Abstract
Objectives
Our aim was to investigate the safety and efficacy of intravenous allogeneic human mesenchymal stem cells (hMSCs) in patients with myocardial infarction (MI).
Background
Bone marrow-derived hMSCs may ameliorate consequences of MI, and have the advantages of preparation ease, allogeneic use due to immunoprivilege, capacity to home to injured tissue, and extensive pre-clinical support.
Methods
We performed a double-blind, placebo-controlled, dose-ranging (0.5, 1.6, and 5 million cells/kg) safety trial of intravenous allogeneic hMSCs (Prochymal, Osiris Therapeutics, Inc., Baltimore, Maryland) in reperfused MI patients (n = 53). The primary end point was incidence of treatment-emergent adverse events within 6 months. Ejection fraction and left ventricular volumes determined by echocardiography and magnetic resonance imaging were exploratory efficacy end points.
Results
Adverse event rates were similar between the hMSC-treated (5.3 per patient) and placebo-treated (7.0 per patient) groups, and renal, hepatic, and hematologic laboratory indexes were not different. Ambulatory electrocardiogram monitoring demonstrated reduced ventricular tachycardia episodes (p = 0.025), and pulmonary function testing demonstrated improved forced expiratory volume in 1 s (p = 0.003) in the hMSC-treated patients. Global symptom score in all patients (p = 0.027) and ejection fraction in the important subset of anterior MI patients were both significantly better in hMSCs versus placebo subjects. In the cardiac magnetic resonance imaging substudy, hMSC treatment, but not placebo, increased left ventricular ejection fraction and led to reverse remodeling.
Conclusions
Intravenous allogeneic hMSCs are safe in patients after acute MI. This trial provides pivotal safety and provisional efficacy data for an allogeneic bone marrow-derived stem cell in post-infarction patients. (Safety Study of Adult Mesenchymal Stem Cells [MSC] to Treat Acute Myocardial Infarction; NCT00114452)
Keywords: magnetic resonance imaging, echocardiography, allogeneic, mesenchymal stem cells
Cell-based therapies for myocardial infarction (MI) are currently under evaluation and are emerging as a promising new therapy (1). Trials indicate that intracoronary delivery of bone marrow mononuclear cells (BMCs) improves ejection fraction (2–4) and other clinical markers (3,5,6). The use of BMCs is limited, however, by the need to obtain a bone marrow aspirate from each individual patient, and may be further complicated by host factor variability in the quality and ability of the BMCs to achieve the desired effects (7).
Pre-cultured bone marrow-derived human mesenchymal stem cells (hMSCs) represent an alternative approach to cardiovascular cell therapy that has a number of advantages when compared with autologous BMCs (8). First, MSCs can be infused intravenously post-MI due to their ability to home to areas of injury within the myocardium, stimulated, at least in part, by the SDF-1/CXCL12 axis (9,10). Second, they may be used as an allogeneic graft as they lack various major histocompatibility complex and costimulatory cell-surface antigens, and secrete anti-inflammatory cytokines. Finally, they represent an enriched population of cells with therapeutic properties, as demonstrated in pre-clinical studies (8). While the use of these cells is highly promising and supported by pre-clinical studies, there are also safety concerns regarding their application, including concerns regarding tumor (11), ectopic tissue formation (12), and organ toxicity resulting from unwanted lodgment in the microvasculature (13).
To address these concerns, and to establish a basis for future efficacy trials, we performed a double-blind, placebo-controlled, dose-ranging study of allogeneic adult bone marrow-derived hMSCs in patients after an acute MI. The purpose of this trial was to assess the safety profile of allogeneic hMSCs administered intravenously to patients after acute MI.
Methods
Trial design
This study was a phase I randomized, double-blind, placebo-controlled, dose-escalation, multicenter trial to evaluate the safety of allogeneic bone marrow-derived hMSC administration for patients experiencing a first acute MI. A total of 53 patients enrolled at 10 participating study centers completed the trial through the 6-month time point, with safety and exploratory efficacy end points evaluated at 1-, 2-, 3-, and 6-month follow-up visits. The trial was conducted in compliance with current Good Clinical Practice standards and in accordance with the principles set forth under the Declaration of Helsinki (1989). Institutional review board approvals of the treatment protocol were obtained at all centers before the initiation of patient enrollment. All patients entering the trial agreed to and signed an institutional review board-approved statement of informed consent.
Composition of active investigational agent and placebo suspension
The active investigational agent was hMSCs isolated from bone marrow aspirates obtained from a single unrelated donor who was not human-leukocyte-antigen–matched to recipients. The hMSCs in Prochymal (Osiris Therapeutics, Inc., Baltimore, Maryland) were manufactured in a manner consistent with International Conference on Harmonization and Food and Drug Administration regulatory guidelines. The donor was tested according to the Food and Drug Administration Donor Suitability Guidance before donation. The phenotype of the hMSC population was characterized by a cell-surface profile of CD105+, CD166+, and CD45−. The infused Prochymal formulation consists of 2.5 × 106 hMSCs/ml, 1.9% human serum albumin, and 3.8% dimethyl sulfoxide in PlasmaLyte A (Baxter, Deerfield, Illinois). Patients assigned to placebo groups received a solution of 1.9% human serum albumin with 3.8% dimethyl sulfoxide in PlasmaLyte A. Release testing of the cells included measurement of cell-surface markers CD105 and CD166, absence of CD45, testing for mycoplasma, sterility, endotoxin, identity, purity, and viability and karyotyping to exclude chromosomal abnormalities. The final viability was at least 70% viable MSCs, as determined by Trypan blue (generic) testing. During the course of this cardiac study, the hMSC preparation under evaluation was referred to as “Provacel,” which is the same investigational agent as Prochymal, a highly purified preparation of ex vivo cultured adult hMSCs.
Treatment groups and infusion parameters
All patients received a single intravenous infusion of Prochymal or placebo suspension delivered at a rate of 2 ml/min. The safety and tolerability of Prochymal infusion were evaluated in 3 placebo-controlled dose escalation cohorts of 0.5, 1.6, and 5.0 × 106 hMSCs/kg body weight. Subjects were randomly assigned in a double-blind fashion to each cohort in a 2:1 ratio of Prochymal to placebo. Intravenous tubing was coated with an opaque wrap so as to obscure any identifying features of the infusion. All patients in each of the cohorts met identical inclusion and exclusion criteria. The cohorts were enrolled sequentially. Advancement to the next dose cohort was contingent on an unblinded review of safety data accumulated to that date by an independent Data Safety and Monitoring Board.
Patient population
To be eligible for trial entry, patients must have had a first MI (either ST-segment elevation or non–ST-segment elevation) 1 to 10 days before randomization. All patients were required to have a patent infarct-related artery on coronary angiography at the time of randomization. Angiographic measures included epicardial blood flow and myocardial perfusion, as assessed by Thrombolysis In Myocardial Infarction (TIMI) flow grade (14) and TIMI myocardial perfusion grade (15), respectively.
The index MI for all patients enrolled met the following criteria: 1) elevation of >2× upper limit of normal of creatine kinase-MB or troponin; 2) presence of regional wall motion abnormality; and 3) global left ventricular ejection fraction (LVEF) of ≤60% and ≥30% as determined by 16-segment echocardiogram or ventriculogram.
