. These effects were consistent across the 12-month follow-up period, suggesting that the DA improved both short- and long-term decision making within this group.
The fact that about half of the study participants had already decided how to manage their risk within a month of testing was unexpected. Because the decision about whether or not to obtain a RRM is not typically time sensitive, we anticipated that most carriers would still be considering their options at the time of randomization. However, our finding that the DA was most effective among those who were undecided is consistent with previous research (O’Connor et al., 1999; O’Connor et al., 2003). For example, a decision aid for newly diagnosed breast cancer patients led to reduced decisional conflict only among undecided patients (Goel et al., 2001). Thus, our results along with previous studies strongly suggest that decision aids have their greatest impact among those individuals who are having the greatest difficulty reaching a decision.
Importantly, by 12-months post-randomization DA participants who were initially undecided fared about as well as participants who had quickly reached a decision. The only group that fared significantly worse was the UC participants who were undecided at randomization. For this group, decisional conflict remained high and decision satisfaction low. Further, only about half of this group had reached a management decision by the 12-month follow-up. Given their high degree of decisional conflict and uncertainty, this group could be at risk for adverse psychosocial and behavioral outcomes (Gattellari & Ward, 2005; O’Connor, 1995). Interestingly, women who quickly reached a decision did not benefit at all from added decision support. This raises the possibility that the decisions quickly reached by these women were effective and of high quality despite the apparent lack of deliberation. Of course it is also possible that these women simply modified their attitudes in order to reflect the decision that they had already made.
The low overall rate of RRM in this study is consistent with previous reports documenting low uptake of RRM following testing (Peshkin et al., 2002; Botkin et al., 2003). The DA did not impact on the rate of RRM across the two groups, nor was the impact of the DA on RRM modified by initial management decision status. However, the DA did impact the timing of RRM. Women randomized to UC were more likely to have a RRM prior to the 1-month follow-up assessment. In contrast, women in the DA group were more likely to obtain a RRM between 6 and 12 months post-disclosure. Although only speculation, this could indicate that the DA led to more deliberation prior to obtaining a RRM. This would be consistent with a central goal of decision aids to foster deliberative decision making. Importantly, since the RRM decision is not time sensitive, deliberative decision making may be particularly beneficial in this context. Future research should examine whether decision aids do in fact lead to increased deliberation and whether extra deliberation leads to improved outcomes.
The results of this trial contrast to the earlier randomized trial evaluating a post-disclosure decision aid for mutation carriers. The prior report focused on an in-person, multi-session shared decision making intervention that was delivered approximately two months following testing (van Roosmalen et al., 2004a). Similar to the present study, the DA did not impact on rates of RRM, but did lead to stronger overall treatment preferences. This is comparable to our finding that the DA led to an increased likelihood of reaching a final decision. It is not clear from the prior report whether the DA had a differential effect among carriers who were undecided about management at the time of the intervention.
The results described here strongly suggest that BRCA1/2 mutation carriers can benefit from additional decision support following the receipt of test results. This is consistent with previous studies that have demonstrated benefits for decision aids delivered before (Green et al., 2004; Wakefield et al., 2007), during (van Roosmalen et al., 2004b), and after genetic counseling (van Roosmalen et al., 2004a). However, our study suggests that such decision aids may be of most benefit to those women who are having difficulty reaching a decision. Unlike prior trials among BRCA1/2 carriers (van Roosmalen et al., 2004a), our results demonstrate the potential benefits of a DA used at home rather than in the clinic. This is consistent with studies of women considering BRCA1/2 testing in which a decision aid delivered at home was found to be effective (Wakefield et al., 2007). Advantages to home use include greater disseminability and the possibility of patients using the DA on their own schedule and as many times as they wish. At home decision aids may be most beneficial for decisions that are less time sensitive. Finally, these data indicate that a substantial proportion of BRCA1/2 mutation carriers have ongoing difficulty making management decisions despite extensive genetic counseling. For many, these difficulties extend out to more than a year following the receipt of genetic test results. Although the long-term implications of these difficulties remain to be seen, it is clear from the data reported here that these difficulties can largely be ameliorated through effective adjunct decision support interventions.
