Abstract
The HLA class II DRB1 antigen DR15 is an important prognostic marker in immune-mediated marrow failure states. DR15 has also been associated with favorable outcomes (reduced acute graft-versus-host disease [aGVHD] and relapse) after allogeneic hematopoietic cell transplant. To elucidate the impact of DR15 on transplantation outcomes, we conducted a retrospective study of 2891 recipients of first allogeneic stem cell transplant from HLA-matched sibling donors for the treatment of acute leukemia, chronic myeloid leukemia, or myelodysplastic syndrome (MDS) between 1990 and 2007. All patients received conventional myeloablative conditioning, T-replete grafts, and cyclosporine plus methotrexate-based GVHD prophylaxis. DNA-based HLA typing allowed categorization of 732 patients (25.3%) as positive and 2159 patients (74.7%) as negative for DRB1*15:01 or *15:02 (DR15). There were no significant differences in baseline characteristics between the HLA DR15 positive and negative groups. In univariate analysis, HLA-DR15 status had no impact on neutrophil engraftment, aGVHD, chronic GVHD (cGVHD), treatment-related mortality, relapse, disease-free survival, or overall survival (OS). In multivariate analysis, DR15 status showed no significant difference in aGVHD, cGVHD, OS, or relapse. In conclusion, DR15 status had no impact on major HLA-matched sibling donor hematopoietic cell transplant outcomes in this large and homogenous cohort of patients with leukemia and MDS.
Keywords: DR15, Hematopoietic stem cell transplantation (HSCT), GVHD, Survival, graft-versus-lymphoma
INTRODUCTION
DR15 (common alleles *15:01, *15:02) is a relatively common HLA class IIDRB1 antigen with a population frequency between 20% and 30% in various racial groups [1,2]. DR15 status has emerged as an important marker in immune-mediated marrow failure states such as severe aplastic anemia (SAA), myelodysplastic syndrome (MDS), and paroxysmal nocturnal hemoglobinuria. Serological HLA DR2, subsequently resolved as HLA DR15 (or HLA DR16, respectively) by molecular HLA typing, has been found at a higher frequency in patients with SAA from many racial groups [3–12]. Of the 30 different DR15 alleles, only *15:01 and *15:02 have been shown to have significantly higher frequencies in patients with SAA compared with the normal population. Diazepam-binding inhibitor-related protein-1, a candidate auto-antigen in SAA is presented by DR15 [13]. The presence of the DR15 antigen is not only overrepresented in marrow failure states, but also reported to predict response to immunosuppressive therapy, although this remains controversial [14–16].
Putative mechanistic explanations for the association of HLA DR15 with auto immune marrow failure states include the possibility that DR15 may present an immunodominant myeloid epitope. Both epitope presentation (mediating graft-versus-malignancy effects) and susceptibility to immunosuppressive therapy (reflecting the responsiveness of graft-versus-host disease [GVHD] to treatment) are themes that are commonly explored in allogeneic stem cell transplantation. The role of DR15 status in allogeneic stem cell transplantation was first examined in a cohort of 167 related and unrelated HLA-matched allogeneic transplants for myeloid malignancies [17,18]. HLA DR15 was identified either by molecular or serological methods. Patients with HLA DR15 experienced a significantly lower incidence of acute GVHD (aGVHD) grades II to IV: 23% versus 42% (P = .041) without any significant difference in chronic GVHD (cGVHD) incidence, progression-free survival, or overall survival (OS). In contrast, in a study of 192 HLA-identical sibling transplantations for acute or chronic leukemia or non-Hodgkin lymphoma, Stern et al. [19] showed that the presence of the DR15 antigen resulted in a higher estimated 5-year OS (76% versus 55%; P=.04). Improved survival for DR15 patients was due to a significant decrease in death from relapse (5% for DR15(+) versus 24% for DR15(−); P = .02), whereas no difference was seen for rates of transplantation-related mortality (19% and 21%, respectively; P = .76). Davidson et al. [20] also showed reduced relapse rates and improved OS (74% versus 54%; P = .01) in 286 adult recipients of HLA-matched sibling transplantations. The relevance of DR15 has been disputed by Newell et al. [21] who have shown in a study of 7950 patients with MDS and chronic myelogenous leukemia(CML) that TNF polymorphisms (in linkage disequilibrium with HLA-DR B1) affect transplant outcome in a disease-dependent manner.