A total of 60 subjects were screened for enrollment in the trial, of which 53 were treated with the investigational agent or placebo. The Data Safety and Monitoring Board approved enrollment of each escalating dose cohort, and an additional cohort was enrolled at the highest dose. Patient enrollment occurred between March 2005 and March 2006.
All patients were hemodynamically stable before randomization, defined as the absence over a 24-h period of: 1) the need for parenteral inotropic support; 2) systolic blood pressure <80 mm Hg for longer than 1 h; and 3) resting heart rate >100 beats/min for longer than 1 h. Adequate pulmonary function to meet entry criteria was defined as a forced expiratory volume in 1 s (FEV1) >50% predicted and peripheral artery oxygen saturation ≥97%. At enrollment, all patients had Karnofsky performance status scores of ≥60.
Patients were not enrolled in the trial if revascularization via coronary artery bypass surgery was required or if the physician anticipated further coronary revascularization procedures during the 6-month study period. Patients with clinically significant primary valvular heart disease or evidence of life-threatening arrhythmia on baseline electrocardiogram (ECG) were also excluded.
Patient enrollment and randomization
Patients were enrolled by study investigators. Randomization, stratified by dose cohort, was conducted in a centralized manner and communicated to cellular therapy laboratory personnel. Subjects were randomly assigned to either Prochymal or placebo in a 2:1 ratio within each cohort. Manual randomization was performed using sealed envelopes.
Assessments
Physicians and other clinical personnel remained blinded to treatment assignment for all patients throughout the study period. Primary safety assessments included monitoring and recording of all adverse events (AEs) and serious AEs. Safety laboratory and urine values, regular vital sign measurements, and physical examination results were recorded as well. Peripheral artery blood oxygen saturation during Prochymal infusion was monitored by pulse oximetry. To follow the occurrence of post-infusion arrhythmias, 12-lead ECGs, 24-h ambulatory ECG recording, and telemetry monitoring were performed. Telemetry was performed continuously in the 4 days after the procedure, and patients received 24-h ambulatory monitoring at the 1-, 2-, 3-, and 6-month follow-up visits. Nonsustained ventricular tachycardia (VT) was defined as 3 consecutive beats at a rate of >100 beats/min. Contrast-enhanced computed tomography scans of the chest, abdomen, and pelvis were utilized to identify any evidence of ectopic tissue formation.
A 16-segment echocardiography was performed to assess LVEF. Multiple views were recorded, including the subcostal, parasternal long- and short-axis, and apical 2- and 4-chamber views. Parasternal short-axis views were recorded at the basal (mitral valve level), mid (papillary muscle level), and apical positions. Subject angulation and transducer position were recorded for serial examinations. Contrast administration was used for enhancement of the endocardial border. End-diastolic wall thickness was measured from the parasternal long- and short-axis views. Wall motion analysis was performed using the 16-segment model proposed by the American Society of Echocardiography (16). Each wall segment was scored using a visual grading system (1 = normal, 2 = hypokinetic, 3 = akinetic, 4 = dyskinetic, and 5 = aneurysmal). The wall motion score index, defined as the average wall motion score for all segments divided by the number of segments analyzable, was determined for each reading. The percentage of wall motion abnormalities was obtained by dividing the number of akinetic, dyskinetic, and aneurysmal segments by the total number of segments evaluated. Left ventricular volumes were determined at end-diastole and -systole. Endocardial borders were manually traced from apical 4- and 2-chamber views, and the volumes obtained were used to calculate LVEF using the biplane summation-of-disks method recommended by the American Society of Echocardiography (16).
A cardiac magnetic resonance imaging (MRI) substudy was performed in a subset of patients. MRI was carried out before hMSC or placebo infusion, after primary coronary intervention, and during the 3- and 6-month follow-up visits. An additional 12-month cardiac MRI was obtained and is also reported here. Baseline MRI scans were limited to nonstress evaluations. MRI scans were evaluated offline at an imaging core (the Netherlands). LVEF was measured according to the validated National Heart, Lung, and Blood Institute-Laboratory of Cardiac Energetics laboratory standard (17), using contiguous short-axis slices obtained by cine MRI. End-diastolic and -systolic endocardial traces were used to determine end-systolic and -diastolic left ventricular volumes and total ejection fraction.
To assess pulmonary function, spirometry tests were performed throughout the 6-month period after treatment. These evaluations were carried out according to American Thoracic Society guidelines (18). Predicted values for FEV1 were calculated using published formulae (19).
A 6-min walk test was performed at randomization and during follow-up visits. The procedure for the 6-min walk test followed the American Thoracic Society guidelines (20). Distance walked in 6 min along a 30-m (100-ft) hallway was recorded.
A global assessment of overall patient health was determined by the investigator from subject interviews at day 10 and during the 6-month period after treatment. Global status of the subject was evaluated relative to pre-treatment using the following categorical ratings: improved, unchanged, and worsened.
Data collection
All data were recorded on case report forms and verified by comparison with source documentation by third-party medical monitors. Incidence summaries are reported as subject counts and percent of treatment group. Safety assessments based on the frequency of AEs and on clinically significant abnormal laboratory values were performed. AEs are summarized as the number and percentage of subjects experiencing an AE within each treatment group.
Statistical analysis
Summaries of continuous measures are presented as the mean and SD. Proportional analyses were used to identify differences in premature ventricular contraction (PVC) incidence and global assessment. Statistical significance in proportional differences (Tables 1 and 2) was analyzed using the Fisher exact test (2-tailed). Where indicated, measures of clinical efficacy are reported as the percent change from baseline evaluation. Echocardiographic and 6-min walk data are presented with means and 95% confidence intervals. Comparisons of changes from baseline conditions were analyzed using the Student t test (2-tailed, paired). Statistical differences in averaged group results were compared by Student t test (2-tailed, homoscedastic) with the Bonferroni correction. Where multiple comparisons were performed, analysis of variance (ANOVA) with repeated measures and Student-Newman-Keuls post-hoc testing was employed for within-group analysis and between-group (treatment vs. placebo) comparisons used 3-way ANOVA with terms for group and a group × time interaction term. Where appropriate, post-hoc analysis with the Bonferroni correction was applied. Testing was performed at a 95% significance level.
Table 1.