This study has several limitations. Although the overall sample size of the trial was adequate, only 33 participants opted for RRM. Thus, the study was underpowered to detect effects on overall rates of RRM. Another limitation of the study was our inability to ensure that all participants in the DA group actually viewed the CD. In fact, about one-third of participants reported that they did not view the CD. When this variable was considered in multivariate models, the results did not change substantively (data not shown). Still, given this rate of noncompliance, our use of an intent to treat approach likely underestimated the impact of the DA among those who used it. A related concern was the fact that we did not track usage patterns of the CD. Thus, we do not know whether certain sections of the DA were particularly beneficial. In future studies we will use internet-based approaches to monitor DA usage patterns. Finally, the lack of diversity of the study sample limits the generalizability of these results. In particular, the vast majority of study participants had access to a home computer on which they could view the DA. Although laptop computers were available for loaning to participants who did not have computer access, only one participant required such a loan. Thus, whether these results could be replicated among lower SES participants with less universal computer access remains a question.
Despite these limitations, the present report demonstrates that BRCA1/2 mutation carriers who are having difficulty deciding upon a breast cancer risk management approach can benefit from adjunct decision support in the form of an interactive decision aid. Future research should determine the optimal timing of this decision aid relative to the receipt of test results and evaluate the possibility of more widespread dissemination of this or other decision aids for BRCA1/2 mutation carriers.
FIGURE 4.
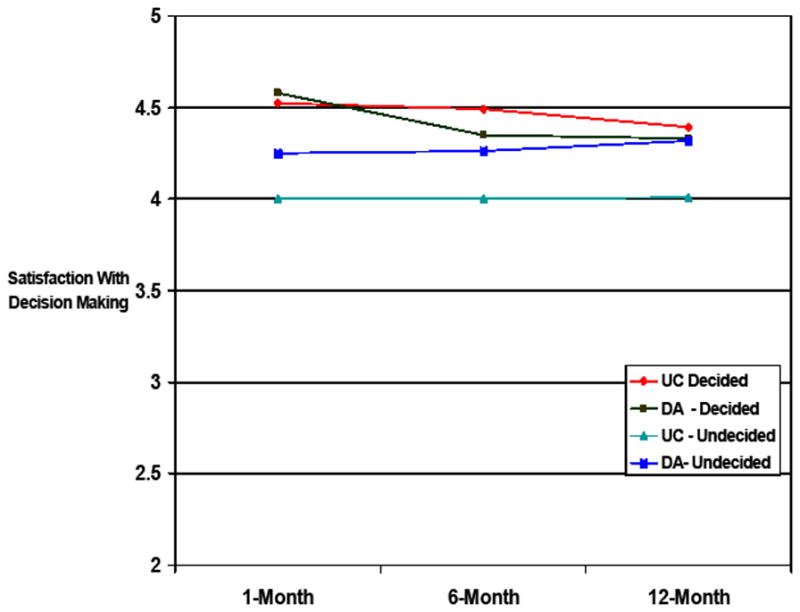
Decisional Satisfaction by Group and Baseline Management Decision
Table 1.
Baseline Group Comparisons
| Characteristic | Usual Care | Decision Aid |
|---|---|---|
| Mean Age (SD) | 43.5 (10.6) | 44.3 (11.2) |
| Education | ||
| < College (%) | 33 (29) | 19 (19) |
| College + (%) | 81 (71) | 81 (81) |
| Religion/Ethnicity | ||
| Jewish (%) | 56 (49) | 49 (49) |
| Non-Jewish (%) | 58 (51) | 51 (51) |
| Employment Status | ||
| Full Time (%) | 55 (48.3) | 48 (48) |
| < Full Time (%) | 59 (51.7) | 52 (52) |
| Race | ||
| Caucasian (%) | 107 (94) | 92 (92) |
| Non-Caucasian (%) | 7 (6) | 8 (8) |
| Marital Status | ||
| Married/Partner (%) | 79 (69) | 64 (64) |
| Single/Widow/Divorced (%) | 35 (31) | 36 (36) |
| Affected with Breast Cancer | ||
| Yes (%) | 44 (39) | 34 (34) |
| No (%) | 69 (61) | 66 (66) |
| Affected with Ovarian Cancer | ||
| Yes (%) | 7 (6) | 15 (15) |
| No (%) | 107 (94) | 85 (85)* |
| Prior Oophorectomy | ||
| Yes (%) | 13 (11.4) | 27 (27) |
| No (%) | 101 (88.6) | 73 (73)** |
| First Degree Relatives with Breast or Ovarian Cancer | ||
| < 2 (%) | 87 (76) | 77 (77) |
| 2+ (%) | 27 (24) | 23 (23) |
| Reached Final Management Decision (at baseline) | ||
| Yes (%) | 56 (49) | 47 (47) |
| No (%) | 58 (51) | 53 (53) |
| Mean Baseline Decisional Conflict (SD) | 31.03 (18.5) | 31.23 (18.4) |
Acknowledgments
Grant support: National Cancer Institute Grant RO1 CA01846.