Confirming that DR15 has a role in immunological outcomes would help further efforts to characterize immunodominant epitopes responsible for graft-versus-lymphoma effects, allow individualization of GVHD prophylaxis, and encourage studies to provide a mechanistic explanation for the unique immunological outcomes related to this specific DR antigen. Therefore, we conducted a retrospective analysis using a large database from the Center for International Blood and Marrow Transplant Research (CIBMTR) to further evaluate the prognostic implications of DR15 on major transplantation outcomes. The current study was confined to HLA-identical sibling transplantations to eliminate any HLA-disparity as a driving force for observed differences.
METHODS
Data Source
Patient, disease, transplantation characteristics, and outcome data reported to the CIBMTR were used for the current study. The CIBMTR is a voluntary working group of over 400 transplantation centers worldwide that contribute data on consecutive hematopoietic transplantations to a Statistical Center at the Medical College of Wisconsin. Computerized error checks, physician review of data, and on-site audits ensure data quality. All patients are followed longitudinally, annually. This study was approved by the Institutional Review Board of the Medical College of Wisconsin.
Inclusion Criteria
Patients with acute myeloid leukemia (AML), acute lymphocytic leukemia (ALL), MDS, or CML who underwent first myeloablative marrow or mobilized peripheral blood stem cell HLA-matched sibling donor transplantation between 1990 and 2007 were included in this analysis. In order to stringently evaluate the impact on GVHD, the study population excluded in vivo and ex vivo T cell depletion and those receiving prophylaxis other than cyclosporine plus methotrexate +/− other. Selection was confined to HLA-identical sibling donor transplantations using marrow or peripheral blood stem cells. To differentiate DR15 from DR16, the serological splits of DR2, we limited eligibility to patients for whom high-resolution (allele-level) typing at the HLA–DRB1 locus was available.
Outcomes
The study evaluated the impact of the DR15 on outcome with grades B-D and C-D aGVHD, cGVHD, relapse, and OS as the primary endpoints and neutrophil engraftment, treatment-related mortality (TRM), and disease-free survival (DFS) as secondary endpoints. The influence of age, disease (“lymphoid” versus “myeloid”), and graft source (marrow versus peripheral blood), which were outcomes specific to the two common alleles (*1501 and *1502), were also planned with secondary analyses to be performed in the event of a positive finding in a primary outcome measure. The incidence of grades B-D aGVHD was determined during the first 100 days after transplantation and was defined according to the CIBMTR scale [22]. Chronic GVHD was defined according to the Seattle criteria [23]. Relapse of malignancy was defined as reported to the CIBMTR by the transplantation center. OS considered death from any cause as the event, and surviving patients were censored at the date of last contact. Neutrophil engraftment was defined as achieving an absolute neutrophil count greater than 500 × 106/L for three consecutive measurements. DFS failure was defined as relapse or death from any cause with patients who were alive and in complete remission censored at the time of last follow-up. TRM was defined as death during a continuous complete remission. Events were summarized by the cumulative incidence estimate with relapse as a competing risk.
Statistical Analysis
Patient-related, disease-related, and transplantation-related factors were compared between the DR15(+) and the DR15(−) groups using the chi-square test for categorical variables and the Mann-Whitney U test for continuous variables. The probabilities of hematopoietic recovery and acute and chronic GVHD were estimated using the cumulative incidence function, treating death without the event as a competing risk. The probability of survival was calculated using the Kaplan-Meier estimator [24]. Risk factors for transplantation outcomes were identified through a stepwise forward/backward model selection procedure. Cox proportional hazard regression models were applied to evaluate the primary outcomes of OS, relapse, grades B-D and C-D aGVHD, and cGVHD. All variables were tested for the proportional hazards assumption. A backward and forward stepwise model building approach was used to stratify variables that violated the proportional hazards assumption. The variable for molecularly defined DR15 status (DR15[+] versus DR15[−]) was forced into the models in all steps of model building and the final model regardless of its statistical significance. Variables that attained a P value ≤.05 were held in the final multivariate models.