Patient Demographics and Primary Baseline Data
| hMSC | Placebo | p Value | |
|---|---|---|---|
| Randomized | 39 | 21 | |
| Completed | 34 (87.2%) | 19 (90.5%) | 1.000 |
| Age, yrs | 59.0 (12.3) | 55.1 (10.2) | 0.244 |
| Male | 28 (82.4%) | 15 (78.9%) | 1.000 |
| Race/ethnicity | |||
| Caucasian | 30 (88.2%) | 16 (84.2%) | 0.691 |
| Hispanic | 3 (8.8%) | 2 (10.5%) | 1.000 |
| Asian | 1 (2.9%) | 1 (5.3%) | 1.000 |
| BMI, kg/m2 | 29.8 (6.7) | 30.3 (4.3) | 0.800 |
| Obesity | 4 (11.8%) | 0 (0%) | 0.284 |
| Diabetes | 6 (17.6%) | 1 (5.3%) | 0.400 |
| Hypertension | 16 (47.1%) | 9 (47.4%) | 1.000 |
| Current smoker | 3 (8.8%) | 2 (10.5%) | 1.000 |
| Former smoker | 1 (2.9%) | 2 (10.5%) | 0.290 |
| Target/index vessel | |||
| LAD | 15 (44.1%) | 9 (47.4%) | 1.000 |
| RCA | 13 (38.2%) | 5 (26.3%) | 0.547 |
| Obtuse marginal | 2 (5.9%) | 1 (5.3%) | 1.000 |
| Diagonal | 2 (5.9%) | 0 (0.0%) | 0.531 |
| Circumflex | 2 (5.9%) | 1 (5.3%) | 1.000 |
| LAD/diagonal | 0 (0.0%) | 1 (5.3%) | 0.359 |
| LAD/RCA | 0 (0.0%) | 1 (5.3%) | 0.359 |
| RCA/circumflex | 0 (0.0%) | 1 (5.3%) | 0.359 |
| Time to reperfusion, h | 9.8 (10.4) | 11.7 (14.2) | 0.578 |
| Baseline LVEF, % | 50.4 (10.6) | 48.7 (9.6) | 0.571 |
| TIMI risk score | 3.6 (2.2) | 2.8 (1.3) | 0.206 |
| TIMI myocardial perfusion grade |
2.3 (1.1) | 2.4(1.1) | 0.828 |
| TIMI flow grade | 2.5 (1.0) | 2.7(1.0) | 0.467 |
| FEV1, % predicted | 74.0 (15.6) | 76.7 (17.6) | 0.566 |
| CK-MB (μg/l) | |||
| Average | 134.6 (125.4) | 160.1 (131.9) | 0.490 |
| Low | 3.2 | 5.3 | |
| High | 429.1 | 457 | |
| ST-segment elevation, % | 79.4 | 68.4 | 1.000 |
Values are presented as n, n (%), or mean (SD).
BMI = body mass index; CK-MB = creatine kinase-MB; FEV1 = forced expiratory volume in 1 s; hMSC = human mesenchymal stem cell; LAD = left anterior descending coronary artery; LVEF = left ventricular ejection fraction; RCA = right coronary artery; TIMI = Thrombolysis In Myocardial Infarction.
Table 2.
AEs and Rehospitalization Summary
| hMSC | Placebo | p Value | |
|---|---|---|---|
| Total AEs | 181 | 132 | |
| Average AEs per patient | 5.3 | 7.0 | |
| Subjects with at least 1 AE | 33 (97.1) | 19 (100.0) | 1.000 |
| Cardiac disorders | 15 (44.1) | 9 (47.4) | 0.999 |
| Gastrointestinal disorders | 9 (26.5) | 4 (21.1) | 0.749 |
| General disorders and administration site conditions (chest pain, fatigue, and so on) |
14 (41.2) | 13 (68.4) | 0.086 |
| Immune system disorders | 2 (5.9) | 0(0) | 0.531 |
| Infections and infestations | 11 (32.4) | 5 (26.3) | 0.760 |
| Rehospitalizations | |||
| Total | 9 | 7 | |
| Average per patient | 0.26 | 0.37 | |
| Subjects requiring at least 1 rehospitalization | 8 (23.5) | 6 (31.6) | 0.535 |
| Average time to rehospitalization, days | 120 | 66 | |
| Arrhythmias | |||
| Arrhythmic events | 7 | 12 | |
| Subjects with at least 1 arrhythmic event | 3 (8.8) | 7 (36.8) | 0.025 |
| Ventricular tachycardia | 1(2.9) | 5 (26.3) | 0.018 |
Values are presented as n or n (%).
AE = adverse event; hMSC = human mesenchymal stem cell.
Results
Patients
Demographic and baseline patient data are listed in Table 1 and Figure 1 and demonstrate similar distributions of age, sex, race, and body mass index; prevalences of obesity, diabetes, smoking, and hypertension; and time post-MI to infusion in the patient groups. Baseline LVEF, TIMI risk score, and FEV1 were also similar at trial entry for those patients assigned to active therapy or placebo infusion.
Figure 1. Time After MI to Infusion of Study Agent.
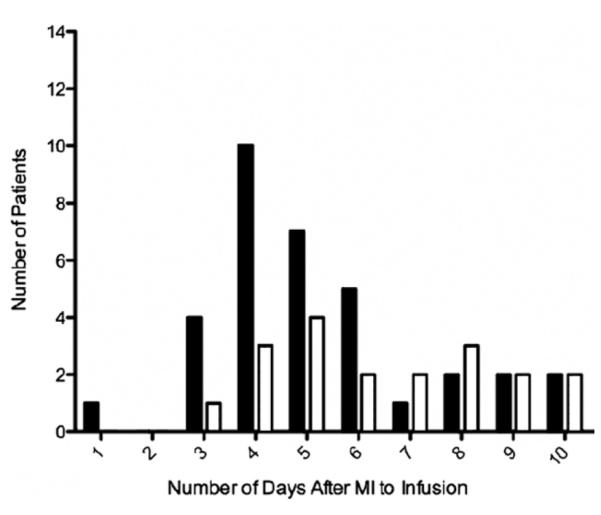
Human mesenchymal stem cells = solid bars; placebo = open bars. MI = myocardial infarction.
AEs
Over the course of the study, 313 AEs were recorded (Table 2). There was no trend within any AE class that indicated a propensity toward an increased incidence in the cell-treated group. No significant trends in AE incidence or in efficacy results were identified among the different dosing cohorts. Accordingly, safety and efficacy data for all 3 hMSC dose cohorts and all placebo cohorts are combined in this report. There was no evidence of increased toxicity with the administration of hMSCs compared with placebo, and administration was well tolerated at all cell dose levels. There were no deaths during the study, and no subjects discontinued from study treatment because of an AE. In addition, no AEs were considered to have a probable relation to study treatment.
There was no evidence of an increased incidence of ectopic tissue growth determined using whole body computed tomography scanning. In a few cases (2 in the cell-treated and 1 in the placebo group), evidence of pre-existing ectopic tissue masses present at baseline were noted by the investigator.
AE rates in hMSC-treated patients were not greater than in placebo-treated patients (5.3 vs. 7.0 AEs per patient, respectively) (Table 2). Furthermore, the serious AE rate was 23.5% (n = 9 events in 8 subjects) in the hMSC group and 31.6% (n = 7 events in 6 subjects) in the placebo group. A similar nonstatistical trend was observed with regard to hospitalization rates (Table 2), which was 31.6% at an average of 66 days after discharge in the placebo group versus 23.5% at an average of 120 days after discharge in the hMSC group.
Arrhythmias
Specific safety monitoring of arrhythmias during the course of the trial revealed improved outcomes in the hMSC-treated group compared with the placebo group. All patients enrolled in the trial underwent ambulatory monitoring to detect the occurrence of any cardiac arrhythmias. The arrhythmia event rate in the patients who received hMSCs was 4-fold lower than that in the patients who received placebo (8.8% vs. 36.8%, p = 0.025, Fisher exact test) (Table 2).