The authors would like to thank Michael Green, Lisa Moss, and Sharon Hecker for their contributions to this research.
Reference List
- Botkin JR, Smith KS, Croyle RT, Baty BJ, Wylie JE, Dutson D, Chan A, Hamann HA, Lerman C, McDonald J, Venne V, Ward JH, Lyon E. Genetic testing for a BRCA1 mutation: prophylactic surgery and screening behavior in women 2 years post testing. American Journal of Medical Genetics. 2003;118A:201–209. doi: 10.1002/ajmg.a.10102. [DOI] [PubMed] [Google Scholar]
- Breast Cancer Linkage Consortium. Cancer risks in BRCA2 mutation carriers. Journal of the National Cancer Institute. 1999;91:1310–1316. doi: 10.1093/jnci/91.15.1310. [DOI] [PubMed] [Google Scholar]
- Chen S, Parmigiani G. Meta-analysis of BRCA1 and BRCA2 penetrance. Journal of Clinical Oncology. 2007;25:1329–1333. doi: 10.1200/JCO.2006.09.1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards W, Newman J. Multiattribute evaluation, vol. 26. Quantitative Applications in the Social Sciences Series. Thousand Oaks, CA: Sage; 1982. [Google Scholar]
- Gattellari M, Ward JE. A community-based randomised controlled trial of three different educational resources for men about prostate cancer screening. Patient Educ Couns. 2005;57:168–182. doi: 10.1016/j.pec.2004.05.011. [DOI] [PubMed] [Google Scholar]
- Goel V, Sawka CA, Thiel EC, Gort EH, O’Connor AM. Randomized trial of a patient decision aid for choice of surgical treatment for breast cancer. Med Decis Making. 2001;21:1–6. doi: 10.1177/0272989X0102100101. [DOI] [PubMed] [Google Scholar]
- Green MJ, Peterson SK, Baker MW, Harper GR, Friedman LC, Rubinstein WS, et al. Effect of a computer-based decision aid on knowledge, perceptions, and intentions about genetic testing for breast cancer susceptibility: a randomized controlled trial. JAMA. 2004;292:442–452. doi: 10.1001/jama.292.4.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrinton LJ, Barlow WE, Yu O, Geiger AM, Elmore JG, Barton MB, et al. Efficacy of prophylactic mastectomy in women with unilateral breast cancer: a cancer research network project. Journal of Clinical Oncology. 2005;23:4275–4286. doi: 10.1200/JCO.2005.10.080. [DOI] [PubMed] [Google Scholar]
- Holmes-Rovner M, Kroll J, Schmitt N, Rovner DR, Breer ML, Rothert ML, et al. Patient satisfaction with health care decisions: the satisfaction with decision scale. Medical Decision Making. 1996;16:58–64. doi: 10.1177/0272989X9601600114. [DOI] [PubMed] [Google Scholar]
- Kauff ND, Satagopan JM, Robson ME, Scheuer L, Hensley M, Hudis CA, et al. Risk-reducing salpingo-oophorectomy in women with a BRCA1 or BRCA2 mutation. New England Journal of Medicine. 2002;346:1609–1615. doi: 10.1056/NEJMoa020119. [DOI] [PubMed] [Google Scholar]
- Kaufman EM, Peshkin BN, Lawrence WF, Shelby R, Isaacs C, Brown K, et al. Development of an interactive decision aid for female BRCA1/BRCA2 carriers. Journal of Genetic Counseling. 2003;12:109–129. doi: 10.1023/A:1022698112236. [DOI] [PubMed] [Google Scholar]
- Keeney RL, Raiffa H. Decisions with multiple objectives: Preferences and value tradeoffs. Cambridge, MA: Cambridge University Press; 1993. [Google Scholar]
- McDonnell SK, Schaid DJ, Myers JL, Grant CS, Donohue JH, Woods JE, et al. Efficacy of contralateral prophylactic mastectomy in women with a personal and family history of breast cancer. Journal of Clinical Oncology. 2001;19:3938–3943. doi: 10.1200/JCO.2001.19.19.3938. [DOI] [PubMed] [Google Scholar]