Other variables tested were: age (in decades), donor–recipient cytomegalovirus (CMV) serostatus (donor and recipient seronegative versus donor and/ or recipient seropositive), disease type (lymphoid versus myeloid), graft source (marrow versus peripheral blood), disease status (early, intermediate, advanced, other/unknown), performance score (≤80% versus ≥90%), race, and year of transplantation (1990–1993 versus 1994–1997 versus 1998–2001 versus 2002–2005 versus 2006–2007). All variables were tested to ensure they met the proportional hazard assumption. Variables that did not meet this assumption were adjusted by stratification. All P values are 2-sided. Analyses were performed using SAS version 9.1 (SAS Institute Inc., Cary, NC).
RESULTS
Patients and Characteristics
A total of 2891 recipients of HLA-matched sibling hematopoietic cell transplantations between 1990 and 2007 were included in this study. Among the study cohort, 732 patients (25.3%) were positive for DR15 (DR15[+]) and 2159 (74.7%) patients were negative for DR15 (DR15[−]). The characteristics of all patients are shown in Table 1. Sixty-seven patients were homozygous for DR15 and were combined with the DR15 heterozygous group. There were no significant differences in baseline transplantation characteristics by DR15 status apart from race and an excess of AML compared to CML in patients with DR15. Hardy-Weinberg equilibrium was evaluated for the distribution of the DR15 antigen within the study population. The observed distribution of DR15 homozygosity did not deviate from Hardy-Weinberg equilibrium (P = .32 in the white cohort), and frequencies were comparable to those observed in the major U.S. race/ethnicity populations by the National Marrow Donor Program [25].
Table 1.
Characteristics of Patients Receiving Myeloablative Transplant from HLA-Identical Sibling for AML, CML, ALL, or MDS, Reported to CIBMTR between 1990 and 2007
| Characteristics of Patients | DR15(+) | DR15(−) | P value |
|---|---|---|---|
| Patient-related | |||
| Number of patients | 732 | 2159 | |
| Number of centers | 156 | 207 | |
| Age at transplantation, median (range), years | 34 (1–66) | 34 (1–71) | .298 |
| Age in decades | .365 | ||
| 0–9 | 42 (6) | 162 (8) | |
| 10–19 | 121 (17) | 347 (16) | |
| 20–29 | 143 (20) | 389 (18) | |
| 30–39 | 153 (21) | 503 (23) | |
| 40–49 | 180 (25) | 490 (23) | |
| 50–59 | 93 (13) | 268 (12) | |
| Gender | .676 | ||
| Male | 418 (57) | 1217 (56) | |
| Female | 314 (43) | 940 (44) | |
| Missing | 0 | 2 (<1) | |
| Race | .006 | ||
| White | 594 (81) | 1729 (80) | |
| African American | 29 (4) | 45 (2) | |
| Asian/Pacific Islander | 53 (7) | 132 (6) | |
| Hispanic | 34 (5) | 161 (7) | |
| Native American | 2 (<1) | 13 (<1) | |
| Other | 13 (2) | 59 (3) | |
| Unknown/missing | 7 (<1) | 20 (<1) | |
| Karnofsky score | .989 | ||
| <90% | 156 (21) | 458 (21) | |
| ≥90% | 567 (77) | 1673 (77) | |
| Missing | 9 (1) | 28 (1) | |
| Disease-related | |||