Consistent with findings related to arrhythmic events in general, similar results were observed with regard to the frequency of VT and number of PVCs recorded for hMSC-versus placebo-treated patients. Only a single patient (2.9%) in the hMSC-treated group, versus 6 episodes in 5 patients (26.3%) in the placebo group, experienced VT (p = 0.018). Subjects treated with hMSCs also had fewer PVCs at all time points after day 10 (Fig. 2). The percentage of patients who experienced more than 10 PVCs per hour for all time points in the trial was also significantly less in those patients who received hMSCs as compared with those who received placebo (10% vs. 24%, p = 0.001). This difference was most pronounced at 1 (6% vs. 32%, p = 0.040) and 2 months (9% vs. 38%, p = 0.043) after MI.
Figure 2. Percentage of Patients With >10 PVCs per Hour.
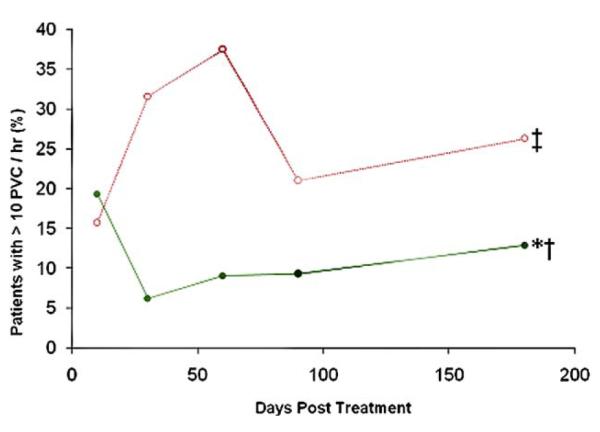
Human mesenchymal stem cells = green line (n = 32 at 3 months, n = 33 at 6 months); placebo = red line (n = 19 at 3 months, n = 16 at 6 months). *p = 0.001 by repeated measure analysis of variance; †p < 0.05 versus days 1 and 90; ‡p = 0.017 versus placebo. PVC = premature ventricular contraction.
Echocardiographic data
Echocardiography revealed improvements in LVEF in hMSC-treated patients (Table 3). Baseline LVEF was similar between patient groups (50.4% in Provacel-treated patients, n = 34, and 48.7% in placebo-treated patients, n = 19; p = 0.561). Overall, patients treated with hMSCs experienced a 5.9 ± 1.8% increase in LVEF at 3 months (p = 0.003 vs. baseline, n = 33) compared with a 4.4 ± 1.8% increase in the placebo group (p = 0.021 vs. baseline, n = 19). This effect was maintained through 6 months, at which time LVEF was increased by 6.7 ± 2.2% over baseline in hMSC-treated patients (p = 0.004 vs. baseline, n = 30). The 6-month echocardiographic ejection fraction was not different between placebo-and cell-treated patients (Table 3).
Table 3.
Ejection Fraction and 6-Min Walk Test
| hMSC Mean (95% CI) |
Placebo Mean (95% CI) |
p Value | |
|---|---|---|---|
| Ejection fraction (all patients), % | |||
| Baseline | 50.4 (46.9–54.0) | 48.7 (44.4–53.1) | 0.561 |
| 3 months | 56.7 (54.0–59.3) | 53.1 (49.8–56.4) | 0.108 |
| 6 months | 56.9 (53.3–60.5) | 56.1 (53.3–58.9) | 0.737 |
| Ejection fraction (anterior MIs), % | |||
| Baseline | 48.3 (42.0–54.5) | 53.2 (48.6–57.8) | 0.225 |
| 3 months | 55.9 (51.7–60.0) | 56.1 (51.6–60.7) | 0.937 |
| 6 months | 55.1 (50.4–59.8) | 57.0 (53.1–60.9) | 0.549 |
| 6-min walk, distance walked, ft | |||
| Baseline | 1,145 (1,037–1,254) | 1,074 (931–1,218) | 0.443 |
| 3 months | 1,437 (1,326–1,548) | 1,310 (1,143–1,477) | 0.220 |
| 6 months | 1,490 (1,381–1,599) | 1,401 (1,217–1,584) | 0.419 |
CI = confidence interval; hMSC = human mesenchymal stem cell; MI = myocardial infarction.
When only patients with anterior wall MIs were examined, the impact of cell therapy relative to placebo was more pronounced (Fig. 3): patients treated with hMSCs had a 7.0 ± 3.5% point improvement in LVEF at 3 months (p = 0.056 vs. baseline, n = 14) and a 7.3 ± 3.4% increase at 6 months relative to baseline (p = 0.044, n = 12). In contrast, increases in ejection fraction in the placebo group, 2.9 ± 2.5% at 3 months and 3.4 ± 3.4% at 6 months, were not statistically significant (p = NS for both time points; n = 9 at 3 months, n = 8 at 6 months).
Figure 3. Increase From Baseline Values in Percent LVEF at 3 and 6 Months Post-Treatment in Patients With Anterior MI.
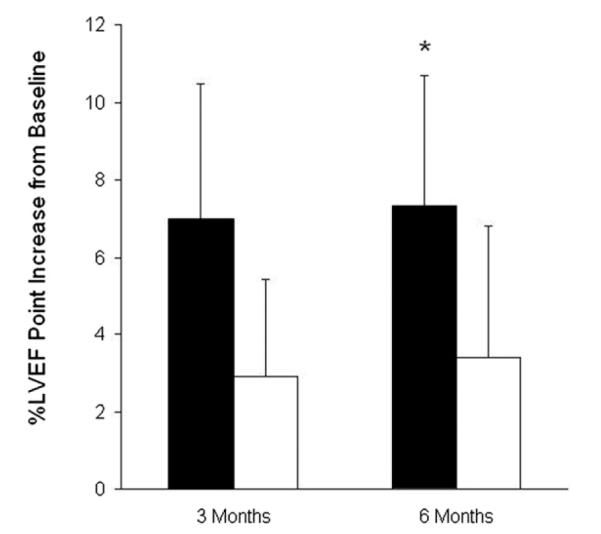
Human mesenchymal stem cells = solid bars (n = 17 at 3 months, n = 15 at 6 months); placebo = open bars (n = 10 at 3 months, n = 9 at 6 months). *p = 0.0436 at 6 months for human mesenchymal stem cell-treated patients versus baseline, analysis of variance. LVEF = left ventricular ejection fraction; MI = myocardial infarction.
There were no significant differences between hMSC-and placebo-treated patients with regard to measures of wall thickness, wall motion score index, and percentage of wall motion abnormalities at baseline. Over the follow-up period, evidence of prevention of wall thinning and reduction in percentage of wall motion abnormalities was observed in hMSC-treated patients (data not shown).