- Metcalfe K, Lynch HT, Ghadirian P, Tung N, Olivotto I, Warner E, et al. Contralateral breast cancer in BRCA1 and BRCA2 mutation carriers. Journal of Clinical Oncology. 2004;22:2328–2335. doi: 10.1200/JCO.2004.04.033. [DOI] [PubMed] [Google Scholar]
- Metcalfe KA, Poll A, O’Connor A, Gershman S, Armel S, Finch A, et al. Development and testing of a decision aid for breast cancer prevention for women with a BRCA1 or BRCA2 mutation. Clinical Genetics. 2007;72:208–217. doi: 10.1111/j.1399-0004.2007.00859.x. [DOI] [PubMed] [Google Scholar]
- Molenaar S, Sprangers MA, Rutgers EJ, Luiten EJ, Mulder J, Bossuyt PM, et al. Decision support for patients with early-stage breast cancer: effects of an interactive breast cancer CDROM on treatment decision, satisfaction, and quality of life. Journal of Clinical Oncology. 2001;19:1676–1687. doi: 10.1200/JCO.2001.19.6.1676. [DOI] [PubMed] [Google Scholar]
- National Comprehensive Cancer Network. [Accessed July 17, 2006];NCCN clinical practice guidelines in oncology. Genetic/familial high-risk assessment: Breast and ovarian. Version 1.2006. 2006 Available at: http://www.nccn.org/professionals/physician_gls/PDF/genetics_screening.pdf.
- O’Connor AM. Validation of a decisional conflict scale. Medical Decision Making. 1995;15:25–30. doi: 10.1177/0272989X9501500105. [DOI] [PubMed] [Google Scholar]
- O’Connor AM, Fiset V, DeGrasse C, Graham ID, Evans W, Stacey D, et al. Decision aids for patients considering options affecting cancer outcomes: evidence of efficacy and policy implications. Journal of the National Cancer Institute Monographs. 1999:67–80. doi: 10.1093/oxfordjournals.jncimonographs.a024212. [DOI] [PubMed] [Google Scholar]
- O’Connor AM, Jacobsen MJ, Stacey D. An evidence-based approach to managing women’s decisional conflict. J Obstet Gynecol Neonatal Nurs. 2002;31:570–581. doi: 10.1111/j.1552-6909.2002.tb00083.x. [DOI] [PubMed] [Google Scholar]
- O’Connor AM, Stacey D, Entwistle V, Llewellyn-Thomas H, Rovner D, Holmes-Rovner M, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database of Systematic Reviews (Online) 2003:CD001431. doi: 10.1002/14651858.CD001431. [DOI] [PubMed] [Google Scholar]
- O’Connor AM, Tugwell P, Wells GA, Elmslie T, Jolly E, Hollingworth G, et al. A decision aid for women considering hormone therapy after menopause: decision support framework and evaluation. Patient Education and Counseling. 1998;33:267–279. doi: 10.1016/s0738-3991(98)00026-3. [DOI] [PubMed] [Google Scholar]
- Peralta EA, Ellenhorn JD, Wagman LD, Dagis A, Andersen JS, Chu DZ. Contralateral prophylactic mastectomy improves the outcome of selected patients undergoing mastectomy for breast cancer. American Journal of Surgery. 2000;180:439–445. doi: 10.1016/s0002-9610(00)00505-5. [DOI] [PubMed] [Google Scholar]
- Peshkin BN, Schwartz MD, Isaacs C, Hughes C, Main D, Lerman C. Utilization of mammography in a clinically-based sample of women following BRCA1/2 testing. Cancer Epidemiology, Biomarkers and Prevention. 2002;11:1115–1118. [PubMed] [Google Scholar]
- Rebbeck TR, Lynch HT, Neuhausen SL, Narod SA, Van’t Veer L, Garber JE, et al. Prophylactic oophorectomy in carriers of BRCA1 or BRCA2 mutations. New England Journal of Medicine. 2002;346:1616–1622. doi: 10.1056/NEJMoa012158. [DOI] [PubMed] [Google Scholar]