| Disease | .001 | ||
| AML | 291 (40) | 747 (35) | |
| ALL | 184 (25) | 516 (24) | |
| CML | 198 (27) | 750 (35) | |
| MDS | 59 (8) | 146 (7) | |
| Disease status at transplantation* | .879 | ||
| Early | 453 (62) | 1361 (63) | |
| Intermediate | 140 (19) | 415 (19) | |
| Advanced | 132 (18) | 366 (17) | |
| Other/unknown | 7 (<1) | 17 (<1) | |
| Transplantation-related | |||
| Common conditioning drug combinations | .974 | ||
| TBI+Cy+/−other | 249 (34) | 742 (34) | |
| TBI+/−other (no Cy) | 54 (7) | 162 (8) | |
| Cy+Bu+/−other (no TBI) | 429 (59) | 1255 (58) | |
| DR sex match | .916 | ||
| M-M | 228 (31) | 679 (31) | |
| M-F | 162 (22) | 466 (22) | |
| F-M | 191 (26) | 537 (25) | |
| F-F | 149 (20) | 471 (22) | |
| Missing | 2 (<1) | 6 (<1) | |
| D-R CMV match | .318 | ||
| Neg-Neg | 361 (49) | 1084 (50) | |
| Neg-Pos | 66 (9) | 242 (11) | |
| Pos-Pos | 103 (14) | 278 (13) | |
| Pos-Neg | 183 (25) | 490 (23) | |
| Missing | 19 (3) | 65 (3) | |
| Graft type | .167 | ||
| Bone marrow | 399 (55) | 1240 (57) | |
| Peripheral blood | 333 (45) | 919 (43) | |
| Year of transplantation | .966 | ||
| 1990–1993 | 7 (<1) | 25 (1) | |
| 1994–1997 | 245 (33) | 705 (33) | |
| 1998–2001 | 243 (33) | 722 (33) | |
| 2002–2005 | 199 (27) | 584 (27) | |
| 2006–2007 | 38 (5) | 123 (6) | |
| GVHD prophylaxis | .966 | ||
| CSA+MTX+/−other | 732 | 2159 | |
| Median follow-up of survivors, mo | 74 (1–226) | 74 (1–220) | |
AML indicates acute myelogenous leukemia; CML, chronic myelogenous leukemia; ALL, acute lymphoblastic leukemia; MDS, myelodysplastic syndrome; CIBMTR, Center for International Blood and Marrow Transplant Research; TBI, total body irradiation; Cy, cyclophosphamide; Bu, busulfan; CMV, cytomegalovirus; GVHD, graft-versus-host disease; CSA, cyclosporine; MTX, methotrexate.
Disease status is categorized as follows:
Early = AML/ALL (CR1), CML (CP1), MDS (RA/RARS/pre-HCT marrow blasts <5%);
Intermediate = AML/ALL (≥CR2), CML (AP or ≥CP2);
Advanced = AML/ALL (REL/PIF), CML in BP, MDS (RAEB/RAEB-t/CMML or marrow blasts ≥5%);
Other/unknown = everything else.
Graft-versus-Host Disease
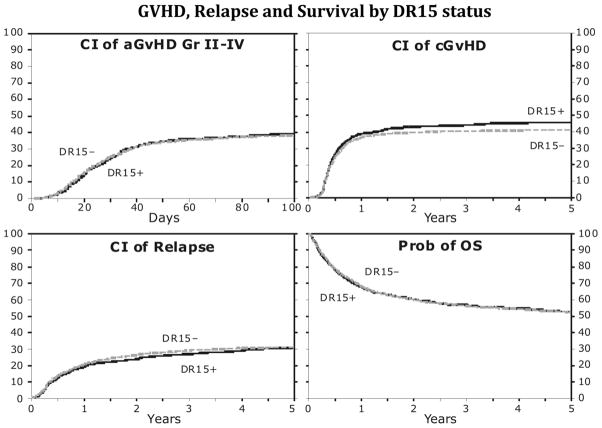
Univariate analysis showed that the probability of grades B-D aGVHD did not differ between the DR15 groups, with 39% and 38% (P = .414) at 100 days after transplantation for the DR15 positive and negative groups, respectively (Figure 1). Grade C-D aGVHD rates did not differ as well (P = .270 at 100 days). The rates of cGVHD at 1 and 3 years were similar between the DR15 groups, but there was a suggestion of a difference at 5 years, with 46% and 41% (P = .044) for the DR15 positive and negative groups, respectively (Figure 1).
Figure 1.
There was no statistically significant difference between the two groups in the cumulative incidences of aGVHD, cGVHD, relapse and overall survival. CI, cumulative incidence; aGVHD, acute graft-versus-host disease; cGVHD, chronic graft-versus-host disease; OS, overall survival.