MRI substudy
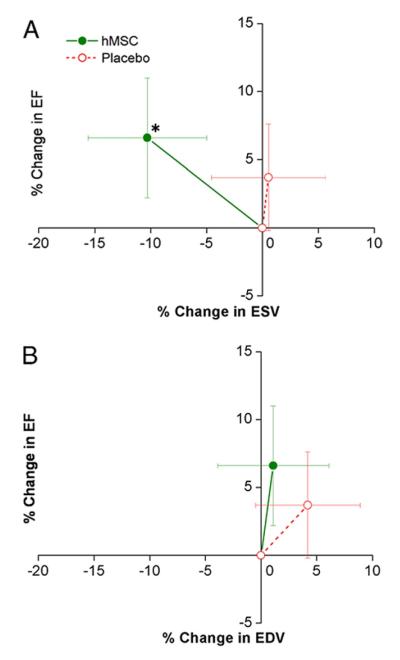
Cardiac MRI was performed in a subgroup of patients (n = 20 hMSC and n = 14 placebo). Baseline LVEF was similar in hMSC and placebo patients (47.3 ± 3.3% and 45.2 ± 3.4%, respectively). MRI evaluation revealed a progressive increase in LVEF relative to baseline at 3 and 6 months in hMSC-but not in placebo-treated patients (Table 4, Fig. 4). Moreover, this difference continued through the 12-month follow-up evaluation, at which time LVEF had increased by 5.2 ± 1.9% (p = 0.003, by repeated measure ANOVA). LVEF in the placebo-treated patients did not increase, and the difference in change between groups was not significant. MRI also allows precise measurement of left ventricular chamber volumes: when plotted relative to ejection fraction, hMSC patients exhibited evidence of reverse remodeling with no increase in left ventricular end-diastolic volume and a decline in left ventricular end-systolic volume, whereas placebo patients demonstrated evidence of left ventricular chamber enlargement (Table 4, Figs. 5A and 5B). The changes in ejection fraction and end-systolic volume did not correlate with baseline end-systolic volume (r < 0.316, p = 0.10).
Table 4.
MRI Findings at Baseline and Absolute Changes at 3, 6, and 12 Months (Mean ± SEM)
| Baseline |
Change at 3 Months |
Change at 6 Months |
Change at 12 Months |
|||||
|---|---|---|---|---|---|---|---|---|
| hMSC | Placebo | hMSC | Placebo | hMSC | Placebo | hMSC | Placebo | |
| Ejection fraction, % | 47.3 ± 1.7 | 45.1 ± 1.7 | 2.6 ± 1.7 | −0.1 ± 1.8 | 2.2 ± 1.6 | −0.7 ± 1.9 | 5.2 ± 1.9* | 1.8 ± 1.5 |
| End-diastolic left ventricular volume, ml | 180.6 ± 11.5 | 202.0 ± 14.7 | 1.9 ± 5.0 | 7.9 ± 12.0 | 9.3 ± 6.5 | 14.0 ± 9.3 | 2.8 ± 8.1 | 10.2 ± 9.9 |
| End-systolic left ventricular volume, ml |
95.3 ± 7.1 | 112.6 ± 10.4 | −3.2 ± 3.2 | 5.8 ± 9.5 | 2.8 ± 4.6 | 10.2 ± 8.2 | −4.3 ± 6.6 | 2.4 ± 6.6 |
p = 0.005 versus baseline.
hMSC = human mesenchymal stem cell; MRI = magnetic resonance imaging.
Figure 4. Impact of hMSC Treatment on LVEF Evaluated by Cardiac MRI.
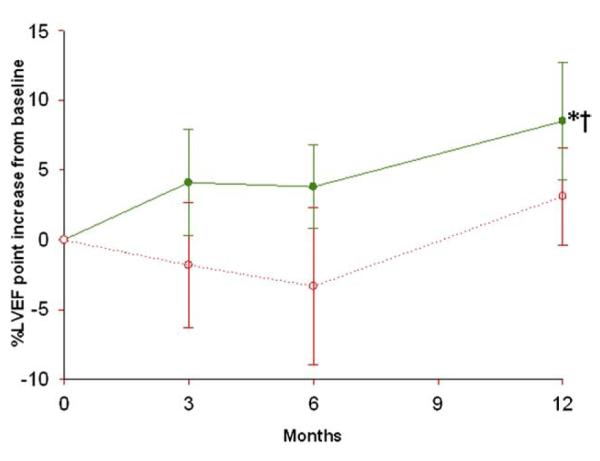
Human mesenchymal stem cells (hMSCs) = green line (n = 21 at 3 months, n = 18 at 6 and 12 months); placebo = red line (n = 13 at 3 months, n = 11 at 6 and 12 months). *p = 0.003 by repeated measure analysis of variance; †p = 0.005 versus baseline. Abbreviations as in Figure 3.
Figure 5. Impact of hMSC Treatment on LV Remodeling.
Changes in left ventricular (LV) ejection fraction (EF) are plotted against the changes in LV end-systolic volume (ESV) (A) and end-diastolic volume (EDV) (B) during follow-up. Human mesenchymal stem cell (hMSC) patients (n = 21 at 3 months, n = 18 at 6 and 12 months) exhibit evidence of reverse remodeling with no increase in LV EDV and a decline in LV ESV, whereas placebo patients (n = 13 at 3 months, n = 11 at 6 and 12 months) demonstrate evidence of LV chamber enlargement. *p = 0.005 versus baseline.
Pulmonary function
Pulmonary function tests were performed to monitor subjects for potential adverse changes related to study treatment. Figure 6 shows the average change in FEV1 percent predicted in trial subjects over time. Compared with patients receiving placebo, those treated with hMSCs showed a greater increase in percent predicted FEV1, relative to baseline values, from 3 days through the 6 months post-infusion. At 10 days, this difference in the increase in percent predicted FEV1 observed for hMSC versus placebo patients was statistically significant (p = 0.01, Bonferroni). Furthermore, the 16% improvement in hMSC-treated patients at 6 months post-infusion was statistically significant when compared with the baseline values (p = 0.003, by repeated measure ANOVA).
Figure 6. Difference From Baseline in FEV1 % Predicted.
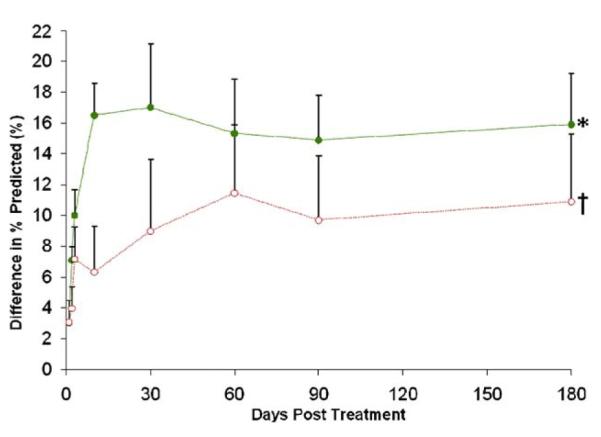
Human mesenchymal stem cells = green line (n = 31); placebo = red line (n = 18). Error bars represent standard error of the mean. *p = 0.003 by repeated measure analysis of variance; †p = 0.01 versus placebo. FEV1 = forced expiratory volume in 1 s.
6-min walk
Six-min walk data are presented in Table 3. As indicated, walk duration did not differ between the 2 groups.
Global assessment
The overall health of study subjects was followed by investigators during the course of the trial for signs of improvement or deterioration. In this global assessment of patient health, hMSC-treated patients received higher scores than did patients in the placebo group at all time points analyzed (Fig. 7). Significantly more patients in the hMSC group were judged to have improved overall condition at 6 months as compared with those receiving placebo (42% vs. 11%, p = 0.027, Fisher exact test). While the proportion of hMSC-treated patients reported by their physicians as improved remained consistently high throughout the study, the proportion of patients in the placebo group that were judged improved steadily decreased, from 47% at 10 days to 11% at 6 months (p = 0.016, Fisher exact test).
Figure 7. Percentage of Patients With Improved Global Assessment.