- Schwartz MD, Benkendorf J, Lerman C, Isaacs C, Ryan-Robertson A, Johnson L. Impact of educational print materials on knowledge, attitudes, and interest in BRCA1/BRCA2: testing among Ashkenazi Jewish women. Cancer. 2001;92:932–940. doi: 10.1002/1097-0142(20010815)92:4<932::aid-cncr1403>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Schwartz MD, Peshkin BN, Hughes C, Main D, Isaacs C, Lerman C. Impact of BRCA1/BRCA2 mutation testing on psychologic distress in a clinic-based sample. J Clin Oncol. 2002;20:514–520. doi: 10.1200/JCO.2002.20.2.514. [DOI] [PubMed] [Google Scholar]
- Schwartz MD, Peshkin BN, Tercyak KP, Taylor KL, Valdimarsdottir H. Decision making and decision support for hereditary breast-ovarian cancer susceptibility. Health Psychol. 2005;24:S78–S84. doi: 10.1037/0278-6133.24.4.S78. [DOI] [PubMed] [Google Scholar]
- Smith KL, Robson ME. Update on hereditary breast cancer. Current Oncology Reports. 2006;8:14–21. doi: 10.1007/s11912-006-0004-x. [DOI] [PubMed] [Google Scholar]
- Tilanus-Linthorst M, Verhoog L, Obdeijn IM, Bartels K, Menke-Pluymers M, Eggermont A, et al. A BRCA1/2 mutation, high breast density and prominent pushing margins of a tumor independently contribute to a frequent false-negative mammography. International Journal of Cancer. 2002;102:91–95. doi: 10.1002/ijc.10666. [DOI] [PubMed] [Google Scholar]
- van Roosmalen MS, Stalmeier PF, Verhoef LC, Hoekstra-Weebers JE, Oosterwijk JC, Hoogerbrugge N, et al. Randomized trial of a shared decision-making intervention consisting of trade-offs and individualized treatment information for BRCA1/2 mutation carriers. Journal of Clinical Oncology. 2004a;22:3293–3301. doi: 10.1200/JCO.2004.05.066. [DOI] [PubMed] [Google Scholar]
- van Roosmalen MS, Stalmeier PF, Verhoef LC, Hoekstra-Weebers JE, Oosterwijk JC, Hoogerbrugge N, et al. Randomised trial of a decision aid and its timing for women being tested for a BRCA1/2 mutation. British Journal of Cancer. 2004b;90:333–342. doi: 10.1038/sj.bjc.6601525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk RJ, Spann SJ, Cass AR, Hawley ST. Patient education for informed decision making about prostate cancer screening: a randomized controlled trial with 1-year follow-up. Ann Fam Med. 2003;1:22–28. doi: 10.1370/afm.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakefield CE, Meiser B, Homewood J, Peate M, Taylor A, Lobb E, Kirk J, Young M, Williams R, Dudding T, Tucker K and the AGenDA Collaborative Group. A randomized controlled trial of a decision aid for women considering genetic testing for breast and ovarian cancer risk. Breast Cancer Research and Treatment. 2007 doi: 10.1007/s10549-007-9539-2. Published Online ahead of print. [DOI] [PubMed] [Google Scholar]
- Waljee JF, Rogers MA, Alderman AK. Decision aids and breast cancer: do they influence choice for surgery and knowledge of treatment options? Journal of Clinical Oncology. 2007;25:1067–1073. doi: 10.1200/JCO.2006.08.5472. [DOI] [PubMed] [Google Scholar]
- Whelan T, Levine M, Willan A, Gafni A, Sanders K, Mirsky D, et al. Effect of a decision aid on knowledge and treatment decision making for breast cancer surgery: a randomized trial. JAMA. 2004;292:435–441. doi: 10.1001/jama.292.4.435. [DOI] [PubMed] [Google Scholar]