Multivariate analysis found significant associations between grade B-D aGVHD and increasing patient age (P<.0001), CMV positive recipient (P = .0109), peripheral blood stem cell (PBSC) grafts (P<.0001), and Karnofsky performance score <90 (P = .0078). After adjusting for the significant covariates, no association was found based on the presence of DR15 (P = .782; Table 2). Statistically significant associations were found between grade C-D aGVHD and increasing patient age (P = .0008), PBSC grafts (P = .0003), Karnofsky performance score <90 (P = .0072), and transplantations before 1995 (P = .0038). After adjustment for the significant covariates, no association was found based on the presence or absence of DR15 (P = .548; Table 2). Chronic GVHD was significantly associated with increasing patient age (P < .0001), female donors (P < .0001), and transplantations before 1995 (P=.0136). After adjusting for the significant covariates, no association was found based on DR15 positivity (P = .276; Table 2). This suggests that the marginally significant difference detected at 5 years in the univariate analysis was due to uncontrolled confounding.
Table 2.
Multivariate Analysis Results
| Outcome | DR15(+)*
|
DR15(−)
|
P value |
|---|---|---|---|
| RR (95% CI) | |||
| Acute GVHD B-D† | 1.00 | 1.02 (0.89–1.16) | .782 |
| Acute GVHD C-D‡ | 1.00 | 0.94 (0.78–1.14) | .548 |
| Chronic GVHD§ | 1.00 | 0.93 (0.81–1.06) | .276 |
| Relapse|| | 1.00 | 1.04 (0.89–1.21) | .612 |
| Overall survival¶ | 1.00 | 1.05 (0.93–1.19) | .441 |
RR indicates relative risk; CI, confidence interval; GVHD, graft-versus-host disease.
Reference group.
Significant covariates: increasing patient age (P < .0001), cytomegalovirus (CMV) positive recipient (P = .0109), peripheral blood stem cell (PBSC) grafts (P < 0.0001), and Karnofsky performance score <90 (P =.0078).
Significant covariaties: increasing patient age (P =.0008), PBSC grafts (P =.0003), Karnofsky performance score <90 (P =.0072), and transplantations before 1995 (P =.0038).
Significant covariates: increasing patient age (P < .0001), female donors (P < .0001), and transplantations before 1995 (P =.0136).
Significant covariates: CMV positive recipients (P = .0392) and lymphoid disease (P =.0020).
Significant covariates: increasing patient age (P < .0001), CMV positive recipients (P=.0136), lymphoid disease (P < .0001), intermediate or advanced disease (P < .0001), PBSC (P =.0014), lower Karnofsky performance score (P < .0001), non-white patients (P = .0058), and transplantations before 1995 (P =.0145).
Relapse
The probability of relapse was not different between the 2 groups, with 31% and 32% relapse at 5 years (P = .944) in the DR15 positive and negative groups, respectively (Figure 1).
Multivariate analysis found significant associations between relapse rates and CMV positive recipients (P = .0392) and lymphoid disease (P = .0020). After adjusting for the significant covariates, no association was found between relapse and presence of DR15 (P = .612; Table 2).
Overall Survival
Similar probability of OS at 52% at 5 years was observed for both the DR15 positive and negative cohorts (Figure 1).
Multivariate analysis found significant associations between OS and increasing patient age (P < .0001), CMV positive recipients (P=.0136), lymphoid disease (P < .0001), intermediate or advanced disease (P < .0001), PBSC (P = .0014), lower Karnofsky performance score (P < .0001), non-white patients (P = .0058), and transplantations before 1995 (P = .0145). After adjusting for all significant covariates, no association was found between OS and the presence of DR15 (P = .441; Table 2).
A bias assessment was performed for included versus excluded cases based on the availability of molecular typing. This found no significant difference in the probability of OS at 1, 3, and 5 years posttransplantation, between the 2859 included and 3470 excluded cases. Any potential selection bias based on the availability of DRB1 molecular typing did not influence the outcomes of the study.
Engraftment
The probability of neutrophil engraftment did not differ between the groups, with 97% of patients engrafting by day 100 (P = .568) in both the DR15 positive and negative groups. Multivariate analyses were not performed on the secondary outcomes due to the lack of difference in the univariate analysis.