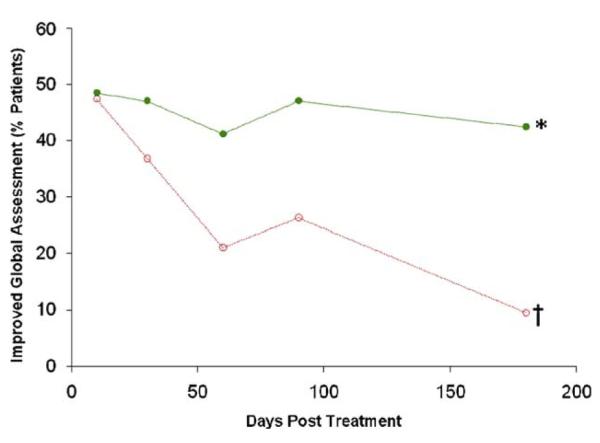
Human mesenchymal stem cells = green line; placebo = red line. *p = 0.027 between groups, Fisher exact test; †p = 0.016 versus day 10, Fisher exact test.
Dose-response
When analyzed for dose-dependent effects, the parameter that exhibited clear dose-responsiveness was PVC count, which did not differ between the placebo and low-dose cell groups, but was evident in the mid- and high-dose groups. All other parameters examined did not exhibit dose-responsiveness.
Discussion
This double-blind, placebo-controlled, randomized, dose-ranging phase I study reports on the first use of allogeneic bone marrow-derived hMSCs for the treatment of patients after acute MI. The study met its primary objective demonstrating safety of intravenous infusion of allogeneic hMSCs during the infusion and during short- and longer-term follow-up. In addition, the results of this study provide provocative, preliminary indications that this therapy has clinical efficacy. In 4 specific areas of pre-specified safety monitoring—cardiac arrhythmias, pulmonary function, cardiac performance, and patient global symptomatic status—the treated patients exhibited significantly improved outcomes relative to the placebo group, consistent with a therapeutic benefit.
The present results advance the growing field of cell-based therapies for adult organ injury. Supported by findings in animal models, a number of clinical trials have been conducted using autologous BMCs administered by intracoronary infusion to patients after MI (3,6,21). While a full consensus is lacking (22), the totality of findings from these clinical trials supports the hypothesis that BMC infusion yields a small but significant increase in ejection fraction (2). Importantly, the REPAIR-AMI (Reinfusion of Enriched Progenitor Cells and Infarct Remodeling in Acute Myocardial Infarction) study also suggests that this therapeutic approach has clinical benefits, including a reduction in the composite of mortality, revascularization, and heart failure hospitalizations (3).
There are significant limitations to the development of BMCs as a therapy for patients with cardiac disease. First, the active cellular constituent of bone marrow that is the agent of repair is not well characterized. Second, it is widely estimated that therapeutically active bone marrow constituents likely represent only 1 in 10,000 bone marrow cells (9). Third, obtaining bone marrow requires an invasive procedure, and fourth, concerns exist that patients most likely to be affected by coronary atherosclerosis are also the most likely to have impaired bone marrow function (7).
The use of allogeneic hMSCs has a number of important advantages. They likely represent an enriched population of cells with therapeutic capacity. They are readily prepared from healthy donors and may be used as an allogeneic, and thus “off-the-shelf” agent. They are easy to administer, as evidenced by the intravenous approach used in this study. Finally, and most compelling, there are a wealth of preclinical data in rodent (23) and larger animal (24–29) models supporting their efficacy in cardiac repair.
This study assessed ejection fraction and left ventricular chamber volumes in hMSC-treated and placebo patients using echocardiography and, in a substudy, cardiac MRI. In the entire study group, ejection fraction increased at 3 months after therapy and remained elevated at 6 months. A similar increase was observed in the BOOST (Bone Marrow Transfer to Enhance ST-Elevation Infarct Regeneration) study, albeit at longer follow-up periods. In addition, the BOOST study also revealed a catch-up phenomenon similar to that observed in our echocardiographic study, in which ejection fraction in the placebo group eventually reaches the level seen in the cell-treated group (21). In the important subgroup of anterior MI patients, the increase in ejection fraction in the treated group was more evident relative to the placebo patients. Importantly, these data are provisional, but do suggest that future studies should focus attention on patients with larger infarctions.
When we used the more sensitive cardiac MRI technique in a subset of patients, only treated patients showed a significant increase in LVEF, which was sustained throughout 12 months of follow-up. In addition, cardiac MRI suggested that hMSC-treated patients exhibit evidence of favorable, reverse remodeling in contrast to placebo patients who exhibited progressive chamber enlargement. These differences were not significant, possibly due to the low number of test subjects, but the findings support a direct cardiac effect of intravenous hMSCs, with additional experimental work required to substantiate this concept (5). Concerns have been raised that murine MSCs cultured in excess of 8 passages develop reduced telomere length, develop chromosomal abnormalities, and induce tumors in animal models (11,30). To address this issue, the cells administered in this study were scrutinized for normal karyotype and were prepared with 5 passages. Extensive pre-clinical testing was performed, which failed to reveal tumor formation in cell-treated animals. Importantly, this study specifically incorporated chest, abdomen, and pelvic computed tomography scanning, and, during the study period, no evidence of ectopic tissue formation was observed. All patients in this study are also undergoing an additional 18 months of follow-up, 24 months total.
Pre-clinical studies indicate that a large proportion of infused cells initially distribute to the lungs after administration (31), raising potential concerns regarding compromised pulmonary function. The results of this study showed no evidence of a pulmonary safety risk after infusion of hMSCs. Instead, the data presented here reveal improved pulmonary function in the hMSC-treated patients, compared with baseline status. The timing of improvement in lung function, beginning shortly after administration, suggests a locally active beneficial hMSC-mediated effect on pulmonary airways.
This study extends the use of allogeneic hMSC therapy to patients with recent acute MI and is the first report of the use of these cells in that setting. Systemic delivery of allogeneic hMSCs has been evaluated previously and is currently being studied in patients with graft versus host disease (32), osteogenic imperfecta (33), and glycogen storage diseases (34).
While the present results provide reassurance regarding the safety of allogeneic hMSCs for cardiac disease, significant work is required to understand the mechanism of action of this approach. hMSCs are reported to differentiate into cardiac myocytes in vitro and, as such, may contribute to replacing lost myocytes after MI (35). hMSCs may also contribute to cardiac repair after MI through the release of locally acting factors (36), which could inhibit infarct scar expansion and stimulate vasculogenesis, cardiogenesis, and the recruitment of additional cells to the injured area. Additionally, hMSCs have the potential to stimulate endogenous healing by recreating cardiac stem cell niches (35,37,38). Work is underway to further delineate the mechanism of action of hMSC therapy after cardiac injury.
This study offers some potential clinical insights. As the primary goal of the study, potential safety concerns are alleviated by the present findings. Importantly, the intravenous infusion did not compromise, but appeared to improve lung function, and ectopic tissue formation did not occur. Finally, this work forms the basis for future clinical trials and mechanistic studies aimed at establishing the therapeutic possibility of infusing hMSCs post-acute MI to improve left ventricular function and reduce infarct-related injury. Should this effect be established, morbidity and/or mortality would be favorably impacted.
