Abstract
A novel mechanism has been defined for controlling PPARγn activity in response to thiazolidinedione ligands, in which deacetylation of PPARγ by SirT1 remodels the transcriptional complex. This change favors expression of genes associated with increased energy utilization and insulin sensitization in white adipose tissue, and is required for a portion of the beneficial effects of thiazolidinediones. More broadly, PPARγ acetylation and other recently identified regulatory modifications are clarifying the mechanisms by which thiazolidinediones exert their antidiabetic effects in fat cells and other tissues.
PPARγ is a ligand activated transcription factor first studied for its importance in adipogenesis. Since then, it has become recognized that PPARγ also mediates diverse effects in non-adipose tissues including liver, skeletal muscle, brain, bone, and blood vessels. PPARγ is thought to be a fatty acid and lipid sensor, which when activated can stimulate expression of genes that promote insulin action, fatty acid storage and glucose metabolism. Other beneficial effects of PPARγ activity may be garnered by inhibition of pro-inflammatory and pro-oxidant gene expression. The therapeutic importance of PPARγ in providing glycemic control is underscored by the actions of the thiazolidinedione (TZD) class of drugs which can markedly improve insulin sensitivity in patients with type 2 diabetes. The beneficial actions of TZDs are thought to be centered on their ability to act as high affinity ligands for PPARγ, where they act in place of endogenous PPARγ ligands to functionally convert PPARγ from a transcriptional repressor to a transcriptional activator. This transition occurs by the disassociation of a corepressor, NCoR, from the PPARγ complex and its replacement by an activator complex. Since the discovery that TZD drugs have potent antidiabetic activity, the mechanism through which they exert their beneficial effects has held significant clinical importance.
In addition to the classic model of ligand-mediated activation, PPARγ has been recently shown to be regulated by a series of post-translational modifications, many of which can be effected by TZDs. One such modification is sumoylation, which has been reported to stabilize the association of PPARγ with the corepressor complex and inhibit PPARγ transcriptional activity.1 In white adipose tissue (WAT), TZDs promote PPARγ transcriptional activity in part by promoting expression of fibroblast growth factor 21 (FGF21), a PPARγ target gene, which acts in a feed-forward mechanism to inhibit PPARγ sumoylation.2 Rather than simply promoting general activating or repressing activities of PPARγ, other modifications have since been identified which regulate subsets of PPARγ target genes. This is exemplified by the effects of cyclin-dependent kinase 5 (CDK5)-mediated phosphorylation at serine 273 (S273) of PPARγ, which results in decreased expression of a small set of PPARγ targets including adiponectin3, an insulin sensitizing adipokine. Importantly, S273 phosphorylation is promoted by factors associated with obesity and high fat diet (free fatty acids and inflammatory cytokines) and inhibited by the binding of TZDs and other novel non-agonist PPARγ ligands.3, 4 Such activities could at least partly account for the antidiabetic effects of TZDs. Now, in the August 3, 2012 issue of Cell, work by Qiang et al. has defined a distinct mechanism for PPARγ regulation (see Figure), whereby its acetylation differentially affects the expression of genes that control energy expenditure in adipocytes and controls the “browning” of subcutaneous inguinal WAT.5
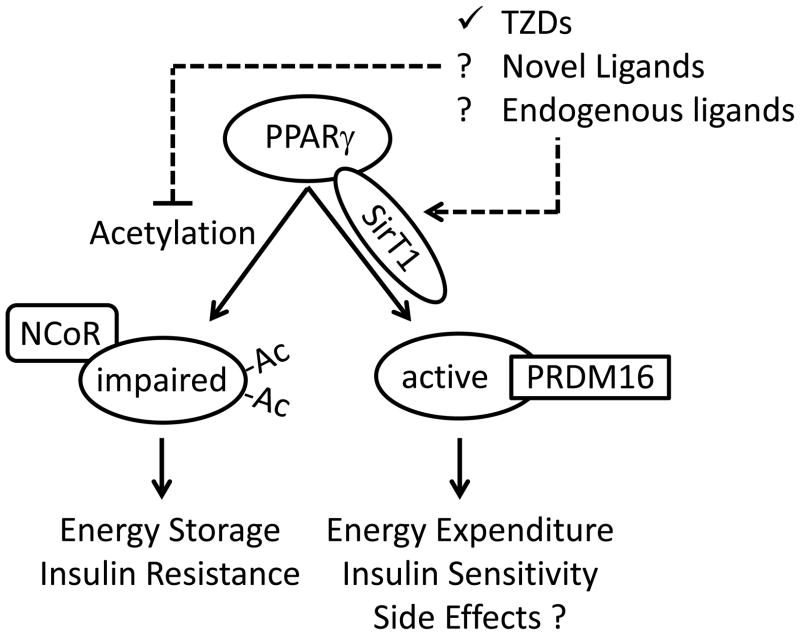
Figure.