Treatment-Related Mortality
The probability of TRM did not differ between the groups, with 23% and 22% at 5 years (P = .677) in the DR15 positive and negative groups, respectively. Multivariate analyses were not performed for secondary outcomes due to the lack of a difference in the univariate analysis.
Disease-Free Survival
The probability of DFS did not differ between the groups, with 46% DFS at 5 years (P = .785) for both the DR15 positive and negative groups. Multivariate analyses were not performed on the secondary outcomes due to the lack of difference in the univariate analysis.
DISCUSSION
Previous studies have examined the role of DR15 in HLA-matched hematopoietic cell transplants with conflicting results. The current study used a large homogeneous dataset from the CIBMTR with differences in study populations, which could explain the discrepant observations. The studies by Battiwalla et al. [17,18] found that DR15-reduced aGVHD included unrelated donor transplantations but without high-resolution typing for all patients, a setting in which aGVHD could be more pronounced. The study by Stern et al. [19], which showed that DR15 presence was associated with reduced DFS, was confined to HLA-identical siblings but had more PBSC grafts, more advanced disease, inclusion of lymphomas, inclusion of reduced intensity conditioning, and diverse GVHD prophylaxis. The study by Davidson et al. [20], which also found an association between DFS and DR15, did not report multivariate analysis, but the study population was apparently very similar to the population studied by Stern.
Our study used careful selection criteria to avoid HLA-disparity, excluded low-resolution DRB1 typing, and defined a homogenous cohort of patients (myeloablative, T-lymphocyte replete transplantations with uniform cyclosporine plus methotrexate-based GVHD prophylaxis). In this setting, DR15 status did not account for significant differences in clinical outcomes such as survival, relapse, or GVHD. This was noted on univariate analysis and confirmed on multivariate analysis. Confining the univariate analysis to myeloid malignancies did not alter these findings. Multivariate analysis was restricted to the primary outcomes defined for the study: OS, relapse, grades B-D and C-D aGVHD, and cGVHD, due to lack of significance for any of the secondary outcomes in the univariate analysis.
Our study was confined to HLA-identical sibling transplantations, and the role, if any, of DR15 in unrelated or mismatched transplantations cannot be determined. Alternative explanations for immunobiologic outcomes related to DR15 need to be considered. DR15 is associated with the DRB1*1501-DRB5*0101-DQB1*0602 haplotype. Linkage disequilibrium with other non-HLA immunogenetic factors, such as TNF-α, could also account for immunological outcomes attributed to DR15 [21]. Although our findings reduce the prospect for DR15-based therapeutic intervention in the allogeneic transplantation setting, they do not diminish the influence of this unique HLA class II antigen in the nontransplantation setting for marrow failure states.
In conclusion, in a large dataset with a homogenous population, HLADR15 presence does not impact clinical outcomes in HLA-identical myeloablative allogeneic transplant for AML, ALL, MDS, or CML.
Footnotes
Disclaimer: The views expressed in this article do not reflect the official policy or position of the National Institute of Health, the Department of the Navy, the Department of Defense, or any other agency of the U.S. Government.
Authorship Statement: M.B., M.F., and S.S. designed the study and wrote the manuscript. K.E., P.L., and S.S. analyzed data and provided statistical input. P.S., G.A., P.H., T.K., R.M., B.S., M.D.A., M.C., W.D., B.G., T.H., N.K., M.L., A.L., W.S., M.A., A.U.I., and C.C. reviewed and provided critical input for the manuscript.