As this study was a clinical trial, we were unable to label the cells so as to ascertain the extent to which they trafficked to the heart. Some pre-clinical studies suggest that the level of cell retention in the heart after intravenous infusion is very low (24). There are also data that suggest recently infarcted tissue releases injury signals that facilitate the trafficking of cells to the damaged area (8). To the extent that minimal cells traffic to the heart, paracrine factors released from the cells may be pivotal in their mechanism of action (36,39). Future experimental work will be required to resolve the mechanistic issues surrounding the use of MSCs and, in particular, the role played by cell delivery route and cell retention within the heart.
Conclusions
This study was conducted to assess the safety of hMSCs after MI, and utilized a rigorous double-blind, placebo-controlled, dose-ranging study design. Importantly, the study met its primary objective and demonstrated safety both with regard to acute infusions of hMSCs as well as long-term absence of ectopic tissue formation. Specific safety monitoring indicated that cell-treated patients, in fact, had improved outcomes with regard to cardiac arrhythmias, pulmonary function, left ventricular function, and symptomatic global assessment. These findings support the conduct of more extensive studies assessing the value of allogeneic hMSCs for the treatment of cardiovascular disorders.
Supplementary Material
Acknowledgments
The investigators would like to thank those who agreed to participate in this trial and the study contributors, including members of the Data Safety and Monitoring Board (Online Appendix). Additionally, they would like to thank Michelle Williams and Candy Lee from Osiris Therapeutics Inc., who provided editorial support.
The study design was developed by Drs. Hare, Gerstenblith, and Schulman, and revised on the basis of discussions with the sponsor (Osiris Therapeutics, Inc., Baltimore, Maryland). The sponsor had no role in data collection, but participated in data analysis and interpretation. Drs. Hare, Gerstenblith, and Schulman also received support from the Johns Hopkins University School of Medicine General Clinical Research Center and National Institutes of Health Specialized Center for Cell-based Therapy (SCCT) grant U54 HL081028. Dr. DeMaria has received research funding/grants from Lantheus, Acusphere, CV Therapeutics, Cardiovascular Biotherapeutics, Angioblast Systems, Inc., Philips Medical Systems, and General Electric Medical Systems; and he has equity interests/stock options in Cardionet. Dr. Hermiller has served as a consultant for BSC. Stephen G. Ellis, MD, served as Guest Editor for this article.
Abbreviations and Acronyms
- AE
adverse event
- BMC
bone marrow mononuclear cell
- FEV1
forced expiratory volume in 1 s
- hMSC
human mesenchymal stem cell
- LVEF
left ventricular ejection fraction
- MI
myocardial infarction
- MRI
magnetic resonance imaging
- PVC
premature ventricular contraction
- VT
ventricular tachycardia
Footnotes
APPENDIX For a list of participating centers and members of the Data Safety and Monitoring Board, please see the online version of this article.
REFERENCES
- 1.Boyle AJ, Schulman SP, Hare JM. Is stem cell therapy ready for patients? Stem cell therapy for cardiac repair—ready for the next step. Circulation. 2006;114:339–52. doi: 10.1161/CIRCULATIONAHA.105.590653. [DOI] [PubMed] [Google Scholar]
- 2.Abdel-Latif A, Bolli R, Tleyjeh IM, et al. Adult bone marrow-derived cells for cardiac repair: a systematic review and meta-analysis. Arch Intern Med. 2007;167:989–97. doi: 10.1001/archinte.167.10.989. [DOI] [PubMed] [Google Scholar]
- 3.Schachinger V, Erbs S, Elsasser A, et al. Intracoronary bone marrow-derived progenitor cells in acute myocardial infarction. N Engl J Med. 2006;355:1210–21. doi: 10.1056/NEJMoa060186. [DOI] [PubMed] [Google Scholar]
- 4.van der LA, Hirsch A, Nijveldt R, et al. Bone marrow cell therapy after acute myocardial infarction: the HEBE trial in perspective, first results. Neth Heart J. 2008;16:436–9. doi: 10.1007/BF03086194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dill T, Schachinger V, Rolf A, et al. Intracoronary administration of bone marrow-derived progenitor cells improves left ventricular function in patients at risk for adverse remodeling after acute ST-segment elevation myocardial infarction: results of the Reinfusion of Enriched Progenitor cells And Infarct Remodeling in Acute Myocardial Infarction study (REPAIR-AMI) cardiac magnetic resonance imaging substudy. Am Heart J. 2009;157:541–7. doi: 10.1016/j.ahj.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 6.Schaefer A, Meyer GP, Fuchs M, et al. Impact of intracoronary bone marrow cell transfer on diastolic function in patients after acute myocardial infarction: results from the BOOST trial. Eur Heart J. 2006;27:929–35. doi: 10.1093/eurheartj/ehi817. [DOI] [PubMed] [Google Scholar]
- 7.Assmus B, Fischer-Rasokat U, Honold J, et al. Transcoronary transplantation of functionally competent BMCs is associated with a decrease in natriuretic peptide serum levels and improved survival of patients with chronic postinfarction heart failure: results of the TOPCARE-CHD registry. Circ Res. 2007;100:1234–41. doi: 10.1161/01.RES.0000264508.47717.6b. [DOI] [PubMed] [Google Scholar]
- 8.Schuleri KH, Boyle AJ, Hare JM. Mesenchymal stem cells for cardiac regenerative therapy. Handb Exp Pharmacol. 2007;180:195–218. doi: 10.1007/978-3-540-68976-8_9. [DOI] [PubMed] [Google Scholar]
- 9.Chamberlain G, Fox J, Ashton B, Middleton J. Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells. 2007;25:2739–49. doi: 10.1634/stemcells.2007-0197. [DOI] [PubMed] [Google Scholar]
- 10.Zhuang Y, Chen X, Xu M, Zhang LY, Xiang F. Chemokine stromal cell-derived factor 1/CXCL12 increases homing of mesenchymal stem cells to injured myocardium and neovascularization following myocardial infarction. Chin Med J (Engl) 2009;122:183–7. doi: 10.3901/jme.2009.07.183. [DOI] [PubMed] [Google Scholar]
- 11.Rubio D, Garcia-Castro J, Martin MC, et al. Spontaneous human adult stem cell transformation. Cancer Res. 2005;65:3035–9. doi: 10.1158/0008-5472.CAN-04-4194. [DOI] [PubMed] [Google Scholar]
- 12.Breitbach M, Bostani T, Roell W, et al. Potential risks of bone marrow cell transplantation into infarcted hearts. Blood. 2007;110:1362–9. doi: 10.1182/blood-2006-12-063412. [DOI] [PubMed] [Google Scholar]
- 13.Vulliet PR, Greeley M, Halloran SM, MacDonald KA, Kittleson MD. Intra-coronary arterial injection of mesenchymal stromal cells and microinfarction in dogs. Lancet. 2004;363:783–4. doi: 10.1016/S0140-6736(04)15695-X. [DOI] [PubMed] [Google Scholar]