Post-translational modified of PPARγ by acetylation impairs its transcriptional activity. In the current report, acetylation promotes energy storage through maintenance of the white adipocyte phenotype and gene expression program, and in response to high fat diets may promote insulin resistance. TZDs increase the association of PPARγ with SirT1 thus promoting deacetylation of PPARγ. Deacetylation of PPARγ by SirT1 or mutation of the acetylated lysines in PPARγ promotes disassociation of the co-repressor NCoR and association with PRDM16. This promotes browning of subcutaneous white adipocytes by stimulating them to adopt a brown adipose tissue gene expression profile, resulting in increased energy expenditure in WAT and insulin sensitivity. However, increased PPARγ activity promoted by TZDs also causes unwanted side effects. It remains unclear if endogenous ligands or new non-agonist PPARγ ligands can prevent PPARγ acetylation, or if deacetylation of PPARγ contributes to side effects caused by TZDs.
Adipose tissue includes two functionally distinct subtypes. White adipocytes provide a repository for energy storage, whereas brown adipocytes are characterized by high mitochondrial density and uncoupling protein activity which makes these cells effective at dissipating energy as heat. While white and brown adipocytes are functionally distinct, some white adipocytes in certain depots can be induced to take on a brown-like phenotype (i.e. browning). Notably, UCP1 expression is much higher in inguinal WAT than in visceral WAT making the inguinal WAT depot more susceptible to taking on a brown-like phenotype.6 Brown-like white adipocytes exhibit increased expression of genes characteristic of brown adipose tissue (BAT) with coincidently decreased expression of genes characteristic of WAT. Mechanistic hints on how this may occur are suggested by the recent identification of white adipocytes (so called beige adipocytes) that respond to cyclic AMP stimulation by increasing UCP1 expression and respiration rates while exhibiting a gene expression profile distinct from both white and brown adipocytes.7 This change in WAT promotes energy expenditure, a process that could potentially be beneficial in diet-induced obesity. Moreover, decreased expression of some WAT genes during browning includes genes associated with insulin resistance. The ability of TZDs to promote browning of WAT and decrease expression of genes associated with insulin resistance suggests that this effect may contribute to the antidiabetic activity of these drugs.8, 9
Insulin sensitizing effects consistent with the browning of white adipocytes have also been linked with increases in the deacetylating activity SirT1, an Nad+-dependent deacetylase.10, 11 Factors that induce SirT1 activity include calorie restriction and exercise, suggesting that SirT1 might serve as an energy state sensor and may promote insulin sensitizing effects.12 Recognizing that TZDs and activators of SirT1 have similar effects on insulin sensitization and browning of white adipocytes, the current study hypothesized that SirT1 might be mediating these effects by regulating PPARγ activity. Consistent with this hypothesis, they found that SirT1 associates with PPARγ and deacetylates two specific lysines, K268 and K293. Importantly, the SirT1:PPARγ association was ligand dependent as SirT1 failed to interact with a non-ligand binding mutant of PPARγ (P467L), and P467L mutant PPARγ was hyperacetylated when expressed in adipocytes. These data suggest that SirT1 acts as a ligand-dependent PPARγ deacetylase. This model was further supported through demonstration of reduced PPARγ acetylation in vivo using three genetically distinct models: a) mice overexpressing SirT1, b) mice lacking expression of a negative regulator of SirT1 (Dbc1), and c) wild type mice treated with a SirT1 activator (resveratrol). Notably, PPARγ acetylation was increased and association with SirT1 was decreased in the inguinal WAT of mice fed a high fat diet, whereas, the acetylation of PPARγ was reduced in high fat diet fed mice that were treated with a TZD (rosiglitazone) or in response to increased SirT1 expression and activity. Importantly, PPARγ acetylation was reduced in pieces of human subcutaneous adipose tissue that were incubated with resveratrol. Therefore, SirT1-mediated PPARγ deacetylation could represent a regulatory modification promoting insulin sensitization in response to intrinsic (endogenous ligands) or extrinsic factors (such as TZDs). It is notable that human subjects bearing the P467L mutation in PPARγ exhibit early onset type II diabetes, insulin resistance and hypertension13 and mice carrying the equivalent mutation are susceptible to both insulin resistance and hypertension.14, 15 Whether this is due to hyperacetylation of the mutant (or wildtype) PPARγ remains to be determined.