Financial disclosure: This work was supported by the National Institute of Health (NIH) intramural research programs of the NHLBI and NCI. CIBMTR is supported by Public Health Service Grant/Cooperative Agreement U24-CA76518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute(NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); a Grant/Cooperative Agreement 5U01HL069294 from NHLBI and NCI; a contract HHSH234200637015C with Health Resources and Services Administration (HRSA/DHHS); two grants N00014-10-1-0204 and N00014-1-1-0339 from the Office of Naval Research; and grants from Allos, Inc.; Amgen, Inc.; Angioblast; anonymous donation to the Medical College of Wisconsin; Ariad; Be the Match Foundation; Blue Cross and Blue Shield Association; Buchanan Family Foundation; Caridian BCT; Celgene Corporation; CellGenix, GmbH; Children’s Leukemia Research Association; Fresenius-Biotech North America, Inc.; Gamida Cell Teva Joint Venture Ltd.; Genentech, Inc.; Genzyme Corporation; GlaxoSmithKline; Kiadis Pharma; The Leukemia & Lymphoma Society; The Medical College of Wisconsin; Millennium Pharmaceuticals, Inc.; Milliman USA, Inc.; Miltenyi Biotec, Inc.; National Marrow Donor Program; Optum Health Care Solutions, Inc.; Otsuka America Pharmaceutical, Inc.; Seattle Genetics; Sigma-Tau Pharmaceuticals; Soligenix, Inc.; Swedish Orphan Biovitrum; THERAKOS, Inc.; and Wellpoint, Inc.
References
- 1.Mori M, Beatty PG, Graves M, Boucher KM, Milford EL. HLA gene and haplotype frequencies in the North American population: the National Marrow Donor Program Donor Registry. Transplantation. 1997;64:1017–1027. doi: 10.1097/00007890-199710150-00014. [DOI] [PubMed] [Google Scholar]
- 2.Middleton D, Menchaca L, Rood H, Komerofsky R. New allele frequency database. Tissue Antigens. 2003;61:403–407. doi: 10.1034/j.1399-0039.2003.00062.x. http://www.allelefrequencies.net. [DOI] [PubMed] [Google Scholar]
- 3.Rugman FP, Ashby D, Davies JM. Does HLA-DR predict response to specific immunosuppressive therapy in aplastic anaemia? Br J Haematol. 1990;74:545–546. doi: 10.1111/j.1365-2141.1990.tb06352.x. [DOI] [PubMed] [Google Scholar]
- 4.Nakao S, Yamaguchi M, Saito M, et al. HLA-DR2 predicts a favorable response to cyclosporine therapy in patients with bone marrow failure. Am J Hematol. 1992;40:239–240. doi: 10.1002/ajh.2830400319. [DOI] [PubMed] [Google Scholar]
- 5.Chapuis B, Von Fliedner VE, Jeannet M, et al. Increased frequency of DR2 in patients with aplastic anaemia and increased DR sharing in their parents. Br J Haematol. 1986;63:51–57. doi: 10.1111/j.1365-2141.1986.tb07494.x. [DOI] [PubMed] [Google Scholar]
- 6.Nakao S, Takamatsu H, Chuhjo T, et al. Identification of a specific HLA class II haplotype strongly associated with susceptibility to cyclosporine-dependent aplastic anemia. Blood. 1994;84:4257–4261. [PubMed] [Google Scholar]
- 7.Nimer SD, Ireland P, Meshkinpour A, Frane M. An increased HLA DR2 frequency is seen in aplastic anemia patients. Blood. 1994;84:923–927. [PubMed] [Google Scholar]
- 8.Saunthararajah Y, Nakamura R, Nam JM, et al. HLA-DR15 (DR2) is overrepresented in myelodysplastic syndrome and aplastic anemia and predicts a response to immunosuppression in myelodysplastic syndrome. Blood. 2002;100:1570–1574. [PubMed] [Google Scholar]
- 9.Marsh JC, Gordon-Smith EC. Insights into the autoimmune nature of aplastic anaemia. Lancet. 2004;364:308–309. doi: 10.1016/S0140-6736(04)16736-6. [DOI] [PubMed] [Google Scholar]
- 10.Song EY, Park S, Lee DS, Cho HI, Park MH. Association of human leukocyte antigen-DRB1 alleles with disease susceptibility and severity of aplastic anemia in Korean patients. Hum Immunol. 2008;69:354–359. doi: 10.1016/j.humimm.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 11.Shao W, Tian D, Liu C, Sun X, Zhang X. Aplastic anemia is associated with HLA-DRB1*1501 in northern Han Chinese. Int J Hematol. 2000;71:350–352. [PubMed] [Google Scholar]