- 14.The TIMI Study Group The Thrombolysis In Myocardial Infarction (TIMI) trial. Phase I findings. N Engl J Med. 1985;312:932–6. doi: 10.1056/NEJM198504043121437. [DOI] [PubMed] [Google Scholar]
- 15.Gibson CM, Cannon CP, Murphy SA, et al. Relationship of TIMI myocardial perfusion grade to mortality after administration of thrombolytic drugs. Circulation. 2000;101:125–30. doi: 10.1161/01.cir.101.2.125. [DOI] [PubMed] [Google Scholar]
- 16.Schiller NB, Shah PM, Crawford M, et al. Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two-Dimensional Echocardiograms 1. J Am Soc Echocardiogr. 1989;2:358–67. doi: 10.1016/s0894-7317(89)80014-8. [DOI] [PubMed] [Google Scholar]
- 17.Kwong RY, Schussheim AE, Rekhraj S, et al. Detecting acute coronary syndrome in the emergency department with cardiac magnetic resonance imaging. Circulation. 2003;107:531–7. doi: 10.1161/01.cir.0000047527.11221.29. [DOI] [PubMed] [Google Scholar]
- 18.Crapo RO, Morris AH, Gardner RM. Reference spirometric values using techniques and equipment that meet ATS recommendations. Am Rev Respir Dis. 1981;123:659–64. doi: 10.1164/arrd.1981.123.6.659. [DOI] [PubMed] [Google Scholar]
- 19.Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26:319–38. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 20.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166:111–7. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 21.Meyer GP, Wollert KC, Lotz J, et al. Intracoronary bone marrow cell transfer after myocardial infarction: eighteen months’ follow-up data from the randomized, controlled BOOST (BOne marrOw transfer to enhance ST-elevation infarct regeneration) trial. Circulation. 2006;113:1287–94. doi: 10.1161/CIRCULATIONAHA.105.575118. [DOI] [PubMed] [Google Scholar]
- 22.Bartunek J, Dimmeler S, Drexler H, et al. The consensus of the task force of the European Society of Cardiology concerning the clinical investigation of the use of autologous adult stem cells for repair of the heart. Eur Heart J. 2006;27:1338–40. doi: 10.1093/eurheartj/ehi793. [DOI] [PubMed] [Google Scholar]
- 23.Dai W, Hale SL, Martin BJ, et al. Allogeneic mesenchymal stem cell transplantation in postinfarcted rat myocardium: short- and long-term effects. Circulation. 2005;112:214–23. doi: 10.1161/CIRCULATIONAHA.104.527937. [DOI] [PubMed] [Google Scholar]
- 24.Freyman T, Polin G, Osman H, et al. A quantitative, randomized study evaluating three methods of mesenchymal stem cell delivery following myocardial infarction. Eur Heart J. 2006;27:1114–22. doi: 10.1093/eurheartj/ehi818. [DOI] [PubMed] [Google Scholar]
- 25.Kraitchman DL, Heldman AW, Atalar E, et al. In vivo magnetic resonance imaging of mesenchymal stem cells in myocardial infarction. Circulation. 2003;107:2290–3. doi: 10.1161/01.CIR.0000070931.62772.4E. [DOI] [PubMed] [Google Scholar]
- 26.Silva GV, Litovsky S, Assad JA, et al. Mesenchymal stem cells differentiate into an endothelial phenotype, enhance vascular density, and improve heart function in a canine chronic ischemia model. Circulation. 2005;111:150–6. doi: 10.1161/01.CIR.0000151812.86142.45. [DOI] [PubMed] [Google Scholar]
- 27.Amado LC, Schuleri KH, Saliaris AP, et al. Multimodality noninvasive imaging demonstrates in vivo cardiac regeneration after mesenchymal stem cell therapy. J Am Coll Cardiol. 2006;48:2116–24. doi: 10.1016/j.jacc.2006.06.073. [DOI] [PubMed] [Google Scholar]
- 28.Amado LC, Saliaris AP, Schuleri KH, et al. Cardiac repair with intra-myocardial injection of allogeneic mesenchymal stem cells following myocardial infarction. Proc Natl Acad Sci U S A. 2005;102:11474–9. doi: 10.1073/pnas.0504388102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Quevedo HC, Hatzistergos KE, Oskouei BN, et al. Allogeneic mesenchymal stem cells restore cardiac function in chronic ischemic cardiomyopathy via trilineage differentiating capacity. Proc Natl Acad Sci U S A. 2009;106:14022–7. doi: 10.1073/pnas.0903201106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Burns JS, Abdallah BM, Guldberg P, Rygaard J, Schroder HD, Kassem M. Tumorigenic heterogeneity in cancer stem cells evolved from long-term cultures of telomerase-immortalized human mesenchymal stem cells. Cancer Res. 2005;65:3126–35. doi: 10.1158/0008-5472.CAN-04-2218. [DOI] [PubMed] [Google Scholar]
- 31.Chin BB, Nakamoto Y, Bulte JW, Pittenger MF, Wahl R, Kraitchman DL. 111In oxine labelled mesenchymal stem cell SPECT after intravenous administration in myocardial infarction. Nucl Med Commun. 2003;24:1149–54. doi: 10.1097/00006231-200311000-00005. [DOI] [PubMed] [Google Scholar]
- 32.Ringden O, Uzunel M, Rasmusson I, et al. Mesenchymal stem cells for treatment of therapy-resistant graft-versus-host disease. Transplantation. 2006;81:1390–7. doi: 10.1097/01.tp.0000214462.63943.14. [DOI] [PubMed] [Google Scholar]
- 33.Horwitz EM, Gordon PL, Koo WK, et al. Isolated allogeneic bone marrow-derived mesenchymal cells engraft and stimulate growth in children with osteogenesis imperfecta: implications for cell therapy of bone. Proc Natl Acad Sci U S A. 2002;99:8932–7. doi: 10.1073/pnas.132252399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koc ON, Day J, Nieder M, Gerson SL, Lazarus HM, Krivit W. Allogeneic mesenchymal stem cell infusion for treatment of metachromatic leukodystrophy (MLD) and Hurler syndrome (MPS-IH) Bone Marrow Transplant. 2002;30:215–22. doi: 10.1038/sj.bmt.1703650. [DOI] [PubMed] [Google Scholar]
- 35.Mazhari R, Hare JM. Mechanisms of action of mesenchymal stem cells in cardiac repair: potential influences on the cardiac stem cell niche. Nat Clin Pract Cardiovasc Med. 2007;4(Suppl 1):S21–6. doi: 10.1038/ncpcardio0770. [DOI] [PubMed] [Google Scholar]
- 36.Mirotsou M, Zhang Z, Deb A, et al. Secreted frizzled related protein 2 (Sfrp2) is the key Akt-mesenchymal stem cell-released paracrine factor mediating myocardial survival and repair. Proc Natl Acad Sci U S A. 2007;104:1643–8. doi: 10.1073/pnas.0610024104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Anversa P, Kajstura J, Leri A, Bolli R. Life and death of cardiac stem cells: a paradigm shift in cardiac biology. Circulation. 2006;113:1451–63. doi: 10.1161/CIRCULATIONAHA.105.595181. [DOI] [PubMed] [Google Scholar]
- 38.Urbanek K, Cesselli D, Rota M, et al. Stem cell niches in the adult mouse heart. Proc Natl Acad Sci U S A. 2006;103:9226–31. doi: 10.1073/pnas.0600635103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gnecchi M, He H, Melo LG, et al. Early beneficial effects of bone marrow-derived mesenchymal stem cells overexpressing Akt on cardiac metabolism after myocardial infarction. Stem Cells. 2009;27:971–9. doi: 10.1002/stem.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.