The physiological significance of PPARγ deacetylation was investigated with regard to the browning effects on white adipocytes mediated by TZDs and SirT1 activity. Factors that increased SirT1 activity and the deacetylation of PPARγ led to an increase in expression of genes typical of BAT and a concomitant decrease in expression of genes characteristic of WAT, whereas the converse was true of factors that reduced SirT1 activity. Importantly, BAT development and function was not grossly altered in Sirt1−/− mice, suggesting that it does not play a unique role in this tissue. Rather, SirT1-dependent deacetylation of PPARγ correlated with a more brown-like phenotype of subcutaneous WAT induced by cold-exposure in mice lacking Dbc1 or in mice over-expressing SirT1, essentially replicating the browning effect that TZDs had previously been shown to induce in WAT.
That these SirT1-dependent effects were mediated through modification of PPARγ was strikingly demonstrated using PPARγ mutants that could not be acetylated (2KR) or which mimicked constitutive acetylation (KQ). Like the effects of reduced SirT1 activity, the constitutive acetylation mutant suppressed expression of BAT genes, markedly blunted the induction of BAT genes by rosiglitazone, and enhanced expression of WAT genes. Conversely, the non-acetylated mutant caused enhanced expression of BAT-like genes with greater potency than did wild type PPARγ. Thus, acetylated PPARγ enforces a white adipocyte (energy storage) phenotype, whereas PPARγ in its deacetylated state promotes a browning (energy utilization) effect on subcutaneous white adipocytes.
How does deacetylation of PPARγ affect its transcriptional activity? Ultimately, two changes in PPARγ complex formation provide likely explanations. First, mutation of either K268 or K293 disrupted association between PPARγ and its co-repressor, NCoR, suggesting that deacetylation may contribute to a switch in PPARγ activity from transcriptional repression toward transcriptional activation. Second, factors that reduced PPARγ acetylation (including TZD treatment) increased PPARγ association with Prdm16, a coactivator that was previously shown to promote browning in WAT.16 Indeed, the non-acetylated PPARγ mutant associated with Prdm16 in the absence of the deacetylating factors normally needed to promote Prdm16 association with wild type PPARγ. The authors propose that deacetylation of PPARγ by SirT1 leads to the selective association of PPARγ with PRDM16, thus promoting a brown-like profile of gene expression. Although the data are consistent with this hypothesis, the authors did not directly test whether the brown-like phenotype resulting from PPARγ deacetylation was PRDM16-dependent.
Another relevant question is how PPARγ acetylation relates to other mechanisms regulating its function. A potential relationship between acetylation and S273 phosphorylation is suggested by molecular modeling which places K268 and K298 proximal to the cleft containing the CDK5 phosphorylation site at S273. Indeed, Qiang et al found that a K293Q mutation enhanced S273 phosphorylation of PPARγ, suggesting that acetylation at this site may favor increased phosphorylation at S273, thus reinforcing impaired PPARγ activity. Despite this correlation and the observation that deacetylation and lack of S273 phosphorylation are both promoted by TZDs, it is presently unclear if there may be settings were these sites are differently affected by their cognate regulators (SirT1 and CDK5, respectively). However, some similarity in the outcome of these activities is suggested by the observation that both the non-acetylated PPARγ mutant and the non-phosphorylated S273A PPARγ mutant each promote increased adiponectin expression compared to wild type PPARγ. By contrast, expression of several brown adipocyte genes enhanced by the non-acetylated PPARγ mutant were not affected by the S273A mutation of PPARγ, suggesting that at least in white adipocytes post-translational modification of these sites may have differential effects on PPARγ-mediated gene expression. Further studies will be necessary to fully understand the range of post-translational modifications that control insulin-sensitivity by PPARγ.
Since SirT1-mediated deacetylation of PPARγ is dependent on ligand binding, it is reasonable to hypothesize that at least some effects of endogenous or exogenous (TZD) ligands may derive from their ability to promote PPARγ deacetylation. But many questions remain. Will the same effects on PPARγ acetylation be preserved with a new class of PPARγ ligands such as SR1664 which retain many beneficial effects of TZDs?4 It will also be important to determine if post-translational modifications of PPARγ regulate its activity outside of adipose tissue. For example, TZDs exert beneficial effects, including improved vascular function and lower arterial pressure, which appear to be at least partially independent of improved glycemic control. In this regard, high fat diet-induced vascular dysfunction and hypertension results from expression of PPARγ mutants like P467L specifically in vascular endothelium or vascular smooth muscle.17–20 It will now be important to determine if P467L PPARγ is hyperacetylated in vascular tissue as it is in adipocytes, and whether the status of this and other post-translational modifications affect PPARγ-dependent phenotypes in non-adipocytes.