- 12.Maciejewski JP, Follmann D, Nakamura R, et al. Increased frequency of HLA-DR2 in patients with paroxysmal nocturnal hemoglobinuria and the PNH/aplastic anemia syndrome. Blood. 2001;98:3513–3519. doi: 10.1182/blood.v98.13.3513. [DOI] [PubMed] [Google Scholar]
- 13.Feng X, Chuhjo T, Sugimori C, et al. Diazepam-binding inhibitor-related protein 1: a candidate autoantigen in acquired aplastic anemia patients harboring a minor population of paroxysmal nocturnal hemoglobinuria-type cells. Blood. 2004;104:2425–2431. doi: 10.1182/blood-2004-05-1839. [DOI] [PubMed] [Google Scholar]
- 14.Oguz FS, Yalman N, Diler AS, Oguz R, Anak S, Dorak MT. HLA-DRB1*15 and pediatric aplastic anemia. Haematologica. 2002;87:772–774. [PubMed] [Google Scholar]
- 15.Usman M, Adil SN, Moatter T, Bilwani F, Arian S, Khurshid M. Increased expression of HLA DR2 in acquired aplastic anemia and its impact on response to immunosuppressive therapy. J Pak Med Assoc. 2004;54:251–254. [PubMed] [Google Scholar]
- 16.Ilhan O, Beksaç M, Koç H, et al. HLA-DR frequency in Turkish aplastic anemia patients and the impact of HLA-DR2 positivity in response rate in patients receiving immunosuppressive therapy. Blood. 1995;86:2055. [PubMed] [Google Scholar]
- 17.Battiwalla M, Hahn T, Radovic M, et al. Human leukocyte antigen (HLA) DR15 is associated with reduced incidence of acute GVHD in HLA-matched allogeneic transplantation but does not impact chronic GVHD incidence. Blood. 2006;107:1970–1973. doi: 10.1182/blood-2005-05-1958. [DOI] [PubMed] [Google Scholar]
- 18.Battiwalla M, Hahn T, McCarthy PL., Jr HLA DR15 and immunobiologic outcomes. Biol Blood Marrow Transplant. 2007;13:371. doi: 10.1016/j.bbmt.2006.12.439. [DOI] [PubMed] [Google Scholar]
- 19.Stern M, Passweg J, Tiercy JM, et al. Human leukocyte antigen DR15 is associated with reduced relapse rate and improved survival after human leukocyte antigen-identical sibling hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2006;12:1169–1175. doi: 10.1016/j.bbmt.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 20.Davidson JA, Tate DG, Poulton KV, et al. HLA-DR15, reduced relapse rate and improved survival after HLA identical sibling hemopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2007;13:493–494. doi: 10.1016/j.bbmt.2006.12.442. [DOI] [PubMed] [Google Scholar]
- 21.Newell LF, Gooley T, Hansen JA, Stirewalt DL, Petersdorf EW, Deeg HJ. Tumor necrosis factor polymorphism affects transplantation outcome in patients with myelodysplastic syndrome but not in those with chronic myelogenous leukemia, independent of the presence of HLA-DR15. Biol Blood Marrow Transplant. 2010;16:1700–1706. doi: 10.1016/j.bbmt.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rowlings PA, Przepiorka D, Klein JP, et al. IBMTR Severity Index for grading acute graft-versus-host disease: retrospective comparison with Glucksberg grade. Br J Haematol. 1997;97:855–864. doi: 10.1046/j.1365-2141.1997.1112925.x. [DOI] [PubMed] [Google Scholar]
- 23.Shulman HM, Sullivan KM, Weiden PL, et al. Chronic graft-versus-host syndrome in man. A long-term clinicopathologic study of 20 Seattle patients. Am J Med. 1980;69:204–217. doi: 10.1016/0002-9343(80)90380-0. [DOI] [PubMed] [Google Scholar]
- 24.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Amer Statist Assn. 1958;53:457–481. [Google Scholar]
- 25.Maiers M, Gragert L, Klitz W. High-resolution HLA alleles and haplotypes in the United States population. Hum Immunol. 2007;68:779–788. doi: 10.1016/j.humimm.2007.04.005. [DOI] [PubMed] [Google Scholar]