Finally, TZDs are now recognized to produce rare but significant adverse effects, including heart failure, bone fracture, and to a lesser degree, heart attack or bladder cancer. Importantly, these unwanted effects are largely responsible for the decline in the rate of new TZD prescriptions. This further establishes the need to define the spectrum of regulatory activities, including post-translational modifications such as sumoylation, phosphorylation, and acetylation that are affected by TZDs and the next generation of PPARγ ligands in the hope that those governing their beneficial effects may be discretely targeted. Although there is still much to be learned, these novel modes of PPARγ regulation clearly represent fertile ground to explore in the effort to identify new antidiabetic drugs with improved safety profiles.
Acknowledgments
Funding:
NIH grants HL048058, HL061446, HL062984, HL084207 to CDS, and from the Johnson Scholars Program at the University of Iowa to FWQ. The authors also gratefully acknowledge the generous research support of the Roy J. Carver Trust.
References
- 1.Floyd ZE, Stephens JM. Control of peroxisome proliferator-activated receptor gamma2 stability and activity by SUMOylation. Obes Res. 2004;12:921–928. doi: 10.1038/oby.2004.112. [DOI] [PubMed] [Google Scholar]
- 2.Dutchak PA, Katafuchi T, Bookout AL, Choi JH, Yu RT, Mangelsdorf DJ, Kliewer SA. Fibroblast growth factor-21 regulates PPARγ activity and the antidiabetic actions of thiazolidinediones. Cell. 2012;148:556–567. doi: 10.1016/j.cell.2011.11.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Choi JH, Banks AS, Estall JL, Kajimura S, Bostrom P, Laznik D, Ruas JL, Chalmers MJ, Kamenecka TM, Bluher M, Griffin PR, Spiegelman BM. Anti-diabetic drugs inhibit obesity-linked phosphorylation of PPARγ by Cdk5. Nature. 2010;466:451–456. doi: 10.1038/nature09291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choi JH, Banks AS, Kamenecka TM, Busby SA, Chalmers MJ, Kumar N, Kuruvilla DS, Shin Y, He Y, Bruning JB, Marciano DP, Cameron MD, Laznik D, Jurczak MJ, Schurer SC, Vidovic D, Shulman GI, Spiegelman BM, Griffin PR. Antidiabetic actions of a non-agonist PPARγ ligand blocking Cdk5-mediated phosphorylation. Nature. 2011;477:477–481. doi: 10.1038/nature10383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qiang L, Wang L, Kon N, Zhao W, Lee S, Zhang Y, Rosenbaum M, Zhao Y, Gu W, Farmer SR, Accili D. Brown Remodeling of White Adipose Tissue by SirT1-Dependent Deacetylation of Pparγ. Cell. 2012;150:620–632. doi: 10.1016/j.cell.2012.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cousin B, Cinti S, Morroni M, Raimbault S, Ricquier D, Penicaud L, Casteilla L. Occurrence of brown adipocytes in rat white adipose tissue: molecular and morphological characterization. J Cell Sci. 1992;103:931–942. doi: 10.1242/jcs.103.4.931. [DOI] [PubMed] [Google Scholar]
- 7.Wu J, Bostrom P, Sparks LM, Ye L, Choi JH, Giang AH, Khandekar M, Virtanen KA, Nuutila P, Schaart G, Huang K, Tu H, van Marken Lichtenbelt WD, Hoeks J, Enerback S, Schrauwen P, Spiegelman BM. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell. 2012;150:366–376. doi: 10.1016/j.cell.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vernochet C, Peres SB, Davis KE, McDonald ME, Qiang L, Wang H, Scherer PE, Farmer SR. C/EBPα and the corepressors CtBP1 and CtBP2 regulate repression of select visceral white adipose genes during induction of the brown phenotype in white adipocytes by peroxisome proliferator-activated receptor gamma agonists. Mol Cell Biol. 2009;29:4714–4728. doi: 10.1128/MCB.01899-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilson-Fritch L, Nicoloro S, Chouinard M, Lazar MA, Chui PC, Leszyk J, Straubhaar J, Czech MP, Corvera S. Mitochondrial remodeling in adipose tissue associated with obesity and treatment with rosiglitazone. J Clin Invest. 2004;114:1281–1289. doi: 10.1172/JCI21752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Banks AS, Kon N, Knight C, Matsumoto M, Gutierrez-Juarez R, Rossetti L, Gu W, Accili D. SirT1 gain of function increases energy efficiency and prevents diabetes in mice. Cell Metab. 2008;8:333–341. doi: 10.1016/j.cmet.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Timmers S, Konings E, Bilet L, Houtkooper RH, van de Weijer T, Goossens GH, Hoeks J, van der Krieken S, Ryu D, Kersten S, Moonen-Kornips E, Hesselink MK, Kunz I, Schrauwen-Hinderling VB, Blaak EE, Auwerx J, Schrauwen P. Calorie restriction-like effects of 30 days of resveratrol supplementation on energy metabolism and metabolic profile in obese humans. Cell Metab. 2011;14:612–622. doi: 10.1016/j.cmet.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Milne JC, Lambert PD, Schenk S, Carney DP, Smith JJ, Gagne DJ, Jin L, Boss O, Perni RB, Vu CB, Bemis JE, Xie R, Disch JS, Ng PY, Nunes JJ, Lynch AV, Yang H, Galonek H, Israelian K, Choy W, Iffland A, Lavu S, Medvedik O, Sinclair DA, Olefsky JM, Jirousek MR, Elliott PJ, Westphal CH. Small molecule activators of SIRT1 as therapeutics for the treatment of type 2 diabetes. Nature. 2007;450:712–716. doi: 10.1038/nature06261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barroso I, Gurnell M, Crowley VE, Agostini M, Schwabe JW, Soos MA, Maslen GL, Williams TD, Lewis H, Schafer AJ, Chatterjee VK, O’Rahilly S. Dominant negative mutations in human PPARγ associated with severe insulin resistance, diabetes mellitus and hypertension. Nature. 1999;402:880–883. doi: 10.1038/47254. [DOI] [PubMed] [Google Scholar]
- 14.Tsai YS, Kim HJ, Takahashi N, Kim HS, Hagaman JR, Kim JK, Maeda N. Hypertension and abnormal fat distribution but not insulin resistance in mice with P465L PPARγ. J Clin Invest. 2004;114:240–249. doi: 10.1172/JCI20964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gray SL, Nora ED, Grosse J, Manieri M, Stoeger T, Medina-Gomez G, Burling K, Wattler S, Russ A, Yeo GS, Chatterjee VK, O’Rahilly S, Voshol PJ, Cinti S, Vidal-Puig A. Leptin deficiency unmasks the deleterious effects of impaired peroxisome proliferator-activated receptor gamma function (P465L PPARγ) in mice. Diabetes. 2006;55:2669–2677. doi: 10.2337/db06-0389. [DOI] [PubMed] [Google Scholar]
- 16.Seale P, Conroe HM, Estall J, Kajimura S, Frontini A, Ishibashi J, Cohen P, Cinti S, Spiegelman BM. Prdm16 determines the thermogenic program of subcutaneous white adipose tissue in mice. J Clin Invest. 2011;121:96–105. doi: 10.1172/JCI44271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beyer AM, de Lange WJ, Halabi CM, Modrick ML, Keen HL, Faraci FM, Sigmund CD. Endothelium-specific interference with peroxisome proliferator activated receptor gamma causes cerebral vascular dysfunction in response to a high-fat diet. Circ Res. 2008;103:654–661. doi: 10.1161/CIRCRESAHA.108.176339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Halabi CM, Beyer AM, de Lange WJ, Keen HL, Baumbach GL, Faraci FM, Sigmund CD. Interference with PPARγ Function in Smooth Muscle Causes Vascular Dysfunction and Hypertension. Cell Metabolism. 2008;7:215–226. doi: 10.1016/j.cmet.2007.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ketsawatsomkron P, Lorca RA, Keen HL, Weatherford ET, Liu X, Pelham CJ, Grobe JL, Faraci FM, England SK, Sigmund CD. PPARgamma Regulates Resistance Vessel Tone Through a Mechanism Involving RGS5-Mediated Control of PKC and BKCa Channel Activity. Circ Res. 2012 Sep 7; doi: 10.1161/CIRCRESAHA.112.271577. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pelham CJ, Ketsawatsomkron P, Groh S, Grobe JL, de Lange WJ, Ibeawuchi S-RC, Keen HL, Weatherford ET, Faraci FM, Sigmund CD. Cullin-3 Regulates Vascular Smooth muscle Function and Aterial Pressure via PPARγ and RhoA-Rho-Kinase. Cell Metabolism. 2012;16:462–472. doi: 10.1016/j.cmet.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]